
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 26 October 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1043809
This article is part of the Research Topic Quality and Nutrition of Meat and Meat Products: Emphasis on Muscle Protein Structure, Activity, Modification, and Functionality View all 5 articles
Along with the future food market developing world widely, the personalized nutrition and rational function food design are found to be urgently attracted. Oil in a water (O/W) emulsion system has an excellent ability to maintain nutraceuticals and thus plays a promising role in producing future functional foods. Understanding the interfacial related mechanisms involved are essential for improving the quality of food products. Protein can effectively reduce interfacial tension and stable immiscible phases. The interfacial properties of proteins directly affect the emulsion qualities, which have gradually become a prospective topic. This review will first briefly discuss the interfacial-related fundamental factors of proteins. Next, the paper thoroughly overviewed current physical and chemical strategies tailored to improving the interfacial and emulsion properties of proteins. To be summarized, a higher flexibility could allow protein to be more easily unfolded and adsorbed onto the interface but could also possibly form a softer interfacial film. Several physical strategies, such as thermal, ultrasound and especially high-pressure homogenization are well applied to improve the interfacial properties. The interfacial behavior is also altered by various green chemical strategies, such as pH adjustment, covalent modification, and low molecular weight (LMW) surfactant addition. These strategies upgraded emulsion properties by increasing adsorption load, accelerating diffusion and adsorption rate, associated with lowering interfacial tension, and promoting interfacial protein interactions. Future researches targeted at elucidating interfacial-bulk protein interactions, unraveling interfacial behavior through in silico tools, exploring connection between interfacial-industrial processing properties, and clarifying the interfacial-sensory-digestive relationships of O/W emulsions is needed to develop emulsion applications.
Emulsion-based colloidal systems are ubiquitous in the food sector and show great potential in protecting and transporting nutraceuticals, enhancing the bioaccessibility of lipophilic bioactive substances and creating health-promoting functional foods (1). Along with the widespread development of the food market worldwide, personalized nutrition and functional food design are urgently needed; thus, emulsion-type food products attract increasing attention (2). It has been indicated that emulsion-type products are suitable for producing 3D print ink (3), biofilms (4), or personalized functional foods (5). Since the quality of emulsion-type products are closely related to emulsifiers’ interfacial behavior, in the food field, oil in water (O/W) emulsion-based interface science has been prospective and thriving in recent years.
The O/W emulsion is a metastable and thermodynamic system consisted with mainly three components, including oil, water and emulsifier. During emulsification, the lipid phase dispersed in an immiscible continuous aqueous phase driven by mechanical shear forces. To maintain the stability, it is necessary for emulsifiers to optimally cover the O/W interface and reduce the interfacial tension (6). As one kind of the major emulsifiers in food systems, proteins play a great role in stabilizing emulsion droplets by typically three steps: 1. diffusing from bulk aqueous toward the O/W interface; 2. after reaching the interface, proteins rearranging their native structure to orient the hydrophobic segments toward the non-aqueous phase, which was hidden inside the center of the coil structure; and 3. these adsorbed proteins tended to interact with neighboring molecules to form a quasi-two-dimensional network with elasticity and viscosity, which could prevent emulsion destabilization such as coalescence or flocculation afterward (7).
As mentioned, the formation of an interfacial layer depends on a complex phenomenon resulted from protein structural rearrangement, denaturation, disulfide bridge formation and intermolecular entanglement (8). The interaction among adsorbed protein layer, which also leads to a higher interfacial elastic modulus, is considered a predominant parameter in inhibiting coalescence and maintaining the stabilization of oil droplets (9, 10). One has concluded that the relationship between mesoscopic-level interfacial properties and macroscopic-level emulsion stability is not always straightforward (11). For example, no direct correlation was indicated between interfacial elasticity and emulsion stability (12). However, the interfacial behavior provides valuable information on emulsion quality. It should also be noted that both interfacial rheology and droplet interaction contributed to the bulk response (13).
Despite a few classic historical studies, this review mostly focused on works published in the recent 5 years, specifically concentrated on the single protein macromolecule tailored interfacial behaviors, which could be modified by low molecular weight (LWM) additives such as phenolic compounds, glucosamine, phospholipids, saponins or small peptides. Although the interface science is a hot topic in food field for recent years, many scientists focused on discussing the effects of a single processing on the interfacial behavior of protein. The comprehensive review and comparison of different treatments are still lacking. To the best of our knowledge, this is the first time that the protein characteristics, improvement strategies and the deep mechanisms are thoroughly reviewed and critically compared. In addition, the potential trends in the future are also raised with great cautions. Since multicomponent and particle-like emulsifiers have been fully reviewed recently (6, 14), to simplify the topic, the interfacial properties of Pickering dispersions and mixture macromolecular emulsifiers such as protein-polysaccharide or protein-protein complexes would not be discussed. In the present work, we introduced how protein features would affect its O/W interfacial and emulsion properties. Then, the physical treatments, including heating, high-pressure treatment, high-pressure homogenization, high-intensity ultrasound and others, for improving interfacial activities were thoroughly summarized. Furthermore, we highlighted the chemical-based application giving rise to promoting emulsion properties of protein. The potential mechanisms for the improvement are summarized and illustrated in Figure 1. Future research trends in O/W interface science in the food field are raised.
The nature of biopolymer proteins dominates their interfacial fate and determines the interfacial film thickness, curvature, protein–protein interactions and thus rheological properties. Such interfacial behavior determines the emulsion properties such as oil droplet distribution, stability and destability, including gravitational-led separation, coalescence, Ostwald ripening, droplet aggregation, bridge and depletion flocculation (15). Here we discussed some protein related characteristics of protein about how they affect emulsion properties by modulating interfacial behaviors, such as amphiphilicity, flexibility, primary sequence, protein aggregation, and concentrations.
The reason why protein could work as a great emulsifier is because both polar and nonpolar amino acid residues distribution, giving protein an amphiphilic nature. The amphiphilicity, namely, the distribution of hydrophobic and hydrophilic residues, greatly affects the adsorption behavior of proteins: an overly high hydrophilicity leads to an insufficient driving force to overcome adsorption entropy, while an overly high hydrophobicity might lead to less surface activity (6). In other words, enough hydrophobic residues are important for protein to come together with oil phase and interact with each other to form strong interfacial film (16), while the number of hydrophilic residues determines the molecular forces between protein-water and steric hindrance within droplets (derived from dangling hydrophilic groups) (17). Briefly, a more balanced polar and nonpolar group distribution is favored for interfacial adsorption and emulsion stabilization afterward, which is determined by the protein sequence (18). Xiong et al. (19) verified the pea protein isolate subunits (i.e., isoforms of vicilins) well balanced with hydrophobic and hydrophilic groups displayed better interfacial activity. Also, after enhancing the amphiphilic balance of chicken liver protein with succinylation, the proteins exhibited better emulsion properties and resulted emulsions with lower particle sized droplets (20).
Conformational flexibility refers to the ability for proteins to undergo conformational or even subunits rearrangement, which is negatively related to the hydrogen bonds between protein-water and the hydrophobic interactions and disulfide bonds among protein (21). It is considered to be a more important parameter than hydrophobicity in determining emulsion performance. Since flexibility is probably the most determinate characteristic influencing O/W interfacial properties, the structures of some typical proteins discussed in this work are depicted and listed in Table 1. The effects of flexibility of proteins on the interfacial properties highly depend on their native structure: (1) for globular protein, it is well acknowledged that globular proteins could rearrange in the interface and interact laterally to form a highly stable quasi-two-dimensional network (22). Many works performed in globular protein such as β-lactoglobulin (21), ovalbumin (23), and soy globulin proteins (24) have drawn a conclusion that an increased flexibility of globular protein leads to a higher rate for spreading at the biphase interface and faster adsorption, therefore favored emulsifying ability; (2) for flexible protein (i.e., β-casein), some classical works performed before have noticed a complete flexible conformation with low rigidity attributed to a thick but less dense interface film predominated by viscous property instead of elasticity, which negatively affected emulsion properties (25, 26). Unlike globular β-lactoglobulin, random coiled Na-casein forms a rather softer protective membrane, while the former is considered to form a cohesive and elastic matrix within the interface (27). To compare how conformational flexibility of protein impact interfacial behavior of β-lactoglobulin, the protein was treated with pH 7.0/pH 7.0+100 mM NaCl/pH 9 and their interfacial shear and dilatational properties were studied. This proved that at pH 9.0, the protein state in the bulk phase exhibited higher flexibility, therefore enhanced its rearranging and film forming ability by improving pronounced hydrophobic interactions and lowering interfacial molecular densities (28). Kieserling et al. (29) proved that the improved structural flexibility by using high hydrostatic pressure would increase the density of the interfacial film. Similarly, the higher molecular flexibility induced by phosphorylation and glycosylation also led to a much higher emulsion activity index (EAI) and emulsion stability index (ESI) owing to the larger adsorption ratio on the oil droplet surface (30, 31). Li et al. (24) established the correlation between molecular flexibility and emulsion ability as affected by the Maillard reaction and found that the correlation coefficient was relatively high (0.92). Accordingly, if the regular helix subunits dominate the secondary structure, the protein fraction shows a lower adsorption capacity (10). It has also been found that subunits of hexamer cruciferin protein with more hypervariable regions, extended loop, and more solvent-exposed surfaces would have better interface stability (32).
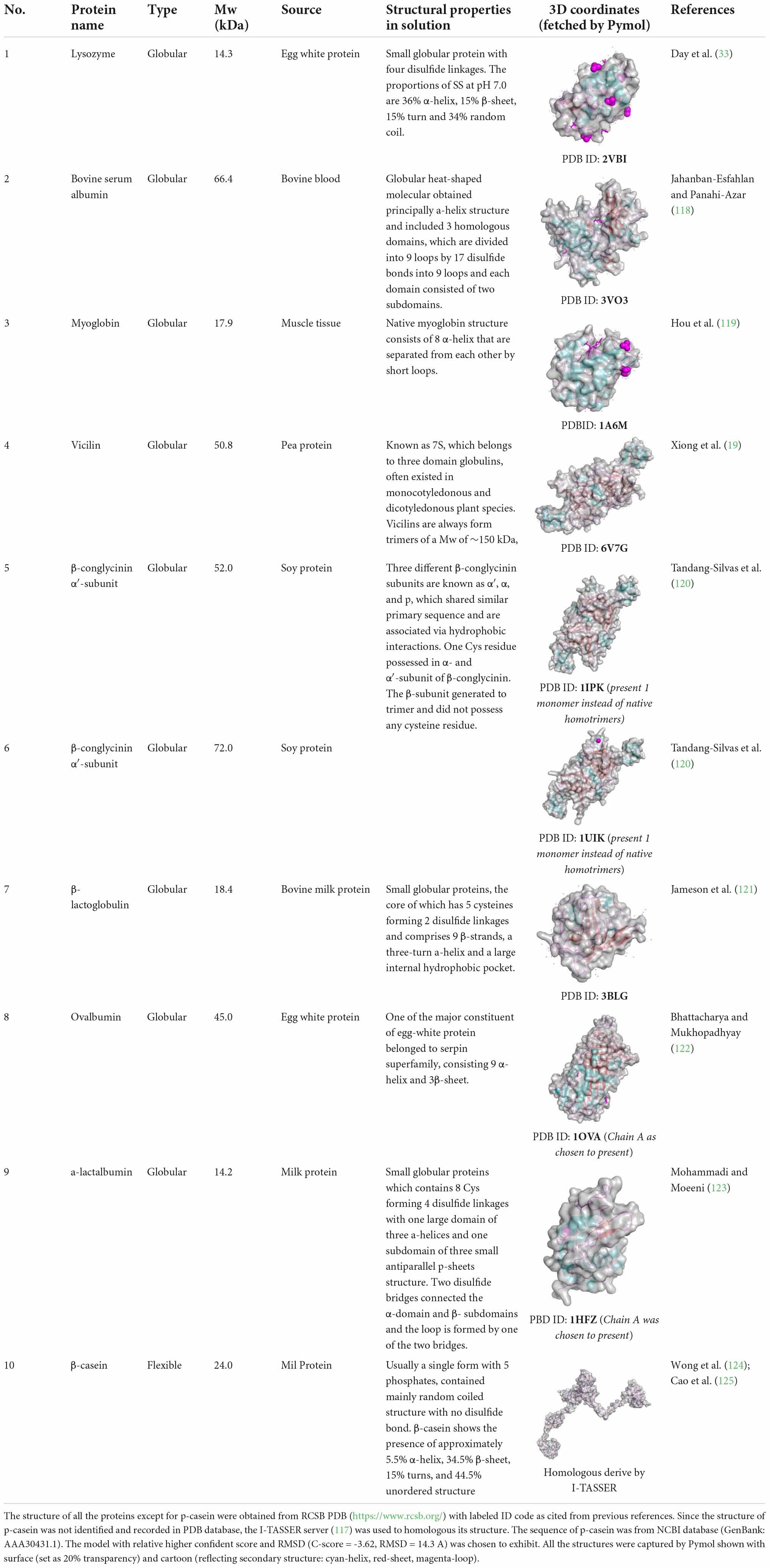
Table 1. Detailed information about globular and flexible structural proteins mentioned in this work.
The primary sequence not only affects interfacial activity by posing different advanced structures but also directly changes protein interfacial performance. For example, a long hydrophilic or hydrophobic fragment could poorly affect interfacial activity of protein (19). Bovine serum albumin (BSA) and lysozyme are both globular-like proteins share high homology similarity, yet the former exhibited better emulsion performance (33). The main reason for this difference is credited to the varied distribution of cysteine residues. The cysteine residues in BSA are distributed in neighbor, while in lysozyme, they are located at the beginning and end of the sequence. Therefore, during adsorption, lysozyme exhibited higher internal cohesion and therefore a lower emulsion ability. Similarly, although monomeric human serum albumin (HAS) and BSA shared comparable structures in the bulk phase, because of different oligomerization stabilities, BSA was found to be more easily expanded and rearranged in the interface than HSA (18). Lajnaf et al. (34) ascribed higher efficiency in reducing the surface tension of camel β-casein than bovine β-casein to the higher content of Ile in the primary sequence, which bringing in more methyl group and thus increased hydrophobicity. Because of varied sequence, whey and casein protein prone to forming disulfide linked and hydrophobicity mediated aggregates, respectively, which resulted in different adsorption behavior (35). The primary sequence also determined the electrical charges and partitioning of peptides from cod bone, therefore causing different interfacial behavior and electrostatic repulsion forces between emulsion droplets (36).
Many works have been conducted recently to reveal the relationship between protein aggregation level and emulsion ability. As summarized by these studies, the aggregation-induced interfacial behavior changes are controversial. Pea protein obtained a much higher necessary protein concentration and longer adsorption time than whey protein, which is attributed to its larger molecular weight and supramolecular structures (37). For whey protein isolates, a high level of denaturation and aggregation in bulk phase leaded to a wider level of droplet distribution but showed minor effects on the diffusion rate. A combination of native aggregated whey protein isolate (WPI) resulted in the best interfacial performance, since native WPI dominated the interfacial adsorption process, while aggregated WPI could facilitate the viscosity of the continuous phase and inhibit phase separation (38). Zhou et al. (39) found the WPI exhibited better emulsion abilities than aggregates because of the interfacial film formed with denser and more brittle (quasi-) 2d structure, which could improve interfacial compactness by Marangoni-like effect. In contrast, soy β-conglycinin aggregates induced by ethanol treatment exhibited greatly improved emulsion performance compared to native protein due to the facilitated formation of bridged emulsions (40). Similarly, by forming soluble aggregates as induced by high pressure homogenization and heating, kidney bean protein obtained much higher emulsifying ability and activity (41). The predominant state of β-lactoglobulin (dimer or monomer) under various pH crucially affects the interfacial properties by changing phenolics binding location and thus altering the interfacial partition of protein (42).
The emulsion and interfacial properties of proteins are highly concentration dependent (39). A change in surface hydrophobicity and interfacial behavior (saturation level, adsorption rate, surface tension, interface denaturation, and diffusion) often emerges as a function of protein concentration (43). As reported, at lower protein concentrations (<1%), the protein adsorption toward O/W interface showed diffusion-controlled manner, while at higher concentrations (1–2%) protein adsorption was only diffusion-dependent because of the prevention of protein migration (44). A low protein concentration always led to flocculation due to an incompletely covered droplet surface, and the threshold concentration for stabilizing an emulsion was dependent on the protein species. Dridi et al. (27) mentioned that the interfacial properties are dependent on protein concentration at rather lower protein content and insensitive to agitation conditions because of limited coalescence process, while it is more reliable on emulsification power under high protein content. The protein concentration affects the interfacial protein profile of pea protein isolates. At a rather low concentration, the contents of pea protein adsorbed in the interface were ranked by aggregates > vicilin > legumin > convicillin, while at saturated adsorption, the protein content was ranked by vicilin > legumin > aggregates > convicillin (45). By using a novel microfluidic device, it is checked at a low protein concentration that the adsorption time and interface are vital to coalescence stability (46).
The protein species and corresponding parameters for all the physical and chemical treatments used for improving the interfacial properties of proteins are summarized in Tables 2, 3, respectively.
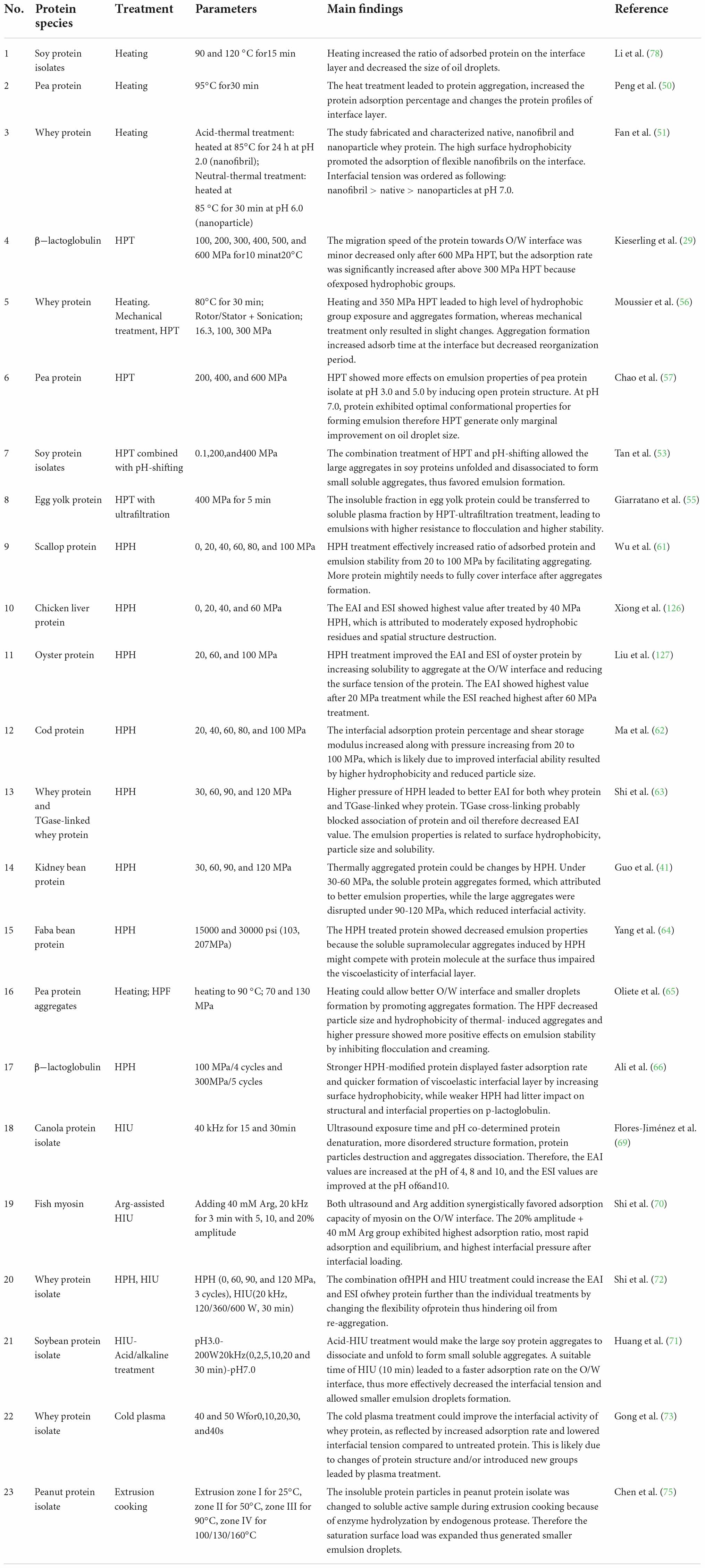
Table 2. Recent studies concentrated on physical treatment extensively applied to improve the interfacial and emulsion properties of proteins.

Table 3. Recent studies concentrated on chemical treatment extensively applied to improve the interfacial and emulsion properties of proteins.
Thermal treatments have long been implemented in improving the emulsion properties of proteins through increasing interfacial adsorbed content and modifying advanced molecular structure (47). Particularly, it is shown that the preheating treatment could facilitate the emulsion activity of protein, which leading to smaller droplet size and lower interfacial tension of droplets (48). Besides emulsion activity, thermal treatment could enhance emulsion properties by leading protein aggregation. As suggested by Li et al. (49) and Peng et al. (50), although the increased molecular size potentially lowered the diffusion and rearrangement rate, it also allowed larger steric hindrance formation at the droplet surface, exhibiting higher interfacial viscoelasticity. Thermal treatment gave rise to the interfacial properties of plant derived protein because of thermally induced disulfide bond cross-linking, surface hydrophobic group exposure and especially protein aggregation (49). Similarly, heated pea proteins (95°C) exhibited a higher extent of protein aggregation and a much higher percentage of adsorbed protein at 0.1–0.5% protein concentrations (50). Thermal treatment also changed the interfacial properties through modifying the morphological state of the aggregates. By fabricating native whey proteins to form particle- and fibril-like aggregates, proteins exhibited effectively improved emulsion ability. The different morphologies also led to altered responses of the interfacial properties to pH (51).
As one of the most attractive non-thermal technologies, high-pressure treatment (HPT) (100–600 MPa) is often regarded as a cold pasteurization process, which is also capable to improve the interfacial properties of food proteins (52). The effective parameter of HPT varied as species-dependent manner. Some works suggested a ≥ 200 MPa pressure is available in improving interfacial properties, such as in soy protein isolates (400 MPa) (53), meat protein (200 MPa) (54), egg yolk protein (400 MPa) (55) and β-lactoglobulin (600 MPa) (29). However, in many cases, the increase in interfacial properties can only be observed in moderate pressure. For example, compared to 16 and 350 MPa-treated whey protein isolates, the 100 MPa pressurized protein exhibited better interfacial properties, reflected by the most closely packed interfacial film and highest interface protein loading content (56). One of the mechanisms for HPT in improving protein interfacial activities is by forming a pressure-induced molten globule state in the bulk water phase, which lagged the adsorption period but increased the affinity toward the hydrophobic oil phase (29). Yang et al. (1) considered the improvement is attributed to depolymerization of interfacial protein through HPT, while in egg yolk granule, the transformation from granule phosvitin to the soluble fraction favored the emulsion properties (55). To be addressed, the environment also decided the efficiency of HPT. For example, HPT (200–600 MPa) could only enhance the emulsion stability of pea protein isolates at pH 3.0 but merely affected the emulsion quality at pH 7.0 (57).
High pressure homogenizer is usually consisted with a displacement pump and a homogenizer valve, which processed mixed dispersions to small droplets using high static pressure (10–100 MPa) (58). Although it is generally acknowledged that high-pressure homogenization (HPH) and high-pressure fluidization (HPF) have excellent performance during emulsion preparation even on an industrial scale, some studies have shown that treating protein with HPH and HPF directly at pre-emulsion stage could also contribute to a better interfacial property and emulsion quality (58–60). HPH provides both shear and cavitation forces to decrease particle size, changing structural properties and thus increasing emulsion capacity. Therefore, it is also widely used in improving emulsion properties of animal-derived protein including scallop protein (best at 100 MPa) (61), alkali-treated cod protein (best at 100 MPa) (62), TGase-induced whey protein isolate (best at 120 MPa) (63) and plant-derived protein like kidney bean proteins (best at 60 MPa) (41) and faba bean protein (decreased by HPH under 15 and 30 psi) (64). Many cases in globular proteins proved the HPH could facilitate the development of O/W interfaces because of forming soluble aggregates by either changing protein molecular conformation or disrupting the pre-formed large aggregates (41, 65). However, there is a study indicated that the soluble supramolecular aggregates induced by HPH might compete with native protein molecules at the interface, thus impair the quality of the interfacial network (64). Except for promoting soluble aggregates formation, HPH could also increase the migration rate and interfacial storage modulus of proteins by changing the tertiary structure and rearranging peptide chains (62). The result from Ali et al. (66) also indicated that since hydrophobicity of β-lactoglobulin was increased by HPH pretreatment, the adsorption rate and interfacial film formation of proteins are improved.
In food field, the low frequency (20–100 kHz) high intensity (10–1,000 W cm–2) ultrasound is recently employed for the modification of proteins (67). Previous work has reviewed that High intensity ultrasound (HIU) treatment is applicable in producing highly stabilized protein emulsions (67). The pretreatment of protein with HIU also potentially affects its emulsion abilities as determined by HIU mode, treatment period, ultrasound frequency, and intensity (68–70). Since the sonication might lead to highly exposed hydrophobic groups in proteins, it could effectively increase oil adsorption capacity and emulsion stability (69). It should be emphasized that HIU is often synergistically utilized with other treatments in improving emulsion properties of protein, such as HPH (63), amino acid fortification (70), and acid treatment (71). To be specific, combing HPH with HIU improved the interfacial properties of whey protein (improved by 13.97%), among which the former could solely increase the EAI by 8.54% (120 MPa) and the latter could solely enhance the EAI by 7.63% (600 W) (72). Similar to HPH, HIU-acid treatment improved the emulsifying properties of the soy protein isolate by dissociating aggregates, unfolding structure, and forming soluble aggregate (71). Also, through facilitating hydrophobic groups exposure and promoting more ordered structure formation, HIU-Arg addition contributed to myosin with better interfacial activity (70).
In addition to the promising tools discussed above, some other novel technologies, such as cold plasma and extrusion treatment, are also applied in improving the interfacial properties of proteins. Gong et al. (73) treated whey protein isolates with cold plasma and found that the interfacial elastic modulus was significantly strengthened for plasma-treated samples (10 s at 50 W power). Similar results on emulsion stability have been reported on peanut protein treated with dielectric barrier discharge cold plasma at 35 V and 2 A for 1, 2, 3, and 4 min (74). The enzymatic proteolysis of peanut protein isolate induced by extrusion pretreatment was able to reduce the saturated interfacial loads and enhance emulsion performance by hydrolyzing proteins into soluble fragments and generating surface active peptides (75). The extrusion cooking treatment is also applied in soy protein isolate modification. The larger aggregates formed by this treatment and the further disruption leaded by homogenization favored emulsion stability (76).
The effects of pH on protein interfacial properties have been studied in many species, including faba bean (9), poppy seed protein (77), egg white/yolk protein (78), and whey protein (28). Under various pH values, the legume proteins suggested distinctive interfacial behavior since the surface charges, hydrophobicity and unfolding level were remarkably changed. It is concluded that pH has more influence on interfacial shear viscoelasticity than dilatational properties of protein. The effects of pH on protein emulsion changed depend on protein source. At a rather acid pH (i.e., 2.5 or 3.0), legume and poppy seed protein could form the strongest interface film and even with a faster rate, which might be induced by the more opened advanced structures (13, 77). Similarly, Tian et al. (79) found that β-conglycinin tended to form emulsions under acidic pH with decreased droplet diameters, leading to a faster diffusion rate and a higher ability for protein structural rearrangement compared with near isoelectric point. The stability of the Na-casein emulsion was maintained against coalescence for at least 1 month after lowering the pH to 1.8, which is likely attributed to the compaction of the monolayer and the increase in elasticity of the interfacial film (27). Whey protein exhibited increased interfacial stability at pH 9.0 than at pH 7.0 because of higher electrostatic repulsion and more hydrophobic structure formation (28). At approximately pI (pH 5.0–7.0), the egg yolk protein obtained a rather low ESI, which is attributed to the presence of insoluble yolk granular proteins of large size (78). Although many reports exhibited a better interfacial activity of protein at a rather extreme pH, it is also pointed out that at near pI conditions, proteins would form films with higher dilatational elasticity because of favored formation of multiple layers, shrinkage of hydrophilic segments, and higher intermolecular attractive interactions (80). Similar results were also reported in microalgae proteins; as the pH increased far from pI, the interfacial modulus decreased due to increased electrostatic repulsions between adsorbed proteins (81).
The pH-shifting process is also well-known as isoelectric solubilization/precipitation process, during which the alkalization/acidification and neutralization steps are commonly utilized to improve the interfacial and emulsion properties of many types of proteins derived from plants, milk, or eggs. Therein, the neutralization step is the main difference compared with the method of adjusting pH. Using alkali-heat treatment could, thus improving the flexibility of α-zein by deamidating, which resulted in higher amphiphilicity and emulsion ability (82). Researchers treated chia protein at pH 10 as well as 12 and recovered at pH 4.5, which profoundly affected the interfacial properties by changing structural properties of native protein (83). Acid treatment (pH 2.5) was proven to improve the adsorption rate to the O/W interface and allow the formation of a stronger interfacial film of soybean glycinin (84). For insoluble microalgae Chlorella protothecoides protein, acid-thermal hydrolyzed protein fragments could mix with protein aggregates in the interface, thus forming more stabilized layers (81). Jiang et al. (85) found that dephenol treatment could promote the improvement dominated by alkali shifting (pH 12.0) of canola protein isolates because of eliminating of endogenous phenolic hindrances, increasing conformational flexibility and surface activity, and improving dispersion.
One of the most attractive topics is how the formation of protein-phenolic complexes improves the interfacial properties of proteins because of multifunctional effects (increased emulsifying ability, oxidation stability, and other bioactivities). This topic has also been fully comprehended by Farooq et al. (86). It is widely studied to produce phenolic-protein complexes to improve the initial interfacial activity of native whey, such as phytic acid (87), lotus seedpod proanthocyanidin (88), and cinnamaldehyde (89). Pei et al. (87) prepared an anionic phytic acid-whey protein complex and proved that the complex obtained higher cream resistance and a thicker cream layer than whey protein alone, which resulted from the faster adsorption rate, the higher ability to lower the interfacial tension and the increased electrostatic bridging between droplets. The covalent cross-linking formation at the O/W interface between whey and cinnamaldehyde favored the interfacial properties of the protein by promoting protein accumulation at the oil droplet surface (89). Felix et al. (90) proved a similar impact of cinnamaldehyde on protein isolates stabilized O/W interface, which is attributed to the increased homogeneity and surface rheological properties. Pan et al. (91) prepared chlorogenic acid-protein covalent complex under alkali conditions, resulting in higher adsorption ability onto the interface and more compact interfacial layers. However, for the flaxseed protein-phenolic complex system, complex formation could only strengthen the interfacial layer at low protein concentrations, while the complex exhibited a browning color because of alkaline-induced polymerization of phenolic compounds (92).
Enzyme catalyzation was also applied in improving protein emulsion properties by changing surface charges, increasing flexibility, modifying structure, or inducing self-assembly. A moderate TGase treatment (treated for 60 min) could improve the emulsion properties of faba bean protein isolates by increasing the net surface charge (93). Yu et al. (94) used TGase to cross-link Alcalase + trypsin hydrolyzed whey protein isolates, resulting in better emulsion properties, which is assumed to be due to increased flexibility and rearranged hydrophobic and hydrophilic amino acid residues. Solely hydrolyzed sodium caseinate by commercial enzymes could potentially self-assemble into network-like supramolecular particles, which drastically improved the stability of the emulsion by 400% (95). TGase also catalyzed glucosamine with soy protein, resulting in a stretched structure, softer state and higher flexibility compared to native soy protein isolates, thereby improving the ability to lower surface tension (31). HIU has been combined with dextran glycosylation to modify buckwheat protein isolates, which tended to be more closely packed and form thicker interfacial film (96).
Generally, LMW surfactant is capable of displacing protein from the O/W droplet surface by an “orogenic” mechanism, thus undermining the stability of emulsions. However, at a proper ratio of surfactant to protein, the combination of these two could lead to a more compact interfacial layer (97). Therefore, the impacts of LMW surfactant on the interfacial properties of proteins are controversial, and it is found to be related to the native structure, concentration and types of both surfactant and polymers. The LMW surfactant consists of a hydrophilic head and one or several hydrophobic tails. Directly adding natural LMW surfactants such as saponins (98), phospholipids (99), sucrose esters (100), monoglycerides (101), and small peptides (102) could enhance the interfacial properties of proteins. The interfacial activity of these LMW surfactants differs because of various structural features (Figure 2):
• Saponins: Some saponins consist of a hydrophobic aglycone structure and hydrophilic sugar residues, and the adjacent sugar residues in the interface could form hydrogen bonds to stabilize an interfacial film Böttcher et al. (103).
• Phospholipid: Some phospholipids show high surface activity due to the hydrophilic phosphate moiety head and hydrophobic fatty acid tail (6).
• Sucrose ester: Sucrose fatty acid ester is commonly used as a non-ionic surfactant because of the contribution of both the hydrophilic sucrose head and the hydrophobic fatty acid tail (100).
• Monoglyceride: Monoglyceride could act as an emulsifier in the food industry due to the hydrophilic glycerol backbone attached to the hydrophobic fatty acyl chain (104).
• Small peptide: To achieve better emulsifying activity, the small peptide emulsifier should exhibit amphiphilic properties by consisting of a hydrophobic and a hydrophilic region independent of the secondary structure, and these regions better show an axial state (105).
Soy saponin addition loosened the rigid conformation of the protein and improved resilience to external deformation of both β-conglycinin (7S) and glycinin (11S), which further led to long-term stability (up to 42 days) (98, 106). Böttcher et al. (103) demonstrated that saponin-β-lactoglobulin complex formation, which is mainly governed by hydrogen bonds and/or hydrophobic interactions, contributing to a stronger and higher viscoelastic interface. A study performed previously applied HIU to produce chickpea protein and ginseng saponin complex, which significantly increased interfacial adsorption and smaller droplets compared to solely protein molecules (107). However, by studying the interfacial co-adsorption aging of Quillaja saponin with pea protein, it is suggested that the mixed saponin-protein interface exhibited a more fluid-like behavior instead of an elastic one, indicating that the effects are highly dependent on the type of complex formed (8).
Adding a low level of lecithin (0.5–1.0%) would help to decrease the O/W interfacial tension of mussel water-soluble protein, while a high level of lecithin (1.5–2.0%) led to unfavorable protein aggregation and unfolding because of competitive adsorption (99). Wang et al. (108) also indicated that soy lecithin affected emulsions stabilized by whey protein isolate in a concentration-dependent manner, among which a low lecithin concentration (0.25–0.75%) endowed better emulsion formation by synergistically improving interfacial properties (e.g., making a rather rigid interfacial film). Lecithin (final concentration of 2%) also synergistic with gelatin in solubilizing oil-loaded emulsions (109).
The competition behavior induced by monoglycerides is determined by both their content and protein subunits. Li et al. (110) indicated that the addition of monoglycerides affected the network formation of β-conglycinin in a synergistic or competitive adsorption manner when the protein concentration was low (4%) or high (>6%), respectively, as reflected by drastically changes in the interfacial elastic modulus G’. Additionally, 0.1 wt% monoglyceride is capable of displacing acid and basic soy protein subunits from the interface, thus reducing the amount of adsorbed protein but not β-subunits (45).
To be addressed, bioinformatics tools have been extensively utilized in predicting the emulsion properties of peptides from rich resources. Hydrolysed peptides are derived from potato (105), soy (111), and seaweed (112). As predicted, the peptide had the highest amphiphilic score, and proper length obtained the best interfacial and emulsifying activity. Peptide could act as emulsifier in two modes: constituting inner interfacial membrane through partitioning and weakly bonding to inner interface and forming a surrounding external layer (113). Introducing amphiphilic peptides into protein systems or simply hydrolyzing proteins to a certain level could change their interfacial and emulsion activities. It has been reported that the addition of zein hydrolysate effectively improved the emulsifying stability of myofibrillar protein by helping to form a more compact and massive interfacial membrane (102). However, another study proved that hydrolyzed peptides showed a disadvantageous impact on EAI and ESI of sunflower protein, which was likely due to the lower ability of peptides to reduce interfacial tension (114).
Compared to the above LMW emulsifiers, some other types are also noticed with limited attention. Zhao et al. (100) studied the effects of the content of sucrose ester on the interfacial properties of sodium caseinate. A lower concentration (<0.05%) led to co-adsorption at the interface, while a higher concentration (>0.05%) of the interfacial interactions between sodium caseinate and sucrose ester governed competitive adsorption. Although BSA (66 KDa) and lysozyme (14.3 KDa) are both globular proteins, the combination of ionic LMW surfactant [C12mim] Br showed co-adsorption behavior on lysozyme but competitive adsorption on BSA, which indicated that the function of LMW surfactant also varies depending on molecular weight (115).
In contrast to covalently bonded protein-phenolic conjugates, some studies have focused on the effects of non-covalently bonded protein-phenol complexes on the interfacial properties of proteins. It has been reported that sunflower protein-chlorogenic acid complex formation, which is mainly driven by hydrogen bonds, resulted in a higher ability to decrease interfacial tension than protein formation alone (116). The addition of alkaline amino acids, such as arginine and lysine, was reported to facilitate many functionalities of proteins, including interfacial activity and thus emulsion properties. Both arginine and lysine increased the adsorbed protein and penetration rate but decreased the diffusion rate of interfacial pork myosin, leading to improved EAI and ESI (49). A higher level of phosphorylation resulted in a reduction in particle size and loosening of the protein structure because of interactions between phosphate groups and amino acid residues, therefore improving the emulsion ability (30).
Applying these above treatments to modify protein structure and physicochemical properties effectively improved interfacial properties through the following pathways, as illustrated in Figure 1:
(1) Aggregates formation: a. Thermal treatment could enhance protein aggregation to a larger size. Although the increased molecular size potentially lowered the diffusion and rearrangement rate, it also allowed larger steric hindrance formation at the droplet surface, exhibiting higher viscoelasticity. b. Treatments such as HPH and HIU led to soluble aggregate formation by cavitation and turbulence functions, which contributed to better interfacial film formation; (2) Reduced molecule size: A smaller sized-protein polymer could be more easily adsorbed onto the O/W interface with a higher migration and diffusion rate, which often results from HPH and HIU enhancement.
The faster speed would let oil droplets stabilize before destabilization, such as coalescence and flocculation; (3) Amphiphilicity modification: Many treatments are reported to be able to modify advanced structures, especially leading to hydrophobic group exposure, such as HPT, pH shifting, and TGase catalysis. A more balanced polar and nonpolar group distribution surely favors interfacial adsorption and film formation; (4) Increasing flexibility: Higher flexibility contributed to faster penetration and rearrangement rates, which led to better interfacial properties. The relatively easier way to adjust the flexibility of proteins is to change the pH. The altered electrostatic repulsion force would tune flexibility around a certain range. In addition, HPT could drive proteins to form a molten globule state, thus increasing flexibility. Glycosylation and phosphorylation were also reported to promote flexibility by introducing new groups; (5) Co-adsorption and stabilization: Although natural LMW surfactants may displace proteins from the interface, under proper conditions, they can synergistically lower the interfacial tension and guarantee the stability of surface layer-covered droplets; (6) Facilitating interfacial protein interactions: One of the main benefits of increasing the surface hydrophobicity of proteins is strengthening protein–protein interactions and forming more compact and elastic networks at the interface. Phenolic compounds grafting also attained similar results because of phenolic-mediated cross-links.
To be emphasized, inappropriate or excessive parameters of such treatments could also deteriorate interfacial activity by forming a softer interfacial film, aggregating to retard diffusion or penetration rate, impeding structure stretching after adsorption, and competitively adsorbing to the interface. Therefore, proper processing conditions are inevitable for effectively facilitating protein interfacial properties. Since each single treatment has been more deeply revealed for decades, a trend focused on associating two or even more treatments to spontaneously enhance interfacial activity and thus emulsion properties is gradually attracting more attention. For example, HIU treatment is suitable to be combined with catalysis and hydrolysis processes to yield synergistic results. pH-shifting treatment is always followed by heating to produce fiber- or particle-like protein agglomerations and change their interfacial behavior. At the end of this review, we address the fact that since the relationship between emulsion properties and interfacial behavior is not considered simply straightforward, the correlations between these two functionalities need to be more clearly elucidated.
In this review, the key natural characteristics of proteins in determining interfacial behaviors and properties were firstly summarized, and their effects on emulsion properties were discussed. Secondly, the improving strategies tailored to improving the interfacial activity of proteins were reviewed in two subsections according to distinctive mechanisms: physical treatments (mainly including thermal treatment, high-pressure treatment, high-pressure homogenization, and high-intensity ultrasound) and chemical modifications (mainly including pH adjustment, covalent modification, and LMW surfactant combinations).
Although the interfacial proteins have been attracted attentions and widely studied, most of researches are prone to delineating the changes of structural and emulsifying properties of these proteins. Mainly processing properties are focused and deeply revealed. However, as the topics caring food nutrition and human health are urgently needed, a lot of work could be conducted at this field. We have proposed some hot topics related to interfacial protein film following:
(1) Relation between interfacial protein and proteins in continuous phase should be more addressed, especially in meat protein system. In meat protein field, since the emulsion product is not typical O/W emulsion, the quality of emulsified meat is not only dependent on inner interfacial film properties (whether they are regular, dense, or compact), but also largely decided by the interaction between interface and sub-interface layer and also the rigidity as well as strength if covered oil droplets. Many works required to be conducted to better unveil the mechanism regarding this aspect.
(2) As bioinformatics and in silico tools like molecular dynamics simulation are rapidly developed, more computational investigation toward interfacial behavior of proteins should be performed. Based on these novel approaches, more detailed information about molecular forces and steric hindrance impact of proteins will be clarified and the interfacial behavior of a single protein molecule could be elucidated.
(3) It is still remained unknown that how does the microscopic interfacial properties give light on upgrading of large-scale industrial processing. To achieve this goal, diversity of the protein species including globular, flexible, and fiber-like molecule, should be implemented in measuring interfacial stage behavior and emulsion analysis, such as emulsion stability, emulsion activity, and emulsion-gel quality. These databases could provide a platform to highlight the practical meaning of interfacial properties.
(4) Although many scientists have already noticed the interfacial region was closely related with flavor release, bioactive protector, and gastrointestinal behavior of emulsions, the information of oral taste, digestion and bioactive delivery properties of O/W emulsions as a function of protein interfacial behavior is still lacking. Therefore, extra attention should also be paid to interpreting how interfacial properties influence sensory perception and human acceptance by altering emulsion rheological properties, oral processing behavior and product texture. Also, the relationships between interfacial properties and digestive and bioaccessibility characteristics also urgently need to be fully understood.
HZ: investigation-equal, validation-equal, visualization- equal, and writing—original draft-equal. XZ: conceptualization-lead, funding acquisition-lead, project administration-lead, resources-lead, supervision-lead, writing—original draft-equal, and writing—review and editing-lead. XC: administration-supporting and supervision-supporting. XX: conceptualization-supporting, funding acquisition-supporting, project administration-supporting, and supervision-supporting. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (32101987) and the Natural Science Foundation of Jiangsu Province of China (BK20210405). The Fundamental Research Funds for the Central Universities (serial number: KYQN2022002). The Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang Q, Sui Z, Lu W, Corke H. Soybean lecithin-stabilized oil-in-water (O/W) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. (2021) 338:128071. doi: 10.1016/j.foodchem.2020.128071
2. McClements DJ. Future foods: a manifesto for research priorities in struc-tural design of foods. Food Funct. (2020) 11:1933–45. doi: 10.1039/C9FO02076D
3. Li, X, Fan L, Liu Y, Li J. New insights into food O/W emulsion gels: strategies of reinforcing mechanical properties and outlook of being applied to food 3D printing. Crit Rev Food Sci Nutr. (2021). doi: 10.1080/10408398.2021.1965953
4. Nielsen CK, Kjems J, Mygind T, Snabe T, Schwarz K, Serfert Y, et al. Antimicrobial effect of emulsion-encapsulated isoeugenol against bio-films of food pathogens and spoilage bacteria. Int J Food Microbiol. (2017) 242:7–12. doi: 10.1016/j.ijfoodmicro.2016.11.002
5. Jeon WY, Yu JY, Kim HW, Park HJ. Production of customized food through the insertion of a formulated nanoemulsion using coaxial 3D food print-ing. J Food Eng. (2021) 311:110689. doi: 10.1016/j.jfoodeng.2021.110689
6. McClements DJ, Gumus CE. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: molecular and physicochemical basis of functional performance. Adv Colloid Interface Sci. (2016) 234:3–26. doi: 10.1016/j.cis.2016.03.002
7. Pugnaloni LA, Dickinson E, Ettelaie R, Mackie AR, Wilde PJ. Competitive adsorption of proteins and low-molecular-weight surfactants: computer simulation and microscopic imaging. Adv Colloid Interface Sci. (2004) 107:27–49. doi: 10.1016/j.cis.2003.08.003
8. Reichert CL, Salminen H, Utz J, Badolato Bönisch G, Schäfer C, Weiss J. Aging behavior of Quillaja saponin-pea protein interfaces. Colloid Interface Sci Commun. (2017) 21:15–8. doi: 10.1016/j.colcom.2017.10.003
9. Felix M, Romero A, Carrera-Sanchez C, Guerrero A. Assessment of interfacial viscoelastic properties of Faba bean (Vicia faba) protein-adsorbed O/W layers as a function of pH. Food Hydrocoll. (2019) 90:353–9. doi: 10.1016/j.foodhyd.2018.12.036
10. Rahmati NF, Koocheki A, Varidi M, Kadkhodaee R. Adsorption of Speckled Sugar bean protein isolate at oil-water interface: effect of ionic strength and pH. Int J Biol Macromol. (2017) 95:1179–89. doi: 10.1016/j.ijbiomac.2016.11.008
11. Félix M, Carrera C, Romero A, Bengoechea C, Guerrero A. Rheolog-ical approaches as a tool for the development and stability behaviour of protein-stabilized emulsions. Food Hydrocoll. (2020) 104:105719. doi: 10.1016/j.foodhyd.2020.105719
12. Chen M, Sagis LMC. The influence of protein/phospholipid ratio on the physicochemical and interfacial properties of biomimetic milk fat globules. Food Hydrocoll. (2019) 97:105179. doi: 10.1016/j.foodhyd.2019.105179
13. Felix M, Romero A, Carrera-Sanchez C, Guerrero A. A comprehen-sive approach from interfacial to bulk properties of legume protein-stabilized emul-sions. Fluids. (2019) 4:65. doi: 10.3390/fluids4020065
14. Ravera F, Dziza K, Santini E, Cristofolini L, Liggieri L. Emulsifica-tion and emulsion stability: the role of the interfacial properties. Adv Colloid Interface Sci. (2021) 288:102344. doi: 10.1016/j.cis.2020.102344
15. Moschakis T, Murray BS, Dickinson E. Microstructural evolution of viscoelastic emulsions stabilised by sodium caseinate and xanthan gum. J Colloid Interface Sci. (2005) 284:714–28. doi: 10.1016/j.jcis.2004.10.036
16. Chang C, Tu S, Ghosh S, Nickerson MT. Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res Int. (2015) 77:360–7. doi: 10.1016/j.foodres.2015.08.012
17. Dickinson E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. (2019) 96:209–23. doi: 10.1016/j.foodhyd.2019.05.021
18. Aguilera-Garrido A, Del Castillo-Santaella T, Yang Y, Galisteo-González F, Gálvez-Ruiz MJ, Molina-Bolívar JA, et al. Applications of serum albumins in deliv-ery systems: differences in interfacial behaviour and interacting abilities with poly-saccharides. Adv Coll Interface Sci. (2021) 290:102365. doi: 10.1016/j.cis.2021.102365
19. Xiong T, Ye X, Su Y, Chen X, Sun H, Li B, et al. Identification and quantification of proteins at adsorption layer of emulsion stabilized by pea protein isolates. Colloids Surf B Biointerfaces. (2018) 171:1–9. doi: 10.1016/j.colsurfb.2018.05.068
20. Lu Y, Pan D, Xia Q, Cao J, Zhou C, He J, et al. Impact of pH-dependent succinylation on the structural features and emulsifying properties of chicken liver protein. Food Chem. (2021) 358:129868. doi: 10.1016/j.foodchem.2021.129868
21. Tang C, Shen L. Dynamic adsorption and dilatational properties of BSA at oil/water interface: role of conformational flexibility. Food Hydrocoll. (2015) 43:388–99. doi: 10.1016/j.foodhyd.2014.06.014
22. Freer EM, Yim KS, Fuller GG, Radke CJ. Interfacial rheology of globular and flexible proteins at the hexadecane/water Interface: comparison of shear and dilatation deformation. J Phys Chem B. (2004) 108:3835–44. doi: 10.1021/jp037236k
23. Xu YT, Yang T, Liu LL, Tang CH. One-step fabrication of multi-functional high internal phase pickering emulsion gels solely stabilized by a softer globular protein nanoparticle: S-Ovalbumin. J Colloid Interface Sci. (2020) 580:515–27. doi: 10.1016/j.jcis.2020.07.054
24. Li R, Wang X, Liu J, Cui Q, Wang X, Chen S, et al. Relation-ship between molecular flexibility and emulsifying properties of soy protein isolate-glucose conjugates. J Agric Food Chem. (2019) 67:4089–97. doi: 10.1021/acs.jafc.8b06713
25. Dickinson E. Structure and composition of adsorbed protein layers and the re-lationship to emulsion stability. J Chem Soc Faraday Trans. (1992) 88:2973–83. doi: 10.1039/ft9928802973
26. Erni P, Windhab EJ, Fischer P. Emulsion drops with complex interfac-es: globular versus flexible proteins. Macromol Mater Eng. (2011) 296:249–62. doi: 10.1002/mame.201000290
27. Dridi W, Harscoat-Schiavo C, Monteil J, Faure C, Leal-Calderon F. Monodisperse oil-in-water emulsions stabilized by proteins: how to master the average droplet size and stability, while minimizing the amount of proteins. Langmuir. (2018) 34:9228–37. doi: 10.1021/acs.langmuir.8b02029
28. Schestkowa H, Wollborn T, Westphal A, Maria Wagemans A, Fritsching U, Drusch S. Conformational state and charge determine the interfacial stabiliza-tion process of beta-lactoglobulin at preoccupied interfaces. J Colloid Interface Sci. (2019) 536:300–9. doi: 10.1016/j.jcis.2018.10.043
29. Kieserling H, Giefer P, Uttinger MJ, Lautenbach V, Nguyen T, Sevenich R, et al. Structure and adsorption behavior of high hydrostatic pressure-treated β-lactoglobulin. J Colloid Interface Sci. (2021) 596:173–83. doi: 10.1016/j.jcis.2021.03.051
30. Tang S, Yu J, Lu L, Fu X, Cai Z. Interfacial and enhanced emulsify-ing behavior of phosphorylated ovalbumin. Int J Biol Macromol. (2019) 131:293–300. doi: 10.1016/j.ijbiomac.2019.03.076
31. Zhang A, Cui Q, Yu Z, Wang X, Zhao X. Effects of transglutaminase glycosylated soy protein isolate on its structure and interfacial properties. J Sci Food Agric. (2021) 101:5097–105. doi: 10.1002/jsfa.11155
32. Withana-Gamage TS, Hegedus DD, McIntosh TC, Coutu C, Qiu X, Wanasundara JPD. Subunit composition affects formation and stabilization of O/W emulsions by 11S seed storage protein cruciferin. Food Res Int. (2020) 137:109387. doi: 10.1016/j.foodres.2020.109387
33. Day L, Zhai J, Xu M, Jones NC, Hoffmann SV, Wooster TJ. Conformational changes of globular proteins adsorbed at oil-in-water emulsion inter-faces examined by synchrotron radiation circular Dichroism. Food Hydrocoll. (2014) 34:78–87. doi: 10.1016/j.foodhyd.2012.12.015
34. Lajnaf R, Zouari A, Trigui I, Attia H, Ayadi MA. Effect of different heating temperatures on foaming properties of camel milk proteins: a comparison with bovine milk proteins. Int Dairy J. (2020) 104:104643. doi: 10.1016/j.idairyj.2020.104643
35. Silva M, Zisu B, Chandrapala J. Interfacial and emulsification properties of sono-emulsified grape seed oil emulsions stabilized with milk proteins. Food Chem. (2020) 309:125758. doi: 10.1016/j.foodchem.2019.125758
36. Zhao Q, Wu C, Yu C, Bi A, Xu X, Du M. High stability of bilayer nano-emulsions fabricated by Tween 20 and specific interfacial peptides. Food Chem. (2021) 340:127877. doi: 10.1016/j.foodchem.2020.127877
37. Hinderink EBA, Schröder A, Sagis L, Schroën K, Berton-Carabin CC. Physical and oxidative stability of food emulsions prepared with pea protein fractions. LWT. (2021) 146:111424. doi: 10.1016/j.lwt.2021.111424
38. Dapueto N, Troncoso E, Mella C, Zúñiga RN. The effect of denatura-tion degree of protein on the microstructure, rheology and physical stability of oil-in-water (O/W) emulsions stabilized by whey protein isolate. J Food Eng. (2019) 263:253–61. doi: 10.1016/j.jfoodeng.2019.07.005
39. Zhou X, Sala G, Sagis LMC. Bulk and interfacial properties of milk fat emulsions stabilized by whey protein isolate and whey protein aggregates. Food Hydrocoll. (2020) 109:106100. doi: 10.1016/j.foodhyd.2020.106100
40. Peng L, Xu Y, Li X, Tang C. Improving the emulsification of soy β-conglycinin by alcohol-induced aggregation. Food Hydrocoll. (2020) 98:105307. doi: 10.1016/j.foodhyd.2019.105307
41. Guo Z, Huang Z, Guo Y, Li B, Yu W, Zhou L, et al. Effects of high-pressure homogenization on structural and emulsifying properties of thermally soluble aggregated kidney bean (Phaseolus vulgaris L.) proteins. Food Hydrocoll. (2021) 119:106835. doi: 10.1016/j.foodhyd.2021.106835
42. Zhang Q, Li H, Cen C, Zhang J, Wang S, Wang Y, et al. Ultrason-ic pre-treatment modifies the pH-dependent molecular interactions between β-lactoglobulin and dietary phenolics: conformational structures and interfacial proper-ties. Ultrason Sonochem. (2021) 75:105612. doi: 10.1016/j.ultsonch.2021.105612
43. Ellouze M, Lajnaf R, Zouari A, Attia H, Ayadi MA, Vial C. Camel α-lactalbumin at the oil-water interface: effect of protein concentration and pH change on surface characteristics and emulsifying properties. Colloids Surf B Bioinerf. (2020) 189:110654. doi: 10.1016/j.colsurfb.2019.110654
44. Guo Q, Mu TH. Emulsifying properties of sweet potato protein: effect of protein concentration and oil volume fraction. Food Hydrocolloids. (2011) 25:98–106. doi: 10.1016/j.foodhyd.2010.05.011
45. Chen W, Liang G, Li X, He Z, Zeng M, Gao D, et al. Impact of soy proteins, hydrolysates and monoglycerides at the oil/water in-terface in emulsions on interfacial properties and emulsion stability. Colloids Surf B Biointerface. (2019) 177:550–8. doi: 10.1016/j.colsurfb.2019.02.020
46. Muijlwijk K, Colijn I, Harsono H, Krebs T, Berton-Carabin C, Schroën K. Coalescence of protein-stabilised emulsions studied with microfluidics. Food Hydrocoll. (2017) 70:96–104. doi: 10.1016/j.foodhyd.2017.03.031
47. Kim DA, Cornec M, Narsimhan G. Effect of thermal treatment on in-terfacial properties of β-lactoglobulin. J Colloid Interface Sci. (2005) 285:100–9. doi: 10.1016/j.jcis.2004.10.044
48. Ma W, Wang J, Wu D, Xu X, Du M, Wu C. Effects of preheat treatment on the physicochemical and interfacial properties of cod proteins and its re-lation to the stability of subsequent emulsions. Food Hydrocoll. (2021) 112:106338. doi: 10.1016/j.foodhyd.2020.106338
49. Li Q, Zheng J, Ge G, Zhao M, Sun W. Impact of heating treatments on physical stability and lipid-protein co-oxidation in oil-in-water emulsion prepared with soy protein isolates. Food Hydrocoll. (2020) 100:105167. doi: 10.1016/j.foodhyd.2019.06.012
50. Peng W, Kong X, Chen Y, Zhang C, Yang Y, Hua Y. Effects of heat treatment on the emulsifying properties of pea proteins. Food Hydrocoll. (2016) 52:301–10. doi: 10.1016/j.foodhyd.2015.06.025
51. Fan Y, Peng G, Pang X, Wen Z, Yi J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT. (2021) 149:111904. doi: 10.1016/j.lwt.2021.111904
52. Khan NM, Mu TH, Zhang M, Arogundade LA. The effects of pH and high hydrostatic pressure on the physicochemical properties of a sweet potato pro-tein emulsion. Food Hydrocoll. (2014) 35:209–16. doi: 10.1016/j.foodhyd.2013.05.011
53. Tan M, Xu J, Gao H, Yu Z, Liang J, Mu D, et al. Effects of combined high hydrostatic pressure and pH-shifting pretreatment on the structure and emulsifying properties of soy protein isolates. J Food Eng. (2021) 306:110622. doi: 10.1016/j.jfoodeng.2021.110622
54. Yang H, Wang H, Tao F, Li W, Cao G, Yang Y, et al. Structural basis for high-pressure improvement in depolymerization of inter-facial protein from RFRS meat batters in relation to their solubility. Food Res Int. (2021) 139:109834. doi: 10.1016/j.foodres.2020.109834
55. Giarratano M, Duffuler P, Chamberland J, Brisson G, House JD, Pouliot Y, et al. Combination of high hydrostatic pressure and ultrafiltration to generate a new emulsifying ingredient from egg yolk. Molecules. (2020) 25:1184. doi: 10.3390/molecules25051184
56. Moussier M, Bosc V, Michon C, Pistre V, Chaudemanche C, Huc-Mathis D. Multi-scale understanding of the effects of the solvent and process on whey protein emulsifying properties: application to dairy emulsion. Food Hydrocoll. (2019) 87:869–79. doi: 10.1016/j.foodhyd.2018.08.052
57. Chao D, Jung S, Aluko RE. Physicochemical and functional properties of high pressure-treated isolated pea protein. Innov Food Sci Emerg Technol. (2018) 45:179–85. doi: 10.1016/j.ifset.2017.10.014
58. Håkansson A. Emulsion formation by homogenization: current understanding and future perspectives. Annu Rev Food Sci Technol. (2019) 10:239–58. doi: 10.1146/annurev-food-032818-121501
59. Wang Y, Jiang S, Zhao Y, Zeng M. Physicochemical and rheological changes of oyster (Crassostrea gigas) protein affected by high-pressure homogenization. LWT. (2020) 134:110143. doi: 10.1016/j.lwt.2020.110143
60. Han T, Wang M, Wang Y, Tang L. Effects of high-pressure homogeni-zation and ultrasonic treatment on the structure and characteristics of casein. LWT. (2020) 130:109560. doi: 10.1016/j.lwt.2020.109560
61. Wu D, Wu C, Wang Z, Fan F, Chen H, Ma W, et al. Effects of high pressure homogenize treatment on the physicochemical and emulsifying proper-ties of proteins from scallop (Chlamys farreri). Food Hydrocoll. (2019) 94:537–45. doi: 10.1016/j.foodhyd.2019.04.003
62. Ma W, Wang J, Wu D, Xu X, Wu C, Du M. Physicochemical prop-erties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocoll. (2020) 100:105429. doi: 10.1016/j.foodhyd.2019.105429
63. Shi R, Ma C, Li J, Wang K, Qayum A, Wang C, et al. Characterization of TGase-induced whey protein isolate: impact of HPHP pretreat-ment. J Food Eng. (2020) 282:110025. doi: 10.1016/j.jfoodeng.2020.110025
64. Yang J, Liu G, Zeng H, Chen L. Effects of high pressure homogeniza-tion on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. (2018) 83:275–86. doi: 10.1016/j.foodhyd.2018.05.020
65. Oliete B, Potin F, Cases E, Saurel R. Modulation of the emulsifying properties of pea globulin soluble aggregates by dynamic high-pressure fluidization. Innov Food Sci Emerg Technol. (2018) 47:292–300. doi: 10.1016/j.ifset.2018.03.015
66. Ali A, Le Potier I, Huang N, Rosilio V, Cheron M, Faivre V, et al. Effect of high pressure homogenization on the structure and the interfacial and emulsifying properties of β-lactoglobulin. Int J Pharm. (2018) 537:111–21. doi: 10.1016/j.ijpharm.2017.12.019
67. O’sullivan JJ, Park M, Beevers J, Greenwood RW, Norton IT. Applications of ultrasound for the functional modification of proteins and nanoemul-sion formation: a review. Food Hydrocoll. (2017) 71:299–310. doi: 10.1016/j.foodhyd.2016.12.037
68. Cheng Y, Donkor PO, Ren X, Wu J, Agyemang K, Ayim I, et al. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual fre-quency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocoll. (2019) 89:434–42. doi: 10.1016/j.foodhyd.2018.11.007
69. Flores-Jiménez NT, Ulloa JA, Silvas JEU, Ramírez JCR, Ulloa PR, Rosales PUB, et al. Effect of high-intensity ul-trasound on the compositional, physicochemical, biochemical, functional and struc-tural properties of canola (Brassica napus L.) protein isolate. Food Res Int. (2019) 121:947–56. doi: 10.1016/j.foodres.2019.01.025
70. Shi T, Liu H, Song T, Xiong Z, Yuan L, McClements DJ, et al. Use of l-arginine-assisted ultrasonic treatment to change the molecular and interfacial characteristics of fish myosin and enhance the physical stability of the emulsion. Food Chem. (2021) 342:128314. doi: 10.1016/j.foodchem.2020.128314
71. Huang L, Ding X, Li Y, Ma H. The aggregation, structures and emulsi-fying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. (2019) 279:114–9. doi: 10.1016/j.foodchem.2018.11.147
72. Shi R, Liu Y, Hu J, Gao H, Qayum A, Bilawal A, et al. Combination of high-pressure homogenization and ultrasound improves physiochemical, interfacial and gelation properties of whey protein isolate. Innov Food Sci Emerg Technol. (2020) 65:102450. doi: 10.1016/j.ifset.2020.102450
73. Gong W, Guo X, Huang H, Li X, Xu Y, Hu J. Structural characteri-zation of modified whey protein isolates using cold plasma treatment and its applica-tions in emulsion oleogels. Food Chem. (2021) 356:129703. doi: 10.1016/j.foodchem.2021.129703
74. Ji H, Dong S, Han F, Li Y, Chen G, Li L, et al. Effects of die-lectric barrier discharge (DBD) cold plasma treatment on physicochemical and func-tional properties of peanut protein. Food Bioprocess Technol. (2018) 11:344–54. doi: 10.1007/s11947-017-2015-z
75. Chen L, Chen J, Yu L, Wu K, Zhao M. Emulsification performance and interfacial properties of enzymically hydrolyzed peanut protein isolate pretreated by extrusion cooking. Food Hydrocoll. (2018) 77:607–16. doi: 10.1016/j.foodhyd.2017.11.002
76. Mozafarpour R, Koocheki A, Milani E, Varidi M. Extruded soy protein as a novel emulsifier: structure, interfacial activity and emulsifying property. Food Hydrocoll. (2019) 93:361–73. doi: 10.1016/j.foodhyd.2019.02.036
77. Aslan Türker D, Göksel Saraç M, Yetiman AE, Doğan M. Interfacial properties of poppy seed protein (Papaver somniferum L.) as an alternative protein source at oil/water interface: influence of pH on stability, morphology and rheology. Eur Food Res Technol. (2021) 247:2545–56. doi: 10.1007/s00217-021-03806-x
78. Li J, Wang C, Li X, Su Y, Yang Y, Yu X. Effects of pH and NaCl on the physicochemical and interfacial properties of egg white/yolk. Food Biosci. (2018) 23:115–20. doi: 10.1016/j.fbio.2017.12.004
79. Tian Y, Taha A, Zhang P, Zhang Z, Hu H, Pan S. Effects of protein concentration, pH, and NaCl concentration on the physicochemical, interfacial, and emulsifying properties of β-conglycinin. Food Hydrocoll. (2021) 118:106784. doi: 10.1016/j.foodhyd.2021.106784
80. Berton-Carabin CC, Sagis L, Schroën K. Formation, structure, and functionality of interfacial layers in food emulsions. Annu Rev Food Sci Technol. (2018) 9:551–87. doi: 10.1146/annurev-food-030117-012405
81. Dai L, Bergfreund J, Reichert CL, Fischer P, Weiss J. Shear rheolog-ical properties of acid hydrolyzed insoluble proteins from Chlorella protothecoides at the oil-water interface. J Colloid Interface Sci. (2019) 551:297–304. doi: 10.1016/j.jcis.2019.05.029
82. Dong S, Xu H, Ma J, Gao Z. Enhanced molecular flexibility of α-zein in different polar solvents. J Cereal Sci. (2020) 96:103097. doi: 10.1016/j.jcs.2020.103097
83. López DN, Boeris V, Spelzini D, Bonifacino C, Panizzolo LA, Abirached C. Adsorption of chia proteins at interfaces: kinetics of foam and emulsion formation and destabilization. Colloids Surf B Biointerfaces. (2019) 180:503–7. doi: 10.1016/j.colsurfb.2019.04.067
84. Abirached C, Medrano A, Añón MC, Panizzolo LA. Effect of acid modification of soy glycinin on its interfacial and emulsifying properties. J Am Oil Chem Soc. (2018) 95:313–23. doi: 10.1002/aocs.12003
85. Jiang J, Nie Y, Sun X, Xiong YL. Partial removal of phenolics cou-pled with alkaline pH shift improves canola protein interfacial properties and emulsion in in vitro digestibility. Foods. (2021) 10:1283. doi: 10.3390/foods10061283
86. Farooq S, Abdullah, Zhang H, Weiss J. A comprehensive review on po-larity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Compr Rev Food Sci Food Saf. (2021) 20:4250–77. doi: 10.1111/1541-4337.12792
87. Pei Y, Wan J, You M, Mcclements DJ, Li B. Impact of whey protein complexation with phytic acid on its emulsification and stabilization properties. Food Hydrocoll. (2018) 87:90–6. doi: 10.1016/j.foodhyd.2018.07.034
88. Chen Y, Zhang R, Xie B, Sun Z, McClements DJ. Lotus seedpod proanthocyanidin-whey protein complexes: impact on physical and chemical stability of β-carotene-nanoemulsions. Food Res Int. (2020) 127:108738. doi: 10.1016/j.foodres.2019.108738
89. Chen E, Cao L, McClements DJ, Liu S, Li B, Li Y. Enhancement of physicochemical properties of whey protein-stabilized nanoemulsions by interfacial cross-linking using cinnamaldehyde. Food Hydrocoll. (2018) 77:976–85. doi: 10.1016/j.foodhyd.2017.11.047
90. Felix M, Yang J, Guerrero A, Sagis LMC. Effect of cinnamaldehyde on interfacial rheological properties of proteins adsorbed at O/W interfaces. Food Hydrocoll. (2019) 97:105235. doi: 10.1016/j.foodhyd.2019.105235
91. Pan X, Fang Y, Wang L, Shi Y, Xie M, Xia J, et al. Covalent interaction between rice protein hydrolysates and chlorogenic acid: improving the stability of oil-in-water emulsions. J Agric Food Chem. (2019) 67:4023–30. doi: 10.1021/acs.jafc.8b06898
92. Pham LB, Wang B, Zisu B, Adhikari B. Complexation between flax-seed protein isolate and phenolic compounds: effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. (2019) 94:20–9. doi: 10.1016/j.foodhyd.2019.03.007
93. Liu C, Damodaran S, Heinonen M. Effects of microbial transglutaminase treatment on physiochemical properties and emulsifying functionality of faba bean protein isolate. LWT. (2019) 99:396–403. doi: 10.1016/j.lwt.2018.10.003
94. Yu X, Liu C, Lu M, Liu Y, Yin J, Zhang Y. Impact of enzymatic hydrolysis followed by transglutaminase-induced cross-linking on decreasing antigen-icity and reserving partial interfacial properties of whey protein isolate. Food Funct. (2019) 10:1653–60. doi: 10.1039/C8FO01880D
95. Ewert J, Glück C, Zeeb B, Weiss J, Stressler T, Fischer L. Modifica-tion of the interfacial properties of sodium caseinate using a commercial peptidase preparation from Geobacillus stearothermophilus. Food Hydrocoll. (2018) 81:60–70. doi: 10.1016/j.foodhyd.2018.02.036
96. Xue F, Wu Z, Tong J, Zheng J, Li C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci Biotechnol Biochem. (2017) 81:1891–8. doi: 10.1080/09168451.2017.1361805
97. Jiang J, Jin Y, Liang X, Piatko M, Campbell S, Lo SK, et al. Synergetic interfacial adsorption of protein and low-molecular-weight emulsifiers in aerated emulsions. Food Hydrocoll. (2018) 81:15–22. doi: 10.1016/j.foodhyd.2018.02.038
98. Zhu L, Xu Q, Liu X, Xu Y, Yang L, Wang S, et al. Oil-water interfacial behavior of soy β-conglycinin-soyasaponin mixtures and their ef-fect on emulsion stability. Food Hydrocoll. (2020) 101:105531. doi: 10.1016/j.foodhyd.2019.105531
99. Zou H, Zhao N, Li S, Sun S, Dong X, Yu C. Physicochemical and emulsifying properties of mussel water-soluble proteins as affected by lecithin concen-tration. Int J Biol Macromol. (2020) 163:180–9. doi: 10.1016/j.ijbiomac.2020.06.225
100. Zhao Q, Liu D, Long Z, Yang B, Fang M, Kuang W, et al. Ef-fect of sucrose ester concentration on the interfacial characteristics and physical prop-erties of sodium caseinate-stabilized oil-in-water emulsions. Food Chem. (2014) 151:506–13. doi: 10.1016/j.foodchem.2013.11.113
101. Du L, Li S, Jiang Q, Tan Y, Liu Y, Meng Z. Interfacial interaction of small molecular emulsifiers tea saponin and monoglyceride: relationship to the for-mation and stabilization of emulsion gels. Food Hydrocoll. (2021) 117:106737. doi: 10.1016/j.foodhyd.2021.106737
102. Li Y, Kong B, Liu Q, Xia X, Chen H. Improvement of the emulsify-ing and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process Biochem. (2017) 53:116–24. doi: 10.1016/j.procbio.2016.11.010
103. Böttcher S, Scampicchio M, Drusch S. Mixtures of saponins and beta-lactoglobulin differ from classical protein/surfactant-systems at the air-water interface. Colloids Surf A Physicochem Eng Aspects. (2016) 506:765–73. doi: 10.1016/j.colsurfa.2016.07.057
104. Wang FC, Marangoni AG. Internal and external factors affecting the stability of glycerol monostearate structured emulsions. RSC Adv. (2015) 5:93108–16. doi: 10.1039/C5RA18748F
105. García-Moreno PJ, Jacobsen C, Marcatili P, Gregersen S, Overgaard MT, Andersen ML, et al. Emulsifying peptides from potato protein predicted by bioinformatics: stabilization of fish oil-in-water emulsions. Food Hydrocoll. (2020) 101:105529. doi: 10.1016/j.foodhyd.2019.105529
106. Zhu L, Xu Q, Liu X, Xu Y, Yang L, Wang S, et al. Soy glycinin-soyasaponin mixtures at oil-water interface: interfacial behavior and O/W emulsion stability. Food Chem. (2020) 327:127062. doi: 10.1016/j.foodchem.2020.127062
107. Xu L, Yan W, Zhang M, Hong X, Liu Y, Li J. Application of ultra-sound in stabilizing of Antarctic krill oil by modified chickpea protein isolate and gin-seng saponin. LWT. (2021) 149:111803. doi: 10.1016/j.lwt.2021.111803
108. Wang S, Shi Y, Tu Z, Zhang L, Wang H, Tian M, et al. Influ-ence of soy lecithin concentration on the physical properties of whey protein isolate-stabilized emulsion and microcapsule formation. J Food Eng. (2017) 207:73–80. doi: 10.1016/j.jfoodeng.2017.03.020
109. Zhang T, Ding M, Zhang H, Tao N, Wang X, Zhong J. Fish oil-loaded emulsions stabilized by synergetic or competitive adsorption of gelatin and surfactants on oil/water interfaces. Food Chem. (2020) 308:125597. doi: 10.1016/j.foodchem.2019.125597
110. Li W, Wang Y, Li J, Jiao Y, Chen J. Synergistic and competitive ef-fects of monoglycerides on the encapsulation and interfacial shear rheological behavior of soy proteins. Food Hydrocoll. (2019) 89:631–6. doi: 10.1016/j.foodhyd.2018.11.023
111. Tong X, Cao J, Sun M, Liao P, Dai S, Cui W, et al. Physical and oxidative stability of oil-in-water (O/W) emulsions in the presence of protein (peptide): characteristics analysis and bioinformatics predic-tion. LWT. (2021) 149:111782. doi: 10.1016/j.lwt.2021.111782
112. Yesiltas B, Gregersen S, Lægsgaard L, Brinch ML, Olsen TH, Marcatili P, et al. Emul-sifier peptides derived from seaweed, methanotrophic bacteria, and potato proteins identified by quantitative proteomics and bioinformatics. Food Chem. (2021) 362:130217. doi: 10.1016/j.foodchem.2021.130217
113. Schwarzländer M, Wagner S, Ermakova YG, Belousov VV, Radi R, Beckman JS, et al. The ‘mitoflash’ probe cpYFP does not respond to superoxide. Nature. (2014) 514:E12–4. doi: 10.1038/nature13858
114. Dabbour M, He R, Mintah B, Xiang J, Ma H. Changes in functionali-ties, conformational characteristics and antioxidative capacities of sunflower protein by controlled enzymolysis and ultrasonication action. Ultrason Sonochem. (2019) 58:104625. doi: 10.1016/j.ultsonch.2019.104625
115. Cao C, Zhou Z, Zheng L, Huang Q, Du F. Dilational rheology of dif-ferent globular protein with imidazolium-based ionic liquid surfactant adsorption layer at the decane/water interface. J Mol Liq. (2017) 233:344–51. doi: 10.1016/j.molliq.2017.02.121
116. Karefyllakis D, Altunkaya S, Berton-Carabin CC, van der Goot AJ, Nikifo-ridis CV. Physical bonding between sunflower proteins and phenols: impact on interfacial properties. Food Hydrocoll. (2017) 73:326–34. doi: 10.1016/j.foodhyd.2017.07.018
117. Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. (2015) 12:7–8. doi: 10.1038/nmeth.3213
118. Jahanban-Esfahlan A, Panahi-Azar V. Interaction of glutathione with bo-vine serum albumin: spectroscopy and molecular docking. Food Chem. (2016) 202:426–31. doi: 10.1016/j.foodchem.2016.02.026
119. Hou Y, Niemi AJ, Peng X, Ilieva N. Myoglobin ligand gate mechanism analysis by a novel 3D visualization technique. J Math Chem. (2019) 57:1586–97. doi: 10.1007/s10910-019-01021-4
120. Tandang-Silvas MRG, Fukuda T, Fukuda C, Prak K, Cabanos C, Kimura A, et al. Conservation and diver-gence on plant seed 11S globulins based on crystal structures. Biochim Biophys Acta Proteins Proteomics. (2010) 1804:1432–42. doi: 10.1016/j.bbapap.2010.02.016
121. Jameson GB, Adams JJ, Creamer LK. Flexibility, functionality and hydrophobicity of bovine β-lactoglobulin. Int Dairy J. (2002) 12:319–29. doi: 10.1016/S0958-6946(02)00028-6
122. Bhattacharya M, Mukhopadhyay S. Structural and dynamical insights into the molten-globule form of ovalbumin. J Phys Chem B. (2012) 116:520–31. doi: 10.1021/jp208416d
123. Mohammadi F, Moeeni M. Study on the interactions of trans-resveratrol and curcumin with bovine α-lactalbumin by spectroscopic analysis and molecular docking. Mater Sci Eng C. (2015) 50:358–66. doi: 10.1016/j.msec.2015.02.007
124. Wong BT, Zhai J, Hoffmann SV, Aguilar MI, Augustin M, Wooster TJ, et al. Conformational changes to deamidated wheat gliadins and β-casein upon adsorption to oil–water emulsion interfaces. Food Hydrocoll. (2012) 27:91–101. doi: 10.1016/j.foodhyd.2011.08.012
125. Cao X, He Y, Kong Y, Mei X, Huo Y, He Y, et al. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocoll. (2019) 94:63–70. doi: 10.1016/j.foodhyd.2019.03.006
126. Xiong G, Jiang X, Xie F, Fan Y, Xu X, Zhang M, et al. Effect of high-pressure homogenization on structural changes and emulsifying properties of chicken liver proteins isolated by isoelectric solubilization/precipitation. LWT. (2021) 151:112092. doi: 10.1016/j.lwt.2021.112092
127. Liu Z, Guo Z, Wu D, Fei X, Ei-Seedi HR, Wang C. High-pressure homogenization influences the functional properties of protein from oyster (Crassostrea gigas). LWT. (2021) 151:112107. doi: 10.1016/j.lwt.2021.112107
128. Östbring K, Matos M, Marefati A, Ahlström C, Gutiérrez G. The effect of ph and storage temperature on the stability of emulsions stabilized by rapeseed proteins. Foods. (2021) 10:1657. doi: 10.3390/foods10071657
129. Pei Y, Wan J, You M, McClements DJ, Li Y, Li B. Impact of whey protein complexation with phytic acid on its emulsification and stabilization properties. Food Hydrocolloids. (2019) 87:90–6.
130. Böttcher S, Keppler JK, Drusch S. Mixtures of Quillaja saponin and beta-lactoglobulin at the oil/water-interface: adsorption, interfacial rheology and emulsion properties. Colloids Surf A. (2017) 518:46–56. doi: 10.1016/j.colsurfa.2016.12.041
Keywords: interfacial properties, protein emulsifier, oil in water emulsion, physical strategies, chemical strategies
Citation: Zhang H, Zhao X, Chen X and Xu X (2022) Thoroughly review the recent progresses in improving O/W interfacial properties of proteins through various strategies. Front. Nutr. 9:1043809. doi: 10.3389/fnut.2022.1043809
Received: 14 September 2022; Accepted: 10 October 2022;
Published: 26 October 2022.
Edited by:
Rui Liu, Yangzhou University, ChinaReviewed by:
Changyu Zhou, Ningbo University, ChinaCopyright © 2022 Zhang, Zhao, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Zhao, emhhb3h1ZUBuamF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.