
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 06 December 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1042558
This article is part of the Research Topic The 4th NACAN Summit Proceedings: Nutrition and Food for a Healthy Life View all 11 articles
 Dongxia Wang1,2
Dongxia Wang1,2 Ning Wang1
Ning Wang1 Juan Zhou1
Juan Zhou1 Gang Luo1
Gang Luo1 Yanyan Li3
Yanyan Li3 Wei Yu3
Wei Yu3 Hongxing Tan3
Hongxing Tan3 Gang Liu4*†
Gang Liu4*† Jun Wang5*†
Jun Wang5*† Liping Hao1
Liping Hao1Introduction: Trace element metabolism disorders are often secondary to disorders of glucose metabolism in diabetes. Although 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] could ameliorate abnormal glucose metabolism in the development of diabetes, the effect on trace element metabolism is unclear. The objective of this study was to evaluate the influence of 1,25(OH)2D3 on urinary excretions of trace elements in Zucker diabetic fatty (ZDF) rats.
Methods: At 6 weeks of age, male ZDF (n = 40) rats were subdivided into four groups: diabetic model (ZDF), low-dose (ZDF + VL, 2 μg/kg⋅bw), middle-dose (ZDF + VM, 8 μg/kg⋅bw) and high-dose (ZDF + VH, 16 μg/kg⋅bw) 1,25(OH)2D3 groups. Another 10 Zucker lean (ZL) rats served as a control group. All rats were given vitamin D deficient Purina #5008 chow and the intervention groups were given the corresponding dose of 1,25(OH)2D3 by gavage on alternate days for 7 weeks. Microalbuminuria (MALB) and urinary creatinine concentration were detected by a biochemical autoanalyzer. Urine trace element concentrations were measured using inductively coupled plasma mass spectrometry (ICP-MS) and were corrected by urinary creatinine.
Results: Throughout the intervention phase, MALB, UACR and urinary creatinine levels in the ZDF group were significantly higher than those in the ZL group, and showed a gradual increase with the prolongation of the intervention time. These changes were reversed in a dose-dependent manner after 1,25(OH)2D3 intervention (P < 0.05). Correspondingly, most of the urinary trace element excretions in the ZDF rats were significantly increased compared with the ZL group, and 1,25(OH)2D3 intervention significantly reduced the urinary copper (Cu), zinc (Zn), selenium (Se) and molybdenum (Mo) levels in the ZDF rats (P < 0.05), especially in the medium and high dose groups.
Conclusion: 1,25(OH)2D3 had improvement effects on urinary Cu, Zn, Se, and Mo excretions in ZDF rats, suggesting that it may be related to the reduction of diabetic renal impairment and renal oxidative damage.
Type 2 diabetes mellitus (T2DM) is an endocrine metabolic disease characterized by chronic hyperglycemia and has become a global public health problem that poses a serious threat to human health. According to the latest data published by the International Diabetes Federation, 537 million adults aged 20–79 years have diabetes worldwide in 2021, with a prevalence of 10.5%, of which about 90% are T2DM (1). Among the abovementioned diabetic patients, about 140.9 million are from China, accounting for about a quarter of the global figure. In addition, a nationally representative epidemiological survey showed that the prevalence of diabetes among adults in China was as high as 12.8% in 2017, much higher than 10.9% in 2013 (2). Therefore, the prevention and treatment of T2DM is an important scientific problem that needs to be addressed urgently.
Abnormal glucose metabolism is now recognized as the most fundamental pathophysiological feature in the development of T2DM (3). In addition, the relationship between diabetes and disorders of trace element metabolism has received much attention, largely because most trace elements are involved in glucose metabolism as important components of certain enzymes and hormones (4, 5). It has been shown that trace element metabolism disorders in diabetic patients are often secondary to disorders of glucose metabolism (6). Some studies reported that whole blood levels of zinc, manganese, and chromium were significantly lower while the urinary levels of the same elements were found to be higher in the T2DM patients than in their healthy age-matched counterparts (5). Recent studies found that serum magnesium levels are decreased and serum copper, zinc, and selenium levels are elevated in patients with T2DM (6). In conclusion, we find that the results regarding the association of trace elements and the risk of T2DM are inconsistent.
Vitamin D, an essential fat-soluble vitamin, is mostly converted from 7-dehydrocholesterol in the skin by ultraviolet light exposure. It further undergoes two hydroxylation reactions in the liver and kidney to produce 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] before becoming biologically active, and active vitamin D exerts its physiological effects mainly through binding to the Vitamin D Receptor (VDR) (7). Given that VDR is widely distributed in most tissues and cells of the body, vitamin D deficiency is not only associated with disorders of calcium and phosphorus metabolism but also with cancer, autoimmune diseases, metabolic and cardiovascular diseases (diabetes, hypertension, renal diseases, etc.) (8, 9). Evidence from numerous observational studies suggests that vitamin D deficiency is prevalent in patients with T2DM and that the incidence of diabetes is negatively correlated with serum 25(OH)D concentration (10–12). A cross-sectional study based on a Chinese diabetic population showed that 83.5% of patients with T2DM had vitamin D deficiency [serum 25(OH)D concentration < 20 ng/mL], and the prevalence of vitamin D deficiency was more severe in patients with combined diabetic nephropathy than in those without diabetic nephropathy (93.1% vs. 78.9%) (13).
To simulate vitamin D deficiency or insufficiency in T2DM patients, Zucker diabetic fatty (ZDF) rats were fed with vitamin D deficient Purina #5008 chow to establish a vitamin D deficient T2DM animal model. Previous study revealed that vitamin D deficiency accelerated and exacerbated the dysregulation of glucose metabolism in non-obese T2DM rats by increasing insulin resistance and that vitamin D deficiency might be a key factor in the pathogenesis of T2DM (14). The present study further focused on the key role of 1,25(OH)2D3 supplementation in a vitamin D deficient model of diabetes. No evidence is available regarding the effect of vitamin D supplementation on urinary trace element metabolism in diabetes. The aim of the present study is to clarify whether 1,25(OH)2D3 supplementation has a protective effect against disorders of trace element excretion in vitamin D-deficient ZDF rats.
1,25(OH)2D3 (purity ≥ 97%) was obtained from Cayman Chemical (Item No. 71820, USA). Microalbuminuria (MALB) and creatinine assay kits were supplied by Shenzhen Mindray Biomedical Electronics Co., Ltd. (Shenzhen, China), which were used via an automatic biochemical analyzer (BS-200, Mindray, Shenzhen, China). All other reagents were analytical grade.
Five-week-old male ZDF (n = 40) rats and age-matched male Zucker lean (ZL; n = 10) rats from Charles River Laboratory (Beijing, China) were maintained on the vitamin D-deficient Purina 5008 chow (500 IU vitamin D3/kg) and tap water ad libitum. Animals were kept on a regular 12:12 h light-dark cycle at a controlled temperature (22 ± 2°C) and relative humidity (55 ± 5%). All experimental procedures involving animals complied with the Regulations on the Administration of Laboratory Animals in China and were approved by the Animal Ethical Committee of Tongji Medical College (Approval NO. S432).
At the age of 6 weeks, male ZDF rats were subdivided into four groups (n = 10): diabetic model (ZDF), low-dose (ZDF + VL, 2 μg/kg⋅bw), middle-dose (ZDF + VM, 8 μg/kg⋅bw), and high-dose (ZDF + VH, 16 μg/kg⋅bw) 1,25(OH)2D3 groups. Another 10 ZL rats served as a control group. 1,25(OH)2D3 was dissolved in corn oil and administered by gavage on alternate days for 7 weeks. The vehicle groups (ZL and ZDF) were only gavaged with corn oil.
At the treatment of 1,4,6,7 weeks, the Zucker rats were housed in metabolic cages and their 24 h urine was dynamically collected at least 3 mL per rat to detect indicators of renal function and minerals. Sodium azide was added to all urine samples at a volume ratio of 10 μL/mL before freezing to protect the urine samples from contamination. When fasting blood glucose (FBG) ≥ 16.7 mmol/L, the model of diabetes was established. At the end of the experiment, the rats were weighed after an overnight fast of 8 h. Blood was collected from the eyes, serum was obtained at least 1 mL per rat from blood samples that has been left at room temperature for 2 h and then centrifuged at 4,000 r/min for 15 min, and the kidneys were removed and weighed. All samples were stored at –80°C for subsequent processing.
FBG was measured by glucose oxidase method according to the manufacturer’s instructions (Biosino Bio-technology and Science Inc., Beijing, China). Serum fasting insulin (FINS) was assayed by double antibody sandwich ELISA using corresponding kit (Millipore Corporation, Billerica, USA). HOMA-IR and HOMA-β were calculated from fasting blood glucose and fasting serum insulin levels with the following formulae:
Serum triglyceride (TG) concentration was detected by phosphoglyceride oxidase-peroxidase method (GPO-PAP). The levels of total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) in serum were measured by the cholesterol oxidase-peroxidase method (CHOD-PAP). The assay kits of lipid indicators mentioned above were supplied by Biosino Bio-technology and Science Inc. Serum adiponectin was detected using ELISA kit (R&D Systems Inc., Minneapolis, USA). All procedures were carried out in strict accordance with the manufacturer’s protocol.
24 h urinary collections were taken at 1, 4, 6, and 7 weeks of treatment, respectively. MALB and urinary creatinine concentration were detected by a Mindray BS-200 biochemical autoanalyzer. Subsequently, urea microalbuminuria creatinine ratio (UACR) was calculated via the following equation:
Twenty-four hour urine samples were collected at 1, 4, 6, and 7 weeks of treatment and stored at –80°C for the dynamic analysis of urinary trace element excretion. Firstly, the urine samples were centrifuged at 4,000 rpm, 4°C for 10 min before being diluted. We mixed 400 μL urine sample and 20 μL of 60% nitric acid solution together and placed them in a refrigerator at 4°C for overnight acidification. Then added 3,580 μL of 1% nitric acid solution to dilute the urine sample 10 times. After centrifuging at 4,000 rpm, 4°C for 10 min, the urine samples were assayed. All samples were measured in triplicate.
Urinary trace element concentrations in the studied samples were detected by inductively coupled plasma mass spectrometer (ICP-MS) at Agilent 7700 (Agilent Technologies, Tokyo, Japan). The obtained data on urinary trace element contents were corrected with urine creatinine concentration and expressed as μg/g UCR.
Data of urine metal concentration were displayed as mean ± SEM or median (IQR). If the data obeyed normal distribution, the statistical significance of comparisons between multiple groups was analyzed by one-way ANOVA, followed by the Least Significant Difference (LSD) multiple range test if the variances were constant, otherwise the Dunnett’s T3 procedure. If the data didn’t follow a normal distribution, we performed a non-parametric (Kruskal-Wallis H) test. All statistical analyses were conducted by SPSS 21.0. P-value < 0.05 was considered to show a statistically significant difference.
ZDF rats were fed with Purina #5008 chow for 7 weeks to establish a spontaneous T2DM rat model, and 1,25(OH)2D3 was administered at the same time. As shown in Figure 1, there was no significant difference in the initial body weight (week 0) of the rats in each group. With the prolongation of the intervention time, the body weight of the rats in each group gradually increased. From the first week of intervention to the end of the experiment, the body weight of the rats in the diabetes group (ZDF) was significantly higher than that in the control group (ZL). While the body weight of the rats in the high-dose 1,25(OH)2D3 intervention group (ZDF + VH) was significantly lower than that in the ZDF group (P < 0.05). At the 7th week of intervention, the body weight of the rats in the ZDF group increased by 28% compared with the ZL group, while compared with the ZDF group, the figure decreased by 18% in the ZDF + VH group (P < 0.05). Meanwhile, the results showed that the weight of kidneys and kidney index in the ZDF group were significantly increased compared with those in the ZL group, and the 1,25(OH)2D3 dose groups effectively reversed the above changes.
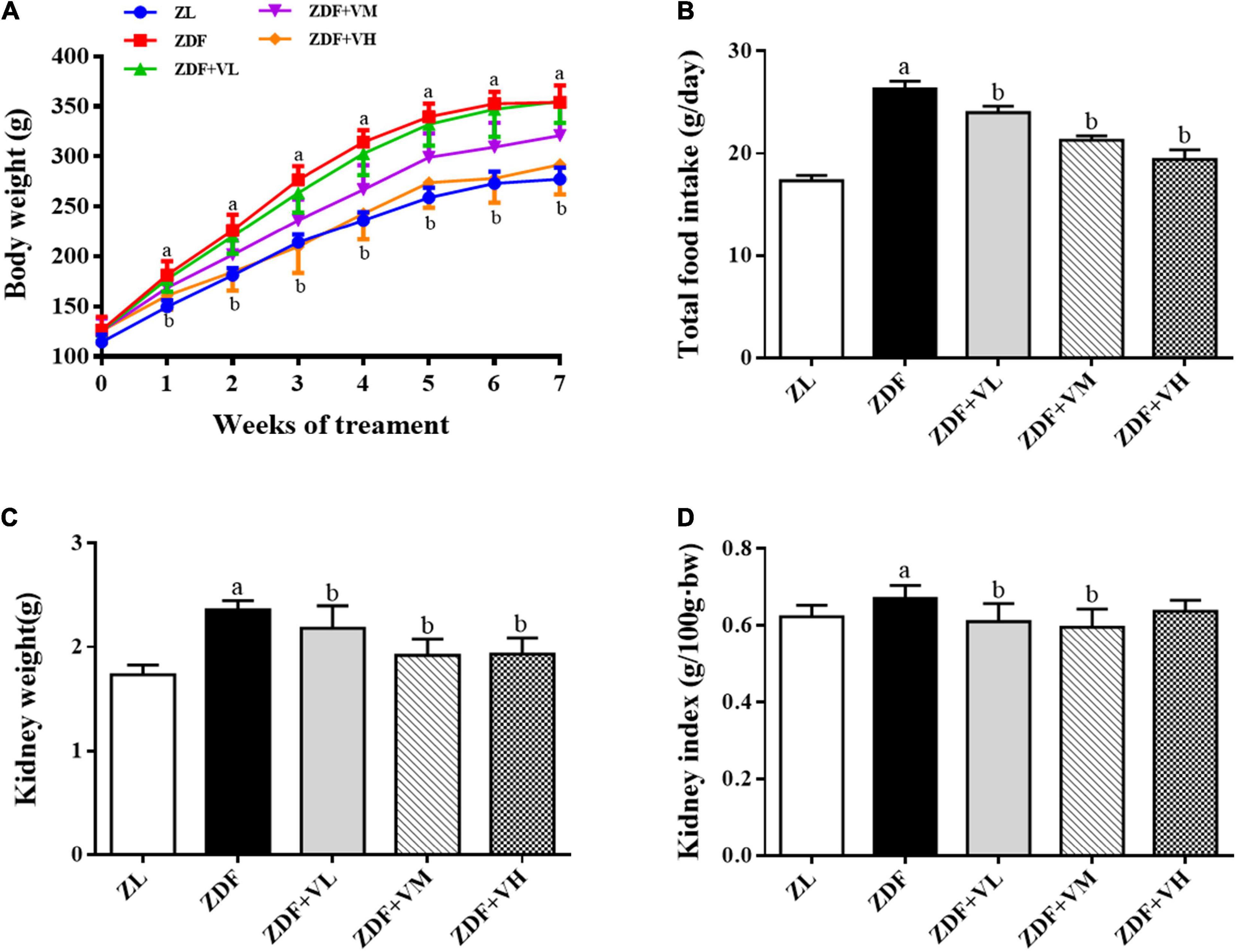
Figure 1. Effects of 1,25(OH)2D3 on the body weight, food intake, and kidney index in Zucker rats. (A) Variation trends of body weight as weeks of 1,25(OH)2D3 treatment. (B) Total food intake. (C) Kidney weight. (D) Kidney index. ZL, Zucker lean group; ZDF, Zucker diabetic fatty group; ZDF + VL, ZDF + low-dose 1,25(OH)2D3 group (2 μg/kg⋅bw); ZDF + VM, ZDF + middle-dose 1,25(OH)2D3 group (8 μg/kg⋅bw); ZDF + VH, ZDF + high-dose 1,25(OH)2D3 group (16 μg/kg⋅bw). Data are presented as mean ± SD (n ≥ 7). aP < 0.05 vs. the ZL group, bP < 0.05 vs. the ZDF group.
As shown in Table 1, the levels of fasting blood glucose, serum insulin, and HOMA-IR in the ZDF group were significantly higher than those in the ZL group. Although the serum insulin in each 1,25(OH)2D3 intervention group was at a high level, the fasting blood glucose and HOMA-IR in each dose group were significantly lower than those in the ZDF group, and the function of HOMA-β was significantly enhanced (P < 0.05). It indicated that 1,25(OH)2D3 played an important role in increasing insulin sensitivity, and the improvement effect was most promising in the middle and high dose groups.
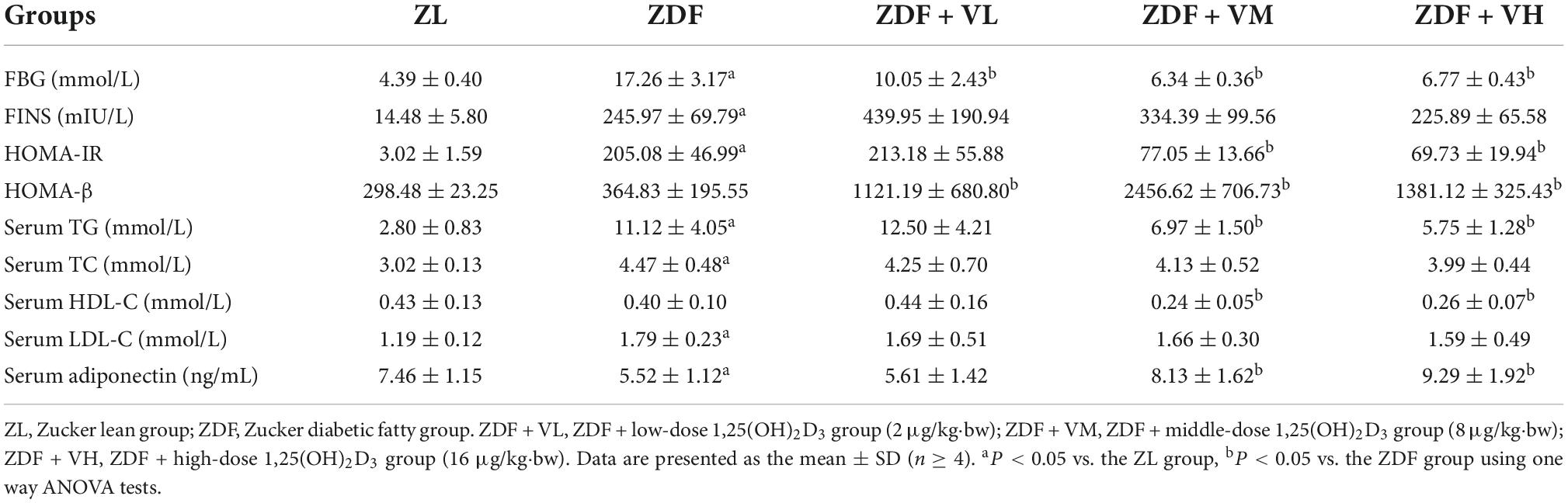
Table 1. Effects of 1,25(OH)2D3 on insulin sensitivity and parameters of lipid metabolism in Zucker rats.
Compared with the ZL group, the serum TG, TC, and LDL-C levels of the ZDF group were increased by 297, 48, and 50%, respectively (P < 0.05). On the contrary, the serum TG levels of the ZDF + VM and ZDF + VH groups were decreased by 37 and 48% than the ZDF group (P < 0.05), meanwhile the serum TC and LDL-C concentrations showed a dose-dependent decrease. Adiponectin is an adipokine produced by adipose tissue, which is involved in glucose and lipid metabolism and inflammatory response. The serum adiponectin level in the ZDF group was 26% lower than that in the ZL group (P < 0.05). However, the serum adiponectin levels in the ZDF + VM and ZDF + VH groups were increased by 47 and 68%, respectively, compared with the ZDF group (P < 0.05). The above results indicate that medium and high doses of 1,25(OH)2D3 supplementation can improve the abnormal lipid metabolism in spontaneous diabetic rats, and its lipid-lowering effect is mainly manifested by a significant reduction in TG levels and an increase in adiponectin levels.
To investigate whether 1,25(OH)2D3 has a nephroprotective effect on ZDF rats, we used UACR and urinary creatinine levels as indicators to evaluate the progress of DN. As shown in Figure 2, the MALB, UACR, and urine creatinine contents in the ZDF group were significantly higher than those in the ZL group during the whole intervention period, and the gap was gradually widened with the extension of the intervention time. These changes were reversed in a dose-dependent manner after 1,25(OH)2D3 intervention (P < 0.05).
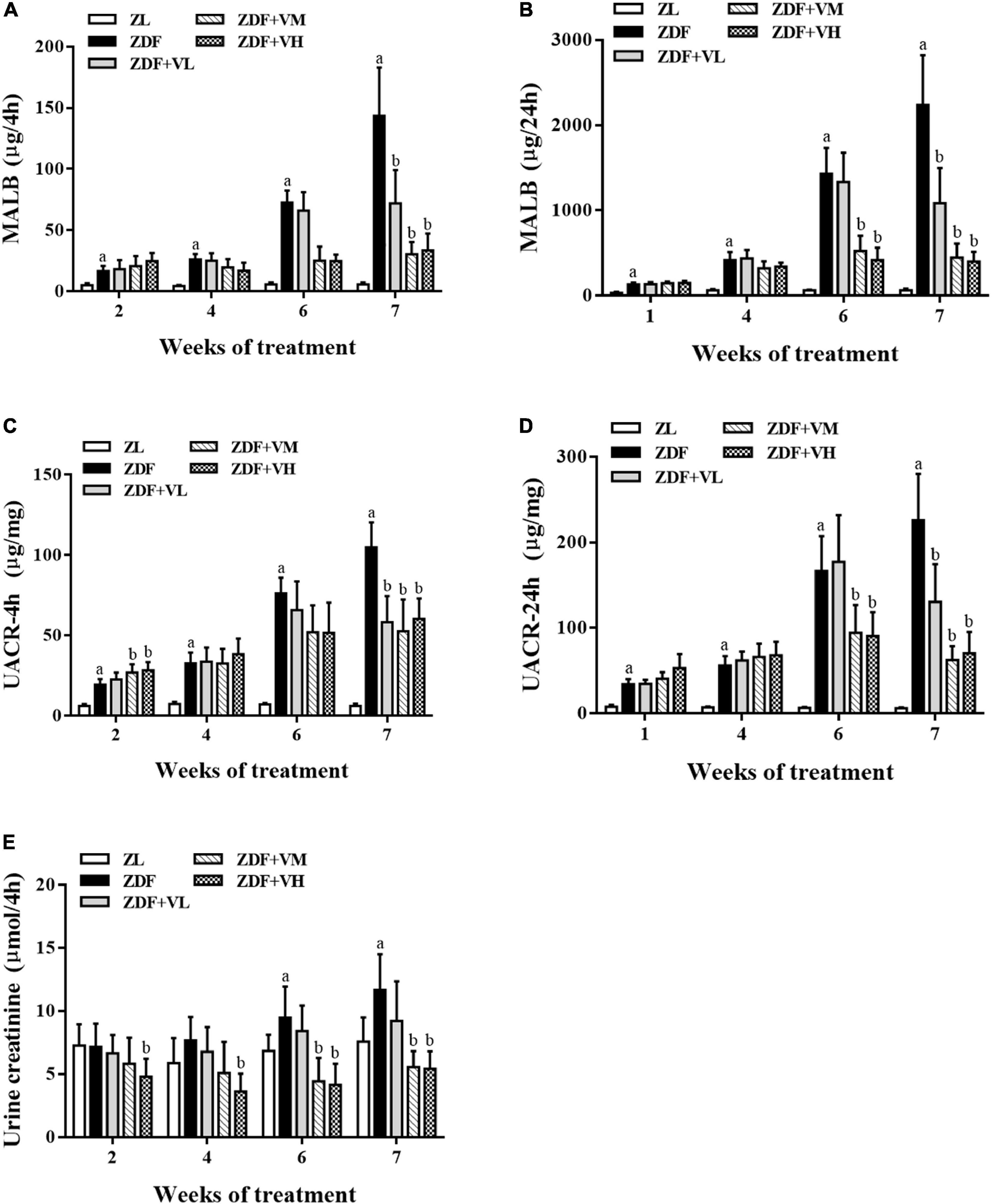
Figure 2. Variation trend of renal function indicators as weeks of 1,25(OH)2D3 treatment. (A,B) Four hour-MALB and 24 h-MALB contents. (C,D) Four hour-UACR and 24 h-UACR contents. (E) Four hour-Urine creatinine content. ZL, Zucker lean group; ZDF, Zucker diabetic fatty group; ZDF + VL, ZDF + low-dose 1,25(OH)2D3 group (2 μg/kg⋅bw); ZDF + VM, ZDF + middle-dose 1,25(OH)2D3 group (8 μg/kg⋅bw); ZDF + VH, ZDF + high-dose 1,25(OH)2D3 group (16 μg/kg⋅bw). Data are presented as mean ± SD (n ≥ 4). aP < 0.05 vs. the ZL group, bP < 0.05 vs. the ZDF group.
In this study, 23 trace element concentrations in urine were dynamically determined by ICP-MS and corrected with urinary creatinine concentrations in μg/g UCR, except Tl, Sb, Cd, Sn, and U which were in ng/g UCR. As seen in Table 2, at 1 week of intervention, urinary levels of Cu, Zn, Se, Mo, As, W, Ti, Co, Cr, Ni, Sr, and Cd were significantly increased in the ZDF group of rats compared to the ZL group, and 1,25(OH)2D3 intervention increased the levels of Se, Tl, Ti, Cr, Al, Mn, Sr, Cd, and Ba in urine compared with the ZDF group (P < 0.05).
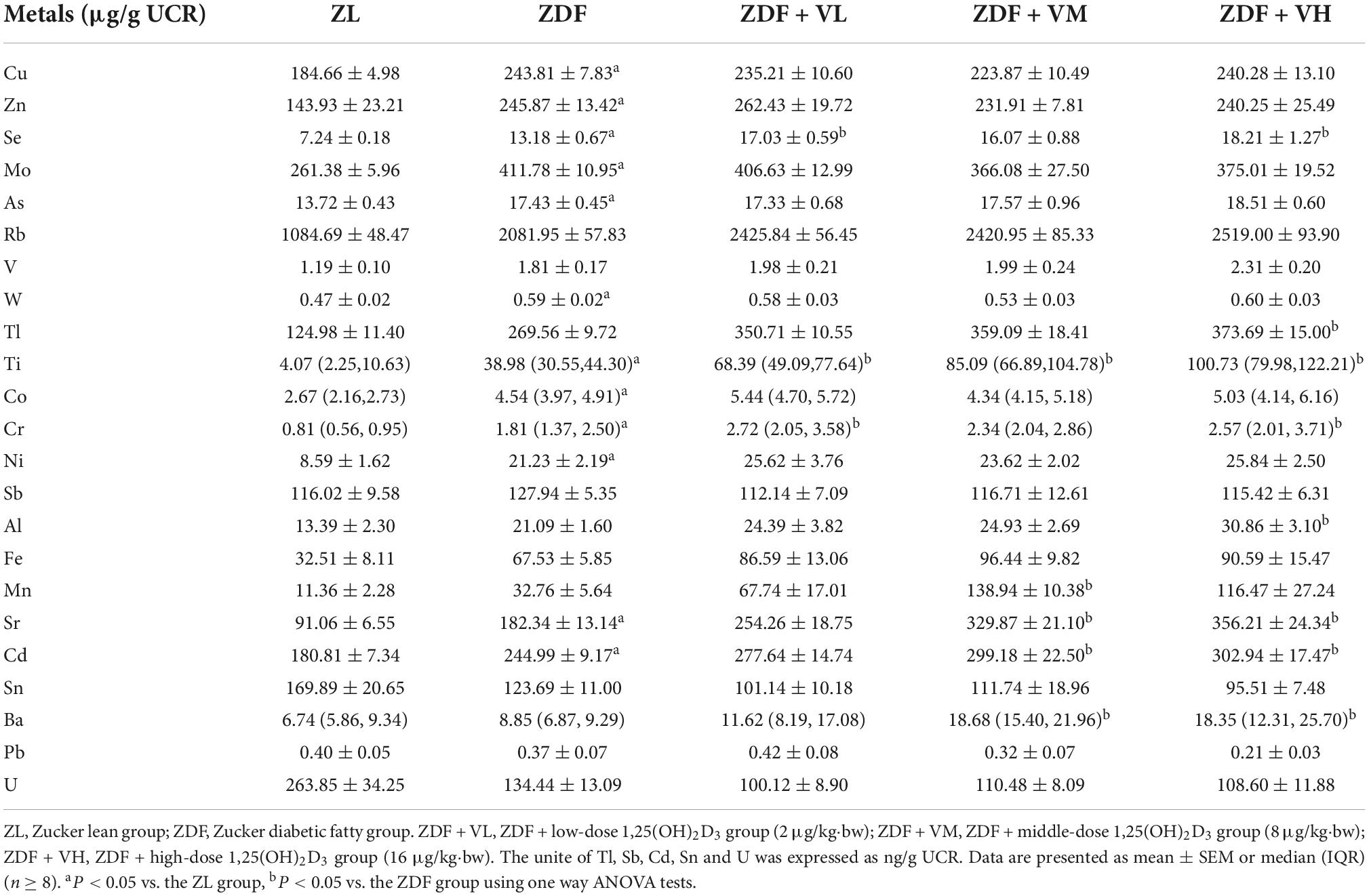
Table 2. Effects of 1,25(OH)2D3 on urine metal concentration of Zucker rats in the first week of treatment.
As seen from Table 3, after 4 weeks of intervention, the urinary levels of Zn, Se, Mo, As, Rb, V, W, Tl, Ti, Co, Cr, Ni, Sb, Al, Fe, Mn, Cd, Ba, and U in the ZDF group of rats were significantly increased compared to the ZL group. And the urinary levels of Se, Mo, As, Rb, W, Ni, Sb, Sn, and U after 1,25(OH)2D3 intervention were significantly decreased compared to the ZDF group, while Sr and Ba levels were increased compared to the ZDF group (P < 0.05).
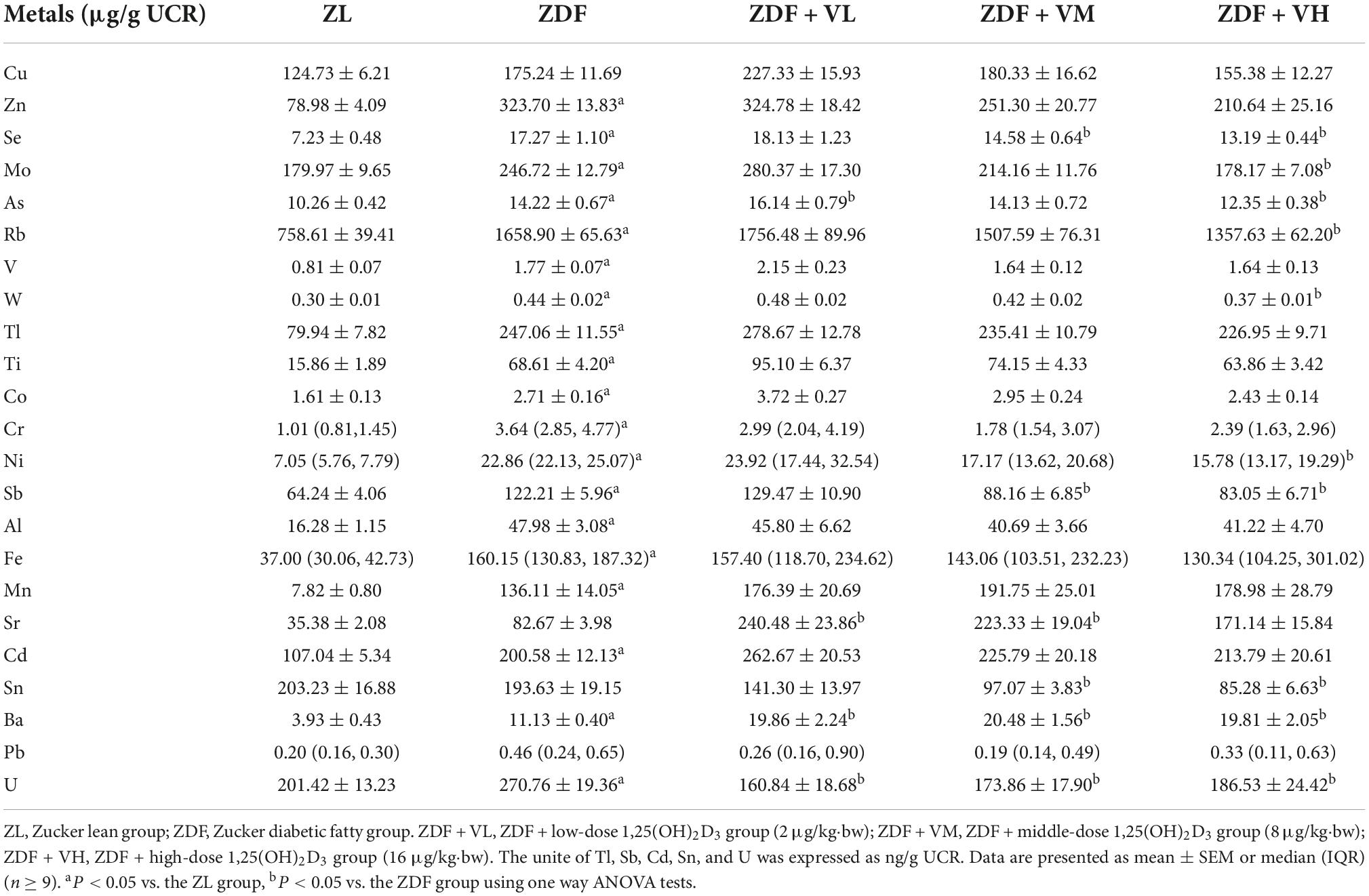
Table 3. Effects of 1,25(OH)2D3 on urine metal concentration of Zucker rats in the fourth week of treatment.
As seen from Table 4, after 6 weeks of intervention, the urinary levels of Cu, Zn, Se, Mo, As, Rb, V, W, Tl, Ti, Co, Cr, Ni, Sb, Al, Fe, Mn, Cd, Sn, Pb, and U in the ZDF group of rats were significantly increased compared to the ZL group. And the urinary levels of Cu, Zn, Mo, As, Rb, Cr, and U after 1,25(OH)2D3 intervention decreased compared with the ZDF group, while Sr level increased compared with the ZDF group (P < 0.05).
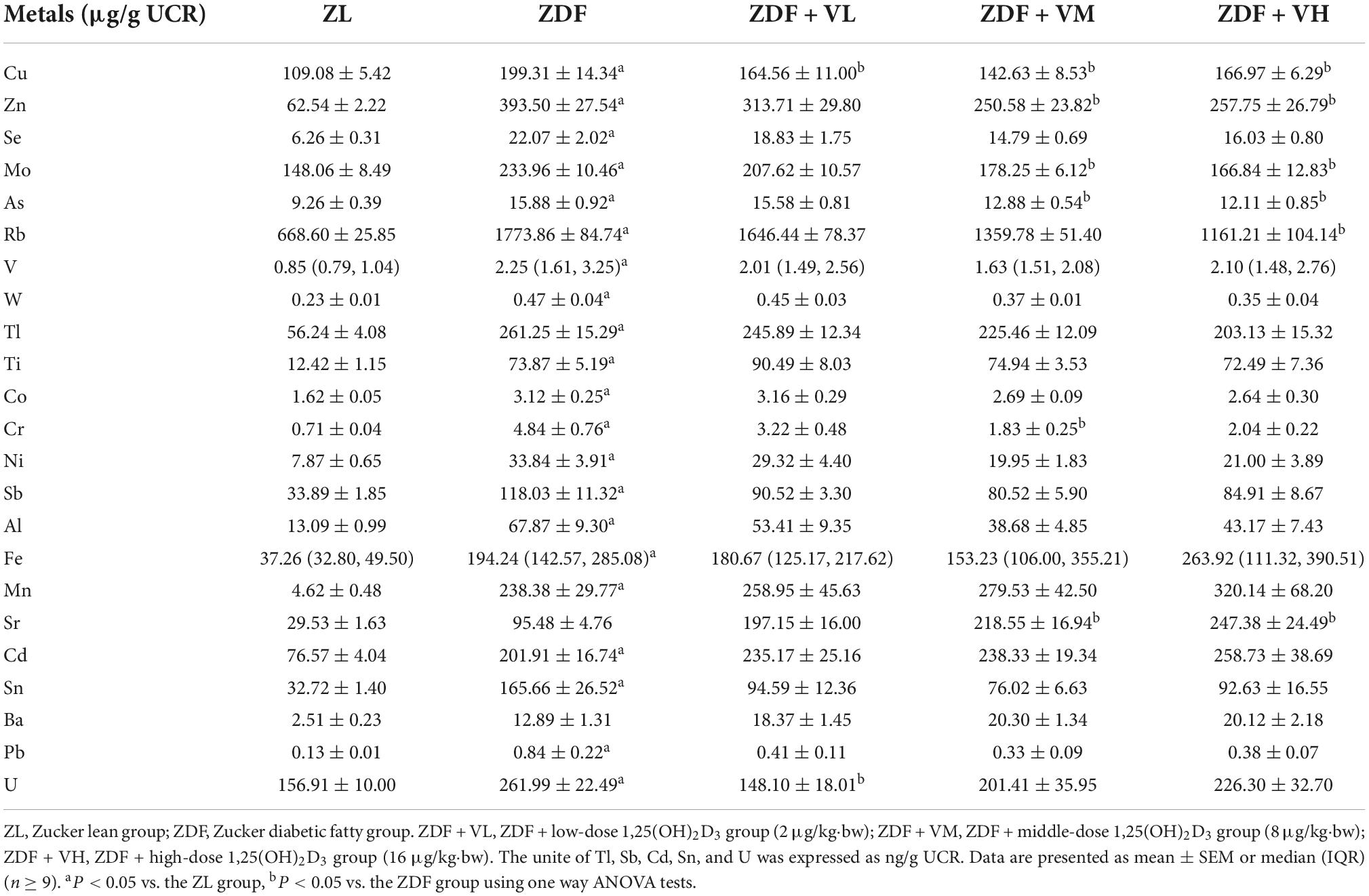
Table 4. Effects of 1,25(OH)2D3 on urine metal concentration of Zucker rats in the sixth week of treatment.
As can be seen from Table 5, after 7 weeks of intervention, the urinary levels of Cu, Zn, Se, Mo, As, Rb, V, W, Tl, Ti, Co, Cr, Ni, Sb, Al, Fe, Mn, Cd, and Sn in the ZDF group of rats were significantly increased compared to the ZL group, and the urinary levels of Cu, Zn, Se, Mo after 1,25(OH)2D3 intervention decreased compared to the ZDF group, while Sr level increased compared to the ZDF group after 1,25(OH)2D3 intervention (P < 0.05).
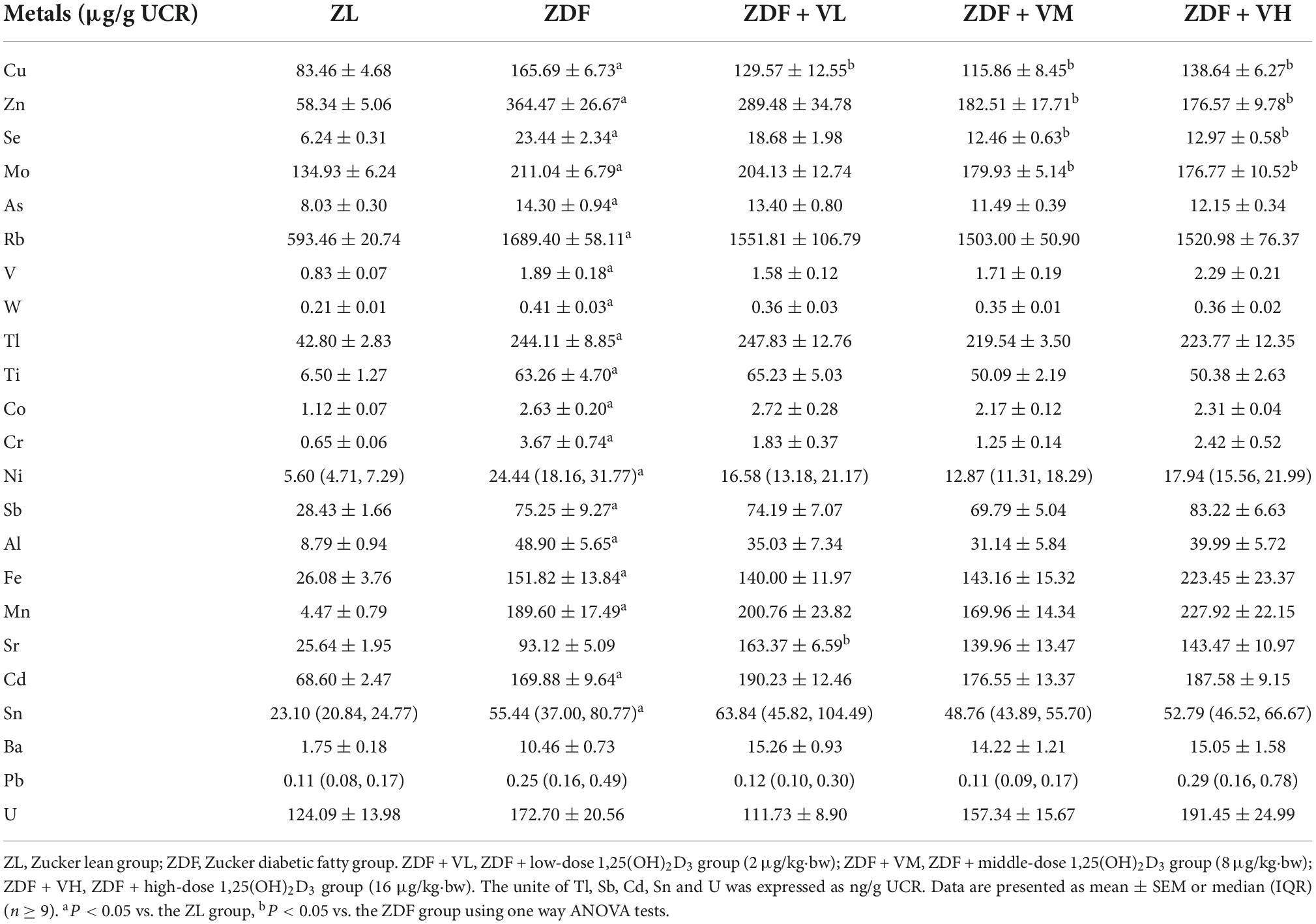
Table 5. Effects of 1,25(OH)2D3 on urine metal concentration of Zucker rats in the seventh week of treatment.
From Tables 2–5, it was found that the trend of Cu, Zn, Se, and Mo levels in the urine of spontaneously diabetic rats after 1,25(OH)2D3 intervention was the most obvious. These four trace elements were thus plotted to visualize their dynamic changes with the extension of the intervention time. As shown in Figures 3A–D, the urinary levels of Cu, Zn, Se, and Mo in the ZDF group increased significantly (P < 0.05) compared with the ZL group at 1st week of intervention. At 4th week of intervention, the urinary levels of Cu, Zn, Se, and Mo in rats in each dose group of 1,25(OH)2D3 showed a tendency to decrease compared with the ZDF group. And with the extension of the intervention time, the differences of the above four urinary trace elements levels in the rats of ZDF + VM and ZDF + VH groups gradually increased compared with the ZDF group, which to some extent coincided with the trend of urine volume. Additionally, we also measured the urinary levels of calcium (Ca) and magnesium (Mg). As displayed in Figures 3E,F, the urinary levels of Ca and Mg in the ZDF group increased compared with the ZL group during the whole intervention, but the differences were not significant for urinary Ca. Relatively, the urinary levels of Ca and Mg in rats in each dose group of 1,25(OH)2D3 maintained high levels even upper than the ZDF group.
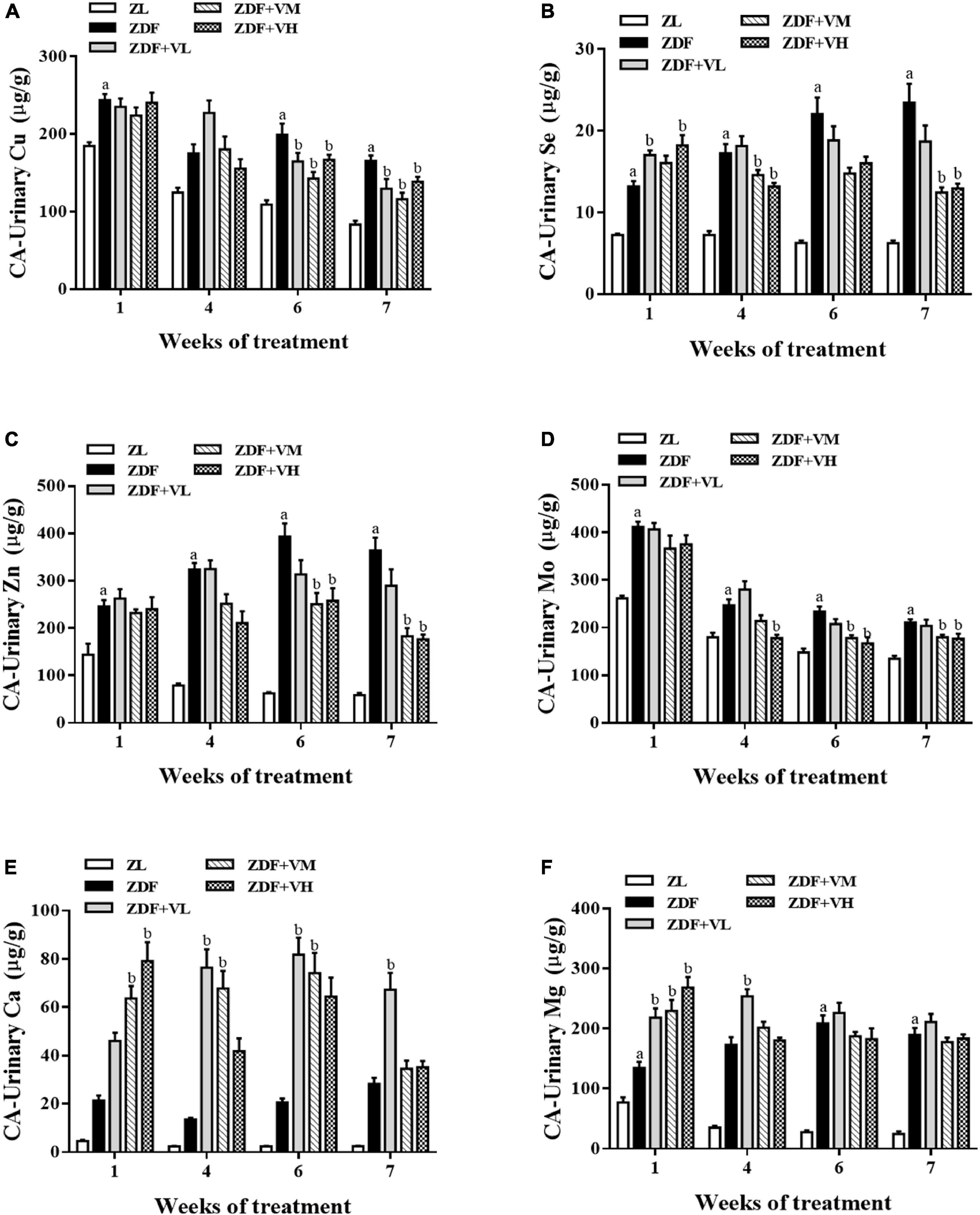
Figure 3. Effect of 1,25(OH)2D3 on urinary mineral levels in Zucker rats. (A) Urinary Cu/UCR. (B) Urinary Se/UCR. (C) Urinary Zn/UCR. (D) Urinary Mo/UCR. (E) Urinary Ca/UCR. (F) Urinary Mg/UCR. ZL, Zucker lean group; ZDF, Zucker diabetic fatty group; ZDF + VL, ZDF + low-dose 1,25(OH)2D3 group (2 μg/kg⋅bw); ZDF + VM, ZDF + middle-dose 1,25(OH)2D3 group (8 μg/kg⋅bw); ZDF + VH, ZDF + high-dose 1,25(OH)2D3 group (16 μg/kg⋅bw). Data are presented as mean ± SEM (n ≥ 9). aP < 0.05 vs. the ZL group, bP < 0.05 vs. the ZDF group.
The present study provides evidence that 1,25(OH)2D3 improves kidney index, insulin sensitivity, abnormal glucose and lipid metabolism, renal function injury and urine trace element metabolism disorder in spontaneous diabetic rats. We found that spontaneous diabetes could lead to renal function damage by increasing MALB, UACR, urine creatinine contents, and exacerbate the disorder of most trace elements in urine. As predicted, we showed that 1,25(OH)2D3 treatment significantly attenuated renal damage and decreased urine Cu, Zn, Se, and Mo contents. However, given that there are few studies on vitamin D reducing urinary trace elements in diabetic patients or models, more studies are needed to confirm this conclusion in the future.
Evidence has reported that MALB is a marker of early kidney damage in DN. In our study, the continuous increase of MALB content confirmed the occurrence and development of renal dysfunction in spontaneous diabetic rats, accompanied by the continuous increase of UACR and urinary creatinine content. Importantly, treatment with 1,25(OH)2D3 decreased the impairment of renal function. Similarly, 1,25(OH)2D3 has been reported to ameliorate renal dysfunction in rats with chronic renal failure (17) or may contribute to delay the deterioration in glomerular function and reduce the occurrence of ESRD in patients with diabetes (18). Besides, our previous research has revealed the positive effect of 1,25(OH)2D3 on renal morphological, pathological changes and oxidative stress damage (19). Considering that trace elements are closely related to diabetes, the levels of trace elements in urine were further detected. It was found that the metabolism of trace elements in the urine of spontaneous diabetic rats was disordered, and the intervention of 1,25(OH)2D3 could significantly reverse the urinary Cu, Zn, Se, and Mo contents of ZDF rats. The results also suggested that the disturbance of trace element metabolism in ZDF rats might be related to kidney damage.
Both a cross-sectional study and a multisite, multiethnic cohort study indicated that increased urinary excretion of zinc was associated with elevated risk of diabetes (20, 21). Some scholars have studied the effect of STZ-induced insulin-dependent diabetes on urinary excretion of zinc, copper and iron, and found that the onset of diabetes symptoms was associated with a rapid and sustained increase in the daily excretion of these three trace elements in urine, which was significantly reduced by insulin treatment (22). Combined with our study, it can be indicated that 1,25(OH)2D3 intervention can achieve the efficacy of insulin therapy to a certain extent. Another study indicated that diabetes can alter levels of copper, zinc, magnesium, and lipid peroxidation, and it also highlighted that disturbance in mineral metabolism was more pronounced in diabetic patients with specific complications (23). Gong later suggested that an imbalance in copper homeostasis may be a key event in triggering the development of diabetic nephropathy (24). Our present study further found that 1,25(OH)2D3 may reduce diabetic kidney injury by restoring urinary copper levels.
Currently, urinary trace element levels are expressed in different ways. Data from a study in Northeast China showed an increase in urinary Zn levels, a decrease in the Zn/Cu ratio, and a decrease in urinary Se levels in patients with T2DM (25). A cross-sectional study has indicated significantly higher urinary Zn levels and no significant changes in urinary Cu, Mo, and Se levels in diabetic patients as compared to healthy subjects (26). However, it is worth noting that these studies present trace element concentrations rather than contents. In a STZ-induced mild diabetic rat model, 24-h urinary excretion of zinc, copper, iron, calcium, and magnesium was found to be positively correlated with urine volume (27), whose daily mineral excretion was determined by multiplying the daily urine volume by the concentration of each mineral in the urine. In most studies, however, such results are calculated by the ratio of the concentration of minerals in urine to the concentration of urinary creatinine. Our research used the latter method, while suggesting that the excretions of minerals in urine were proportional to the amount of urine. The reason could be that hyperglycemia may cause hyperosmolar urine or impair tubular reabsorption of trace elements which may further hamper tubular function and lead to hyperfiltration.
In a STZ-induced diabetic rat model, it showed increased levels of Fe and Cu and decreased levels of Zn and Mg in liver and kidney tissues, decreased contents of 24 h urinary Cu and increased contents of 24 h urinary Zn and Mg (28). This study suggests that impaired trace element metabolism may be associated with disturbances in oxidative homeostasis in liver and kidney tissues. More importantly, vitamin D supplementation modulated blood and tissue zinc concentrations, hepatic glutathione, and blood biochemical parameters in diabetic rats (29). However, studies on whether vitamin D supplementation modulates urinary trace elements in diabetic rats are relatively rare. Compared with previous studies, this study used ICP-MS to dynamically detect the concentrations of more than 20 trace elements in urine, and at the same time normalized and corrected the concentration of urine creatinine. It has made a comprehensive and systematic evaluation of the preventive effect of 1,25(OH)2D3 on urinary trace elements disorders in diabetic rats. Zinc (Zn) is an essential trace element in many enzymes and is involved in antioxidant defense. Numerous studies have shown that zinc supplementation has a protective effect against diabetic renal damage by promoting metallothionein synthesis (30, 31) and regulating oxidative stress (32–34). Meanwhile, triethylenetetramine (TETA) is a Copper (II)-selective chelation that inhibits copper-mediated oxidative stress and restores antioxidant defenses. A growing body of research suggests that TETA may reduce the chronic complications of diabetes by enhancing antioxidant defense mechanisms (35, 36).
Since copper and zinc are important components of superoxide dismutase (SOD), selenium is a component of the antioxidant enzyme GSH-Px, both of which are major natural antioxidants capable of scavenging excess free radicals from the body. Therefore, we speculate that renal function damage in spontaneous diabetic rats induces an increase in the excretion of urinary trace elements, which in turn causes trace element metabolism disorders. However, 1,25(OH)2D3 intervention may improve the metabolic disorder of trace elements by reducing the renal function damage in ZDF rats, among which copper, zinc, and selenium are most closely related to oxidative stress. Ito further pointed out that increased urinary copper excretion in patients with advanced diabetic nephropathy may be due to the copper-albumin and ceruloplasmin-copper complexes through the impaired glomeruli (37). Considering that the related mechanism of 1,25(OH)2D3 improving specific trace elements in urine is unknown, future research is warranted to explore the underlying mechanisms. Except for urine trace elements, we also focused the urinary levels of Ca and Mg. In brief, 1,25(OH)2D3 may accelerate urinary Ca and urinary Mg excretion in the early period, which is less pronounced with the extension of the intervention. This phenomenon needs to be further confirmed by other relevant studies.
Eventually, from our study, we speculate that 1,25(OH)2D3 supplementation may have a positive effect in diabetic rats that are deficient or inadequate in vitamin D status. Conversely, if vitamin D levels are adequate in diabetic rats, 1,25(OH)2D3 intervention may have no significant improvement. This is an important hypothesis that we need to validate in our next study.
In summary, our research confirmed that vitamin D-deficient diabetic rats suffered from renal injury and disturbance of trace element metabolism, manifested by increased production of MALB, UACR, urine creatinine content, and urinary excretion of trace elements. Interestingly, we found that 1,25(OH)2D3 reversed renal impairment and improved urinary excretions of Cu, Zn, Se, and Mo in ZDF rats. Our study indicated that 1,25(OH)2D3 may be a potential therapeutic strategy for correcting trace element disorders in DN patients in the near future.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
This animal study was reviewed and approved by the Animal Ethical Committee of Tongji Medical College.
DW: conceptualization, performing the experiment, and writing—original draft preparation. NW and JZ: performing the experiment. GLu: interpreting the results. YL: conceptualization. WY and HT: data analysis. GLi and JW: funding acquisition. LH: revising the manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Science and Technology Project of Hebei Education Department (grant no. QN2022016), the National Natural Science Foundation of China (grant no. 81703226), and the Hubei Key Laboratory of Kidney Disease Pathogenesis and Intervention (grant no. SB202107).
We thank Xiaohao Liu from Beijing Normal University for helping to polish English language.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; MALB, microalbuminuria; T2DM, type 2 diabetes mellitus; UACR, urea microalbuminuria creatinine ratio; ZDF, Zucker diabetic fatty; ZL, Zucker lean.
1. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels: International Diabetes Federation (2019).
2. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland china using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ. (2020) 369:m997. doi: 10.1136/bmj.m997
3. DeFronzo R, Ferrannini E, Groop L, Henry R, Herman W, Holst J, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. (2015) 1:15019. doi: 10.1038/nrdp.2015.19
4. Chen M, Lin P, Tsou C, Wang J, Lin W. Selected metals status in patients with noninsulin-dependent diabetes mellitus. Biol Trace Elem Res. (1995) 50:119–24. doi: 10.1007/bf02789414
5. Kazi T, Afridi H, Kazi N, Jamali M, Arain M, Jalbani N, et al. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. (2008) 122:1–18. doi: 10.1007/s12011-007-8062-y
6. Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, et al. Alterations of serum trace elements in patients with type 2 diabetes. J Trace Elem Med Biol. (2017) 40:91–6. doi: 10.1016/j.jtemb.2016.12.017
7. Holick M. The vitamin d deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
8. Thacher T, Clarke B. Vitamin D insufficiency. Mayo Clin Proc. (2011) 86:50–60. doi: 10.4065/mcp.2010.0567
9. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. (2010) 78:140–5. doi: 10.1038/ki.2010.17
10. Shen L, Zhuang Q, Ji H. Assessment of vitamin D levels in type 1 and type 2 diabetes patients: results from metaanalysis. Mol Nutr Food Res. (2016) 60:1059–67. doi: 10.1002/mnfr.201500937
11. Gagnon C, Lu Z, Magliano D, Dunstan D, Shaw J, Zimmet P, et al. Serum 25-hydroxyvitamin d, calcium intake, and risk of Type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian diabetes, obesity and lifestyle study). Diabetes Care. (2011) 34:1133–8. doi: 10.2337/dc10-2167
12. Lucato P, Solmi M, Maggi S, Bertocco A, Bano G, Trevisan C, et al. Low Vitamin d levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. Maturitas. (2017) 100:8–15. doi: 10.1016/j.maturitas.2017.02.016
13. Peng Y, Li L. Serum 25-hydroxyvitamin d level and diabetic nephropathy in patients with type 2 diabetes mellitus. Int Urol Nephrol. (2015) 47:983–9. doi: 10.1007/s11255-015-0983-3
14. Park S, Kim D, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing Ppar-gamma expression in nonobese type 2 diabetic rats. J Nutr Biochem. (2016) 27:257–65. doi: 10.1016/j.jnutbio.2015.09.013
15. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/bf00280883
16. Reaven G. Homa-beta in the Ukpds and adopt. Is the natural history of type 2 diabetes characterised by a Progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diab Vasc Dis Res. (2009) 6:133–8. doi: 10.1177/1479164109336038
17. Yang X, Wan J, Yuan J, Dong R, Da J, Sun Z, et al. Effects of calcitriol on peripheral endothelial progenitor cells and renal renovation in rats with chronic renal failure. J Steroid Biochem Mol Biol. (2021) 214:105956. doi: 10.1016/j.jsbmb.2021.105956
18. Nakhoul N, Thawko T, Farber E, Dahan I, Tadmor H, Nakhoul R, et al. The therapeutic effect of active vitamin D supplementation in preventing the progression of diabetic nephropathy in a diabetic mouse model. J Diabetes Res. (2020) 2020:7907605. doi: 10.1155/2020/7907605
19. Wang D, Li Y, Wang N, Luo G, Wang J, Luo C, et al. 1α,25-dihydroxyvitamin D3 prevents renal oxidative damage via the Parp1/Sirt1/Nox4 pathway in zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. (2020) 318:E343–56. doi: 10.1152/ajpendo.00270.2019
20. Wang X, Karvonen-Gutierrez C, Herman W, Mukherjee B, Harlow S, Park S. Urinary metals and incident diabetes in midlife women: study of women’s health across the nation (Swan). BMJ Open Diabetes Res Care. (2020) 8:e001233. doi: 10.1136/bmjdrc-2020-001233
21. Velmurugan G, Swaminathan K, Veerasekar G, Purnell J, Mohanraj S, Dhivakar M, et al. Metals in urine in relation to the prevalence of pre-diabetes, diabetes and atherosclerosis in rural India. Occup Environ Med. (2018) 75:661–7. doi: 10.1136/oemed-2018-104996
22. Lau A, Failla M. Urinary excretion of zinc, copper and iron in the streptozotocin-diabetic rat. J Nutr. (1984) 114:224–33. doi: 10.1093/jn/114.1.224
23. Walter R Jr, Uriu-Hare J, Olin K, Oster M, Anawalt B, Critchfield J, et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. (1991) 14:1050–6. doi: 10.2337/diacare.14.11.1050
24. Gong D, Lu J, Chen X, Reddy S, Crossman D, Glyn-Jones S, et al. A copper(Ii)-selective chelator ameliorates diabetes-evoked renal fibrosis and albuminuria, and suppresses pathogenic Tgf-beta activation in the kidneys of rats used as a model of diabetes. Diabetologia. (2008) 51:1741–51. doi: 10.1007/s00125-008-1088-7
25. Zhou Q, Guo W, Jia Y, Xu J. Serum and urinary selenium status in patients with the pre-diabetes and diabetes in northeast China. Biol Trace Elem Res. (2019) 191:61–9. doi: 10.1007/s12011-018-1604-7
26. Flores C, Puga M, Wrobel K, Garay Sevilla M, Wrobel K. Trace elements status in diabetes mellitus type 2: possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res Clin Pract. (2011) 91:333–41. doi: 10.1016/j.diabres.2010.12.014
27. Gomez T, Bequer L, Mollineda A, Molina J, Alvarez A, Lavastida M, et al. Concentration of zinc, Copper, iron, calcium, and magnesium in the serum, tissues, and urine of streptozotocin-induced mild diabetic rat model. Biol Trace Elem Res. (2017) 179:237–46. doi: 10.1007/s12011-017-0962-x
28. Ozcelik D, Tuncdemir M, Ozturk M, Uzun H. Evaluation of trace elements and oxidative stress levels in the liver and kidney of streptozotocin-induced experimental diabetic rat model. Gen Physiol Biophys. (2011) 30:356–63. doi: 10.4149/gpb_2011_04_356
29. Kechrid Z, Hamdi M, Nazıroğlu M, Flores-Arce M. Vitamin D supplementation modulates blood and tissue zinc, liver glutathione and blood biochemical parameters in diabetic rats on a zinc-deficient diet. Biol Trace Elem Res. (2012) 148:371–7. doi: 10.1007/s12011-012-9383-z
30. Tang Y, Yang Q, Lu J, Zhang X, Suen D, Tan Y, et al. Zinc Supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. (2010) 21:237–46. doi: 10.1016/j.jnutbio.2008.12.010
31. Ozcelik D, Naziroglu M, Tuncdemir M, Celik O, Ozturk M, Flores-Arce M. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res. (2012) 150:342–9. doi: 10.1007/s12011-012-9508-4
32. Zhang X, Zhao Y, Chu Q, Wang Z, Li H, Chi Z. Zinc modulates high glucose-induced apoptosis by suppressing oxidative stress in renal tubular epithelial cells. Biol Trace Elem Res. (2014) 158:259–67. doi: 10.1007/s12011-014-9922-x
33. Yang F, Li B, Dong X, Cui W, Luo P. The beneficial effects of zinc on diabetes-induced kidney damage in murine rodent model of type 1 diabetes mellitus. J Trace Elem Med Biol. (2017) 42:1–10. doi: 10.1016/j.jtemb.2017.03.006
34. Barman S, Pradeep S, Srinivasan K. Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative-stress-mediated molecular markers in streptozotocin-induced experimental rats. J Nutr Biochem. (2018) 54:113–29. doi: 10.1016/j.jnutbio.2017.11.008
35. Lu J, Gong D, Choong S, Xu H, Chan Y, Chen X, et al. Copper(Ii)-selective chelation improves function and antioxidant defences in cardiovascular tissues of rats as a model of diabetes: comparisons between triethylenetetramine and three less copper-selective transition-metal-targeted treatments. Diabetologia. (2010) 53:1217–26. doi: 10.1007/s00125-010-1698-8
36. Cooper G. Selective divalent copper chelation for the treatment of diabetes mellitus. Curr Med Chem. (2012) 19:2828–60. doi: 10.2174/092986712800609715
Keywords: 1α, 25-dihydroxyvitamin D3, diabetes, ZDF rats, renal function, urinary Cu, urinary Zn, urinary Se, urinary Mo
Citation: Wang D, Wang N, Zhou J, Luo G, Li Y, Yu W, Tan H, Liu G, Wang J and Hao L (2022) Urine trace element disorder along with renal function injury in vitamin D deficient diabetic rats and intervention effect of 1α,25-dihydroxyvitamin D3. Front. Nutr. 9:1042558. doi: 10.3389/fnut.2022.1042558
Received: 12 September 2022; Accepted: 22 November 2022;
Published: 06 December 2022.
Edited by:
Zhi Chai, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Guoxun Chen, The University of Tennessee, Knoxville, United StatesCopyright © 2022 Wang, Wang, Zhou, Luo, Li, Yu, Tan, Liu, Wang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Liu, Y2FyZGlvMjAwNEAxNjMuY29t; Jun Wang, anVud2FuZ3doQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.