
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 13 October 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1039056
This article is part of the Research Topic Benefits of Bioactive Plant-Based Compounds Supplementation on Cardiovascular and Metabolic Health View all 9 articles
 Yunjiao Yang1,2†
Yunjiao Yang1,2† Wen Deng3†
Wen Deng3† Yanmei Wang1,2
Yanmei Wang1,2 Tongyi Li1,2
Tongyi Li1,2 Yiding Chen1,2
Yiding Chen1,2 Cong Long1,2
Cong Long1,2 Qing Wen1,2
Qing Wen1,2 Yue Wu1,2
Yue Wu1,2 Qiu Chen1*
Qiu Chen1*Background/Aim: Omega-3 fatty acids (OM3-FA), a promising treatment for high triglycerides, have gradually attracted public attention. However, some studies showed that their application presented tricky problems, like increasing low-density lipoprotein cholesterol (LDL-C) levels. This study aimed to systematically evaluate the effect of OM3-FA or their combination with statins on the lipid profile in patients with hypertriglyceridemia.
Materials and methods: This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) guidelines. PubMed, Embase, Web of science, and Cochrane library were searched up to May 15, 2022. The random-effects model was applied to calculate the mean difference (MD) and associated 95% confidence intervals (CI).
Results: This meta-analysis included 32 studies with 15,903 subjects. When OM3-FA was used as monotherapy compared with placebo, it significantly decreased TG (MD: −39.81, 95% CI: −54.94 to −24.69; p < 0.001), TC (MD: −2.98, 95% CI: −5.72 to −0.25, p = 0.03), very low-density lipoprotein cholesterol (VLDL-C) (MD: −25.12, 95% CI: −37.09 to −13.14; p < 0.001), and non-high-density lipoprotein cholesterol (non-HDL-C) levels (MD: −5.42, 95% CI: −8.06 to−2.78; p < 0.001), and greatly increased LDL-C (MD: 9.10, 95% CI: 4.27 to 13.94; p < 0.001) and HDL levels (MD: 1.60, 95% CI: 0.06 to 3.15; p = 0.04). Regarding apolipoprotein B (Apo-B) and apolipoprotein AI (Apo-AI), no significant effect was identified. When OM3-FA was combined with statins, significant reductions were observed in the concentrations of TG (MD: −29.63, 95% CI: −36.24 to −23.02; p < 0.001), TC (MD: −6.87, 95% CI: −9.30 to −4.45, p < 0.001), VLDL-C (−20.13, 95% CI: −24.76 to −15.50; p < 0.001), non-HDL-C (MD: −8.71, 95% CI: −11.45 to −5.98; p < 0.001), Apo-B (MD: −3.50, 95% CI: −5.37 to −1.64; p < 0.001), and Apo-AI (MD: −2.01, 95% CI: −3.07 to −0.95; p < 0.001). However, the combined therapy did not exert significant changes on the levels of high-density lipoprotein cholesterol (HDL-C) and LDL-C compared to control group.
Conclusion: The use of OM3-FA either as monotherapy or in combination with statins may potentially reduce the levels of TG, TC, VLDL-C, non-HDL-C, Apo-B, and Apo-AI while increasing the levels of LDL-C and HDL-C. Nevertheless, the effects of OM3-FA observed in this review should be interpreted with caution due to the high heterogeneity between the included studies.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42022329552].
Hypertriglyceridemia, one of the most prevalent diseases, is still a significant public health problem worldwide. A series of complications caused by hypertriglyceridemia are eroding people’s health. Studies showed that the incidence of cardiovascular events remains high in patients with cardiovascular disease even after primary or secondary preventive treatment. A growing body of literature demonstrated that hypertriglyceridemia as an independent risk factor for atherosclerotic cardiovascular disease (ASCVD) might explain this problem (1–6). In addition, hypertriglyceridemia is closely related to obesity, type 2 diabetes, nephrotic syndrome, chronic renal insufficiency, hypothyroidism, fatty liver, and ischemic stroke (7–9). Moreover, hypertriglyceridemia is an essential cause of pancreatitis. Pedersen et al. reported that mild-to-moderate hypertriglyceridemia from 177 mg/dl and above is associated with a high risk of acute pancreatitis (10). Therefore, it is so important to control triglycerides in a reasonable range. However, how to choose an appropriate treatment is still a problem to be solved.
In recent years, omega-3 fatty acids (OM3-FA) have gradually attracted public attention as a promising way to treat high triglycerides. A large proportion of scientific research has shown that OM3-FA could reduce triglyceride levels (11–14). Two meta-analyses evaluating the effects of OM3-FA on highly active antiretroviral therapy (HAART) associated hypertriglyceridemia in HIV/AIDS patients showed that OM3-FA significantly reduced triglyceride levels (15, 16). Moreover, OM3-FA has been approved in the United States to control hypertriglyceridemia (17). However, it was reported that the application of OM3-FA increased low-density lipoprotein cholesterol (LDL-C) (14, 18, 19), an essential risk factor for cardiovascular disease, and decreased apolipoprotein AI (Apo-AI) (18, 20, 21), a good lipoprotein. Therefore, a dilemma was posed regarding the use of OM3-FA. In addition, it is unclear whether such a problem exists in patients with hypertriglyceridemia treated with OM3-FA in combination with statins. A previous meta-analysis, including patients with hypertriglyceridemia (≥150 mg/dl), evaluated the effect of OM3-FA on triglycerides (22). Although this analysis demonstrated that OM3-FA could reduce triglycerides, the quality of literature was low, and the amount of sample size was not enough. Besides, the study was limited in assessing levels of lipids other than triglycerides. In recent years, many large randomized controlled, double-blind trials of OM3-FA for hypertriglyceridemia have been published (19, 20, 23, 24), providing further strong support for the determination of clinical evidence. In addition, no systematic review or meta-analysis has reported the effect of OM3-FA added to statins on serum lipid profile in patients with hypertriglyceridemia.
Based on the above existing problems, we conducted this systematic review and meta-analysis for the following purposes: first, to systematically evaluate the efficacy of OM3-FA, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) for patients with hypertriglyceridemia but not in AIDS-associated hypertriglyceridemia to provide reliable evidence for clinical practice; Second, to investigate whether OM3-FA affect other lipid levels in patients with hypertriglyceridemia; Third, to evaluate the effect of OM3-FA added to statins on lipid profile in patients with hypertriglyceridemia.
This meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (25). The PRISMA checklist is available in Supplementary material 1. We registered this meta-analysis on the PROSPERO database1 with the Registration Number (CRD42022329552).
Two reviewers (YY and WD) independently searched databases including PubMed, Cochrane library, Embase, and Web of science from their inception to May 15, 2022. In addition, we also searched the Clinical Trials,2 unpublished gray literature, and references cited in the eligible studies. The key search terms are as follows: (“Omega-3 fatty acid” OR “n-3 Oil” OR “n-3 OM3-FA” OR “n-3 PUFA” OR “n-3 polyunsaturated fatty Acid” OR “eicosapentaenoic acid” OR “docosahexaenoic acids”) AND (“hypertriglyceridemia” OR “hyperlipidemia” OR “hyperlipemia”) AND (“randomized controlled trial” OR “RCT” OR “randomized clinical trial” OR “randomly”) Full details of the search strategy are available in Supplementary material 2.
Population: This study only included adults with triglyceride levels ≥150 mg/dl, without restriction on gender. A study was also eligible if the study participants had mixed hyperlipidemia, obesity, and metabolic syndrome as long as the triglyceride level was ≥150 mg/dl. Intervention: OM3-FA monotherapy or combined therapy of statins plus OM3-FA. The type of OM3-FA considered in this review included EPA and/or DHA. The OM3-FA should only be taken orally in the form of capsule with no minimum/maximum dose restriction. Comparison: For the OM3-FA monotherapy, the control group must be the placebo. Regarding the combined therapy of statins plus OM3-FA, the control group must also use statins with or without a placebo. There are no restrictions on the types of statins used. Outcomes: The primary efficacy parameter was triglyceride (TG) percentage change from baseline to end of the study; Secondary endpoints included total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C, very low-density lipoprotein cholesterol (VLDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (Apo-B) and Apo-AI percentage change from baseline to end of the study. Study design: Randomized controlled trials with parallel design published in English. There was no restriction on the sample size and the intervention time.
Population: Patients with HIV/AIDS-associated hypertriglyceridemia, uncontrolled diabetes (HbA1c > 9%), thyroid disease, symptomatic heart disease, immunologic disease, and severe liver and kidney disease were excluded. Intervention and comparison: OM3-FA monotherapy vs. other triglyceride-lowering drugs, such as fibrates, ezetimibe, gemfibrozil, nicotinic acid, and bile acid sequestrants. Outcomes: Studies in which lipid profile’s mean and standard deviation were not reported or could not be obtained by formula transformation were excluded. Study design: Trials with cross-over design, observational studies, reviews, case reports, abstracts, and protocols were excluded.
Two reviewers (YW and TL) extracted data independently. They settled through negotiation or consulted a third reviewer (QC) if there were any discrepancies. After searching the four databases, the software (Endnote X9) was used to remove duplicates. Qualified records were further extracted for relevant data. The specific extraction contents are as follows: first author, year of publication, the country where the study was conducted, study design, trial registration number, duration of treatment, the sample size of experimental group and control group, type and dose of OM3-FA, age, gender, and body mass index (BMI) of study subjects, the diet of the participants, reported endpoints. The data from the highest one was extracted for studies in which multiple dosages of OM3-FA were reported simultaneously. We emailed the corresponding author for more details on literature that lacked enough information. If no response was received, we only analyzed the available data.
The risk of bias was assessed by two reviewers (YC and CL) independently based on the Cochrane collaboration tool (26). Any disagreements were settled by consensus or by the third reviewer. Each study was classified as high, unclear, or low risk of bias for seven aspects (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of study outcome examination, selective reporting, completeness of study outcome information, and other sources of bias).
Stata17.0 and Review Manager 5.3 software were used to perform the meta-analysis. Since all outcomes were continuous variables, we standardized the extracted data to obtain the mean and standard deviation. When the units of lipid levels reported as mmol/L, we uniformly converted mmol/L to mg/dl according to the unit conversion method (triglyceride: 1 mg/dL = 0.01129 mmol/L, cholesterol: 1 mg/dL = 0.02586 mmol/L). We calculated the mean and standard deviation using specific formulas for the studies reporting values with median and interquartile ranges (27, 28). Data presented as standard error (SE) were converted to standard deviation (SD) by the equation SD = SE × . When the literature data form was reported as Median (minimum-maximum) and mean with 95% confidence interval (CI) or the articles only described the mean and standard deviation at baseline and post-treatment, we used appropriate methods described in the Cochrane Handbook for Systematic Reviews of Interventions3 for transformation. Due to the differences in baseline characteristics among the included trials, a random-effect model was used to perform the meta-analysis. Heterogeneity among studies was evaluated by Cochran Q and I2 statistics, and the p-value of < 0.1 and I2 > 50% indicates significant heterogeneity. Besides, we performed subgroup analysis according to the intervention time (<12 weeks or ≥12 weeks), baseline TG levels (150–200, 200–500, and ≥500 mg/dl), and the dosage (<4 or ≥4 g/days) and kind (EPA, DHA, EPA+DHA) of OM3-FA. However, subgroup analyses based on fatty acid species were not performed for the combined therapy of OM3-FA and statins, because EPA and DHA were used in all studies except in one study that only used EPA. Sensitivity analysis was performed to check the robustness of the results. Funnel plot and Egger’s test were conducted to assess publication bias for outcomes involving ten or more studies. A p-value < 0.1 was defined as significant publication bias. Then, the trim-and-fill computation was used to estimate the effect of publication bias on interpreting the results. This meta-analysis expressed the results as mean difference (MD) and 95% CI, and the p < 0.05 was considered statistically significant.
A total of 2,850 papers were identified by searching four databases. No additional eligible articles were identified from other sources. Duplicate articles (n = 1761) were removed by Endnote X9, and another 1,089 articles were approved to be further screened. After reading titles and abstracts, 155 were left for full-text reading, and then 123 articles were excluded for various reasons. Finally, 32 studies with 15,903 participants were enrolled in this meta-analysis (11–14, 18–21, 23, 24, 29–50). The flowchart of study selection is shown in Figure 1.
Among the 32 included studies, there were 16 multicenter randomized controlled trials (11, 12, 14, 18–21, 23, 24, 29, 38, 41–43, 45, 46). All RCTs were double-blind except for six trials (13, 31, 35, 46, 47, 49). The study duration varied from 4 to 48 weeks. The mean age of the participants ranged from 46.7 to 65 years. The mean BMI of the subjects ranged from 24.3 to 35.2 kg/m2. The dosage of EPA/DHA ranged from 1.24 to 4 g/days. The dosage of EPA was 0.6–4 g/days. The dosage of DHA was 3–4 g/days. The trials were conducted in the following regions: Worldwide (n = 4), USA (n = 9), Korea (n = 6), Australia (n = 5), Iran (n = 2), India (n = 1), German (n = 1), UK (n = 1), Japan (n = 1), Norway (n = 1), and China (n = 1). These studies were published between 1993 and 2022. The mean baseline TG level in the experimental group ranged from 154.2 to 699 mg/dl. The placebo controls used in most studies were corn oil and olive oil. The detailed characteristics of included studies are summarized in Table 1.
Regarding selection bias, ten studies (11–14, 18, 19, 34, 35, 38, 43) were determined as “low risk” because they reported their specific randomization strategies and allocation concealment method. The remaining articles only described random assignment but did not point out the specific random sequence method and how to realize allocation concealment. Therefore, they were identified as “unclear risk” Concerning performance bias, 26 studies used a double-blind design, which was identified as “low risk” and three studies (13, 31, 35) used a single-blind method, which was also identified as “low risk” according to the corresponding aspect. Two articles (47, 49) were considered “unclear risk” because of not specifying whether blinding was used. Only one study (46) was deemed “high risk” because of the open-label design. Most studies registered for the clinical trial protocol, and even those not registered reported all the expected results. There were a few trials (13, 20, 35, 45) with patients dropping out, but the number was balanced between the experimental and control groups, and not enough affected the results. Besides, no factors were found that could affect the test results in all studies. Hence, total studies were identified as “low risk” for the detection bias, follow-up bias, and reporting bias. However, several studies (30, 31, 34, 44, 49) in which differences in baseline characteristics were significant between the experimental group and control group could be considered as “high risk” for the other bias. The summary and graph are shown in Figure 2.
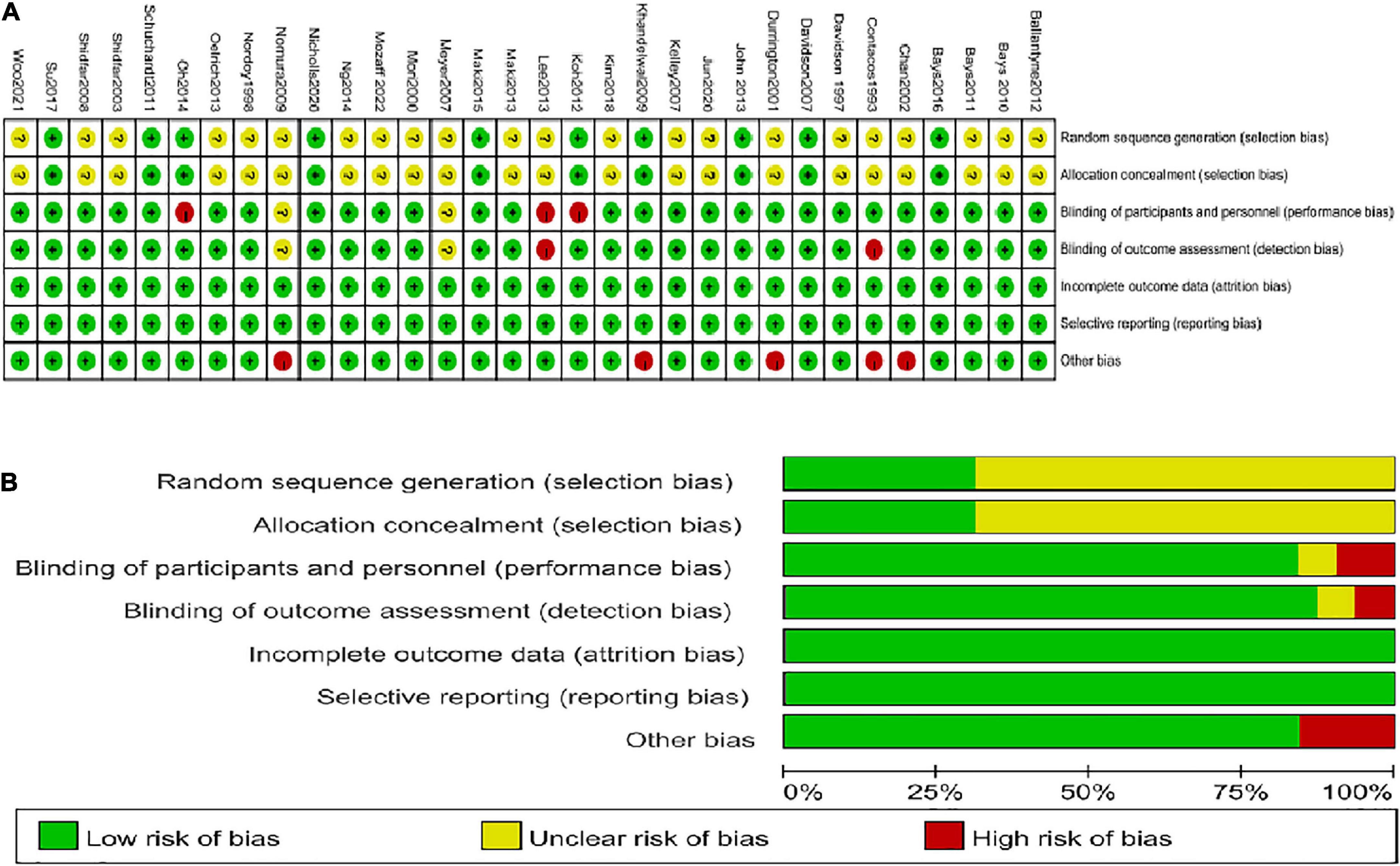
Figure 2. Summary of quality evaluation based on the Cochrane’s Risk of Bias Tool. (A) Risk of bias summary for each risk of bias item for each included study; (B) Risk of bias graph for each risk of bias item presented as percentages across all included studies.
Nineteen studies (20 groups) with 13,612 participants reported the effect of OM3-FA on TG. The pooled analysis showed that OM3-FA exerted a significant reduction in TG concentrations compared to placebo (MD: −39.81, 95% CI: −54.94 to −24.69; p < 0.001), but significant heterogeneity was identified (I2 = 96.4%, phe < 0.001) (Figure 3A). Fourteen studies with 2291 individuals reported the effect of OM3-FA combined with statins on TG. Similar effect was identified (MD: −29.63, 95% CI: −36.24 to −23.02; p < 0.001), which also accompanied by obvious heterogeneity (I2 = 80.3%, phe < 0.001) (Figure 3B). All subgroup analyses showed that OM3-FA effectively reduced TG, regardless of whether OM3-FA monotherapy or combined therapy of statins plus OM3-FA (Tables 2, 3).

Figure 3. The effect of OM3-FA on TG. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
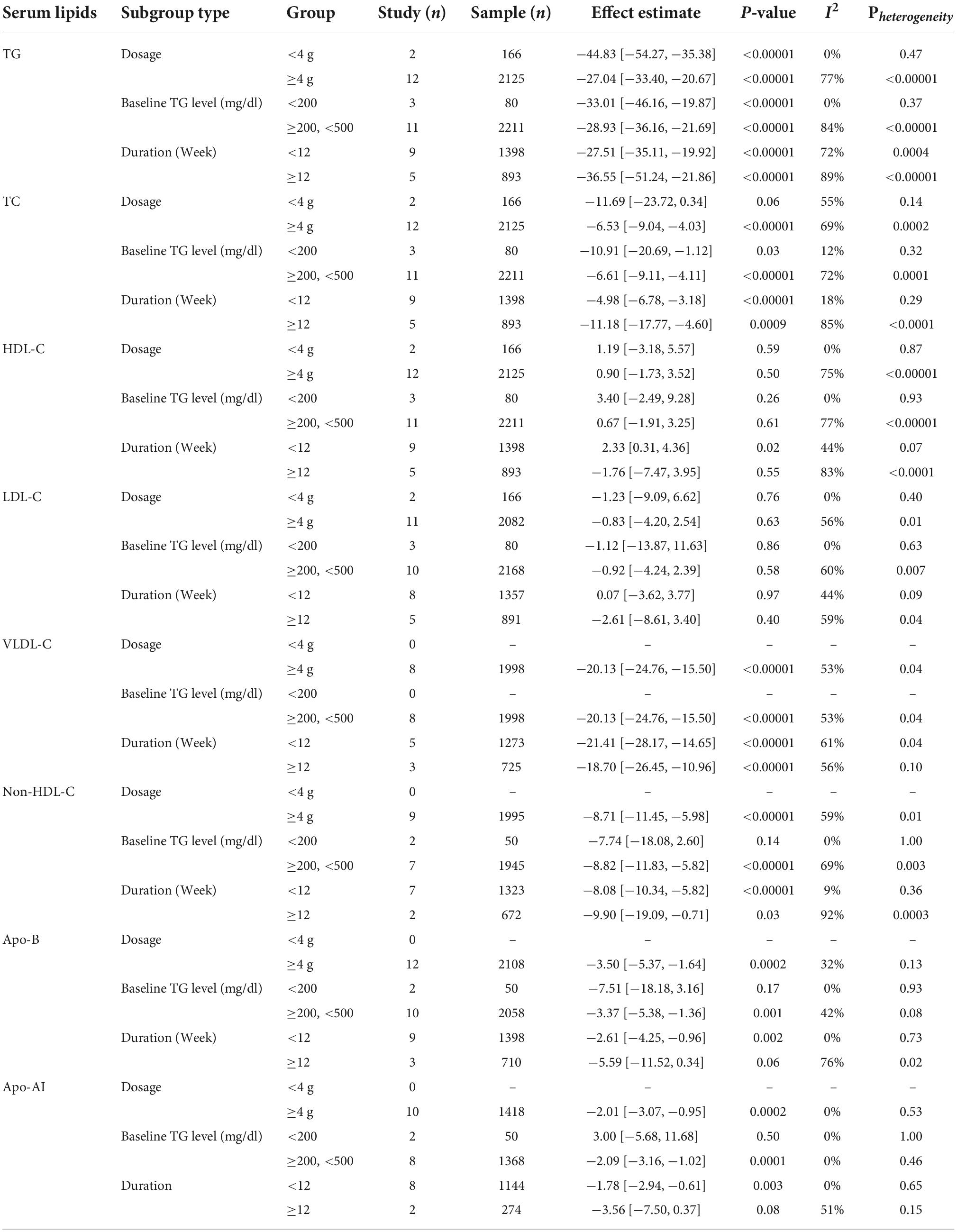
Table 3. Subgroup analysis of the effect of combined therapy of statins plus OM3-FA on lipid profile.
Sixteen studies with 13,018 participants reported the effect of OM3-FA on TC. The overall analysis showed that OM3-FA significantly reduced TC level (MD: −2.98, 95% CI: −5.72 to −0.25, p = 0.03; I2 = 64.4%, phe < 0.001) (Figure 4A). Fourteen studies with 2,291 patients reported the effect of OM3-FA added to statins on TG. The combined result showed that the TC reduction was more significant (MD: −6.87, 95% CI: −9.30 to −4.45, p < 0.001; I2 = 66.7%, phe < 0.001) (Figure 4B). In subgroup analyses, with respect to OM3-FA monotherapy, we found that the TC reduction was statistically significant only when the dose of OM3-FA was ≥4 g (MD: −4.72, 95% CI: −8.70 to −0.73, p = 0.02; I2 = 72%, phe = 0.007), the baseline TG level of participants was ≥500 mg/dL (MD: −10.01, 95% CI: −15.52 to −4.50, p = 0.0004; I2 = 51%, phe = 0.13), EPA and DHA were used together (MD: −4.55, 95% CI: −6.95 to −2.15, p < 0.0002; I2 = 36%, phe = 0.12). However, when DHA was used alone, it increased the level of TC (MD: 14.78, 95% CI: 4.61 to 24.95, p = 0.004; I2 = 0%, phe = 0.82) (Table 2). Regarding the combined therapy of statins plus OM3-FA, dose-based subgroup analysis showed that the TC reduction was not significant if the dose was <4 g (MD: −11.69, 95% CI: −23.72 to 0.34, p = 0.06; I2 = 55%, phe = 0.14) (Table 3).
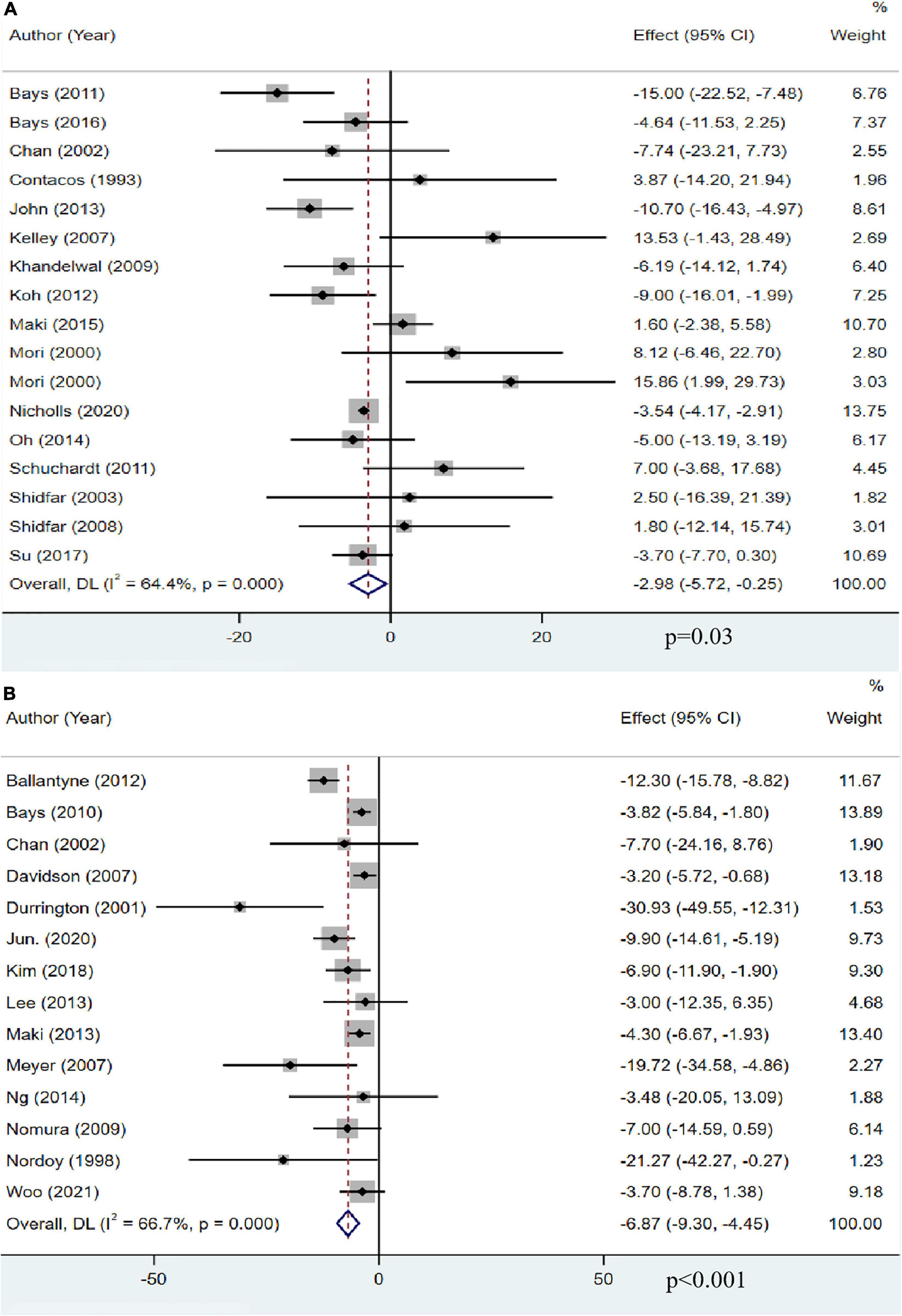
Figure 4. The effect of OM3-FA on TC. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
A total of 18 studies, including 13,555 participants, investigated the effect of OM3-FA on HDL-C. The pooled analysis showed that OM3-FA increased the concentration of HDL-C compared with placebo (MD: 1.60, 95% CI: 0.06 to 3.15; p = 0.04), with significant heterogeneity (I2 = 56.1%, phe = 0.002) (Figure 5A). However, by removing one article (11) which favored that OM3-FA significantly increased HDL-C levels, no significant heterogeneity was identified (I2 = 0%, phe = 0.51). Fourteen studies with 2,291 patients assessed the effect of OM3-FA added to statins on HDL-C, and the pooled result demonstrated that no significant impact was identified (MD: 0.96, 95% CI: −1.37 to 3.30; p = 0.42), with apparent heterogeneity (I2 = 71%, phe < 0.001) (Figure 5B). By removing two articles that favored that OM3-FA reduced HDL-C levels (41, 44), the pooled result was reversed (MD: 2.50, 95% CI: 0.90 to 4.10, p = 0.002), without significant heterogeneity (I2 = 32%, phe = 0.13). In the subgroup analyses, we found that OM3-FA monotherapy significantly increased HDL-C levels only in the case of EPA and DHA used together (MD: 1.22, 95% CI: 0.70 to 1.73, p < 0.00001; I2 = 0%, phe = 0.73) and participants with a baseline TG level less than 500 mg/dL (Table 2). When OM3-FA was used with statins, subgroup analysis showed that the HDL-C level increased when the intervention duration was <12 weeks (MD: 2.33, 95% CI: 0.31 to 4.36, p = 0.02; I2 = 44%, phe = 0.07) (Table 3).
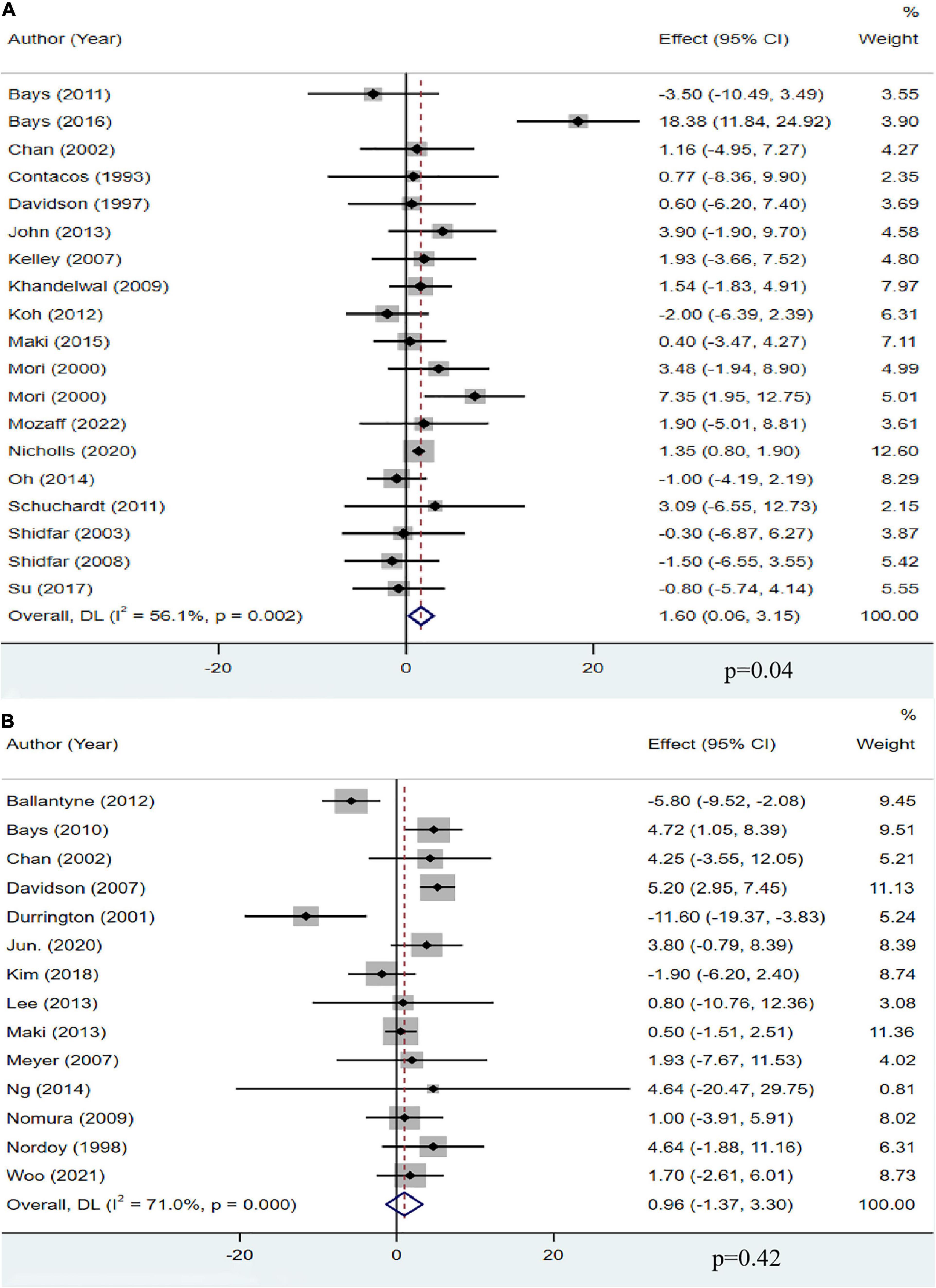
Figure 5. The effect of OM3-FA on HDL-C. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
A total of 19 studies with 13,612 objects described the effect of OM3-FA on LDL-C. The pooled result showed that OM3-FA significantly increased LDL-C levels compared to the control group (MD: 9.10, 95% CI: 4.27 to 13.94; p < 0.001), with large heterogeneity (I2 = 75.8%, phe < 0.001) (Figure 6A). Thirteen studies with 2,248 patients assessed the effect of OM3-FA in combination with statins on LDL-C. However, the level of LDL-C was not increased compared to the control group (MD: −0.85, 95% CI: −3.90 to 2.19, p = 0.58; I2 = 49.3%, phe = 0.023), which is quite different from the result when OM3-FA was used alone (Figure 6B). In subgroup analyses, we found that OM3-FA had no significant effect on LDL-C if the participants had a baseline TG level of <200 mg/dL (MD: 3.81, 95% CI: −4.80 to 12.42, p = 0.39; I2 = 38%, phe = 0.16) or ≥500 mg/dL (MD: 13.42, 95% CI: 0.05 to 26.79, p = 0.05; I2 = 71%, phe = 0.01) (Table 2). None of the subgroups demonstrated an increase in LDL-C levels for the combined therapy of OM3-FA and statins (Table 3).
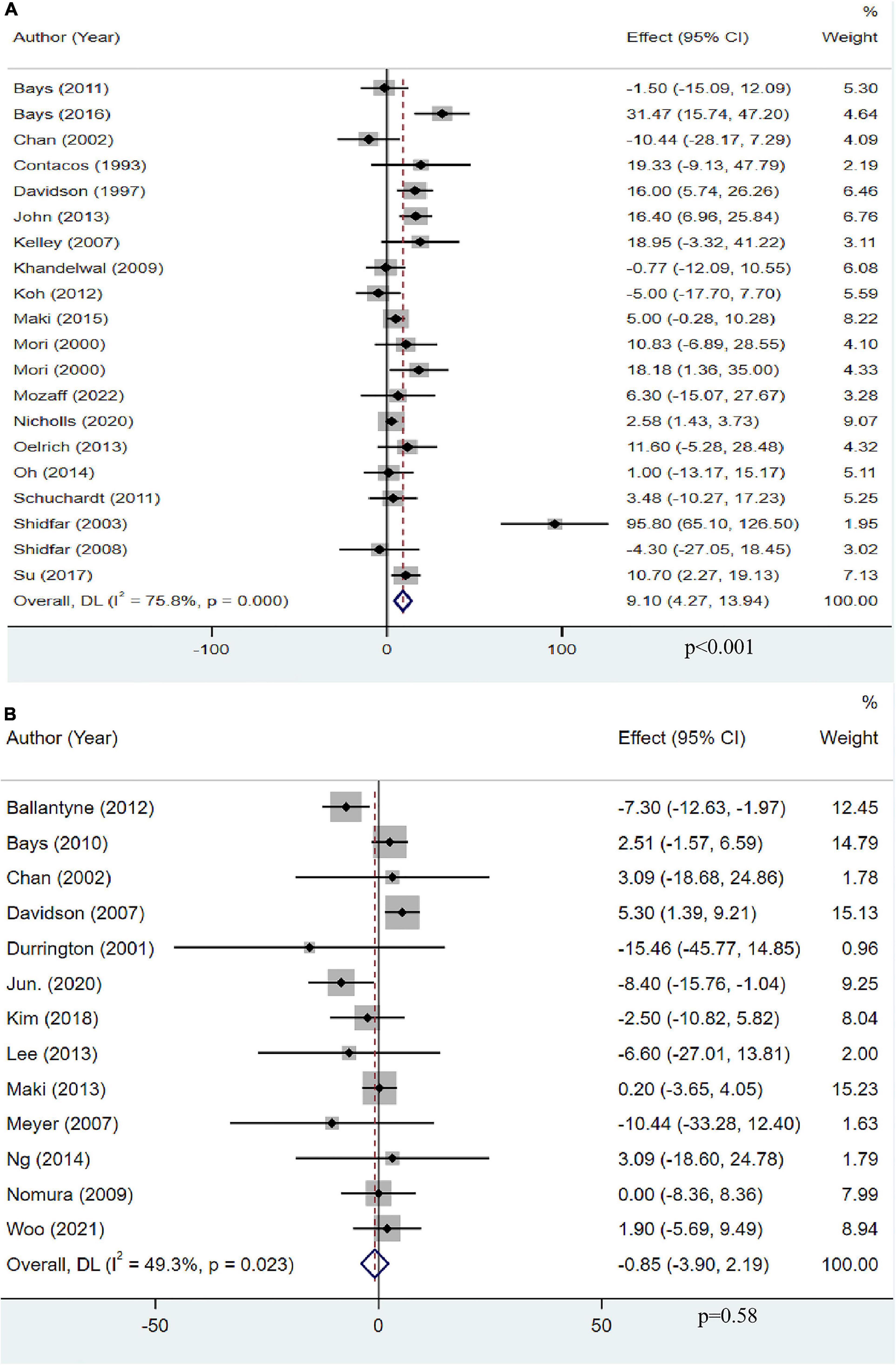
Figure 6. The effect of OM3-FA on LDL-C. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
Only four articles with 952 participants reported the effect of OM3-FA monotherapy on VLDL-C. The pooled result revealed that OM3-FA significantly decreased VLDL-C levels (MD: −25.12, 95% CI: −37.09 to −13.14; p < 0.001), with large heterogeneity (I2 = 62.4%, phe = 0.046) (Figure 7A). Eight studies with 1,998 patients assessed the effect of OM3-FA added to statins on VLDL-C. Similar effect was identified (MD: −20.13, 95% CI: −24.76 to −15.50; p < 0.001), with obvious heterogeneity (I2 = 53.5%, phe = 0.035) (Figure 7B). In subgroup analysis, when the dosage of OM3-FA was <4 g, the pooled result of two studies showed no significant effect on VLDL-C (MD: −23.00, 95% CI: −50.94 to 4.94, p = 0.11; I2 = 85%, phe = 0.009) (Table 2). For the combined therapy of statins plus OM3-FA, the subgroup analyses were consistent with the overall effect (Table 3).
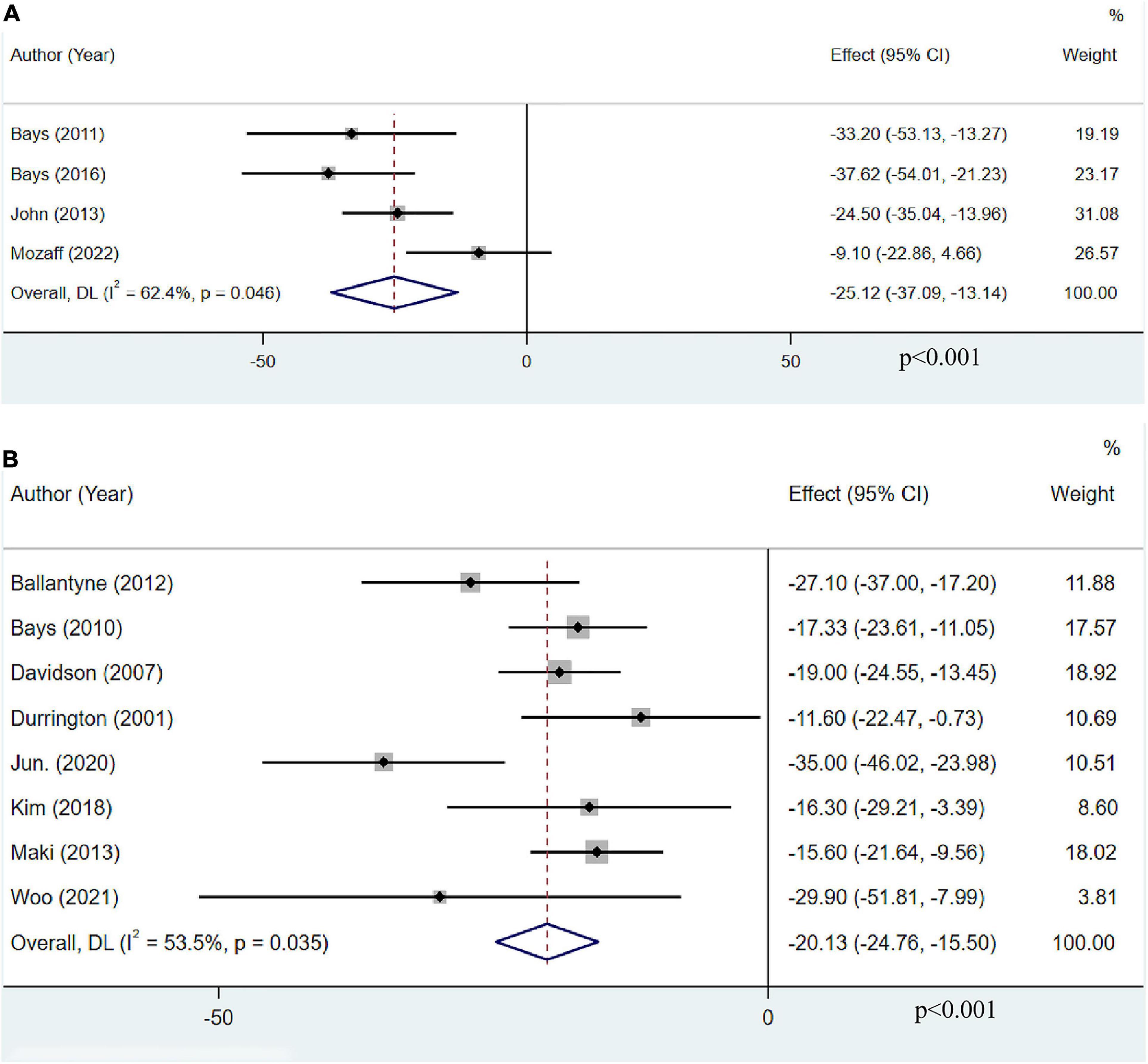
Figure 7. The effect of OM3-FA on VLDL-C. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
Eleven studies, including 13,179 participants, investigated the effects of OM3-FA on non-HDL-C. The pooled result demonstrated that OM3-FA significantly reduced non-HDL-C level (MD: −5.42, 95% CI: −8.06 to −2.78; p < 0.001), with large heterogeneity (I2 = 60.5%, p = 0.005) (Figure 8A). Nine studies with 1,995 patients assessed the effect of OM3-FA added to statins on non-HDL-C. the effect of lowering non-HDL-C was more obvious (MD: −8.71, 95% CI: −11.45 to −5.98; p < 0.001), with significant heterogeneity (I2 = 59%, p = 0.012) (Figure 8B). However, subgroup analyses showed that non-HDL-C reduction was statistically significant only when the dose of OM3-FA was ≥4 g (MD:−7.32, 95% CI: −10.67 to −3.96, p < 0.0001; I2 = 55%, phe = 0.05), EPA and DHA were used together (MD:−5.41, 95% CI: −6.56 to −4.25, p < 0.00001; I2 = 4%, phe = 0.39), the baseline TG level of participants was ≥500 mg/dL (MD:−9.39, 95% CI: −13.84 to −4.94, p < 0.0001; I2 = 39%, phe = 0.18) and the duration of treatment was ≥12 weeks (MD: −7.83, 95% CI: −11.48 to −4.18, p < 0.0001; I2 = 59%, phe = 0.04) (Table 2). When OM3-FA was combined with statins, all subgroup analyses showed statistically significant reductions in non-HDL-C levels except when participants had baseline TG levels of less than 200 mg/dL (MD: −7.74, 95% CI: −18.08 to 2.60, p = 0.14; I2 = 0%, phe = 1.00) (Table 3).
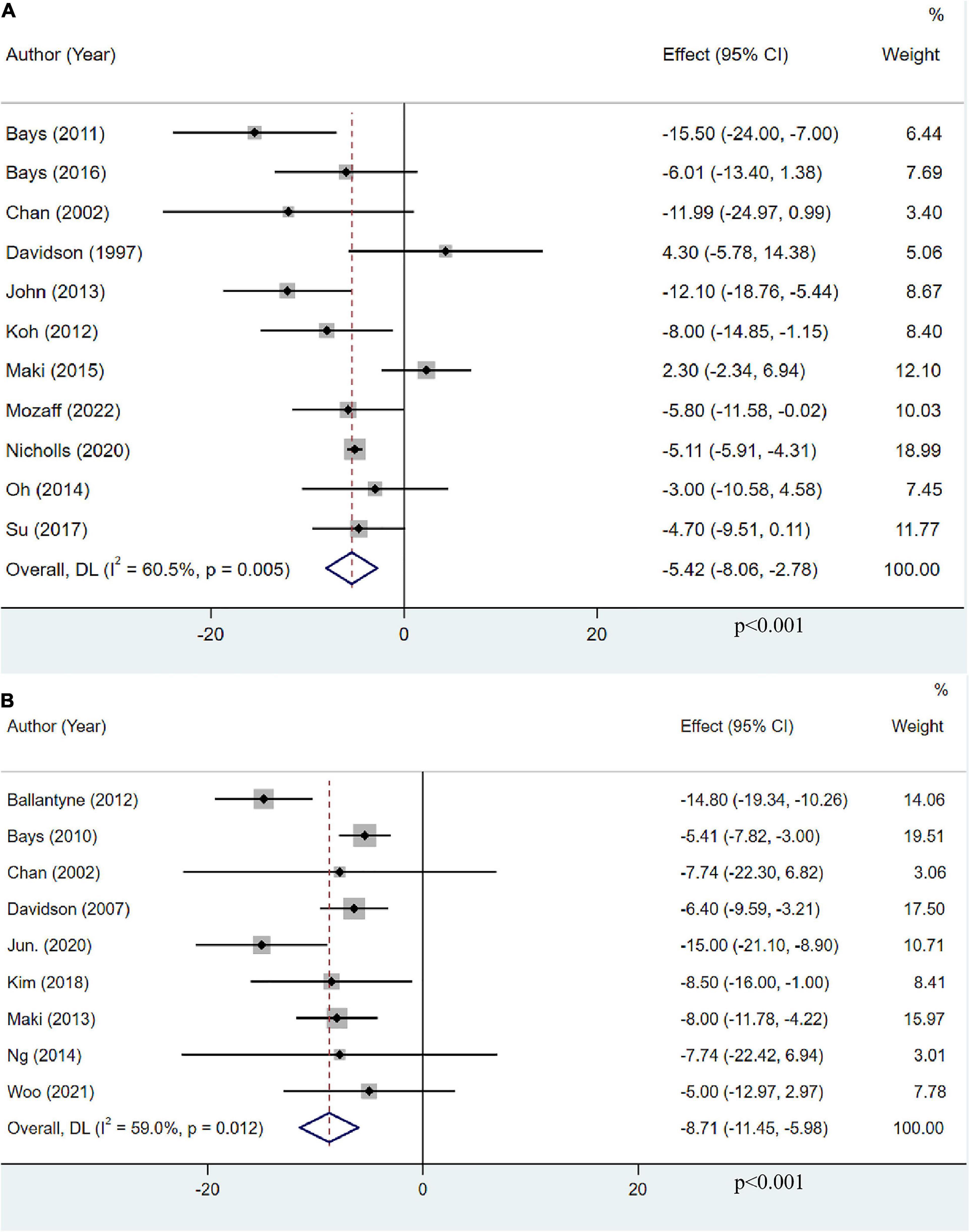
Figure 8. The effect of OM3-FA on non-HDL-C. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
Eleven studies with 12,563 participants explored the effects of OM3-FA on Apo-B. The pooled result demonstrated that OM3-FA had no significant effect on Apo-B compared with control group (MD: −2.44, 95% CI: −5.42 to 0.54; p = 0.11), with low heterogeneity (I2 = 38%, p = 0.096) (Figure 9A). Twelve studies with 2,108 patients assessed the effect of OM3-FA added to statins on Apo-B. However, as opposed to OM3-FA alone, the combination exerted a significant reduction in Apo-B level (MD: −3.50, 95% CI: −5.37 to −1.64; p < 0.001) without significant heterogeneity (I2 = 32%, p = 0.135) (Figure 9B). In subgroup analyses, we found that the effect of OM3-FA on reducing Apo-B was significant if the treatment duration was <12 weeks (MD: −5.19, 95% CI: −9.70 to −0.69, p = 0.02; I2 = 0%, phe = 0.58) (Table 2). When OM3-FA were added to statins, all subgroups showed significant reductions in Apo-B levels except when participants’ baseline TG levels were less than 200 mg/dL (MD: −7.51, 95% CI: −18.18 to 3.16, p = 0.17; I2 = 0%, phe = 0.93) (Table 3).
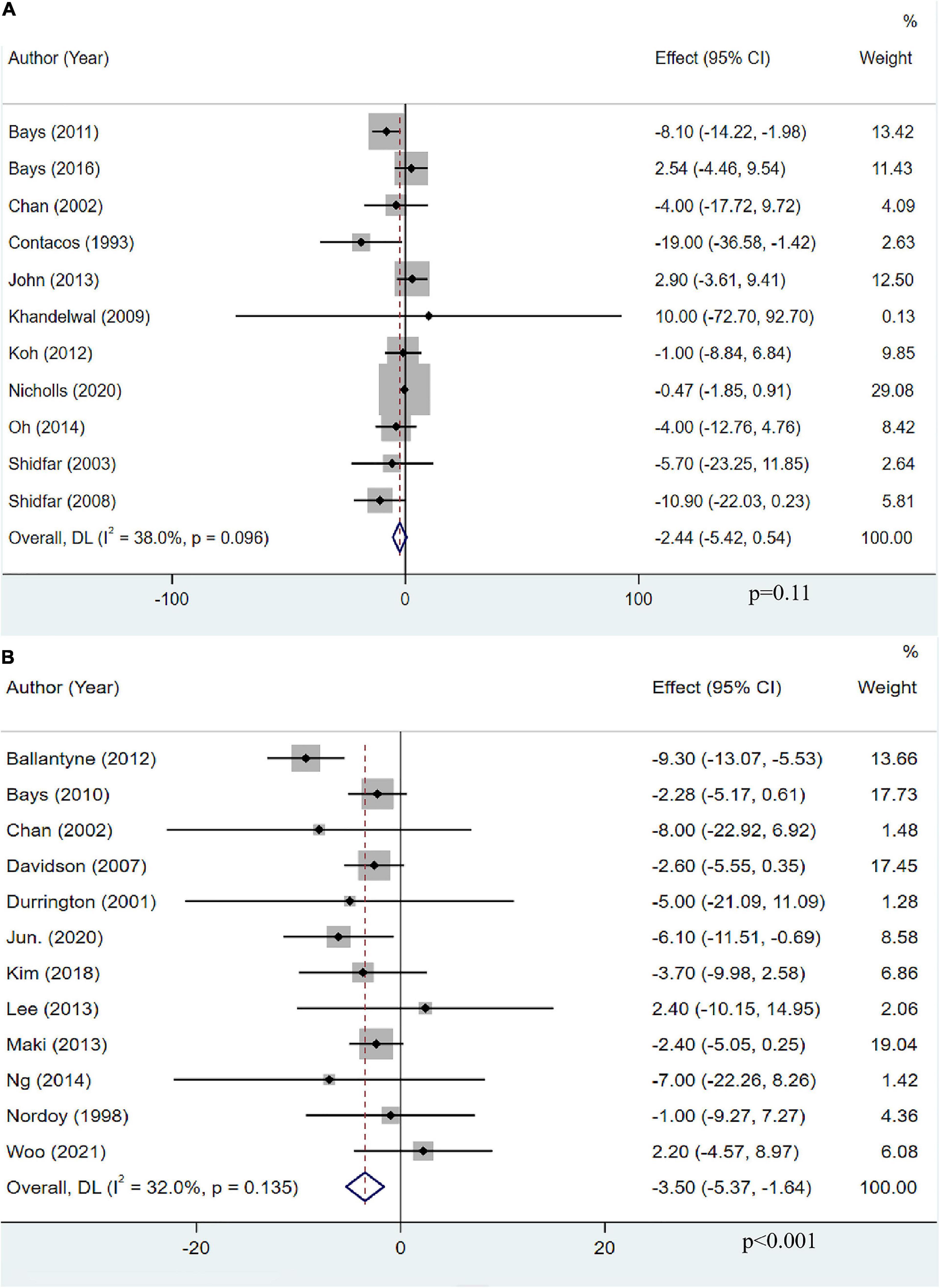
Figure 9. The effect of OM3-FA on Apo-B. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
Apolipoprotein AI levels were assessed as an outcome measure in nine studies with 684 participants. The pooled result demonstrated that OM3-FA had no significant effect on Apo-AI (MD: −0.33, 95% CI: −4.37 to 3.71; p = 0.87), with obvious heterogeneity (I2 = 63.8%, p = 0.005) (Figure 10A). Ten studies with 1,418 patients assessed the effect of OM3-FA added to statins on Apo-AI. Intriguingly, combination therapy exerted a significant reduction in Apo-AI level (MD: −2.01, 95% CI: −3.07 to −0.95; p < 0.001), without heterogeneity (I2 = 0%, p = 0.533) (Figure 10B). When OM3-FA was used alone, the results of all subgroup analyses were consistent with the overall result (Table 2). For combination therapy, the results of all subgroup analyses were consistent with the overall results except when participants had baseline TG levels of less than 200 mg/dL (MD: 3.00, 95% CI: −5.68 to 11.68, p = 0.50; I2 = 0%, phe = 1.00) (Table 3).
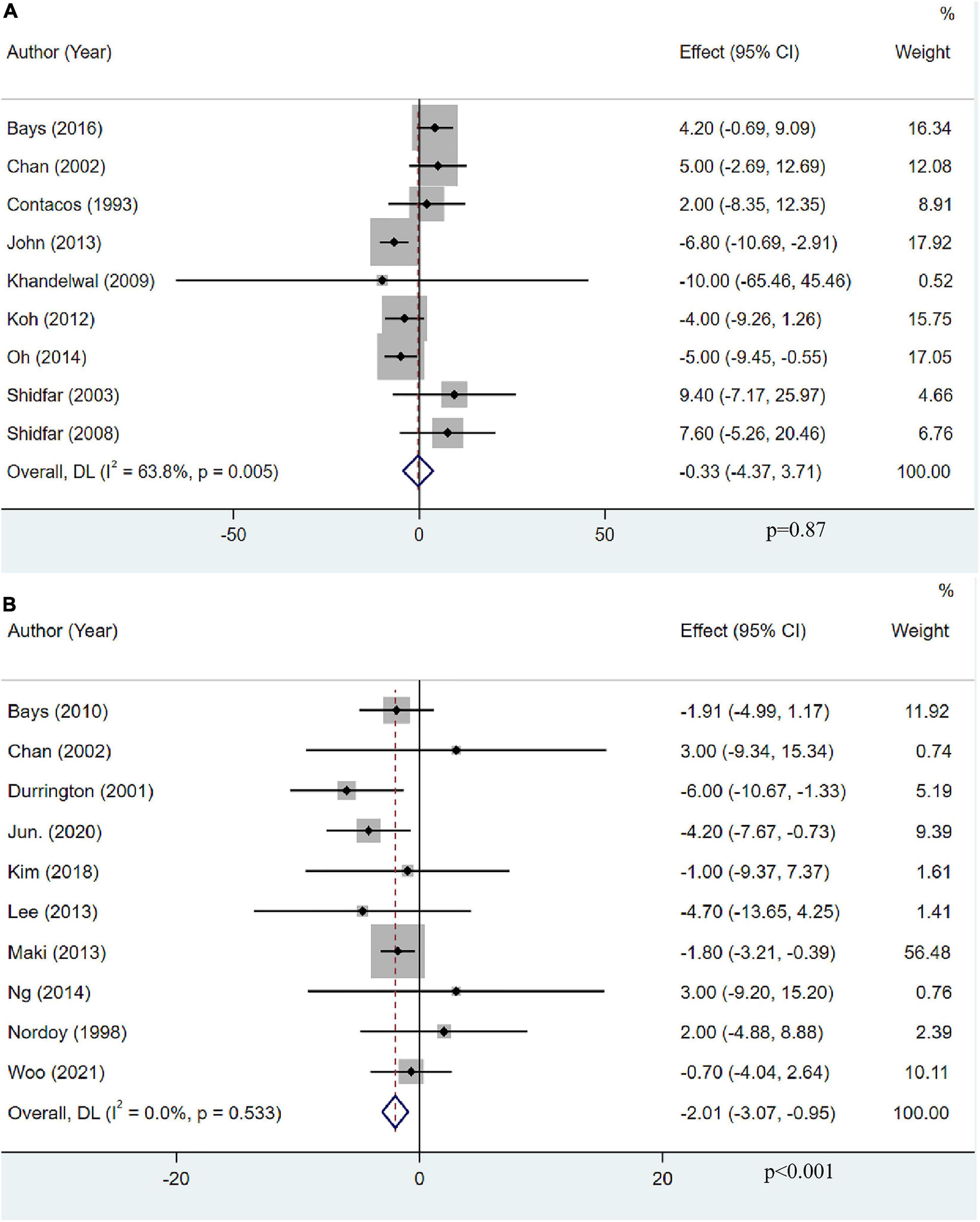
Figure 10. The effect of OM3-FA on Apo-AI. (A) OM3-FA monotherapy; (B) Combined therapy of statins plus OM3-FA.
Sensitivity analyses were performed, in which one study was removed, and the others analyzed to estimate whether the results could have been affected markedly by a single study. The sensitivity analyses’ results indicated no reversals and significant fluctuations in all outcomes except for the effect of OM3-FA monotherapy on HDL-C. After removing one study (11), the sensitivity analysis showed that the 95% CI of the HDL-C narrowed significantly (Supplementary material 3; Figure 3).
The results of funnel plots and Egger’s tests showed that there might be a publication bias for several outcomes, specifically including the effect of OM3-FA monotherapy on TG (Egger’s test, p = 0.071) and LDL-C (Egger’s test, p = 0.028) and the impact of combined therapy of statins plus OM3-FA on TG (Egger’s test, p = 0.044) and TC (Egger’s test, p = 0.049) levels. Consequently, a trim-and-fill method was conducted. Results showed no trimming was performed, and the results were unchanged for the effect on LDL-C with OM3-FA monotherapy and the impact on TG and TC with combined therapy of OM3-FA added to statins. The result was also not reversed for the efficacy of OM3-FA monotherapy on TG after filling in four studies. Therefore, the results of our meta-analysis are all robust. Details were available in Supplementary material 4.
This meta-analysis aimed to evaluate the effects of OM3-FA monotherapy or combined therapy of statins plus OM3-FA on TG and other lipid profiles in patients with hypertriglyceridemia. Through the analysis based on the 32 RCTs, the findings are as follows: first, the efficacy of lowering TG was definite whether OM3-FA monotherapy or combined therapy of statins plus OM3-FA; Second, the levels of TC, VLDL-C, and non-HDL-C also showed a significant reduction both with OM3-FA monotherapy and integrated treatment of OM3-FA and statins. Third, OM3-FA monotherapy elevated LDL-C and HDL-C levels, while combined therapy of statins plus OM3-FA exerted no significant effect on LDL-C and HDL-C. Fourth, the concentrations of Apo-B and Apo-AI were not significantly affected by OM3-FA monotherapy but were significantly reduced by the combined therapy of OM3-FA and statins. Fifth, subgroup analysis demonstrated that DHA significantly increased the level of TC.
Compared to previous studies, a meta-analysis assessing the effect of OM3-FA on type 2 diabetes showed that OM3-FA lowered TG and VLDL-C levels, raised LDL-C levels (51), which was consistent with the results of our research. However, it showed no significant effect on TC, which was different from our results. We speculated that it might be due to the different populations included. In addition, our study showed that OM3-FA at doses ≥4 g or <4 g was effective in reducing triglyceride levels, the same as the results of a meta-analysis investigating the effects of OM3-FA on HIV-associated hypertriglyceridemia (15).
The results found that both OM3-FA monotherapy and combined therapy of OM3-FA with statins reduced TC levels. However, when combined with statins, the reduction in TC was more potent, and we suspected that it was not simply because the statins lowered LDL-C. It is well known that LDL-C, HDL-C, and VLDL-C are all included in TC. This meta-analysis showed that OM3-FA monotherapy increased LDL-C and HDL-C, which did not happen for the combined treatment. Besides, the effect on VLDL-C was similar between two treatment modalities; this undoubtedly increases the gap in total cholesterol reduction. Similarly, both VLDL-C and LDL-C are included in non-HDL-C; therefore, when OM3-FA was added to statins, the reduction in non-HDL-C was also more apparent. However, why does OM3-FA increase LDL-C levels? The following reasons may be explained. Lu. et al. (52) found that OM3-FA could amplify the propensity of very low-density lipoprotein (VLDL) to be converted to low-density lipoprotein (LDL). OM3-FA also diminished hepatic triglyceride-rich lipoprotein (TRL) secretion and enhanced TRL to LDL conversion (53). Moreover, it is reported that DHA enhances VLDL lipolysis, leading to greater conversion to LDL and increasing larger, more buoyant LDL particles (54). Two other studies also suggested that DHA-containing supplements significantly elevated LDL-C (55, 56). In addition, subgroup analysis showed that DHA significantly increased the level of TC, probably also because DHA increased LDL-C levels. Regarding the reason why LDL-C did not increase when OM3-FA was combined with statins, we thought it might be because the LDL-C lowering effect of statins outweighed the impact of OM3-FA in raising LDL-C.
Regarding the effect of OM3-FA on HDL-C, individual studies were found to impact the overall result significantly. After removing one article (11), the heterogeneity of OM3-FA monotherapy on HDL-C was reduced from 56 to 0%. We speculated that this trial could be the source of heterogeneity by combining sensitivity analysis results. However, the outcome of this study was consistent with the pooled results. Therefore, this did not affect the interpretation of the effect of OM3-FA on HDL-C. When OM3-FA was used in combination with statins, the pooled results showed no significant effect on HDL-C; However, when two studies were removed (41, 44), the results were reversed, and heterogeneity was also significantly reduced. Hence, we reviewed these two articles. One study (41) used only EPA, and the other (44) showed significant differences in baseline TG levels between the experimental and control groups (336.7 and 407.6 mg/dL, respectively). Therefore, we speculate that these might be the sources of heterogeneity and thus affect the overall results. The above analyses suggest that OM3-FA are tended to increase HDL-C levels. Nonetheless, the impact of combined therapy of statins plus OM3-FA on HDL-C still needs to be confirmed by large randomized multicenter well-designed trials.
Apolipoprotein B and Apo-AI are the main surface proteins on LDL and HDL particles, respectively (57). The Apo-B/Apo-AI ratio is an essential indicator of atherosclerosis. Our results showed that OM3-FA monotherapy had no significant effect on Apo-B and Apo-AI. However, when combined with statins, the levels of Apo-B and Apo-AI were significantly reduced compared to the control group. It was reported that statins affect lipid metabolism primarily by inhibiting cholesterol biosynthesis, which in turn increases the number of hepatic Apo-B receptors, and the clearance of all Apo B-containing LDL and VLDL is accelerated when OM3-FA is co-administrated (58). However, why the level of Apo-AI decreased after OM3-FA was added to statins needs to be further explored. Since combination therapy with OM3-FA and statins reduced Apo-B and Apo-AI simultaneously, the ratio may not change much. Therefore, we thought that combination therapy for the treatment of hypertriglyceridemia is still desirable.
We thought some implications from this meta-analysis could be obtained to guide clinical practice. First, when OM3-FA is used alone, if the patient’s LDL-C is already at a high level, it is not recommended because of the risk of further increasing LDL-C. Second, OM3-FA can be added to statins to treat residual hypertriglyceridemia, thus lowering TG without increasing LDL-C levels. Third, because of the risk of raising total cholesterol, DHA should be used in combination with EPA.
This meta-analysis has several strengths. First, compared with the previous meta-analysis, we included more literature with a larger sample size, making our results more robust. Second, nearly half of the included articles were multicenter, randomized, double-blind experiments, which made our results more accurate. Third, our study included populations from all over the world, which increased the generalizability of the results. Fourth, most of the included studies provided dietary guidance and advice to the participants, which significantly reduced the influence of dietary habits on the findings. Fifth, the studies we included all had a parallel design, which significantly reduced the influence of the study method on the results. Lastly, our study not only investigated the effect of OM3-FA on different blood lipid profiles but also evaluated the effect of the combination of OM3-FA and statins on lipid profiles, to some extent, which provided the necessary guidance for the correct use of OM3-FA.
However, several limitations of our study cannot be overlooked. First, significant heterogeneity was identified in most of the results even though we performed subgroup analyses according to intervention time, baseline TG level of participants, and type and dosage of OM3-FA. Fortunately, sensitivity analysis found that individual studies did not affect most outcomes. Second, the statins used varied across articles. Some studies did not indicate the type of statins and only generally referred to statins treatment, which greatly limited the subgroup analysis based on the type of statin. Third, we failed to investigate further the dose-response relationship of the effects of OM3-FA on lipid profiles.
Based on the current study, there are some recommendations for future studies on OM3-FA. The dose-response relationship of OM3-FA needs to be further investigated to provide a suitable and effective starting dose. The adverse effects of OM3-FA compared with placebo also require further evaluation. This study only evaluated the impact of OM3-FA on lipid profile, and whether there are some advantages of OM3-FA compared with other triglyceride-lowering drugs also needs to be further explored to provide an appropriate option for patients with hypertriglyceridemia.
In conclusion, the results of our meta-analysis indicated that OM3-FA monotherapy could decrease the concentrations of TG, TC, VLDL-C, and non-HDL-C and increase the levels of LDL-C and HDL-C, without significant effects on Apo-B and Apo-AI. The combined therapy of statins plus OM3-FA could exert significant reductions in TG, TC, VLDL-C, non-HDL-C, Apo-B, and Apo-AI levels, with no significant impact on LDL-C and HDL-C. Nevertheless, the effects of OM3-FA observed in this review should be interpreted with caution due to the high heterogeneity between the included studies.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
YY and WD conceived the study, designed the search strategy, conducted the study selection, interpreted the results, and drafted the manuscript. YW and TL extracted the data and performed the statistical analyses. YC and CL evaluated the risk of bias of included studies. QW and YW processed the pictures and tables. QC provided the guidance and resolved disagreements. All authors read and approved the final version of the manuscript.
This work was supported by the project of construction and regional promotion and application of the intelligent management innovation system of TCM for metabolic diseases based on Internet of Things Technology (CKY2021088).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1039056/full#supplementary-material
1. Simha V. Management of hypertriglyceridemia. BMJ (Clin Res ed.). (2020) 371:m3109. doi: 10.1136/bmj.m3109
2. Klempfner R, Erez A, Sagit BZ, Goldenberg I, Fisman E, Kopel E, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. (2016) 9:100–8. doi: 10.1161/circoutcomes.115.002104
3. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab. (2018) 103:3019–27. doi: 10.1210/jc.2018-00470
4. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes Metab. (2019) 21:366–71. doi: 10.1111/dom.13537
5. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. (2019) 40:537–57. doi: 10.1210/er.2018-00184
6. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. (2007) 115:450–8. doi: 10.1161/circulationaha.106.637793
7. Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Residual hypertriglyceridemia and estimated atherosclerotic cardiovascular disease risk by statin use in U.S. Adults with diabetes: national health and nutrition examination survey 2007-2014. Diabetes Care. (2019) 42:2307–14. doi: 10.2337/dc19-0501
8. Berglund L, Brunzell J, Sacks FM. Patient information page from The Hormone Foundations. Patient guide to the assessment and treatment of hypertriglyceridemia (high triglycerides). J Clin Endocrinol Metab. (2012) 97:31a–2a. doi: 10.1210/jcem.97.9.zeg31a
9. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. (2008) 300:2142–52. doi: 10.1001/jama.2008.621
10. Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. (2016) 176:1834–42. doi: 10.1001/jamainternmed.2016.6875
11. Bays HE, Hallén J, Vige R, Fraser D, Zhou R, Hustvedt SO, et al. Icosabutate for the treatment of very high triglycerides: a placebo-controlled, randomized, double-blind, 12-week clinical trial. J Clin Lipidol. (2016) 10:181e–91e. doi: 10.1016/j.jacl.2015.10.012
12. Maki KC, Geohas JG, Dicklin MR, Huebner M, Udani JK. Safety and lipid-altering efficacy of a new omega-3 fatty acid and antioxidant-containing medical food in men and women with elevated triacylglycerols. Prostaglandins Leukot Essent Fatty Acids. (2015) 99:41–6. doi: 10.1016/j.plefa.2015.05.002
13. Oh PC, Koh KK, Sakuma I, Lim S, Lee Y, Lee S, et al. Omega-3 fatty acid therapy dose-dependently and significantly decreased triglycerides and improved flow-mediated dilation, however, did not significantly improve insulin sensitivity in patients with hypertriglyceridemia. Int J Cardiol. (2014) 176:696–702. doi: 10.1016/j.ijcard.2014.07.075
14. Su TC, Hwang JJ, Huang KC, Chiang FT, Chien KL, Wang KY, et al. A randomized, double-blind, placebo-controlled clinical trial to assess the efficacy and safety of ethyl-ester omega-3 fatty acid in Taiwanese hypertriglyceridemic patients. J Atheroscler Thromb. (2017) 24:275-89. doi: 10.5551/jat.34231
15. Oliveira JM, Rondó PH. Omega-3 fatty acids and hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: systematic review and meta-analysis. HIV Clin Trials. (2011) 12:268–74. doi: 10.1310/hct1205-268
16. Vieira ADS, Silveira G. Effectiveness of n-3 fatty acids in the treatment of hypertriglyceridemia in HIV/AIDS patients: a meta-analysis. Cien Saude Colet. (2017) 22:2659–69. doi: 10.1590/1413-81232017228.21752015
17. Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American heart association. Circulation. (2019) 140:e673–91. doi: 10.1161/cir.0000000000000709
18. Kastelein JJP, Maki K, Susekov A, Ezhov M, Nordestgaard B, Kling D, et al. Management of severe hypertriglyceridemia with a novel omega-3 free-fatty acid formulation: subgroups in the evolve trial. J Clin Lipidol. (2013) 7:271–2. doi: 10.1016/j.jacl.2013.03.072
19. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. (2020) 324:2268-80. doi: 10.1001/jama.2020.22258
20. Jun JE, Jeong IK, Yu JM, Kim SR, Lee IK, Han KA, et al. Efficacy and safety of omega-3 fatty acids in patients treated with statins for residual hypertriglyceridemia: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab J. (2020) 44:78-90. doi: 10.4093/dmj.2018.0265
21. Maki KC, Orloff DG, Nicholls SJ, Dunbar RL, Roth EM, Curcio D, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. (2013) 35:.e1–3. doi: 10.1016/j.clinthera.2013.07.420
22. Lewis A, Lookinland S, Beckstrand RL, Tiedeman ME. Treatment of hypertriglyceridemia with omega-3 fatty acids: a systematic review. J Am Acad Nurse Pract. (2004) 16:384–95. doi: 10.1111/j.1745-7599.2004.tb00388.x
23. Mozaffarian D, Maki KC, Bays HE, Aguilera F, Gould G, Hegele RA, et al. Effectiveness of a novel ω-3 krill oil agent in patients with severe hypertriglyceridemia: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2141898. doi: 10.1001/jamanetworkopen.2021.41898
24. Woo JS, Hong SJ, Cha DH, Kim KS, Kim MH, Lee JW, et al. Comparison of the efficacy and safety of atorvastatin 40 mg/ω-3 fatty acids 4 g fixed-dose combination and atorvastatin 40 mg monotherapy in hypertriglyceridemic patients who poorly respond to atorvastatin 40 mg monotherapy: an 8-week, multicenter, randomized, double-blind phase III study. Clin Ther. (2021) 43:1419–30. doi: 10.1016/j.clinthera.2021.07.001
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
26. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed.). (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Statist Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
28. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
29. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. (2011) 108:682–90. doi: 10.1016/j.amjcard.2011.04.015
30. Chan DC, Watts GF, Mori TA, Barrett PH, Beilin LJ, Redgrave TG. Factorial study of the effects of atorvastatin and fish oil on dyslipidaemia in visceral obesity. Eur J Clin Invest. (2002) 32:429–36. doi: 10.1046/j.1365-2362.2002.01001.x
31. Contacos C, Barter PJ, Sullivan DR. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb. (1993) 13:1755–62. doi: 10.1161/01.atv.13.12.1755
32. Davidson MH, Maki KC, Kalkowski J, Schaefer EJ, Torri SA, Drennan KB. Effects of docosahexaenoic acid on serum lipoproteins in patients with combined hyperlipidemia: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. (1997) 16:236–43. doi: 10.1080/07315724.1997.10718680
33. Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. (2007) 86:324–33. doi: 10.1093/ajcn/86.2.324
34. Khandelwal S, Demonty I, Jeemon P, Lakshmy R, Mukherjee R, Gupta R, et al. Independent and interactive effects of plant sterols and fish oil n-3 long-chain polyunsaturated fatty acids on the plasma lipid profile of mildly hyperlipidaemic Indian adults. Br J Nutr. (2009) 102:722–32. doi: 10.1017/s0007114509297170
35. Koh KK, Quon MJ, Shin KC, Lim S, Lee Y, Sakuma I, et al. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis. (2012) 220:537–44. doi: 10.1016/j.atherosclerosis.2011.11.018
36. Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. (2000) 71:1085–94. doi: 10.1093/ajcn/71.5.1085
37. Oelrich B, Dewell A, Gardner CD. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol and LDL subfractions in hypertriglyceridemic adults. Nutr Metab Cardiovasc Dis. (2013) 23:350–7. doi: 10.1016/j.numecd.2011.06.003
38. Schuchardt JP, Neubronner J, Kressel G, Merkel M, von Schacky C, Hahn A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: results from a six month randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids. (2011) 85:381–6. doi: 10.1016/j.plefa.2011.07.006
39. Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterr Health J. (2008) 14:305–13.
40. Shidfar F, Keshavarz A, Jallali M, Miri R, Eshraghian M. Comparison of the effects of simultaneous administration of vitamin C and omega-3 fatty acids on lipoproteins, apo A-I, apo B, and malondialdehyde in hyperlipidemic patients. Inte J Vitamin Nutr Res. (2003) 73:163–70. doi: 10.1024/0300-9831.73.3.163
41. Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. (2012) 110:984–92. doi: 10.1016/j.amjcard.2012.05.031
42. Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega-3-acid ethyl esters on non–high-density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. (2010) 85:122–8. doi: 10.4065/mcp.2009.0397
43. Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. (2007) 29:1354–67. doi: 10.1016/j.clinthera.2007.07.018
44. Durrington PN, Bhatnagar D, Mackness MI, Morgan J, Julier K, Khan MA, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. (2001) 85:544–8. doi: 10.1136/heart.85.5.544
45. Kim CH, Han KA, Yu J, Lee SH, Jeon HK, Kim SH, et al. Efficacy and safety of adding omega-3 fatty acids in statin-treated patients with residual hypertriglyceridemia: ROMANTIC (Rosuvastatin-OMAcor iN residual hyperTrIglyCeridemia), a randomized, double-blind, and placebo-controlled trial. Clin Ther. (2018) 40:83-94. doi: 10.1016/j.clinthera.2017.11.007
46. Lee MW, Park JK, Hong JW, Kim KJ, Shin DY, Ahn CW, et al. Beneficial effects of omega-3 fatty acids on low density lipoprotein particle size in patients with type 2 diabetes already under statin therapy. Diabetes Metab J. (2013) 37:207–11. doi: 10.4093/dmj.2013.37.3.207
47. Meyer BJ, Hammervold T, Rustan AC, Howe PRC. Dose-dependent effects of docosahexaenoic acid supplementation on blood lipids in statin-treated hyperlipidaemic subjects. Lipids. (2007) 42:109–15. doi: 10.1007/s11745-006-3014-4
48. Ng TW, Ooi EM, Watts GF, Chan DC, Barrett PH. Atorvastatin plus omega-3 fatty acid ethyl ester decreases very-low-density lipoprotein triglyceride production in insulin resistant obese men. Diabetes Obes Metab. (2014) 16:519–26. doi: 10.1111/dom.12243
49. Nomura S, Inami N, Shouzu A, Omoto S, Kimura Y, Takahashi N, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. (2009) 20:16–22. doi: 10.1080/09537100802409921
50. Nordøy A, Bonaa KH, Nilsen H, Berge RK, Hansen JB, Ingebretsen OC. Effects of simvastatin and omega-3 fatty acids on plasma lipoproteins and lipid peroxidation in patients with combined hyperlipidaemia. J Intern Med. (1998) 243:163–70. doi: 10.1046/j.1365-2796.1998.00297.x
51. Hartweg J, Perera R, Montori V, Dinneen S, Neil HA, Farmer A. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. (2008) 2008:Cd003205. doi: 10.1002/14651858.CD003205.pub2
52. Lu G, Windsor SL, Harris WS. Omega-3 fatty acids alter lipoprotein subfraction distributions and the in vitro conversion of very low density lipoproteins to low density lipoproteins. J Nutr Biochem. (1999) 10:151–8. doi: 10.1016/s0955-2863(98)00094-1
53. Ooi EM, Watts GF, Ng TW, Barrett PH. Effect of dietary fatty acids on human lipoprotein metabolism: a comprehensive update. Nutrients. (2015) 7:4416–25. doi: 10.3390/nu7064416
54. Davidson MH. Omega-3 fatty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr Opin Lipidol. (2013) 24:467–74. doi: 10.1097/MOL.0000000000000019
55. Jacobson TA, Glickstein SB, Rowe JD, Soni PN. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol. (2012) 6:5–18. doi: 10.1016/j.jacl.2011.10.018
56. Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgraduate Med. (2014) 126:7–18. doi: 10.3810/pgm.2014.11.2828
57. Parish S, Peto R, Palmer A, Clarke R, Lewington S, Offer A, et al. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. (2009) 30:2137–46. doi: 10.1093/eurheartj/ehp221
Keywords: Omega-3 fatty acids, statins, triglyceride, low-density lipoprotein cholesterol, hypertriglyceridemia, meta-analysis
Citation: Yang Y, Deng W, Wang Y, Li T, Chen Y, Long C, Wen Q, Wu Y and Chen Q (2022) The effect of omega-3 fatty acids and its combination with statins on lipid profile in patients with hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 9:1039056. doi: 10.3389/fnut.2022.1039056
Received: 07 September 2022; Accepted: 27 September 2022;
Published: 13 October 2022.
Edited by:
Azizah Ugusman, National University of Malaysia, MalaysiaReviewed by:
Nur Aishah Che Roos, National Defence University of Malaysia, MalaysiaCopyright © 2022 Yang, Deng, Wang, Li, Chen, Long, Wen, Wu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Chen, Y2hlbnFpdTEwMDVAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.