
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 03 November 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1037851
This article is part of the Research Topic Dietary and Nutritional Indices and Chronic Diseases View all 22 articles
 Marieh Salavatizadeh1,2
Marieh Salavatizadeh1,2 Samira Soltanieh1,2
Samira Soltanieh1,2 Hossein Poustchi3
Hossein Poustchi3 Zahra Yari4
Zahra Yari4 Maryam Shabanpur5
Maryam Shabanpur5 Asieh Mansour6
Asieh Mansour6 Mohammad E. Khamseh2*
Mohammad E. Khamseh2* Fariba Alaei-Shahmiri2
Fariba Alaei-Shahmiri2 Azita Hekmatdoost1*
Azita Hekmatdoost1*Background: This study was conducted to evaluate possible associations between Dietary Total Antioxidant Capacity (DTAC) and odds of non-alcoholic fatty liver disease (NAFLD) in people with type-2 diabetes mellitus (T2DM).
Materials and methods: We recruited two hundred people with T2DM, and evaluated their liver steatosis using Fibroscan. Dietary intakes of participants were assessed using a validated food frequency questionnaire. DTAC was computed via ferric reducing antioxidant power (FRAP).
Results: In the crude model, no statistically significant association was found between DTAC and the odds of NAFLD in people with diabetes. However, after adjustment for potential confounders including age, gender, diabetes duration, smoking status, physical activity, BMI, waist circumference, and energy, the most reduced adjusted OR was indicated for the third tertile vs. the first one (OR: 0.28, 95% CI: 0.09–0.81, P = 0.02), meaning that diabetic patients in the third tertile of DTAC had 72% decreased risk of NAFLD in comparison to those in the first one. The relationship was remained significant after additional adjustment for HOMA-IR, HbA1c, serum Triglyceride (TG), and low-density lipoprotein-cholesterol (LDL) levels (OR: 0.29, 95% CI: 0.09–0.93, P = 0.03). Importantly, a dose-response pattern was demonstrated for DTAC and risk of NAFLD (P = 0.04).
Conclusion: Higher DTAC was related with a decreased risk of NAFLD in individuals with diabetes.
Non-alcoholic fatty liver disease (NAFLD), an obesity complication commonly overlooked, is defined by the deposition of more than 5% fat in the liver not resulting from other identifiable factors such as alcohol consumption or viral hepatitis, ranging from hepatic steatosis to fibrosis and related cirrhosis (1, 2). Non-alcoholic steatohepatitis (NASH) is a more severe form of NAFLD featured by inflammation, hepatocyte necrosis, and regularly fibrosis (3). As a major cause of liver disease globally, NAFLD and NASH give rise to a substantial burden (4). A recent meta-analysis of 86 studies in 2016, estimated the prevalence of NAFLD in general population worldwide at 25.24%, whereas several meta-analyses demonstrated that in 2017 the prevalence was twofold (54–59.67%) in people with type 2 diabetes mellitus (T2DM) (2, 5, 6). The link between NAFLD and T2DM has been clearly identified, and can be described by IR and hyperinsulinemia resulting in defective lipid metabolism and the accumulation of fatty acids in the liver (7). Moreover, in people suffering from T2DM and NASH, the process of oxidative stress is increased, compared to T2DM patients without NAFLD (8, 9). T2DM furthermore enhances the susceptibility to advanced NAFLD including NASH, liver fibrosis, and hepatocellular carcinoma (4). Therefore, it seems vital to identify and monitor these high-risk patients.
The gold standard procedure for distinguishing NAFLD is liver biopsy, however, is impossible to apply an invasive and expensive technique in such a great number of T2DM patients (10). Consequently, it is of benefit in identifying people suffering from NAFLD using simple methods, in order to detect those likely to progress to NASH or advanced liver disease (10). Nutrition is known as a main modifiable environmental factor in the development and management of NAFLD (11). A few researchers have clarified that antioxidant intake plays a crucial role in protecting against oxidative damage and relevant inflammatory complications in people with NAFLD (12). Currently, dietary total antioxidant capacity (DTAC) is regarded as a useful index to investigate the whole antioxidant capacity of foods (13). In comparison to a simple sum of certain dietary antioxidants, DTAC provides the cumulative capability of the total dietary antioxidants (13). Accumulating evidence suggests that DTAC is inversely associated with adverse health consequences such as cardiovascular diseases, T2DM, cancer deaths, and obesity (14–17). A recent case-control study declared that high DTAC is significantly related to decreased risk of NAFLD (12). Additionally, patients with greater DTAC indicated lower odds of NAFLD in comparison to lower amounts of DTAC (18).
Overall, the present study focused on the changes in blood glucose, lipid profile, transaminases, and IR among T2DM patients with and without NAFLD in order to present potential prognostic markers for identifying diabetic patients being at high risk of NAFLD. Furthermore, people with T2DM need to specifically be considered in the assessment of potential dietary prevention strategies for NAFLD. Nevertheless, to the best of the authors’ knowledge, the association between DTAC and the development of NAFLD in T2DM patients has not yet been investigated. Thus, the present study was conducted to evaluate probable association between DTAC and odds of NAFLD in T2DM patients.
This cross-sectional study was conducted between April 2021 and February 2022 and was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences (NO: IR.SBMU.NNFTRI.REC.1399.061). Eligible volunteers were selected by the use of consecutive-sampling method and provided with informed written consent, prior to study commencement.
The study population was recruited from people with T2DM referred to the diabetes clinic affiliated with the Institute of Diabetes and Metabolism, Iran University of Medical Sciences, Tehran, Iran. Participants in the NAFLD group (n = 133) were individuals aged between 18 and 70 years old with confirmed T2DM for over 2 years and CAP (Controlled attenuation parameter) score > 270 dB/m. The non-NAFLD group (n = 67) included individuals with 18–70 years of age and diagnosis of T2DM for over 2 years, as well as CAP score ≤ 270 dB/m. In addition, no participants in the NAFLD or non-NAFLD group was on insulin therapy. Body mass index (BMI) ≥ 23 kg/m2 was another inclusion criterion for participants in the both study groups. NAFLD was diagnosed using the findings of Fibroscan performed by an expert physician.
People with a history of any type of pathologically confirmed cancer, under chemotherapy or radiotherapy (due to cancer), drug use, chronic inflammatory disease, heart failure, myocardial infarction, and kidney disease were not included in the study. Moreover, Participants were excluded for recently weight-loss diet, taking weight-loss medications, pregnancy, lactation, more than 10% weight reduction during the last 6 months, history of hepatic diseases such as hepatitis, autoimmune disease, biliary disease, hereditary disorders of the liver including Wilson’s disease and hemochromatosis, and using toxins or drugs affecting the liver such as NSAIDs, anti-inflammatory drugs, etc. Participants with a clear drinking history (≥ 21 units/week in men and ≥ 14 units/week in women) were not included in the research. All of the studied patients reported that they do no drink alcohol at all. The flowchart of participants selection is shown in Figure 1.
Required information about age, sex, smoking status, duration of diabetes, and use of supplements were collected via a standard questionnaire. In order to measure CAP score, transient elastography (TE) equipped with M and XL probes (Fibroscan®) was used. A digital scale (Seca, Germany) was used to evaluate individuals’ weight (kg), unshod and to the nearest 100 g. Height was measured using a tape measure and in a standing position to the nearest 0.5 cm. Finally, BMI was calculated by dividing weight (kg) by the square of height (meters). In order to determine the level of physical activity during the last 7 days, the International Physical Activity Questionnaire (IPAQ) short form was applied, and it was expressed as metabolic equivalent task (MET)-min/week (19). The validity and reliability of this questionnaire have been already examined in Iranian adult women. Blood pressure was measured on the basis of standard protocols using an automatic sphygmomanometer (OMRON, Mannheim, Germany).
Venous blood samples were collected after 10–12 h of overnight fasting. Enzymatic colorimetric method was used to measure the levels of fasting blood glucose. Serum levels of Triglyceride (TG), Total Cholesterol (TC), and high-density lipoprotein (HDL) were calculated by the use of enzymatic assays and standard biochemical kits (Pars Azmun Co., Iran). Between- and within-run coefficient of variations were less than 6.2%. Low-density lipoprotein (LDL) was calculated by modified version of Friedewald equation (20). ECLIA method and Roche Diagnostics kits (Roche Cobas 6000 analyzer) were used to measure serum insulin. HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) was calculated by the following equation: [fastinginsulin(μU/mL)×fastingglucose(mmol/L)]/22.5 (21). QUICKI (Quantitative Insulin Sensitivity Check Index) was computed as 1/[log(fastinginsulin[cpsbreak]inμU/mL)+log (fastingglucoseinmg/dL)] (22). TyG (Triglyceride and glucose) index was determined as Ln[TG(mg/dL)×fasting[cpsbreak]glucose(mg/dL)/2] (23).
Dietary intakes of participants during the last year were examined using a semi-quantitative food frequency questionnaire (FFQ) with 147 item which its reliability and validity in Iranian population have been previously evaluated (24). An expert dietitian, being totally unaware of the participants’ situation (regarding having NAFLD), administered the FFQ via face-to-face interviews. Subjects reported how often they consumed each food item during the last year and household measures were used to convert usual intakes to grams per day (25). Subsequently, Nutritionist 4 software (First Databank Inc., Hearst Corp., San Bruno, CA, USA) modified for Iranian foods determined the amount of total energy and dietary nutrients consumed every day. In the present study, DTAC was calculated via ferric reducing antioxidant power (FRAP). In order to obtain the total antioxidant capacity (TAC) (in mmol) of each food, published databases with antioxidant capacity of foods measured by the use of FRAP, were applied. When TAC of food items was unavailable, the amount of the closest comparable food was allocated (26). In order to calculate DTAC (in mmol), the daily intake of each food item over all products and units of antioxidant content (derived from an antioxidant index database) were summed (27).
The normality of data was assessed using the Kolmogorov–Smirnov test and the histogram chart. By the use of the independent Student’s t-test and Mann–Whitney test, general characteristics with normal and abnormal distributions were compared between the study groups, respectively, and for qualitative variables, X2 test was applied. Next, subjects were categorized into tertiles of DTAC. General characteristics, biochemical parameters, and dietary intakes across tertiles of DTAC were evaluated by the use of Kruskal–Wallis test and analysis of covariance (ANCOVA) for continuous variables and X2 test for categorical variables.
Quantitative and qualitative variables were reported as mean ± SD (standard deviation) and percentages, respectively. To assess the association between biochemical factors and NAFLD in diabetic patients, binary logistic regression in different models was used. In addition, multiple logistic regression models were applied to determine unadjusted and adjusted ORs for DTAC. In all analyses, the first tertile of biochemical factors or DTAC was regarded as the reference category. A broad range of confounders was controlled to examine whether the association was independent of them. All the statistical analyses were conducted using SPSS (SPSS Inc., version 25). P values less than 0.05 were considered as significant.
Overall characteristics of individuals across the study groups are presented in Table 1. No statistically significant difference was found in age between the NAFLD and non-NAFLD groups. Among the diabetic patients with NAFLD and without NAFLD, 58.64 and 44.77 % were women, respectively. In terms of BMI, subjects in the NAFLD group significantly presented greater BMI compared with those in the non-NAFLD group. Besides, diabetic patients with NAFLD (NAFLD group) higher levels of TC, TG, LDL, transaminases, HbA1c, and TyG index, in comparison to those without NAFLD. Moreover, IR was more prevalent among them. The two groups, however, showed no significant differences in current smoking, diabetes duration, blood pressure, physical activity, FBS, HDL, and DTAC.

Table 1. Baseline characteristics of type 2 diabetic patients with or without non-alcoholic fatty liver disease (NAFLD)†.
Among categories of DTAC, a significant difference was found in terms of age, diastolic blood pressure (DBP), and insulin levels. However, BMI, physical activity, blood pressure, diabetes duration, and biochemical parameters did not differ significantly across categories of DTAC. We found that individuals in the top tertile had significantly higher intakes of monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), dietary fiber, fruits, vegetables, low-fat dairy products, and poultry. Additionally, the intake of whole grains, legumes, nuts, seeds, green/black tea, and coffee was significantly higher in them (Table 2).
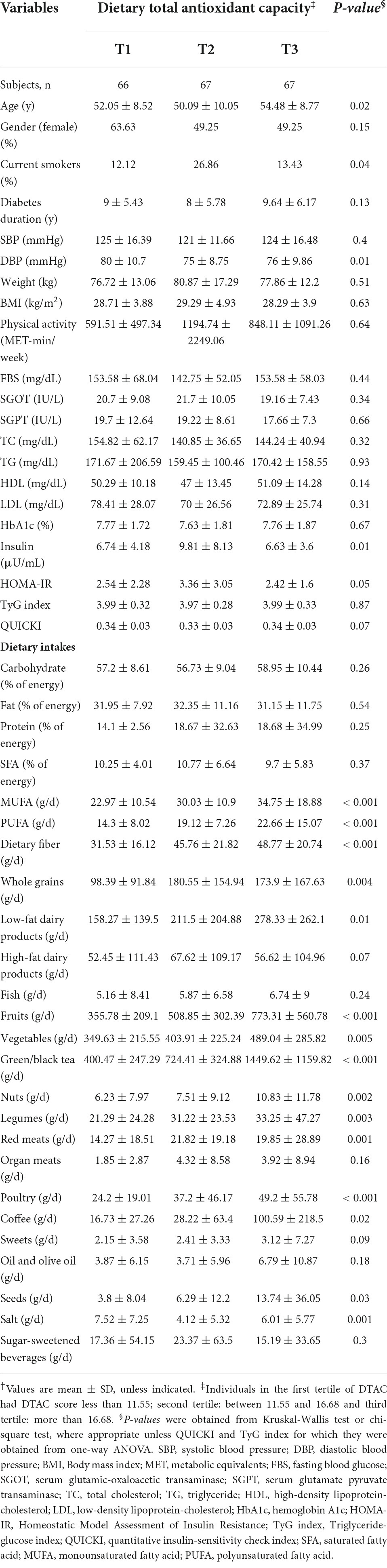
Table 2. Characteristics, biochemical parameters, and dietary intakes of study participants across tertiles of dietary total antioxidant capacity†.
Multiple logistic regression analysis was applied to examine whether certain biochemical factors were independently and significantly associated with the presence of NAFLD in people with diabetes (Table 3). For all T2DM patients, LDL-C, HbA1c, HOMA-IR, and TyG index were significantly associated with greater odds of having NAFLD, according to the fully adjusted model. On the other hand, the analysis showed that high QUICKI was a protective factor for NAFLD. Specifically, those with LDL (OR = 3.08, 95% CI: 1.16–8.16, P = 0.02) levels in tertile 3 had significantly increased odds for NAFLD, independently of age, gender, diabetes duration, smoking status, physical activity, BMI, waist circumference, HOMA-IR, HbA1c, and energy. Additionally, compared to tertile 1, HbA1c levels in tertile 3 were independently associated with a significantly increased odd for NAFLD (OR = 2.92, 95% CI: 1.14–7.45, P = 0.02). Similarly, T2DM patients with higher amounts of HOMA-IR (> 2.7) had significantly 5.33 times the odds (OR = 5.33, 95% CI: 1.83–15.5, P = 0.002) of NAFLD compared to T2DM patients with lower amounts (< 1.62) after controlling for the potential confounders. Also, diabetic patients with higher levels of QUICKI had reduced OR in NAFLD than those with lower levels (OR = 0.18, 95% CI: 0.06–0.54, P = 0.002). Another parameter independently associated with an increased odds of having NAFLD was TyG index (OR: 2.99, 95% CI: 1.22–7.32; P = 0.01).
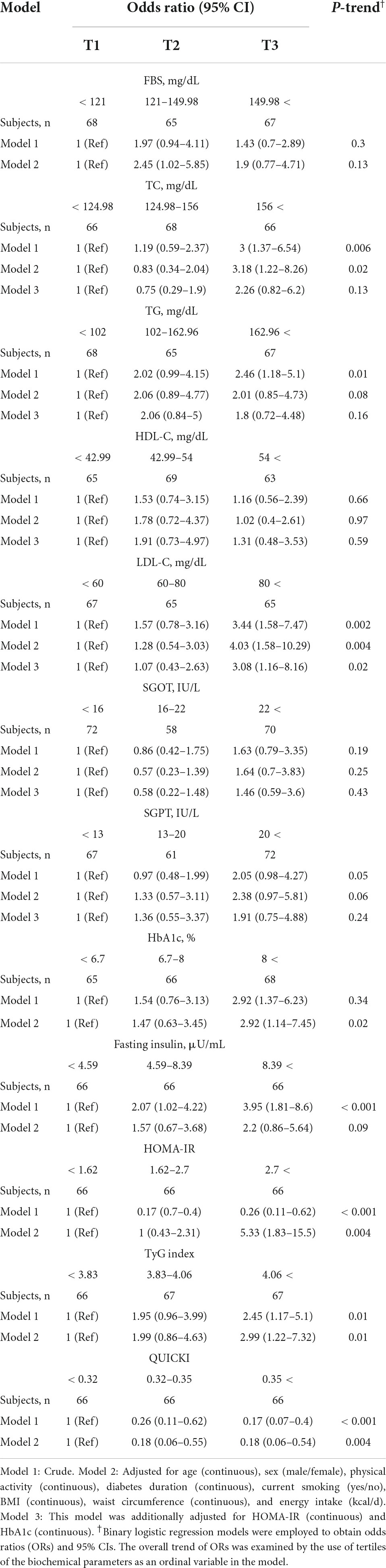
Table 3. Crude and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) for non-alcoholic fatty liver disease (NAFLD) in participants with type 2 diabetes across tertiles of biochemical parameters.
Multivariable-adjusted odds ratios and 95% CIs for NAFLD by tertiles of DTAC are indicated in Table 4. In the crude model, DTAC was not significantly associated with the odds of NAFLD in people with diabetes. However, after adjustment for potential confounders including age, gender, diabetes duration, smoking status, physical activity, BMI, waist circumference, and energy, the lowest adjusted OR was observed for the last tertile vs. the first one (OR: 0.28, 95% CI: 0.09–0.81, P = 0.02), meaning that diabetic patients in the highest tertile of DTAC had 72 % decreased risk of NAFLD compared with those in the lowest tertile. The association remained significant after additional adjustment for HOMA-IR, HbA1c, TG, and LDL levels (OR: 0.29, 95% CI: 0.09–0.93, P = 0.03). Importantly, a dose-response pattern was demonstrated for DTAC and risk of NAFLD (P = 0.04).
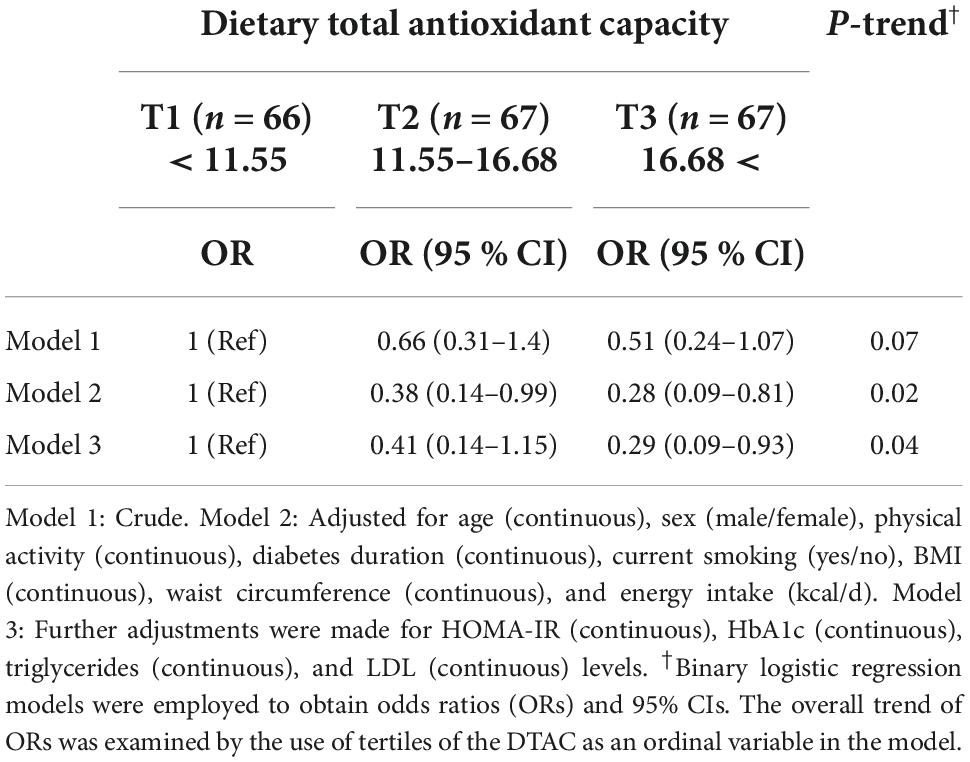
Table 4. Crude and multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) for non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes patients across tertiles of dietary total antioxidant capacity.
The present cross-sectional study investigated the associations between DTAC and NAFLD in people with diabetes. According to our results, patients in the NAFLD group (diabetes with NAFLD) had a higher BMI and serum levels of TC, TG, LDL, transaminases and HbA1C than the non-NAFLD group (diabetes without NAFLD). These patients also showed higher degrees of IR. The risk of NAFLD was significantly higher in the third tertile of LDL and HbA1c compared to the first tertile. The risk of NAFLD also increased significantly with increasing IR and decreasing insulin sensitivity. After fully adjustment for age, sex, physical activity, diabetes duration, current smoking, BMI, waist circumference, energy intake, Triglycerides, LDL, HbA1c, and HOMA-IR, risk of NAFLD was significantly lower in patients with higher DTAC. However, no significant difference was observed in DTAC score between the study groups. To the best of our knowledge, this is the first study reporting the inverse associations between DTAC and odds of NAFLD in people with diabetes.
Dietary total antioxidant capacity (DTAC) is a measure of the antioxidant capacity of the diet and is considered a good indicator of diet quality (28). An inverse association has been reported between DTAC and numerous chronic diseases including cardiovascular diseases (29), diabetes (16, 30), metabolic syndrome (31) and NAFLD (32, 33). Similar metabolic abnormalities are common among these disorders, which are exacerbated by combination of diabetes and NAFLD. Previously, an inverse association of antioxidant-rich dietary patterns, including the Dietary Approaches to Stop Hypertension (DASH) diet (34–36), plant-based diet (37, 38), with the risk of NAFLD and diabetes has been reported separately. All of these diets are rich in antioxidant compounds and therefore have antioxidant effects.
Oxidative stress is involved in the pathogenesis of NAFLD by promoting IR, dyslipidemia (39). Antioxidant-rich foods/diets might be effective in reinforcing antioxidant defense by reducing lipid peroxidation, cellular and organ damage, and insulin sensitivity (40). Increasing the antioxidant capacity of cells improves glucose and lipid metabolism and reduces the risk of NAFLD (39, 40).
The findings of this study showed that the risk of NAFLD in people with diabetes increased significantly with increasing IR. Study groups were significantly different in terms of insulin resistance and sensitivity, although this difference was not found among DTAC tertiles. IR is a mediator in the effect of oxidative stress on lipid profile (41). Another factor that may play a role in DTAC and NAFLD risk reduction is fiber, which contributes to weight loss, improved insulin sensitivity, dyslipidemia, and glycemic control (42).
Although dietary antioxidants play a role in reducing obesity and related disorders by inhibiting fat absorption, stimulating adipose tissue catabolism, inhibiting the proliferation and differentiation of adipocytes (43), no significant difference in weight was observed between the three tertiles of DTAC. However, it has been suggested that the association of DTAC with cardiovascular risk factors is independent of weight (44).
In this study, no difference was observed between DTAC between NAFLD and non-NAFLD groups, which is contrary to previous findings in diabetic patients (45) and NAFLD patients (33). However, in these studies, the comparisons were made between patients and healthy individuals, while in our study, diabetic people with and without NAFLD were compared. Oxidative stress reduces insulin secretion by damaging the mitochondria of pancreatic beta cells and causes diabetes (46, 47).
The present study has several limitations. First, due to the cross-sectional nature of the study design, cause and effect relationships could not be confirmed. The use of United States Department of Agriculture (USDA) food composition table was another limitation of the study due to incomplete database for the content of Iranian food antioxidant. The validity of DTAC obtained from the FFQ in the Iranian diet has not been examined previously. Also, the use of FFQ is associated with recall bias.
In conclusion, higher DTAC was associated with a decreased odds of having NAFLD in people with diabetes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Shahid Beheshti University of Medical Sciences, Tehran, Iran. The patients/participants provided their written informed consent to participate in this study.
MS conceived and designed the study, performed the statistical analysis, interpreted the data, and drafted the manuscript. SS and AM contributed to the sampling selection schedule. ZY and MSh drafted the manuscript. HP contributed to the data interpretation. MK, FA-S, AH, and MSh made critical revisions to the manuscript. MK and AH contributed to the study supervision. All authors have read and approved the final version to be published.
Funding for this study was provided by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant ID: 26839).
We express their appreciation to the participants of the study for their enthusiastic support and to the staff of the Institute of Diabetes and Metabolism of the Iran University of Medical Sciences for their valuable help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moore MP, Cunningham RP, Dashek RJ, Mucinski JM, Rector RS. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity. (2020) 28:1843–52. doi: 10.1002/oby.22964
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
3. Barb D, Bril F, Kalavalapalli S, Cusi K. Plasma fibroblast growth factor 21 is associated with severity of nonalcoholic steatohepatitis in patients with obesity and type 2 diabetes. J Clin Endocrinol Metab. (2019) 104:3327–36. doi: 10.1210/jc.2018-02414
4. Lange NF, Graf V, Caussy C, Dufour J-F. PPAR-targeted therapies in the treatment of non-alcoholic fatty liver disease in diabetic patients. Int J Mol Sci. (2022) 23:4305. doi: 10.3390/ijms23084305
5. Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine. (2017) 96:e8179. doi: 10.1097/MD.0000000000008179
6. Atan NAD, Koushki M, Motedayen M, Dousti M, Sayehmiri F, Vafaee R, et al. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. (2017) 10(Suppl. 1):S1.
7. Alsabaani AA, Mahfouz AA, Awadalla NJ, Musa MJ, Al Humayed SM. Non-alcoholic fatty liver disease among type-2 diabetes mellitus patients in Abha City, South Western Saudi Arabia. Int J Environ Res Public Health. (2018) 15:2521. doi: 10.3390/ijerph15112521
8. Klisic A, Isakovic A, Kocic G, Kavaric N, Jovanovic M, Zvrko E, et al. Relationship between oxidative stress, inflammation and Dyslipidemia with fatty liver index in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. (2018) 126:371–8. doi: 10.1055/s-0043-118667
9. Casoinic F, Sampelean D, Buzoianu AD, Hancu N, Baston D. Serum levels of oxidative stress markers in patients with type 2 diabetes mellitus and non-alcoholic steatohepatitis. Roman J Intern Med. (2016) 54:228–36. doi: 10.1515/rjim-2016-0035
10. Silaghi CA, Silaghi H, Colosi HA, Craciun AE, Farcas A, Cosma DT, et al. Prevalence and predictors of non-alcoholic fatty liver disease as defined by the fatty liver index in a type 2 diabetes population. Clujul Med. (2016) 89:82–8. doi: 10.15386/cjmed-544
11. Talenezhad N, Mirzavandi F, Rahimpour S, Amel Shahbaz AP, Mohammadi M, Hosseinzadeh M. Empirically derived dietary pattern and odds of non-alcoholic fatty liver diseases in overweight and obese adults: a case-control study. BMC Gastroenterol. (2022) 22:158. doi: 10.1186/s12876-022-02222-z
12. Salehi-Sahlabadi A, Mokari A, Elhamkia M, Farahmand F, Jabbari M, Hekmatdost A. Dietary total antioxidant capacity and risk of non-alcoholic fatty liver disease: a case-control study. J Res Health Sci. (2020) 20:e00486. doi: 10.34172/jrhs.2020.18
13. Kim D, Han A, Park Y. Association of dietary total antioxidant capacity with bone mass and osteoporosis risk in Korean women: analysis of the Korea national health and nutrition examination survey 2008-2011. Nutrients. (2021) 13:1149. doi: 10.3390/nu13041149
14. Mirzababaei A, Shiraseb F, Abaj F, Khosroshahi RA, Tavakoli A, Koohdani F, et al. The effect of dietary total antioxidant capacity (DTAC) and Caveolin-1 gene variant interaction on cardiovascular risk factors among overweight and obese women: a cross-sectional investigation. Clin Nutr. (2021) 40:4893–903. doi: 10.1016/j.clnu.2021.07.013
15. Zamani B, Daneshzad E, Azadbakht L. Dietary total antioxidant capacity and risk of gastrointestinal cancers: a systematic review and meta-analysis of observational studies. Arch Iran Med. (2019) 22:328–35. doi: 10.1017/S1368980019001125
16. Mancini FR, Affret A, Dow C, Balkau B, Bonnet F, Boutron-Ruault M-C, et al. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia. (2018) 61:308–16. doi: 10.1007/s00125-017-4489-7
17. Zujko ME, Witkowska AM, Waśkiewicz A, Piotrowski W, Terlikowska KM. Dietary antioxidant capacity of the patients with cardiovascular disease in a cross-sectional study. Nutr J. (2015) 14:1–13. doi: 10.1186/s12937-015-0005-4
18. Sohouli M, Fatahi S. Associations between dietary total antioxidant capacity and risk of nonalcoholic fatty liver disease (NAFLD) in adults: a case-control study. Nutr Food Sci Res. (2020) 7:1–7. doi: 10.29252/nfsr.7.3.1
19. Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of international physical activity questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. (2012) 18:1073–80.
20. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18:499–502. doi: 10.1093/clinchem/18.6.499
21. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. (2000) 23:57–63. doi: 10.2337/diacare.23.1.57
22. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. (2000) 85:2402–10. doi: 10.1210/jcem.85.7.6661
23. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
24. Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. (2005) 81:55–63. doi: 10.1093/ajcn/81.1.55
25. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy. (1999) 7:42–58.
26. Zhou S, Wang X, Tan Y, Qiu L, Fang H, Li W. Association between vitamin C intake and glioma risk: evidence from a meta-analysis. Neuroepidemiology. (2015) 44:39–44. doi: 10.1159/000369814
27. Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. (2010) 9:1–11. doi: 10.1186/1475-2891-9-3
28. Kourlaba G, Panagiotakos DB. Dietary quality indices and human health: a review. Maturitas. (2009) 62:1–8. doi: 10.1016/j.maturitas.2008.11.021
29. Bahadoran Z, Golzarand M, Mirmiran P, Shiva N, Azizi F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran lipid and glucose study. Nutr Metab. (2012) 9:70. doi: 10.1186/1743-7075-9-70
30. van der Schaft N, Schoufour JD, Nano J, Kiefte-de Jong JC, Muka T, Sijbrands EJ, et al. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: the Rotterdam study. Eur J Epidemiol. (2019) 34:853–61. doi: 10.1007/s10654-019-00548-9
31. Hermsdorff HHM, Puchau B, Volp ACP, Barbosa KB, Bressan J, Zulet M, et al. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr Metab. (2011) 8:1–8. doi: 10.1186/1743-7075-8-59
32. Salehi-Sahlabadi A, Mokari A, Elhamkia M, Farahmand F, Jabbari M, Hekmatdoost A. Dietary total antioxidant capacity and risk of non-alcoholic fatty liver disease: a case-control study. J Res Health Sci. (2020) 20:e00486.
33. Sohouli MH, Fatahi S, Sayyari A, Olang B, Shidfar F. Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (NAFLD) in adults: a case–control study. J Nutr Sci. (2020) 9:e48. doi: 10.1017/jns.2020.39
34. Liese AD, Bortsov A, Günther AL, Dabelea D, Reynolds K, Standiford DA, et al. Association of DASH diet with cardiovascular risk factors in youth with diabetes mellitus: the SEARCH for diabetes in Youth study. Circulation. (2011) 123:1410–7. doi: 10.1161/CIRCULATIONAHA.110.955922
35. Liese AD, Nichols M, Sun X, D’Agostino RB Jr., Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. (2009) 32:1434–6. doi: 10.2337/dc09-0228
36. Doustmohammadian A, Clark CC, Maadi M, Motamed N, Sobhrakhshankhah E, Ajdarkosh H, et al. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol Cohort study (AmolCS). Sci Rep. (2022) 12:1–9. doi: 10.1038/s41598-022-06035-8
37. Alferink LJ, Erler NS, de Knegt RJ, Janssen HL, Metselaar HJ, Darwish Murad S, et al. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur J Epidemiol. (2020) 35:1069–85. doi: 10.1007/s10654-020-00627-2
38. Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern Med. (2019) 179:1335–44. doi: 10.1001/jamainternmed.2019.2195
39. de Oliveira DG, de Faria Ghetti F, Moreira APB, Hermsdorff HHM, de Oliveira JM, de Castro Ferreira LEVV. Association between dietary total antioxidant capacity and hepatocellular ballooning in nonalcoholic steatohepatitis: a cross-sectional study. Eur J Nutr. (2019) 58:2263–70.
40. Liu W, Baker SS, D Baker R, Zhu L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr Drug Targets. (2015) 16:1301–14. doi: 10.2174/1389450116666150427155342
41. Videla LA, Rodrigo R, Araya J, Poniachik J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol Med. (2006) 12:555–8. doi: 10.1016/j.molmed.2006.10.001
42. Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. (2008) 19:71–84. doi: 10.1016/j.jnutbio.2007.02.009
43. Barnard ND, Scialli AR, Turner-McGrievy G, Lanou AJ, Glass J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med. (2005) 118:991–7. doi: 10.1016/j.amjmed.2005.03.039
44. Mozaffari H, Daneshzad E, Surkan PJ, Azadbakht L. Dietary total antioxidant capacity and cardiovascular disease risk factors: a systematic review of observational studies. J Am Coll Nutr. (2018) 37:533–45. doi: 10.1080/07315724.2018.1441079
45. Zujko ME, Witkowska AM, Górska M, Wilk J, Kretowski A. Reduced intake of dietary antioxidants can impair antioxidant status in type 2 diabetes patients. Pol Arch Med Wewn. (2014) 124:599–607. doi: 10.20452/pamw.2497
46. Franzini L, Ardigò D, Zavaroni I. Dietary antioxidants and glucose metabolism. Curr Opin Clin Nutr Metab Care. (2008) 11:471–6. doi: 10.1097/MCO.0b013e328303be79
Keywords: NAFLD, diabetes, diet, DTAC, antioxidant
Citation: Salavatizadeh M, Soltanieh S, Poustchi H, Yari Z, Shabanpur M, Mansour A, Khamseh ME, Alaei-Shahmiri F and Hekmatdoost A (2022) Dietary total antioxidant capacity is inversely associated with the odds of non-alcoholic fatty liver disease in people with type-2 diabetes. Front. Nutr. 9:1037851. doi: 10.3389/fnut.2022.1037851
Received: 06 September 2022; Accepted: 13 October 2022;
Published: 03 November 2022.
Edited by:
Sorayya Kheirouri, Tabriz University of Medical Sciences, IranReviewed by:
Yoshihiro Miyamoto, National Cerebral and Cardiovascular Center, JapanCopyright © 2022 Salavatizadeh, Soltanieh, Poustchi, Yari, Shabanpur, Mansour, Khamseh, Alaei-Shahmiri and Hekmatdoost. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azita Hekmatdoost, YV9oZWttYXQyMDAwQHlhaG9vLmNvbQ==; Mohammad E. Khamseh, a2hhbXNlaC5tQGl1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.