- 1Research Laboratory: Education, Motricity, Sport and Health, Sfax, Tunisia
- 2Higher Institute of Sport and Physical Education of Sfax, University of Sfax, Sfax, Tunisia
- 3Department of Training and Movement Science, Institute of Sport Science, Johannes Gutenberg-University Mainz, Mainz, Germany
- 4UFR SESS-STAPS, Paris-East Créteil University, LIRTES (EA 7313), Créteil, France
- 5Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Genoa, Italy
- 6National Research Council, Institute of Clinical Physiology, Lecce, Italy
- 7Sport and Exercise Science, School of Allied Health, Human Services and Sport, La Trobe University, Melbourne, VIC, Australia
- 8Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
- 9Centre for Intelligent Healthcare, Coventry University, Coventry, United Kingdom
- 10Department of Health, Exercise Science Research Center Human Performance and Recreation, University of Arkansas, Fayetteville, AR, United States
- 11Department of Nutrition, Faculty of Pharmacy and Medical Sciences, University of Petra, Amman, Jordan
- 12Department of Psychiatry, Ministry of Health, Manama, Bahrain
- 13Department of Psychiatry, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain
- 14Laboratory for Industrial and Applied Mathematics, Department of Mathematics and Statistics, York University, Toronto, ON, Canada
Religious fasting is practiced by people of all faiths, including Christianity, Islam, Buddhism, Jainism, as well as Hinduism, Judaism, and Taoism. Individual/clinical, public, global, and planetary health has traditionally been studied as separate entities. Nevertheless, religious fasting, in conjunction with other religious health assets, can provide several opportunities, ranging from the individual to the population, environmental, and planetary levels, by facilitating and supporting societal transformations and changes, such as the adoption of healthier, more equitable, and sustainable lifestyles, therein preserving the Earth's systems and addressing major interconnected, cascading, and compound challenges. In this review, we will summarize the most recent evidence on the effects of religious fasting, particularly Orthodox and Ramadan Islamic fasting, on human and public health. Further, we will explore the potential effects of religious fasting on tackling current environmental issues, with a special focus on nutrition/food restriction and planetary health. Finally, specific recommendations, particularly around dietary intake during the fasting rituals, will be provided to ensure a sustainable healthy planet.
Introduction
Human life expectancy is substantially higher today as opposed to 100 years ago, mainly attributed to advances in both preventive and clinical medicine and public health policies (1). However, increased longevity is associated with a growth in non-communicable and disabling diseases of old age such as neurodegenerative diseases, cardiovascular disease, and cancer. Furthermore, the western diet and lifestyle, characterized by unhealthy diet and sedentariness, has engendered many, so-called diseases of civilization, including obesity-associated metabolic syndrome, coronary heart disease, hypertension, stroke, type 2 diabetes, chronic liver disease, autoimmune disease, epithelial cell cancers, and osteoporosis, which are scarce or non-existent in hunter-gatherers and other non-westernized populations (2). As a consequence of civilization, life expectancy is expected, for the first time, to fall (3, 4). Moreover, the current western dietary pattern, high in animal-based and low in plant-based foods, is harmful; not only to personal health (5, 6), but also to public, global, and planetary health (7, 8). In this context, previous studies show meal consumption is linked to premature mortality and type 2 diabetes (9–11). Moreover, meat has been recognized as the food with the highest influence on greenhouse gas (GHG) emissions and land usage (12). In this context, producing 1,000 kcal of lamb or beef creates 14 and 10 kg of GHG emissions, respectively, compared to 1 and 3 kg for lentils or tofu (13). Additionally, meat production is deleterious for aquifers (14); one serving of beef or pork requires 1211 and 469 L of water, respectively, but one serving of dry beans, tofu, or tomatoes requires 220, 57, and 30 L, respectively (13). Several studies have found that reducing meat consumption can reduce GHG emissions, as well as land, water, and energy use, while concomitantly improving health outcomes (12). Therefore, an extension of a healthy life, while tackling transnational environmental challenges, is of utmost importance.
Religious fasting, or faith-based fasting, is predominantly practiced to satisfy prescribed religious requirements and is defined as a nutritional model characterized by a variance in the degree of caloric restriction and abstinence from specific foods (15). Interestingly, religious fasting could help to improve individual health as well as the community, and planet. Fasting rituals are followed by billions of individuals worldwide and their effects may differ from one religious community to another (16). It should be acknowledged that 83% of the world's population self-identified as adhering to a religion in 2010, and this number is projected to rise to 87% by 2050 (17–19). In addition, Christians are the world's largest religious community, with Islam coming in second and being the fastest-growing faith (20). Skirbekk et al. (19) reported that countries with a greater proportion of religiously affiliated people are facing more environmental risks and are less prepared for those risks. Therefore, targeting the largest religious groups (i.e., Christians, Muslims) via specific recommendations may contribute to a “healthy planet, supporting healthy people.”
Using a comprehensive search, we will summarize in this review the latest evidence on the effects of religious fasting, particularly Orthodox and Ramadan Islamic fasting, on human health. Additionally, the potential effects of religious fasting on public and planetary health will be discussed and specific recommendations will be provided.
Methods
A two-step search process was conducted to identify (i) systematic reviews, with/or without meta-analyses, or scoping reviews and (ii) experimental studies evaluating the effects of any type of religious fasting on health outcomes in apparently healthy individuals (not physically active individuals and athletes) and patients.
In the first step, to identify systematic reviews, with/or without meta-analyses, or scoping reviews, a systematic literature search was conducted in five databases (PubMed, Web of Science, Cochrane, EBSCOhost, Scielo) on August 10th 2022. An additional search on Google Scholar was conducted on August 15th 2022. The following terms were used (see Table S1): “Religious fasting,” “faith-based fasting,” “human,” “health,” “scoping review,” “systematic review,” and “meta-analysis.” Appropriate Boolean operators were used to join the various keywords. Field tags, wild-card options (i.e., truncated words), and medical subject headings (MeSH) terms were also incorporated where appropriate. In the search strategy, no language or date limits were applied. The full search strategy for all databases is presented in Table S1. References of included papers were manually verified for a comprehensive search for relevant reviews. Personal files were also searched.
In the second step, experimental studies were searched on August 10th 2022 in Google Scholar using the following terms: “Religious fasting,” “faith-based fasting,” “human” and “health.” To identify experimental studies published after the date of the last search of the most recent comprehensive review, date limits were applied.
Results
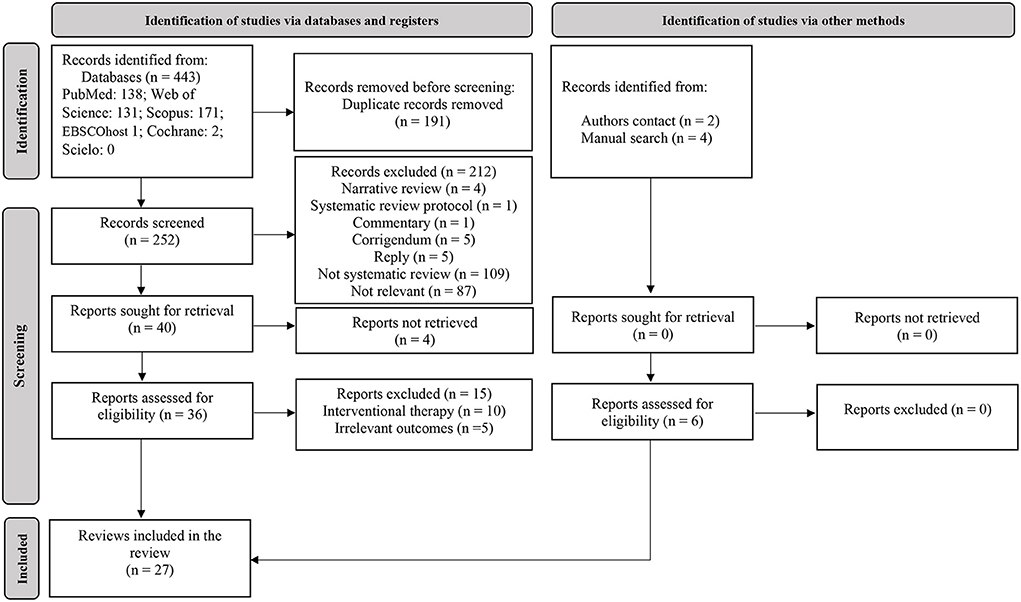
For the first-step, the predefined search strategies yielded a preliminary pool of 443 possible papers. A total of 191 duplicates were removed. Then, 252 published papers were screened by titles and abstracts for eligibility, of which 36 published studies met the inclusion criteria. After a careful review of the 36 full texts, 27 reviews (systematic review with or without a meta-analysis) were included (Figure 1). Of the 27 included reviews, only one systematic review (21) and a scoping review (22) evaluated the effects of Orthodox fasting on health/nutritional outcomes. The remainder reviews (n = 25) focused on the effects of Ramadan fasting on anthropometric and metabolic markers (23–25), glucometabolic parameters (26–29), salivary flow rate, inflammatory and metabolic variables (30), immunity, inflammatory markers and infectious events (31–33), blood pressure and cardiovascular events (34–36), liver function (37), renal function and chronic kidney diseases (38, 39), body mass (i.e., body weight) and body composition (40–42), intestinal microbiome changes (43), hormones regulating appetite and satiety (44), psychiatric parameters (45), sleep quality (46) and pregnancy outcomes (47). The characteristics and the main outcomes of the included reviews are presented in Table S2.
For the second-step, 53 research articles evaluating the effect of Ramadan fasting on anthropometric parameters (n = 10), hydration status (n = 11), renal function (n = 12), metabolic health (n = 9), liver function (n = 4), markers of inflammation, immunity and oxidative stress (n = 2), the gut microbiome (n = 3), and sleep (n = 2) were included. Other empirical studies evaluating the effects of Orthodox fasting (n = 13), Daniel (n = 3) and Buddhist (n = 2) fasting were included.
Religious fasting, nutrition and individual health
In many religions, fasting is a fundamental practice with both spiritual and physical advantages (48). The health implications of religious fasting have been the topic of several scientific investigations, with the majority of studies conducted in the previous three decades. However, these implications have typically been studied at the individual level, with few studies exploring other domains across wellbeing from a transdisciplinary perspective. Besides the individual level of wellbeing (subjective hedonic and psychosocial wellbeing, “a balanced mind and a healthy body,” and spiritual wellbeing), fasting impacts the community's well-being (“social or collective wellbeing,” social capital, cohesion, connectedness, and “social identity” – a sense of belonging to a community), and the environmental and planetary wellbeing (“connection with nature,” nature-based mindfulness, “environmental and planetary wellbeing,” and nurturing environments). Over time, and throughout history, several religious actors and institutions, including trusted religious leaders, faith-based organizations, and faith communities, at all levels and of any religious creed/belief, have played a major role in the delivery of healthcare services and provisions and have contributed to shaping health emergency preparedness and response, as well as to mobilizing community-led action and catalyzing community partnership.
The effects of the religious Islamic fasting of Ramadan, the Greek Orthodox Christianity fasting, and the Biblical-based Daniel Fast, on subjects' dietary intake and health-related outcomes, will be outlined. Other forms of religious fasting (i.e., Buddhism, Daniel fast, Judaism) are only briefly overviewed in this review given the paucity of health-related data available.
Islamic Ramadan fasting
During Ramadan fasting, one of the five pillars of the Islamic religion, more than 1.8 billion healthy pubescent Muslims worldwide must abstain from eating, drinking, and other specific behaviors (e.g., smoking, sexual intercourses, and all sensory pleasures) from dawn to sunset, during a period that lasts 29 or 30 days (lunar month) (15, 49). The daily fasting time length depends on the geographical location (latitude) and season. For example, in the north of the UK, daylight lasts <6 h in winter and about 19 h in midsummer (50).
During this month, water and food consumption are exclusively nocturnal and the typical dietary practice during Ramadan fasting consists of consuming one substantial meal after sunset and one lighter meal before dawn (51); however, an additional meal is sometimes consumed before sleeping (52). Interestingly, Ramadan fasting is similar to Alternate-Day Fasting in that the 12-h fast is followed by a 12-h feast. However, Ramadan fasting varies from Alternate-Day Fasting in that drinking water is not permitted throughout the 12-h fast (51, 53).
Food choices/preferences
Globally, there is large variability in dietary pattern between cultures in countries where individuals are observing Ramadan. Therefore, it is not surprising to observe different food preferences, specific to each culture and/or country. For example, Pirsaheb et al. (54) found, in a study of 160 Iranian subjects, there was a higher consumption of vegetables and fruits during Ramadan, whereas the consumption of dairy products, meat, and cereals decreased significantly. Contrarywise, in a study of 366 Ghanaian adolescents, it was found that the consumption of milk and vitamin A-rich fruits increased during Ramadan, while there was a lower consumption of dark leafy vegetables, legumes, and nuts (55). The authors also found that meat (beef, mutton, and chevon) consumption patterns remained relatively stable compared to before the month of Ramadan (~3 days/week), while poultry (chicken, guinea fowl, turkey) intake decreased marginally during Ramadan (55).
During a year-round study (56), including the month of Ramadan, Lebanese adults (n = 62), completed multiple (9–12, 14) 24-h dietary recalls. Dietary intake was estimated using food groups as well as energy, macro, and micronutrient consumption. The authors found that the intakes of cereals, cereal-based products, pasta, eggs, seeds and nuts, milk and dairy, and oils and fats were lower, while dried fruit, vegetables, cakes, pastries, and Arabic sweets, and sugar-sweetened-beverages intakes were higher during Ramadan as compared to the remainder of the year (56). In addition, the intake of poultry, meat, fish and seafood remained unchanged during vs. the remainder of the year (56). Nashvak et al. (57) evaluated the consumption of food groups before and during Ramadan in 160 healthy men from Iran. The authors found that, compared to before Ramadan, the consumption of bread and cereals, dairy products, meat, and vegetables decreased, while the consumption of fruit, fat, and oil increased during Ramadan.
Anthropometric parameters
During the month of Ramadan, food and fluid intake becomes less frequent and, therefore, fluctuations in some anthropometric variables are to be expected.
The first published meta-analysis evaluating the effect of Ramadan observance on body mass was by Kul et al. (23). The authors found that Ramadan fasting decreases body mass (small effect). In addition, the subgroup analysis indicated that the decrease in body mass was sex-specific as it was observed in men but not in women.
The aforementioned meta-analysis (23) was updated by Sadeghirad et al. (40); in the updated version, the authors showed Ramadan fasting elicited a statistically significant decrease in body mass (−1.24 kg by the end of Ramadan). Again, the decrease in body mass was significant in both sexes (−1.51 kg in men and −0.92 kg in women). However, the body mass loss during Ramadan was continent-specific, as it was greater among Asian populations compared with Africans and Europeans. Furthermore, the body mass loss observed at follow-up (2–6 weeks after Ramadan) was less pronounced, albeit body mass was still statistically significantly lower compared to before Ramadan (−0.72 kg).
The first systematic review with meta-analysis evaluating the effects of Ramadan fasting on body composition was conducted by Fernando et al. (41). The authors found that, from before to the end of Ramadan, there was a significant reduction in fat percentage in overweight or obese individuals, but not in those of normal weight. Similarly, Ramadan fasting yielded a significant loss of fat-free mass, but was about 30% less than the loss of absolute fat mass. After the end of Ramadan (2–5 weeks), there was a return toward, or to, pre-Ramadan measurements in body mass and body composition.
In another systematic review and meta-analysis, Jahrami et al. (42) concluded Ramadan fasting yielded a significant, but small reduction in body mass; this effect size translates into a difference in means of −1.022 kg. The regression analyses, used to explain sources of heterogeneity in the results, revealed that the decrease in body mass was directly associated with fasting time and correlated with country and season.
Regarding recent empirical studies, Agagündüz et al. (58) found, in 27 healthy Turkish participants (11 males, and 16 females) aged 27.6 ± 1.69 yrs, a significant decrease in body mass, body mass index (BMI), and fat-free mass. Similarly, Mengi Çelik et al. (59) reported a decrease in body mass and BMI during Ramadan in 32 (12 males 20 females) Turkish healthy adults aged 19 and 32 years. Similar findings were reported in 49 Indonesian healthy adults (15 males, 34 females) (60). Hasan et al. (61) found that Ramadan fasting decreased BMI and waist circumference in 55 overweight and obese participants (22 females and 33 males). Alsowaid et al. (62) enrolled only female individuals (n = 20) who were healthy students at the University of Bahrain. The authors found that Ramadan fasting decreased body mass, BMI, fat mass, and waist circumference (62). The decrease in body mass, BMI, and waist circumference could be attributed to the increased utilization of stored body fat as an energy substrate and/or the decreased energy intake as a result of fasting (63). Nevertheless, decreased total water intake during the month of Ramadan, possibly leading to a state of hypohydration, may also explain the decrease in body mass and BMI (63, 64).
Recently, Al-Maiman et al. (65) evaluated the effects of Ramadan fasting on anthropometric parameters of patients with type 1 (n = 42) and type 2 (n = 62) diabetes from Saudi Arabia, aged ≥ 20 years, and who fasted a minimum of 15 days during Ramadan. The authors concluded that Ramadan fasting decreased body mass and BMI in patients with type 2 diabetes. A significant decrease in body mass and BMI, with no significant effect of Ramadan fasting on body fat, visceral fat, and muscle mass was observed in Saudi patients (n = 20) with chronic kidney disease (CKD) (66). In contrast, Oueslati et al. (67) found, in 55 Tunisian type 2 diabetes (mean age=54.5 ± 10.1 yrs) who fasted 29.3 ± 2.3 days during the month of Ramadan, a significant decrease in body mass, BMI, waist circumference, fat body mass. The body mass loss was significantly correlated with the number of fasting days (r = 0.348, p = 0.009).
Badran et al. (68) found, in 98 patients with non-alcoholic fatty liver disease, that Ramadan fasting decreased significantly body mass and BMI, while waist circumference, hip circumference, waist/hip ratio, and triceps skin fold thickness remained unchanged.
It should be acknowledged that there are cases where body mass and body mass index may increase during the month of Ramadan, likely as a result of excess intake of fat, sugar, and energy, along with increased sedentariness (69).
Hydration status
Optimal hydration status is beneficial for physical and cognitive functioning, as well as health (70). Fluid restriction during daylight hours of the month of Ramadan may influence the hydration status of fasters. Indeed, previous reports have indicated that total water intake was less during Ramadan compared to before Ramadan (71–73), possibly leading to altered kidney function. Fortunately, fluid balance can be maintained by maximizing urinary concentration and decreasing obligatory urine output (74, 75). However, Leiper et al. (76) reported a prolonged state of hypohydration induces a degree of stress on kidney function, which may negatively affect the efficiency of the water-conserving mechanism. Hydration status can be assessed using urine (e.g., urine osmolality, urine-specific gravity) or blood (e.g., plasma/serum osmolality) markers, among others, such as the estimation of the total body water (64).
Ramadan et al. (77) evaluated the effect of Ramadan fasting on hydration status, assessed using plasma osmolarity, in 13 healthy Kuwaiti adults who were either sedentary (n = 7) or physically active (n = 6). The authors found plasma osmolarity increased significantly in sedentary individuals, suggesting a state of hypohydration (77). However, plasma osmolarity remained unchanged in the physically active group, possibly explained by an adequate water intake after the break of the fast (77).
Ibrahim et al. (73) found, in 18 healthy men (mean age = 22.0 ± 2.4 yrs), a significant increase in urine specific-gravity measures, with mean values higher than 1.020, indicative of a state of hypohydration during Ramadan. Conversely, serum osmolality levels decreased significantly during Ramadan, but remained within normal ranges. It appears the homeostatic mechanisms may have kept serum osmolality at normal levels (73). The lack of change in urine osmolality during Ramadan was also observed in 16 male Sudanese students aged 20–22 years (78). Husain et al. (79) found, in 21 Malaysian healthy individuals (12 males and 9 females), that water intake and urine output volumes during the fasting and non-fasting control periods were approximately the same. Again, the authors concluded fluid balance was maintained throughout the month of Ramadan (79).
Only three studies, with mixed findings, evaluated the effects of Ramadan fasting on the hydration status of patients. While Dikme and Dikme (80) reported the absence of the effect of Ramadan fasting on serum osmolality in 62 Turkish patients admitted to the emergency department, Azwany et al. (81) showed a significant increase in urine osmolality at the end of Ramadan vs. pre-Ramadan. In the latter study (81), urine osmolality remained within normal ranges. In patients (n = 31) with CKD (grades 2–4), Hassan et al. (82) found a reduction in the total body water as estimated using an impedancemeter, suggesting a state of hypohydration.
Collectively, information obtained to date does not suggest hydration status is adversely affected by Ramadan fasting. Moreover, changes in urine/plasma biomarkers are usually relatively small, with values remaining within the normal reference range. It appears fasting during Ramadan can be safely maintained for healthy individuals; however, future studies on patients with underlying chronic conditions are warranted.
Renal function
Optimal kidney function is of paramount importance for wellbeing and health (83). Creatinine clearance and the plasma/serum concentrations of creatinine, urea, and uric acid are all potential measures of renal function (84). Increases in serum urea and creatinine have been observed in sedentary men during Ramadan (77). However, possibly because of adaptations of renal function, the serum uric acid concentration is lower in physically active than in sedentary men (77). Other studies on healthy individuals have reported slight modifications in the levels of renal function markers, albeit with no clinical significance (85–87). To the best of our knowledge, no previous study has reported impairment of renal function in healthy individuals as a consequence of Ramadan fasting. Nevertheless, the impact of Ramadan fasting on renal function in patients with CKD is a subject of debate.
The first published systematic review evaluating the effects of Ramadan on renal function in patients with CKD was conducted by Bragazzi (38). After identifying and summarizing the results of 26 studies published between the years 1989 and 2013, the author concluded that recipients of kidney allograft can safely fast during the month of Ramadan (38). However, evidence regarding safety in patients with nephrolithiasis and CKD is equivocal. In addition, the authors pointed out the scarcity of information about Ramadan fasting falling in hot seasons (38). Finally, given the lack of evidence-based guidelines and protocols, which correctly address the issue of the impact of Ramadan fasting on CKD patients, the authors recommended the publication of randomized clinical trials.
In his systematic review and meta-analysis, Bragazzi (39) concluded patients with CKD can safely fast during Ramadan because glomerular filtration rates (GFR) do not significantly change. Additionally, sensitivity analyses revealed no impact of seasonality (39).
Other research articles have been published in an effort to better understand and evaluate the effects of Ramadan fasting in CKD patients. Mbarki et al. (88) evaluated the renal function in 60 CKD patients who fasted for the entire month of Ramadan. The authors found that 11.7% of CKD patients had an increase of serum creatinine by 442.1 μmol/L on the initial serum creatinine level, and a 25% reduction of GFR, indicating the development of acute renal failure (ARF). The study by NasrAllah and Osman (89) showed compared to non-fasting CKD patients (control group), there were significant adverse effects in the fasted CKD patients [estimated GFR (eGFR) = 27.7 mL/min/1.73 m2] and an increase of 60.4% in the serum creatinine after 1 week of Ramadan fasting. In addition, 3 months after the end of Ramadan, plasma creatinine remained high in 23% of the fasting CKD patients, with no significant difference vs. the non-fasting CKD patients' control group (89). The authors concluded the increase in creatinine levels was likely due to CKD progression, opposed to fasting (89). In a prospective study that enrolled 65 CKD (stage 3) patients, an increase of serum creatinine by ≥ 26.5 μmol/L in 33% of patients was reported (90). Chowdhury et al. (91) found in 68 CKD stage 3 diabetic patients who fasted ~19 h, a significant difference in proteinuria and ARF risk compared to 61 same-category patients who did not fast.
A significant improvement in eGFR (from 29.7 mL/min/1.73 m2 to 32.7 mL/min/1.73 m2 after fasting) in diabetic individuals was reported (92). Despite non-optimal hydration status and decreased serum basal B-type natriuretic peptide, Hassan et al. (82) found no significant differences in eGFR between fasting and non-fasting CKD patients (stages 2-4). In Turkish patients (45 fasters and 49 non-fasters CKD, stages 3-5), Kara et al. (93) found no significant changes in eGFR between non-fasters and fasters; however, individuals above the age of 72 appeared to be at a higher risk of renal function impairment than CKD patients under the age of 64. Recently, a retrospective study of 1,199 patients, who were not exempt from Ramadan fasting for 2 years (2016 and 2017), showed fasting significantly reduced the risk of developing ARF, particularly in patients with comorbidities (94). The authors concluded that Ramadan fasting conferred no negative effects on the majority of patients with comorbid disorders (94). Similarly, following Ramadan fasting, no significant impairment in renal function was found in autosomal dominant polycystic disease patients with early CKD (95). Baloglu et al. (96) found that hypertension and fasting days during Ramadan are strong predictors of ARF, where ARF developed in 27/117 patients with stage 2-3 CKD, with an average age of 60 years. The authors recommended adequate hydration and regular check-ups in order to lower the risk of developing ARF during Ramadan (96). According to an Egyptian-based study (97), serum creatinine increased significantly after Ramadan fasting, although eGFR remained unchanged in individuals without CKD. Conversely, serum creatinine in CKD patients was lowered, and eGFR improved significantly during Ramadan, most likely as a result of better blood pressure control in hypertensive CKD patients (97).
In summary, Ramadan fasting appears to have no adverse effect on the renal function of healthy individuals. However, findings in CKD patients are mixed and it is difficult to determine the reasons for discrepancies in the identified studies. The differences between the studied populations in terms of CKD severity, hydration levels during non-fasting hours, fasting days, fasting length, observation period, and other lifestyle factors such as physical activity level may explain the contradictory findings. Fasting during the month of Ramadan tends to be more harmful in patients with increasing degrees of renal impairment; however, this is not universally true in all aforementioned papers. Recently, Malik et al. (98) recommended that stable CKD non-dialysis (up to Stage 3) patients may be able to fast with close monitoring, while hemodialysis and peritoneal dialysis patients are considered very high risk.
Metabolic health
Metabolic health is a broad term covering numerous elements of cellular, cardiovascular, and cardiorespiratory health and wellbeing (99). Anthropometrics, blood pressure, and blood-based indices, such as blood glucose and serum lipids can all be utilized in clinical settings to assess metabolic health (99). One well-established method is to use diagnostic criteria of metabolic syndrome (MetS) (100), a multidimensional disorder that predisposes people to serious health concerns such as atherosclerotic heart disease (101) and type 2 diabetes (102).
Kul et al. (23) conducted the first systematic review and meta-analysis evaluating the effects of Ramadan fasting on blood levels of lipids and fasting glucose, considering gender differences. The authors found that compared to pre-Ramadan levels, fasting during Ramadan significantly decreases LDL-cholesterol and fasting blood glucose levels in both sexes (23). In females, total cholesterol (TC) and triglyceride (TG) levels did not change, while HDL-cholesterol levels increased during vs. before Ramadan. In males, there was a significant decrease in total cholesterol, LDL-cholesterol and TGs.
The previous systematic review and meta-analysis was updated by Mirmiran et al. (26). The authors concluded that Ramadan fasting has no significant effect on circulating triglycerides, total cholesterol and LDL-cholesterol in apparently healthy individuals. Conversely, Ramadan fasting results in decreased levels of HDL-cholesterol and very low-density lipoprotein cholesterol (VLDL-C), as well as increased LDL-cholesterol levels.
Faris et al. (28) evaluated the effect of Ramadan fasting on glucometabolic markers (i.e., fasting glucose, insulin, insulin resistance, leptin, adiponectin) in healthy non-athletic individuals. The authors concluded that fasting during Ramadan decreases significantly fasting glucose, with a minimal non-significant effect on insulin, insulin resistance, leptin, and adiponectin. The authors concluded Ramadan fasting has no adverse metabolic impacts, and could help improve glucometabolic markers, particularly fasting glucose levels, in healthy individuals.
Faris et al. (27) conducted a meta-analysis evaluating the effect of Ramadan fasting on MetS components among healthy Muslims. Results revealed that Ramadan fasting decreases significantly waist circumference, fasting blood glucose, serum triacylglycerol and systolic blood pressure, while it increases HDL-cholesterol (27).
Recently, Jahrami et al. (29) conducted a meta-analysis to evaluate the effect of Ramadan fasting on cardiometabolic risk factors in healthy adults. The authors found that Ramadan fasting significantly decreases total cholesterol, LDL-cholesterol, VLDL-C, TGs and diastolic blood pressure, while it increases HDL-cholesterol (29). Resting heart rate did not change during compared to before Ramadan (29). The authors concluded Ramadan fasting positively impacts cardiometabolic risk factors, which may provide healthy persons short-term protection against cardiovascular diseases (29). The beneficial effect of Ramadan fasting on systolic and diastolic blood pressure was originally proposed in the meta-analysis by Al-Jafar et al. (36).
The effect of Ramadan fasting on the main hormones regulating appetite and satiety (i.e., leptin, and adiponectin) was investigated in a systematic review and meta-analysis by Gaeini et al. (44). Accordingly, a significant decrease in leptin levels was observed after Ramadan fasting. Ramadan fasting had no significant effect on the levels of adiponectin. In addition, the sub-group analysis demonstrated a greater decrease in leptin levels among normal-weight individuals compared to those of overweight/obese subjects. The authors concluded Ramadan fasting may decrease leptin levels, particularly in normal-weight individuals.
The beneficial effects of Ramadan fasting on glycaemic parameters in patients with type 2 diabetes was also shown by Aydin et al. (24). In their systematic review and meta-analysis, the authors found Ramadan fasting significantly decreases fasting plasma glucose (24). Additionally, the subgroup analysis revealed the decrease in fasting blood glucose was observed in the monotherapy (single oral antidiabetics) group, the oral combination therapy (multi oral antidiabetics) group, and the multi-treatment (oral antidiabetics plus insulin or diet modification) group (24). However, postprandial plasma glucose, glycated hemoglobin, and fructosamine levels remained unchanged during vs. before Ramadan (24). BMI decreased significantly in the oral combination therapy group alone (24). The authors concluded Ramadan fasting has no significant negative effects on postprandial plasma glucose and fructosamine levels. Interestingly, BMI and fasting plasma glucose were positively impacted by Ramadan fasting (24). Other recent empirical studies reported improvement in the metabolic profile in diabetics during Ramadan (103, 104).
In summary, Ramadan fasting improves the metabolic health of healthy individuals and type 2 diabetes patients. However, according to an epidemiological study of 13 countries with large Muslim populations in northern Africa, Asia, and the Middle East, hypoglycaemic episodes increased in people with diabetes (types 1 and 2) during Ramadan (105). Fortunately, the results of the systematic review and meta-analysis of Tahapary et al. (25) showed patients with diabetes who fasted during Ramadan had a low incidence of hypoglycemia and no studies reported fatal hypoglycemic events. Supporting this, Almulhem et al. (35) concluded there is insufficient evidence to link Ramadan fasting to an increased or decreased risk of cardiovascular events in diabetics. It is worth noting that Ramadan is challenging when it falls close to the summer (when daylight hours are longer) (106). Thus, contemporary recommendations for patients with diabetes seeking to participate in fasting during Ramadan should be followed (106, 107). Recent guidelines, authored by the International Diabetes Federation and the Diabetes and Ramadan International Alliance (IDF-DAR), pertaining to the management of patients with diabetes during the month of Ramadan have been developed (107). In brief, a health assessment, pre-Ramadan, should be conducted, taking place around 6–8 weeks prior to commencing Ramadan. This approach would permit health care professionals to obtain a detailed medical history, in addition to performing a risk assessment, thus facilitating the classification of diabetic patients into a low-risk (i.e., fasting is probably safe), moderate-risk (i.e., fasting safety is uncertain), or high-risk (i.e., fasting is probably unsafe) group (107). This classification system will underpin all subsequent recommendations, which will include guidance related to the safety of fasting, strategies/approaches for modifications or adjustments in dosage or treatment regimen, Ramadan-focused education, and dietary guidance (107). Following the above steps, individuals who elect to fast must adhere to diabetes management guidelines during Ramadan fasting, inclusive of changes to glycemic monitoring schedules and adjustments in medication dosing. Finally, a follow-up post-Ramadan is advised. This will assist healthcare professionals in identifying and understanding critical information about the individual's Ramadan successes and challenges, ensuring that subsequent participation in Ramadan fasting is more successful (107). Moreover, this process must be replicated each Ramadan because fasting safely at one-time point does not guarantee the same level of success the following year (107).
It should be acknowledged that utilizing analogs of basal insulin during Ramadan is recommended due to the relatively lower risk of hypoglycemia, as compared to regular human insulin (108, 109). For instance, for once-daily basal insulin analogs, the IDF-DAR guidelines advocate a reduced (15–30%) dose, administered at the break of fast (i.e., iftar) (107). Further, recent empirical evidence has highlighted the safety and effectiveness of insulin glargine 300 U/mL, a second-generation basal insulin analog, in patients with type 2 diabetes who fasted during Ramadan (110, 111). Further specific recommendations for type 1 and type 2 diabetics who observe Ramadan are also provided in the recent guidelines (107).
Structured diabetes education is important for diabetic patients who will observe Ramadan (112, 113). Previous studies concluded diabetic patients who benefited from a structured diabetes education (i) improved their blood glucose levels/glycemic control (112, 113), (ii) reduced the incidence of hypoglycemic events (112, 113) and hyperglycemic crises (113), (iii) decreased their body mass (112), and (iv) increased their acceptance and frequency of glycemia measurements (113). Interestingly, Bravis et al. (112) reported glycated hemoglobin reduction was sustained 12 months after attending the structured diabetes education.
The Diabetes and Ramadan International Alliance recommended practicing regular light-to-moderate exercise during Ramadan for patients with diabetes (114). In that regard, Attarawih prayer, which includes movements such as kneeling, bowing and rising, should be included in the daily exercise routine of diabetic patients (114).
Public health authorities should prioritize structured diabetes education for patients with diabetes who will fast during the month of Ramadan.
Liver function
The liver is a vital organ with multiple functions ranging from detoxification of drugs and toxic chemicals, regulation of red blood cells, and maintaining energy metabolism and bile acid homeostasis (115). The liver function tests typically include alanine transaminase (ALT) and aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), serum bilirubin, prothrombin time (PT), the international normalized ratio (INR) and albumin (116).
Faris et al. (37) conducted a systematic review and meta-analysis to evaluate the effects of Ramadan fasting on liver function tests in healthy people, and to examine the impact of different covariates using subgroup analysis and meta-regression. Faris et al. (37) found that Ramadan fasting induced a significant positive effect on markers of liver damage including AST, ALP, and bilirubin. The authors concluded Ramadan fasting could confer short-term protection against liver steatosis in healthy individuals (37), possibly attributed to the improvement in the metabolic profile (e.g., increased HDL-cholesterol and decreased TC and LDL-cholesterol) and the decrease of body mass, waist circumference and body fat percentage (37). However, the small number of included studies, particularly those assessing GGT, warrant further research.
The effects of Ramadan fasting on salivary liver function tests were investigated by Besbes et al. (30) who concluded salivary ALP increased, whilst AMP decreased significantly during vs. before Ramadan.
Recently, the positive effect of Ramadan fasting on liver function in 70 healthy Iranian individuals was reported (117). Similar to healthy individuals, Ramadan fasting improved non-invasive measures for non-alcoholic steatohepatitis severity assessment (118, 119). Furthermore, it appears to have no negative effect on the liver function of obese males (120).
Collectively, Ramadan fasting appears to protect liver function in healthy individuals and improves non-invasive measures in patients with non-alcoholic steatohepatitis. However, more rigorous studies on patients are needed during Ramadan.
Markers of inflammation, immunity and oxidative stress
The imbalance between the generation of reactive oxygen species and the availability of antioxidants or radical scavengers results in oxidative stress (121). Excess reactive oxygen species can either oxidize biomolecules or structurally change proteins and genes, resulting in signaling cascades that can contribute to inflammatory-related diseases (121).
It well-known that patients with chronic diseases are exempt from fasting during Ramadan. However, while some of these patients are eager to celebrate this time of year with their peers, there are no guidelines to assist physicians in dealing with the concerns of those with infectious diseases who choose to fast during Ramadan. In their systematic review, Bragazzi et al. (31) concluded that (1) patients with diabetes who are at risk of developing infectious complications should not fast, (2) Ramadan fasting has little effect on diarrheal patients (31), (3) HIV is a challenge, and patients should be advised to take ad hoc drug combinations, and to not eat fatty meals that could interfere with the treatment (31), (4) Ramadan has no effect on anti-helminthic therapy effectiveness (31), and (5) patients with active ulcers should not fast, because they are more likely to develop complications (31).
The second systematic review on the topic was conducted by Adawi et al. (32). The authors synthesized 45 studies and found that: (i) Ramadan fasting can mildly influence the immune system, with generally transient alterations, returning to baseline pre-Ramadan values immediately afterward; (ii) Ramadan fasting during the second trimester of pregnancy is safe, not resulting in negative fetal outcomes or maternal oxidative status alterations; (iii) Ramadan fasting can enhance lipid profile and mitigate against oxidative stress in cardiovascular patients; (iv) Ramadan fasting is safe among asthmatic patients as well as in patients with HIV/AIDS and autoimmune disorders, and (v) Ramadan fasting can lead to increased immunological markers in psychiatric patients.
Faris et al. (33) performed a systematic review and meta-analysis to examine changes in inflammatory and oxidative stress markers in healthy individuals before and after Ramadan. The authors found that fasting during Ramadan resulted in very small reductions in interleukin (IL)-1, C-reactive protein (CRP)/high sensitivity-CRP, and malondialdehyde (MDA), and small reductions in tumor necrosis factor-α and IL-6. Faris et al. (33) concluded that fasting during Ramadan protects healthy people against elevated inflammatory and oxidative stress markers. This could be attributed to the reduction caloric intake, and the associated decrease in body mass (predominately body fat).
In their systematic review, Besbes et al. (30) concluded that salivary immunoglobulin-A, playing an important role in mucosal immunity, decreased during the last week of Ramadan. It is worth noting that because there is no salivary IgA concentration threshold (122), a decrease in those levels does not necessarily imply that the participant is more susceptible to oral infection onset.
In obese and overweight subjects (n = 114), Makdour et al. (123) showed that Ramadan fasting ameliorates the genetic expression of antioxidant and anti-inflammatory and metabolic regulatory genes, which may confer protection against oxidative stress and its metabolic-related disorders in non-diabetic obese individuals. Compared to non-fasting obese males (n = 14), improvement in systemic inflammation biomarkers in obese males fasting during Ramadan (n = 14) has been reported (120).
The gut microbiome
The human gastrointestinal microbiome, which contains millions of organisms (e.g., bacteria, viruses, fungi, parasites), can be influenced by various environmental factors (e.g., diet). Conversely, various studies have shown that adverse changes in the intestinal microbiome can be associated with the development of various chronic diseases such as metabolic diseases (124), liver diseases (125, 126), neurodegenerative disorders (127), cancer (128), and others. Some findings have revealed that fasting diets can also cause changes in the microbiome (129, 130). In a recent systematic review, Mousavi et al. (43) reported that Ramadan fasting improves health parameters through positive effects on some bacterial strains such as Akkermansia muciniphila and Bacteroide.
The positive effects of Ramadan fasting on gut microbiota were recently demonstrated by Chen et al. (131). The authors investigated the influence of fasting during Ramadan on the gut metabolic profiles in fecal samples obtained from healthy Chinese (n = 16) and Pakistani (n = 18) subjects, using liquid chromatography-mass spectrometry-based metabolomics analysis (131). Chen et al. (131) concluded fasting leads to changes in metabolite profiles specific to each ethnic group in a manner dependent on dietary components. Additionally, these changes are correlated with dynamic shifts in microbiota composition and diversity which, in conjunction with dietary changes during Ramadan fasting, lead to the enrichment or depletion of various functional metabolites (131).
Another study showed that Ramadan fasting resulted in significant beta diversity and enrichment in the Bacteroidetes phylum (132). The increase in the Bacteroidetes phylum after Ramadan fasting is important since a decrease in the Bacteroidetes/Firmicutes ratio has been found to play a key role in the development of obesity (133).
Su et al. (130) concluded that Ramadan fasting provokes substantial remodeling of the gut microbiome in healthy non-obese individuals and that this remodeling involves the upregulation of Lachnospiraceae species. It should be acknowledged that Lachnospiraceae and Ruminococcaceae are both bacteria that produce butyric acid, which helps to reduce oxidative stress, inflammation, and the risk of colon cancer (134–136).
In summary, Ramadan fasting appears to be beneficial for the gut microbiome of healthy individuals; however, future studies on patients are warranted.
Mental health
Good mental health is defined as a state of wellbeing that allows a person to cope with the normal stresses of life and function productively (137). Prevention of mental disorders (e.g., bipolar disorders) has emerged as an essential component of modern clinical psychiatry (137). Mental health can be assessed using psychometric interviews or specific questionnaires (137). One systematic review and meta-analysis evaluated the effects of Ramadan fasting on mental health in individuals without psychiatric disorders (45). The authors concluded that Ramadan observance was associated with lower stress, anxiety, and depression (45). However, given the small number of included studies (n = 5), the findings of Berthelot et al. (45) should be interpreted with caution. Additionally, the authors reported that no previous studies were carried out in psychiatric patients and recommended further studies should include these populations (45).
Current evidence suggests Ramadan fasting may not be considered a non-pharmacological therapy enhancing the mental health of healthy individuals, but promising results exist.
Sleep
Sleep plays a vital role in maintaining good overall health (138). Prolonged sleep loss is a risk factor for the development of non-communicable diseases (NCDs) such as diabetes, obesity, hypertension, heart disease and stroke and may contribute, in the long term, to premature death (139).
To date, the systematic review and meta-analysis of Faris et al. (46) is the only to estimate the effect size of Ramadan fasting on sleep duration and daytime sleepiness. The results revealed sleep duration decreased from 7.2 h per night before Ramadan to 6.4 h during Ramadan, while the Epworth sleepiness scale score, indicative of daytime sleepiness, increased slightly from 6.1 before Ramadan to 7.0 during Ramadan. Therefore, Faris et al. (46) concluded Ramadan fasting decreases sleep duration and increases daytime sleepiness levels. The increased night-time social activities (e.g., meetings in the coffee, prayer such as Attarawih, Quran reading group) and the large amount of food consumed after the break of fast, possibly delaying sleep, may explain these findings.
It should be acknowledged that almost all studies assessed sleep parameters using subjective tools. Therefore, the previous results should be interpreted with caution.
Recently, Mengi Çelik et al. (59) assessed sleep quality before and during the month of Ramadan, using the Pittsburgh Sleep Quality Index Questionnaire, in 32 (12 males 20 females) healthy Turkish adults. The authors found no significant effect of Ramadan fasting on sleep quality in healthy individuals. A similar result was observed in female healthy students from Bahrain (62). Alzhrani et al. (140) reported that sleep duration remained unchanged from before to during Ramadan in 115 adults (96 females and 19 males) form Saudi Arabia.
Collectively, Ramadan fasting appears to negatively affect sleep parameters. Future studies, with higher methodological rigor, evaluating strategies (e.g., sleep hygiene education, supplementation) aiming at improving sleep assessed using objective tools (e.g., polysomnography) are warranted.
Pregnancy outcomes
Despite being exempt, many pregnant Muslim women choose to fast during Ramadan. However, a sub-optimal diet during pregnancy could impact birth weight. Glazier et al. (47) investigated whether Ramadan fasting by pregnant women affects perinatal outcomes including perinatal mortality, preterm birth and small for gestational age infants, stillbirth, gestational diabetes, hypertensive disorders of pregnancy, congenital abnormalities, serious neonatal morbidity, birth weight, preterm birth, placental weight, neonatal death and maternal death. The authors concluded Ramadan fasting had no negative effects on birth weight, but there is insufficient evidence on potential effects on other perinatal outcomes (47). As a result, future studies on this topic are needed.
Greek orthodox Christian fasting
The Christian Orthodox Church (COC) recommends that those who follow a long-term organized fasting diet must refrain from meat, dairy products, and eggs for 180–200 days every year while increasing their consumption of grains, fruits, legumes, vegetables, fish, seafood and olive oil. COC fasting consists of three main fasting periods: 40 days before Christmas (from 15 November to 24 December), 48 days before Easter (from Clean Monday to Holy Saturday), 14 days before Assumption (from 1 to 14 August), the fasting period before Holy Apostles (lasting 0-30 days depending on Easter feast), and three other daily feasts (5 January, 29 August, 14 September), in addition to every Wednesday and Friday (141).
Food choices
The COC fasting regime could be described as a periodical vegetarian diet, in which fish are occasionally and seafood always permitted (142), sharing numerous similarities with the typical Mediterranean diet (21, 143, 144). Therefore, it is not surprising previous studies reported increases in the consumption of fruits, vegetables and legumes in fasting individuals according to COC recommendations (142, 143, 145–147).
COC fasting and health-related indices
One systematic review (21) and one scoping review (22) evaluating the effects of COC fasting on health-related indices has been published. Interestingly, almost all included studies in those reviews were conducted in Greece.
Koufakis et al. (21) concluded that COC fasting has a beneficial effect on the lipid profile, with the decrease of TC and LDL-cholesterol levels being a consistent finding across trials (i.e., up to 17.8 and 31.4%, respectively). This was previously attributed to the decrease in the intake of energy and saturated fatty acids (148–150). However, the impact of COC fasting on HDL-cholesterol is controversial (21), with some studies reporting a significant decrease in HDL-cholesterol (151, 152), whilst others reported a lack of change (143, 153). It should be acknowledged that the decrease in HDL-cholesterol was previously observed after vegetarian diet intervention (154).
Conclusions on the influence of COC fasting on body mass, glucose homeostasis, blood pressure, antioxidant factors, iron status and relative hematological parameters cannot be drawn since relevant evidence is scarce and/or yields contradictory findings (21). Koufakis et al. (21) noted that any potential negative effect of COC fasting, primarily due to the reduced intake of vitamin D and B12 and minerals (mostly calcium), warrants additional investigation. Additionally, the authors pointed out that incorporating this dietary pattern on a daily clinical basis as a health-promoting diet should be rigorously appraised and individually assessed, because current evidence is inconclusive, necessitating further investigations (21).
In their recent scoping review, Kokkinopoulou and Kafatos (22) suggested a beneficial effect of COC fasting on the lipid profile, marked by a decrease in total cholesterol and LDL-cholesterol. Additionally, the authors showed COC fasting reduced body mass, BMI and systolic blood pressure. Therefore, The COC fasting diet pattern may be recommended for the prevention of chronic diseases, as well as for persons who desire to adopt a plant-based diet for a healthier and/or more sustainable way of life (22). Although there is evidence in favor of health advantages, the COC fasting dietary recommendations, similar to any other dietary patterns, should always be followed under individualized supervision on effective meal planning (22). Nevertheless, long-term follow-up studies should be conducted to assess whether the favorable impacts of COC fasting on health markers are maintained over time (22).
A recent study by Kokkinopoulou et al. (155) investigated the relationship between COC fasting recommendations and cancer risk, specifically focusing on fiber, vegetables, fruit, and red and processed meat consumption. The authors found the diet of fasters to be healthy and follows the World Cancer Research Fund Cancer Recommendations (156), which is in favor of decreasing the chance of developing colorectal cancer compared to their non-fasting counterparts. Furthermore, eating more vegetables and fruits and eating less overall processed meat could reduce the risk of MetS.
In another study (157), plasma adiponectin, biochemical, and anthropometric data were collected from 55 COC fasters and 42 time-restricted eating (TRE) controls (all women, mean age = 47.8 years) at three different time points: baseline, the end of the dietary intervention (7 weeks), and 5 weeks after participants resumed their usual dietary habits (12 weeks from baseline). In the COC fasting group, adiponectin levels increased significantly at 12 weeks compared to baseline, and body fat mass decreased significantly between baseline and 12 weeks and between 7 and 12 weeks. Throughout the investigation, an inverse relationship between adiponectin and waist circumference values was detected in the same group. The authors concluded that COC fasting has beneficial metabolic effects linked to improved adiponectin levels.
Other recent studies showed the beneficial effect of COC fasting on metabolic health indices (148, 149, 158). For example, it has been shown that COC fasting has superior lipid-lowering effects compared to the TRE pattern in overweight adults (148). In addition, the favorable long-term effects of COC on irisin levels (involved in the adipose tissue browning) in overweight, metabolically healthy, adults have been shown (158).
Interestingly, in the study of Papazoglou et al. (16), participants were 60 Greek Orthodox volunteers (30 with dyslipidemia and 30 without) who followed the COC fasting for 7 weeks, and 15 young (non-dyslipidemic) Muslim individuals who observed Ramadan fasting. The serum blood tests of study participants were measured pre- and post-fasting for biochemical (iron, ferritin, vitamin B12, calcium, LDL-cholesterol, HDL-cholesterol, TC, TG, and fasting glucose) and hematological (hemoglobin, hematocrit) (16). The results showed that COC fasting resulted in significant decreases in fasting glucose, HDL-cholesterol, LDL-cholesterol, and TC in both dyslipidemic and non-dyslipidemic Orthodox individuals. Hemoglobin, hematocrit, iron, and ferritin levels were significantly higher after COC fasting, although vitamin B12 and calcium levels were significantly lower (16). Further, a subanalysis of dyslipidemic and non-dyslipidemic Orthodox individuals found the former had a greater decrease in cholesterol levels (16). While TG, LDL-cholesterol, and TC levels were all higher in Muslim participants after Ramadan fasting, no significant effect was observed for the remaining assessed blood-based health indices (16). The authors concluded that prevention of calcium and vitamin B12 deficiency during COC fasting by supplement consumption is needed.
The sex-specific changes in lipid concentrations during COC fasting, as well as the potential role of vitamin D status in mediating these variations, have been investigated in a 12-week prospective intervention (159). In this study, biochemical data on serum lipids and vitamin D status were collected at baseline, 7 weeks after COC fasting, and 5 weeks after fasters resumed their standard dietary habits (12 weeks from baseline) (159). Based on 25-hydroxyvitamin D [25(OH)D] concentrations, participants (24 premenopausal females, 53%) were divided into two groups: those with concentrations above and below the median values (159). The study's findings revealed sex-specific differences in the pattern of lipid profile changes following COC implementation (159). Additionally, female participants with 25(OH)D concentrations below the median at each time point showed variability in TC and LDL-cholesterol responses; a 15% reduction was observed during the fasting period, followed by an appreciable increase slightly above baseline values 5 weeks after OF cessation (159). A similar pattern, albeit less pronounced, was observed for HDL-cholesterol concentrations, as well (159). For male participants, an inverse non-significant trend of TC, LDL-cholesterol, and HDL-cholesterol increase during the study period was observed; however, this was only seen in individuals with 25 (OH)D concentrations below the median (159).
Athonian COC fasting, practiced by Athonian monks, is a pescetarian variation of the typical COC fasting (160). Athonian monks must abstain from red meat consumption throughout the year, during both fasting and non-fasting periods (160). Karras et al. (160) showed lower BMI, body fat mass, and homeostatic model assessment-insulin resistance in male Athonian monks (n = 57), compared to a general population practicing the typical COC fasting (n = 43). Additionally, the authors found that secondary hyperparathyroidism, resulting from profound hypovitaminosis D, was observed in the Athonian monks' group.
Nonetheless, larger sample size studies and/or long-term follow-ups are warranted before drawing firm conclusions on the effects of COC fasting on human health.
The effect of COC fasting on cognitive function and emotional wellbeing of healthy adults was evaluated by Spanki et al. (161). Two groups of fasting (n = 105) and non-fasting (n = 107) individuals were evaluated regarding their cognitive performance using the Mini Mental Examination Scale and the presence of anxiety and depression using, the Hamilton Anxiety Scale, and the Geriatric Depression Scale, respectively. The authors concluded that COC fasting has a positive effect on cognition and mood in middle-aged and elderly individuals (161).
There is a growing body of evidence highlighting the beneficial effects of COC fasting on the health of Orthodox individuals. Unfortunately, studies evaluating the effects of COC fasting in patients are scarce. Future COC fasting-based studies in diverse ethnic populations, pregnant and breast-feeding women, and patients are also warranted.
Other types of religious fasting
Health-related indices
The Daniel fast
The Biblical-based Daniel fast, practiced by some Christians, prohibits the consumption of animal products, refined carbohydrates, preservatives, food additives, flavorings, sweeteners, alcohol, and caffeine (162). It is typically observed for 21 days, while fasts of 10 and 40 days have been reported (162).
Bloomer et al. (163) evaluated the effect of Daniel fast on human metabolic and cardiovascular health in 43 healthy individuals. The authors found that total energy, protein, fat, saturated lipids, trans-fat and dietary cholesterol intake decreased while carbohydrates, fibers, and vitamin C intake increased. They also found significant reductions in TC, LDL-cholesterol, HDL-cholesterol, systolic blood pressure, and diastolic blood pressure.
The effect of Daniel fast on glucose homeostasis has also been investigated (164). Following the Daniel fast, a significant decrease in glycemia, fasting blood insulin and HOMA-IR was observed (164). Interestingly, the Daniel fast appears to decrease blood oxidative stress (as measured by MDA), increase antioxidant capacity (as measured by TEAC), and increase nitrate/nitrite levels (165). The improved antioxidant status was attributed to a combination of lower calorie and saturated fat intake and an increase in nutrient- and fiber-rich fruit, whole grain and vegetable consumption (165). In addition, the elimination of dietary additives, preservatives, and processing agents, as well as a reduction in protein consumption (methionine included) may explain the beneficial effect of the Daniel fast on the oxidative stress profile (165).
Unfortunately, given the scarcity of Daniel fast-related studies, no firm conclusions can be drawn on its health impacts.
The Jewish Yom Kippur fast
Yom Kippur occurs on the 10th day of the 7th month of the Hebrew calendar, after Rosh Hashanah (the Jewish New Year), and is distinguished by a prohibition on eating and drinking (166). The Jewish Yom Kippur fast differs from other fasting rituals in that it requires full abstinence from food and water for 25 h (167). The fast begins before sunset the evening before Yom Kippur and finishes after midnight the next day (166). It should be acknowledged that on Yom Kippur, ingestion of a shiur (~ a half mouthful of liquid) is allowed at intervals of between 4- and 9-min, and Jewish fasters can consume (if necessary) periodically 30 cc of food (MBE, 2009). This fast is short to generate major metabolic alterations leading to significant health implications (167). Unfortunately, available human studies regarding the effect of this fasting ritual on fasters' health are lacking, precluding a firm conclusion on its effects on health.
The Buddhist fasting
Buddhist fasting pattern, observed all year, is similar to a standard vegetarian diet that excludes meat and dairy products (occasionally milk). In addition, it is forbidden to consume five pungent vegetables (garlic, garlic chives, Welsh onion, leeks, and asant), processed foods, and alcohol (144). Food consumption varies by country and culture (168, 169). Few studies have evaluated the effects of Buddhist fasting on health-related indices. In a cross-sectional study, Ho-Pham et al. (170) evaluated the effects of a lifelong vegetarian diet on bone mineral density and body composition in a group of postmenopausal women. Participants were 105 Mahayana Buddhist nuns (vegans) and 105 omnivorous women (average age = 62, range = 50–85 yrs) (170). The results showed that although vegans consumed less calcium and protein than omnivores, veganism showed no negative effects on bone mineral density or body composition (170). Additionally, no significant adverse effects have been reported (170).
Lee and Krawinkel (168) compared body composition and nutrient intake data of 54 Buddhist vegetarian nuns and 31 omnivore Catholic nuns from South Korea. The authors found (i) nutrient intake of Korean Buddhist vegetarians was comparable to that of omnivores, and (ii) the intake of some nutrients in Buddhist vegetarians was more favorable than in omnivores (168). Additionally, Buddhist vegetarians had higher fat-free mass, body fat, and BMI than omnivores, and body fat was inversely correlated with the duration of vegetarianism for vegetarians (168). Future studies evaluating the effect of Buddhist fasting in high-risk populations are warranted.
Religious fasting as a public health nutrition strategy
NCDs, often known as chronic diseases, are a growing concern for national governments and society throughout the world due to their high mortality and morbidity rates (171). NCDs are caused by a variety of factors, including behavioral, environmental, genetic, and physiological factors (171). They share four major behavioral risk factors: unhealthy diet, tobacco use, excess alcohol consumption, and sedentariness (172). According to current WHO projections, the total number of NCD deaths will rise to 55 million by 2030 (WHO). In 2012, all countries committed to lowering premature mortality from NCDs by 25% by 2025 (the 25 x 25 target) (173). Furthermore, the reduction in NCD premature mortality is included in the Sustainable Development Goals (SDGs), as SDG target 3.4 is to reduce NCD premature mortality one-third by 2030, compared to 2015 levels, and promote mental health and wellbeing (174). Public health practitioners and policymakers are responsible to ensure the health of all individuals by searching for and implementing effective public health strategies enabling the prevention and management of NCDs.
When examining the religious recommendations related to some types of fasting, some observations can be noticed. First, the consumption of alcohol is prohibited during Islamic, Daniel, and Buddhist fasting, and occasionally permitted during COC fasting. It is worth noting that alcohol consumption is a cause of over 200 diseases and injuries, accounting for 5.3% of premature deaths and 5.1% of the global disease and injury burden (174). Individuals strictly adhering to religious fasting will refrain from alcohol consumption, which will contribute to the improvement of their health status.
Second, during Ramadan fasting, smoking is not permitted during daylight. It should be acknowledged that tobacco smoking is the single leading cause of premature death worldwide (175), leading to the death of more than 8 million people each year (174). More than 7 million of these deaths are directly related to tobacco use (174). While adhering to Ramadan fasting will likely reduce smoking during this month (176, 177), the challenge is to maintain this habit following the break of the fast and after Ramadan (178). In this context, behavioral approaches may be effective to achieve this goal. For example, Ismail et al. (179) demonstrated a religious-based smoking cessation behavioral intervention delivered to Muslims from Malaysia during Ramadan resulted in sustained smoking reduction after Ramadan. Unfortunately, to our knowledge, no data exists on the effects of other religious fasting regimens on smoking habits.
Third, the COC fasting regimen could be described as periodic vegetarian advocacy, with several similarities to the typical Mediterranean diet and incorporating almost all of its cardioprotective and health-promoting mechanisms, such as calorie restriction and a lower intake of dietary cholesterol and fatty acids (180). Consequently, observers of COC fasting will consume a healthy diet, in which macronutrients are consumed in appropriate proportions to support energetic and physiologic needs, without excess intake, while also providing adequate micronutrients and hydration to meet the body's physiologic needs (181). Similarly, the Buddhist diet appears healthy, as it is similar to a standard vegetarian diet (144).
Unfortunately, the diet of Muslim observers (consumed after the break of fast) during Ramadan could be characterized as “unhealthy.” In this context, Khaled and Belbraouet (182) reported that Ramadan observers consume more food rich in saturated fat, sugar, and processed carbohydrates, which may mask/reduce the beneficial effects of Ramadan fasting on health. Shifting toward a healthy diet (i.e., multiple plant-based diets) such as the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH diet) will be beneficial for the health of Muslim observers. Current evidence suggests plant-based diets reduce the risk of NCDs such as obesity, cardiovascular diseases, type 2 diabetes, and some cancers (5). Interestingly, these dietary patterns might include animal products (e.g., dairy, moderate quantities of red meat), and have all been shown to be healthier and associated with less environmental impact than the average diet in the United States (12).
Given the lack of restriction on food choices during Ramadan fasting, and the possible lack of food literacy in many Muslim individuals (183), nutritional education for Muslims before the month of Ramadan is warranted. Additionally, stakeholders involved in public health should actively participate in consumer health programs focused on nutrition awareness.
Fourth, previous studies have revealed that level of physical activity is generally reduced during fasting rituals. For example, Farook et al. (184) observed decreases in the objectively assessed habitual physical activity of adults during the month of Ramadan. Similarly, Lessan et al. (185) reported that the total number of steps walked per day were significantly lower during Ramadan. Also, Buddhists are characterized by a sedentary lifestyle; they were only participating in some “religious” daily light activities (e.g., chanting, maintenance of the temple premises, meditation) (186). However, it is well-known that persistent low levels of physical activity are detrimental to individual health and wellbeing (187, 188) and that adhering to regular a physical activity program is effective in reducing the burden of NCDs (189), as well as premature morbidity and mortality (190). Unfortunately, the results of a recent study (191) revealed religiosity did not play a mediating role in physical activity participation. Therefore, the promotion of physical activity during and outside fasting periods is needed. It should be noted that undertaking physical activity in a fasting state improves body composition (i.e., body fat) and some metabolic indices (e.g., HDL-cholesterol) (192, 193).
Finally, the frequency of emergency department admissions appears not to change (194–198) or even decrease (199) during Ramadan, suggesting no adverse effects regarding the safety of fasting during the month of Ramadan. Nevertheless, data related to the effect of other types of religious fasting on the frequency of emergency department admissions are lacking. Given the healthy diet and the possible intake of water characterizing other religious fasting regimens, it is hypothesized a decrease in the frequency of emergency department admissions will be observed.
Taken together, religious fasting regimens appear safe and beneficial for public health. Individuals involved in the public health sector such as physicians and nutritionists should encourage religious believers to adhere to fasting. Public health stakeholders should encourage public mass media to promote elements found in religious-based fasts as a nutritional public health strategy. Additionally, to optimize health gains from fasting, sessions of nutritional education (e.g., workshops, and seminars) should be organized during and outside the fasting rituals.
Religious fasting and planetary health
The prestigious medical journal, The Lancet, published at the beginning of 2014 a manifesto for planetary health, which was signed by 7,390 scientists from all over the world, primarily from the fields of medicine, public health, health care, environmental science, and ecology (200). The Lancet declared planetary health a new research area in its own right in 2017, requiring multidisciplinary, interdisciplinary, and transdisciplinary efforts to address unprecedented challenges. This relatively new integrating concept focuses on preserving “the health of human civilization and the state of the natural systems on which it depends” (201) and is gaining support from reputable funding and charitable organizations (e.g., the Rockefeller Foundation). Good human health cannot be preserved when the ecological systems that sustain life on Earth are in a poor or unsatisfactory condition (202). Planetary health is also considered a social movement that aims to transform current practices of living and conducting business at all levels (i.e., individual, societal, national, regional, global), in response to threats to human wellbeing, the sustainability of human civilization, and the health of the planet we inhabit and share with other species (200). Current evidence supports the assertion that global warming is human-induced (203). Religious fasting may help in tackling some environmental issues. For example, during some religious fasting patterns (i.e., Muslim, Buddhism, Daniel), alcoholic drinks are not permitted, and in some Muslim countries (e.g., Tunisia), and are even regulated by a law to not be on sale during the month of Ramadan. It should be acknowledged that some alcoholic beverages (e.g., Whiskey, Vodka) are classified by the NOVA system (204) as ultra-processed foods (UPFS). UPFs include multiple industrial processing (e.g., extrusion, molding, and pre-frying) and manufacturing stages, usage of a large variety of components/additives which have potential dual detrimental impacts on the environment and health (205), and the usage of extensive synthetic packaging, a major source of environmental waste production, mostly containing compounds with carcinogenic and endocrine disruptor properties, UPFs seems to have the most harmful impact, not only on health, but also on the environment (206). It appears that decreasing the consumption of alcoholic drinks during religious fasting is beneficial for environmental health.
It is well-known that livestock is responsible for a significant portion of GHG emissions (14). Marinova and Raphaely (207) found that replacing meat (i.e., beef) with a plant-based option (i.e., wheat) generates 113 times less GHG emissions per nutrient. In addition, red meat is detrimental to planetary health through its direct impacts on land use and freshwater withdrawals (202). Meat consumption is also a significant contributor to biodiversity loss (208), and phosphorus depletion, which endangers future plant-based food production (209). Additionally, based on 800 studies, red meat was classified as carcinogenic to humans (Category 1 for processed meat and Category 2a for cooked meat) by the World Health Organization in 2015 (WHO, 2015). Clearly, red meat consumption is an issue that requires immediate attention in this social movement for restoring and preserving planetary health.
Red meat consumption is not permitted in some religious fasting, including COC fasting, Daniel fast, and Buddhist fasting, which may contribute to the planetary health preservation. However, observers of Ramadan are permitted consume red meat. It is, therefore, necessary to find a strategy that will enable the reduction of its consumption by the Muslim community. In this context, UNEP (210) reported religion continues to play a significant role in many societies including education, health, and politics, and could help to address environmental challenges. Indeed, elements from religion can influence public opinion on sustainability by changing adherents' daily practices, and providing ethical orientation and visions for addressing climate, environmental, and ecological crises. For example, Barclay (211) reported a Muslim fishing community from Tanzania implemented sustainable fishing methods resultant of scientific information provided by Islamic leaders on the subject. Fortunately, “Muslim eco-theologians believe that they have a personal and spiritual responsibility to prevent the spread of environmental degradation because Islam encompasses not only humanity but also nature” (212). In addition, the duty to care for and protect nature is rooted in the Roman Catholic Church's Encyclical “Laudato Si” (213), and Pope Francis has publicly spoken of ecological sins when referring to the climate crisis (214). Likewise, the Interfaith Rainforest Initiative, which fights rainforest deforestation and protects indigenous peoples, uses religious texts from member religions (e.g., Buddhism, Catholicism, Hinduism, Islam, Judaism, Protestant) to advocate for protection (215).
In this context, Weder et al. (216) reported that communication based on religious texts is an effective tool for raising public awareness and shaping public opinion on sustainable development and the environment. Making the public aware of the environmental crisis during prayers is recommended. However, this can be achieved only when religious leaderships (e.g., Imam in the Islamic religion, Pope in the Christian religion) are appropriately educated.
Religious leaders should orient their dialogue toward the obligation to foster efforts of religious believers to decrease pollution, which is responsible for approximately 9 million deaths per year (217). For example, encouraging religious believers to limit (when possible) the use of fuel-powered means of transport will contribute to the decrease of ambient-air pollution, as well as tackling sedentariness. It is worth noting that, in the Muslim religion, avoiding means of transport to join a mosque is encouraged.
Religious leaders should pay attention to food security, which exists only when all individuals have access to physically, socially, and economically sufficient, nutritious, and safe food that meets their dietary needs (218). In this context, Islam does not encourage overeating during and outside the month of Ramadan. Therefore, religious leaders should focus their discussions on avoiding overeating, which could help to ensure food security.
Collectively, religious fasting can help tackle current environmental issues due to the restriction/decrease of the intake of some foods harmful to planetary health. Religious-based communication/dialogue must assert landscape values of respect, love, and care for current and future generations, the planet, and other species. Religiosity and, more broadly speaking, spirituality, are topics, generally, narrowly articulated and framed and have rarely been investigated at the intersection of other topics, such as health, from the individual, public, global, and planetary perspectives. Rethinking religiosity/spirituality means dissecting its profound impacts on human life and daily activities, as well as it means reflecting on public, global, and planetary health from “unusual” points of view. Both religion and health should be conceived and understood, not as abstract entities, but rather as interconnected parts of everyday life, like all those “activities that make us human and keep us in motion: worship, travel, work, migration, the quest for health, and encounters with disease and death” (219). Integrating and incorporating elements of religiosity/spirituality and spiritual, religious, and faith-based practices in public, global, and planetary policies, fully respecting cultural traditions, cultivating and fostering religious and interfaith dialogue, and embracing diversity and pluralism as well as shifting toward more sustainable lifestyles, including dietary intake, may contribute to a more just and equitable society. In conclusion, fasting represents a fundamental “religious health asset,” that is to say, “an asset located in or held by a religious entity that can be leveraged for the purposes of development of public health” (220). Religion-based fasting can be, indeed, mobilized to improve individual health, as well as the health of communities, populations, and the planet.
Conclusions
Religious fasting is universally widespread—from Buddhism, Jainism, and Hinduism to Christianity, Islam, Judaism, and Taoism—and not the prerogative of a single religious belief. Individual/clinical, public, global, and planetary health have usually been investigated separately and siloed from each other. Religious fasting, alongside other religious health assets, can provide several opportunities across multiple levels of scale, ranging from the individual to the community, population, environmental, and planetary levels, by facilitating and supporting societal transformations and changes, including the adoption of healthier, more equitable, and sustainable lifestyles, preserving the Earth's systems, and addressing major interrelated, cascading and compound challenges, with tremendous impacts on the “whole health”—from inequities and inequalities to NCDs and the climate crisis (221).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KT, SG, and NB conceived and designed the manuscript. AA, MB, LP, ES, OB, SK, CC, JG, OA, HJ, and HC critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1036496/full#supplementary-material
References
1. Christ A, Latz E. The western lifestyle has lasting effects on metaflammation. Nat Rev Immunol. (2019) 19:267–8. doi: 10.1038/s41577-019-0156-1
2. Carrera-Bastos P, Fontes-Villalba M, O'Keefe JH, Lindeberg S, Cordain L. The western diet and lifestyle and diseases of civilization. Res Rep Clin Cardiol. (2011) 2:15–35. doi: 10.2147/RRCC.S16919
3. Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A Potential decline in life expectancy in the united states in the 21st century. New Engl J Med. (2005) 352:1138–45. doi: 10.1056/NEJMsr043743
5. Hemler EC, Hu FB. Plant-based diets for personal, population, planetary health. Adv Nutr. (2019) 10:S275–83. doi: 10.1093/advances/nmy117
6. Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metabol Syndr Obesity. (2019) 12:2221–36. doi: 10.2147/DMSO.S216791
7. Fresán U, Sabaté J. Vegetarian diets: planetary health and its alignment with human health. Adv Nutr. (2019) 10:S380–8. doi: 10.1093/advances/nmz019
8. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: The EAT-lancet commission on healthy diets from sustainable food systems. Lancet. (2019) 393:447–92. doi: 10.1016/S0140-6736(18)31788-4
9. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, et al. Red meat consumption and risk of Type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. (2011) 94:1088–96. doi: 10.3945/ajcn.111.018978
10. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from two prospective cohort studies. Arch Intern Med. (2012) 172:555–63. doi: 10.1001/archinternmed.2011.2287
11. Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of Type 2 diabetes mellitus: Three cohorts of US men and women. JAMA Intern Med. (2013) 173:1328–35. doi: 10.1001/jamainternmed.2013.6633
12. Nelson ME, Hamm MW, Hu FB, Abrams SA, Griffin TS. Alignment of healthy dietary patterns and environmental sustainability: a systematic review. Adv Nutr. (2016) 7:1005–25. doi: 10.3945/an.116.012567
13. Culinary Institute of America and President and Fellows of Harvard College. Menus of Change 2018 Annual Report. Culinary Institute of America and Harvard TH Chan School of Public Health (2018).
14. Potter JD. Red and processed meat, and human and planetary health. Br Med J. (2017) 357:j2190. doi: 10.1136/bmj.j2190
15. Halawa A. Impact of intermittent dietary restriction on the health-related outcomes of faith-based fasting. J Ethnic Foods. (2020) 7:14. doi: 10.1186/s42779-020-00047-3
16. Papazoglou AS, Moysidis DV, Tsagkaris C, Vouloagkas I, Karagiannidis E, Kartas A, et al. Impact of religious fasting on metabolic and hematological profile in both dyslipidemic and non-dyslipidemic fasters. Eur J Clin Nutr. (2022) 76:891–8. doi: 10.1038/s41430-021-01053-7
17. Hackett C, Stonawski MJ, Potančoková M, Grim BJ, Skirbekk V. The future size of religiously affiliated and unaffiliated populations. Demogr Res. (2015) 32:829–42. doi: 10.4054/DemRes.2015.32.27
18. Stonawski M, Skirbekk V, Hackett C, Potančoková M, Connor P, Grim B. (2015) Global Population Projections by Religion: 2010–2050. Amsterdam: Brill (2015).
19. Skirbekk V, Potančoková M, Hackett C, Stonawski M. Religious affiliation among older age groups worldwide: estimates for 2010 and projections until 2050. J Gerontol. (2018) 73:1439−45. doi: 10.1093/geronb/gbw144
20. Chouraqui JP, Turck D, Briend A, Darmaun D, Bocquet A, Feillet F, et al. Religious dietary rules and their potential nutritional and health consequences. Int J Epidemiol. (2021) 50:12–26. doi: 10.1093/ije/dyaa182
21. Koufakis TSN, Karras AV, Angeloudi E, Zebekakis P, Kotsa K. Effects of orthodox religious fasting on human health: a systematic review. Eur J Nutr. (2017) 56:2439–55. doi: 10.1007/s00394-017-1534-8
22. Kokkinopoulou A, Kafatos A. Impact of christian orthodox church dietary recommendations on metabolic syndrome risk factors: a scoping review. Nutr Res Rev. (2021) 2021:1–15. doi: 10.1017/S0954422421000184
23. Kul S, Savaş E, Öztürk ZA, Karadag G. Does ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health. (2014) 53:929–42. doi: 10.1007/s10943-013-9687-0
24. Aydin N, Kul S, Karadag G, Tabur S, Araz M. Effect of ramadan fasting on glycaemic parameters & body mass index in type II diabetic patients: a meta-analysis. Ind J Med Res. (2019) 150:546–56. doi: 10.4103/ijmr.IJMR_1380_17
25. Tahapary DL, Astrella C, Kristanti M, Harbuwono DS, Soewondo P. The impact of ramadan fasting on metabolic profile among Type 2 diabetes mellitus patients:a meta-analysis. Diabetes Metab Syndr. (2020) 14:1559–70. doi: 10.1016/j.dsx.2020.07.033
26. Mirmiran P, Bahadoran Z, Gaeini Z, Moslehi N, Azizi F. Effects of ramadan intermittent fasting on lipid and lipoprotein parameters: an updated meta-analysis. Nutr Metab Cardiovasc Dis. (2019) 29:906–15. doi: 10.1016/j.numecd.2019.05.056
27. Faris MA, Jahrami HA, Alsibai J, Obaideen AA. Impact of ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic muslim people aged over 15 years: a systematic review and meta-analysis. Br J Nutr. (2020) 123:1–22. doi: 10.1017/S000711451900254X
28. Faris M, Jahrami HA, BaHammam A, Kalaji Z, Madkour M, Hassanein M. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during ramadan on glucometabolic markers in healthy subjects. Diabetes Res Clin Pract. (2020) 165:108226. doi: 10.1016/j.diabres.2020.108226
29. Jahrami HA, Faris IE, Janahi A, Janahi MI, Abdelrahim DN, et al. Does four-week consecutive, dawn-to-sunset intermittent fasting during ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. (2021) 31:2273–301. doi: 10.1016/j.numecd.2021.05.002
30. Besbes A, Khemiss M, Bragazzi N, Ben Saad H. The Impacts of ramadan intermittent fasting on saliva flow-rate and metabolic data: a systematic review. Front Nutr. (2022) 9:873502. doi: 10.3389/fnut.2022.873502
31. Bragazzi NL, Briki W, Khabbache H, Rammouz I, Mnadla S, Demaj T, et al. Ramadan fasting and infectious diseases: a systematic review. J Infect Dev Count. (2015) 9:1186–94. doi: 10.3855/jidc.5815
32. Adawi M, Watad A, Brown S, Aazza K, Aazza H, Zouhir M, et al. Ramadan fasting exerts immunomodulatory effects: insights from a systematic review. Front Immunol. (2017) 8:e01144. doi: 10.3389/fimmu.2017.01144
33. Faris E, Jahrami HA, Obaideen AA, Madkour MI. Impact of diurnal intermittent fasting during Ramadan on inflammatory and oxidative stress markers in healthy people: Systematic review and meta-analysis. J Nutr Intermediary Metab. (2019) 15:18–26. doi: 10.1016/j.jnim.2018.11.005
34. Turin TC, Ahmed S, Shommu NS, Afzal AR, Al Mamun M, Qasqas M, et al. Ramadan fasting is not usually associated with the risk of cardiovascular events: A systematic review and meta-analysis. J Family Community Med. (2016) 23:73–81. doi: 10.4103/2230-8229.181006
35. Almulhem M, Susarla R, Alabdulaali L, Khunti K, Karamat MA, Rasiah T, et al. The effect of ramadan fasting on cardiovascular events and risk factors in patients with Type 2 diabetes: A systematic review. Diabetes Res Clin Pract. (2020) 159:107918. doi: 10.1016/j.diabres.2019.107918
36. Al-Jafar R, Zografou Themeli M, Zaman S, Akbar S, Lhoste V, Khamliche A, et al. Effect of religious fasting in ramadan on blood pressure: results from LORANS(London Ramadan Study) and a meta-analysis. J Am Heart Assoc. (2021) 10:e021560. doi: 10.1161/JAHA.120.021560
37. Faris M, Jahrami HA, Abdelrahim D, Bragazzi N, BaHammam A. The effects of ramadan intermittent fasting on liver function in healthy adults: a systematic review, meta-analysis, and meta-regression. Diabetes Res Clin Pract. (2021) 178:108951. doi: 10.1016/j.diabres.2021.108951
38. Bragazzi NL. Ramadan fasting and chronic kidney disease: a systematic review. J Res Med Sci. (2014) 19:665–76.
39. Bragazzi NL. Ramadan fasting and chronic kidney disease: does estimated glomerular filtration rate change after and before Ramadan? Insights from a mini meta-analysis. Int J Nephrol Renovasc Dis. (2015) 8:53–7. doi: 10.2147/IJNRD.S61718
40. Sadeghirad B, Motaghipisheh S, Kolahdooz F, Zahedi MJ, Haghdoost AA. Islamic fasting and weight loss: a systematic review and meta-analysis. Public Health Nutr. (2014) 17:396–406. doi: 10.1017/S1368980012005046
41. Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients. (2019) 11:E478. doi: 10.3390/nu11020478
42. Jahrami HA, Alsibai J, Clark CCT, Faris MA. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during ramadan on body weight in healthy subjects aged 16 years and above. Eur J Nutr. (2020) 59:2291–316. doi: 10.1007/s00394-020-02216-1
43. Mousavi SN, Rayyani E, Heshmati J, Tavasolian R, Rahimlou M. Effects of ramadan and non-ramadan intermittent fasting on gut microbiome. Front Nutr. (2022) 9:860575. doi: 10.3389/fnut.2022.860575
44. Gaeini Z, Mirmiran P, Bahadoran Z. Effects of ramadan intermittent fasting on leptin and adiponectin: a systematic review and meta-analysis. Hormones. (2021) 20:237–46. doi: 10.1007/s42000-021-00285-3
45. Berthelot E, Etchecopar-Etchart D, Thellier D, Lancon C, Boyer L, Fond G. Fasting interventions for stress, anxiety and depressive symptoms: a systematic review and meta-analysis. Nutrients. (2021) 13:3947. doi: 10.3390/nu13113947
46. Faris M, Jahrami HA, Alhayki FA, Alkhawaja NA, Ali AM, Aljeeb SH, et al. Effect of diurnal fasting on sleep during ramadan:a systematic review and meta-analysis. Sleep Breathing. (2020) 24:771–82. doi: 10.1007/s11325-019-01986-1
47. Glazier JD, Hayes D, Hussain S, D'Souza SW, Whitcombe J, Heazell A, et al. The effect of Ramadan fasting during pregnancy on perinatal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2018) 18:421. doi: 10.1186/s12884-018-2048-y
48. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. (2017) 37:371–93. doi: 10.1146/annurev-nutr-071816-064634
49. Mosaferchi S, Sharif-Paghaleh E, Mortezapour A, Heidarimoghadam R. Letter to the editor:the first ramadan during COVID-19 pandemic:1.8 billion muslims should fast or not? Metabolism. (2020) 108:154253. doi: 10.1016/j.metabol.2020.154253
50. Ahmed I, Maughan RJ, Iqbal Z, Ali K, Naji O, Awan S, et al. Competing in the ramadan fasted state: for spirituality, health and performance. Br J Sports Med. (2022) 2022:105230. doi: 10.1136/bjsports-2021-105230
51. Ibrahim WH, Habib HM, Jarrar AH, Al Baz AS. Effect of ramadan fasting on markers of oxidative stress and serum biochemical markers of cellular damage in healthy subjects. Ann Nutr Metab. (2008) 53:175–81. doi: 10.1159/000172979
52. Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A. Sleep during ramadan intermittent fasting. J Sleep Res. (2001) 10:319–27. doi: 10.1046/j.1365-2869.2001.00269.x
53. Attinà A, Leggeri C, Paroni R, Pivari F, Dei Cas M, Mingione A, et al. Fasting: How to guide. Nutrients. (2021) 13:1570. doi: 10.3390/nu13051570
54. Pirsaheb S, Pasdar Y, Navabi SJ, Rezaei M, Darbandi M, Niazi P. Fasting consequences during ramadan on lipid profile and dietary patterns. J Fast Health. (2013) 1:6–12. doi: 10.22038/JFH.2013.300
55. Ali Z, Abizari AR. Ramadan fasting alters food patterns, dietary diversity and body weight among ghanaian adolescents. Nutr J. (2018) 17:75. doi: 10.1186/s12937-018-0386-2
56. Shatila H, Baroudi M, El Sayed Ahmad R, Chehab R, Forman MR, Abbas N, et al. Impact of ramadan fasting on dietary intakes among healthy adults: a year-round comparative study. Front Nutr. (2021) 8:689788. doi: 10.3389/fnut.2021.689788
57. Nachvak SM, Pasdar Y, Pirsaheb S, Darbandi M, Niazi P, Mostafai R, et al. Effects of ramadan on food intake, glucose homeostasis, lipid profiles and body composition composition. Eur J Clin Nutr. (2019) 73:594–600. doi: 10.1038/s41430-018-0189-8
58. Agagündüz D, Acar-Tek N, Bozkurt O. Effect of intermittent fasting(18/6) on energy expenditure, nutritional status, and body composition in healthy adults. Evidence-Based Complement Alternative Med. (2021) 2021:7809611. doi: 10.1155/2021/7809611
59. Mengi Çelik Ö, Koçak T, Köksal E. Effects of diurnal ramadan intermittent fasting on cardiometabolic risk factors and sleep quality in healthy turkish adults. Ecol Food Nutr. (2022) 2022:1–13. doi: 10.1080/03670244.2022.2089878
60. Adelina R, Paramastri R, Chao JCJ. Comparisons of anthropometric measures, dietary intakes, and lifestyle factors of young adult indonesian muslims during ramadan fasting and in regular days. Int J Med Sci Clin Res Stud. (2022) 2:73–81. doi: 10.47191/ijmscrs/v2-i1-11
61. Hasan H, Madkour M, Awadallah S, Hassanein M, Jahrami H, Faris M. Ramadan intermittent fasting is associated with changes in circulating proprotein convertase subtilisin/kexin Type 9(PCSK9) in metabolically healthy obese subjects. Medicina. (2022) 58:503. doi: 10.3390/medicina58040503
62. Alsowaid L, Perna S, Peroni G, Gasparri C, Alalwan TA, Rondanelli M. Multidimensional evaluation of the effects of ramadan intermittent fasting on the health of female students at the university of bahrain. Arab J Basic Appl Sci. (2021) 28:360–9. doi: 10.1080/25765299.2021.1975403
63. Trabelsi K, Ammar A, Boukhris O, Glenn JM, Clark CCT, Stannard SR, et al. Dietary intake and body composition during ramadan in athletes: a systematic review and meta-analysis with meta-regression. J Am Nutr Assoc. (2022) 2022:1–22. doi: 10.1080/07315724.2021.2000902
64. Trabelsi K, Stannard SR, Chtourou H, Moalla W, Ghozzi H, Jamoussi K, et al. Monitoring athletes' hydration status and sleep patterns during ramadan observance: methodological and practical considerations. Biol Rhythm Res. (2018) 49:337–65. doi: 10.1080/09291016.2017.1368214
65. Al-Maiman RS, Al-Orf SM, Bawazeer NM. Impact of fasting during ramadan on daily habits, diet and body weight of individuals with diabetes: a sample of Saudi Arabia. Malaysian J Med Health Sci. (2022) 18:141–9. doi: 10.47836/mjmhs18.4.20
66. Yousef EA, Atwa MA, Mahmoud MA. Effect of Ramadan fasting on chronic inflammation and body composition in patients with chronic kidney disease. Saudi J Kidney Dis Transplant. (2021) 32:1013–8. doi: 10.4103/1319-2442.338274
67. Oueslati I, Kardi A, Boukhayatia F, Hammami B, Cheikh M, Romdhane NB, et al. Impact of ramadan intermittent fasting on metabolic and inflammatory profiles in Type 2 diabetic patients. J Diabetes Metab Disord. (2022) 21:751–8. doi: 10.1007/s40200-022-01046-8
68. Badran H, Elsabaawy M, Sakr A, Eltahawy M, Elsayed M, Elsabaawy D, et al. Impact of intermittent fasting on laboratory, radiological, and anthropometric parameters in NAFLD patients. Clin Experi Hepatol. (2022) 8:e115056. doi: 10.5114/ceh.2022.115056
69. Das A, Hasmat N, Kumar Ghosh S, Sahu S. Effects of ramadan intermittent fasting and pattern of nutrients intake on BMI and MUAC of a population consisting of Indian Muslims. Biol Rhythm Res. (2021) 52:1260–9. doi: 10.1080/09291016.2019.1700328
70. Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration and health. Nutr Rev. (2010) 68:439–58. doi: 10.1111/j.1753-4887.2010.00304.x
71. Bouhlel E, Salhi Z, Bouhlel H, Mdella S, Amamou A, Zaouali M, et al. Effect of Ramadan fasting on fuel oxidation during exercise in trained male rugby players. Diabetes Metab. (2006) 32:617–24. doi: 10.1016/S1262-3636(07)70317-8
72. Trabelsi K, Shephard RJ, Boukhris O, Ammar A, El-Abed K, Khanfir S, et al. Effects of Ramadan fasting on athletes' hematological indices: a systematic review. Tunis Med. (2019) 97:1104–13.
73. Ibrahim NSI, Hardinsyah H, Setiawan B. Hydration status and liver function of young men before and after ramadan fasting. J Gizi Dan Pangan. (2018) 13:33–8. doi: 10.25182/jgp.2018.13.1.33-38
74. Berbari AE, Daouk NA, Mallat SG, Jurjus AR. Ramadan Fasting in Health and Disease. In Special Issues in Hypertension. Milano: Springer. (2012). doi: 10.1007/978-88-470-2601-8_26
75. Meo SA, Hassan A. Physiological changes during fasting in ramadan. J Pakistan Med Assoc. (2015) 65:S6–14.
76. Leiper JB, Molla AM, Molla AM. Effects on health of fluid restriction during fasting in ramadan. Eur J Clin Nutr. (2003) 57:S30–8. doi: 10.1038/sj.ejcn.1601899
77. Ramadan J, Telahoun G, Al-Zaid NS, Barac-Nieto M. Responses to exercise, fluid, and energy balances during ramadan in sedentary and active males. Nutrition. (1999) 15:735–9. doi: 10.1016/S0899-9007(99)00145-8
78. Mustafa KY, Mahmoud NA, Gumaa KA, Gader AM. The effects of fasting in ramadan. 2. Fluid and electrolyte balance. Br J Nutr. (1978) 40:583–9. doi: 10.1079/BJN19780162
79. Husain R, Duncan MT, Cheah SH, Ch'ng SL. Effects of fasting in ramadan on tropical asiatic moslems. Br J Nutr. (1987) 58:41–8. doi: 10.1079/BJN19870067
80. Dikme O, Dikme O. Ramadan fasting and its influence on serum osmolality in emergency patients. J Emerg Med Crit Care. (2016) 2:1–4. doi: 10.13188/2469-4045.1000007
81. Azwany N, Aziz AL, Mohammad W. The impact of ramadan fasting on hydration status of Type 2 diabetics in kubang kerian, kelantan. J Kesihatan Masyarakat. (2001) 1:31–4.
82. Hassan S, Hassan F, Abbas N, Hassan K, Khatib N, Edgim R, et al. Does ramadan fasting affect hydration status and kidney function in CKD patients? Ann Nutr Metab. (2018) 72:241–7. doi: 10.1159/000486799
83. Vanholder R, Annemans L, Bello AK, Bikbov B, Gallego D, Gansevoort RT, et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin Kidney J. (2021) 14:1719–30. doi: 10.1093/ckj/sfab070
85. Salahuddin M, Javed MH. Effects of ramadan fasting on some physiological and biochemical parameters in healthy and hypertensive subjects in Aurangabad district of Maharashtra, India. J Nutr Fasting Health. (2014) 2:7–13.
86. Asegaonkara SB, Kareemb L, Bavikarc J, Pagdhuned A, Aghadee S, Thoratf AP. Effect of ramadan fasting on renal function markers in healthy adults from Aurangabad. Walawalkar Int Med J. (2014) 1:13–7.
87. Urooj A, Pai Kotebagilu N, Shivanna LM, Anandan S, Thantry AN, Siraj SF. Effect of ramadan fasting on body composition, biochemical profile, and antioxidant status in a sample of healthy individuals. Int J Endocrinol Metab. (2020) 18:e107641. doi: 10.5812/ijem.107641
88. Mbarki H, Tazi N, Najdi A, Tachfouti N, Arrayhani M, Sqalli T. Effects of fasting during ramadan on renal function of patients with chronic kidney disease. Saudi J Kidney Dis Transplant. (2015) 26:320–4. doi: 10.4103/1319-2442.152494
89. NasrAllah MM, Osman NA. Fasting during the month of ramadan among patients with chronic kidney disease: renal and cardiovascular outcomes. Clin Kidney J. (2014) 7:348–53. doi: 10.1093/ckj/sfu046
90. Bakhit AA, Kurdi A.-M., Wadera J.-J., Alsuwaida, et al.-O. Effects of ramadan fasting on moderate to severe chronic kidney disease. A prospective observational study. Saudi Med J. (2017) 38:48–52. doi: 10.15537/smj.2017.1.17566
91. Chowdhury A, Khan H, Lasker SS, Chowdhury TA. Fasting outcomes in people with diabetes and chronic kidney disease in East London during Ramadan 2018: The East London Diabetes in Ramadan survey. Diabetes Res Clin Pract. (2019) 152:166–70. doi: 10.1016/j.diabres.2019.05.022
92. Bernieh B, Al Hakim MR, Boobes Y, Abu Zidan FM. Fasting ramadan in chronic kidney disease patients: clinical and biochemical effects. Saudi J Kidney Dis Transplant. (2010) 21:898–902.
93. Kara E, Sahin OZ, Kizilkaya B, Ozturk B, Pusuroglu G, Yildirim S, et al. Fasting in Ramadan is not associated with deterioration of chronic kidney disease: A prospective observational study. Saudi J Kidney Dis Transpl. (2017) 28:68–75. doi: 10.4103/1319-2442.198140
94. AlAbdan NA, Almohammed OA, Altukhaim MS, Farooqui MA, Abdalla MI, Al Otaibi HQ, et al. Fasting during Ramadan and Acute Kidney Injury(AKI): A retrospective, propensity matched cohort study. BMC Nephrol. (2022) 23:54. doi: 10.1186/s12882-022-02674-1
95. Ekinci I, Erkoc R, Gursu M, Dogan EE, Kilic E, Cebeci E, et al. Effects of fasting during the month of ramadan on renal function in patients with autosomal dominant polycystic kidney disease. Clin Nephrol. (2018) 89:103–12. doi: 10.5414/CN109102
96. Baloglu I, Turkmen K, Kocyigit I, Altunoren O, Demirtas L, Zararsiz G, et al. The effect of ramadan fasting on kidney function in patients with chronic kidney disease. Int Urol Nephrol. (2020) 52:1337–43. doi: 10.1007/s11255-020-02506-x
97. Eldeeb AA, Mahmoud MA, Ibrahim AB, Yousef EA, Sabry AA. Effect of ramadan fasting on arterial stiffness parameters among Egyptian hypertensive patients with and without chronic kidney disease. Saudi J Kidney Dis Transplant. (2020) 31:582–8.
98. Malik S, Bhanji A, Abuleiss H, Hamer R, Shah SH, Rashad R, et al. Effects of fasting on patients with chronic kidney disease during ramadan and practical guidance for healthcare professionals. Clin Kidney J. (2021) 14:1524–34. doi: 10.1093/ckj/sfab032
99. Hyvärinen M, Juppi HK, Taskinen S, Karppinen JE, Karvinen S, Tammelin TH, et al. Metabolic health, menopause, and physical activity-a 4-year follow-up study. Int J Obesity. (2022) 46:544–54. doi: 10.1038/s41366-021-01022-x
100. Grundy SM, Hansen B, Smith SC, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the american heart association/national heart, lung, and blood Institute/American diabetes association conference on scientific issues related to management. Circulation. (2004) 109:551–6. doi: 10.1161/01.CIR.0000112379.88385.67
101. Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. (2004) 110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E
102. Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. (2008) 31:1898–904. doi: 10.2337/dc08-0423
103. Farag HA. Impact of Ramadan Fasting on Metabolic Syndrome Criteria: Among Treated Hypertensive and Diabetic Females of Halabja. Kurdistan Journal of Applied Research (2022). p. 125–32. doi: 10.24017/Science.2022.1.11
104. Timurkaan M, Timurkaan ES, Aslan Y, Kalayci M, Ayyildiz H. Does Ramadan fasting affect glicemic control in patients with diabetes mellitus? effects of fasting on betatrophin and insulin resistance. Medicine. (2022) 11:394–8. doi: 10.5455/medscience.2021.09.303
105. Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of ramadan in 13 countries: results of the epidemiology of diabetes and ramadan 1422/2001(EPIDIAR) study. Diabetes Care. (2004) 27:2306–11. doi: 10.2337/diacare.27.10.2306
106. Mahmood A, Dar S, Dabhad A, Aksi B, Chowdhury TA. Advising patients with existing conditions about fasting during ramadan. Br Med J. (2022) 376:e063613. doi: 10.1136/bmj-2020-063613
107. Hassanein M, Afandi B, Ahmedani MY, Alamoudi RM, Alawadi F, Bajaj HS, et al. Diabetes and Ramadan: practical guidelines 2021. Diabetes Res Linical Pract. (2022) 2022:109185. doi: 10.1016/j.diabres.2021.109185
108. Hassanein M, Al-Arouj M, Hamdy O, Bebakar WMW, Jabbar A, Al-Madani A. Diabetes and ramadan: practical guidelines. Diabetes Res Clin Pract. (2017) 126:303–16. doi: 10.1016/j.diabres.2017.03.003
109. Hassanein M, Al Awadi FF, El Hadidy KES, Ali SS, Echtay A, Djaballah K. The characteristics and pattern of care for the type 2 diabetes mellitus population in the MENA region during Ramadan: An international prospective study(DARMENA T2DM). Diabetes Res Clin Pract. (2019) 151:275–84. doi: 10.1016/j.diabres.2019.02.020
110. Hassanein M, Buyukbese MA, Malek R, Pilorget V, Naqvi M, Berthou B, et al. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: the observational ORION study. Diabetes Res Clin Pract. (2020) 166:108189. doi: 10.1016/j.diabres.2020.108189
111. Malik RA, Elhadd T, Alattar A, Al Shaikh A, Al Randi M, Arora R, et al. Safety and effectiveness of insulin glargine 300 u/ml in participants with type 2 diabetes who fast during ramadan in the gulf region: a subgroup analysis of the real-world orion study. Diabetes Therapy. (2022) 13:569–81. doi: 10.1007/s13300-022-01225-z
112. Bravis V, Hui E, Salih S, Mehar S, Hassanein M, Devendra D. Ramadan education and awareness in diabetes(READ) programme for Muslims with Type 2 diabetes who fast during Ramadan. Diabetic Med. (2010) 27:327–31. doi: 10.1111/j.1464-5491.2010.02948.x
113. Nassar M, Ahmed TM, AbdAllah NH, El Hadidy KES, Sheir RES. The impact of structured diabetes education on glycemic control during Ramadan fasting in diabetic patients in Beni Suef, Egypt. Diab Metab Syndr. (2021) 15:102249. doi: 10.1016/j.dsx.2021.102249
114. Hassanein M, Ahmedani MY. Pre-Ramadan education. Int Diabetes Federation Diabetes. (2016) 2016:61–69
115. Cai X, Young GM, Xie W. The xenobiotic receptors PXR and CAR in liver physiology, an update. Biochim Biophys. (2021) 1867:166101. doi: 10.1016/j.bbadis.2021.166101
116. Lala V, Zubair M, Minter DA. Liver function tests. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK482489/
117. Mohammadian M, Feizollah zadeh S, Rasuli J, Rasouli MA, Alizadeh M. The effect of ramadan fast on serum liver enzyme levels in Iranian adults. J Res Appl Basic Med Sci. (2021) 7:104–7. doi: 10.52547/rabms.7.2.104
118. Ebrahimi S, Gargari BP, Aliasghari F, Asjodi F, Izadi A. Ramadan fasting improves liver function and total cholesterol in patients with nonalcoholic fatty liver disease. Int J Vitamin Nutr Res. (2020) 90:95–102. doi: 10.1024/0300-9831/a000442
119. Mari A, Khoury T, Baker M, Said Ahmad H, Abu Baker F, Mahamid M. The impact of ramadan fasting on fatty liver disease severity: a retrospective case control study from Israel. Israel Med Assoc J. (2021) 23:94–8.
120. Zouhal H, Bagheri R, Ashtary-Larky D, Wong A, Triki R, Hackney AC, et al. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol Behav. (2020) 225:113090. doi: 10.1016/j.physbeh.2020.113090
121. Chatterjee S. Oxidative stress, inflammation, and disease: in oxidative stress and biomaterials. Elsevier. (2016) 2016:35–58. doi: 10.1016/B978-0-12-803269-5.00002-4
122. Develioglu ON, Kucur M, Ipek HD, Celebi S, Can G, Kulekci M. Effects of ramadan fasting on serum immunoglobulin G and M. and salivary immunoglobulin A concentrations. J Int Med Res. (2013) 41:463–72. doi: 10.1177/0300060513476424
123. Madkour M, Hassan R, Sherif N, Awadallah S, Serafi A, Jahrami H, et al. Modulation of anti-oxidant, anti-inflammatory and metabolism-controlling genes expressions by ramadan intermittent fasting: a prospective observational study. Curr Dev Nutr. (2020) 4:1265. doi: 10.1093/cdn/nzaa058_023
124. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
125. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. (2019) 11:e9302. doi: 10.15252/emmm.201809302
126. Kolodziejczyk AA, Federici S, Zmora N, Mohapatra G, Dori-Bachash M, Hornstein S, et al. Acute liver failure is regulated by MYC- and microbiome-dependent programs. Nat Med. (2020) 26:1899–911. doi: 10.1038/s41591-020-1102-2
127. Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
128. Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. (2021) 39:1317–41. doi: 10.1016/j.ccell.2021.08.006
129. Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in Db/Db mice. Diabetes. (2018) 67:1867–79. doi: 10.2337/db18-0158
130. Su J, Wang Y, Zhang X, Ma M, Xie Z, Pan Q, et al. Remodeling of the gut microbiome during ramadan-associated intermittent fasting. Am J Clin Nutr. (2021) 113:1332–42. doi: 10.1093/ajcn/nqaa388
131. Chen S, Ali I, Li X, Long D, Zhang Y, Long R, et al. Shifts in fecal metabolite profiles associated with ramadan fasting among Chinese and Pakistani individuals. Front Nutr. (2022) 9:845086. doi: 10.3389/fnut.2022.845086
132. Ozkul C, Yalinay M, Karakan T. Structural changes in gut microbiome after ramadan fasting: a pilot study. Benef Microbes. (2020) 11:227–33. doi: 10.3920/BM2019.0039
133. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
134. Hamer HM, Jonkers MD, Bast A, Vanhoutvin ALW, Fischer AJG, Kodde A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. (2009) 28:88–93. doi: 10.1016/j.clnu.2008.11.002
135. Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. (2009) 682:39–53. doi: 10.1016/j.mrrev.2009.04.001
136. Bui TPN, Ritari J, Boeren S, de Waard P, Plugge CM, de Vos MW. Production of butyrate from lysine and the amadori product fructoselysine by a human gut commensal. Nat Commun. (2015) 6:10062. doi: 10.1038/ncomms10062
137. Fusar-Poli P, Salazar de Pablo G, De Micheli A, Nieman DH, Correll CU, Kessing LV, et al. What is good mental health? A scoping review. Eur Neuropsychopharmacol. (2020) 31:33–46. doi: 10.1016/j.euroneuro.2019.12.105
138. Hale L, Troxel W, Buysse DJ. Sleep health: an opportunity for public health to address health equity. Annu Rev Public Health. (2020) 41:81–99. doi: 10.1146/annurev-publhealth-040119-094412
139. Cappuccio FP, Miller MA. Sleep and cardio-metabolic disease. Curr Cardiol Rep. (2017) 19:110. doi: 10.1007/s11886-017-0916-0
140. Alzhrani A, Alhussain MH, BaHammam AS. Changes in dietary intake, chronotype, and sleep pattern upon ramadan among healthy adults in Jeddah, Saudi Arabia: A prospective study. Front Nutr. (2022) 9:966861. doi: 10.3389/fnut.2022.966861
141. Lazarou C, Matalas AL. A critical review of current evidence, perspectives and research implications of diet-related traditions of the eastern christian orthodox church on dietary intakes and health consequences. Int J Food Sci Nutr. (2010) 61:739–58. doi: 10.3109/09637481003769782
142. Sarri KO, Kafatos AG, Higgins S. Is religious fasting related to iron status in Greek Orthodox Christians? Br J Nutr. (2005) 94:198–203. doi: 10.1079/BJN20051472
143. Papadaki A, Vardavas C, Hatzis C, Kafatos A. Calcium, nutrient and food intake of Greek orthodox christian monks during a fasting and non-fasting week. Public Health Nutr. (2008) 11:1022–9. doi: 10.1017/S1368980007001498
144. Persynaki A, Karras S, Pichard C. Unraveling the metabolic health benefits of fasting related to religious beliefs:a narrative review. Nutrition. (2017) 35:14–20. doi: 10.1016/j.nut.2016.10.005
145. Sarri KO, Linardakis MK, Bervanaki FN, Tzanakis NE, Kafatos AG. Greek Orthodox fasting rituals: a hidden characteristic of the Mediterranean diet of Crete. Br J Nutr. (2004) 92:277–84. doi: 10.1079/BJN20041197
146. Sarri KO, Linardakis M, Codrington C, Kafatos A. Does the periodic vegetarianism of Greek Orthodox Christians benefit blood pressure? Prevent Med. (2007) 44:341–8. doi: 10.1016/j.ypmed.2006.11.009
147. Kokkinopoulou A, McGowan R, Brogan Y, Armstrong J, Pagkalos I, Hassapidou M, et al. Associations between Christian orthodox church fasting and adherence to the world cancer research fund's cancer prevention recommendations. Nutrients. (2022) 14:1383. doi: 10.3390/nu14071383
148. Karras SN, Koufakis T, Adamidou L, Antonopoulou V, Karalazou P, Thisiadou K, et al. Effects of orthodox religious fasting versus combined energy and time restricted eating on body weight, lipid concentrations and glycaemic profile. Int J Food Sci Nutr. (2021) 72:82–92. doi: 10.1080/09637486.2020.1760218
149. Karras SN, Koufakis T, Adamidou L, Polyzos SA, Karalazou P, Thisiadou K, et al. Similar late effects of a 7-week orthodox religious fasting and a time restricted eating pattern on anthropometric and metabolic profiles of overweight adults. Int J Food Sci Nutr. (2021) 72:248–58. doi: 10.1080/09637486.2020.1787959
150. Koufakis T, Karras SN, Zebekakis P, Kotsa K. Orthodox religious fasting as a medical nutrition therapy for dyslipidemia: where do we stand and how far can we go? Eur J Clin Nutr. (2018) 72:474–9. doi: 10.1038/s41430-018-0113-2
151. Basilakis A, Kiprouli K, Mantzouranis S, Konstantinidis T, Dionisopoulou M, Hackl JM. Nutritional study in Greek-Orthodox monasteries–effect of a 40-day religious fasting. Aktuelle Ernährungsmedizin. (2002) 27:250–5. doi: 10.1055/s-2002-33359
152. Sarri KO, Tzanakis NE, Linardakis MK, Mamalakis GD, Kafatos AG. Effects of greek orthodox christian church fasting on serum lipids and obesity. BMC Public Health. (2003) 3:16. doi: 10.1186/1471-2458-3-16
153. Sarri K, Bertsias G, Linardakis M, Tsibinos G, Tzanakis N, Kafatos A. The effect of periodic vegetarianism on serum retinol and alpha-tocopherol levels. Int J Vitamin Nutr Res. (2009) 79:271–80. doi: 10.1024/0300-9831.79.56.271
154. Wang F, Zheng J, Yang B, Jiang J, Fu Y, Li D. Effects of vegetarian diets on blood lipids: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. (2015) 4:e002408. doi: 10.1161/JAHA.115.002408
155. Kokkinopoulou A, Pagkalos I, Hassapidou M, Kafatos A. Dietary patterns in adults following the christian orthodox fasting regime in Greece. Front Nutr. (2022) 9:803913. doi: 10.3389/fnut.2022.803913
156. World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. A Summary of the Third Expert Report; World Cancer Research Fund/American Institute for Cancer Research. Washington, DC. (2018).
157. Karras SN, Koufakis T, Adamidou L, Dimakopoulos G, Karalazou P, Thisiadou K, et al. Implementation of christian orthodox fasting improves plasma adiponectin concentrations compared with time-restricted eating in overweight premenopausal women. Int J Food Sci Nutr. (2022) 73:210–20. doi: 10.1080/09637486.2021.1941803
158. Karras SN, Koufakis T, Adamidou L, Dimakopoulos G, Karalazou P, Thisiadou K, et al. Effects of christian orthodox fasting versus time-restricted eating on plasma irisin concentrations among overweight metabolically healthy individuals. Nutrients. (2021) 13:1071. doi: 10.3390/nu13041071
159. Karras SN, Koufakis T, Dimakopoulos G, Adamidou L, Karalazou P, Thisiadou K, et al. Vitamin D equilibrium affects sex-specific changes in lipid concentrations during christian orthodox fasting. J Steroid Biochem Mol Biol. (2021) 211:105903. doi: 10.1016/j.jsbmb.2021.105903
160. Karras SN, Koufakis T, Petróczi A, Folkerts D, Kypraiou M, Mulrooney H, et al. Christian Orthodox fasting in practice: A Comparative Evaluation between Greek Orthodox General Population Fasters and Athonian Monks. Nutrition. (2019) 59:69–76. doi: 10.1016/j.nut.2018.07.003
161. Spanki C, Rodopaios NE, Koulouri A, Pliakas T, Papadopoulou SK, Vasara E, et al. The christian orthodox church fasting diet is associated with lower levels of depression and anxiety and a better cognitive performance in middle life. Nutrients. (2021) 13:627. doi: 10.3390/nu13020627
162. Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J. (2010) 9:57. doi: 10.1186/1475-2891-9-57
163. Bloomer RJ, Kabir MM, Canale RE, Trepanowski JF, Marshall KE, Farney TM, et al. Effect of a 21 day daniel fast on metabolic and cardiovascular disease risk factors in men and women. Lipids Health Dis. (2010) 9:94. doi: 10.1186/1476-511X-9-94
164. Trepanowski JF, Kabir MM, Alleman RJ, Bloomer RJ. A 21-day daniel fast with or without krill oil supplementation improves anthropometric parameters and the cardiometabolic profile in men and women. Nutr Metab. (2012) 9:82. doi: 10.1186/1743-7075-9-82
165. Bloomer RJ, Kabir MM, Trepanowski JF, Canale RE, Farney TM. A 21 day daniel fast improves selected biomarkers of antioxidant status and oxidative stress in men and women. Nutr Metab. (2011) 8:17. doi: 10.1186/1743-7075-8-17
166. Venegas-Borsellino C, Martindale RG. From religion to secularism: the benefits of fasting. Curr Nutr Rep. (2018). doi: 10.1007/s13668-018-0233-2
168. Lee Y, Krawinkel M. Body composition and nutrient intake of buddhist vegetarians. Asia Pac J Clin Nutr. (2009) 18:265–71.
169. Chen CW, Lin YL, Lin TK, Lin CT, Chen BC, Lin CL. Total cardiovascular risk profile of Taiwanese vegetarians. Eur J Clin Nutr. (2008) 62:138–44. doi: 10.1038/sj.ejcn.1602689
170. Ho-Pham LT, Nguyen PLT, Le TTT, Doan T, Tran NT, Le TA, et al. Veganism, bone mineral density, and body composition: a study in buddhist nuns. Osteoporosis Int. (2009) 20:2087–93. doi: 10.1007/s00198-009-0916-z
171. Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and prevention strategies for non-communicable diseases(NCDs) and their risk factors. Front Public Health. (2020) 8:574111. doi: 10.3389/fpubh.2020.574111
172. World Health Organization. (2020). WHO Global Meeting to Accelerate Progress on SDG Target 3.4 on Noncommunicable Diseases and Mental Health. Muscat: World Health Organization.
173. Tool AB. NCD Countdown 2025: Accountability for the 25 x 25 NCD Mortality Reduction Target. Diabetes (2010).
174. World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000-2025. World Health Organization (2018).
175. GBD. Tobacco Collaborators 2021 Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990-2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet. (2019) 397:2337–2360.
176. Pratt R, Ojo-Fati O, Adam A, Sharif H, Kahin A, Mahamud A, et al. Text message support for smoking cessation during ramadan:a focus group study with somali immigrant muslim men. Nicotine Tobacco Res. (2020) 22:1636–9. doi: 10.1093/ntr/ntz187
177. Ünal, M, Öztürk O, Öztürk G, Fidanci I, Atayoglu AT, et al. Effect of fasting on smoking addiction: a multicentered primary care research. J Addict Nurs. (2021). doi: 10.1097/JAN.0000000000000414
178. Mughal F. Smoking reduction during ramadan. Br J Gene Pract. (2017) 67:254. doi: 10.3399/bjgp17X691061
179. Ismail S, Abdul Rahman H, Abidin EZ, Isha ASN, Abu Bakar S, Zulkifley NA, et al. The effect of faith-based smoking cessation intervention during ramadan among malay smokers. Qatar Med J. (2016) 2:16. doi: 10.5339/qmj.2016.16
180. Koufakis T, Karras SN, Zebekakis P, Kotsa K. Orthodox religious fasting: A vital subset of the Mediterranean diet. Mediterranean Diet. (2020) 2020:73–8. doi: 10.1016/B978-0-12-818649-7.00007-2
181. Cena H, Calder PC. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients. (2020) 12:E334. doi: 10.3390/nu12020334
182. Khaled BM, Belbraouet S. Effect of ramadan fasting on anthropometric parameters and food consumption in 276 type 2 diabetic obese women. Int J Diabetes Dev Ctries. (2009) 29:62–8. doi: 10.4103/0973-3930.53122
183. Benajiba N, Mahrous L, Bernstein J, Aboul-Enein BH. Food labeling use by consumers in arab countries: a scoping review. J Community Health. (2020) 45:661–74. doi: 10.1007/s10900-019-00750-6
184. Farooq A, Chamari K, Sayegh S, El Akoum M, Al-Mohannadi AS. Ramadan daily intermittent fasting reduces objectively assessed habitual physical activity among adults. BMC Public Health. (2021) 21:1912. doi: 10.1186/s12889-021-11961-9
185. Lessan N, Saadane I, Alkaf B, Hambly C, Buckley AJ, Finer N, et al. The effects of ramadan fasting on activity and energy expenditure. Am J Clin Nutr. (2018) 107:54–61. doi: 10.1093/ajcn/nqx016
186. Prasad MRC, Asha GF. Assessment of lifestyle and health status of buddhist monks. Int J Health Sci Res. 10:25–36. Available online at: https://www.ijhsr.org/IJHSR_Vol.10_Issue.12_Dec2020/4.pdf
187. World Health Organization. Global Strategy on Diet, Physical Activity and Health: A Framework to Monitor and Evaluate Implementation. World Health Organization (2006).
188. Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. (2010) 35:725–40. doi: 10.1139/H10-079
189. Ballin M, Nordström P. Does exercise prevent major non-communicable diseases and premature mortality? A critical review based on results from randomized controlled trials. J Intern Med. (2021) 290:1112–29. doi: 10.1111/joim.13353
190. Hall KS, Hyde ET, Bassett DR, Carlson SA, Carnethon MR, Ekelund U, et al. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. Int J Behav Nutr Phys Act. (2020) 17:78. doi: 10.1186/s12966-020-00978-9
191. Noh YE, Lim BH, Danaee M. Motives for physical activity participation: does religious faith play a mediating role? Int J Sport Exerc Psychol. (2022) 2022:1–20. doi: 10.1080/1612197X.2022.2090986
192. Trabelsi K, Abed K, Stannard SR, Jammoussi K, Zeghal KM, Hakim A. Effects of fed- versus fasted-state aerobic training during ramadan on body composition and some metabolic parameters in physically active men. Int J Sport Nutr Exerc Metab. (2012) 22:11–8. doi: 10.1123/ijsnem.22.1.11
193. Trabelsi K, Stannard SR, Ghlissi Z, Maughan RJ, Kallel C, Jamoussi K, et al. Effect of fed- versus fasted state resistance training during ramadan on body composition and selected metabolic parameters in bodybuilders. J Int Soc Sports Nutr. (2013) 10:23. doi: 10.1186/1550-2783-10-23
194. Hammami R, Bahloul A, Charfeddine S, Gargouri R, Ellouze T, Abid L, et al. Cardiovascular Disease and Ramadan. A Literature Review. (2022). doi: 10.1016/J.ANCARD.2021.09.011
195. Pekdemir M, Ersel M, Yilmaz S, Uygun M. No significant alteration in admissions to emergency departments during ramadan. J Emerg Med. (2010) 38:253–6. doi: 10.1016/j.jemermed.2008.03.013
196. Halasa W. Effect of ramadan fasting on emergency walk-in-clinics in Jordan. Br J Gene Pract. (2014) 64:388. doi: 10.3399/bjgp14X680833
197. Cevik Y, Corbacioglu SK, Cikrikci G, Oncul V, Emektar E. The effects of ramadan fasting on the number of renal colic visits to the emergency department. Pakistan J Med Sci. (2016) 32:18–21. doi: 10.12669/pjms.321.8248
198. Al Assaad RG, Bachir R, El Sayed JM. Impact of ramadan on emergency department visits and on medical emergencies. Euro J Emerg Med. (2018) 25:440–4. doi: 10.1097/MEJ.0000000000000485
199. Sagy I, Zeldetz V, Halperin D, Abu Tailakh M, Novack V. The effect of ramadan fast on the incidence of renal colic emergency department visits. QJM. (2017) 110:571–6. doi: 10.1093/qjmed/hcx079
200. Horton R, Beaglehole R, Bonita R, Raeburn J, McKee M, Wall S. From public to planetary health: a manifesto. Lancet. (2014) 383:847. doi: 10.1016/S0140-6736(14)60409-8
201. Whitmee S, Haines A, Beyrer C, Boltz F, Capon AG, de Souza Dias BF, et al. Safeguarding human health in the anthropocene epoch: report of the rockefeller foundation-lancet commission on planetary health. Lancet. (2015) 386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1
202. Marinova D, Bogueva D. Planetary health and reduction in meat consumption. Sustainable Earth. (2019) 2:3. doi: 10.1186/s42055-019-0010-0
203. Cook J, Oreskes N, Doran PT, Anderegg WRL, Verheggen B, Maibach EW, et al. Consensus on consensus: a synthesis of consensus estimates on human-caused global warming. Environ Res Lett. (2016) 11:e048002. doi: 10.1088/1748-9326/11/4/048002
204. Monteiro CA, Cannon G, Levy R, Moubarac JC, Jaime P, et al. NOVA. The Star Shines Bright. World Nutrition. (2016) 7:28–38.
205. Anastasiou K, Baker P, Hadjikakou M, Hendrie GA, Lawrence M. A conceptual framework for understanding the environmental impacts of ultra-processed foods and implications for sustainable food systems. J Clean Prod. (2022) 368:133155. doi: 10.1016/j.jclepro.2022.133155
206. Seferidi P, Scrinis G, Huybrechts I, Woods J, Vineis P, Millett C. The neglected environmental impacts of ultra-processed foods. Lancet Planetary Health. (2020) 4:e437–8. doi: 10.1016/S2542-5196(20)30177-7
207. Marinova D, Raphaely T. Impact of Vegetarian Diets on the Environment. In Vegetarian Nutrition and Wellness. CRC Press. (2018). doi: 10.1201/b22003-2
208. Raphaely T, Marinova D. Impact of Meat Consumption on 6.0 Health and Environmental Sustainability. Hershey: IGI Global. (2016). doi: 10.4018/978-1-4666-9553-5
209. White S, Cordell D. Global Phosphorus Security – Diet, Sustainability and Food for Thought. Meat the Future: How Cutting Meat (2015).
210. UNEP. How All Religious Faiths Advocate for Environmental Protection. (2019). Available online at: https://www.unep.org/news-and-stories/story/how-all-religious-faiths-advocate-environmental-protection
211. Barclay E. African Fishermen Find Way of Conservation in the Koran. The Christian Science Monitor (2007).
212. Djoundourian SS. Middle East and North Africa: Civil society and environmental activism in the Arab world. The Routledge Handbook of Environmental Movements. (2022). doi: 10.4324/9780367855680-8
213. Camosy C, Guy Consolmagno S, Dalton AM, Harvie T, Haught JF, Jacobs B, et al. Integral Ecology for a More Sustainable World: Dialogues with Laudato Si'. Lexington Books (2019).
215. Platzer M. Faith-Based Organizations the United Nations. In Kury H, Redo S, editors. Crime Prevention and Justice in 2030. Cham: Springer (2021). doi: 10.1007/978-3-030-56227-4_32
216. Weder F, Karmasin M, Krainer L. Sustainability communication as critical perspective in media communication studies - an introduction. In Weder F, Krainer L, Karmasin M, editors. The Sustainability Communication Reader: A Reflective Compendium. Wiesbaden: Springer Nature. (2021). doi: 10.1007/978-3-658-31883-3
217. Fuller R, Landrigan PJ, Balakrishnan K, Bathan G, Bose-O'Reilly S, Brauer M, et al. Pollution and health: a progress update. Lancet Planetary Health. (2022). doi: 10.1016/S2542-5196(22)00090-0
218. FAO. Global Strategic Framework for Food Security & Nutrition (GSF); Committee on World Food Security (2014).
219. Holman SR. Beholden: Religion, Global Health, Human Rights. Oxford: Oxford University Press. (2015). doi: 10.1093/acprof:oso/9780199827763.001.0001
220. ARHAP. ‘Appreciating Assets: The Contribution of Religion to Universal Access in Africa.' ARHAP, Report for the World Health Organization. Cape Town (2006).
Keywords: faith-based fasting, sustainability, nutrition, lifestyle, global health, public health
Citation: Trabelsi K, Ammar A, Boujelbane MA, Puce L, Garbarino S, Scoditti E, Boukhris O, Khanfir S, Clark CCT, Glenn JM, Alhaj OA, Jahrami H, Chtourou H and Bragazzi NL (2022) Religious fasting and its impacts on individual, public, and planetary health: Fasting as a “religious health asset” for a healthier, more equitable, and sustainable society. Front. Nutr. 9:1036496. doi: 10.3389/fnut.2022.1036496
Received: 04 September 2022; Accepted: 26 October 2022;
Published: 24 November 2022.
Edited by:
Prisco Piscitelli, Istituto Scientifico Biomedico Euro Mediterraneo (ISBEM), ItalyReviewed by:
Theocharis Koufakis, Aristotle University of Thessaloniki, GreeceMehmet Akif Buyukbese, Independent Researcher, Gaziantep, Turkey
Copyright © 2022 Trabelsi, Ammar, Boujelbane, Puce, Garbarino, Scoditti, Boukhris, Khanfir, Clark, Glenn, Alhaj, Jahrami, Chtourou and Bragazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Luigi Bragazzi, cm9iZXJ0b2JyYWdhenppQGdtYWlsLmNvbQ==
 Khaled Trabelsi
Khaled Trabelsi Achraf Ammar
Achraf Ammar Mohamed Ali Boujelbane2
Mohamed Ali Boujelbane2 Luca Puce
Luca Puce Sergio Garbarino
Sergio Garbarino Egeria Scoditti
Egeria Scoditti Omar Boukhris
Omar Boukhris Cain C. T. Clark
Cain C. T. Clark Omar A. Alhaj
Omar A. Alhaj Haitham Jahrami
Haitham Jahrami Nicola Luigi Bragazzi
Nicola Luigi Bragazzi