- 1Department of Medicine and Therapeutics, The Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
- 2Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, VIC, Australia
- 3School of Public Health, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4Department of Epidemiology and Biostatistics, Wuhan University, Wuhan, China
- 5Nankai University School of Medicine, Tianjin, China
- 6Department of Microbiology, Faculty of Applied Science, Taiz University, Taiz, Yemen
- 7Faculty of Medicine and Health, School of Population Health, University of New South Wales, Sydney, NSW, Australia
- 8Population Wellbeing and Environment Research Lab (PowerLab), Wollonggong, NSW, Australia
- 9The George Institute for Global Health, Newtown, NSW, Australia
- 10Department of Family Medicine, McGill University, Montreal, QC, Canada
- 11China Centre for Health Development Studies, Peking University, Beijing, China
- 12Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong, China
- 13Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 14Department of Pharmacy Administration and Clinical Pharmacy, Xi'an Jiaotong University, Xi'an, China
- 15School of Public Health, Xuzhou Medical University, Xuzhou, China
- 16Department of Orthopaedics, Wenzhou Medical University, Wenzhou, China
- 17Department of Endocrinology, University of Science and Technology of China, Hefei, China
- 18Institute of Child and Adolescent Health, Peking University, Beijing, China
- 19Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
- 20Institute for Health and Environment, Chongqing University of Science and Technology, Chongqing, China
- 21School of the Environment, Yale University, New Haven, CT, United States
- 22Department of Rheumatology and Immunology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 23Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, China
Background and aims: The disease burden attributable to metabolic risk factors is rapidly increasing in China, especially in older people. The objective of this study was to (i) estimate the pattern and trend of six metabolic risk factors and attributable causes in China from 1990 to 2019, (ii) ascertain its association with societal development, and (iii) compare the disease burden among the Group of 20 (G20) countries.
Methods: The main outcome measures were disability-adjusted life-years (DALYs) and mortality (deaths) attributable to high fasting plasma glucose (HFPG), high systolic blood pressure (HSBP), high low-density lipoprotein (HLDL) cholesterol, high body-mass index (HBMI), kidney dysfunction (KDF), and low bone mineral density (LBMD). The average annual percent change (AAPC) between 1990 and 2019 was analyzed using Joinpoint regression.
Results: For all six metabolic risk factors, the rate of DALYs and death increased with age, accelerating for individuals older than 60 and 70 for DALYs and death, respectively. The AAPC value in rate of DALYs and death were higher in male patients than in female patients across 20 age groups. A double-peak pattern was observed for AAPC in the rate of DALYs and death, peaking at age 20–49 and at age 70–95 plus. The age-standardized rate of DALYs increased for HBMI and LBMD, decreased for HFPG, HSBP, KDF, and remained stable for HLDL from 1990 to 2019. In terms of age-standardized rate of DALYs, there was an increasing trend of neoplasms and neurological disorders attributable to HFPG; diabetes and kidney diseases, neurological disorders, sense organ diseases, musculoskeletal disorders, neoplasms, cardiovascular diseases, digestive diseases to HBMI; unintentional injuries to LBMD; and musculoskeletal disorders to KDF. Among 19 countries of Group 20, in 2019, the age-standardized rate of DALYs and death were ranked fourth to sixth for HFPG, HSBP, and HLDL, but ranked 10th to 15th for LBMD, KDF, and HBMI, despite the number of DALYs and death ranked first to second for six metabolic risk factors.
Conclusions: Population aging continuously accelerates the metabolic risk factor driven disease burden in China. Comprehensive and tight control of metabolic risk factors before 20 and 70 may help to mitigate the increasing disease burden and achieve healthy aging, respectively.
Introduction
Increasing life expectancy has led to a global burden of late-life disease and research has recently been focused on ways of avoiding this trend in the general population (1). In China the period from 1950 to 2019 has seen the total fertility rate decrease from 5.91 to 1.43, yet life expectancy has increased from 49.6 to 74.7 years for men and from 52.6 to 80.8 years for women (2). The summary exposure values of metabolic risk factors increased from 14.90 to 22.14 in China from 1990 to 2019 with an annualized rate of change of 1.37% (3).
Understanding the contributions of metabolic risk factors to disease over time is vital to enabling healthy extended lifespans (4). According to a Global Burden of Disease (GBD) study from 2019, metabolic risk factors include high fasting plasma glucose (HFPG), high systolic blood pressure (HSBP), high low-density lipoprotein (HLDL) cholesterol, high body-mass index (HBMI), kidney dysfunction (KDF), and low bone mineral density (LBMD) (3). Metabolic risk factors have become the leading cause of ischemic heart disease in developing countries (5). The associations between type 2 diabetes with different cardiovascular diseases, including peripheral arterial disease, ischemic stroke, stable angina, heart failure, and non-fatal myocardial infarction have been established (6). Around 31.7% and 23.3% of patients with hypertension had blood pressure below 140/90 mm Hg and below 130/80 mm Hg, contributing to heart attack, stroke, and chronic kidney disease (7). HBMI accounted for 4.0 million deaths globally, more than two-thirds of which were due to cardiovascular disease, and nearly 40% of these occurred in people who were not obese (8). LBMD-associated increase in fracture risk affects virtually all skeletal sites, especially among older women and patients treated with glucocorticoid (9, 10). Occurring in a continuum with acute and chronic kidney disease, people with KDF are 5–10 times more likely to die prematurely, largely due to cardiovascular disease and cancer (11, 12). The principal target of lipid-modification therapies is to lower LDL cholesterol to prevent cardiovascular death, although recent research focuses on triglyceride-rich lipoproteins in addition to LDL as the causal risk factor for atherosclerosis (13).
Over the past three decades, the role of metabolic aging in extending a healthy lifespan has been increasingly acknowledged with population aging (14). Available evidence supports the idea that decreased nutrient signaling extends longevity and anabolic signaling accelerates aging (15). Previous study has investigated the mortality, morbidity, and risk factors in China (16). Therefore, our study further analyzed the disease burden of metabolic risk factors in the era of population aging. The Group of 20 (G20) may provide significant research insights that are highly relevant to the Chinese context, as the mean population age in China was lower than most countries in G20 (2).
The objectives of this study were to (1) investigate the pattern of disease burden driven by six metabolic risk factors disaggregated by age and sex, (2) characterize the temporal trend of the six metabolic risk factors, (3) ascertain the temporal trend of metabolic risk attributable cause, (4) determine the association between societal development and metabolic risk factors, and (5) compare metabolic risk factors in China with those of G20 countries.
Methods
Data sources and data extraction
Global Burden of Disease study (GBD) 2019 was established to quantify the health loss caused by diseases, injuries, and risk factors, including 369 diseases, injuries, and 87 risk factors [including six metabolic risk factors–HFPG, HSBP, HLDL, HBMI, KDF, and LBMD (Case definition in Supplementary material)] (3, 17). This study was produced as part of the GBD Collaborator Network and in accordance with the GBD Protocol (IHME ID. 1775-GBD2019-012021). The study collected original data from the Global Health Data Exchange (GHDx), including age-sex-year disability adjusted life years (DALYs), years lived with disability (YLDs), years of life lost (YLLs), and death of six metabolic risk factors in terms of absolute number, age-standardized rate, and crude rate per 100,000 population.
Inclusion and exclusion criteria
According to the comparative risk assessment conceptual framework, the GBD 2019 study established a causal web of hierarchically organized risks or causes that contributed to health outcomes. A set of behavioral, environmental, occupational, and metabolic risk factors-outcome pairs were constructed based on evidence rules. The study included 23, 66, and 61 metabolic risk factor-level 2, 3, and 4 outcome pairs, and 108 risk-most detailed outcome pairs (The GBD metabolic risk factor attributable cause hierarchy in Supplementary material) (3). The study excluded GBD behavioral, environmental, and occupational risk factors attributable to cause hierarchy.
Population attributable fraction and socio-demographic index
The population attributable fractions (PAFs) were used to quantify the contribution of risk factors to the burden of disease (Estimation of six metabolic risk factors in Supplementary material) (18). The sociodemographic index (SDI) is a composite indicator of socio-demographic development status, which is strongly correlated with health outcomes. It is the geometric mean of 0 to 1 indices of total fertility rate in those under 25 years old, mean education for those aged 15 years or older, and lag-distributed income per capita. The national SDIs for China between 1990 and 2019 ranged from 0.433 to 0.686 (17).
Statistical analysis
The number and rate of DALYs, YLDs, YLLs, and deaths with 95% uncertainty intervals (UIs) of the six metabolic risk factors were reported according to age and sex from 1990 to 2019. Age-standardized rates of DALYs and death were plotted against SDI between 1990 and 2019 with a simple correlation. Temporal trend changes were determined using a Joinpoint regression model. Average annual percent change (AAPC) was calculated for the entire period analyzed, and annual percent change (APC) was calculated for each segmented line regression. The temporal trends were defined according to the statistical significance of the AAPC compared to zero. Any AAPC or APC with a 95% CI overlapping with zero was considered stable. All statistical analyses were performed using Joinpoint Regression Program (version 4.8.0.1, Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute), with P-values < 0.05 considered statistically significant.
Results
Trend of disease burden driven by six metabolic risk factors according to age and year
We first addressed how metabolic risk factor driven disease burden changed with increasing age. Overall, people aged 65–69 and 80–84 years had the highest number of DALYs and deaths, respectively (Figures 1A, B). The rate of DALYs and death increased with age, especially for people older than 60 and 70 for DALYs and deaths, respectively (Figures 1C, D). Notably, the disease burden attributable to KDF and LBMD increased rapidly with age (Supplementary Figures 1E, F, 2E, F). The disease burden was higher in male patients than female patients across different age groups for each (Supplementary Figures 1A–F, 2A–F) and all of the metabolic risk factors (Figures 1E–H). Besides, a double-peak pattern was observed for AAPC in the rate of DALYs, deaths, and YLLs, peaking at age 20 to 49 and at age 70 to 95 plus (Figures 1E, G, H); while a trumpet pattern was found for AAPC in the rate of YLDs (Figure 1F). A double-peak pattern was also observed for AAPC in the rate of HFPG, HLDL, HSBP (Supplementary Figures 1B–D, 2B–D).
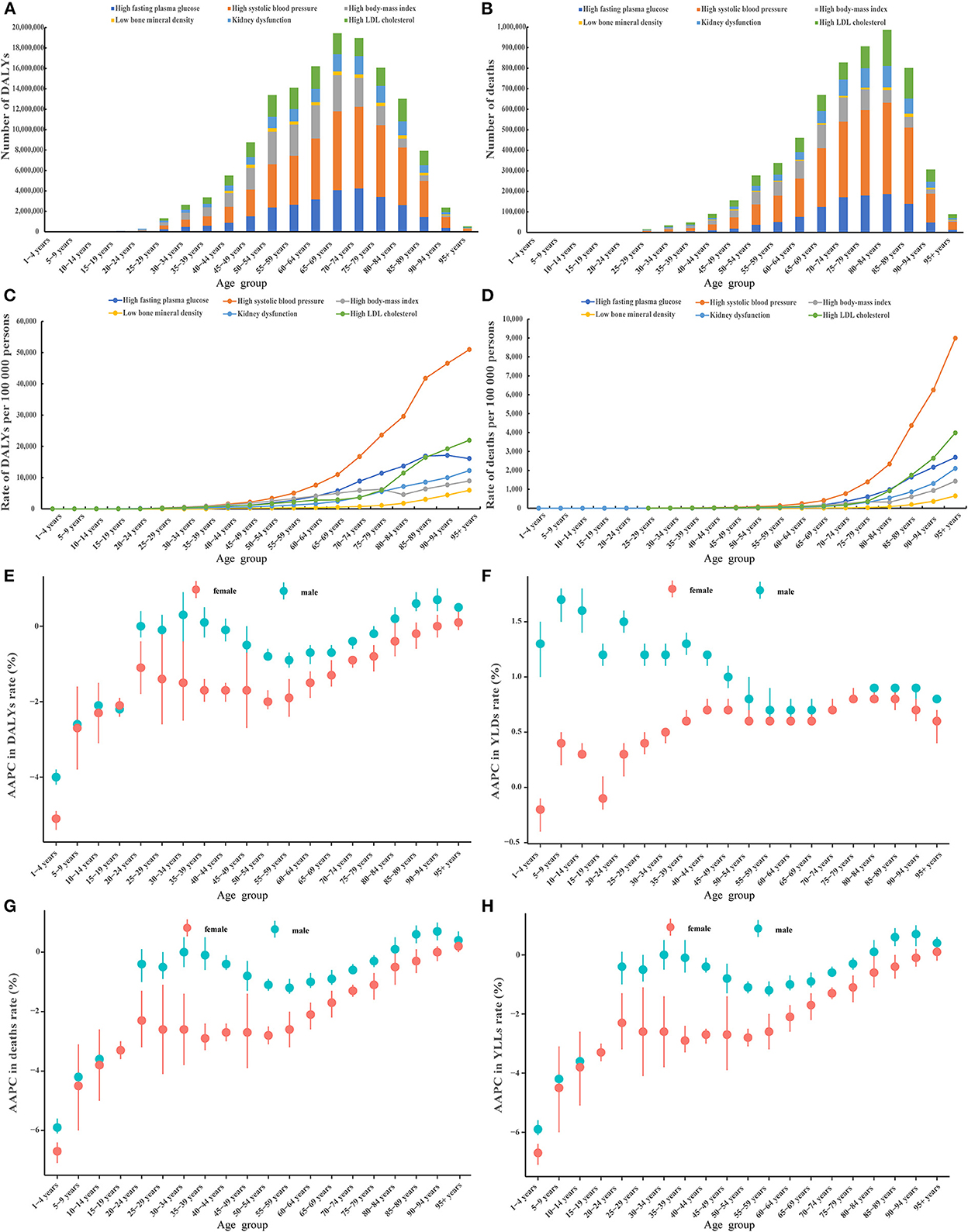
Figure 1. Cross-sectional and longitudinal trend of the disease burden attributable to six metabolic risk factors throughout the human lifespan. Number of DALYs attributable to six metabolic risk factors across 20 age groups in 2019 (A), number of deaths attributable to six metabolic risk factors across 20 age groups in 2019 (B), rate of DALYs attributable to six metabolic risk factors across 20 age groups in 2019 (C), rate of deaths attributable to six metabolic risk factors across 20 age groups in 2019 (D), AAPC in the rate of DALYs attributable to metabolic risk factors across 20 age groups, 1990–2019 (E), AAPC in the rate of YLDs attributable to metabolic risk factors across 20 age groups, 1990–2019 (F), AAPC in the rate of deaths attributable to metabolic risk factors across 20 age groups, 1990–2019 (G), AAPC in the rate of YLLs attributable to metabolic risk factors across 20 age groups, 1990–2019 (H). AAPC, average annual percent change; DALYs, disability-adjusted life years; YLDs, years lived with a disability; YLLs, years of life lost.
We further determined how the disease burden changed with age. A strong increasing trend was found across the 16 age groups, especially in people older than 60 (Supplementary Figure 3). Amongst all subjects aged 15 to 94, individuals aged 60–74 contributed the greatest numbers of DALYs and the 75–94 age group contributed the greatest number of deaths (Supplementary Figures 4, 5).
We next ascertained if the gender differences in the six metabolic risk factors existed. The numbers and age-standardized rate of DALYs and deaths were higher in male than female patients for five out of six metabolic risk factors (except LBMD) (Supplementary Figure 6, Supplementary Table 1). The age-standardized rate of YLDs was higher in male than female patients for five out of six metabolic risk factors (except HFPG), while the age-standardized rate of YLLs was higher in female than male patients for all six metabolic risk factors (Supplementary Figure 7).
Temporal trend of metabolic risk factors from 1990 to 2019
From 1990 to 2019, the number of DALYs and deaths attributable to the six metabolic risk factors showed rapid growth, with the highest rate for HBMI (DALYs: 4.0; death: 4.2) with no sex-based difference (Table 1, Supplementary Figure 8, Supplementary Table 1). The age standardized rate of DALYs increased for HBMI (1.28) and LBMD (0.18), decreased for HFPG (−0.41), HSBP (−0.89), KDF (−0.61), and remained stable for HLDL. The age standardized rate of death increased for HBMI (1.03), LBMD (0.38), HLDL (0.45), decreased for HSBP (−0.78) and remained stable for HFPG (−0.41) and KDF (−0.10) (Table 1, Supplementary Figure 8). A sex difference was identified in the trend of the age-standardized rate of DALYs for HFPG and HLDL, the age-standardized rate of death for HFPG, HSBP, KDF, and HLDL (Supplementary Table 1).
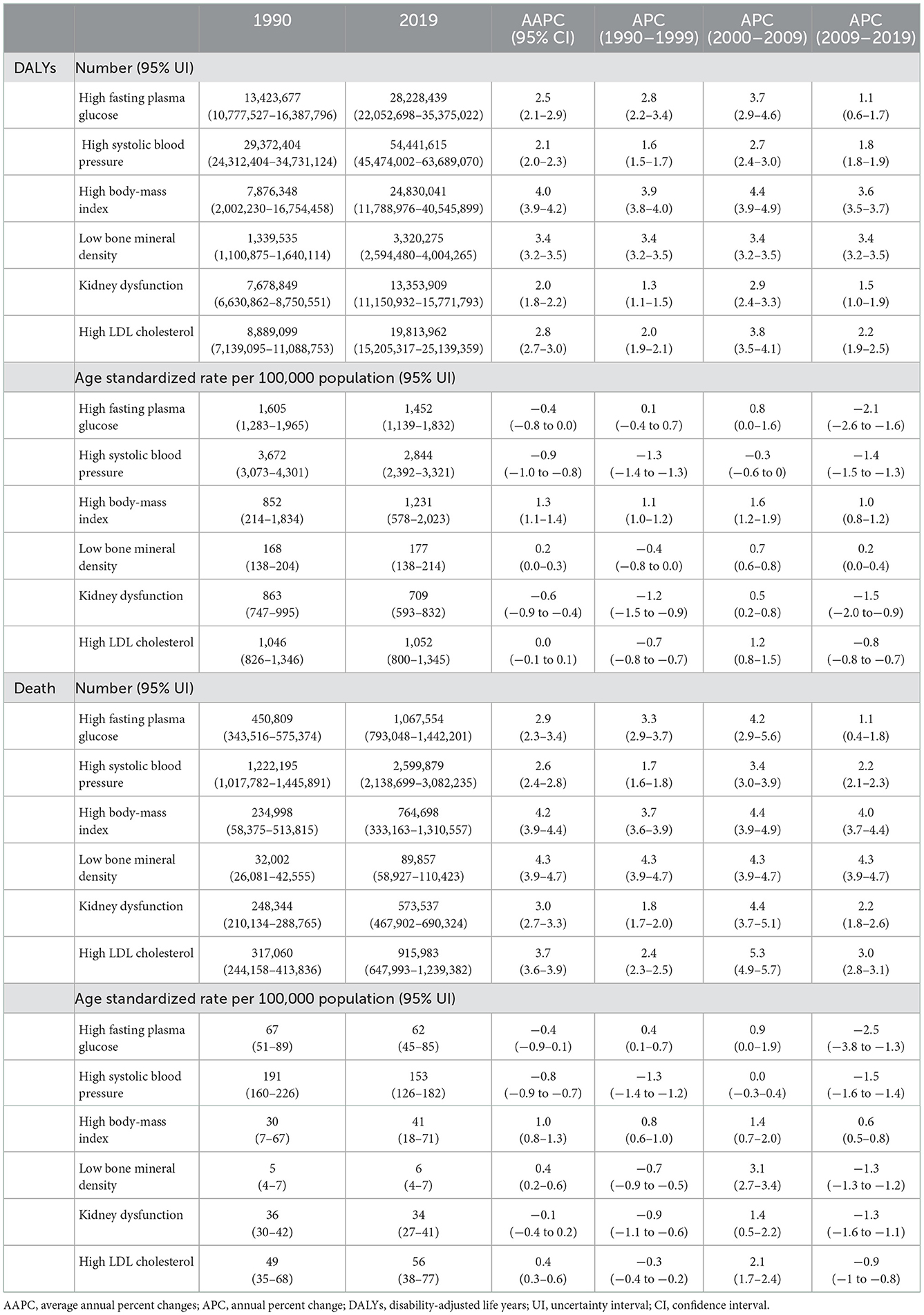
Table 1. Trends in the number and age-standardized rate of DALYs and death attributable to six metabolic risk factors for both sexes in China, 1990–2019.
For the 22 level 2 causes, the number of DALYs and death increased from 1990 to 2019, with the highest speed in HBMI attributable neurological disorders (DALYs: 6.7; death: 6.8) (Table 2). In terms of age-standardized rate of DALYs, there was an increasing trend of neoplasms and neurological disorders attributable to HFPG, diabetes and kidney diseases, neurological disorders, sense organ diseases, musculoskeletal disorders, neoplasms, cardiovascular diseases, digestive diseases attributable to HBMI, unintentional injuries attributable to LBMD, and musculoskeletal disorders attributable to KDF (Table 2). In terms of age-standardized rate of death, there was an increasing trend of neoplasms attributable to HFPG, cardiovascular diseases attributable to HSBP, neurological disorders, diabetes and kidney diseases, neoplasms, cardiovascular diseases attributable to HBMI, unintentional injuries attributable to LBMD (Table 2). A similar trend was also observed in level 3, level 4, and detailed causes (Supplementary Tables 2–4).
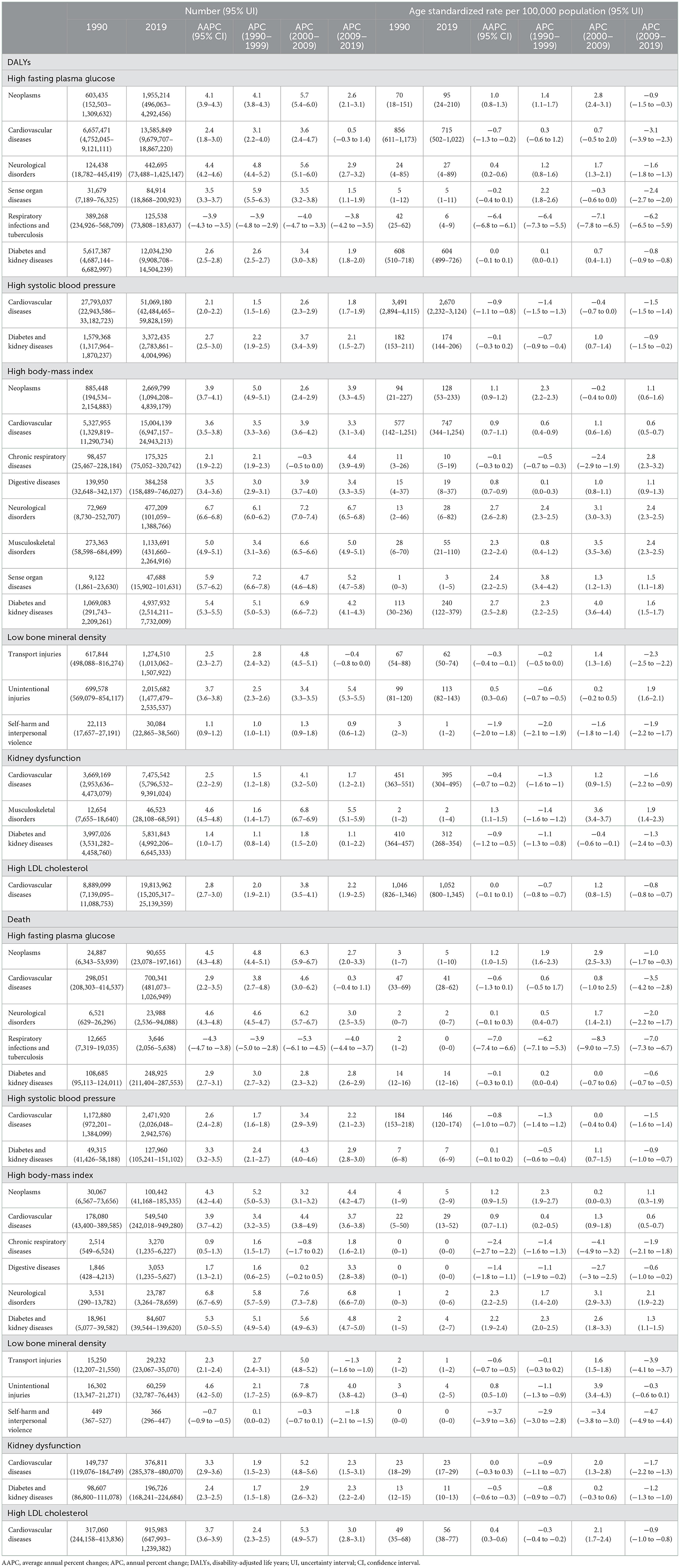
Table 2. Trends in the number and age-standardized rate of DALYs and death for level 2 causes attributable to six metabolic risk factors in China, 1990–2019.
Temporal trend of the effect of metabolic risk factors on cause from 1990 to 2019
In 2019, in terms of age-standardized rate of DALYs, HBMI contributed to 29.4, 15.1, 3.7% of diabetes and kidney diseases, cardiovascular diseases, neoplasms, HFPG to 73.8, 14.5, 2.8, 2.5% of diabetes and kidney diseases, cardiovascular diseases, neoplasms, neurological disorders. HLDL contributed to 21.3% of cardiovascular diseases, HSBP to 54.1%, 21.4% of cardiovascular diseases, diabetes and kidney diseases, KDF to 38.3%, 8.0% of diabetes and kidney diseases, cardiovascular diseases, and LBMD to 10.3%, 7.2% of unintentional injuries, transport injuries (Supplementary Table 5). Similar results were found for the contribution of the six metabolic risk factors to disease burden in terms of age-standardized rate of death (Supplementary Table 5).
Analysis of the PAF of age standardized DALYs rate indicated a significant increasing contribution of HFPG to neoplasms (2.1), respiratory infections and tuberculosis (0.7), cardiovascular diseases (0.7), neurological disorders (0.5), diabetes and kidney diseases (0.4), increasing contribution of HSBP to cardiovascular diseases (0.5), diabetes and kidney diseases (0.3), increasing contribution of HBMI to digestive diseases (4.0), chronic respiratory diseases (3.8), diabetes and kidney diseases (3.0), neurological disorders (2.8), musculoskeletal disorders (2.5), sense organ diseases (2.5), cardiovascular diseases (2.3), neoplasms (2.2), increasing contribution of LBMD to unintentional injuries (3.4), self-harm and interpersonal violence (2.0), transport injuries (1.0), increasing contribution of HLDL to cardiovascular diseases (1.4), increasing contribution of KDF to cardiovascular diseases (1.0) and musculoskeletal disorders (1.5), but a significant decreasing contribution to diabetes and kidney diseases (−0.5) (Table 3, Supplementary Figure 9). Analysis of PAF of age standardized death rate also showed a similar trend (Table 3, Supplementary Figure 10).
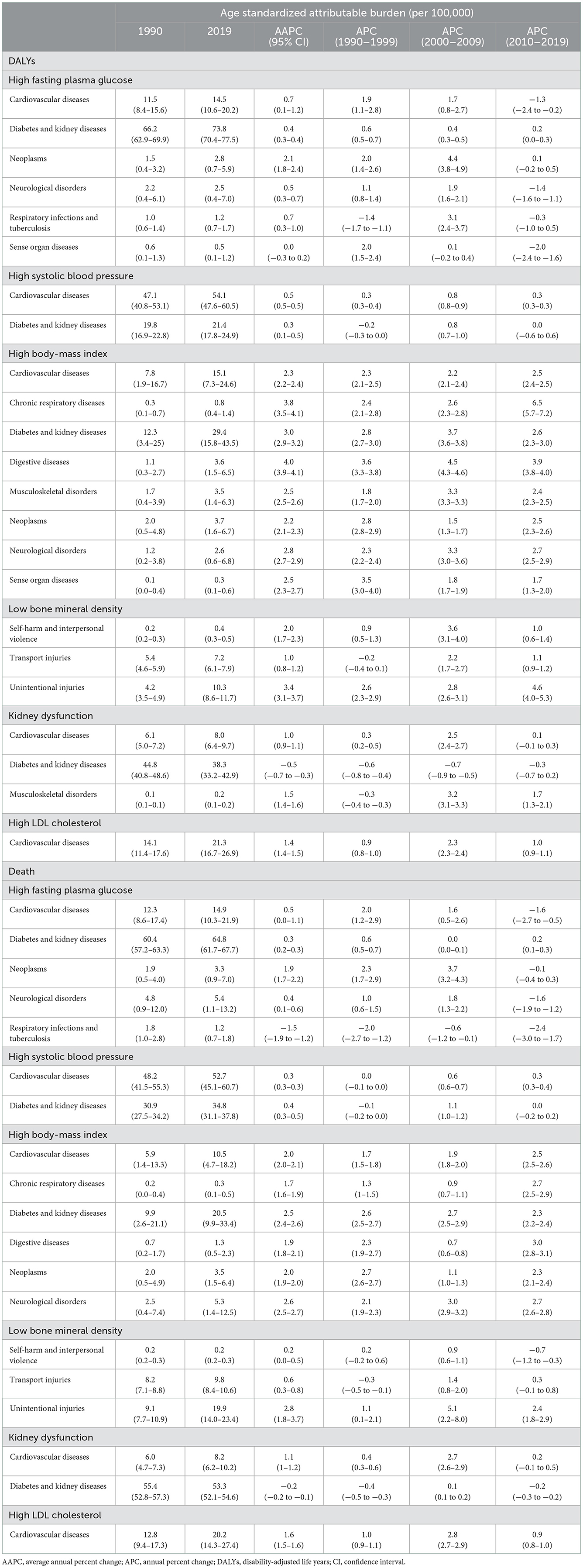
Table 3. Trends in the population attributable fraction on the age-standardized rate of DALYs and death of level 2 causes attributable to six metabolic risk factors, 1990–2019.
Association between metabolic risk factor and SDI
There was a positive correlation between the SDI level and the number of six metabolic risk factors attributable DALYs, YLDs, deaths, and YLLs (Supplementary Table 6, Supplementary Figure 11). The SDI level showed a positive correlation with the age-standardized rate of DALYs for HBMI, LBMD, and HLDL, but a negative correlation with DALYs for HSBP and KDF. The SDI level showed a positive correlation with the age-standardized rate of deaths for HBMI, KDF, and HLDL, but a negative correlation with HSBP. The SDI level was positively correlated with the age-standardized rate of YLDs for all six metabolic risk factors except LBMD. The SDI level was positively correlated with the age-standardized rate of deaths YLLs for HBMI, and HLDL, but was negatively correlated with HFPG, HSBP, and KDF (Supplementary Table 6, Supplementary Figure 11).
Overall burden ranking in Group of 20 (G20) countries
Among 19 countries of Group 20, in 2019, China ranked 1st, 1st, 1st, 2nd, 2nd, 2nd for HSBP, HBMI, HLDL, HFPG, LBMD, KDF in terms of number of DALYs and ranked 1st, 1st, 1st, 1st, 2nd, 2nd for HSBP, HBMI, HLDL, KDF, HFPG, LBMD in terms of number of deaths, respectively (Figure 2, Supplementary Table 7). The age-standardized rate of DALYs for HFPB, HSBP, HLDL, LBMD, KDF, HBMI ranked 4th, 6th, 6th, 10th, 11th, 15th, respectively (Figure 2, Supplementary Table 7). The age standardized rate of death for LBMD, HSBP, HLDL, KDF, HFPG, HBMI ranked 4th, 5th, 5th, 10th, 12th, 15th, respectively (Figure 2, Supplementary Table 7).
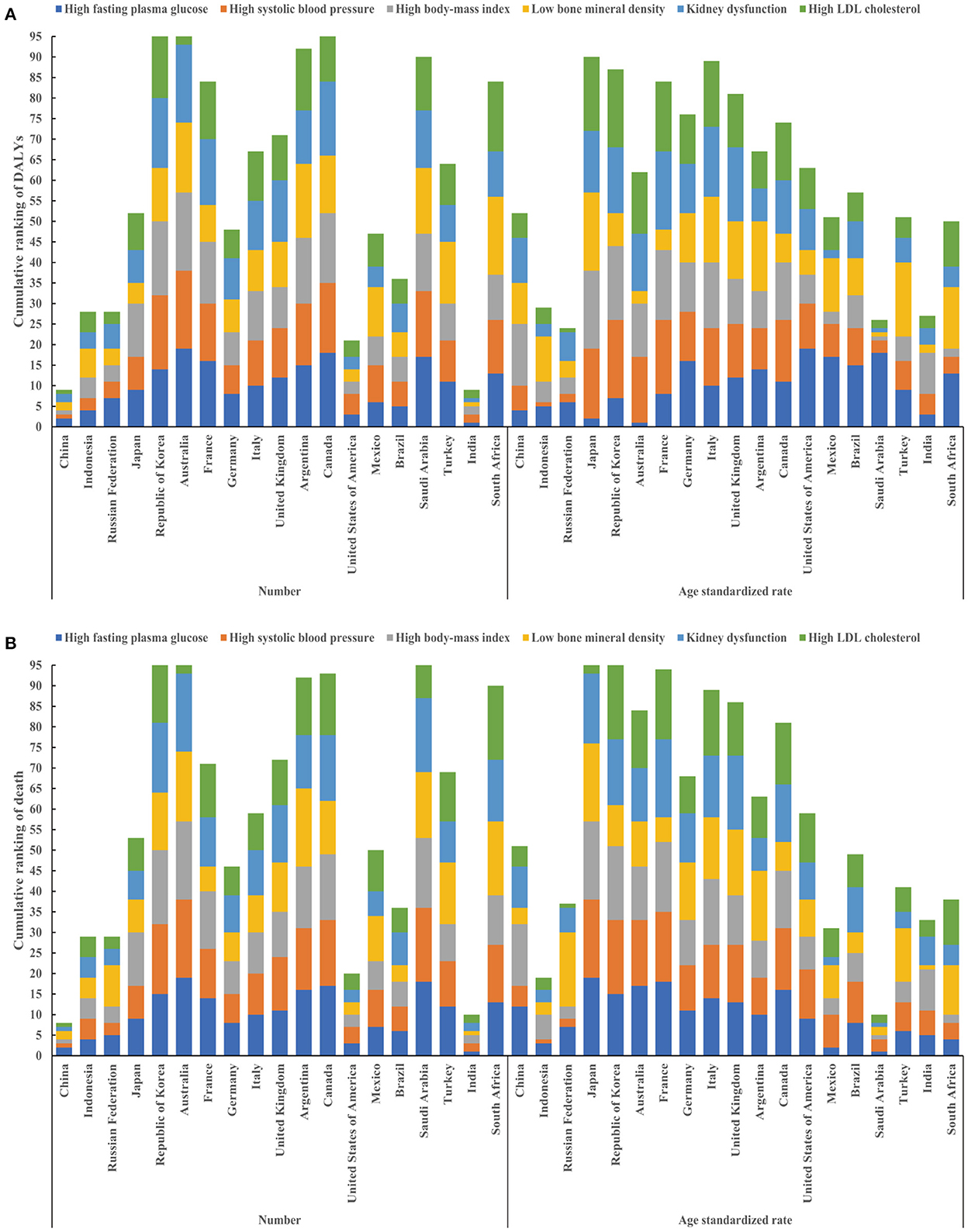
Figure 2. Cumulative ranking of DALYs and deaths attributable to six metabolic risk factors in 19 countries of Group 20 (excluding the European Union). Cumulative ranking of number (left) and age standardized rate (right) of DALYs attributable to six metabolic risk factors in 19 countries of Group 20 (A); Cumulative ranking of number (left) and age standardized rate (right) of deaths attributable to six metabolic risk factors in 19 countries of Group 20 (B). DALYs, disability-adjusted life years.
Discussion
Our findings support the view that mitigation of metabolic risk factors provides a unique opportunity to achieve better health in the future. At first, 36 independent drivers of global health, especially 5 metabolic risk factors, were expected to deteriorate without comprehensive management, although most of them were forecast to improve by 2040 (19). In China, the population aged >65 is increasing rapidly and those aged >65 and >80 may reach 400 million and 150 million, respectively, before 2050 (20). Most importantly, our data support the view that risk factor driven disease burdens increased with age, reaching a peak for individuals aged >60.
Our results call for early and tight control of metabolic risk factors starting before the first peak (age 20–49) targeting adolescence. As a pivotal point in the life course characterized by openness to change, adolescence offers a unique window of opportunity to promote the adoption of a healthy lifestyle (diet and physical activity). However, this window of opportunity has largely been overlooked in behavior and policy research (21). Growing up in an era of “toxic” food environment, the current generation of adolescents may face unprecedented disease burdens from metabolic risk factors in their later life (22). The pace of change in the nutritional habits of adolescents poses a great threat to their health. National and individual efforts should identify key meanings and context of their food choices and seek to improve their food environments and choices by harnessing widely shared adolescent values that go beyond nutrition or health (23). Our findings also provided us an impetus to pharmacologically target key metabolic pathways linked to longevity before the second peak factors with the hope of delaying aging and ameliorating age-related diseases (24). On the one hand, a hallmark of aging is metabolism dysfunction, especially glucose homeostasis, negatively regulating energy metabolism and ultimately increasing the organism's susceptibility to disease. On the other hand, metabolic dysfunction occurs increasingly with age, including modulation of mitochondrial function, a decline in insulin sensitivity, and alterations in substrate utilization, which are associated with obesity, hyperglycemia, dyslipidemia, and insulin resistance (24).
These findings highlighted that male gender is a biological variable due to higher disease burden attributable to metabolic risk factors across all age groups. Free testosterone in men may be associated with a risk for major adverse cardiovascular events as men with lower sex hormone–binding globulin concentrations have a higher risk for myocardial infarction (25). The greater increase in disease burden among males will require more attention from health systems. Our data also support the view that menopause transition contributes to the increase in metabolic disease burden of old age (26), based on the fact that the AAPC in the rate of YLDs decreased for male patients but increased for female patients from 1 to 49 years before reaching a plateau. The reported findings underline the potential benefits of monitoring women's health during midlife and emphasize the critical window for early intervention strategies. Postmenopausal women, especially those younger than 60, should be fully advised on the benefits and harms of different types and timing of hormone therapy (27).
Our data imply that an integrative metabolic strategy should prioritize HBMI and LBMD due to the increased age-standardized rates of DALYs and death. Maintaining a BMI of 20.0–25.0 kg/m2 can help minimize all-cause mortality, and a minimum of 150 min weekly leisure moderate to vigorous physical activity was associated with the most health benefits (28, 29). Phentermine–topiramate and glucagon-like peptide-1 receptor agonists (GLP-1RA), particularly semaglutide, seem to be the best drugs for controlling weight in overweight and obese adults (30). Men and women with LBMD, consistent with osteoporosis or osteopenia, showed a significantly increased risk of fractures and mortality (31). Nonetheless, indiscriminate use of vitamin D supplementation to prevent fracture and osteoporosis seems should be avoided (32). Treatment with bisphosphonates or denosumab, and teriparatide or abaloparatide should be considered for individuals at high and imminent risk of fracture, respectively (33).
Integrative management in old individuals often requires the use of multiple drugs to treat concurrent dyslipidemia, diabetes, and hypertension, and those with metabolic syndrome should be tightly managed. Anti-hypertensive treatment targeting systolic blood-pressure 110–130 mmHg rather than 130–150 mmHg led to fewer incidences of cardiovascular events in Chinse older patients with hypertension (34). Compared with the gold standard angiotensin-converting enzyme inhibitor (enalapril) an angiotensin receptor-neprilysin inhibitor (sacubitril/valsartan) not only decreases the risk of cardiovascular death or heart failure hospitalization but also improves symptoms in patients with chronic heart failure with reduced ejection fraction (35). Clinical trials exploring the cardiovascular and renal outcomes of GLP-1RA and sodium-glucose cotransporter-2 inhibitors (SGLT2i) not only change the treatment paradigm of diabetes but also support a more holistic approach beyond glycemic control which emphasize on cardiac and reno-protective effects (36). Although statins, ezetimibe, and PCSK9 inhibitors are the standard of care for coronary artery disease, many novel LDL cholesterol–lowering drugs, such as inclisiran recently approved by the FDA, may transform the care of patients who are at risk of life-threatening coronary events (37, 38). KDF is harmful but treatable if individuals at risk are identified at an early stage. More individuals than ever before are experiencing KDF and nephrosclerosis-age-associated histologic changes, as observed in 2.7, 58, and 73% of biopsies from donors aged < 30, 60–69,70 plus, respectively (39). There is partial but limited success for kidney diseases treated with immunosuppressive agents, antihypertensives, and diuretics. Sodium-glucose co-transporter-2 (SGLT2) inhibition should be used as a foundational therapy for chronic kidney disease as SGLT2 inhibitors might substantially slow the progression of chronic kidney disease in people with type 2 diabetes (40, 41). New candidate therapeutic drugs targeting the glomerular filtration barrier may help to correct defects within or between cells of the glomerular filtration barrier and maintain its integrity (42). Most importantly, a mechanistic link has been observed among obesity, kidney dysfunction, and hypertension (43). Namely, excessive adiposity, which is a major driver of kidney diseases and prolonged obesity, and progressive renal injury, often leads to the development of treatment-resistant hypertension.
Our findings identified the precise diseases and risk factors that are most in need of attention. For example, our data showed that KDF-driven musculoskeletal disorders at level 2 cause and gout at level 3 cause. We also ascertained the trend of the contribution of risk factors, which may help formulate feasible and quantitative health policy. Analysis of population-attributable fractions indicated the increasing contribution of HSBP to cardiovascular diseases and of HFPG to neoplasms. A 5-mmHg decrement in systolic blood pressure decreases major cardiovascular events by ~10%, irrespective of previous cardiovascular disease (44). The risk of incident colorectal cancer associated with HFPG may help to identify people at high risk for future colorectal cancer (45). In addition, the evolutionary pattern of disability and death with societal development, as measured by the SDI, may help prioritize metabolic factors while formulating health policy. In addition to physical equipment, the government may also need to improve the availability of phentermine–topiramate and GLP-1 receptor agonists in adults who are overweight and obese as there is an increasing trend for HBMI and HLDL with the increase of SDI (46). In addition to the early detection of chronic kidney disease and interventions aiming to reduce urine protein excretion, more efforts are needed to delay and recover the progression of kidney function because there is increasing disability and decreasing death for KDF with the increase of SDI (47).
Our data revealed that China was the largest contributor to disease burden due to metabolic risk factors among 19 G20 countries. This presents a public health challenge and an opportunity for China to improve future health worldwide. Systematic management of individuals with metabolic syndromes must be stressed again as the age-standardized rate of DALYs and death in China ranked fourth to sixth among G20 countries for HFPG, HSBP, and HLDL. Although the age-standardized rate of DALYs and death in China ranked 10th to 15th for LBMD, KDF, and HBMI, this does not automatically imply that these indices are well-controlled. Since China gained more increments in life expectancy compared to other G20 countries, which may have led to a lower disease burden from age-related LBMD, KDF, and HBMI in the past three decades (2).
These findings are not entirely surprising if one follows the concept that metabolic aging is remolding future health structures. At the same time, these findings are interesting as they foster the concept of prevention always triumphs over remediation during adolescence and integrative strategy for key risk factors and individuals. Nonetheless, these findings are subject to two major limitations. First, the study did not include urban-rural and province-level data, which may be useful to formulate provincial-, urban-, and rural-specific health policies. Second, the study is subject to the same limitation as the GBD 2019 studies (2, 3).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This manuscript was produced as part of the GBD Collaborator Network in accordance with the GBD Protocol (IHME ID. 1775-GBD2019-012021). Data released from the Global Health Data Exchange query did not require informed patient consent. This study used an anonymized publicly available data set with no identifiable information about the survey participants.
Author contributions
JC, KHH, YZJ, L-LL, SM, HS, A-MW, and SWX provided data or critical feedback on data sources. YZJ and A-MW developed methods of computational machinery and managed the estimation or publication process. AB, JC, EC, XQF, KHH, KAH, YZJ, L-LL, SM, FW, ZYW, DZW, A-MW, SWX, and ZYZ provided critical feedback on methods or results. AB, JC, EC, KAH, JJH, YZJ, L-LL, YMS, L-ST, FW, ZYW, DZW, A-MW, and CWZ drafted the work or revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The Global Burden of Disease study was funded by the Bill and Melinda Gates Foundation. The funders had no role in study design, data collection, and analysis, nor the decision to publish or preparation of the manuscript.
Acknowledgments
We thank the staff at the Institute for Health Metrics and Evaluation and its collaborators who prepared publicly available and impactful data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1035439/full#supplementary-material
References
1. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. (2018) 561:45–56. doi: 10.1038/s41586-018-0457-8
2. Collaborators GBDD. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1160–203. doi: 10.1016/S0140-6736(20)30977-6
3. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
4. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. (2019) 571:183–92. doi: 10.1038/s41586-019-1365-2
5. Wang W, Hu M, Liu H, Zhang X, Li H, Zhou F, et al. Global Burden of Disease Study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab. (2021) 33:1943–56. doi: 10.1016/j.cmet.2021.08.005
6. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. (2015) 3:105–13. doi: 10.1016/S2213-8587(14)70219-0
7. Brouwers S, Sudano I, Kokubo Y, Sulaica EM. Arterial hypertension. Lancet. (2021) 398:249–61. doi: 10.1016/S0140-6736(21)00221-X
8. Collaborators G2O, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
9. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. (2016) 374:254–62. doi: 10.1056/NEJMcp1513724
10. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. (2020) 16:437–47. doi: 10.1038/s41574-020-0341-0
11. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394:1949–64. doi: 10.1016/S0140-6736(19)32563-2
12. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
13. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
14. Hofmann SM, Landgraf R. Research in metabolic ageing - a tale of mice versus humans? Nat Rev Endocrinol. (2022) 18:7–8. doi: 10.1038/s41574-021-00597-9
15. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. (2013) 153:1194–217. doi: 10.1016/j.cell.2013.05.039
16. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
17. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
18. Brooks-Pollock E, Danon L. Defining the population attributable fraction for infectious diseases. Int J Epidemiol. (2017) 46:976–82. doi: 10.1093/ije/dyx055
19. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
20. Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev. (2015) 24(Pt B):197–205. doi: 10.1016/j.arr.2015.08.003
21. Hargreaves D, Mates E, Menon P, Alderman H, Devakumar D, Fawzi W, et al. Strategies and interventions for healthy adolescent growth, nutrition, and development. Lancet. (2021) 399:198–210. doi: 10.1016/S0140-6736(21)01593-2
22. Norris SA, Frongillo EA, Black MM, Dong Y, Fall C, Lampl M, et al. Nutrition in adolescent growth and development. Lancet. (2021) 399:172–84. doi: 10.1016/S0140-6736(21)01590-7
23. Neufeld LM, Andrade EB, Suleiman AB, Barker M, Beal T, Blum LS, et al. Food choice in transition: adolescent autonomy, agency, and the food environment. Lancet. (2021) 399:185–97. doi: 10.1016/S0140-6736(21)01687-1
25. Yeap BB, Marriott RJ, Antonio L, Raj S, Dwivedi G, Reid CM, et al. Associations of serum testosterone and sex hormone-binding globulin with incident cardiovascular events in middle-aged to older men. Ann Intern Med. (2021) 175:159–70. doi: 10.7326/M21-0551
26. Khoudary SRE, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the american heart association. Circulation. (2020) 142:e506–32. doi: 10.1161/CIR.0000000000000912
27. Gartlehner G, Patel SV, Feltner C, Weber RP, Long R, Mullican K, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US preventive services task force. JAMA. (2017) 318:2234–49. doi: 10.1001/jama.2017.16952
28. Bakker EA, Lee DC, Hopman MTE, Oymans EJ, Watson PM, Thompson PD, et al. Dose-response association between moderate to vigorous physical activity and incident morbidity and mortality for individuals with a different cardiovascular health status: a cohort study among 142,493 adults from the Netherlands. PLoS Med. (2021) 18:e1003845. doi: 10.1371/journal.pmed.1003845
29. Global BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. (2016) 388:776–86. doi: 10.1016/S0140-6736(16)30175-1
30. Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li J, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet. (2021) 399:259–69. doi: 10.1016/S0140-6736(21)01640-8
31. Hauger AV, Bergland A, Holvik K, Stahle A, Emaus N, Strand BH. Osteoporosis and osteopenia in the distal forearm predict all-cause mortality independent of grip strength: 22-year follow-up in the population-based Tromso Study. Osteoporos Int. (2018) 29:2447–56. doi: 10.1007/s00198-018-4653-z
32. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. (2018) 6:847–58. doi: 10.1016/S2213-8587(18)30265-1
33. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
34. Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. (2021) 385:1268–79. doi: 10.1056/NEJMc2117463
35. Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/valsartan: neprilysin inhibition 5 years after PARADIGM-HF. JACC Heart Fail. (2020) 8:800–10. doi: 10.1016/j.jchf.2020.06.020
36. American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44(Suppl 1.): S111–24. doi: 10.2337/dc21-S009
37. Musunuru K. Treating coronary artery disease: beyond statins, ezetimibe, and PCSK9 inhibition. Annu Rev Med. (2021) 72:447–58. doi: 10.1146/annurev-med-080819-044918
38. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two Phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. (2020) 382:1507–19. doi: 10.1056/NEJMoa1912387
39. O'Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. (2017) 28:407–20. doi: 10.1681/ASN.2015121308
40. Mark PB, Sattar N. Implementation, not hesitation, for SGLT2 inhibition as foundational therapy for chronic kidney disease. Lancet. (2022) 400:1745–7. doi: 10.1016/S0140-6736(22)02164-X
41. Nuffield Department of Population Health Renal Studies G, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. (2022) 400:1788–801. doi: 10.1016/S0140-6736(22)02074-8
42. Daehn IS, Duffield JS. The glomerular filtration barrier: a structural target for novel kidney therapies. Nat Rev Drug Discov. (2021) 20:770–88. doi: 10.1038/s41573-021-00242-0
43. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. (2019) 15:367–85. doi: 10.1038/s41581-019-0145-4
44. Blood Pressure Lowering Treatment Trialists C. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. (2021) 397:1625–36. doi: 10.1016/S0140-6736(21)00590-0
45. Itoh H, Kaneko H, Okada A, Yano Y, Morita K, Seki H, et al. Fasting plasma glucose and incident colorectal cancer: analysis of a nationwide epidemiological database. J Clin Endocrinol Metab. (2021) 106:e4448–e58. doi: 10.1210/clinem/dgab466
46. Teufel F, Seiglie JA, Geldsetzer P, Theilmann M, Marcus ME, Ebert C, et al. Body-mass index and diabetes risk in 57 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 685 616 adults. Lancet. (2021) 398:238–48. doi: 10.1016/S0140-6736(21)00844-8
Keywords: disease burden, metabolic risk factors, temporal trend, aging, China
Citation: Jin YZ, So H, Cerin E, Barnett A, Mubarik S, Hezam KA, Feng XQ, Wang ZY, Huang JJ, Zhong CW, Hayat KH, Wang F, Wu A-M, Xu SW, Zou ZY, Lim L-L, Cai J, Song YM, Tam L-S and Wu DZ (2023) The temporal trend of disease burden attributable to metabolic risk factors in China, 1990–2019: An analysis of the Global Burden of Disease study. Front. Nutr. 9:1035439. doi: 10.3389/fnut.2022.1035439
Received: 02 September 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Devin Wahl, Colorado State University, United StatesReviewed by:
Qingyu Dou, Sichuan University, ChinaSuman Chakrabarty, West Bengal State University, India
Copyright © 2023 Jin, So, Cerin, Barnett, Mubarik, Hezam, Feng, Wang, Huang, Zhong, Hayat, Wang, Wu, Xu, Zou, Lim, Cai, Song, Tam and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongze Wu,  ZG9uZ3plX3d1QDE2My5jb20=; Lai-shan Tam,
ZG9uZ3plX3d1QDE2My5jb20=; Lai-shan Tam,  bHN0YW1AY3Voay5lZHUuaGs=
bHN0YW1AY3Voay5lZHUuaGs=
 Yingzhao Jin
Yingzhao Jin Ho So
Ho So Ester Cerin
Ester Cerin Anthony Barnett
Anthony Barnett Sumaira Mubarik
Sumaira Mubarik Kamal Hezam
Kamal Hezam Xiaoqi Feng
Xiaoqi Feng Ziyue Wang10,11
Ziyue Wang10,11 Junjie Huang
Junjie Huang Chenwen Zhong
Chenwen Zhong Khezar Hayat
Khezar Hayat Fang Wang
Fang Wang Ai-Min Wu
Ai-Min Wu Suowen Xu
Suowen Xu Zhiyong Zou
Zhiyong Zou Lee-Ling Lim
Lee-Ling Lim Jiao Cai
Jiao Cai Yimeng Song
Yimeng Song Lai-shan Tam
Lai-shan Tam Dongze Wu
Dongze Wu