- 1Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 2Nutrition and Metabolic Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 3Nutrition Department, Faculty of Paramedicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 4Division of Immunology and Allergy, Department of Pediatrics, Abuzar Children's Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 5Department of Biostatistics and Epidemiology, School of Public Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 6Air Pollution and Respiratory Disease Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Existing asthma treatments are associated with side effects and limitations, which has led to an interest in alternative and complementary therapies. Given the anti-inflammatory properties of pomegranate, the present study aimed to determine the impact of pomegranate extract supplementation on lung function parameters evaluated through spirometry, high-sensitivity C-reactive protein (hs-CRP), pro-oxidant antioxidant balance, and interleukin 35) (IL-35) in participants with mild and moderate allergic asthma (based on forced expiratory volume in 1 second (FEV1) and clinical symptoms). Participants with mild and moderate allergic asthma (n = 64) were randomly assigned to two groups: the intervention group, which received two pomegranate extract capsules (500 mg/day), or the control group for eight weeks. Also, the physician prescribed similar drugs to the participants in the study. Independent samples T-test and Mann–Whitney U were used to compare the quantitative outcomes between the intervention group and the comparison group. The Wilcoxon test and the paired T-test were applied for within-group comparisons. A p-value <0.05 was considered significant. At the end of the study, the change levels of IL-35 in the intervention group increased significantly compared to the control group. In terms of the lung function parameters, FEV1/ forced vital capacity (FVC) (FEV1/FVC) ratio enhanced significantly in the intervention group compared to the control group. Also, the pomegranate extract significantly improved forced expiratory flow 25–75% (FEF25−75%), FEV1/FVC ratio, and FEV1 in the intervention group. No significant changes in FEV1 values were observed between the two groups at the end of the study. Also, no significant changes were seen in other indicators. It seems that pomegranate extract can improve lung function parameters and IL-35 expression in mild and moderate allergic asthma.
Clinical trial registration: https://www.irct.ir/trial/45612; identifier: IRCT20200205046384N1.
Introduction
Asthma is a multifaceted and complicated illness caused by the interaction between environmental exposure, host factors, and genetic susceptibility (1). It is one of the most common chronic respiratory diseases (2), affecting more than 300 million people worldwide (3).
Inflammation, increased airway responsiveness, remodeling alternations, excessive mucus secretions, and reversible airway obstruction are the prominent features of asthma (4, 5). Immune system cells and airway structural cells emit a variety of cytokines and inflammatory mediators, which contribute to airway inflammation in people with asthma (5). Continuous respiratory tract inflammation is accompanied by a mucosal influx of mast cells, T lymphocytes, eosinophils, and the diffusion of lipid mediators and pro-inflammatory cytokines (6). The airways produce a diversity of inflammatory mediators due to exposure to environmental oxidants, including reactive oxygen species (7). Pro-oxidant antioxidant balance (PAB) assessment simultaneously evaluates pro-oxidant load and antioxidant capacity, showing us an indicator of redox status (8).
One of the most prominent indicators of the inflammatory process is C-reactive protein (CRP) (9). The serum high-sensitivity CRP (hs-CRP) levels can represent levels of CRP that are not detectable using usual methods and can assess even mild inflammation in many diseases, including cardiovascular disease and diabetes (10). The association between asthma control and serum levels of hs-CRP in adult patients has been investigated, and the results have been encouraging (11, 12). In addition, interleukin 35 (IL-35) appears to suppress asthma's allergic inflammation (13). Recent evidence suggests the role of IL-35 in the pathophysiology of asthma and considers IL-35 to be a critical factor in regulating cellular relationships, differentiation, and inflammation (13).
Spirometry is one of the lung function tests to evaluate the response of patients with asthma to treatment, and clinical symptoms (14). Among the different variables measured by spirometry test, forced expiratory volume in 1 second (FEV1), indicating the severity of airway obstruction, is the most commonly used (14, 15) and also the most reproducible pulmonary function parameter (15). The severity of asthma symptoms is categorized as mild, moderate, and severe, based on FEV1 values (14).
The health benefits of pomegranate, as a rich source of polyphenols, in animals and humans have been extensively studied (16–20). It has been found that polyphenols have anti-inflammatory and antioxidant features in the human body (21). According to a study on the impact of ellagic acid (one of the main ingredients of pomegranate) on asthmatic mice, the results showed that ellagic acid had significant anti-inflammatory activity in asthma and might be regarded as a therapeutic agent for allergic asthma (22). In a study that examined the effect of pomegranate juice supplementation on inflammatory markers in patients with diabetes, it was demonstrated that taking 250 mL of pomegranate juice daily for 12 weeks led to a significant reduction in IL-6 and serum hs-CRP levels (23).
The significant increase in asthma incidence in the last few decades and the geographical variation in both the magnitude of the increases and base prevalence rates confirm the hypothesis that environmental alterations are involved in the present asthma epidemic (24).
The incidence of asthma in Iran is reported to be higher than the global and regional average due to the transition to urban and industrial life, specific climatic conditions, and industrial pollution in the country. According to the Iranian Asthma and Allergy Association, the prevalence of asthma in Iran is between 5 and 15% (25), while in Ahvaz, the number of people with asthma is more than twice the national average (26).
In general, given that asthma exerts a significant effect not only on the quality of life related to a person's health status but also through absenteeism in the workplace and early retirement, it will impose high costs on the economy and society (27). Also, existing asthma treatments are associated with side effects and limitations, which has led to an interest in alternative and complementary therapies (28). Therefore, given the anti-inflammatory properties of pomegranate, the present study aimed to determine the impact of pomegranate extract on the lung function parameters evaluated through spirometry, hs-CRP, PAB, and IL-35 in participants with mild and moderate allergic asthma.
Methods
Design of trial
The current study was approved by the Ahvaz Jundishapur University of Medical Sciences ethics committee (Registration No. IR.AJUMS.REC.1398.905) and registered at the Iranian Registry of Clinical Trials (IRCT20200205046384N1). The present study applied a randomized controlled trial using a double-blind design.
The formula for comparing averages with β = 0.2, α = 0.05, S1 = 29.8, S2 = 0.71, μ1 = 16.13, μ2 = 0.9 was applied to compute the sample size considering the PAB variable levels based on a previous study (29):
According to the formula mentioned above, at least 30 patients were required to participate in the intervention and control groups. Assuming 15% removal or violation of protocols, 35 people were assigned to each group. The participants signed an informed consent form at the beginning of the study. The researchers randomly classified participants using computer-generated blocked randomization based on asthma severity and clinical symptoms (prepared by a biostatistics specialist). To follow the double-blind scheme, a person was asked to number the capsule bottles. Each participant was given a code based on the permuted block. Each participant completed a questionnaire on demographic information and personal characteristics, such as age and age of onset of asthma, at the beginning and end of the study. Also, participants' weight, type, and dose of the drugs used were recorded at the beginning and end of the study. Height was also measured at the beginning of the study. Furthermore, participants in both groups were asked not to alter their physical activity and energy received throughout the study. Physical activity was assessed at the beginning and end of the study through the international physical activity questionnaire. Allergic asthma was confirmed by measuring the total serum immunoglobulin E (IgE) levels. Since the serum levels of IgE are more reliable than other tests for allergic asthma, such as a skin prick test, we used the serum levels of IgE to confirm allergic asthma (30). The serum levels of IgE ≥ 30 international units (IU) are known as allergic patients (31). Therefore, individuals with serum IgE levels ≥ 30 participated in the present study. The questionnaires were completed, and blood samples were taken at the beginning and end of the study (after 8 weeks). Participants were asked not to consume pomegranate juice, pomegranate paste, and pomegranate-containing products throughout the study. They were also asked to avoid certain foods if they were allergic to them, not to eat processed foods such as sausages, and not to take nutritional supplements. Moreover, to evaluate the participants' diet (total calories and received macronutrients) at the beginning and end of the study, the 24-h dietary recall on 3 days (2 weekdays and 1 weekend day) was recorded. Participants should not suffer from any disease other than asthma and should not take any medication other than asthma medications. The physician prescribed similar drugs to the participants in the study.
Regular use of the capsules was followed up by a phone call, and the participants were asked to return the capsule bottles at the end of the study.
Participants
The research participants comprised 30 men and 40 women, ranging from 18 to 65 years. Participants with persistent allergic asthma were categorized into two groups (mild and moderate) based on their severity (FEV1 ≥ 60 %) and clinical symptoms using the Global Initiative for Asthma (GINA) diagnostic criteria for asthma by asthma and allergy specialist. In mild persistent asthma, symptoms are experienced more than once a week and less than once a day. The disease's symptoms may affect the patient's activity and sleep. Experiencing nocturnal symptoms occurs more than twice a month, and FEV1 is ≥ 80%. In moderate persistent asthma, symptoms are experienced daily and may affect the patient's activity and sleep. Experiencing nocturnal asthma symptoms occurs more than once a week, and FEV1 is 60–80% (14).
Participants were selected from asthma and allergy center in Ahvaz, Iran. A description of the trial was given to the participants, and their written consent was obtained to participate in the study. The inclusion criteria of the research were people aged 18–65 years with persistent allergic asthma (mild and moderate), IgE ≥ 30 IU, and body mass index below 30 kg/m2. We did not include unwillingness, lactation, pregnancy, taking mineral/multivitamin supplements during the last 2 months, malignancy, smoking, diabetes, other pulmonary diseases, and autoimmune diseases.
Intervention
The pomegranate extract capsule and the placebo (rusk powder) were prepared by the Institute of Medicinal Plants, Karaj, Alborz Province, Iran. The pomegranate extract capsule and the placebo were identical in size and color. Each capsule of pomegranate extract (consumed twice a day for 8 weeks) contained 250 mg of pomegranate seed extract, 2.1 μg of ellagic acid, 118.4 μg of punicalagin alpha, and 53 μg of punicalagin beta (measured using the high-performance liquid chromatography method). Ghavipour et al. showed that consuming 250 mg of pomegranate extract twice a day for 8 weeks reduced oxidative stress and some blood biomarkers of inflammation in patients with rheumatoid arthritis (20). Therefore, in the study, we applied the same duration of follow-up in both study arms.
Outcomes
The measurement of PAB, hs-CRP, IL-35, and spirometry tests involved the primary outcomes of this research at the beginning and the end of the 8th week.
Blood sample collection
At the beginning of the study and the end of the 8th week, venous blood samples (6 mL) were taken from all participants. The centrifuged blood samples were stored at – 80°C in a freezer.
Laboratory analyses
IL-35 concentration and serum hs-CRP levels were measured by the ZellBio enzyme-linked immunosorbent assay (ELISA) kit (made in Germany) and the CRP high-sensitive ELISA (product code: DM E-4600, brand: LDN), respectively.
PAB assessment
A corrected PAB was used based on a method explained in a previous study (32). To prepare a standard solution, different ratios of 250 μM of hydrogen peroxide (0–100%) were mixed with uric acid (3 mM) in NaOH (10 mM). Tetramethylbenzidine (TMB) powder (60 mg) was dissolved in dimethyl sulfoxide (DMSO) (10 mL). To ready TMB cation, TMB/DMSO (400 μL) was mixed in acetate buffer (20 mL, pH 4.5, 0.05 M), and then fresh chloramine T solution (70 μL, 100 mM) was added to this 20 mL. The solution was blended finely and incubated at room temperature (in a dark place) for 2 h. Subsequently, a solution of peroxidase enzyme (25 units) was added to 20 mL of a cationic solution of TMB and distributed as 1 mL before storage at – 20°C. To provide the TMB solution, TMB/DMSO (200 μL) was mixed in acetate buffer (10 mL, 0.05 M, pH 5.8), and by mixing TMB cation (1 mL) with TMB solution (10 mL), the working solution was provided. The working solution was incubated in a dark environment at room temperature for 2 min and then applied instantly. Next, 10 μL of each sample, blank (distilled water) or standard, was blended with the working solution (200 μL) in each well and incubated in a dark environment at 37°C for 12 min. At the end of the incubation, 2N hydrogen chloride (100 μL) was added to each well and assessed by an ELISA reader (450 nm, reference wavelength of 570 nm or 620 nm). A standard curve was obtained from the standard sample values.
Hamidi-Koliakos (HK) units were applied to express PAB values, indicating the percentage of hydrogen peroxide in the standard solution. According to the values acquired from the above-mentioned standard curve, unknown sample values were evaluated.
Lung function
Asthma is clinically recognized via physical examination, patient's history, and spirometry tests. Spirometry tests are useful tools to assess lung function to diagnose asthma and follow the treatment process of the disease (33). Also, spirometry tests show an accurate picture of the asthma severity and the degree of airway obstruction (15). To evaluate the impact of pomegranate extract on the spirometry tests, forced expiratory flow 25–75% (FEF25 − 75%), forced vital capacity (FVC), FEV1 and FEV1/FVC ratio were evaluated at the beginning and end of the study. Before performing spirometry, the patient's identification was checked, their height without shoes or boots and weight were measured, and their age and sex were recorded. Measurement posture was as follows: the participants should sit straight and put their feet on the ground. Also, they were asked to wear loose clothing. We asked the patients not to use long-acting bronchodilators and other bronchodilators 24 and 8 h before the spirometry test, respectively. A Spirolab spirometry device (MIR Company, Italy) was used to evaluate the lung function parameters.
Statistical analysis
Statistical analysis was performed using the SPSS software version 22 (SPSS Inc. Chicago, IL, USA). The normality of continuous variables was examined using Shapiro-Wilk's W-test. Continuous variables are reported as mean (confidence interval (CI) 95%). Categorical data are expressed as numbers (percentages). Independent samples T-test or Mann–Whitney U was used to compare PAB, FEV1/FVC ratio, FVC, FEF25 − 75%, FEV1, IL-35, and serum hs-CRP levels between the intervention group (pomegranate extract supplementation) and comparison group (control), whenever needed. The Wilcoxon test and the paired T-test were applied for within-group comparison. Also, the False Rate Discovery approach was performed for correction by multiple comparisons. Moreover, subgroup analysis was done based on asthma severity. A p-value <0.05 was considered significant.
Results
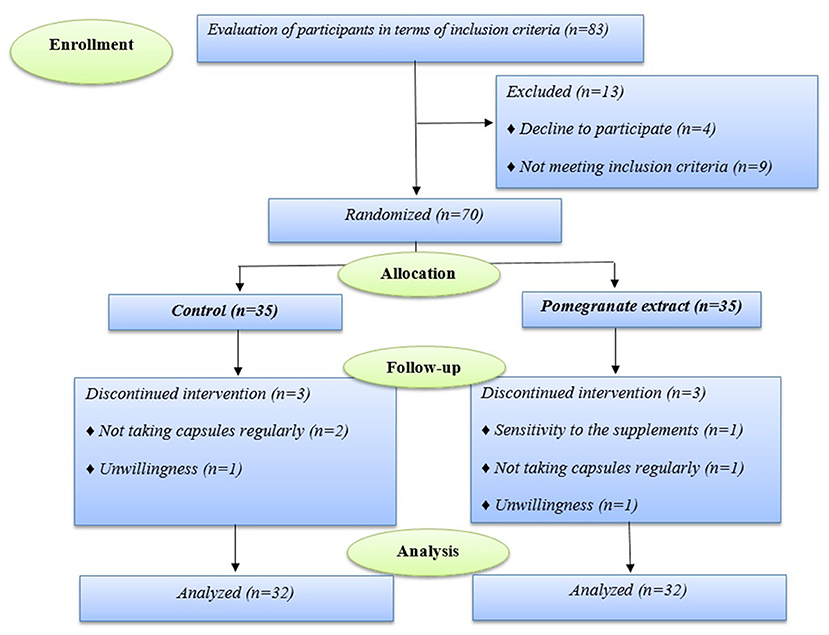
During the follow-up period, 64 of 70 participants who enrolled in the trial completed the study. Six of the enrolled ones discontinued the study (in the pomegranate group, one subject because of sensitivity to the supplements, one person did not take capsules regularly, and one person was excluded from the study due to unwillingness, and in the control group, two subject due to did not take capsules regularly, and one person was excluded because of unwillingness) (Figure 1).
Baseline characteristics
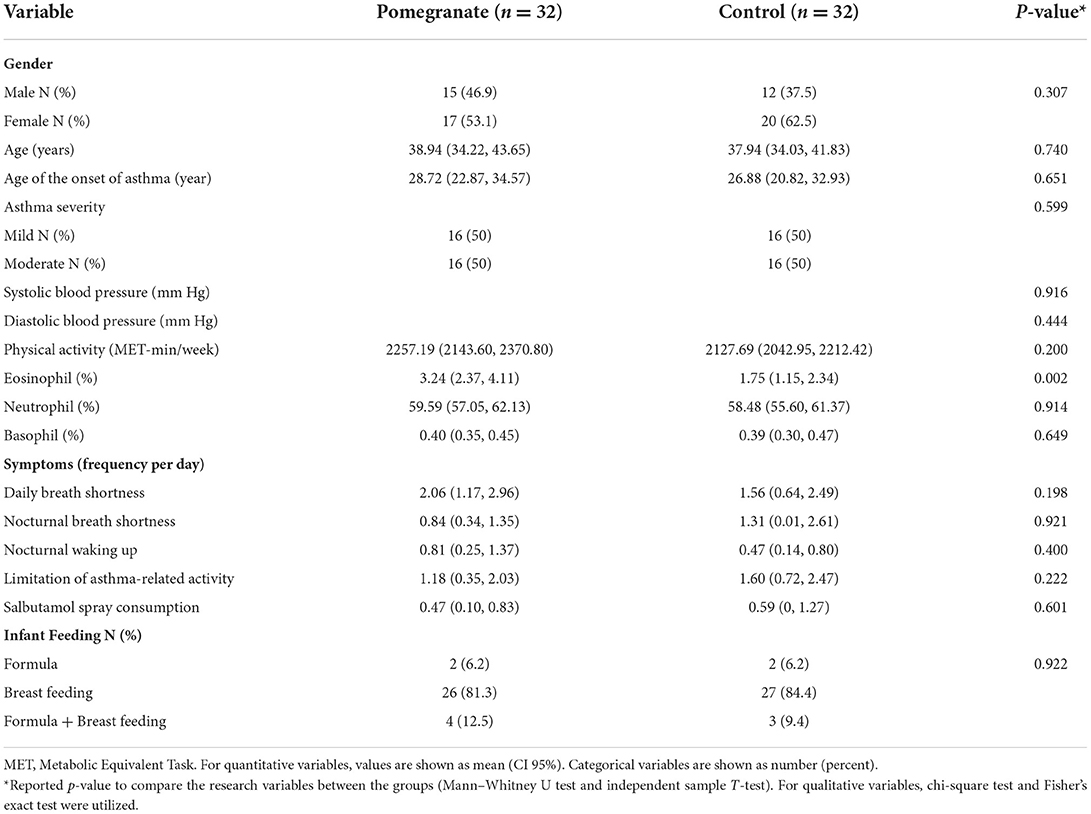
The mean and standard deviation of participants' age was 38.44 ± 11.90 years. In addition, 50% of the participants had mild asthma, and the rest had moderate asthma. Men and women constituted 42.18 and 57.81% of the sample, respectively. No statistically significant differences in dietary and anthropometric variables (data not reported), asthma severity, physical activity, and clinical features were observed between the two groups, as indicated in Table 1 (p > 0.05). Furthermore, no significant differences were observed between the two groups regarding the medication taken (Symbicort, Budesonide, Tiova, and Salbutamol spray) and the age of onset of asthma (p > 0.05).
IL-35, hs-CRP, and PAB
The results of IL-35 and serum hs-CRP levels are presented in Figures 2–5 and Table 2. At the beginning of the study, the differences between the intervention group and the control group were not statistically significant based on IL-35 and serum hs-CRP levels (p = 0.055 and p = 0.277, respectively). At the end of the study, there was no statistically significant difference between the two groups in terms of IL-35 (p = 0.172). However, the change levels of IL-35 between the two groups were statistically significant (p = 0.026). In terms of IL-35, in the intervention group, a statistically significant increase was seen when the data at the beginning and end of the study were compared (p = 0.031).
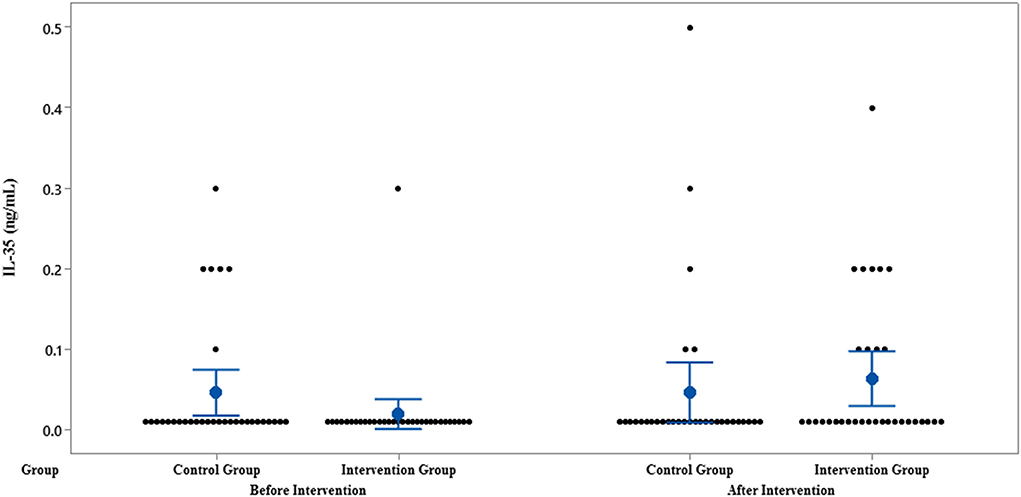
Figure 2. Interval plot of IL-35 pre- and post-intervention with 95% CI for the mean plus the dots for IL-35 values, in each group.
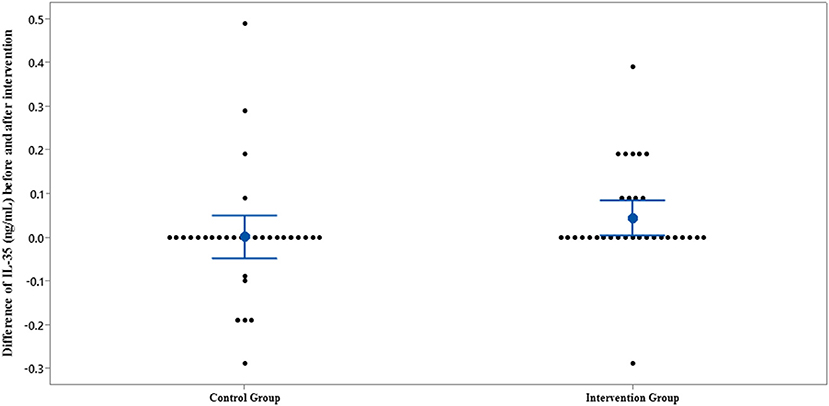
Figure 3. Interval plot of difference between IL-35 pre- and post-intervention with 95% CI for the mean plus the dots for IL-35 values, in each group.
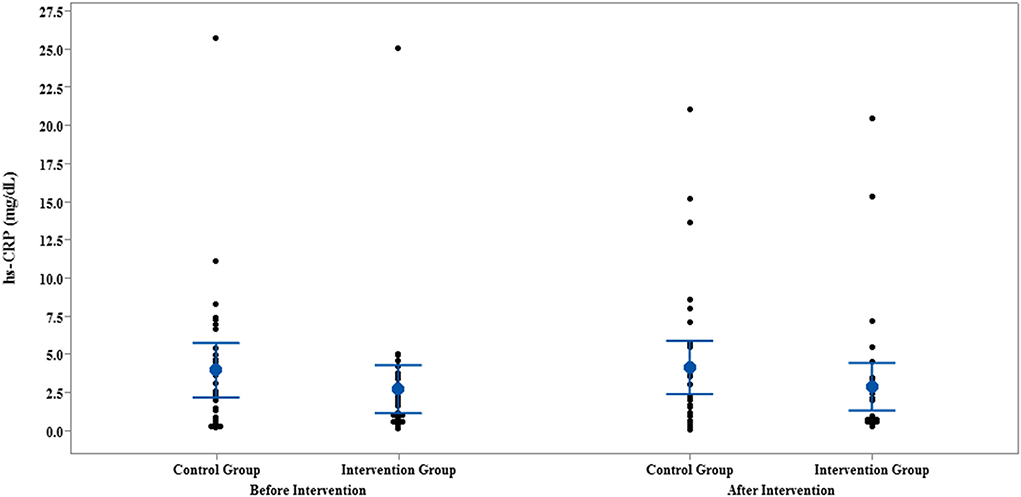
Figure 4. Interval plot of hs-CRP pre- and post-intervention with 95% CI for the mean plus the dots for hs-CRP values, in each group.
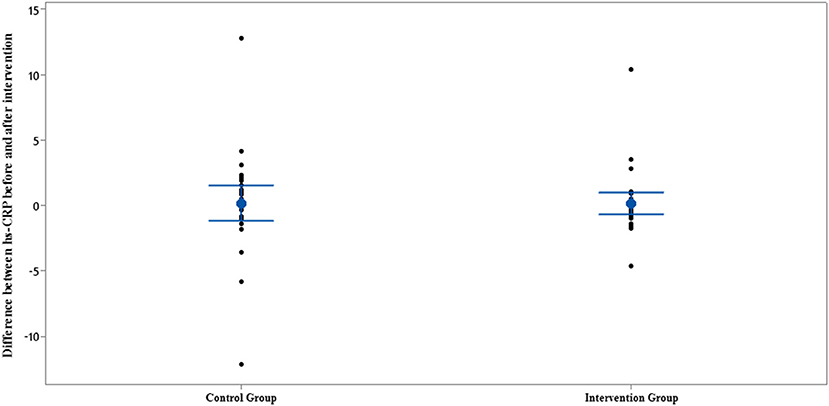
Figure 5. Interval plot of difference between hs-CRP pre- and post-intervention with 95% CI for the mean plus the dots for hs-CRP values, in each group.
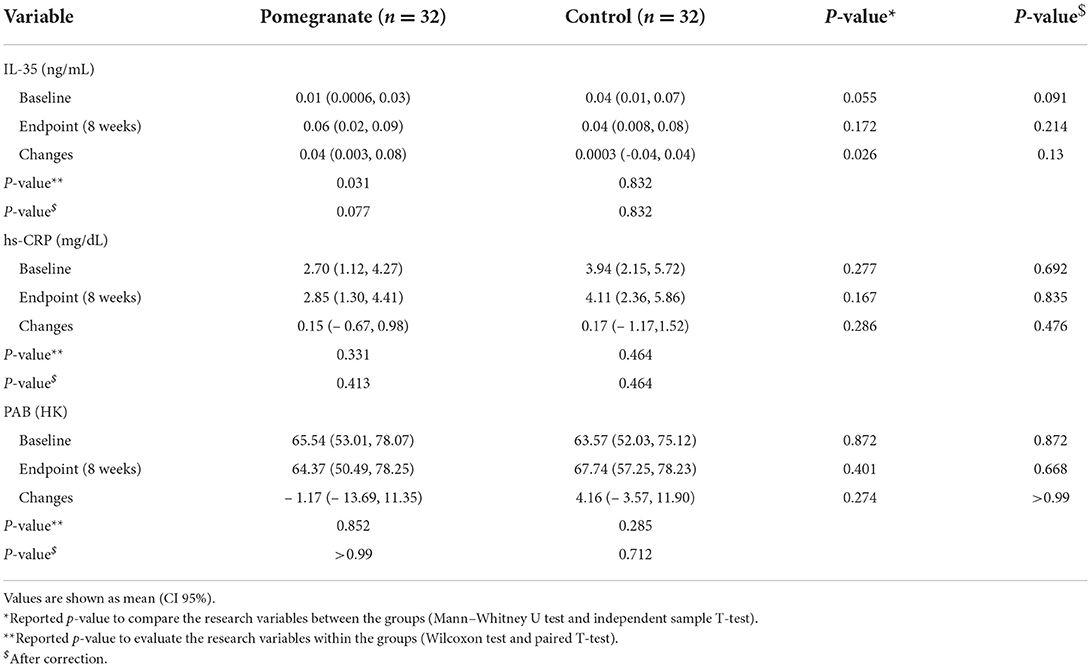
Table 2. Comparison of IL-35, hs-CRP and PAB levels between the two studies groups (intervention/control).
In terms of serum hs-CRP levels, at the end of the study, no statistically significant differences were detected in the intra/intergroup comparison (p > 0.05).
The comparison of PAB in the control and intervention groups is presented in Figures 6, 7 and Table 2. As can be seen from Figures 6, 7 and Table 2, in terms of PAB, there were no statistically significant differences at the beginning and end of the study between the control and intervention groups (p = 0.872 and p = 0.401, respectively).

Figure 6. Interval plot of PAB pre- and post-intervention with 95% CI for the mean plus the dots for PAB values, in each group.
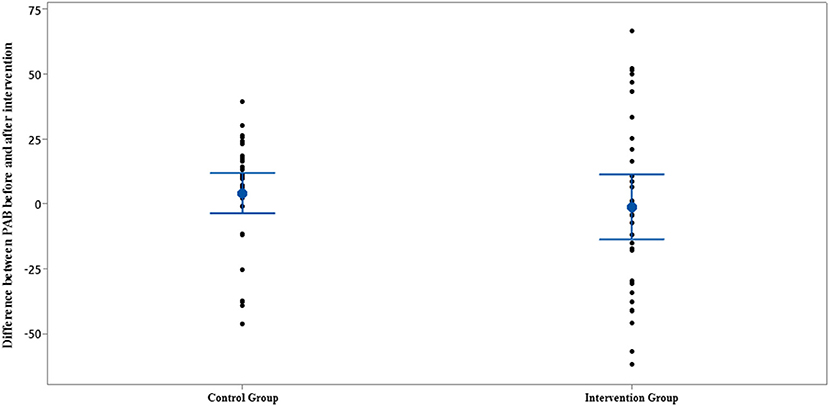
Figure 7. Interval plot of difference between PAB pre- and post-intervention with 95% CI for the mean plus the dots for PAB values, in each group.
The results of subgroup analysis based on asthma severity showed that the changes in IL-35 were statistically significant only in the intervention group with mild asthma severity (p = 0.03). Also, the change levels of IL-35 were statistically significant in mild asthma severity between the intervention and control groups (p = 0.02). Regarding hs-CRP and PAB, no significant differences were observed in both mild and moderate asthma severity (p > 0.05) (Table 3).
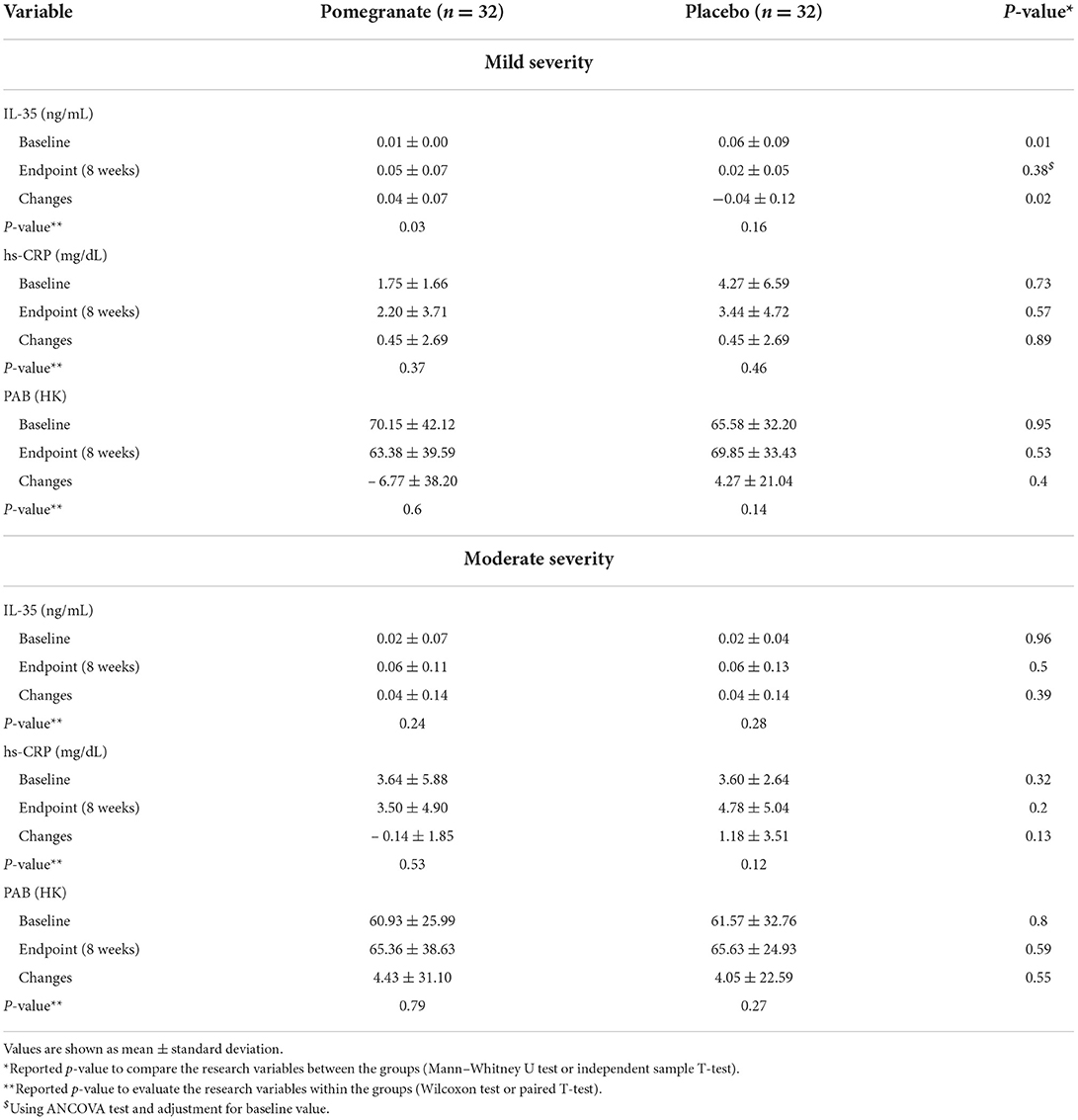
Table 3. Comparison of IL-35, hs-CRP and PAB levels between the two studies groups (intervention/control).
Lung function
FEF25 − 75%, FEV1/FVC, FEV1, and FVC were examined at the beginning and end of the study. The results indicated that FEF25 − 75%, FEV1/FVC ratio, and FEV1 increased in the intervention group (p = 0.012, p = 0.001, and p = 0.031, respectively). Supplementation with pomegranate extract could only exert a statistically significant difference in FEV1/FVC ratio when comparing the control and intervention groups (p = 0.028). Additionally, the levels of change in the FEV1/FVC ratio between the two groups were statistically significant (p = 0.023) (Table 4).
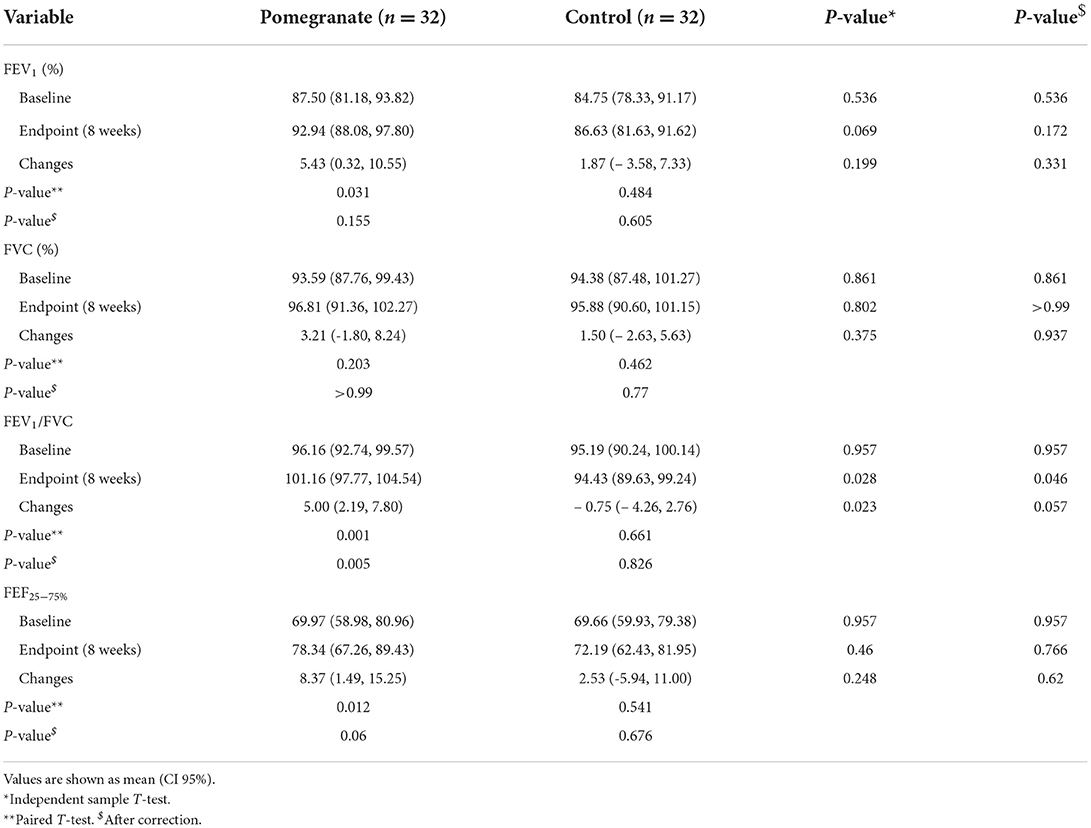
Table 4. The comparison of changes in the spirometry tests between the intervention and control groups.
Based on the asthma severity, there were statistically significant changes in FEV1, FEV1/FVC, and FEF25 − 75% in the intervention group with mild asthma severity (p = 0.02, p = 0.006, and p = 0.03, respectively). Also, the change levels in FEV1/FVC ratio were significant between the intervention and control groups in mild asthma severity (p = 0.008) (Table 5).
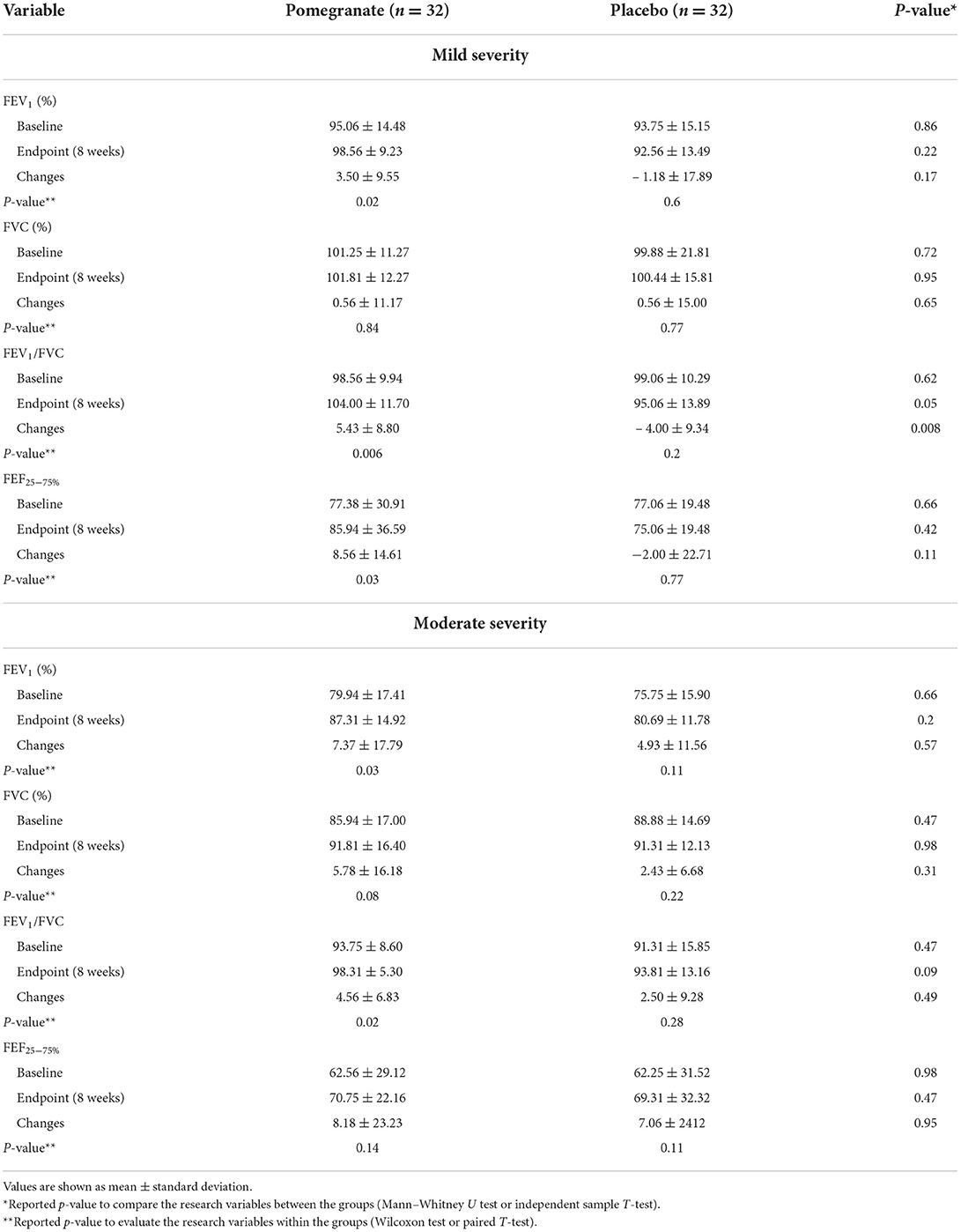
Table 5. The comparison of changes in the spirometry tests between the intervention and control groups.
In moderate asthma severity, there were statistically significant changes in FEV1 and FEV1/FVC in the intervention group (p = 0.03 and p = 0.02, respectively). However, no statistically significant differences were observed between the two groups in terms of spirometry tests in moderate asthma severity (p > 0.05) (Table 5).
Discussion
The effectiveness of ellagic acid, one of the main components of pomegranate, in alleviating allergic inflammation of the airways was shown in a mouse model (34). Asthma is an inflammatory illness that causes the lung cells to be overstimulated (35). It is characterized by increased airway responsiveness, inflammation, and structural changes. Many cytokines and other inflammatory mediators are released by immune system cells and airway structural cells, leading to respiratory tract inflammation in patients with asthma (5). Few studies have investigated the association between components of pomegranate and inflammatory factors. Bachoual et al. examined the influence of pomegranate peel extract on inflammation of lungs in mice. According to their results, pomegranate peel can inhibit myeloperoxidase (MPO) activity and thus decrease lung inflammation (36).
To the best of our knowledge, this study was the first double-blind, randomized trial on the impacts of pomegranate extract on lung function parameters, IL-35, hs-CRP, and PAB in patients with mild and moderate persistent allergic asthma. Our findings indicated that 500 mg/day of pomegranate supplementation ameliorated IL-35 and some spirometry test parameters in the intervention group.
At the end of the current research, a significant increase in IL-35, which has anti-inflammatory properties, was found in the intervention group. In 2007, IL-35 was recognized as an anti-inflammatory and immunosuppressive cytokine secreted by regulatory T cells (37). This interleukin acts on the original T cells, turning them into a family of regulatory T cells (Treg) called IL-35-producing Treg (iTR35). These cells start to secrete IL-35. IL-35 decreases the immune response in allergic diseases, including asthma (37, 38). A study found that in mice whose expression of IL-35 was suppressed, the production of IL-17, hypersensitivity of the allergic airways, and the number of macrophages, neutrophils, and eosinophils were increased, probably indicating the anti-inflammatory role of this cytokine (39).
Wang et al. indicated that increased oxidative stress could induce apoptosis in Treg cells (40). As previously mentioned, pomegranate is rich in antioxidants. As a result, pomegranate extract may effectively reduce the apoptosis of Treg cells and increase IL-35 by reducing oxidative stress.
Evidence suggests that various mechanisms involve eosinophils in asthma exacerbation, including interaction with neutrophils (41). It has also been shown that ellagic acid effectively decreases the number of neutrophils and eosinophils in mouse models (42). In this study, it was also shown that pomegranate extract caused a significant decrease in the levels of changes in eosinophils and neutrophils in the intervention group compared to the control group (unpublished data).
Some fruits, including pomegranate, contain polyphenols such as ellagic acid and ellagitannin, and it was discovered that these fruits are rich in antioxidant and anti-inflammatory properties (43). Rasheed et al. found that pomegranate extract suppresses expression of pro-inflammatory cytokines in human cells by inhibiting nuclear factor (NF)-κB and mitogen-activated protein (MAP) kinase (44). Taheri Rouhi et al. proved that pomegranate juice and pomegranate seed could decrease inflammatory biomarkers such as tumor necrosis factor-α (TNF-α) and IL-6 in rats with diabetes mellitus (45).
As mentioned previously, supplementation with pomegranate extract did not significantly influence serum hs-CRP levels in the present study. A study on the impact of unsweetened pomegranate juice (240 mL) for 2 months on people with type 2 diabetes showed that pomegranate juice had no significant effect on serum hs-CRP levels (46). The findings of another study on the influences of pomegranate supplementation on the metabolic status of patients with type 2 diabetes proved that pomegranate supplementation did not exert a significant effect on serum hs-CRP levels (47). However, in a study on the impact of pomegranate juice (250 mL for 12 weeks) on inflammatory factors in patients with diabetes, Sohrab et al. showed that pomegranate juice consumption could significantly reduce serum hs-CRP levels (23). One possible mechanism of the influence of pomegranate on the reduction of serum hs-CRP levels is the expression suppression of pro-inflammatory cytokines by pomegranate, which is rich in polyphenols such as punicalagin and ellagic acid (44). One of the reasons for the difference between the result of Sohrab et al. and the current study may be the duration of the two studies (12 vs. 8 weeks). Despite significant changes in lung function parameters in the intervention group and significant changes in FEV1/FVC ratio between the two groups, no significant changes in serum hs-CRP were observed. A study showed no significant difference in serum hs-CRP levels in patients with different levels of asthma control based on GINA and FEV1. Additionally, it showed no relationship between the degree of systemic inflammation estimated by hs-CRP and other clinical indicators of asthma, such as FEV1 and GINA criteria (48). Therefore, asthma may be controlled, or spirometry tests may be improved, but the serum hs-CRP levels may not be changed. In the present study, as mentioned above, some spirometry tests improved in the intervention group, but hs-CRP levels did not show significant changes. These findings are supported by previous studies (49–51). Furthermore, the present study proved a small but significant change in IL-35, an anti-inflammatory factor, between the two groups; however, no significant change in serum hs-CRP levels was found. A study by Oflar et al. showed no significant correlation between IL-35 and serum hs-CRP (52).
Tannins and flavonoids are the other pomegranate compounds with antioxidant properties (53). Oxidative stress can trigger and exacerbate airway inflammation in patients with asthma (54). Our findings demonstrated that pomegranate supplementation (500 mg per day) for 8 weeks did not cause a significant decrease in the PAB values. Conversely, in terms of the within-group comparison, there was a decrease in PAB in the intervention group; however, it was not statistically significant. One reason for not observing a significant change in this parameter is that PAB may be an acute indicator of oxidative stress rather than a long-term indicator (32). Furthermore, Nobakht et al. demonstrated that the oxidation level is not related to the severity of the disease defined based on spirometry tests (55). To the best of our knowledge, no study has been conducted on the impact of pomegranate extract on PAB in people with asthma; however, in a study on the effect of 240 mL of sugar-free pomegranate juice on antioxidant factors in type 2 diabetes for 8 weeks, it was shown that the consumption of pomegranate juice had no significant effect on total antioxidant capacity (56). Conversely, a study on the impact of pomegranate juice (100 mL, 3 times/week for 1 year) on hemodialysis showed that it could reduce oxidative stress (57). According to the study mentioned above, long-term consumption of pomegranate can decrease oxidative stress.
As mentioned above, supplementation with pomegranate extract improved lung function parameters such as FEF25 − 75%, FEV1, and FEV1/FVC in patients with allergic asthma. Compared with the control group, supplementation only improved the FEV1/FVC ratio in the intervention group. In addition to FEV1, airway obstruction can be detected using a decrease in FEV1/FVC ratio (58). In this study, a significant increase in FEV1/FVC ratio in the intervention group indicates a reduction in airway obstruction. To date, no study has investigated the influence of pomegranate extract on spirometry tests in patients with asthma; however, a study showed that antioxidant vitamins such as vitamins C, E, and beta-carotene could affect lung health. The present study found that people in the lower quartiles of the serum antioxidant vitamins had less FEV1 and FVC compared to those in the upper quartiles in terms of these vitamins (59). Another study discovered that a daily intake of 40 mg more vitamin C was related to 25 mL more FEV1 and 23.3 mL more FVC (60). Dietary intake, as the primary source of non-enzymatic antioxidants, including vitamin C, seems to be correlated with a reduced risk of obstructive pulmonary disease, and an increase in vitamin C intake of 50 mg per day in men is associated with more FEV1 by 70.7 mL (61). Pomegranate contains antioxidants and is an excellent source of vitamin C, which is likely why it can improve spirometry test parameters.
Strengths of the present study included a randomized, double-blind, placebo-controlled trial design, the first randomized clinical trial to evaluate the effects of pomegranate extract in allergic asthma in a human model, and a stratified blocked randomization design based on asthma severity. The study duration was one of the limitations of the present study. Also, other inflammatory parameters such as TNF-α, IL-6, IL-4, IL-5 and IL-13 were not measured because of limited financial resources. Furthermore, we could not measure plasma polyphenol derivatives' levels of pomegranate extract in the participants.
Conclusion
In conclusion, it seems that pomegranate extract can improve lung function parameters and IL-35 expression. To confirm the results of this study, it is recommended that studies with a longer duration should be conducted. It is also recommended that other inflammatory pathways (such as NF-κB, MAP kinase, and MPO activity) through which pomegranate extract may improve lung function parameters in asthma should be investigated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences has approved this clinical trial (No. IR.AJUMS.REC.1398.905). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZS and SH have made substantial contributions to conceptualize and to design the work. ZS and FA participated in the acquisition of data. SH was supervisor of this study. Medical experts of the current research were FA and MH. The statistical data were analyzed by EM. The manuscript was written by ZS and SH and edited by MZ. All authors contributed to the article and approved the submitted version.
Funding
The sponsor of this study was Vice Chancellor for Research of Ahvaz Jundishapur University of Medical Sciences [grant number: NRC-9823]. This article is extracted from the Ph.D. thesis of the ZS of the present study.
Acknowledgments
The researchers appreciate Ahvaz Jundishapur University of Medical Sciences for its financial aid and all the participants, and those who helped us in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatrics. (2019) 7:246. doi: 10.3389/fped.2019.00246
2. Park S-Y, Kim J-H, Kim H-J, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy Asthma Immunol Res. (2018) 10:387–96. doi: 10.4168/aair.2018.10.4.387
3. Boonpiyathad T, Sözener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. (2019) 46:101333. doi: 10.1016/j.smim.2019.101333
4. Ishmael FT. The inflammatory response in the pathogenesis of asthma. J Osteopathic Med. (2011) 111:11–7.
5. Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediat Inflammation. (2015) 2015:1–8. doi: 10.1155/2015/879783
6. Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther. (2018) 186:98–113. doi: 10.1016/j.pharmthera.2018.01.004
7. Dut R, Dizdar E, Birben E, Sackesen C, Soyer O, Besler T, et al. Oxidative stress and its determinants in the airways of children with asthma. Allergy. (2008) 63:1605–9. doi: 10.1111/j.1398-9995.2008.01766.x
8. Parizadeh SM, Azarpazhooh MR, Moohebati M, Nematy M, Ghayour-Mobarhan M, Tavallaie S, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. (2011) 46:333–40. doi: 10.1007/s11745-010-3517-x
9. Jat K, Sankar J, Lakshmy R, Kabra S. Role of High-Sensitivity C Reactive Protein (hs-CRP) in Assessement of Asthma Control in Children. (2021).
10. Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Osteopathic Med. (2005) 105:409–16.
11. Zietkowski Z, Tomasiak-Lozowska MM, Skiepko R, Mroczko B, Szmitkowski M, Bodzenta-Lukaszyk A. High-sensitivity C-reactive protein in the exhaled breath condensate and serum in stable and unstable asthma. Respir Med. (2009) 103:379–85. doi: 10.1016/j.rmed.2008.10.003
12. Qian FH, Zhang Q, Zhou LF, Liu H, Huang M, Zhang XL, et al. High-sensitivity C-reactive protein: a predicative marker in severe asthma. Respirology. (2008) 13:664–9. doi: 10.1111/j.1440-1843.2008.01314.x
13. Gao P, Su Z, Lv X, Zhang J. Interluekin-35 in asthma and its potential as an effective therapeutic agent. Mediat Inflammation. (2017) 2017:1–8. doi: 10.1155/2017/5931865
14. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respiratory J. (2008) 31:143–78. doi: 10.1183/09031936.00138707
15. Enright PL, Lebowitz MD, Cockroft DW. Physiologic measures: pulmonary function tests. Am J Respiratory Crit Care Med. (2012) 149:S9–S18. doi: 10.1164/ajrccm/149.2_Pt_2.S9
16. Seeram NP, Zhang Y, McKeever R, Henning SM, Lee R-p, Suchard MA, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food. (2008) 11:390–4. doi: 10.1089/jmf.2007.650
17. Mertens-Talcott S U, Jilma-Stohlawetz P, Rios J, Hingorani L. Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. (2006) 54:8956–61. doi: 10.1021/jf061674h
18. Makled MN, El-Awady MS, Abdelaziz RR, Atwan N, Guns ET, Gameil NM, et al. Pomegranate protects liver against cecal ligation and puncture-induced oxidative stress and inflammation in rats through TLR4/NF-κB pathway inhibition. Environ Toxicol Pharmacol. (2016) 43:182–92. doi: 10.1016/j.etap.2016.03.011
19. Sun W, Yan C, Frost B, Wang X, Hou C, Zeng M, et al. Pomegranate extract decreases oxidative stress and alleviates mitochondrial impairment by activating AMPK-Nrf2 in hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Sci Rep. (2016) 6:1–12. doi: 10.1038/srep34246
20. Ghavipour M, Sotoudeh G, Tavakoli E, Mowla K, Hasanzadeh J, Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur J Clin Nutr. (2017) 71:92–6. doi: 10.1038/ejcn.2016.151
21. Hosseini B, Saedisomeolia A, Wood LG, Yaseri M, Tavasoli S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: a randomized controlled clinical trial. Complement Ther Clin Pract. (2016) 22:44–50. doi: 10.1016/j.ctcp.2015.12.003
22. Zhou E, Fu Y, Wei Z, Yang Z. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. (2014) 5:2106–12. doi: 10.1039/C4FO00384E
23. Sohrab G, Nasrollahzadeh J, Zand H, Amiri Z, Tohidi M, Kimiagar M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: a randomized, placebo-controlled trial. J Res Med Sci Official J Isfahan Univ Med Sci. (2014) 19:215.
24. Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. (2009) 181:E181–90. doi: 10.1503/cmaj.080612
25. Mohajjel Aghdam A, Hasankhani H, Gharemohammadlu R, Esmaeily M. Relation of patients self-efficacy with control of asthma symptoms. J Gorgan Univ Med Sci. (2013) 15:70–76.
26. Raji H, Riahi A, Borsi SH, Masoumi K, Khanjani N, AhmadiAngali K, et al. Acute effects of air pollution on hospital admissions for asthma, COPD, and bronchiectasis in Ahvaz, Iran. Int J Chron Obstruct Pulmon Dis. (2020) 15:501. doi: 10.2147/COPD.S231317
27. Honkoop PJ, Loijmans RJ, Termeer EH, Snoeck-Stroband JB, van den Hout WB, Bakker MJ, et al. Symptom-and fraction of exhaled nitric oxide–driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. (2015) 135:682–88. doi: 10.1016/j.jaci.2014.07.016
28. MacRedmond R, Dorscheid DR. Conjugated linoleic acid (CLA): is it time to supplement asthma therapy? Pulmonary Pharmacol Therapeutics. (2011) 24:540–48. doi: 10.1016/j.pupt.2011.03.005
29. Nikbakht-Jam I, Khademi M, Nosrati M, Eslami S, Foroutan-Tanha M, Sahebkar A, et al. Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: a randomized, placebo-controlled clinical trial. Eur J Integr Med. (2016) 8:307–12. doi: 10.1016/j.eujim.2015.12.008
30. Sunyer J, Antó JM, Sabrià J, Roca J, Morell F, Rodríguez-Roisin R, et al. Relationship between serum IgE and airway responsiveness in adults with asthma. J Allergy Clin Immunol. (1995) 95:699–706. doi: 10.1016/S0091-6749(95)70175-3
31. Zilaee M, Hosseini SA, Jafarirad S, Abolnezhadian F, Cheraghian B, Namjoyan F, et al. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: a double-blind, randomized placebo-controlled trial. Respir Res. (2019) 20:1–11. doi: 10.1186/s12931-019-0998-x
32. Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F, et al. Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. (2008) 41:375–80. doi: 10.1016/j.clinbiochem.2007.12.008
33. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. (2007) 120:S94–S138. doi: 10.1016/j.jaci.2007.09.029
34. de Freitas Alves C, Angeli GN, Favarin DC, Lemos de Andrade E, Lazo Chica JE, Faccioli LH, et al. The effects of proresolution of ellagic acid in an experimental model of allergic airway inflammation. Mediat Inflam. (2013) 2013:1–9. doi: 10.1155/2013/863198
35. Bahraini M, Hosseini S, Cheraghian B, Haddadzadeh Shoushtari M. Effect of saffron on anti-inflammatory and oxidative stress in asthma. Int J Pharmaceutical Phytopharmacol Res. (2020) 10:179–84.
36. Bachoual R, Talmoudi W, Boussetta T, Braut F, El-Benna J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem Toxicol. (2011) 49:1224–8. doi: 10.1016/j.fct.2011.02.024
37. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. (2007) 450:566–9. doi: 10.1038/nature06306
38. Castellani ML, Anogeianaki A, Felaco P, Toniato E, De Lutiis M, Shaik B, et al. IL-35, an anti-inflammatory cytokine which expands CD4+ CD25+ Treg Cells. J Biol Regulat Homeostatic Agents. (2010) 24:131–35.
39. Whitehead GS, Wilson RH, Nakano K, Burch LH, Nakano H, Cook DN. IL-35 production by inducible costimulator (ICOS)–positive regulatory T cells reverses established IL-17–dependent allergic airways disease. J Allergy Clin Immunol. (2012) 129:207–215. doi: 10.1016/j.jaci.2011.08.009
40. Wang B, Sun J, Ma Y, Wu G, Shi Y, Le G. Increased oxidative stress and the apoptosis of regulatory T cells in obese mice but not resistant mice in response to a high-fat diet. Cell Immunol. (2014) 288:39–46. doi: 10.1016/j.cellimm.2014.02.003
41. Nakagome K, Nagata M. Involvement and possible role of eosinophils in asthma exacerbation. Front Immunol. (2018) 9:2220. doi: 10.3389/fimmu.2018.02220
42. Rogerio AP, Fontanari C, Borducchi É, Keller AC, Russo M, Soares EG, et al. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol. (2008) 580:262–70. doi: 10.1016/j.ejphar.2007.10.034
43. Danesi F, Ferguson LR. Could pomegranate juice help in the control of inflammatory diseases? Nutrients. (2017) 9:958. doi: 10.3390/nu9090958
44. Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. J Inflamm. (2009) 6:1–12. doi: 10.1186/1476-9255-6-1
45. Taheri Rouhi SZ, Sarker M, Rahman M, Rahmat A, Alkahtani SA, Othman F. The effect of pomegranate fresh juice vs. pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complement Altern Med. (2017) 17:1–13. doi: 10.1186/s12906-017-1724-1
46. Babaeian S, Ebrahimi-Mameghani M, Niafar M, Sanaii S. The effect of unsweetened pomegranate juice on insulin resistance, high sensitivity c-reactive protein and obesity among type 2 diabetes patients. J Ardabil Univ Med Sci. (2013) 13:7–15.
47. Jandari S, Hatami E, Ziaei R, Ghavami A, Yamchi AM. The effect of pomegranate (Punica granatum) supplementation on metabolic status in patients with type 2 diabetes: a systematic review and meta-analysis. J Complement Therapies Med. (2020) 52:102478. doi: 10.1016/j.ctim.2020.102478
48. Sigari N, Ghasri H. Correlation between hs-CRP and asthma control indices. Tanaffos. (2013) 12:44.
49. Kilic H, Karalezli A, Hasanoglu HC, Erel O, Ates C. The relationship between hs-CRP and asthma control test in asthmatic patients. Allergol Immunopathol. (2012) 40:362–7. doi: 10.1016/j.aller.2011.10.002
50. Melosini L, Dente FL, Bacci E, Bartoli ML, Cianchetti S, Costa F, et al. Asthma control test (ACT): comparison with clinical, functional, and biological markers of asthma control. J Asthma. (2012) 49:317–23. doi: 10.3109/02770903.2012.661008
51. Büyüköztürk S, Gelincik AA, Genç S, Koçak H, Öneriyidogan Y, Erden S, et al. Acute phase reactants in allergic airway disease. Tohoku J Exp Med. (2004) 204:209–13. doi: 10.1620/tjem.204.209
52. Oflar E, Sahin MH, Demir B, Ertugrul AS, Oztas DM, Beyaz MO, et al. Interleukin-35 levels in patients with stable coronary artery disease. Arq Bras Cardiol. (2022) 118:400–8. doi: 10.36660/abc.20200945
53. Zarfeshany A, Asgary S, Javanmard SH. Potent health effects of pomegranate. Adv Biomed Res. (2014) 3:1–8. doi: 10.4103/2277-9175.129371
54. Cho YS, Moon H-B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res. (2010) 2:183–87. doi: 10.4168/aair.2010.2.3.183
55. Nobakht M. Gh BF, Oskouie AA, Aliannejad R, Rezaei-Tavirani M, Tavallaie S, Baghban AA, et al. Pro-oxidant–antioxidant balance in Iranian veterans with sulfur mustard toxicity and different levels of pulmonary disorders. Drug Chem Toxicol. (2016) 39:362–6. doi: 10.3109/01480545.2015.1122033
56. Babaeian amini S, Ebrahimi Mameghani M, Niafar M. The effect of pomegranate juice (no added sugar) consumption on fast blood sugar, lipid profile, antioxidant factors in Type 2 diabetes Sci Magazine Yafte. (2016) 18:95–103.
57. Shema-Didi L, Sela S, Ore L, Shapiro G, Geron R, Moshe G, et al. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radical Biol Med. (2012) 53:297–304. doi: 10.1016/j.freeradbiomed.2012.05.013
58. Glady CA, Aaron SD, Lunau M, Clinch J, Dales RE, A. spirometry-based algorithm to direct lung function testing in the pulmonary function laboratory. Chest. (2003) 123:1939–46. doi: 10.1378/chest.123.6.1939
59. Schünemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. (2001) 163:1246–55. doi: 10.1164/ajrccm.163.5.2007135
60. Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, Lewis SA, et al. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med. (1995) 151:1383–7. doi: 10.1164/ajrccm.151.5.7735589
Keywords: asthma, high-sensitivity C-reactive protein, interleukin-35, lung function, pomegranate
Citation: Shateri Z, Hosseini SA, Abolnezhadian F, Maraghi E, Haddadzadeh Shoushtari M and Zilaee M (2022) Pomegranate extract supplementation improves lung function parameters and IL-35 expression in participants with mild and moderate persistent allergic asthma: A randomized, double-blind, placebo-controlled trial. Front. Nutr. 9:1026343. doi: 10.3389/fnut.2022.1026343
Received: 23 August 2022; Accepted: 26 September 2022;
Published: 18 October 2022.
Edited by:
Sara Manti, University of Messina, ItalyReviewed by:
Fernando Augusto Lima Marson, Sao Francisco University, BrazilYuquan Chen, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Shateri, Hosseini, Abolnezhadian, Maraghi, Haddadzadeh Shoushtari and Zilaee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed Ahmad Hosseini, c2V5ZWRhaG1hZGhvc3NlaW5pQHlhaG9vLmNvbQ==
 Zainab Shateri
Zainab Shateri Seyed Ahmad Hosseini2,3*
Seyed Ahmad Hosseini2,3* Elham Maraghi
Elham Maraghi