
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 October 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1025897
This article is part of the Research Topic Advances in Natural Polysaccharides and Oligosaccharides: Purification Techniques, Analysis Methods, and Physiochemical Properties View all 44 articles
Pectic-polysaccharides are considered as one of the most abundant bioactive components in okra, which possess various promising health-promoting effects. However, the knowledge regarding the structure-bioactivity relationship of okra pectic-polysaccharides (OPP) is still limited. In this study, effects of various degrees of esterification (DEs) on in vitro antioxidant and immunostimulatory activities of OPP were analyzed. Results displayed that OPP with high (42.13%), middle (25.88%), and low (4.77%) DE values were successfully prepared by mild alkaline de-esterification, and their primary chemical structures (compositional monosaccharide and glycosidic linkage) and molecular characteristics (molecular weight distribution, particle size, and rheological property) were overall stable. Additionally, results showed that the notable decrease of DE value did not significantly affect antioxidant activities [2,2’-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) and nitric oxide (NO) radical scavenging abilities as well as ferric reducing antioxidant power (FRAP)] of OPP, suggesting that the DE was not closely related to its antioxidant activity. In fact, the slight decrease of antioxidant activity of OPP after the alkaline de-esterification might be attributed to the slight decrease of uronic acid content. Nevertheless, the immunostimulatory effect of OPP was closely related to its DE, and a suitable degree of acetylation was beneficial to its in vitro immunostimulatory effect. Besides, the complete de-acetylation resulted in a remarkable reduction of immune response. The findings are beneficial to better understanding the effect of DE value on antioxidant and immunomodulatory activities of OPP, which also provide theoretical foundations for developing OPP as functional foods or health products.
Pectic-polysaccharides are complex heteropolysaccharides existed in the primary cell walls of vegetables and fruits, which are predominantly composed of homogalacturonan (HG), rhamnogalacturonan I (RG-I), and rhamnogalacturonan II (RG-II) domains (1). Recently, pectic-polysaccharides extracted from vegetables and fruits have attracted increasing attention to be developed into functional food ingredients owing to their various health-promoting properties, such as antioxidant, anti-inflammatory, immunomodulatory, anti-tumor, anti-hyperlipidemic, anti-hyperglycemic, anti-obesity, and prebiotic properties (1–3). A great number of studies have demonstrated that the ratio of HG/RG-I, molecular mass, branched chain length, degree of esterification (DE), glycosidic linkage, and compositional monosaccharide of pectic-polysaccharides are critical chemical structures for their health beneficial effects (1–3). Nevertheless, the knowledge regarding the precise structure-biological activity relationships of pectic-polysaccharides is still limited because of a lack of pure samples and fine structure analysis. Therefore, it is important to uncover the relationship between the precise structures of pectic-polysaccharides and their biological activities, which is beneficial to better promoting the application of pectic-polysaccharides in the functional food industry.
Abelmoschus esculentus L. Moench, known as okra or lady’s finger, is a vital edible and medicinal plant in China. It is native to the Africa but can now be found throughout tropical and subtropical areas of the world (4). Okra is not only consumed as a delicious vegetable, but also utilized as a folk medicine for the treatment of various diseases (5). Due to its promising health benefits, such as antioxidant, immunomodulatory, anti-diabetic, anti-cancer, anti-hypertensive, and anti-microbial effects, okra has attracted increasing attention to be developed and utilized as functional foods in recent years (4). Lots of studies have demonstrated that pectic-polysaccharides, proteins, flavonoids, and phenolic acids exist as the major bioactive components in okra, which contribute to its various beneficial properties (4, 6). Especially, pectic-polysaccharides are considered as one of the most abundant bioactive components in okra, which play a critical role in its biological activities (6). Indeed, the backbone of okra pectic-polysaccharides (OPP) is identified as →4)-α-D-GalAp-(1→2,4)-α-L-Rhap-(1→, confirming that the RG I segment is rich in okra (7–9). Besides, the HG segment is also found in okra (6). Indeed, OPP also have a low degree of methyl esterification and a high degree of acetylation (10, 11). Generally, pectic-polysaccharides are complex biomacromolecules, their physicochemical or structural features can directly impact the biological functions. Several studies have indicated that the molecular mass of OPP significantly affect their antioxidant, prebiotic, and anti-inflammatory activities (12, 13), as well as immune stimulating activity (14). Besides, a recent study has shown that biological activities of OPP can be improved through the degradation by ultrasound assisted H2O2/Vc reaction, and the in vitro antioxidant and immunostimulatory effects of OPP are related to its molecular mass, branched chain length, and DE (9). Furthermore, the DE value has gained much attention in the investigation of pectic-polysaccharides, because the DE value can obviously affect biological activities and functional properties of pectic-polysaccharides, such as inhibitory effect on α-amylase, modulation of gut microbial composition, immunoregulatory effect, gel property, and emulsifying ability (15–18). Indeed, the mild alkaline de-esterification has been considered as one of the most important methods to reduce the esterification of pectic-polysaccharides (15, 19). However, the potential relationships between DE value and biological activity of OPPare still unclear, which require to be systematically investigated.
Therefore, in order to further clarify the potential structure-bioactivity relationship of OPP, effects of various degrees of esterification on in vitro antioxidant capacities and immunostimulatory activities of OPP were investigated in the present study.
Okra fruits of Abelmoschus esculentus cv. Wufu used in this study were harvested from Chengdu, Sichuan Province, China. Monosaccharide standards, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), sodium nitroprusside (SNP), vitamin C (Vc), griess reagent, lipopolysaccharide (LPS), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The preparation of purified OPP was performed as previously reported (20). Briefly, the crude water-soluble polysaccharides from okra fruit powders were extracted by ultrasound assisted-extraction (650 W, 24 kHz, Scientz, Ningbo, China) as previously reported (20). Afterward, the supernatants were sequentially precipitated (three volumes of 95% ethanol), redissolved, dialyzed (molecular mass cutoff, 3.5 kDa), and separated by a DEAE anion exchange column (5 × 50 cm) to prepare purified OPP. Moreover, the modification of OPP was carried out to improve its in vitro biological activities as previously reported (9). Briefly, 50.0 mL of OPP solutions (10.0 mg/mL) were mixed with ascorbic acid and H2O2 at the final concentrations of 20.0 and 40.0 mM, respectively, and then degraded by ultrasound (650 W, 24 kHz, Scientz, Ningbo, China) at the power of 520 W for 0.5 h (9). Finally, the degraded product of okra pectic-polysaccharides (DOPP) with promoted biological functions was obtained.
Furthermore, the preparation of DOPP with various DE values was carried out according to a previous study with a few modifications (19). In brief, the mild alkaline de-esterification was carried out by stirring 0.5% (w/v) of DOPP in NaOH solution at basic pH values of 11.0 and 13.0 for 0.5 h under 4°C, respectively. At the end of reaction, the sample solution was acidified to pH = 6.0 by adding HCl (1 M). Then, after dialysis (molecular mass cutoff, 3.5 kDa) and freeze-drying in turn, okra pectic-polysaccharides with a middle DE value (DOPP-MDE) and a low DE value (DOPP-LDE) were obtained. Indeed, the yields of DOPP-MDE and DOPP-LDE were measured to be 95.73 and 90.82%, respectively. Correspondingly, the original DOPP was named DOPP-HDE, which possessed a relatively high DE value.
Total polysaccharides, uronic acids, and proteins of OPP with various DE values were detected by colorimetric methods as previously reported (21). Molecular weight (Mw), molecular weight distribution (Mw/Mn), and radius of gyration (Rg) as well as rheological property of DOPP-HDE, DOPP-MDE, and DOPP-LDE were also measured as previously reported (9, 22). In brief, a TSKgel GMPWXL column (300 × 7.8 mm, i.d.) was utilized for the separation of DOPP-HDE, DOPP-MDE, and DOPP-LDE, respectively. Both multi-angle laser light scattering detection and refractive index detection (Wyatt Technology Co., Santa Barbara, CA, USA) were applied for the analysis of DOPP-HDE, DOPP-MDE, and DOPP-LDE, respectively. The apparent viscosities of DOPP-HDE, DOPP-MDE, and DOPP-LDE were measured by a Discovery Hybrid Rheometer-1 (DHR-1, TA Instruments, New Castle, DE, USA). For the investigation of primary chemical structures, the monosaccharide compositions, FT-IR spectra, and 1H NMR spectra of OPP with various DE values were analyzed. In brief, monosaccharide compositions of DOPP-HDE, DOPP-MDE, and DOPP-LDE were analyzed by HPLC (Thermo Fisher Scientific, Waltham, MA, USA) as previously reported (23). A C18 column (150 × 4.6 mm, 5 μm, Thermo Fisher Scientific, Waltham, MA, USA) was carried out for the separation of monosaccharides, and the signals were recorded at 245 nm. Additionally, 1H NMR spectra of DOPP-HDE, DOPP-MDE, and DOPP-LDE were also recorded on a Bruker Ascend 600 MHz spectrometer (Bruker, Rheinstetten, Germany) as previously reported (24, 25). Furthermore, the FT-IR spectra of DOPP-HDE, DOPP-MDE, and DOPP-LDE were also analyzed according to a previous reported method (26, 27). Indeed, the DE value was estimated based on the FT-IR spectra at 1,700–1,750 cm–1 (about 1,730 cm–1) and 1,600–1,640 cm–1 (about 1,635 cm–1), which was estimated based on the following equation:
The ferric reducing antioxidant power (FRAP), ABTS radical scavenging ability, and nitric oxide (NO) radical scavenging ability of OPP with various DE values were evaluated according to previously reported methods (26). In brief, for the determination of ABTS radical scavenging ability, the ABTS radical cation working solution (200 μL) was mixed with 20 μL of each sample (2.0–10.0 mg/mL) in a 96-well microplate to react at 30°C for 20 min; for the determination of NO radical scavenging ability, each sample (450 μL, 2.0–10.0 mg/mL) was mixed with 50 μL of SNP (10 mM) to react at 25°C for 3 h, and then 250 μL of Griess reagent was added. Besides, the IC50 values (mg/mL) of DOPP-HDE, DOPP-MDE, and DOPP-LDE for scavenging free radicals could be determined on the basis of a logarithmic regression curve. Additionally, for the determination of FRAP, 100 μL of each sample (2.0–10.0 mg/mL) was mixed with 100 μL of potassium ferricyanide (1%, w/w) at 50°C for 20 min, and then 100 μL of trichloroacetic acid (10%, w/v) was added and centrifugated. Finally, both distilled water (100 μL) and ferric chloride (20 μL) were added into the supernatant (100 μL). The absorbance of the mixture was recorded at 593 nm. Vc was used as a positive control in each experiment.
Immunostimulatory activities of OPP with various DE values were evaluated by using an in vitro model of RAW 264.7 macrophages according to a previously reported method (9). In brief, effects of OPP with various DEs at the concentrations ranged from 5 to 320 μg/mL on the proliferation of RAW 264.7 macrophages were determined by the MTT colorimetric method. Additionally, the production of NO and release of cytokines [interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)] from RAW 264.7 macrophages were also detected as previously reported (9). After the RAW 264.7 macrophage was stimulated with DOPP-HDE, DOPP-MDE, and DOPP-LDE at the concentrations ranged from 5 to 320 μg/mL, the NO production was measured by Griess reagent. Besides, the release of IL-6 and TNF-α from RAW 264.7 macrophages were measured by ELISA kits according to the manufacturer’s procedures (eBioscience, San Diego, CA, USA).
Each experiment was carried out in triplicate. Data are presented as mean ± standard deviation. Statistical analysis was performed by using a two-tailed Student’s t-test and one-way analysis of variance followed by a Duncan’s test, respectively.
The chemical compositions of OPP with different DE values are summarized in Table 1. Total polysaccharides in DOPP-HDE, DOPP-MDE, and DOPP-LDE were detected to be 92.59, 92.26, and 93.21%, respectively, indicating that the contents of total polysaccharides were not affected by mild alkaline de-esterification. However, total uronic acids in DOPP-HDE, DOPP-MDE, and DOPP-LDE slightly decreased from 33.51 to 27.51% by mild alkaline de-esterification, which might be due to the fact that the elimination reaction could induce the hydrolysis of pectic-polysaccharides by splitting their backbone (15, 28). Additionally, minor proteins were found in DOPP-HDE, DOPP-MDE, and DOPP-LDE, which were similar with a previous study (9).
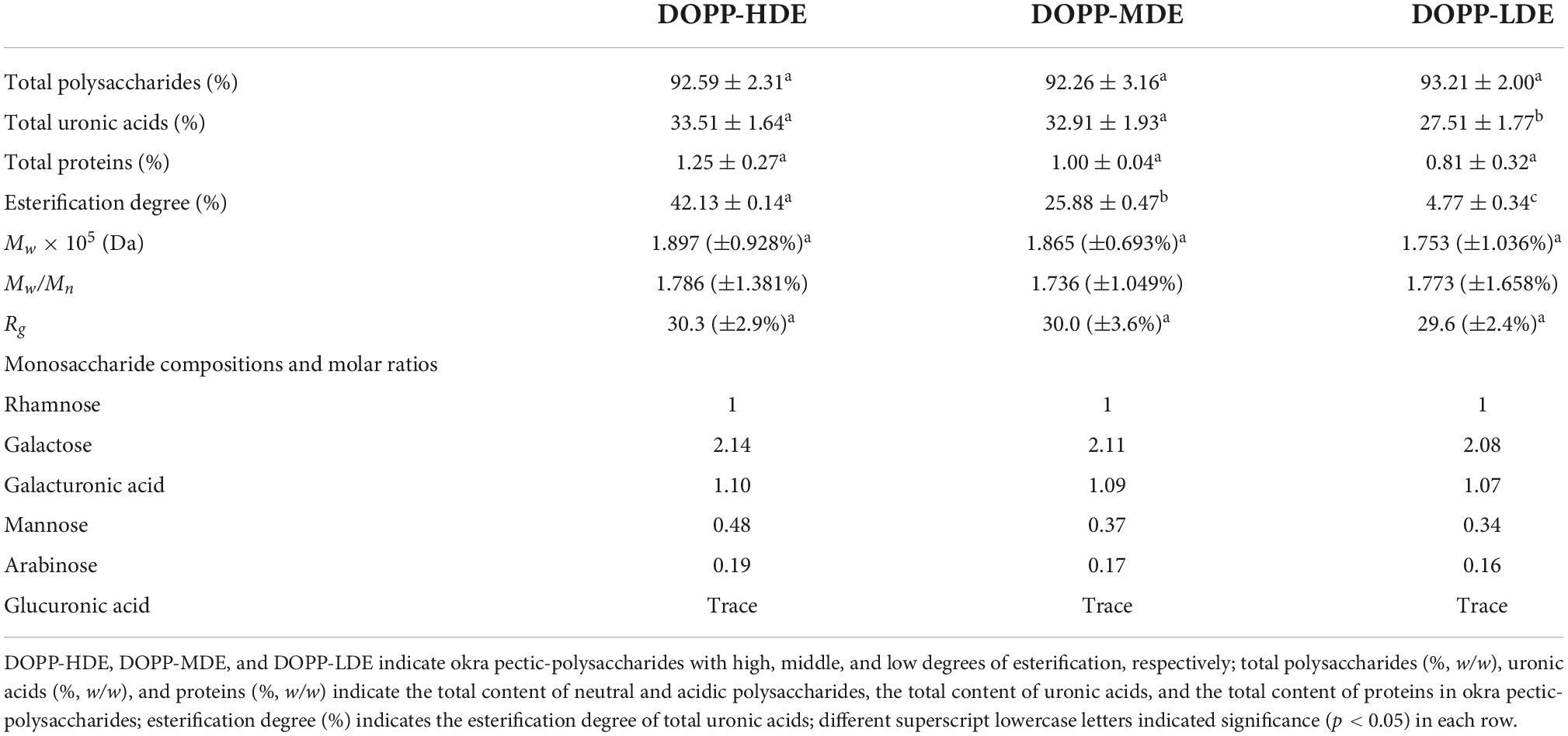
Table 1. Chemical composition, molecular weight (Mw), polydispersity (Mw/Mn), radius of gyration (Rg), and constituent monosaccharide of okra pectic-polysaccharides (OPP) with various degrees of esterification.
Furthermore, in order to confirm the primary chemical structures of OPP with different DE values, the monosaccharide compositions, FT-IR spectra, and 1H NMR spectra were systematically analyzed. As shown in Figure 1A, similar FT-IR spectra were found in DOPP-HDE, DOPP-MDE, and DOPP-LDE, indicating that the major chemical groups of OPP were stable after the treatment of mild alkaline de-esterification. The typical absorption bands of pectic-polysaccharides, including 3466.43, 2938.97, 1730.13, 1635.43, 1411.29, and 1150.36 cm–1, were found in all tested samples (28, 29). However, as shown in Figure 1A, the peak areas of absorption band at around 1730.13 cm–1 related to esterified functional groups remarkably changed after the treatment of mild alkaline de-esterification (28). Indeed, the DE values of DOPP-HDE, DOPP-MDE, and DOPP-LDE were estimated to be 42.13, 25.88, and 4.77% based on the peak areas of absorption bands at around 1730.13 and 1635.43 cm–1 (Table 1), respectively, indicating that OPP with various DE values were successfully prepared. Additionally, as shown in Figure 1B, the same types of monosaccharides were found in DOPP-HDE, DOPP-MDE, and DOPP-LDE, and galacturonic acid, rhamnose, and galactose were determined as the major monosaccharides as previously reported (9). Indeed, similar molar ratios of constituent monosaccharides were also found in all samples (Table 1), suggesting that the primary chemical structures of OPP, except the DE, were relatively stable after the treatment of mild alkaline de-esterification. Furthermore, 1H NMR spectra of OPP with various DE values were also analyzed for the confirmation of their chemical structures (Figure 2). More specifically, the signal at around 2.09 ppm in DOPP-HDE was assigned to acetyl groups (10, 11), which might locate on O-2 or O-3 of galacturonosyl residues and O-3 of rhamnosyl residues (11). The intensity of this signal obviously decreased in DOPP-MDE or even disappeared in DOPP-LDE, indicating that the degree of acetylation of galacturonosyl or rhamnosyl residues in OPP could be decreased by mild alkaline de-esterification. Additionally, the signal at around 3.81 ppm in DOPP-HDE was assigned to methoxyl groups (10, 11). This signal could be also found in DOPP-MDE and DOPP-LDE, suggesting that methoxyl groups bonded to carboxyl groups of galacturonic acid could still exist in OPP under the mild alkaline de-esterification conditions. Similar phenomena were also found in previous studies that the methoxyl group from the esterified units of galacturonic acids could not be completely removed under the mild alkaline conditions (15, 28). Collectively, these results indicated that the decrease of DE value in DOPP-MDE and DOPP-LDE compared to DOPP-HDE might be mainly attributed to the complete de-acetylation and the partial de-methylation.
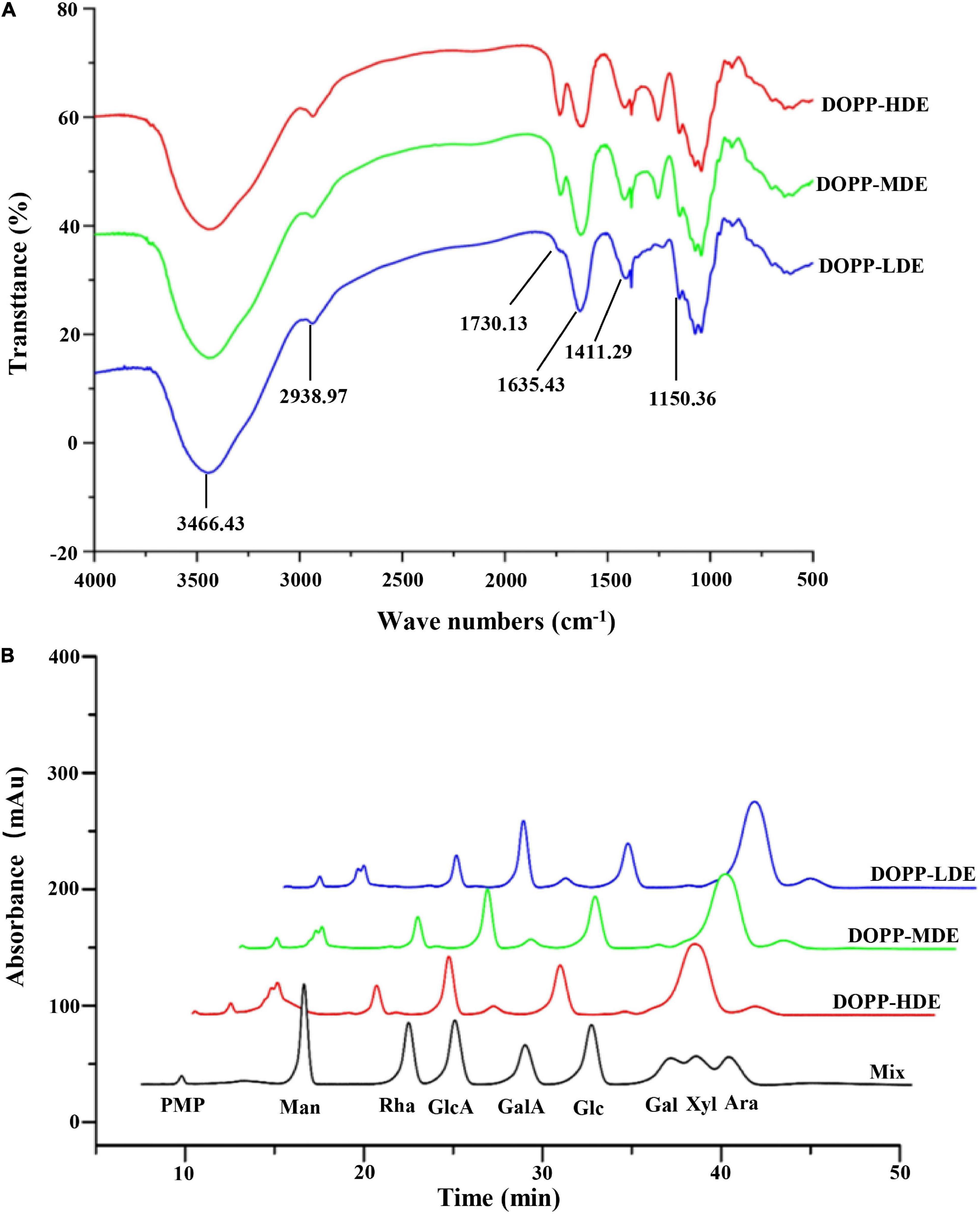
Figure 1. FT-IR spectra (A) and high-performance liquid chromatograms of compositional monosaccharides (B) of okra pectic-polysaccharides (OPP) with various degrees of esterification. DOPP-HDE, DOPP-MDE, and DOPP-LDE indicate OPP with high, middle, and low degrees of esterification, respectively; Mix indicates the monosaccharide standards, which was analyzed by HPLC under the same conditions of samples. PMP, 1-phenyl-3-methyl-5-pyrazolone; Man, mannose; Rha, rhamnose; GlcA, glucuronic acid; GalA, galacturonic acid; Glc, glucose; Gal, galactose; Xyl, xylose; Ara, arabinose.
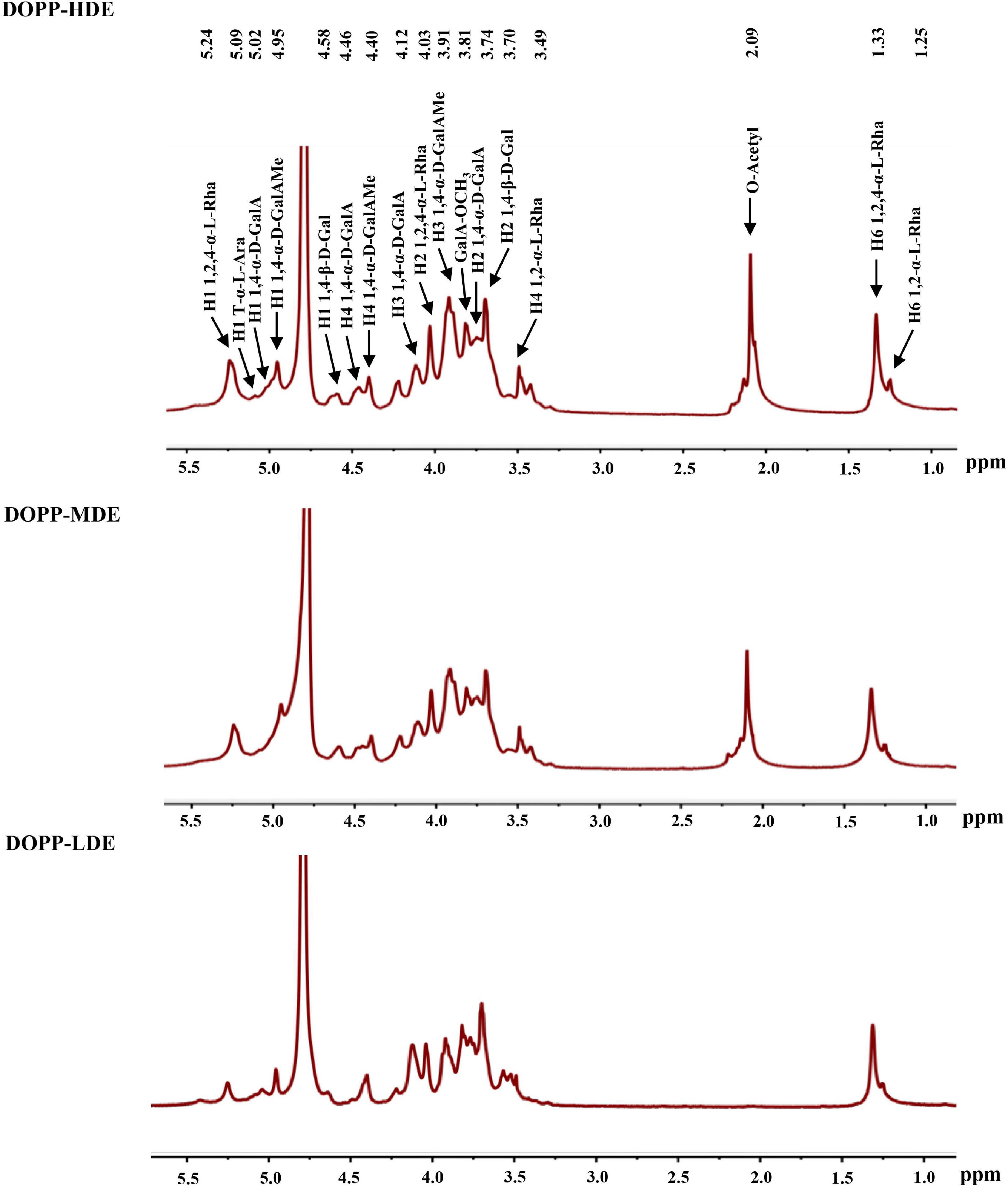
Figure 2. 1H NMR spectra of okra pectic-polysaccharides (OPP) with various degrees of esterification. The sample codes were the same in Figure 1.
Moreover, the typical signals, including 1,4-α-D-GalAp (H-1, 5.02 ppm), 1,4-α-D-GalAMep (H-1, 4.95 ppm), 1,2-α-L-Rhap (H-6, 1.25 ppm), 1,2,4-α-L-Rhap (H-1/H-6, 5.24/1.33 ppm), 1,4-β-D-Galp (H-1, 4.58 ppm), could be found in DOPP-HDE, DOPP-MDE, and DOPP-LDE. These results suggested that the RG-I backbone with galactan side chains existed as the main pectic-polysaccharides in DOPP-HDE, DOPP-MDE, and DOPP-LDE (7, 9, 10, 28), and the primary chemical structures of OPP, except the DE, were overall stable after the treatment of mild alkaline de-esterification.
Macromolecular characteristics of pectic-polysaccharides, such as molecular mass, molecular mass distribution, and particle size, have significant impacts on their biological properties and applications in the functional food industry (9, 30, 31). Consequently, macromolecular characteristics of OPP with various DE values were measured and compared. As shown in Figure 3A, similar size exclusion chromatography (SEC) profiles were found in OPP with various DE values, which exhibited a single symmetrical peak. Results showed that the retention time of DOPP-HDE, DOPP-MDE, and DOPP-LDE were almost the same, suggesting that the molecular mass and molecular mass distribution of OPP were overall stable after the treatment of mild alkaline de-esterification. Indeed, as shown in Table 1, the molecular masses of DOPP-HDE, DOPP-MDE, and DOPP-LDE were detected to be 1.897 × 105, 1.865 × 105, and 1.753 × 105 Da, respectively, suggesting that the mild alkaline de-esterification could slightly (no significant difference) degrade the molecular weight of OPP. This phenomenon was similar with previous studies that β-elimination reaction could cause the degradation of molecular mass (15, 28). Besides, the molecular mass distributions of OPP with different DE values were similar, which ranged from 1,736 to 1,786. Additionally, corresponding with the changes in molecular mass, the particle size of DOPP-HDE also slightly (no significant difference) decreased from 30.3 to 29.6 nm after the treatment of mild alkaline de-esterification.
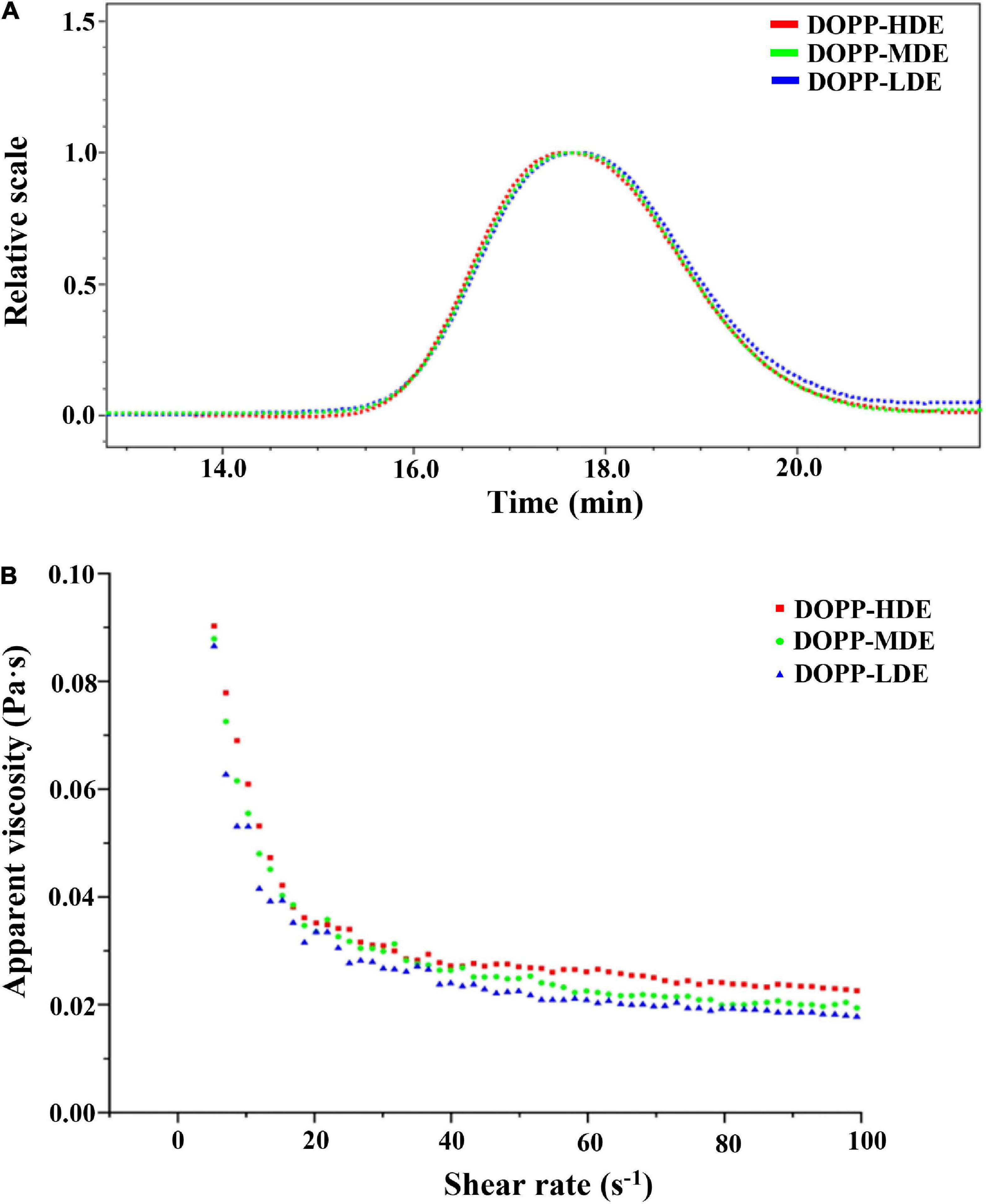
Figure 3. Size exclusion chromatograms (A) and dependences of apparent viscosity on the shear rate (B) of okra pectic-polysaccharides (OPP) with various degrees of esterification. The sample codes were the same in Figure 1.
Rheological property is considered as one of the most important factors that affect the biological functions and food applications of pectic-polysaccharides (28, 32). Figure 3B displays the dependences of apparent viscosity on shear rate of OPP (40 mg/mL) with different DE values. As expected, the apparent viscosities of DOPP-HDE, DOPP-MDE, and DOPP-LDE affected by the shear rate. More specifically, the apparent viscosities of each sample declined with the increase of the shear rate ranged from 0.01 to 50 s–1, indicating that each sample solution exhibited non-Newtonian shear thinning fluid behavior (32). In addition, the apparent viscosities of each sample declined slightly with the increase of the shear rate ranged from 50 to 100 s–1, exhibiting Newtonian flow fluid behavior (9, 21). This rheological character of OPP might be due to the fact that the chains of pectin were arranged in an orderly manner along the fluid direction with the increase of the shear rate, and the interactions between the adjacent chains and the viscosity decreased (33). Furthermore, compared with DOPP-HDE, the apparent viscosities of DOPP-MDE and DOPP-LDE slightly reduced, indicating that the obvious decrease of DE value did not cause a sharp decrease of apparent viscosity. This result is different from a previous study that the pectin with a lower degree of esterification is often accompanied by a decrease of viscosity (34). In this study, DOPP-LDE with a lower degree of esterification also exhibited a higher apparent viscosity which might be attributed to the fact that the viscosity of the sample was affected by several factors, such as molecular mass, chain conformation, monosaccharide composition (9, 21, 32). Collectively, although the DE value of OPP was significantly decreased, its apparent viscosity was relatively stable after the treatment of mild alkaline de-esterification.
The antioxidant activity has been demonstrated as one of the most important biological functions of okra (4), and OPP have remarkable in vitro antioxidant capacities against different free radicals (9, 21, 26, 35). Several studies have shown that the antioxidant activities of crude OPP may be related to their molecular mass, chain conformation, uronic acid, and DE as well as conjugated polyphenols (21, 26, 35, 36). A recent study also showed that the antioxidant activity of a purified okra pectic-polysaccharide might be closely related to the combination effect of molecular mass and DE (9). However, whether the DE can directly affect the antioxidant activity of OPP is still not clear. Therefore, in the present study, in order to evaluate the precise degree of esterification on in vitro antioxidant activity of OPP, three OPP with high, middle, and low degrees of esterification were prepared and their antioxidant activities were evaluated.
Figure 4 displays the FRAPs and ABTS radical scavenging capacities as well as NO radical scavenging capacities of OPP with high, middle, and low DE values. Results showed that OPP with various DE values exhibited remarkable antioxidant activities with a dose-dependent manner. For the FRAP, the absorbance values of DOPP-HDE, DOPP-MDE, and DOPP-LDE at 593 nm were detected to be 0.52 ± 0.01, 0.50 ± 0.02, and 0.43 ± 0.01 at the concentration of 10 mg/mL, respectively, which were lower than that of Vc (0.95 ± 0.02). Additionally, in terms of ABTS radical scavenging activity, the IC50 values of DOPP-HDE, DOPP-MDE, and DOPP-LDE were detected to be 7.76 ± 0.31, 8.40 ± 0.32, and 10.24 ± 0.72 mg/mL, respectively, which were higher than that of Vc (0.04 mg/mL). Furthermore, in terms of NO radical scavenging activity, the IC50 values of DOPP-HDE, DOPP-MDE, and DOPP-LDE were detected to be 8.43 ± 0.21, 9.02 ± 0.49, and 10.64 ± 0.38 mg/mL, respectively, which were also higher than that of Vc (0.62 ± 0.02 mg/mL). Surprisingly, results showed that DOPP-LDE with the lowest DE value (4.77%) among three samples exhibited the lowest antioxidant activities in the present study. Besides, although the DE value of DOPP-MDE (25.88%) was significantly lower than that of DOPP-HDE (42.13%), the in vitro antioxidant activities of DOPP-HDE and DOPP-MDE were similar. Therefore, these results indicated that the remarkable decrease of DE value (mainly degree of acetylation) of OPP by mild alkaline de-esterification did not obviously affect their antioxidant activity, suggesting that the DE was not closely correlated to the antioxidant activity of OPP. This phenomenon is quite different from previous studies that the lower DE of pectic-polysaccharides is closely related to their higher antioxidant activity (37, 38). In fact, the antioxidant activity of pectic-polysaccharides is assigned to their hydrogen-donating abilities, and several studies have shown that the presence of free uronic acids in the pectic-polysaccharides can activate the hydrogen atom of the anomeric carbon (39, 40). However, in this study, the decrease of DE value was mainly attributed to the de-acetylation of galacturonosyl residues in OPP (Figure 2), which might not obviously affect the rate of unmethylated uronic acids. Besides, the antioxidant capacity of pectic-polysaccharides is often closely related to their molecular mass and uronic acids (12, 15, 36, 41). Therefore, in this study, according to the structural differences measured as abovementioned, the slight decrease of antioxidant activity of DOPP-LDE compared to DOPP-HDE might be due to the slight decrease of uronic acid content (Table 1).
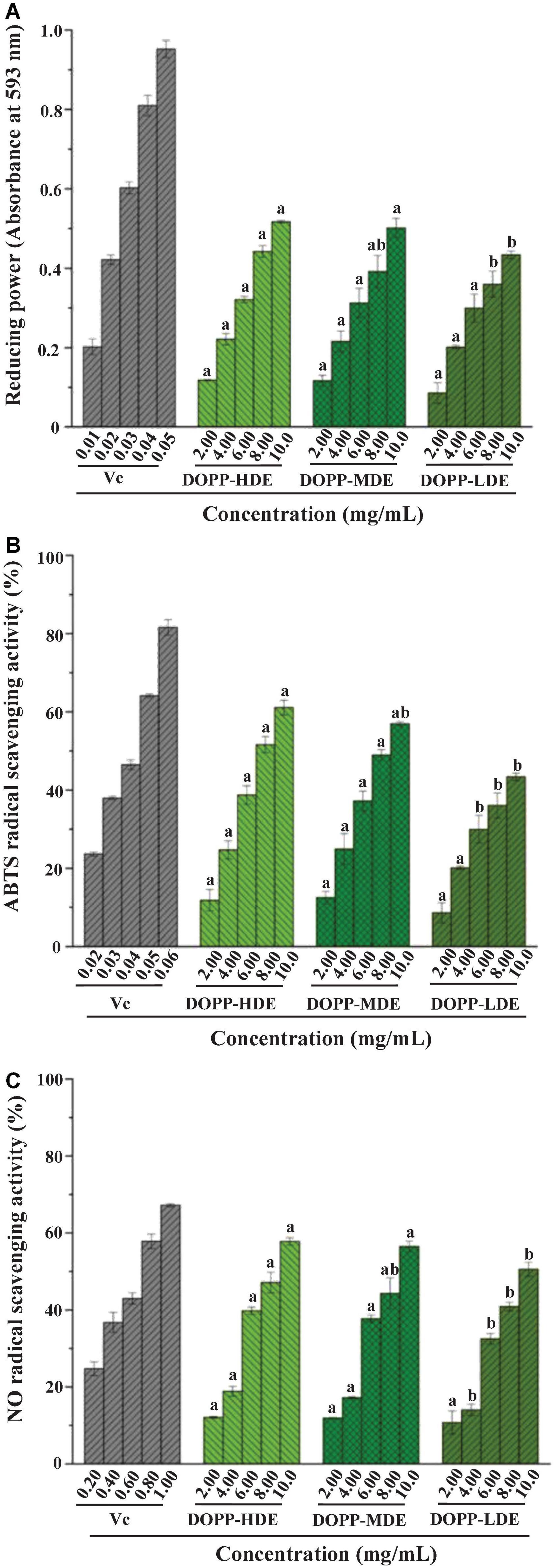
Figure 4. Ferric reducing antioxidant power (FRAP) (A), 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical scavenging activity (B), and nitric oxide (NO) radical scavenging activity (C) of okra pectic-polysaccharides (OPP) with various degrees of esterification. The sample codes were the same in Figure 1; the error bars are standard deviations; significant (p < 0.05) differences among OPP with various degrees of esterification are shown by data bearing different letters (a-b); statistical significances were carried out by ANOVA and Ducan’s test.
Immunity refers to the protection effects of biological organisms against foreign bacteria, viruses, and other harmful substances. A large number of studies have demonstrated that dietary polysaccharides from edible and medicinal plants can maintain the human health by regulating the immune system (42, 43). Generally, the immunostimulatory effects of dietary polysaccharides are associated with their molecular mass, branched chain length, uronic acid, chain conformation, and glycosidic linkage (44, 45). In fact, several studies have shown that pectic-polysaccharides isolated from different parts of okra possess remarkable in vitro and in vivo immunostimulatory effects (14, 46–48). A previous study also showed that the immunostimulatory effect of a purified okra pectic-polysaccharide was closely related to the combination effect of molecular mass, branched chain length, and DE (9). However, whether the DE can directly affect the immunostimulatory effect of OPP remains unclear.
Therefore, the RAW 264.7 macrophage was applied as a cell model for the determination of immunostimulatory effects of OPP with various DE values. Figure 5 displays the immunostimulatory effects of DOPP-HDE, DOPP-MDE, and DOPP-LDE. As shown in Figure 5A, all tested samples could slightly promote the proliferation of RAW 264.7 macrophages at the concentrations ranged from 5 to 320 μg/mL, indicating that DOPP-HDE, DOPP-MDE, and DOPP-LDE had no toxicity effects. Furthermore, macrophages can exert their functions by secreting NO and various cytokines, such as IL-6 and TNF-α (49). NO is a biologically active cell messenger that plays a critical role in killing pathogenic microorganisms and tumor cells; TNF-α is active in regulating inflammation and autoimmunity; IL is involved in the immune response in the host that plays a key role in maintaining homeostasis. As shown in Figures 5B–D, OPP with different DE values could remarkably promote the release of NO, IL-6, and TNF-α from RAW 264.7 macrophages in a dose-dependent manner, respectively. Interestingly, OPP with various DE values exhibited notably different effects on the release of NO, IL-6, and TNF-α from RAW 264.7 macrophages. More specifically, the higher productions of NO, IL-6, and TNF-α from RAW 264.7 macrophages were found in DOPP-MDE compared to DOPP-HDE, while the lower productions of NO, IL-6, and TNF-α were found in DOPP-LDE compared to DOPP-HDE. Collectively, according to the structural differences among DOPP-HDE, DOPP-MDE, and DOPP-LDE, these results indicated that the immunostimulatory effect of OPP was closely related to its DE. A previous study also showed that the DE played a key role in the immunostimulatory effect of pectic-polysaccharides from Asparagus officinalis L., and a relatively high DE value was associated with the relatively high immunostimulatory effect (50). Indeed, the acetyl groups of a purified polysaccharide from Polygonatum cyrtonema might also benefit its immunostimulatory effect (51). In addition, several studies have demonstrated that the acetylation modification of natural polysaccharides can enhance their immunostimulatory effects (52, 53), while removing the acetyl groups resulted in the remarkable decrease of immunostimulatory functions (54, 55). Therefore, the findings in the present study indicate that OPP with a DE value of 25.88% possess remarkable in vitro immunostimulatory effect, and the complete de-acetylation in DOPP-LDE results in a remarkable reduction of immune response. Nevertheless, the precise structure-immunostimulatory activity relationship of OPP and related mechanism of action are required to be deeply uncovered in the future.
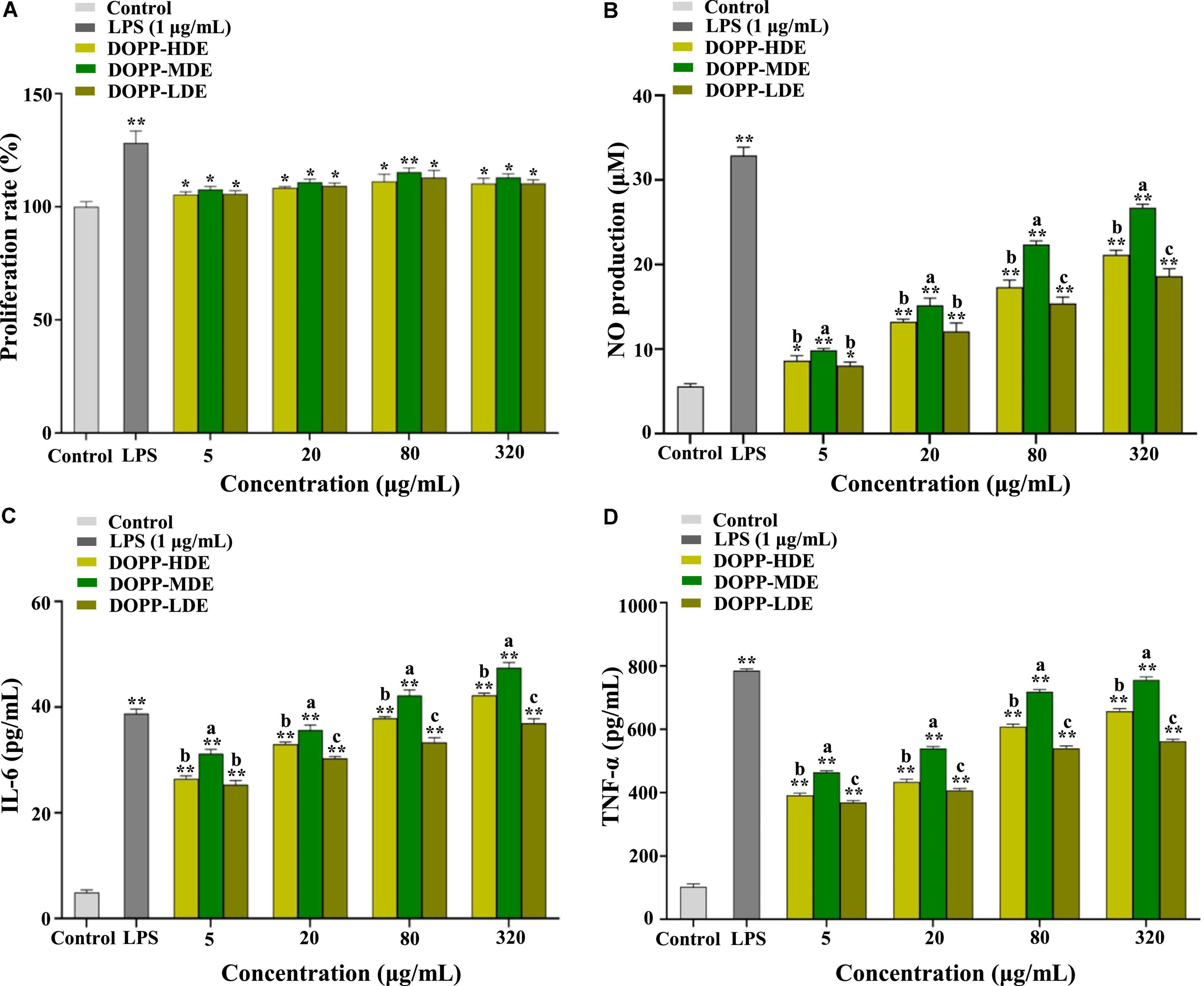
Figure 5. Effects of okra pectic-polysaccharides (OPP) with various degrees of esterification on proliferation (A), nitric oxide (NO) production (B), interleukin-6 (IL-6) production (C), and tumor necrosis factor-α (TNF-α) production (D) of RAW 264.7 macrophages. The sample codes were the same in Figure 1; the error bars are standard deviations; significant differences of cell proliferation and release of NO, IL-6, and TNF-α in LPS, DOPP-HDE, DOPP-MDE, and DOPP-LDE vs. control are shown by *p < 0.05, **p < 0.01. Significant differences (p < 0.05) of release of NO, IL-6, and TNF-α among DOPP-HDE, DOPP-MDE, and DOPP-LDE are shown by data bearing different letters (a–c).
Pectic-polysaccharides are regarded as one of the most abundant bioactive components in okra. However, the knowledge about the precise structure-bioactivity relationships of OPP is still limited. Therefore, in order to further clarify the potential structure-bioactivity relationship of okra pectic-polysaccharides, effects of various degrees of esterification on in vitro antioxidant capacities and immunostimulatory activities of OPP were investigated. Results showed that the decrease of DE was mainly attributed to the de-acetylation of OPP according to the 1H NMR spectra analysis. In addition, results showed that the DE value was not related to the antioxidant activity of OPP. However, the immunostimulatory effect of OPP was closely related to its DE value, and a suitable DE value is beneficial to its in vitro immunostimulatory effect. Collectively, the findings are beneficial to revealing the effect of esterification degree on antioxidant activity and immunomodulatory activity of OPP. However, due to the limitations of in vitro models, it is necessary to evaluate the bioactivities of okra pectic-polysaccharide and its structure dependent relationships in animal models in the future.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
WL and JL: investigation, formal analysis, resources, software, and writing – original draft. JW: investigation, formal analysis, and validation. YH: investigation and validation. Y-CH: resources and software. D-TW: data curation, methodology, formal analysis, funding acquisition, and writing – review and editing. LZ: methodology, formal analysis, supervision, resources, and writing – review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Open Research Project Programme of the State Key Laboratory of Quality Research in Chinese Medicine, University of Macau (No. SKL-QRCM-OP21001) and the Scientific Research Foundation of Chengdu University (2081921047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhang, S, Waterhouse GIN, Xu F, He Z, Du Y, Lian Y, et al. Recent advances in utilization of pectins in biomedical applications: a review focusing on molecular structure-directing health-promoting properties. Crit Rev Food Sci Nutr. (2021) 1–34. doi: 10.1080/10408398.2021.1988897
2. Jin M-Y, Li M-Y, Huang R-M, Wu X-Y, Sun Y-M, Xu Z-L. Structural features and anti-inflammatory properties of pectic polysaccharides: a review. Trends Food Sci Technol. (2021) 107:284–98. doi: 10.1016/j.tifs.2020.10.042
3. Yu CX, Wu DM, Zhu K, Hou L, Xiao H, Ding T, et al. Challenges of pectic polysaccharides as a prebiotic from the perspective of fermentation characteristics and anti-colitis activity. Carbohydr Polym. (2021) 270:118377. doi: 10.1016/j.carbpol.2021.118377
4. Agregán R, Pateiro M, Bohrer BM, Shariati MA, Nawaz A, Gohari G, et al. Biological activity and development of functional foods fortified with okra (Abelmoschus esculentus). Crit Rev Food Sci Nutr. (2022) 1–16. doi: 10.1080/10408398.2022.2026874
5. Dantas TL, Alonso Buriti FC, Florentino ER. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants. (2021) 10:1683. doi: 10.3390/plants10081683
6. Zhu X-M, Xu R, Wang H, Chen J-Y, Tu Z-C. Structural properties, bioactivities, and applications of polysaccharides from okra [Abelmoschus esculentus (L.) Moench]: a review. J Agric Food Chem. (2020) 68:14091–103. doi: 10.1021/acs.jafc.0c04475
7. Liu J, Zhao Y, Wu Q, John A, Jiang Y, Yang J, et al. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. (2018) 242:211–6. doi: 10.1016/j.foodchem.2017.09.051
8. Zhang T, Xiang J, Zheng G, Yan R, Min X. Preliminary characterization and anti-hyperglycemic activity of a pectic polysaccharide from okra (Abelmoschus esculentus (L.) Moench). J Funct Foods. (2018) 41:19–24. doi: 10.1016/j.jff.2017.12.028
9. Wu D-T, He Y, Fu M-X, Gan R-Y, Hu Y-C, Peng L-X, et al. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. (2022) 122:107085. doi: 10.1016/j.foodhyd.2021.107085
10. Kpodo FM, Agbenorhevi JK, Alba K, Bingham RJ, Oduro IN, Morris GA, et al. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. (2017) 72:323–30. doi: 10.1016/j.foodhyd.2017.06.014
11. Alba K, Laws AP, Kontogiorgos V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. (2015) 43:726–35. doi: 10.1016/j.foodhyd.2014.08.003
12. Yeung YK, Kang Y-R, So BR, Jung SK, Chang YH. Structural, antioxidant, prebiotic and anti-inflammatory properties of pectic oligosaccharides hydrolyzed from okra pectin by Fenton reaction. Food Hydrocoll. (2021) 118:106779. doi: 10.1016/j.foodhyd.2021.106779
13. Wu D-T, He Y, Yuan Q, Wang S, Gan R-Y, Hu Y-C, et al. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocoll. (2022) 132:107897. doi: 10.1016/j.foodhyd.2022.107897
14. Trakoolpolpruek T, Moonmangmee S, Chanput W. Structure-dependent immune modulating activity of okra polysaccharide on THP-1 macrophages. Bioact Carbohydr Diet Fibre. (2019) 17:100173. doi: 10.1016/j.bcdf.2018.10.002
15. Liang W-L, Liao J-S, Qi J-R, Jiang W-X, Yang X-Q. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. (2022) 375:131806. doi: 10.1016/j.foodchem.2021.131806
16. Wu Q, Fan L, Tan H, Zhang Y, Fang Q, Yang J, et al. Impact of pectin with various esterification degrees on the profiles of gut microbiota and serum metabolites. Appl Microbiol Biotechnol. (2022) 106:3707–20. doi: 10.1007/s00253-022-11926-x
17. Bai Y, Atluri S, Zhang Z, Gidley MJ, Li E, Gilbert RG. Structural reasons for inhibitory effects of pectin on α-amylase enzyme activity and in-vitro digestibility of starch. Food Hydrocoll. (2021) 114:106581. doi: 10.1016/j.foodhyd.2020.106581
18. Beukema M, Jermendi É, Oerlemans MMP, Logtenberg MJ, Akkerman R, An R, et al. The level and distribution of methyl-esters influence the impact of pectin on intestinal T cells, microbiota, and Ahr activation. Carbohydr Polym. (2022) 286:119280. doi: 10.1016/j.carbpol.2022.119280
19. Hua X, Yang H, Din P, Chi K, Yang R. Rheological properties of deesterified pectin with different methoxylation degree. Food Biosci. (2018) 23:91–9. doi: 10.1016/j.fbio.2018.03.011
20. Nie XR, Fu Y, Wu DT, Huang TT, Jiang Q, Zhao L, et al. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okra (Abelmoschus esculentus). Molecules. (2020) 25:1155. doi: 10.3390/molecules25051155
21. Nie X-R, Li H-Y, Du G, Lin S, Hu R, Li H-Y, et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int J Biol Macromol. (2019) 139:459–67. doi: 10.1016/j.ijbiomac.2019.08.016
22. Wu D-T, An L-Y, Liu W, Hu Y-C, Wang S-P, Zou L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res Int. (2022) 156:111185. doi: 10.1016/j.foodres.2022.111185
23. Ji X, Guo J, Pan F, Kuang F, Chen H, Guo X, et al. Structural elucidation and antioxidant activities of a neutral polysaccharide from arecanut (Areca catechu L.). Front Nutr. (2022) 9:853115. doi: 10.3389/fnut.2022.853115
24. Wu DT, Feng KL, Huang L, Gan RY, Hu YC, Zou L. Deep eutectic solvent-assisted extraction, partially structural characterization, and bioactivities of acidic polysaccharides from lotus leaves. Foods. (2021) 10:2330. doi: 10.3390/foods10102330
25. Ji X, Guo J, Ding D, Gao J, Hao L, Guo X, et al. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J Food Meas Charact. (2022) 16:2191–200. doi: 10.1007/s11694-022-01288-3
26. Yuan Q, Lin S, Fu Y, Nie X-R, Liu W, Su Y, et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int J Biol Macromol. (2019) 127:178–86. doi: 10.1016/j.ijbiomac.2019.01.042
27. Wu D-T, Liu W, Yuan Q, Gan R-Y, Hu Y-C, Wang S-P, et al. Dynamic variations in physicochemical characteristics of oolong tea polysaccharides during simulated digestion and fecal fermentation in vitro. Food Chem X. (2022) 14:100288. doi: 10.1016/j.fochx.2022.100288
28. Bai L, Zhu P, Wang W, Wang M. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: the case of okra polysaccharide. Food Hydrocoll. (2020) 102:105586. doi: 10.1016/j.foodhyd.2019.105586
29. Ji X, Cheng Y, Tian J, Zhang S, Jing Y, Shi M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem Biol Technol Agric. (2021) 8:54. doi: 10.1186/s40538-021-00255-2
30. Yin L, Fu S, Wu R, Wei S, Yi J, Zhang L-M, et al. Chain conformation of an acidic polysaccharide from green tea and related mechanism of α-amylase inhibitory activity. Int J Biol Macromol. (2020) 164:1124–32. doi: 10.1016/j.ijbiomac.2020.07.125
31. Guo R, Tian S, Li X, Wu X, Liu X, Li D, et al. Pectic polysaccharides from purple passion fruit peel: a comprehensive study in macromolecular and conformational characterizations. Carbohydr Polym. (2020) 229:115406. doi: 10.1016/j.carbpol.2019.115406
32. Hou Z, Chen S, Ye X. High pressure processing accelarated the release of RG-I pectic polysaccharides from citrus peel. Carbohydr Polym. (2021) 263:118005. doi: 10.1016/j.carbpol.2021.118005
33. Yan J-K, Wang C, Qiu W-Y, Chen T-T, Yang Y, Wang W-H, et al. Ultrasonic treatment at different pH values affects the macromolecular, structural, and rheological characteristics of citrus pectin. Food Chem. (2021) 341:128216. doi: 10.1016/j.foodchem.2020.128216
34. Hu W, Ye X, Chantapakul T, Chen S, Zheng J. Manosonication extraction of RG-I pectic polysaccharides from citrus waste: optimization and kinetics analysis. Carbohydr Polym. (2020) 235:115982. doi: 10.1016/j.carbpol.2020.115982
35. Chen Z-L, Wang C, Ma H, Ma Y, Yan J-K. Physicochemical and functional characteristics of polysaccharides from okra extracted by using ultrasound at different frequencies. Food Chem. (2021) 361:130138. doi: 10.1016/j.foodchem.2021.130138
36. Wang C, Yu Y-B, Chen T-T, Wang Z-W, Yan J-K. Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench). Food Chem. (2020) 320:126647. doi: 10.1016/j.foodchem.2020.126647
37. Chen R, Luo S, Wang C, Bai H, Lu J, Tian L, et al. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides. Food Hydrocoll. (2021) 121:107016. doi: 10.1016/j.foodhyd.2021.107016
38. Naqash F, Masoodi FA, Gani A, Nazir S, Jhan F. Pectin recovery from apple pomace: physico-chemical and functional variation based on methyl-esterification. Int J Food Sci Tech. (2021) 56:4669–79. doi: 10.1111/ijfs.15129
39. Wang Z-B, Pei J-J, Ma H-L, Cai P-F, Yan J-K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydr Polym. (2014) 109:49–55. doi: 10.1016/j.carbpol.2014.03.057
40. Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev. (2016) 2016:5692852. doi: 10.1155/2016/5692852
41. Wu D-T, Fu M-X, Guo H, Hu Y-C, Zheng X-Q, Gan R-Y, et al. Microwave-assisted deep eutectic solvent extraction, structural characteristics, and biological functions of polysaccharides from sweet tea (Lithocarpus litseifolius) leaves. Antioxidants. (2022) 11:1578. doi: 10.3390/antiox11081578
42. Yin M, Zhang Y, Li H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front Immunol. (2019) 10:145. doi: 10.3389/fimmu.2019.00145
43. Mohan K, Muralisankar T, Uthayakumar V, Chandirasekar R, Revathi N, Ramu Ganesan A, et al. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits – A comprehensive review. Carbohydr Polym. (2020) 238:116185. doi: 10.1016/j.carbpol.2020.116185
44. Mzoughi Z, Majdoub H. Pectic polysaccharides from edible halophytes: insight on extraction processes, structural characterizations and immunomodulatory potentials. Int J Biol Macromol. (2021) 173:554–79. doi: 10.1016/j.ijbiomac.2021.01.144
45. Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure–function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym. (2015) 132:378–96. doi: 10.1016/j.carbpol.2015.05.079
46. Chen H, Jiao H, Cheng Y, Xu K, Jia X, Shi Q, et al. In vitro and in vivo immunomodulatory activity of okra (Abelmoschus esculentus L.) polysaccharides. J Med Food. (2016) 19:253–65. doi: 10.1089/jmf.2015.3513
47. Chen Y, Zhou R, He L, Wang F, Yang X, Teng L, et al. Okra polysaccharide-2 plays a vital role on the activation of RAW264.7 cells by TLR2/4-mediated signal transduction pathways. Int Immunopharm. (2020) 86:106708. doi: 10.1016/j.intimp.2020.106708
48. Zheng W, Zhao T, Feng W, Wang W, Zou Y, Zheng D, et al. Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohydr Polym. (2014) 106:335–42. doi: 10.1016/j.carbpol.2014.02.079
49. Huang LX, Shen MY, Morris GA, Xie JH. Sulfated polysaccharides: immunomodulation and signaling mechanisms. Trends Food Sci. Technol. (2019) 92:1–11. doi: 10.1016/j.tifs.2019.08.008
50. Wang NF, Jia GG, Wang XF, Liu Y, Li ZJ, Bao HH, et al. Fractionation, structural characteristics and immunomodulatory activity of polysaccharide fractions from asparagus (Asparagus officinalis L.) skin. Carbohydr Polym. (2021) 256:117514. doi: 10.1016/j.carbpol.2020.117514
51. Zhao P, Zhou H, Zhao C, Li X, Wang Y, Wang Y, et al. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr Polym. (2019) 214:44–52. doi: 10.1016/j.carbpol.2019.03.014
52. Yang Y, Chen J, Lei L, Li F, Tang Y, Yuan Y, et al. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem Toxicol. (2019) 125:38–45. doi: 10.1016/j.fct.2018.12.036
53. Liu X, Xie J, Jia S, Huang L, Wang Z, Li C, et al. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int J Biol Macromol. (2017) 98:576–81. doi: 10.1016/j.ijbiomac.2017.02.028
54. Martins VMR, Simões J, Ferreira I, Cruz MT, Domingues MR, Coimbra MA. In vitro macrophage nitric oxide production by Pterospartum tridentatum (L.) Willk. inflorescence polysaccharides. Carbohydr Polym. (2017) 157:176–84. doi: 10.1016/j.carbpol.2016.09.079
Keywords: Abelmoschus esculentus, pectic-polysaccharides, structure-activity relationship, degree of esterification, immunostimulatory effect
Citation: Li W, Li J, Wang J, He Y, Hu Y-C, Wu D-T and Zou L (2022) Effects of various degrees of esterification on antioxidant and immunostimulatory activities of okra pectic-polysaccharides. Front. Nutr. 9:1025897. doi: 10.3389/fnut.2022.1025897
Received: 23 August 2022; Accepted: 06 October 2022;
Published: 20 October 2022.
Edited by:
Xiaolong Ji, Zhengzhou University of Light Industry, ChinaReviewed by:
Junyi Yin, Nanchang University, ChinaCopyright © 2022 Li, Li, Wang, He, Hu, Wu and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding-Tao Wu, d3VkaW5ndGFvQGNkdS5lZHUuY24=; Liang Zou, em91bGlhbmdAY2R1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.