- 1School of Biological Engineering, Sichuan University of Science and Engineering (SUSE), Zigong, Sichuan, China
- 2Liquor-Making Biotechnology and Application Key Laboratory of Sichuan Province, Sichuan University of Science and Engineering (SUSE), Zigong, Sichuan, China
- 3College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease and its global incidence is estimated to be 24%. Beer, wine, and Chinese baijiu have been consumed worldwide including by the NAFLD population. A better understanding of the effects of these alcoholic beverages on NAFLD would potentially improve management of patients with NAFLD and reduce the risks for progression to fibrosis, cirrhosis, and hepatocellular carcinoma. There is evidence suggesting some positive effects, such as the antioxidative effects of bioactive flavor compounds in beer, wine, and baijiu. These effects could potentially counteract the oxidative stress caused by the metabolism of ethanol contained in the beverages. In the current review, the aim is to evaluate and discuss the current human-based and laboratory-based study evidence of effects on hepatic lipid metabolism and NAFLD from ingested ethanol, the polyphenols in beer and wine, and the bioactive flavor compounds in baijiu, and their potential mechanism. It is concluded that for the potential beneficial effects of wine and beer on NAFLD, inconsistence and contrasting data exist suggesting the need for further studies. There is insufficient baijiu specific human-based study for the effects on NAFLD. Although laboratory-based studies on baijiu showed the antioxidative effects of the bioactive flavor compounds on the liver, it remains elusive whether the antioxidative effect from the relatively low abundance of the bioactivate compounds could outweigh the oxidative stress and toxic effects from the ethanol component of the beverages.
1. Introduction
Alcoholic beverages not only have been consumed for thousands of years for social, ceremonial, behavioral, and ritual purposes but also are widely consumed. Around 43% of adults (aged 15 years or older) reported consuming alcohol globally in 2016 (1). The average global alcohol consumption was equivalent to approximately 6.43 L of pure ethanol per capita of the adult population in 2015 (2). In 2016, 46% of the total alcohol consumption was beers (34.3%) and wines (11.7%), and 44.8% was spirits (1). South-east Asia region consumed 87.9% of the total spirit globally (1). The major spirit consumed in China (one of the most populated areas of South-east Asia region) is baijiu. Despite its popularity, alcohol consumption ranks as the third most important preventable cause of the disease (3), the fifth-leading risk factor for premature death and disability globally (4), and accounted for 5.1% of the global burden of disease expressed in DALYs (disability-adjusted life years) (1). Excessive alcohol consumption, referring to daily consumption of greater than three drinks (one drink is equivalent to 14 g of pure ethanol), is associated with increased risk of various diseases (5–9), cancers (10–12), and all-cause mortality (13).
On the other hand, however, there are studies demonstrating that low alcohol consumption is not associated with an increased risk of some cancers (14–17). Moreover, some studies from recent years have indicated that low to moderate alcohol consumption, typically 2–3 drinks (approximately 28–42 g of ethanol) per day for men and 1–2 drinks (approximately 14–28 g of ethanol) per day for women, is associated with some beneficial health effects, such as lower risks for cardiovascular disease, dementia, and insulin resistance (18–21). Moderate alcohol consumption is also associated with reduced all-cause mortality (6, 10, 13), and the association is often formed a J-shape relationship (10, 13). Furthermore, some flavor compounds in alcoholic beverages, such as phenolic acids (in beers and wines), organic acids, esters, and terpenoids (22) (in baijiu), may also have additional impacts on health. For instance, the Copenhagen prospective population studies (23) have shown that wine intake is associated with better beneficial effects on all-cause mortality than those from purely alcohol consumption. It was hypothesized that the additional beneficial effects may have come from numerous phenolic compounds present in wine, such as phenolic acids, flavan-3-ols, and anthocyanins.
Non-alcoholic fatty liver disease (NAFLD), one of the most common causes of chronic liver disease worldwide, is clinically diagnosed with the presence of liver fat accumulation ≥5%, determined by radiological imaging techniques, in absence of other known causes (e.g., alcohol, drugs, and virus) (24). The prevalence of NAFLD is increasing constantly, and the current global incidence of NAFLD is estimated to be 24%, with Asia (27%) USA (24%), and Europe (23%) (25). Its prevalence is increasing at a fast pace. In the US alone, for instance, it was projected that the number of patients with NAFLD will increase from 83.1 million (in 2015) to around 100.9 million in 2030 (26). In addition, NAFLD is associated with metabolic syndrome, especially type 2 diabetes and enhances the comorbidities (24, 27). Furthermore, NAFLD, if left unmanaged/poorly managed, can progress to non-alcoholic steatohepatitis (NASH). Approximately 40–50% of the patients with NASH may further progress to hepatic fibrosis, with increased risks of cirrhosis and hepatocellular carcinoma (24). Thus, NAFLD is a growing burden for global healthcare systems. Having good strategies to manage and treat NAFLD, therefore, has become important. The diagnosis of NAFLD reveals NAFLD population consisted of either abstainers or low to moderate alcoholic beverage drinkers. A good understanding of the effects of common alcoholic beverage intake on NAFLD could improve daily NAFLD management and improve the condition of comorbidities.
In this review, the aim is to critically evaluate and discuss the current evidence of effects on liver and NAFLD from human-based and laboratory-based studies on beer, wine, and Chinese baijiu, with a focus on the effects and potential mechanism of ethanol in the beverages, the polyphenols in beer and wine, and the bioactive flavor compounds in baijiu.
2. The effects of ethanol on NAFLD
2.1. Evidence from studies
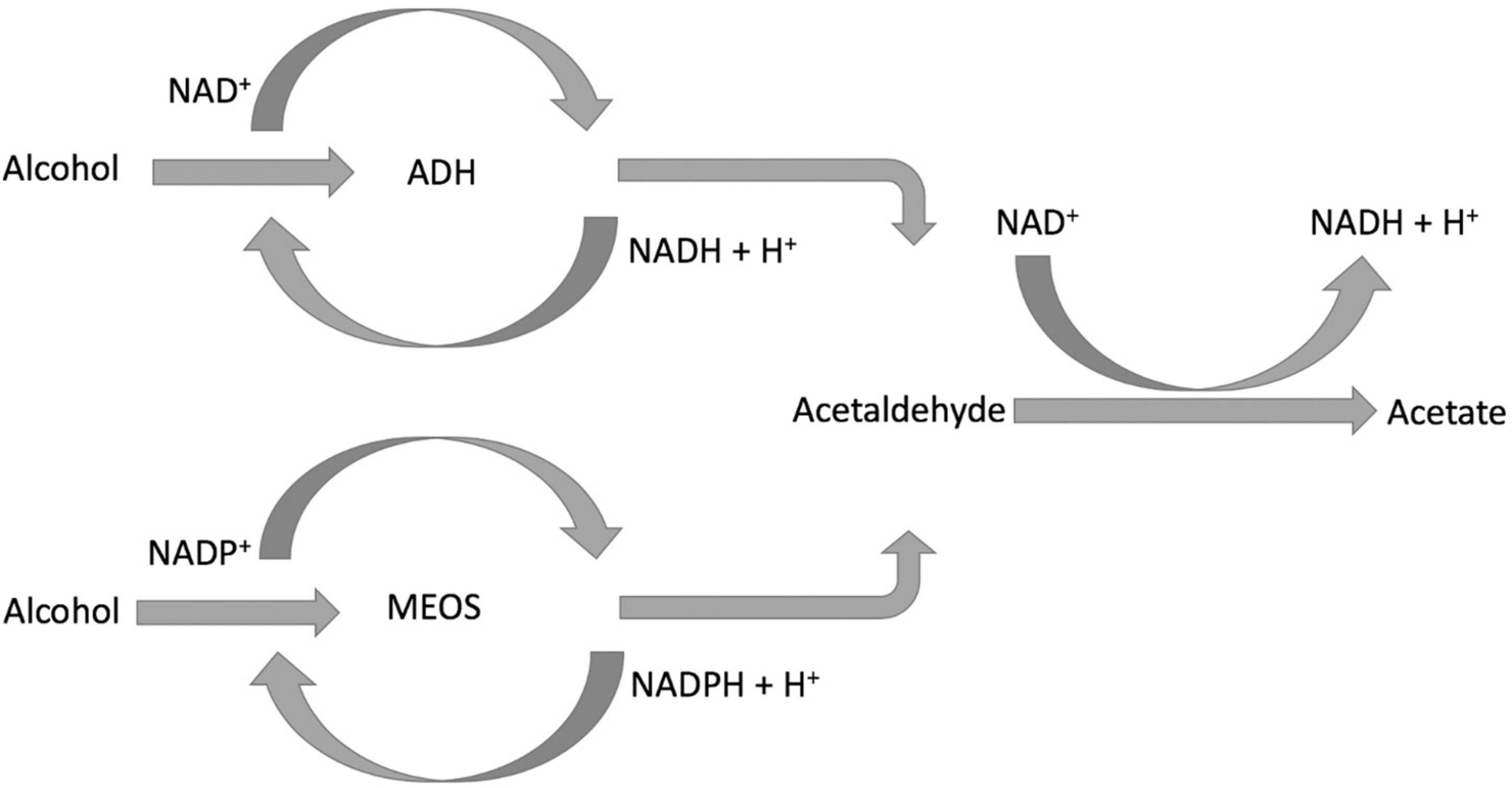
The consumption of equivalent to 50 g of ethanol per day has an estimated excess risk of 46% for liver cancer, the end stage of NAFLD progression, and the consumption of 100 g of ethanol per day has an excess risk of 66%. A meta-analysis of prospective studies by Turati et al. (12) has shown a positive association between heavy alcohol drinking and liver cancers. Moreover, excessive alcohol consumption is linked to an increased incidence of liver diseases (7). Consumption of alcohol equivalent to 30–50 g of ethanol/day for 5–10 years or longer is associated with an increased risk of alcoholic liver disease (ALD) (5). However, in NAFLD populations, alcohol consumption is either none or low to moderate. The effects of low to moderate alcohol consumption on NAFLD from studies are controversial. On the one side, Bagnardi et al. (28) found daily alcohol consumption no greater than 12.5 g showed no association with liver cancers. In addition, moderate alcohol drinking is associated with a reduced risk for NAFLD and NASH (29, 30). Some studies found low to moderate alcohol consumption could improve serum lipid profiles (31, 32), and alcohol consumption equal to or less than 25 g/day could significantly reduce cardiovascular risk in patients with diabetes (33). Animal studies showed a moderate baijiu (a type of spirit) consumption may potentially improve serum lipid profiles while having no significant damage to the liver (31). On the other side, however, there is evidence suggesting alcohol consumption has no safe limit for NAFLD, even low alcohol consumption could still increase the risk of disease progression to advanced stages (34, 35). The putative mechanism of ethanol’s effects on NAFLD, although not entirely clear, involves the impact of decreased NAD+/NADH ratio, oxidation stress, and acetaldehyde on hepatic lipid metabolism. All three factors are the outcome of ethanol’s metabolism in the liver (Figure 1; 36–38).
2.2. The mechanisms
After ingestion, approximately 94–98% of the ethanol is removed by two enzyme systems: alcohol dehydrogenase (ADH) (39, 40) and microsomal ethanol-oxidizing system (MEOS) (36, 41). Although ADH is expressed in the cytosol of both gastric mucosa and hepatocytes, the majority is presented in the liver. MEOS is presented predominantly in the endoplasmic reticulum of hepatocytes; it is also expressed in the intestinal mucosa. ADH is metabolizing the majority of the ingested ethanol, especially when consuming no more than three drinks. MEOS, however, plays an important role in removing ethanol after excessive alcohol consumption (such as binge drinking). It is because, in the presence of high blood alcohol concentration (BAC), the activity of MEOS is induced and increased.
After alcohol consumption, gastric ADH eliminates a small fraction of ingested ethanol before it is absorbed and delivered to the liver through the portal vein. The rate of ethanol elimination by Gastric ADH is affected by gender and age, polymorphisms between ethnic groups, rate of drink, and fed or fasted state. This may contribute to the variation and discrepancy of the results in studies. The majority of ingested ethanol is absorbed by the intestinal mucosa and transported to the liver for clearance. The ethanol oxidization catalyzed by ADH also reduces the coenzyme nicotinamide adenine dinucleotide (NAD+) to NADH. After heavy drinking, ethanol oxidation by ADH decreases the ratio of NAD+/NADH, which could enhance the synthesis of triglyceride and the accumulation of lipids in the liver. For patients with NAFLD, this may potential enhance disease progression. Moreover, the decrease in NAD+/NADH ratio also inhibits the oxidation of acetaldehyde, the accumulation of which impairs mitochondria. Mitochondria impairment may lead to lipid accumulation in the liver.
MEOS (42) is predominantly found in the liver, whose main component is cytochrome P450 (CYP) isoform CYP2E1. CYP2E1 oxidizes ethanol to form acetaldehyde and converts nicotinamide adenine dinucleotide phosphate (NADPH) to NADP+. MEOS has a higher Michaelis–Menten constant (Km) for ethanol than ADH and activates with high BAC. It normally accounts for 20–25% of all alcohol metabolism. Ethanol metabolism facilitated by MEOS also results in the production of various reactive oxygen species (ROS), such as ethoxy radical CH3CH2O•, hydroxyethyl radical CH3C(•)HOH, acetyl radical CH3CHO•, and singlet radical 1O2. After heavy alcohol consumption, the elevated level of ROS generated undergoes covalent bonding to macromolecules on the membrane, subcellular organelles, and subsequently interferes with their biological function (43). In addition, the oxidative stress caused by the ROS on the one hand damages the mitochondria impairing fatty acid beta-oxidation and causing lipid accumulation. On the other hand, oxidative stress on the endoplasmic reticulum (ER) can activate its stress response and enhance fatty acid synthesis.
Acetaldehyde, the direct metabolite of ethanol, is not only IARC classified group 1 carcinogen but also toxic. It is oxidized to acetate by hepatic acetaldehyde dehydrogenase (ALDH), and acetate is further oxidized to CO2. The generation of the elevated level of acetaldehyde and/or its slow removal is harmful. The main ALDH isozyme that metabolizes acetaldehyde in the liver is ALDH2. The polymorphism of ALDH2 results in a low-activity enzyme, which has been presented among the East Asian population (such as Han Chinese and Japanese). This polymorphism of ALDH2 may be a potential factor that causes study results discrepancy. Oxidation of acetaldehyde by ALDH requires the reduction of NAD+ to NADH. Heavy alcohol consumption increases NADH levels and decreases the NAD+/NADH ratio, which could inhibit acetaldehyde oxidation and cause its accumulation. Acetaldehyde can form adducts with DNA, lipids, and proteins; therefore, its accumulation could disrupt normal liver metabolism and may impose a negative impact on the NAFLD population.
3. The effects of beer on NAFLD
3.1. Evidence from clinical, epidemiological, and laboratory studies
Beer is a type of popular fermented beverage, and its consumption alone took 34.3% of total global alcohol consumption in 2016 (1). Low to moderate beer consumption has been shown to reduce the risk of cardiovascular disease compared to abstainers and heavy drinkers, suggesting the potential cardiovascular protection function of its polyphenols. However, its association with liver function is still inconclusive. The underline mechanism, although unclear, is thought to involve but not limited to the antioxidation, anti-inflammation, and lipid modulation properties of the polyphenolic and bitter acids.
There are limited epidemiological studies investigating beer consumption and liver health, and the outcome remains inconclusive. On the one hand, a positive and significant population-based association between beer consumption and liver disease-led mortality was demonstrated in 221 municipalities in the State of Louisiana in the US (44). In a Danish population–based study, 30,630 men and women with more than five drinks/day of all three types of alcohol were associated with an increased risk for liver cirrhosis compared to abstainers or low alcohol drinkers. However, wine drinkers showed lower risk than beer and spirits drinkers (45). On the other hand, in an Eastern French population study, moderate beer consumption was not associated with increased mortality due to cirrhosis (46).
Various clinical studies and laboratory studies on human subjects have been carried out investigating the beneficial biological properties of polyphenols, bitter acids, and other non-alcoholic components in beer and their potential impact on health. In a randomized crossover trial involving 11 healthy middle-aged non-smoking men, beer consumption (equivalent to 40 g of ethanol per day) for 3 weeks did not increase the values of liver enzymes: gamma-glutamyltransferase (GGT), aspartate aminotransferase (AAT), and alanine aminotransferase (ALT) (47). Similar results were obtained from another crossover trial involving 60 healthy Spanish adults (31 men and 29 women), in which the levels of hepatic enzymes (GGT, GOT, and GPT) are unchanged after beer consumption (equivalent to 11 g/day for women and 22 g/day for men) for 1 month (48). However, in the crossover trial involving 10 middle-aged men and 10 post-menopausal women, the levels of both GGT and ALT showed a slight increase (but still within the normal clinical range) after beer consumption (equivalent to 40 g/day for men and 30 g/day for women) for 3 weeks. But interestingly, inflammation markers, C-reactive protein, and fibrinogen were decreased significantly, indicating anti-inflammatory action, after the 3-week beer consumption (49).
In a laboratory study, antioxidant melatonin was detected and measured in 18 brands of beer with different alcohol concentrations (50). In addition, serum samples from seven healthy human subjects were analyzed before and after beer consumption, which showed both melatonin and total antioxidant status increased after beer consumption. This suggests beer consumption may increase the antioxidative capability of human serum attributed to melatonin and other compounds beer contains. This coincides with the results from other studies, which found increased plasma polyphenolic contents and antioxidant capability (51, 52).
In vitro and in vivo studies also showed the beneficial biological effects of polyphenols, bitter acids, and other non-alcoholic components in beer. In a study using an aluminum-induced neurotoxicity murine model, the beer treatment group showed significant lower lipid peroxidation, higher expression of antioxidant enzymes (at mRNA levels), and lower expression (mRNA) of inflammation marker TNFα (53). The authors speculated polyphenols (such as resveratrol) and antioxidants (such as folic acid) in beer may have contributed a part to the antioxidation and anti-inflammation properties of the beer. These studies showed that beer-derived polyphenols may be absorbed and reach the blood circulation to exert biological functions. Two recent studies by Shafreen et al. and Tung et al. further demonstrate that serum polyphenols (come from beer) can bind and interact with serum proteins, such as human serum albumin, plasma circulation fibrinogen, and low-density lipoprotein to exert antioxidant functions (54, 55). In an in vitro study on peripheral blood mononuclear cells by Winkler et al., beer components were shown to increase neopterin production and tryptophan degradation and reduce ROS generation by inhibiting the production of pro-inflammatory cytokine interferon-γ (56).
3.2. The effects and putative mechanisms of the main flavor compounds in beer
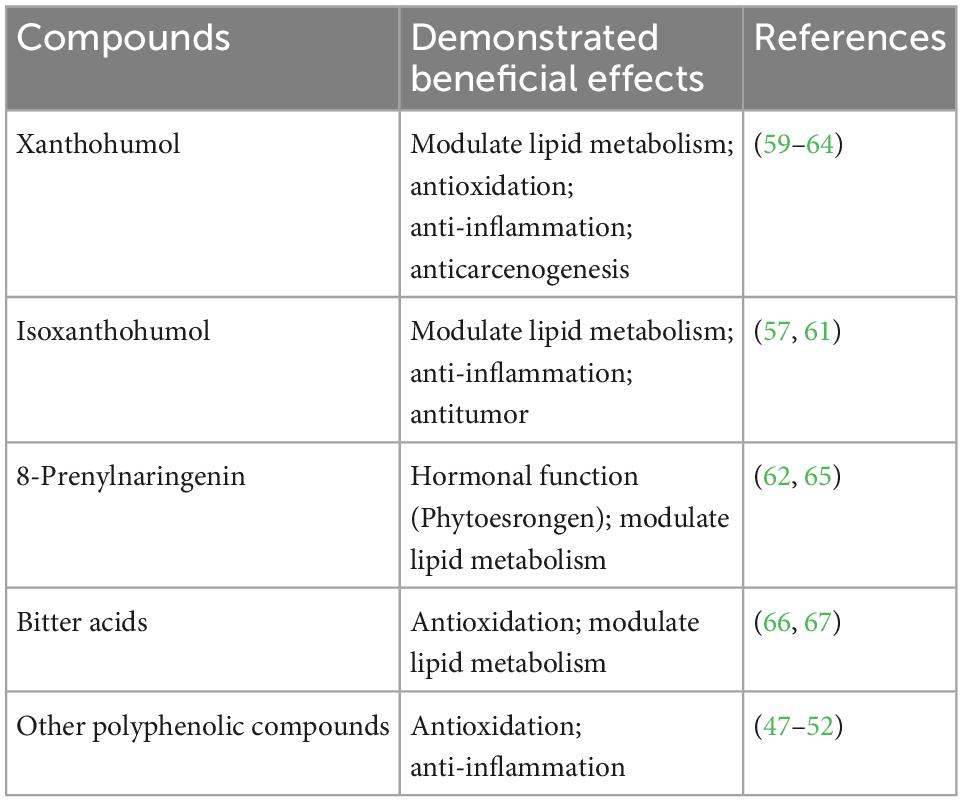
Beer is fermented from cereals and hops (Humulus lupulus), consisting of over 90% of water, carbohydrates, ethanol, (more than 50) polyphenolic compounds, bitter acids (e.g., humulones and lupulones), proteins, B-complex vitamins, and trace amounts of minerals (57, 58). The alcohol concentration of beer varied approximately from 3.5 to 10% (w/v) in different kinds of beers. The main non-alcoholic flavor compounds of beer thought to exert beneficial biological functions are (1) polyphenolic compounds: xanthohumol (around 0.2 mg/L), isoxanthohumol (around 0.6–3.4 mg/L), and phenolic acids (25–29 mg/L); (2) bitter acids: humulones (approximately up to 4 mg/L), lupulones (around 0.012–0.14 mg/L), and iso-humulones (around 10–100 mg/L) (57).
Among the non-alcoholic compounds in beer, the hop-derived phenolic compounds and bitter acids have been shown to modulate hepatic lipid metabolism and process anti-inflammatory, antioxidative, and anticarcinogenesis properties (Table 1). Xanthohumol, of which beer is the main human diet source, is a bioactive multifunctional prenylated flavonoid from the female inflorescence of the hop plant (59, 60). It has been shown with the capability to modulate hepatic lipid metabolism (61, 62). In type 1 diabetic rodent model, insulin deprivation led to down-regulation of fatty acid synthase (FAS), inactivation of Acetyl-CoA Carboxylase (ACC), and inhibition of lipogenesis (61). Xanthohumol was able to activate ACC, increase the expression of FAS, and restore some proportion of lipogenesis, through a mechanism not clearly understood. In mice fed with a high-fat diet, xanthohumol was able to reduce triglycerides and cholesterol content in the liver and skeletal muscle by inhibiting lipogenesis and lipid uptake and promoting β-oxidation (62). The putative mechanism involves xanthohumol activation of AMP-activated protein kinase (AMPK), which then inhibits the expression of sterol regulatory element-binding protein 1c (SREBP-1c), downstream ACC and FAS, down-regulation of the expression of lipid transporter CD36. In addition, xanthohumol was shown to inhibit liver fibrosis in type 1 diabetic rodent model (61). The mechanism, although not clear, is speculated to involve anti-inflammation and antioxidation actions as demonstrated in another study based on the same type 1 diabetic rodent model (63). Furthermore, xanthohumol was shown in a rodent model to protect the liver and the colon from DNA damage, and preneoplastic lesion caused by cooked food mutagen (64), indicating the capability to prevent liver cancer development from more general carcinogens, such as ethanol and acetaldehyde. Xanthohumol can be converted to isoxanthohumol during the brewing process and/or in the stomach. As one of the major flavonoids in normal beers, isoxanthohumol may also involve in modulating hepatic lipid metabolism, anti-inflammation, and antitumor (57, 61). Isoxanthohumol can be further converted to 8-prenylnaringenin by the microbiota in the intestine (57). 8-Prenylnaringenin not only exerts hormonal function as the strongest phytoestrogen but also involves modulating lipid metabolism (62, 65). Landmann et al. showed that normal beer (brewed with the hop) was able to attenuate hepatic lipid accumulation in a binge-drinking mouse model (66). The putative mechanism is shown to be the inhibition of hepatic iNOS induction and lipid peroxidation. The further study by the same group showed that iNOS and lipid peroxidation inhibition may be exerted by iso- α-acids (iso- humulones) from hop (67). The authors speculated the protection effects may also involve other compounds in the hop extracts, such as β-acids (lupulones).
The putative mechanisms that beer flavor compounds involved can be summarized as following three main areas (refer to Figure 2). (1) Modulate hepatic lipid metabolism: down-regulating hepatic lipogenesis, reducing hepatic lipid uptake from circulation, and enhancing β-oxidation; (2) antioxidation: as antioxidant removing ROS, increasing quantity and activity of antioxidant enzymes, and inhibition of lipid peroxidation; (3) anti-inflammation: preventing hepatic inflammation (through JNK/NF-κ B) caused by lipid accumulation induced endoplasmic reticulum stress in hepatocyte and lipid peroxidation. The exact process and how these are integrated remain elusive.
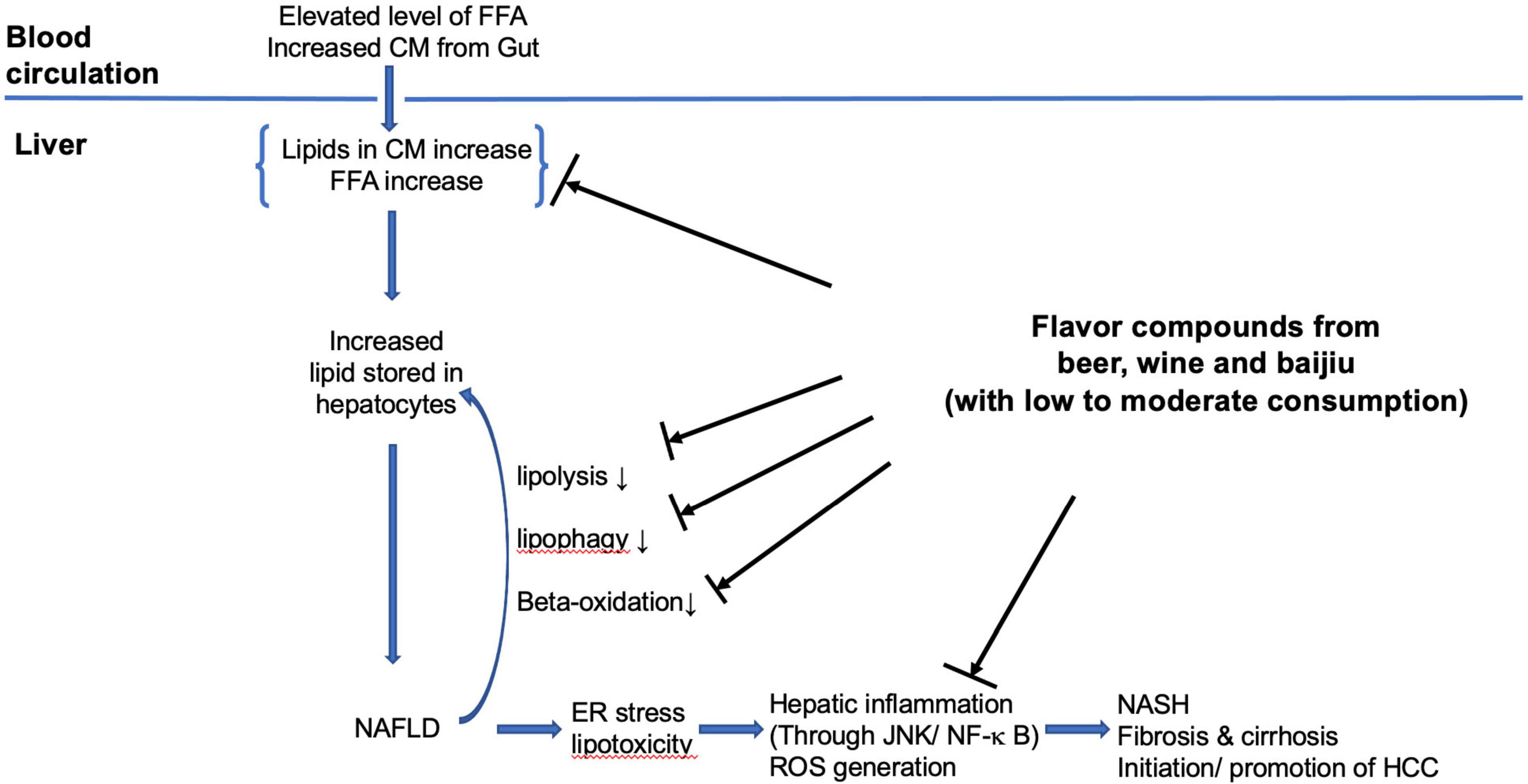
Figure 2. The putative mechanisms of flavor compounds on NAFLD. FFA, free fatty acids; CM, chylomicron remnants; ER, endoplasmic reticulum; HCC, hepatocellular carcinoma.
4. The effects of wine on NAFLD
4.1. Evidence from clinical and epidemiological studies
Wine is a type of popular alcoholic beverage fermented from grape vines. The term French paradox describes the observation of a lower incidence of coronary heart disease in France than in other Western countries, despite similar intake of high levels of saturated fat (68). This was based on epidemiological studies, which suggested the observation was attributed to the beneficial effects of red wine consumption, on the data collected from the MONICA project organized by WHO. Since then, epidemiological studies and human trials have been carried out investigating the potential health benefit of wine. Among them, only limited studies directly investigated the effects of wine on NAFLD prevalence and progression with promising outcomes; however, more data are needed before any conclusion can be drawn. Dunn et al. performed the first epidemiological study investigating the association between modest consumption of wine and NAFLD (69). The study showed a lower NAFLD prevalence in participants who consumed up to four ounces of wine daily when compared to abstainers and participants whose daily consumption of up to 12 ounces of beer, 1 ounce of liquor, or 1 drink of mixed alcoholic drinks. The wine drinkers also demonstrated a lower prevalence of diabetics and other metabolic syndrome features in the study. However, this study did not demonstrate the safety of modest wine drinking in patients with NAFLD. A single-center cohort study by Mitchell et al. showed modest wine consumption (<70 g of ethanol per week without binge consumption) was associated with a significantly lower risk of advanced hepatic fibrosis compared to abstinence among patients with NAFLD (70). Some studies investigated the association between wine consumption and the risk of liver cirrhosis, the effects of wine drinking on hepatic lipid levels, functions, serum cholesterol profiles, and NAFLD co-exist metabolic syndrome. The outcome of the studies was inconsistent and inconclusive. In a prospective study in the Copenhagen area, Becker et al. found an increase in the risk of liver cirrhosis with increasing total alcohol intake for beer, wine, and spirit, but wine consumption showed a lower risk (45). In a large cohort prospective study including 1.3 million middle age UK women, with a mean of 15 years of following up of 401,806 women, the authors found that the risk of liver cirrhosis increased with the total amount of alcohol intake (event with moderate consumption), the increase of risk, in a given weekly intake of alcohol, was also associated with consumption without a meal or daily consumption, regardless whether drinking only wine or more than one type of alcoholic beverages (71). A randomized crossover trial by Beulens et al. showed 4 weeks of red wine consumption (40 g of ethanol per day) did not significantly increase liver fat compared to 4 weeks of consumption of de-alcoholized red wine (72). An interventional cohort study by Rajdl et al. showed, although there was an increase in liver enzymes AST (within the normal reference range) and ALT (slightly exceeded upper threshold), white wine consumption is associated with an increase in antioxidative effects (73). In a prospective randomized trial involving 44 healthy subjects (32 women and 12 men), Kechagias et al. showed an increase in ALT and AST (within an upper reference threshold), decrease in LDL cholesterol, and a trend of hepatic triglyceride content increase for subjects with moderate red wine consumption for 90 days (74). These changes in the red wine consumption group were significantly different when compared with the alcohol abstention group. Taborsky et al. carried out the prospective, multi-center, randomized In Vino Veritas study comparing the effects on healthy subjects between red and white wine consumption (75). The results showed that the changes in total cholesterol, HDL, LDL, triglyceride, liver function, and other markers during the 12-month wine consumption were not varied significantly between red and white wine groups, regardless of the significant difference in the polyphenolic compounds between the two wines. However, when comparing the baseline within each group, both groups showed a significant reduction in LDL for time points at 6 months and 12 months, a significant total cholesterol reduction at 6 months, the red wine group showed a significant HDL reduction at 6 months and a significant total cholesterol reduction at 12 months. Type 2 diabetes is one of the most common metabolic syndromes that co-exist with NAFLD (24). In a 2-year randomized intervention trial, Gepner et al. demonstrated that red wine consumption significantly increased HDL-C levels and decreased the total cholesterol/HDL-C ratio (76). When compared to the non-drinking (water) group, the overall value of metabolic syndrome components was further significantly decreased in the red wine group.
4.2. The effects and putative mechanisms of the phenolic compounds in wine
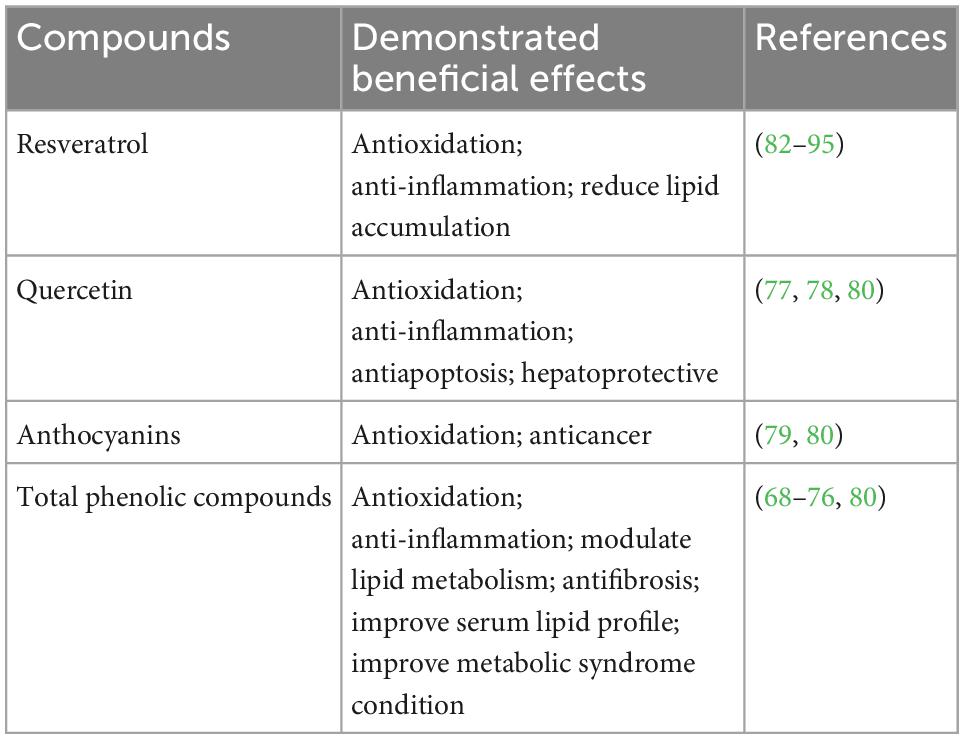
Wine contains water, carbohydrates, organic acids, alcohol, polyphenols, minerals, and B vitamins. The rich phenolic compounds of wine (especially red wine) are thought to provide potential health benefit effects (Table 2). The main phenolic compounds in wine are stilbenes (resveratrol), phenolic acids, and flavonoids (flavan-3-ols, Anthocyanins, quercetin) (77–80). Although the mean level of resveratrol (a type of stilbenes) is 7 mg/L in red wine, the total stilbenes level could be up to 20 mg/L (80, 81). The levels of catechin and epicatechin, as main flavan-3-ols, are approximately 100 and 75 mg/L, respectively. The amount of anthocyanins and quercetin is up to 500 mg/L and around 16 mg/L, respectively (80, 81). Various mechanisms (concerning the polyphenolic compounds in wine) have been proposed for the potential beneficial effects of wine, especially red wine on liver metabolism and NAFLD. These include the antioxidation effects, anti-inflammatory effects, and modulation of lipid metabolism. Resveratrol, one of the most important phenolic compounds in wine, demonstrated the capability to ameliorate antioxidative stress and inflammation and modulated hepatic lipid metabolism (82, 83). Resveratrol is not only an antioxidant, scavenging ROS, HO, peroxyl radicals, and chelating metal ions interacting with ROS (84–86), but also capable of increasing the activity of hepatic antioxidation enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase (87–90). Resveratrol has also been shown to be able to modulate hepatic lipid metabolism by activation of sirtuin 1 (SIRT 1)–AMPK signaling, which on the one hand promotes the fatty acid beta-oxidation by activating peroxisome proliferator-activated receptor α (PPARα), PPARγ co-activator 1α (PGC1α), and their target genes, on the other hand, down-regulates fatty acid synthesis through SREBP-1c inhibition (91). Additionally, resveratrol was shown to reduce intracellular lipid droplets possibly by promoting autophagy in HepG2 cells (92). Resveratrol has also been shown in studies to possess anti-inflammatory properties, such as inhibiting infiltration of macrophage and recruitment of Kupffer cells, reducing TNFα levels (93–95).
The putative mechanisms that phenolic compounds in wine, such as resveratrol involved in can be summarized in three main areas (refer to Figure 2). (1) Antioxidation: as antioxidants scavenging ROS, HO, and peroxyl radicals, increasing quantity and activity of antioxidant enzymes and inhibition of lipid peroxidation; (2) modulating hepatic lipid metabolism: activation of SIRT 1–AMPK signaling leading to inhibition of hepatic lipogenesis and enhancing β-oxidation, promoting lipid autophagy; (3) anti-inflammation: through inhibition of NF-κ B pathways. The exact processing involved is not entirely clear, more studies are needed.
5. The effects of baijiu on NAFLD
5.1. Evidence from laboratory studies
Among the alcoholic beverages consumed globally, 44.8% are spirits. In China, alcohol consumption has been increasing since the 1960s with total recorded consumption reaching equivalent to 5.7 L of pure alcohol per capita in 2016 (Figure 3A). Spirits consumption is 67% of all alcoholic beverages in 2016 (Figure 3B; 1). Baijiu, the main category of spirits consumed in China, is produced by unique multi-strain and solid-state fermentation techniques.
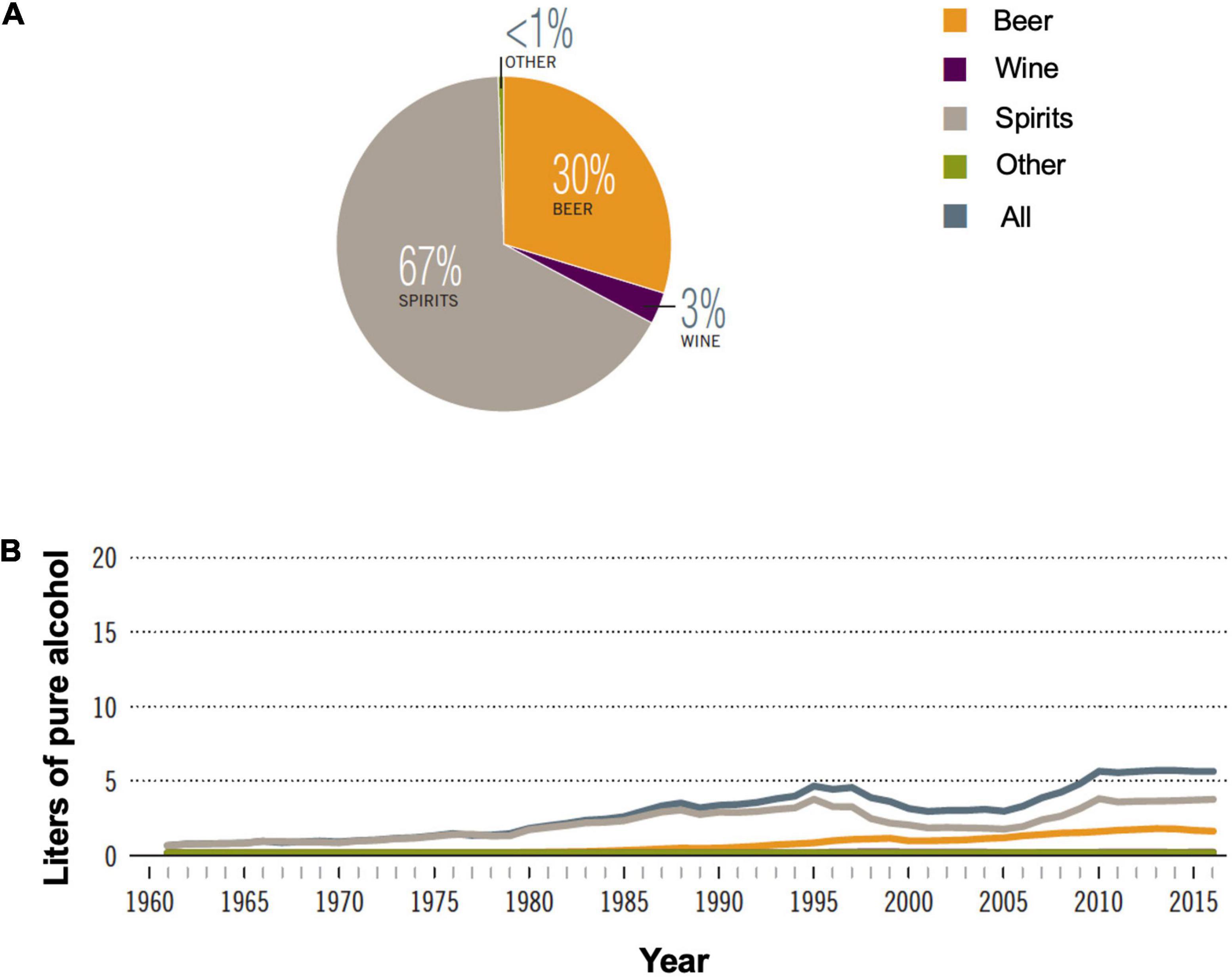
Figure 3. Alcohol consumption in China. (A) Proportion of different alcoholic beverages consumed in 2016; (B) recorded alcohol consumption per capita in adults (age > 15 years), 1961–2016. Adapted with permission from ref (1) under Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 IGO license. (CC BY-NC SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
A study (96) based on the human liver cell line, Hep3B, has shown a non-alcoholic residue of Maotai (a brand of baijiu), is able to up-regulate GST A1, an antioxidant-responsive element. This subsequently promotes antioxidative activity through an ERKs- and p38 K-dependent pathway, which may reduce oxidative stress caused by alcohol metabolism and provide protection to the liver. Subsequent animal studies (97, 98) on Maotai have shown that it has different effects on the liver than that of the same amount of alcohol. It significantly induced various antioxidation factors, heme oxygenase-l, metallothionein, Nrf2, and GCLC. A tetrapeptide from sesame flavor-type baijiu has been shown to promote hepatic antioxidation factors through various mechanisms to counteract the oxidative stress caused by alcohol metabolisms (99). A recent animal study (100) compared the effects of daily consumption of equivalent to approximately three drinks of baijiu or an equivalent amount of pure alcohol solution. Results showed that the baijiu treatment group has significantly less liver injury and steatosis. Further study on approximately 1.5 drinks of baijiu or equivalent pure alcohol solution showed pure alcohol solution treatment group induced significantly higher plasma ALT and hepatic triglyceride levels. The non-alcoholic flavor compounds in baijiu have been shown in an animal study, to be able to attenuate liver damage caused by ethanol potentially through differential impact on host gut microbiota (101).
Animal and in vitro studies have shown baijiu posts less injury to the liver than an equivalent amount of pure alcohol. This coincides with the speculation that the non-alcoholic components, especially biologically active compounds of baijiu may have additional effects on the liver that is apart from alcohol. However, due to the limited evidence, this hypothesis is still controversial. First, more laboratory studies are needed to demonstrate what are the main compounds that could convey these effects (either beneficial or harmful), the mechanisms, and potential interactions between the compounds and ethanol. Second, evidence is needed to show that the amount of compounds in baijiu is sufficient to convey the proposed effects. Moreover, the evidence from the epidemiology and clinical studies is insufficient and inconclusive (102, 103). Better controlled epidemiology and clinical studies, such as randomized controlled trials, would be needed (104).
5.2. The effects and putative mechanisms of the main flavor compounds in baijiu
It is proposed that the non-alcoholic components, such as polyphenols in alcoholic beverages, may have additional beneficial effects (83, 105). Baijiu, a type of distilled spirit produced through solid-state fermentation (106), has contained more than 1,874 kinds of identified flavor compounds (22, 106). Among these compounds, there are at least 138 kinds have been shown to be bioactive (22). It is speculated that the biologically active compounds in baijiu may have some protective effects on the liver from the injury caused by ethanol metabolism when baijiu consumption is low to moderate (Table 3; 22, 100).
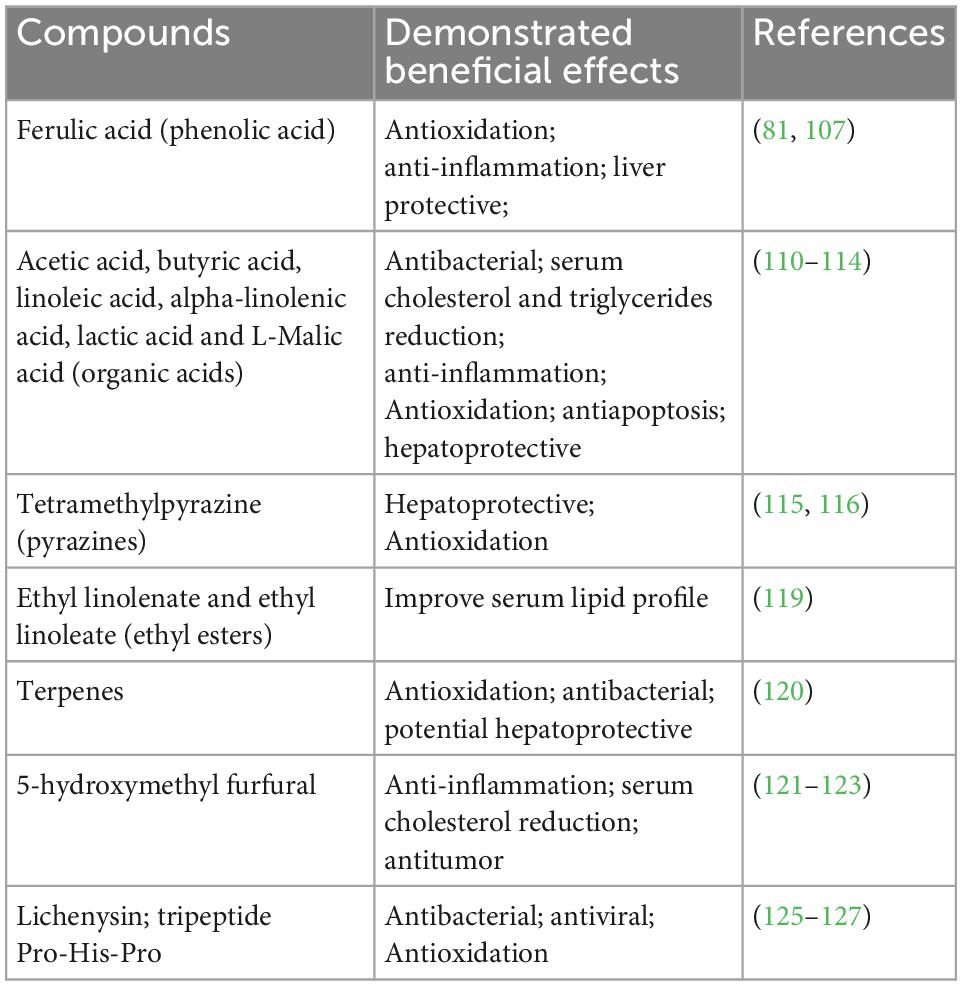
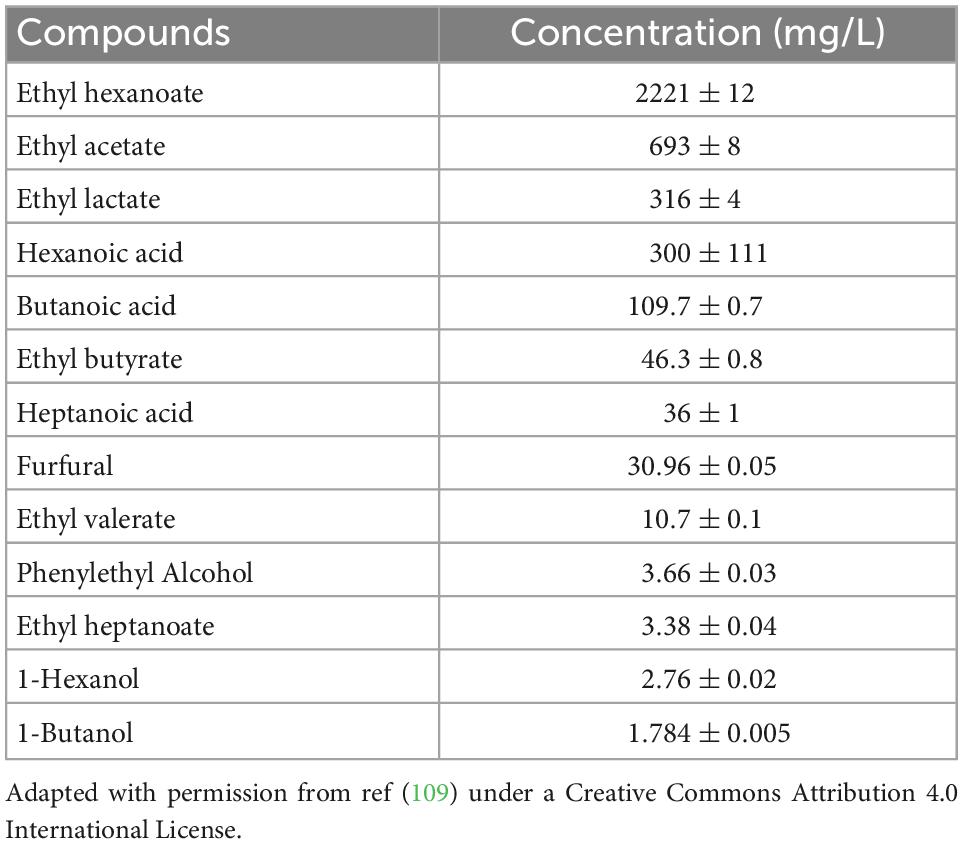
The biological active volatile compounds include phenols, organic acids, esters, terpenes, pyrazines, sulfur compounds, and furan derivants. The non-volatile compounds include polyols, peptides, amino acids, vitamins, and minerals. Various phenols have been identified by techniques, such as GC-MS, HPLC-MS, and GC-TOF-MS. Five of them are shown to have beneficial effects. Ferulic acid, the main ingredient of several Chinese herbals, has been shown to have antioxidation and anti-inflammation effects and may be protective of the liver against the oxidative stress caused by alcohol metabolism (81, 107). Baijiu contains 127 organic acids, which are important to the baijiu flavor. In the sesame-aroma type of baijiu, there are eight acids that have a quantity higher than 10 mg/L (108). In baijiu, Luzhoulaojiao, the quantity of the acid reaches as high as 300 mg/L (Table 4; 109). The acids may potentially have beneficial effects. To date, 16 acids have been reported to be health beneficial. Acetic acid, butyric acid, linoleic acid, alpha-linolenic acid, lactic acid, tartaric acid, and L-Malic acid are typical ones (110–114). The non-saturated acids, such as linoleic and linolenic acids, may improve lipid profiles. The acid may also help regulate liver lipid synthesis. SCFA, such as butyric acid (around 80 mg/L in baijiu), are known to involve in the regulation of energy homeostasis, obesity, immune system, brain function, and colorectal cancer prevention (113). Although the concentration of butyric acid in baijiu is low, it may serve as a source of dietary intake of butyrate to maintain its physiological concentration in the human body.
Pyrazines are a category of biologically active compounds in baijiu and may have health benefits. A pyrazine and its several derivants, such as tetramethylpyrazine, have been detected in Maotai, Laobaigan, Yanghe River Daqu, and Fenjiu. Tetramethylpyrazine is the main active ingredient of Rhizoma Ligustici Chuanxiong, a Chinese herbal that has long been used to treat liver disease and protect the liver from fibrosis (115, 116). It has antioxidation properties and enhances triglyceride degradation. Esters are the major flavor compounds in baijiu. To date, 510 esters have been identified in baijiu (117). For instance, lactic acid ethyl ester is approximately 900 mg/L in soy sauce aroma type baijiu (118). At least five of them have been reported to have beneficial effects. Fatty acid esters, such as ethyl linolenate and ethyl linoleate may have a regulatory property on cholesterol synthesis (119). Alpha-angelica lactone, an important ingredient of Chinese herbal Angelicae sinensis radix and Rhizoma Ligustici Chuanxiong, has been detected in Jiannanchun and Gujinggong. It is shown to protect and regulate the immune system, especially speeding the immune system recovery after chemotherapy. It may potentially regulate liver immune response upon oxidative stress caused by alcohol metabolism and prevent the progression of ALD. Terpenes are a category of important compounds in baijiu. Fifty-two of the seventy-six identified terpenes in baijiu have been reported to be health beneficial (106). They process antioxidation, antiviral, and antibacterial properties, which may potentially be liver protective (120). Its concentration in baijiu can be as high as 3,400–3,600 μg/L. Baijiu also contains sulfur compounds, to date, 73 compounds have been identified. At least six of the identified sulfur compounds have been reported to be beneficial to health. One of their properties is antioxidation, which protects cells from injury from oxidative stress. Furans have also been identified in baijiu, which have antitumor properties. In particular, 5-hydroxymethyl furfural has been shown to inhibit tumor progression and anti-inflammation and is capable to reduce the serum cholesterol level (121–123). Among the non-volatile compounds, peptides are recently identified as bioactive compounds in baijiu. Lichenysin is a lipopeptide identified in Dongjiu (124) with a concentration as high as 112 μg/L. One of its properties is antibacterial activity and antiviral activity (125, 126). Based on structural similarity to surfactin, it is speculated that lichenysin may process antitumor properties through tumor cell G2/M arrest; however, experimental confirmation is needed. A tripeptide Pro-His-Pro (PHP) has been identified in the sesame-aroma type of baijiu Gujinggong (127). An in vitro study on human liver cell line HepG2 cells has demonstrated its ability to up-regulate cellular antioxidation enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase through Nrf2/antioxidant response in the element signaling pathway. Therefore, PHP pre-treatment was able to prevent HepG2 cells from oxidative stress induced by 2,20-azobis (2-methylpropanimidamidine) dihydrochloride.
The proposed mechanisms of the flavor compounds in baijiu involved can be summarized as antioxidation, anti-inflammation, and lipid metabolism modulation (refer to Figure 2). For antioxidation mechanisms, the flavor compounds can act as antioxidants to remove ROS and to up-regulate cellular antioxidation enzyme quantity and activities. For the anti-inflammatory effects, the flavor compounds may alleviate hepatic inflammation by modulating NF-κB-regulated pathways. They can also reduce the hepatic inflammation induced by liver cell endoplasmic reticulum stress caused by intracellular oxidation stress (through antioxidation pathways). For hepatic lipid metabolism modulation, the flavor compounds enhance lipid degradation and β-oxidation and at the same time reduce lipogenesis.
Although the aforementioned compounds in baijiu have potential biological effects, many of them are in low concentrations. For moderate consumption of baijiu, therefore, it is less likely that the intake of each of those low-concentration compounds reaches a sufficient level to exert any effects. Therefore, it is important to identify the main compounds that possess the beneficial effects and potential combinational effects they impose as a whole and elucidate the mechanism underlining the combinational effects.
6. Discussion
It is commonly accepted that excessive alcohol consumption or binge drinking (128) leads to ALD as well as advanced stages of NAFLD. For low to moderate alcohol consumption, controversial evidence exists on ethanol effects on NAFLD from epidemiology and clinical studies, and the mechanism is not entirely clear. The genetic variance of ADH and ALDH among the study population could convey variation in effects on the liver.
In addition, the findings from epidemiology and clinical studies on the effect of the polyphenolic compounds from beer and wine on NAFLD are inconsistent and inconclusive. For epidemiological studies, for instance, there are variations in a range of factors that contribute to the resulting inconsistency. These factors include but are not limited to the drinking patterns (frequency and amount, binge drinking or not, with/without a meal, proportion of wine among total alcohol consumed), variation of wine consumed, biological variation of investigated subjects (ethnic, gender, age, health status, etc.), study duration, and population. Hence, more well-controlled, long-term, randomized trials are needed.
For baijiu, there are very limited epidemiology and clinical studies available, most of the evidence is from laboratory based in vitro and in vivo studies. Different from beer and wine, baijiu contains a much higher concentration of alcohol. One could speculate low to moderate baijiu consumption could result in a much lower intake of bio-activate non-alcoholic flavor compounds for any potential beneficial effects. However, laboratory studies demonstrate the significant effects between baijiu and pure ethanol (100, 102). More laboratory studies are needed to first verify this difference and then elucidate the reason/mechanisms behind it. In addition, more specifically designed, baijiu based epidemiology, and clinical studies are needed to further investigate the effects of baijiu on the liver and the mechanism.
7. Conclusion
The non-alcoholic bioactive flavor compounds in beer, wine, and baijiu have been shown beneficial to NAFLD. The underline mechanism for the beneficial effects is proposed to involve modulation of lipid metabolism, reduction of oxidative stress and damages, and alleviation of inflammation. However, it is inconclusive whether low to moderate consumption of these three types of beverages is beneficial to NAFLD. For patients with NAFLD, it is recommended to abstain, although a low level of alcohol consumption may be alright. For normal people, the recommendation is either abstaining or consuming low to moderate alcohol without binge drinking.
Author contributions
YZ: conceptualization and drafting of the manuscript. JH: manuscript writing. ZH: conceptualization, manuscript draft review, and modification. All authors contributed to the article and approved the submitted version.
Funding
This research was kindly supported by the Science and Technology Bureau of Sichuan Province (China) project grant (2019YJ0461), Sichuan Provincial Academician (Expert) Workstation (China) project grant (2018YSGZZ03), and Sichuan University of Science and Engineering (China) seeding grant (2017RCL72).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. WHO. Global Information Sytem on Alcohol and Health (GISAH). Geneva: World Health Organization (2016).
3. Singal A, Anand B. Recent trends in the epidemiology of alcoholic liver disease. Clin Liver Dis. (2013) 2:53–6. doi: 10.1002/cld.168
4. Lim S, Vos T, Flaxman A, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2224–60. doi: 10.1016/S0140-6736(12)61766-8
5. Poli A, Marangoni F, Avogaro A, Barba G, Bellentani S, Bucci M, et al. Moderate alcohol use and health: a consensus document. Nutr Metab Cardiovasc Dis. (2013) 23:487–504. doi: 10.1016/j.numecd.2013.02.007
6. Wood A, Kaptoge S, Butterworth A, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. (2018) 391:1513–23. doi: 10.1016/S0140-6736(18)30134-X
7. French S. How to prevent alcoholic liver disease. Exp Mol Pathol. (2015) 98:304–7. doi: 10.1016/j.yexmp.2015.03.007
8. Molina P, Gardner J, Souza-Smith F, Whitaker A. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology. (2014) 29:203–15. doi: 10.1152/physiol.00055.2013
9. Yoon S, Jung J, Lee S, Kim J, Ahn S, Shin E, et al. The protective effect of alcohol consumption on the incidence of cardiovascular diseases: is it real? A systematic review and meta-analysis of studies conducted in community settings. BMC Public Health. (2020) 20:90. doi: 10.1186/s12889-019-7820-z
10. Thun M, Peto R, Lopez A, Monaco J, Henley S, Heath C, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Eng J Med. (1997) 337:1705–14.
11. Scheideler J, Klein W. Awareness of the link between alcohol consumption and cancer across the world: a review. Cancer Epidemiol Biomarkers Prev. (2018) 27:429–37. doi: 10.1158/1055-9965.Epi-17-0645
12. Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. (2014) 25:1526–35. doi: 10.1093/annonc/mdu020
13. Di Castelnuovo A, Costanzo S, Bagnardi V, Donati M, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. (2006) 166:2437–45. doi: 10.1001/archinte.166.22.2437
14. Islami F, Tramacere I, Rota M, Bagnardi V, Fedirko V, Scotti L, et al. Alcohol drinking and laryngeal cancer: overall and dose–risk relation – A systematic review and meta-analysis. Oral Oncol. (2010) 46:802–10. doi: 10.1016/j.oraloncology.2010.07.015
15. Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose–response meta-analysis of published studies. Ann Oncol. (2011) 22:1958–72. doi: 10.1093/annonc/mdq653
16. Tramacere I, Scotti L, Jenab M, Bagnardi V, Bellocco R, Rota M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer. (2010) 126:1474–86. doi: 10.1002/ijc.24936
17. Hamajima N, Hirose K, Tajima K, Rohan T, Calle E, Heath C Jr, et al. Alcohol, tobacco and breast cancer – Collaborative reanalysis of individual data from 53 epidemiological studies, including 58 515 women with breast cancer and 95 067 women without the disease. Br J Cancer. (2002) 87:1234–45. doi: 10.1038/sj.bjc.6600596
18. Ronksley P, Brien S, Turner B, Mukamal K, Ghali W. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and metaanalysis. Br Med J. (2011) 342:d671.
19. Anstey K, Mack H, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. (2009) 17:542–55.
20. Lazarus R, Sparrow D, Weiss S. Alcohol intake and insulin levels. The normative aging study. Am J Epidemiol. (1997) 145:909–16.
21. Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, et al. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (bruneck study). Br Med J. (1996) 313:1040–4. doi: 10.1136/bmj.313.7064.1040
22. Wu J, Huang M, Zheng F, Sun J, Sun X, Li H, et al. Research progress of healthy baijiu. J Food Sci Technol. (2019) 37:17–23.
23. Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein H, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. (2000) 133:411–9.
24. Byrne C, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62(Suppl. 1):S47–64. doi: 10.1016/j.jhep.2014.12.012
25. Younossi Z, Koenig A, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
26. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal A. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. (2018) 67:123–33. doi: 10.1002/hep.29466
27. Friedman S, Neuschwander-Tetri B, Rinella M, Sanyal A. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
28. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. (2013) 24:301–8. doi: 10.1093/annonc/mds337
29. Ajmera V, Terrault N, Harrison S. Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? A critical review. Hepatology. (2017) 65:2090–9. doi: 10.1002/hep.29055
30. Chhimwal J, Patial V, Padwad Y. Beverages and non-alcoholic fatty liver disease (NAFLD): think before you drink. Clin Nutr. (2021) 40:2508–19. doi: 10.1016/j.clnu.2021.04.011
31. Liu Y, Zhou L, Gr Y, Zhang H, Li Y, Tang X, et al. Effect of liquors on serum lipid and key enzymes in lipid metabolismof rats. Mod Prev Med. (2017) 44:4151–5.
32. Rs Z, Ping Z, Bai XQ, Shi J. The influence of drinking frequency to high-density lipoprotein cholesterol level. Natl Med Front China. (2012) 7:95–6.
33. Wu S, Zhang Q, Qi C, Qin T, Tian Q, Jing C, et al. Effect of alcohol consumption on cardio-cerebrovascular events in male diabetic population. Chin J Hypertens. (2011) 19:1065–9.
34. Di Ciaula A, Bonfrate L, Krawczyk M, Frühbeck G, Portincasa P. Synergistic and detrimental effects of alcohol intake on progression of liver steatosis. Int J Mol Sci. (2022) 23:2636. doi: 10.3390/ijms23052636
35. Idalsoaga F, Kulkarni A, Mousa O, Arrese M, Arab J. Non-alcoholic fatty liver disease and alcohol-related liver disease: two intertwined entities. Front Med. (2020) 7:448. doi: 10.3389/fmed.2020.00448
36. Teschke R. Alcoholic liver disease: current mechanistic aspects with focus on their clinical relevance. Biomedicines. (2019) 7:8. doi: 10.3390/biomedicines7030068
37. Teschke R. Alcoholic liver disease: alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines. (2018) 6:106. doi: 10.3390/biomedicines6040106
38. Teschke R. Alcoholic steatohepatitis (ASH) and alcoholic hepatitis (AH): cascade of events, clinical aspects, and pharmacotherapy options. Expert Opin Pharmacother. (2018) 19:779–93. doi: 10.1080/14656566.2018.1465929
39. Ramchandani V. Genetics of alcohol metabolism. In: RR Watson editor. Alcohol, Nutrition and Health Consequences. New York, NY: Springer Science (2013) p. 15–25.
40. Chi Y, Lee S, Lai C, Lee Y, Lee S, Chiang C, et al. Ethanol oxidation and the inhibition by drugs in human liver, stomach and small intestine: quantitative assessment with numerical organ modeling of alcohol dehydrogenase isozymes. Chem Biol Interact. (2016) 258:134–41. doi: 10.1016/j.cbi.2016.08.014
41. Jones A. Alcohol, its absorption, distribution, metabolism, and excretion in the body and pharmacokinetic calculations. WIREs Forensic Sci. (2019) 1:e1340. doi: 10.1002/wfs2.1340
42. Lieber C, DeCarli L, Matsuzaki S, Ohnishi K, Teschke R. The microsomal ethanol oxidizing system (Meos). Methods Enzyme. (1978) 52:355–68.
43. Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. (2014) 20:17756–72.
44. Cohen D, Mason K, Farley T. Beer consumption and premature mortality in louisiana: an ecologic analysis. J Stud Alcohol. (2004) 65:398–403. doi: 10.15288/jsa.2004.65.398
45. Becker U, Gronbaek M, Johansen D, Sorensen T. Lower risk for alcohol-induced cirrhosis in wine drinkers. Hepatology. (2002) 35:868–75. doi: 10.1053/jhep.2002.32101
46. Renaud S, Guéguen R, Siest G, Salamon R. Wine, beer, and mortality in middle-aged men from Eastern France. Arch Intern Med. (1999) 159:1865–70. doi: 10.1001/archinte.159.16.1865
47. Sillanaukee P, van der Gaag M, Sierksma A, Hendriks H, Strid N, Pönniö M, et al. Effect of type of alcoholic beverages on carbohydrate-deficient transferrin, sialic acid, and liver enzymes. Alcohol Clin Exp Res. (2003) 27:57–60. doi: 10.1097/01.Alc.0000047302.67780.Fa
48. Romeo J, González-Gross M, Wärnberg J, Díaz L, Marcos A. Effects of moderate beer consumption on blood lipid profile in healthy Spanish adults. Nutr Metab Cardiovasc Dis. (2008) 18:365–72. doi: 10.1016/j.numecd.2007.03.007
49. Sierksma A, van der Gaag M, Kluft C, Hendriks H. Moderate alcohol consumption reduces plasma c-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. (2002) 56:1130–6. doi: 10.1038/sj.ejcn.1601459
50. Maldonado M, Moreno H, Calvo J. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin Nutr. (2009) 28:188–91. doi: 10.1016/j.clnu.2009.02.001
51. Gorinstein S, Caspi A, Libman I, Leontowicz H, Leontowicz M, Tashma Z, et al. Bioactivity of beer and its influence on human metabolism. Int J Food Sci Nutr. (2007) 58:94–107. doi: 10.1080/09637480601108661
52. Gasowski B, Leontowicz M, Leontowicz H, Katrich E, Lojek A, Ciz M, et al. The influence of beer with different antioxidant potential on plasma lipids, plasma antioxidant capacity, and bile excretion of rats fed cholesterol-containing and cholesterol-free diets. J Nutr Biochem. (2004) 15:527–33. doi: 10.1016/j.jnutbio.2004.03.004
53. Gonzalez-Munoz M, Meseguer I, Sanchez-Reus M, Schultz A, Olivero R, Benedi J, et al. Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem Toxicol. (2008) 46:1111–8. doi: 10.1016/j.fct.2007.11.006
54. Tung W, Rizzo B, Dabbagh Y, Saraswat S, Romanczyk M, Codorniu-Hernandez E, et al. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch Biochem Biophys. (2020) 694:108589. doi: 10.1016/j.abb.2020.108589
55. Shafreen R, Lakshmi S, Pandian S, Park Y, Kim Y, Pasko P, et al. Unraveling the antioxidant, binding and health-protecting properties of phenolic compounds of beers with main human serum proteins: in vitro and in silico approaches. Molecules. (2020) 25:4962. doi: 10.3390/molecules25214962
56. Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Fuchs D. Beer down-regulates activated peripheral blood mononuclear cells in vitro. Int Immunopharmacol. (2006) 6:390–5. doi: 10.1016/j.intimp.2005.09.002
57. Osorio-Paz I, Brunauer R, Alavez S. Beer and its non-alcoholic compounds in health and disease. Crit Rev Food Sci Nutr. (2020) 60:3492–505. doi: 10.1080/10408398.2019.1696278
58. de Gaetano G, Costanzo S, Di Castelnuovo A, Badimon L, Bejko D, Alkerwi A, et al. Effects of moderate beer consumption on health and disease: a consensus document. Nutr Metab Cardiovasc Dis. (2016) 26:443–67. doi: 10.1016/j.numecd.2016.03.007
59. Liu M, Hansen P, Wang G, Qiu L, Dong J, Yin H, et al. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus Lupulus). Molecules. (2015) 20:754–79. doi: 10.3390/molecules20010754
60. Samuels J, Shashidharamurthy R, Rayalam S. Novel anti-obesity effects of beer hops compound xanthohumol: role of AMPK signaling pathway. Nutr Metab. (2018) 15:42. doi: 10.1186/s12986-018-0277-8
61. Lima-Fontes M, Costa R, Rodrigues I, Soares R. Xanthohumol restores hepatic glucolipid metabolism balance in type 1 diabetic wistar rats. J Agric Food Chem. (2017) 65:7433–9. doi: 10.1021/acs.jafc.7b02595
62. Costa R, Rodrigues I, Guardão L, Rocha-Rodrigues S, Silva C, Magalhães J, et al. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J Nutr Biochem. (2017) 45:39–47. doi: 10.1016/j.jnutbio.2017.03.006
63. Costa R, Negrão R, Valente I, Castela Â, Duarte D, Guardão L, et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J Nat Prod. (2013) 76:2047–53. doi: 10.1021/np4002898
64. Ferk F, Huber W, Filipic M, Bichler J, Haslinger E, Misík M, et al. Xanthohumol, a prenylated flavonoid contained in beer, prevents the induction of preneoplastic lesions and DNA damage in liver and colon induced by the heterocyclic aromatic amine amino-3-methyl-imidazo[4,5-F]quinoline (Iq). Mutat Res. (2010) 691:17–22. doi: 10.1016/j.mrfmmm.2010.06.006
65. Trius-Soler M, Marhuenda-Munoz M, Laveriano-Santos E, Martinez-Huelamo M, Sasot G, Storniolo C, et al. Moderate consumption of beer (with and without Ethanol) and menopausal symptoms: results from a parallel clinical trial in postmenopausal women. Nutrients. (2021) 13:2278. doi: 10.3390/nu13072278
66. Landmann M, Sellmann C, Engstler A, Ziegenhardt D, Jung F, Brombach C, et al. Hops (Humulus Lupulus) content in beer modulates effects of beer on the liver after acute ingestion in female mice. Alcohol Alcohol. (2017) 52:48–55. doi: 10.1093/alcalc/agw060
67. Hege M, Jung F, Sellmann C, Jin C, Ziegenhardt D, Hellerbrand C, et al. An iso-α-acid-rich extract from hops (Humulus Lupulus) attenuates acute alcohol-induced liver steatosis in mice. Nutrition. (2018) 45:68–75. doi: 10.1016/j.nut.2017.07.010
68. Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. (1992) 339:1523–6. doi: 10.1016/0140-6736(92)91277-f
69. Dunn W, Xu R, Schwimmer J. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. (2008) 47:1947–54. doi: 10.1002/hep.22292
70. Mitchell T, Jeffrey G, de Boer B, MacQuillan G, Garas G, Ching H, et al. Type and pattern of alcohol consumption is associated with liver fibrosis in patients with non-alcoholic fatty liver disease. Am J Gastroenterol. (2018) 113:1484–93. doi: 10.1038/s41395-018-0133-5
71. Simpson R, Hermon C, Liu B, Green J, Reeves G, Beral V, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK million women study. Lancet Public Health. (2019) 4:e41–8. doi: 10.1016/s2468-2667(18)30230-5
72. Beulens J, van Beers R, Stolk R, Schaafsma G, Hendriks H. The effect of moderate alcohol consumption on fat distribution and adipocytokines. Obesity. (2006) 14:60–6. doi: 10.1038/oby.2006.8
73. Rajdl D, Racek J, Trefil L, Siala K. Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiol Res. (2007) 56:203–12. doi: 10.33549/physiolres.930936
74. Kechagias S, Zanjani S, Gjellan S, Leinhard O, Kihlberg J, Smedby O, et al. Effects of moderate red wine consumption on liver fat and blood lipids: a prospective randomized study. Ann Med. (2011) 43:545–54. doi: 10.3109/07853890.2011.588246
75. Taborsky M, Ostadal P, Adam T, Moravec O, Gloger V, Schee A, et al. Red or white wine consumption effect on atherosclerosis in healthy individuals (in vino veritas study). Bratisl Lek Listy. (2017) 118:292–8. doi: 10.4149/bll_2017_072
76. Gepner Y, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Shelef I, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized controlled trial. Ann Intern Med. (2015) 163:569–79. doi: 10.7326/m14-1650
77. Miltonprabu S, Tomczyk M, Skalicka-Woźniak K, Rastrelli L, Daglia M, Nabavi S, et al. Hepatoprotective effect of quercetin: from chemistry to medicine. Food Chem Toxicol. (2017) 108(Pt B):365–74. doi: 10.1016/j.fct.2016.08.034
78. Auger C, Teissedre P, Gérain P, Lequeux N, Bornet A, Serisier S, et al. Dietary wine phenolics catechin, quercetin, and resveratrol efficiently protect hypercholesterolemic hamsters against aortic fatty streak accumulation. J Agric Food Chem. (2005) 53:2015–21. doi: 10.1021/jf048177q
79. Rivero-Pérez M, Muñiz P, González-Sanjosé M. Contribution of anthocyanin fraction to the antioxidant properties of wine. Food Chem Toxicol. (2008) 46:2815–22. doi: 10.1016/j.fct.2008.05.014
80. Forester S, Waterhouse A. Metabolites are key to understanding health effects of wine polyphenolics. J Nutr. (2009) 139:1824S–31S. doi: 10.3945/jn.109.107664
81. Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. (2021) 13:273. doi: 10.3390/nu13010273
82. Bedê T, de Jesus V, Rosse de Souza V, Mattoso V, Abreu J, Dias J, et al. Effect of grape juice, red wine and resveratrol solution on antioxidant, anti-inflammatory, hepactic function and lipid profile in rats feds with high-fat diet. Nat Prod Res. (2020) 35:5255–60. doi: 10.1080/14786419.2020.1747458
83. Silva P, Fernandes E, Carvalho F. Dual effect of red wine on liver redox status: a concise and mechanistic review. Arch Toxicol. (2015) 89:1681–93. doi: 10.1007/s00204-015-1538-1
84. López-Vélez M, Martínez-Martínez F, Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit Rev Food Sci Nutr. (2003) 43:233–44. doi: 10.1080/10408690390826509
85. Cai Y, Fang J, Ma L, Yang L, Liu Z. Inhibition of free radical-induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim Biophys Acta. (2003) 1637:31–8. doi: 10.1016/S0925-4439(02)00174-6
86. Gülçin I. Antioxidant properties of resveratrol: a structure–activity insight. Innov Food Sci Emerg Technol. (2010) 11:210–8. doi: 10.1016/j.ifset.2009.07.002
87. Yang H, Lee M, Kim Y. Protective activities of stilbene glycosides from acer mono leaves against h2o2-induced oxidative damage in primary cultured rat hepatocytes. J Agric Food Chem. (2005) 53:4182–6. doi: 10.1021/jf050093
88. Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, et al. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. (2007) 80:1033–9. doi: 10.1016/j.lfs.2006.11.044
89. Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. (2008) 8:40. doi: 10.1186/1471-230X-8-40
90. Hassan-Khabbar S, Vamy M, Cottart C, Wendum D, Vibert F, Savouret J, et al. Protective effect of post-ischemic treatment with trans-resveratrol on cytokine production and neutrophil recruitment by rat liver. Biochimie. (2010) 92:405–10. doi: 10.1016/j.biochi.2009.12.009
91. Ajmo J, Liang X, Rogers C, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. (2008) 295:G833–42. doi: 10.1152/ajpgi.90358.2008
92. Tang L, Yang F, Fang Z, Hu C. Resveratrol ameliorates alcoholic fatty liver by inducing autophagy. Am J Chin Med. (2016) 44:1207–20. doi: 10.1142/s0192415x16500671
93. Jeon B, Jeong E, Shin H, Lee Y, Lee D, Kim H, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. (2012) 61:1444–54. doi: 10.2337/db11-1498
94. Chan C, Lee K, Huang Y, Chou C, Lin H, Lee F. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J Gastroenterol Hepatol. (2014) 29:603–13. doi: 10.1111/jgh.12366
95. Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, et al. Resveratrol modulates autophagy and nf-κb activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. (2014) 63:166–73. doi: 10.1016/j.fct.2013.08.036
96. Zhang D, Lu H, Li J, Shi X, Huang C. Essential roles of ERKS and P38k in up-regulation of GST A1 expression by maotai content in human hepatoma cell line Hep3b. Mol Cell Biochem. (2006) 293:161–71. doi: 10.1007/s11010-006-9238-z
97. Yi X, Long L, Yang C, Lu Y, Cheng M. Maotai ameliorates diethylnitrosamine-initiated hepatocellular carcinoma formation in mice. PLoS One. (2014) 9:e93599. doi: 10.1371/journal.pone.0093599
98. Liu J, Cheng M, Shi J, Yang Q, Wu J, Li C, et al. Differential effects between maotai and ethanol on hepatic geneexpression in mice: possible role of metallothionein and heme oxygenase-1 induction by maotai. Exp Biol Med. (2006) 231:1535–41. doi: 10.1177/153537020623100913
99. Wu J, Huo J, Huang M, Zhao M, Luo X, Sun B. Structural characterization of a tetrapeptide from sesame flavor-type baijiu and its preventive effects against aaph-induced oxidative stress in Hepg2 cells. J Agric Food Chem. (2017) 65:10495–504. doi: 10.1021/acs.jafc.7b04815
100. Fang C, Du H, Zheng X, Zhao A, Jia W, Xu Y. Solid-state fermented Chinese alcoholic beverage (baijiu) and ethanol resulted in distinct metabolic and microbiome responses. FASEB J. (2019) 33:7274–88. doi: 10.1096/fj.201802306R
101. Fang C, Zhou Q, Liu Q, Jia W, Xu Y. Crosstalk between gut microbiota and host lipid metabolism in a mouse model of alcoholic liver injury by chronic baijiu or ethanol feeding. Food Funct. (2022) 13:596–608. doi: 10.1039/d1fo02892h
102. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. (2009) 373:2223–33.
103. Rimm E, Klatsky A, Grobbee D, Stampfer M. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. Br Med J. (1996) 312:731–6.
104. Thelle D. Alcohol and heart health: the need for a randomized controlled trial. Eur J Prev Cardiol. (2020) 27:1964–6. doi: 10.1177/2047487320914433
105. Visioli F, Lastra C, Andres-Lacueva C, Aviram M, Calhau C, Cassano A, et al. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. (2011) 51:524–46.
106. Liu H, Sun B. Effect of fermentation processing on the flavor of baijiu. J Agric Food Chem. (2018) 66:5425–32. doi: 10.1021/acs.jafc.8b00692
107. Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep. (2019) 24:e00370. doi: 10.1016/j.btre.2019.e00370
108. Wu T, Zhu S, Sun X, Zhao W, Cui G. Analysis of health factors of meilanchun sesame-flavor liquor. Liquor Making Sci Technol. (2013) 8:125–30.
109. Yao F, Yi B, Shen C, Tao F, Liu Y, Lin Z, et al. Chemical analysis of the Chinese liquor luzhoulaojiao by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Sci Rep. (2015) 5:9553. doi: 10.1038/srep09553
110. Budak N, Aykin E, Seydim A, Greene A, Guzel-Seydim Z. Functional properties of vinegar. J Food Sci. (2014) 79:R757–64. doi: 10.1111/1750-3841.12434
111. Saini R, Keum Y. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance - A review. Life Sci. (2018) 203:255–67. doi: 10.1016/j.lfs.2018.04.049
112. Zhou H, Xin-Yan Y, Yu W, Liang X, Du X, Liu Z, et al. Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Int Rev Immunol. (2022) 41:4–18. doi: 10.1080/08830185.2021.1955876
113. Stilling R, van de Wouw M, Clarke G, Stanton C, Dinan T, Cryan J. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
114. Chi Z, Wang Z, Wang G, Khan I, Chi Z. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit Rev Biotechnol. (2016) 36:99–107. doi: 10.3109/07388551.2014.924474
115. Ge H, Lin P, Luo T, Yan Z, Xiao J, Miao S, et al. Fabrication of ligusticum chuanxiong polylactic acid microspheres: a promising way to enhance the hepatoprotective effect on bioactive ingredients. Food Chem. (2020) 317:126377. doi: 10.1016/j.foodchem.2020.126377
116. Mo Z, Liu Y, Li C, Xu L, Wen L, Xian Y, et al. Protective effect of SFE-Co2 of ligusticum chuanxiong hort against d-galactose-induced injury in the mouse liver and kidney. Rejuvenation Res. (2017) 20:231–43. doi: 10.1089/rej.2016.1870
117. Xu Y, Zhao J, Liu X, Zhang C, Zhao Z, Li X, et al. Flavor mystery of Chinese traditional fermented baijiu: the great contribution of ester compounds. Food Chem. (2022) 369:130920. doi: 10.1016/j.foodchem.2021.130920
118. Fang C, Du H, Jia W, Xu Y. Compositional differences and similarities between typical Chinese baijiu and western liquor as revealed by mass spectrometry-based metabolomics. Metabolites. (2018) 9:2. doi: 10.3390/metabo9010002
119. Spitalniak-Bajerska K, Szumny A, Pogoda-Sewerniak K, Kupczynski R. Effects of N-3 fatty acids on growth, antioxidant status, and immunity of preweaned dairy calves. J Dairy Sci. (2020) 103:2864–76. doi: 10.3168/jds.2019-17001
120. Kiyama R. Estrogenic terpenes and terpenoids: pathways, functions and applications. Eur J Pharmacol. (2017) 815:405–15. doi: 10.1016/j.ejphar.2017.09.049
121. Chow P, Kourghi M, Pei J, Nourmohammadi S, Yool A. 5-Hydroxymethyl-furfural and structurally related compounds block the ion conductance in human aquaporin-1 channels and slow cancer cell migration and invasion. Mol Pharmacol. (2020) 98:38–48. doi: 10.1124/mol.119.119172
122. Lin N, Liu T, Lin L, Lin S, Zang Q, He J, et al. Comparison of in vivo immunomodulatory effects of 5-hydroxymethylfurfural and 5, 5’-oxydimethylenebis (2-furfural). Regul Toxicol Pharmacol. (2016) 81:500–11. doi: 10.1016/j.yrtph.2016.10.008
123. Li W, Qu X, Han Y, Zheng S, Wang J, Wang Y. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-hmf) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int J Mol Sci. (2015) 16:2446–57. doi: 10.3390/ijms16022446
124. Zhang R, Wu Q, Xu Y, Qian M. Isolation, identification, and quantification of lichenysin, a novel nonvolatile compound in Chinese distilled spirits. J Food Sci. (2014) 79:C1907–15. doi: 10.1111/1750-3841.12650
125. Grangemard I, Wallach J, Maget-Dana R, Peypoux F. Lichenysin: a more efficient cation chelator than surfactin. Appl Biochem Biotechnol. (2001) 90:199–210. doi: 10.1385/abab:90:3:199
126. Galie S, Garcia-Gutierrez C, Miguelez E, Villar C, Lombo F. Biofilms in the food industry: health aspects and control methods. Front Microbiol. (2018) 9:898. doi: 10.3389/fmicb.2018.00898
127. Wu J, Sun B, Luo X, Zhao M, Zheng F, Sun J, et al. Cytoprotective effects of a tripeptide from Chinese baijiu against aaph-induced oxidative stress in Hepg2 Cells Via NRF2 Signaling. RSC Adv. (2018) 8:10898–906. doi: 10.1039/c8ra01162a
Keywords: non-alcoholic fatty liver disease, flavor compound, beer, wine, baijiu
Citation: Zhou Y, Hua J and Huang Z (2023) Effects of beer, wine, and baijiu consumption on non-alcoholic fatty liver disease: Potential implications of the flavor compounds in the alcoholic beverages. Front. Nutr. 9:1022977. doi: 10.3389/fnut.2022.1022977
Received: 19 August 2022; Accepted: 07 December 2022;
Published: 06 January 2023.
Edited by:
Mingquan Huang, Beijing Technology and Business University, ChinaReviewed by:
Weizheng Sun, South China University of Technology, ChinaJiajia Song, Southwest University, China
Copyright © 2023 Zhou, Hua and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Huang,  aHpnd3d3QDEyNi5jb20=
aHpnd3d3QDEyNi5jb20=
†These authors have contributed equally to this work
 Yabin Zhou
Yabin Zhou Jin Hua1,3†
Jin Hua1,3†