- National Health Commission Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, Department of Endocrinology and Metabolism, Institute of Endocrinology, The First Hospital of China Medical University, Shenyang, China
Thyroid cancer (TC) is the most frequent endocrine malignancy. The incidence of TC, especially papillary thyroid carcinoma (PTC), has continued to rise all over the world during the past few years, for reasons that are not entirely clear. Though the phenomenon of overdiagnosis is occurring, it is not the sole driver of the substantial increase in incidence. Lifestyle, environmental factors, or complications are considered to be potential risk factors. Among these factors, iodine is a micronutrient that is vital to thyroid function. The effect of iodine intake on PTC has been controversial for many years and the epidemiological or experimental studies provided diametrically opposite conclusions. Combining all these studies, we found that iodine nutrition may affect the overall prevalence, distribution of the histological types, and clinicopathological aggressiveness of TC, especially PTC. However, the available evidence is poor due to the impact of various internal and external related factors. Therefore, this article sums up available results from both epidemiological and experimental studies, future studies are also warranted to expound on the relationship between overall PTC prevalence and iodine intake.
Introduction
Though the global thyroid cancer (TC) incidence has grown remarkably over the past few years (1–3), the mortality rate remains static (4, 5). There are four main kinds of TC: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid carcinoma (MTC), and anaplastic thyroid cancer (ATC) (6). ATC, one of the fatal and rare forms of TC (1–2%) that generally presents as a rapidly growing neck tumor (7), needs early, accurate identification and timely treatment (8). However, most TC especially PTC differentiates well and has a low risk of becoming malignant. Thus, it is necessary to adjust the treatment plan according to the specific situation to avoid overtreatments and identify controllable risk factors to conduct preventive programs. Risk factors including radiation exposure, dietary nutrition, BMI (9), metabolic syndrome (10), environmental pollutants, family history of thyroid nodules, and overdiagnosis have been reported (11). Despite overdiagnosis, environmental/lifestyle factors do contribute to some increase in TC prevalence (12–16).
Dietary iodine intake has also been speculated as a risk factor that may influence the occurrence and development of PTC (17), but inconsistencies in research results have led to great controversy throughout these years. Iodine is a crucial micronutrient and a vital composition for the biosynthesis of thyroid hormone which plays a part in various biochemical and metabolic pathways throughout the human body (18, 19). The thyroid can maintain normal function, and keep thyroid hormone and thyroid stimulating hormone (TSH) in an appropriate ratio through automatic regulation even though daily iodine intake fluctuates widely. A U curve has been come up by many studies (20–22), both chronic iodine deficiency and excess can lead to thyroid dysfunction by interfering with homeostasis (23, 24), which means that the dose-based effects of iodine nutrition on the prevalence of TC need to be considered.
At the population level, the main sources of iodine intake include salt (25), water, milk, and seaweed (26, 27). The thyroid gland actively uptakes about 120 μg of iodine per day, which is distributed to a reservoir in the thyroid that contains about 5,000–10,000 μg of iodine. Monoiodothyronine and diiodothyronine are deiodinated in the periphery and T4 is converted to T3, resulting in the return of 60 μg of iodine per day to the external thyroid reservoir. Approximately 110 μg of iodine (about 97% of daily intake) is excreted in the urine, preserving the normal daily equilibrium (28). Thus urinary iodine concentration (UIC) has been considered a sensitive indicator of recent iodine intake (29–31). The status of iodine nutrition can be divided into four stages based on UIC according to WHO iodine recommendations: UIC < 100 μg/L (insufficient), 100–199 μg/L (adequate), 200–299 μg/L (above requirements), and ≥ 300 μg/L (excessive) (32, 33). To adjust for the influences from dilution of the urine, the proportion of urinary I/Cr is also used to evaluate the iodine status (30). Creatinine-adjusted UIC: < 85 μg/g Cr (deficiency), 85–219 μg/g Cr (adequate), and ≥ 220 μg/g Cr (excessive). So as a satisfying bioindicator of the iodine level (34, 35), UIC and urinary I/Cr ratio have been examined in various epidemiological studies aimed to clarify the association between iodine intake and PTC risk.
Therefore, we overview the standpoints from relevant epidemiological studies and experiments to clarify the correlations between iodine nutrition and PTC.
Epidemiological studies
Effect of iodine intake on thyroid cancer
Mandatory universal salt iodization (USI) has been put into practice since the 1990s (33), which meets the iodine requirements and gained notable success in preventing iodine deficiency in the general population (36). The ensuing question is whether iodine affects the onset of TC. Numerous epidemiological researches have evaluated the relationship between iodine intake and TC, and have presented a variety of views. Although some studies supported that there is no clear association between iodine nutrition and TC (37–39), a large number of studies these years have provided evidence for the relationship. Most studies were affected by many factors such as ethnic differences, diet customs (23), lifestyle, complications, and other environmental factors which can influence the development of TC. For example, the occurrence rate of TC was increased in two areas of high iodine intake: Iceland (40) and Hawaii (41). However, the natural radiation here is higher than in many other areas, so the radiation here can also drive the development of TC, especially in childhood (42, 43). Therefore, these studies cannot offer persuasive evidence to prove that high iodine intake can be a hazardous factor for TC.
Credible evidence was also presented in some epidemiological studies. A 1992 study evaluated the prevalence of TC in patients with goiter in iodine excess and iodine deficiency areas (44), which proved that individuals with excessive iodine intake had a considerably higher risk of TC than individuals in iodine deficiency regions. What’s more, our epidemiological study in 2006 also investigated thyroid diseases over 5 years in three representative regions with insufficient, normal, and excessive iodine intake (45). No cases of TC were diagnosed in insufficient and normal iodine supplementation areas at baseline; however, 10 subjects were found to have PTC in Huanghua, one region with excessive intake of iodine. Between 1999 and 2004, 13 cases of PTC were found in Huanghua but none were identified in the other two areas. A retrospective analysis of the association between daily iodized salt intake and TC conducted by another research group in Hunan province also showed that consuming more than 5 g of iodized salt daily increased the risk of TC (46). Consistent with the above conclusions, a study examining TC trends in populations from three different geographic areas in Thailand between 1990 and 2009 showed an increase in PTC prevalence and a decrease in FTC prevalence as population iodine deficiency levels declined (47).
At the same time, several studies presented other viewpoints. Some studies supported the protective effect of iodine intake on TC risk (48). For example, an ecological study of epidemiology showed that low consumption of iodized salt with mild iodine deficiency may be responsible for the high prevalence of TC in Daishan Country (49). The results of a meta-analysis also suggested that dietary iodine has a protective effect on TC (50). The drawback, however, is the lack of data on iodine intake. French Polynesia, a mild iodine deficiency area, has one of the highest TC occurrence rates in the world, so iodine was suspected to play a part in this phenomenon. In 2012, a case-control study was conducted among the inhabitants of French Polynesia (51), which showed that in this region, higher consumption of seafood and an iodine-rich diet were associated with a reduced risk of TC. However, a limitation of this study that can not be ignored is that their iodine intake was calculated by the amount and composition of the participants’ daily food intake, using a composition table established in metropolitan France, which may be unsuitable for French Polynesia. What’s more, cooking or other factors also have an uncontrollable influence on the final iodine intake, it would be more accurate by measuring 24-h UIC.
What’s more, previous studies have indicated the prevalence of thyroid diseases may raise with both insufficient and overmuch iodine intake (52, 53). A study in 2016 indicated that compared to patients with benign thyroid nodules, TC patients tended to be distributed in UIC < 300 μg/L and UIC ≥ 2500 μg/L, this suggested that UIC may be involved in predicting TC risk in patients with thyroid nodules (54). The study supported that both low and overmuch iodine intakes can be related to TC in the iodine-replete region, so there may exist a U-shaped relationship between iodine intake and TC. Further research in the future also ought to reveal the mechanism of how iodine works and help to guide iodine intake.
A retrospective study based on patients who underwent thyroidectomy at Peking Union Medical College Hospital (PUMCH) from 1986 to 2018 implied that PTC has become the predominant type in TC surgery after USI, while the proportion of other histological subtypes has remained stable during this period (55). Therefore, we focused on the effect of iodine levels on PTC.
Effect of iodine intake on papillary thyroid carcinoma
Current studies reflected that iodine intake has a significant impact on PTC though some studies did not agree with this conclusion (56). One study that followed TC prevalence before and after iodine prevention in Argentina shows that PTC patients increased significantly after iodine supplementation, PTC/FTC ratio also increased significantly (57). Therefore, it is speculated that high iodine intake may be associated with a high prevalence of PTC (58, 59).
The prevalence of thyroid diseases in Shenyang has also raised obviously with the iodine intake increased since USI was implemented in China in 1996 (60). The diagnosis of TC and the proportion of PTC raised notably, and the proportion of FTC and UTC reduced while the ratio of MTC was not changed after USI. This study did find a correlation between iodine intake and TC, especially PTC, but there are also advances in detection technology and overdiagnosis, which need to be further verified. UIC differences between patients with PTC and nodular goiter were not statistically significant in another study (61), while in female PTC patients, extremely excessive iodine intake was independently related to the increased tumor size. This study supported that high iodine intake may be associated with the increase of tumor volume rather than its oncogenesis. Contrary to the above conclusions, a study conducted in a multiethnic group, investigated dietary iodine exposure among TC women in the San Francisco Bay area and women in the general population and concluded that an increase in dietary iodine is most likely associated with a reduced risk of PTC in those “low-risk” women (women with no risk factors) (62).
Iodine intake and combined factors
Nowadays, some studies have indicated that the combined effect of iodine and other factors plays a certain role in the occurrence and development of PTC. For example, Bisphenol A (BPA) is a kind of organic material that is widely applied to manufacturing processes (63). It has been reported that as an effective endocrine disruptor, free BPA can inhibit the expression of thyroid hormone-regulated genes by binding to thyroid hormone receptors (64). One study investigated whether BPA levels and excessive iodine intake were linked to PTC (65). The results indicated that the PTC groups’ UIC and Urinary BPA concentrations (UBC) were higher than those in the control group, which suggested that high levels of UBC and iodine intake may be the predictive factors for PTC. What’s more, BPA and iodine may interact with each other through some common pathways in the process of the occurrence and development of PTC.
A 2020 study tested UIC and thyroid function in patients with PTC, patients with benign thyroid tumors, and healthy individuals (66). The median UIC of the PTC and benign thyroid tumor group was markedly higher than that of healthy control groups. The regression analysis in this study also indicated that thyroglobulin antibody (TgAb) was an independent risk factor for PTC (67). What’s more, the association between TgAb and UIC was noteworthy, indicating that excessive iodine in patients with thyroid tumors may affect TgAb, which may contribute to the development of thyroid damage and subsequent malignancy (such as PTC) (68). Another case-control study in 2021 evaluated the cooperative effect of iodine intake and thyroid function on the risk of developing PTC and papillary thyroid microcarcinoma (PTMC) (69), indicating that excessive iodine intake using creatinine-adjusted UIC and high free T4 levels may have a synergistic effect on PTC and PTMC. Therefore, it is of interest to consider thyroid function in addition to iodine intake to predict the risk of PTC and PTMC. This also suggests that the combined effect of UIC and hormones on PTC risk needs to be verified in future larger studies.
Iodine intake and lymphatic metastasis in papillary thyroid carcinoma
A study in 2014 assessed the median urine iodine (MUI) of participants in Qingdao (70) and found that patients with benign thyroid nodules (MUI = 331.33 μg/l) and patients with PTC (MUI = 466.23 μg/l) had higher iodine intake than people in the control (MUI = 174.30 μg/l), which was in the iodine-replete region. In terms of MUI level, PTC patients with lymph node metastasis were higher than PTC patients without lymph node metastasis. The clinical data of 359 PTC patients who underwent surgical treatment in PUMCH from May 2015 to November 2020 were retrospectively analyzed (71). Consistent with the conclusions of previous studies, they demonstrated that low iodine was a protective factor for central lymph node metastasis in PTC, which indicated that iodine may not only be a promoter of tumorigenesis, but also a predictive factor for the aggressiveness of PTC. Another study also raised the point that high iodine intake does not seem to be a trigger, but may be a weak promoter for PTC progression in women patients, which needs further validation (72). The above data are consistent with most epidemiological studies that show an association between high iodine intake and PTC and its aggressiveness.
Iodine intake and BRAF mutation in papillary thyroid carcinoma
The familiar PTC mutation types include BRAF mutation, RET rearrangement, and RAS mutation. Among these alterations, BRAF mutations occur most frequently in PTC (73–75). Some studies proved that the BRAF V600E mutation plays a part in the biological behaviors of PTMC (≤ 1 cm) and small PTC (1–1.5 cm) (76). However, the correlation between these alterations and iodine intake remains controversial (77). Kowalska’s institution diagnosed an increased prevalence of BRAFV600E alterations in PTC, then they speculated that changes in iodine intake might contribute to the increased prevalence of TC (78). To clarify the above perspective, Guan and her team (79) assessed and compared the prevalence of the T1799A BRAF mutation in 1,032 PTC patients from five areas with different dietary iodine content in China. This study indicated that the frequency of BRAF mutation and the tumorigenesis of PTC are cogently associated with high iodine intake. The BRAF mutation was also confirmed to be a prognostic marker of PTC. Another study in Korea also investigated the correlation between iodine intake and BRAF mutation in PTC patients (80). BRAF mutation was the lowest in the 300–499 μg/L UIC group, which was different from that in the rarely low iodine intake (UIC < 300 μg/L) and excessive iodine intake (UIC ≥ 500 μg/L) groups, confirming that UIC can be used as the predictor of BRAF mutation in PTC. Their results verified the U-shaped curves again.
Some studies hold contrary views. For instance, one 2016 study conducted molecular analyses of two differentiated TCs, PTC, and FTC in two countries with different iodine intake: the iodine-rich country (Japan) and the iodine-poor country (Vietnam) (77). Their study indicated that there was no difference in genetic mutations between patients from iodine-rich and iodine-poor countries, the conclusion may support that iodine status does not influence the genetic changes of PTC and FTC. Another study also investigated the iodine intake of PTC patients with or without BRAFV600E mutation and that of healthy participants in 2018 (81). Though their results indicated that iodine status differs significantly between PTC patients and healthy participants, the correlation between iodine status and BRAF alteration was not statistically significant.
Many epidemiological studies and meta-analyses showed inconsonant conclusions because of dietary information bias, measurement error, and differences in ethnic groups and regions. It is also uncertain whether there is publication bias (82) or other factors influencing thyroid carcinogenesis (83). So definitive epidemiological studies are still warranted in the future.
In vitro studies
Most of the current studies focused on epidemiological investigation, the molecular biological effect of iodine promoting PTC is unclear until now. Here we review the mechanism of iodine-induced biological behavior of PTC cells. Studies have supported the protective function of excessive iodine on thyroid follicular cells through specific pathways. For instance, RET, a proto-oncogene involved in the carcinogenesis of PTC, can be activated by the fusion of the tyrosine kinase domain with the 5′ region of another gene. This process can produce chimeric products, collectively known as RET/PTC (84–86), leading to the activation of the MAPK pathway, which plays a part in driving PTC. So a study once evaluated the effect of high iodine concentrations on RET/PTC3-activated thyroid cells and indicated an antioncogenic role for excess iodine during thyroid oncogenic activation (87). Consistent with this viewpoint, another study in 2014 cultured thyroid follicular cells with doxycycline for 2 days, with or without 10 μM sodium iodide (88), then they found that high iodine inhibited miR-19, the newly discovered regulator of Smad4, which was activated by BRAFV600E, and restored the response to TGF-β signaling via the Notch pathway. This study indicated that iodine has a protective influence on thyroid cells, alleviating microRNA deregulation mediated by the BRAF oncogene, which contributes to the understanding of the physiological role of iodine on PTC. In addition, a recent study found that BRAF kinase can induce autophagy in PTC cells to participate in anti-apoptosis, and promote cell proliferation and migration under high iodine concentration (89), which support the view that high concentration of iodine can inhibit cell proliferation and promote cell apoptosis and migration.
Other studies have shown that excess iodine has adverse effects on thyroid cells. A study found that with a high iodine treatment, the miR-422a/MAPK1 pathway was complicated in the procedures of cell migration and proliferation, thus regulating tumorigenesis (90). With a high iodine concentration (100 μM), the MAPK1 signaling pathway was activated significantly in thyroid follicular epithelial cells, which means that in normal thyroid cells, high iodine may lead to the imbalance of the miR-422a/MAPK1 pathway. Considering that they only conducted functional experiments in two iodine concentrations, more studies are in the future.
Several studies have found that iodine has a double influence on thyroid cells’ behaviors, depending on the iodine concentration. A study assessed the influences of different iodine concentrations on the proliferation and migration of two well-differentiated thyroid cell lines in vitro (91). The results supported that when iodine concentration was at a certain level, it could play a role in promoting the proliferation of thyroid cells. Iodine under 1.0 × 10–3 mM promotes the growth of thyroid cells while iodine higher than this concentration has the opposite effect. Besides, the mRNA level of VEGF-A was up-regulated in thyroid cells cultured in low iodine concentration (1.0 × 10–5, 1.0 × 10–4, and 1.0 × 10–3 mM) and down-regulated in thyroid cells cultured in high iodine concentration (1.0 × 10–2 and 1.0 × 10–1 mM), which indicated that the Akt, Erk, and the cytokine VEGF-A are the important mechanisms. However, iodine concentration in the human thyroid is usually from 1.0 × 10–6 to 1.0 × 10–5 mM, so in the human body, the high level of iodine intake may promote the proliferation and migration of PTC cells. Another study in 2019 also illustrated this dual effect, they investigated how iodine affected the physiological features of TC cells in vitro, including proliferation and apoptosis (92). Compared with the control group, extra-high doses of iodine (1.0 × 10–3 mol/l) inhibited cell proliferation and promoted cell apoptosis, while extra-low doses of iodine (1.0 × 10–4–1.0 × 10–8 mol/l) showed opposing effects. Their results also indicated that the level of SPANXA1 was increased in cells treated with a certain concentration of iodine. The SPANXA1 (93) can also be one of the key genes, which enhanced the process of tumor growth in cells treated with an extra-low dose of iodine. Cell proliferation can be promoted by high expression of SPANXA1 while cell apoptosis can be inhibited by SPANXA1. In addition, PI3K/AKT was supposed to be a key signaling pathway through which SPANXA1 mediates its effects. Thus, SPANXA1 can be a biomarker in PTC and help in guiding dietary plans for patients with TC, which remind us that patients’ iodine intake should be restricted.
Though these studies suggested some possible mechanisms for how iodine affected thyroid carcinogenesis, many other confounding factors cannot be ruled out. The effects of iodine on PTC patients are also complex and influenced by many chemical agents in vivo, so it is hard to clarify the interaction and feedback mechanisms of so many hormones by conducting cell experiments. Therefore, more in vivo studies are needed to clarify the function and mechanism of iodine on PTC.
In vivo studies
Animal studies examining the effect of different levels of iodine intake on the development of PTC were still rare. But earlier studies have shown that the development of iodine deficiency can cause PTC. The long-term effects on the thyroid with low iodine intake were assessed in 98 Sherman albino female rats (94), iodine deficiency was shown to be attributed to the production of tumors in thyroid glands. One study also found that iodine deficiency can cause goiter, hyperplasia, or malignant change as iodine deficiency time goes on (95), which also speculated that iodine deficiency can lead to reduced thyroid hormone synthesis, while the increased TSH drove chronic overstimulation of the thyroid. Proliferating thyroid cells, meanwhile, can also be more susceptible to radiation, chemical carcinogens, and oxidative stress, so more genetic mutations will show up in these cells. In addition, thyroid hyperplasia caused by insufficient iodine can lead to the change of chromosomes in the thyroid and increase the number of aneuploid cells in rats (96). Therefore, it is speculated that chronic stimulation in iodine deficiency may be one of the vital mechanisms of PTC. However, another study proved the U-shaped relationship by investigating the influence of iodine intake on p14ARF and p16INK4a expression of PTC in rats (97). This study suggested that both low and high iodine intake can decrease the expression of p14ARF and p16INK4a and drive tumor development.
The association between human iodine intake and PTC still cannot be explained because iodine deficiency or excess is much more severe in most animal models than in the human diet.
Discussion
Over these years, the occurrence rate of TC, especially PTC is increasing significantly in the world (1). Although overdiagnosis has been reported to increase the prevalence of PTC (98), there has also been a true increase. It is therefore meaningful to illustrate the role of these suspected risk factors, especially iodine intake. TC has been reported in iodine deficiency areas in earlier years. The prevalence of PTC also increased after iodine intake increased due to salt iodization (99) and iodine supplementation. In contrast, iodine was a protective factor for PTC in some studies, which lead to controversy about the correlation between iodine intake and PTC. Previous epidemiologic studies’ results could be influenced by different test standards, study methods, dietary habits, measurement errors, information bias or so many other factors. For example, it is difficult to measure 24-h UIC, the gold standard for iodine intake (31). Therefore, some studies may use random spot urine UIC as an alternative indicator. While the studies in vitro or in vivo cannot reflect the true human iodine status, the evidence is far from sufficient. Hence it is still unclear the true iodine interval that directly induces PTC development, or indirectly contributes to PTC risk through interaction with other factors.
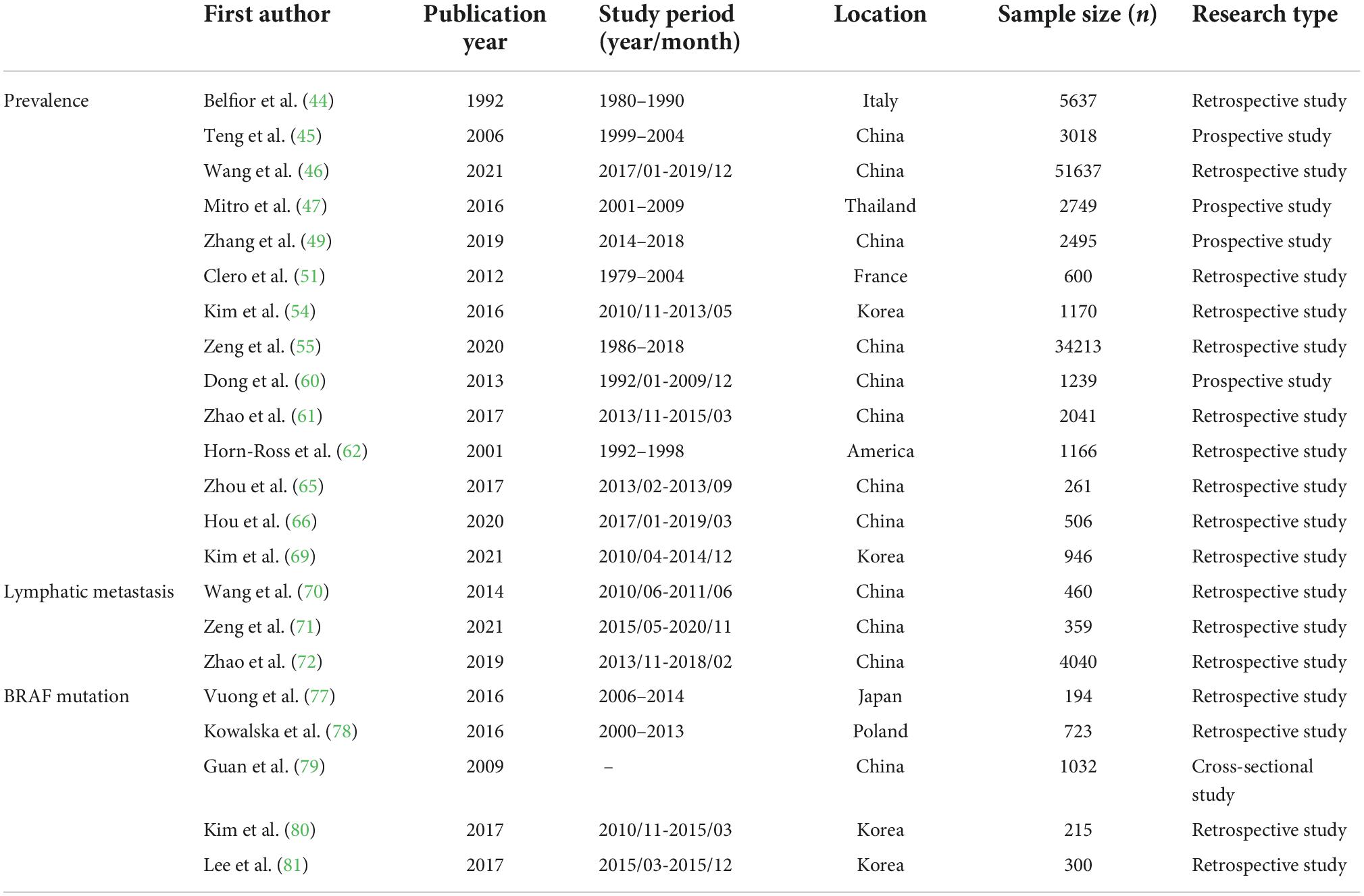
Many previous studies have addressed this controversial issue, showing that different iodine nutritional status has different effects on the development of PTC. We discuss their views in this article and summarize the basic information in Table 1 to help readers think objectively. Taking the present studies into consideration, we speculate that the relationship between iodine nutrition and PTC may be intricate and the effect of iodine should be considered dose-based. Besides, the combined action of more variables are ought to be considered in the research of iodine and PTC.
The relationship between iodine and PTC is complex, we are still unclear about the specific role of iodine, let alone the mechanism of its activities due to the disagreement of current research results. The present studies have yielded mixed results which indicated that iodine intake may influence the development or progression of PTC, change the proportion of several subtypes of TC in the crowd, or affect the invasiveness of PTC especially lymphatic metastasis and BRAF mutation. These data provide further evidence supporting that it makes sense to achieve the appropriate level of iodine intake to satisfy the body’s normal nutritional needs while avoiding either deficient or excessive iodine supplementation.
Author contributions
WT supervised the work. XZ drafted the manuscript. FZ provided the major technical support. QL and CF assisted in the literature review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Research Fund for Public Welfare, National Health and Family Planning Commission of China (Grant No. 201402005) and the Clinical Research Fund of Chinese Medical Association (Grant No. 15010010589).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/s0140-6736(16)30172-6
2. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. (2016) 26:1541–52. doi: 10.1089/thy.2016.0100
3. Us Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, et al. Screening for thyroid cancer: US preventive services task force recommendation statement. JAMA. (2017) 317:1882–7. doi: 10.1001/jama.2017.4011
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
5. Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol. (2015) 27:1–7. doi: 10.1097/CCO.0000000000000148
6. Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol. (2020) 11:102. doi: 10.3389/fendo.2020.00102
7. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. (2017) 13:644–60. doi: 10.1038/nrendo.2017.76
8. Dierks C, Seufert J, Aumann K, Ruf J, Klein C, Kiefer S, et al. Combination of lenvatinib and pembrolizumab is an effective treatment option for anaplastic and poorly differentiated thyroid carcinoma. Thyroid. (2021) 31:1076–85. doi: 10.1089/thy.2020.0322
9. Peterson E, De P, Nuttall R. BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One. (2012) 7:e29177. doi: 10.1371/journal.pone.0029177
10. Rinaldi S, Lise M, Clavel-Chapelon F, Boutron-Ruault MC, Guillas G, Overvad K, et al. Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer. (2012) 131:E1004–14. doi: 10.1002/ijc.27601
11. Davies L, Hoang JK. Thyroid cancer in the USA: current trends and outstanding questions. Lancet Diabetes Endocrinol. (2021) 9:11–2. doi: 10.1016/s2213-8587(20)30372-7
12. Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. (2013) 23:885–91. doi: 10.1089/thy.2013.0045
13. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
14. Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. (2014) 24:472–9. doi: 10.1089/thy.2013.0257
15. Parad MT, Fararouei M, Mirahmadizadeh AR, Afrashteh S. Thyroid cancer and its associated factors: a population-based case-control study. Int J Cancer. (2021) 149:514–21. doi: 10.1002/ijc.33537
16. Liang J, Zhao N, Zhu C, Ni X, Ko J, Huang H, et al. Dietary patterns and thyroid cancer risk: a population-based case-control study. Am J Transl Res. (2020) 12:180–90.
17. Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int J Cancer. (2012) 131:2360–6. doi: 10.1002/ijc.27497
18. Gonzalez A, Paz S, Rubio C, Gutierrez AJ, Hardisson A. Human exposure to iodine from the consumption of edible seaweeds. Biol Trace Elem Res. (2020) 197:361–6. doi: 10.1007/s12011-019-01996-w
19. Pearce EN. Iodine nutrition: recent research and unanswered questions. Eur J Clin Nutr. (2018) 72:1226–8. doi: 10.1038/s41430-018-0226-7
20. Sun X, Shan Z, Teng W. Effects of increased iodine intake on thyroid disorders. Endocrinol Metab. (2014) 29:240–7. doi: 10.3803/EnM.2014.29.3.240
21. Wang B, He W, Li Q, Jia X, Yao Q, Song R, et al. U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur J Endocrinol. (2019) 181:255–66. doi: 10.1530/EJE-19-0212
22. Song J, Zou SR, Guo CY, Zang JJ, Zhu ZN, Mi M, et al. Prevalence of thyroid nodules and its relationship with iodine status in Shanghai: a population-based study. Biomed Environ Sci. (2016) 29:398–407. doi: 10.3967/bes2016.052
23. Eveleigh ER, Coneyworth LJ, Avery A, Welham SJM. Vegans, vegetarians, and omnivores: how does dietary choice influence iodine intake? A systematic review. Nutrients. (2020) 12:1606. doi: 10.3390/nu12061606
24. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. (2010) 24:13–27. doi: 10.1016/j.beem.2009.08.013
25. Iacone R, Iaccarino Idelson P, Russo O, Donfrancesco C, Krogh V, Sieri S, et al. Iodine Intake from food and iodized salt as related to dietary salt consumption in the italian adult general population. Nutrients. (2021) 13:3486. doi: 10.3390/nu13103486
26. Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. (2004) 14:836–41. doi: 10.1089/thy.2004.14.836
27. Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab. (2004) 89:3421–4. doi: 10.1210/jc.2003-032002
28. Koukkou EG, Roupas ND, Markou KB. Effect of excess iodine intake on thyroid on human health. Minerva Med. (2017) 108:136–46. doi: 10.23736/S0026-4806.17.04923-0
29. Prete A, Paragliola RM, Corsello SM. Iodine supplementation: usage “with a grain of salt”. Int J Endocrinol. (2015) 2015:312305. doi: 10.1155/2015/312305
30. Ahn J, Lee JH, Lee J, Baek JY, Song E, Oh HS, et al. Association between urinary sodium levels and iodine status in Korea. Korean J Intern Med. (2020) 35:392–9. doi: 10.3904/kjim.2017.375
31. Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid. (2009) 19:1281–6. doi: 10.1089/thy.2009.0094
32. Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. (2012) 70:553–70. doi: 10.1111/j.1753-4887.2012.00528.x
33. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid. (2020) 30:568–79. doi: 10.1089/thy.2019.0067
34. Jeon MJ, Kim WG, Kwon H, Kim M, Park S, Oh HS, et al. Excessive iodine intake and thyrotropin reference interval: data from the Korean National health and nutrition examination survey. Thyroid. (2017) 27:967–72. doi: 10.1089/thy.2017.0078
35. Huang L, Zhang L, Rao Z, Huang C, Huang H. Dietary iodine intake and urinary iodine concentration during pregnancy in Chengdu, China. Asia Pac J Clin Nutr. (2021) 30:643–50. doi: 10.6133/apjcn.202112_30(4).0011
36. Dold S, Zimmermann MB, Jukic T, Kusic Z, Jia Q, Sang Z, et al. Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: a cross-sectional multicenter study. J Nutr. (2018) 148:587–98. doi: 10.1093/jn/nxy015
37. Poljak NK, Kontić M, Colović Z, Jeroncić I, Luksić B, Mulić R. Iodine intake and epidemiological characteristics of thyroid cancer: comparison between inland and littoral Croatia. Acta Clin Croat. (2011) 50:329–39.
38. Sehestedt T, Knudsen N, Perrild H, Johansen C. Iodine intake and incidence of thyroid cancer in Denmark. Clin Endocrinol. (2006) 65:229–33. doi: 10.1111/j.1365-2265.2006.02580.x
39. Lv C, Yang Y, Jiang L, Gao L, Rong S, Darko GM, et al. Association between chronic exposure to different water iodine and thyroid cancer: a retrospective study from 1995 to 2014. Sci Total Environ. (2017) 609:735–41. doi: 10.1016/j.scitotenv.2017.07.101
40. Williams ED, Doniach I, Bjarnason O, Michie W. Thyroid cancer in an iodide rich area: a histopathological study. Cancer. (1977) 39:215–22. doi: 10.1002/1097-0142(197701)39:1<215::aid-cncr2820390134>3.0.co;2-#
41. Goodman MT, Yoshizawa CN, Kolonel LN. Descriptive epidemiology of thyroid cancer in Hawaii. Cancer. (1988) 61:1272–81. doi: 10.1002/1097-0142(19880315)61:6<1272::aid-cncr2820610636>3.0.co;2-8
42. Reiners C, Drozd V. Editorial: radiation as risk factor, early diagnosis, therapy, and follow-up of differentiated thyroid cancer. Front Endocrinol. (2021) 12:797969. doi: 10.3389/fendo.2021.797969
43. Abend M, Pfeiffer RM, Port M, Hatch M, Bogdanova T, Tronko MD, et al. Utility of gene expression studies in relation to radiation exposure and clinical outcomes: thyroid cancer in the Ukrainian-American cohort and late health effects in a MAYAK worker cohort. Int J Radiat Biol. (2021) 97:12–8. doi: 10.1080/09553002.2020.1748739
44. Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake. Am J Med. (1992) 93:363–9. doi: 10.1016/0002-9343(92)90164-7
45. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. (2006) 354:2783–93. doi: 10.1056/NEJMoa054022
46. Wang Y, Wang J, Chen Z, Ma M, Lin C, He Q, et al. Analysis of the correlation between high iodized salt intake and the risk of thyroid nodules: a large retrospective study. BMC Cancer. (2021) 21:1000. doi: 10.1186/s12885-021-08700-z
47. Mitro SD, Rozek LS, Vatanasapt P, Suwanrungruang K, Chitapanarux I, Srisukho S, et al. Iodine deficiency and thyroid cancer trends in three regions of Thailand, 1990-2009. Cancer Epidemiol. (2016) 43:92–9. doi: 10.1016/j.canep.2016.07.002
48. Hoang T, Lee EK, Lee J, Hwangbo Y, Kim J. Seaweed and iodine intakes and SLC5A5 rs77277498 in relation to thyroid cancer. Endocrinol Metab. (2022) 37:513–23. doi: 10.3803/EnM.2021.1306
49. Zhang YL, Li P, Liu ZY, Yi JP, Chen Y, Zhang M, et al. Does relatively low iodine intake contribute to thyroid cancer? An ecological comparison of epidemiology. Medicine. (2019) 98:e17539. doi: 10.1097/MD.0000000000017539
50. Cao LZ, Peng XD, Xie JP, Yang FH, Wen HL, Li S. The relationship between iodine intake and the risk of thyroid cancer: a meta-analysis. Medicine. (2017) 96:e6734. doi: 10.1097/MD.0000000000006734
51. Clero E, Doyon F, Chungue V, Rachedi F, Boissin JL, Sebbag J, et al. Dietary iodine and thyroid cancer risk in French polynesia: a case-control study. Thyroid. (2012) 22:422–9. doi: 10.1089/thy.2011.0173
52. Chen X, Huang H, Liang B, Zhou J. Abnormal iodine nutrition-induced ER stress upregulates MCP-1 expression through P38/MAPK signaling pathway in thyroid cells. Biol Trace Elem Res. (2019) 191:98–103. doi: 10.1007/s12011-018-1610-9
53. Fan L, Meng F, Gao Y, Liu P. Insufficient iodine nutrition may affect the thyroid cancer incidence in China. Br J Nutr. (2021) 126:1852–60. doi: 10.1017/S0007114521000593
54. Kim HJ, Kim NK, Park HK, Byun DW, Suh K, Yoo MH, et al. Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur J Nutr. (2017) 56:965–71. doi: 10.1007/s00394-015-1144-2
55. Zeng Z, Li K, Kang W, Yu J, Wang X, Zhang Z, et al. Changing patterns of thyroid cancer in different stages of Universal Salt Iodization in Peking Union Medical College Hospital, 1986-2018. Gland Surg. (2020) 9:1338–45. doi: 10.21037/gs-20-346
56. Choi JY, Lee JH, Song Y. Evaluation of iodine status among Korean patients with papillary thyroid cancer using dietary and urinary iodine. Endocrinol Metab. (2021) 36:607–18. doi: 10.3803/EnM.2021.1005
57. Harach HR, Williams ED. Thyroid cancer and thyroiditis in the goitrous region of Salta, Argentina, before and after iodine prophylaxis. Clin Endocrinol. (1995) 43:701–6. doi: 10.1111/j.1365-2265.1995.tb00538.x
58. Yu Z, Yu Y, Wan Y, Fan J, Meng H, Li S, et al. Iodine intake level and incidence of thyroid disease in adults in Shaanxi province: a cross-sectional study. Ann Transl Med. (2021) 9:1567. doi: 10.21037/atm-21-4928
59. Xiu C, He Q, Zhao HJ, Yuan ZN, Guo LH, Wang FQ, et al. Strong correlation of abnormal serum and urinary iodine levels with papillary thyroid cancer: a case-control study. Biomed Environ Sci. (2020) 33:62–7. doi: 10.3967/bes2020.009
60. Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, et al. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit. (2013) 19:49–53. doi: 10.12659/msm.883736
61. Zhao H, Li H, Huang T. High urinary iodine, thyroid autoantibodies, and thyroid-stimulating hormone for papillary thyroid cancer risk. Biol Trace Elem Res. (2018) 184:317–24. doi: 10.1007/s12011-017-1209-6
62. Horn-Ross PL, Morris JS, Lee M, West DW, Whittemore AS, McDougall IR, et al. Iodine and thyroid cancer risk among women in a multiethnic population: the bay area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. (2001) 10:979–85.
63. Murata M, Kang JH. Bisphenol A (BPA) and cell signaling pathways. Biotechnol Adv. (2018) 36:311–27. doi: 10.1016/j.biotechadv.2017.12.002
64. Yuan N, Wang L, Zhang X, Li W. Bisphenol A and thyroid hormones: bibliometric analysis of scientific publications. Medicine. (2020) 99:e23067. doi: 10.1097/MD.0000000000023067
65. Zhou Z, Zhang J, Jiang F, Xie Y, Zhang X, Jiang L. Higher urinary bisphenol A concentration and excessive iodine intake are associated with nodular goiter and papillary thyroid carcinoma. Biosci Rep. (2017) 37:BSR20170678. doi: 10.1042/BSR20170678
66. Hou D, Xu H, Li P, Liu J, Qian Z. Potential role of iodine excess in papillary thyroid cancer and benign thyroid tumor: a case-control study. Asia Pac J Clin Nutr. (2020) 29:603–8. doi: 10.6133/apjcn.202009_29(3).0020
67. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine. (2014) 46:554–60. doi: 10.1007/s12020-013-0121-x
68. Noel JE, Thatipamala P, Hung KS, Chen J, Shi RZ, Orloff LA. Pre-operative antithyroid antibodies in differentiated thyroid cancer. Endocr Pract. (2021) 27:1114–8. doi: 10.1016/j.eprac.2021.06.014
69. Kim K, Cho SW, Park YJ, Lee KE, Lee DW, Park SK. Association between iodine intake. Thyroid function, and papillary thyroid cancer: a case-control study. Endocrinol Metab. (2021) 36:790–9. doi: 10.3803/EnM.2021.1034
70. Wang F, Wang Y, Wang L, Wang X, Sun C, Xing M, et al. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol. (2014) 35:11375–9. doi: 10.1007/s13277-014-2397-8
71. Zeng Z, Li K, Wang X, Ouyang S, Zhang Z, Liu Z, et al. Low urinary iodine is a protective factor of central lymph node metastasis in papillary thyroid cancer: a cross-sectional study. World J Surg Oncol. (2021) 19:208. doi: 10.1186/s12957-021-02302-6
72. Zhao H, Li H, Huang T. High iodine intake and central lymph node metastasis risk of papillary thyroid cancer. J Trace Elem Med Biol. (2019) 53:16–21. doi: 10.1016/j.jtemb.2019.01.015
73. Goh X, Lum J, Yang SP, Chionh SB, Koay E, Chiu L, et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin Otolaryngol. (2019) 44:114–23. doi: 10.1111/coa.13238
74. Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. (2022) 34:9–18. doi: 10.1097/CCO.0000000000000797
75. Rashid FA, Munkhdelger J, Fukuoka J, Bychkov A. Prevalence of BRAF(V600E) mutation in Asian series of papillary thyroid carcinoma-a contemporary systematic review. Gland Surg. (2020) 9:1878–900. doi: 10.21037/gs-20-430
76. Silver JA, Bogatchenko M, Pusztaszeri M, Forest VI, Hier MP, Yang JW, et al. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas </= 1.5 cm. J Otolaryngol Head Neck Surg. (2021) 50:63. doi: 10.1186/s40463-021-00543-9
77. Vuong HG, Kondo T, Oishi N, Nakazawa T, Mochizuki K, Inoue T, et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med. (2016) 5:1883–9. doi: 10.1002/cam4.781
78. Kowalska A, Walczyk A, Kowalik A, Palyga I, Trybek T, Kopczynski J, et al. Increase in papillary thyroid cancer incidence is accompanied by changes in the frequency of the BRAF V600E mutation: a single-institution study. Thyroid. (2016) 26:543–51. doi: 10.1089/thy.2015.0352
79. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. (2009) 94:1612–7. doi: 10.1210/jc.2008-2390
80. Kim HJ, Park HK, Byun DW, Suh K, Yoo MH, Min YK, et al. Iodine intake as a risk factor for BRAF mutations in papillary thyroid cancer patients from an iodine-replete area. Eur J Nutr. (2018) 57:809–15. doi: 10.1007/s00394-016-1370-2
81. Lee JH, Song RY, Yi JW, Yu HW, Kwon H, Kim SJ, et al. Case-control study of papillary thyroid carcinoma on urinary and dietary iodine status in South Korea. World J Surg. (2018) 42:1424–31. doi: 10.1007/s00268-017-4287-x
82. Schneck A. Examining publication bias-a simulation-based evaluation of statistical tests on publication bias. PeerJ. (2017) 5:e4115. doi: 10.7717/peerj.4115
83. Harach HR, Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, argentina model. Endocr Pathol. (2008) 19:209–20. doi: 10.1007/s12022-008-9038-y
84. Li AY, McCusker MG, Russo A, Scilla KA, Gittens A, Arensmeyer K, et al. RET fusions in solid tumors. Cancer Treat Rev. (2019) 81:101911. doi: 10.1016/j.ctrv.2019.101911
85. Santoro M, Moccia M, Federico G, Carlomagno F. RET gene fusions in malignancies of the thyroid and other tissues. Genes. (2020) 11:424. doi: 10.3390/genes11040424
86. Khan MS, Qadri Q, Makhdoomi MJ, Wani MA, Malik AA, Niyaz M, et al. RET/PTC gene rearrangements in thyroid carcinogenesis: assessment and clinico-pathological correlations. Pathol Oncol Res. (2020) 26:507–13. doi: 10.1007/s12253-018-0540-3
87. Fiore AP, Fuziwara CS, Kimura ET. High iodine concentration attenuates RET/PTC3 oncogene activation in thyroid follicular cells. Thyroid. (2009) 19:1249–56. doi: 10.1089/thy.2008.0408
88. Fuziwara CS, Kimura ET. High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid. (2014) 24:453–62. doi: 10.1089/thy.2013.0398
89. Zhang D, Xu X, Li J, Yang X, Sun J, Wu Y, et al. High iodine effects on the proliferation, apoptosis, and migration of papillary thyroid carcinoma cells as a result of autophagy induced by BRAF kinase. Biomed Pharmacother. (2019) 120:109476. doi: 10.1016/j.biopha.2019.109476
90. Wang J, Yang H, Si Y, Hu D, Yu Y, Zhang Y, et al. Iodine promotes tumorigenesis of thyroid cancer by suppressing Mir-422a and up-regulating MAPK1. Cell Physiol Biochem. (2017) 43:1325–36. doi: 10.1159/000481844
91. Xiang J, Wang X, Wang Z, Wu Y, Li D, Shen Q, et al. Effect of different iodine concentrations on well-differentiated thyroid cancer cell behavior and its inner mechanism. Cell Biochem Biophys. (2015) 71:299–305. doi: 10.1007/s12013-014-0198-8
92. Yang X, Sun J, Han J, Sun L, Wang H, Zhang D, et al. Iodine promotes thyroid cancer development via SPANXA1 through the PI3K/AKT signalling pathway. Oncol Lett. (2019) 18:637–44. doi: 10.3892/ol.2019.10391
93. Li J, Bo H, Zhu F, Li Q, Chen T, Lei S, et al. Hypomethylated SPANXA1/A2 promotes the metastasis of head and neck squamous cell carcinoma. Med Oncol. (2020) 37:112. doi: 10.1007/s12032-020-01441-2
94. Axelrad AA, Leblond CP. Induction of thyroid tumors in rats by a low iodine diet. Cancer. (1955) 8:339–67. doi: 10.1002/1097-0142(1955)8:2<339::aid-cncr2820080214>3.0.co;2-m
95. Schaller RTJ, Jk S. Development of carcinoma of the thyroid in iodine-deficient mice. Cancer. (1966) 19:1063–80. doi: 10.1002/1097-0142(196608)19:8<1063::aid-cncr2820190804>3.0.co;2-a
96. Al-Saadi AA, Beierwaltes WH. Chromosomal changes in rat thyroid cells during iodine depletion and repletion. Cancer Res. (1966) 26:676–88.
97. Sun R, Wang J, Li X, Li L, Yang J, Ren Y, et al. Effect of Iodine Intake on p14ARF and p16INK4a expression in thyroid papillary carcinoma in rats. Med Sci Monit. (2015) 21:2288–93. doi: 10.12659/MSM.893486
98. Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. (2017) 24:332–6. doi: 10.1097/med.0000000000000359
Keywords: iodine, papillary thyroid cancer, iodine nutrition, thyroid cancer, epidemiological studies
Citation: Zhang X, Zhang F, Li Q, Feng C and Teng W (2022) Iodine nutrition and papillary thyroid cancer. Front. Nutr. 9:1022650. doi: 10.3389/fnut.2022.1022650
Received: 18 August 2022; Accepted: 30 September 2022;
Published: 20 October 2022.
Edited by:
Weimin Ye, Karolinska Institutet (KI), SwedenReviewed by:
Jiangbo Du, Nanjing Medical University, ChinaFen Huang, Anhui Medical University, China
Copyright © 2022 Zhang, Zhang, Li, Feng and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiping Teng, dHdwQHZpcC4xNjMuY29t
†ORCID: Fan Zhang, orcid.org/0000-0002-0176-2715; Weiping Teng, orcid.org/0000-0002-6445-6192
 Xueqi Zhang
Xueqi Zhang Fan Zhang
Fan Zhang Qiuxian Li
Qiuxian Li Chuyao Feng
Chuyao Feng Weiping Teng
Weiping Teng