- 1School of Liquor and Food Engineering, Guizhou University, Guiyang, China
- 2Laboratory of Brewing Microbiology and Applied Enzymology, Key Laboratory of Industrial Biotechnology of Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi, China
- 3Guizhou Guotai Liquor Group Co., Ltd., Renhuai, China
Volatile thiols give a unique flavor to foods and they have been extensively studied due to their effects on sensory properties. The analytical assay of volatile thiols in food is hindered by the complexity of the matrix, and by both their high reactivity and their typically low concentrations. A new ultraperformance liquid chromatography (UPLC) strategy has been developed for the identification and quantification of volatile thiols in Chinese liquor (Baijiu). 4,4’-Dithiodipyridine reacted rapidly with eight known thiols to form derivatives, which provided a diagnostic fragment ion (m/z 143.5) for tandem mass spectrometry (MS/MS). To screen for new thiols, Baijiu samples were analyzed by means of UPLC–MS/MS screening for compounds exhibiting the diagnostic fragment ion (m/z X→143.5). New peaks with precursor ions of m/z 244, 200 and 214 were detected. Using UPLC with quadrupole-time-of-flight mass spectrometry (UPLC–Q-TOF–MS) and authentic standards, ethyl 2-mercaptoacetate, 1-butanethiol, and 1-pentanethiol were identified in Baijiu for the first time. Commercial Baijiu samples were analyzed with the new method and the distribution of 11 thiols was revealed in different Baijiu aroma-types. The aroma contribution of these thiols was evaluated by their odoractivity values (OAVs), with the result that 7 of 11 volatile thiols had OAVs > 1. In particular, methanethiol, 2-furfurylthiol, and 2-methyl-3-furanthiol had relatively high OAVs, indicating that they contribute significantly to the aroma profile of Baijiu.
Introduction
Baijiu (Chinese liquor) is a locally-produced, distilled alcoholic beverage that has been very popular for thousands of years and is produced using a unique traditional solid-state fermentation process (1, 2). It is typically made from sorghum or a mixture of wheat, barley, corn, rice, and sorghum. Baijiu is produced using traditional spontaneous fermentation processes with an assortment of microbial communities involved (3). The characteristic aroma of Baijiu can vary considerably, resulting from differences in raw materials, production processes, and flavor components. Baijiu is generally classified into 12 aroma types (4). At present, soy sauce aroma-type Baijiu (SSAB) (5, 6), strong aroma-type Baijiu (SAB) (7, 8), light aroma-type Baijiu (LAB) (9, 10), and roasted sesame-like aroma-type Baijiu (RSAB) (11, 12) are the four common Baijiu aroma-types in China.
The popularity of Baijiu arises mainly from its pleasant taste and the odor active compounds in its volatile fraction. More than 1,870 volatile compounds have been identified in Baijiu, including esters, alcohols, ketones, acids, aldehydes, nitrogenous compounds, and sulfur compounds (4). Despite this great complexity, only a small number of compounds are responsible for the majority of the olfactory sensation Baijiu provides. Some sulfur containing compounds are among the most important for Baijiu flavor (13, 14). In particular, volatile thiols, known historically as mercaptans, have the general structure, R-SH, and exhibit important sensory effects to Baijiu, because their concentrations are much higher than their low odor thresholds (12, 15, 16). Therefore, their determination and insights into their concentrations could help to improve the sensory quality of Baijiu and modulate its sensory attributes.
Several methods for analyzing volatile thiols in Baijiu have been developed, all using gas chromatography (GC) (12, 17). However, profiling volatile thiols in Baijiu remains a bottleneck with GC-based methods. The complexity of the Baijiu sample matrix, the typically low concentrations of volatile thiols and their low detection-sensitivity in electron-impact mass spectrometry (EIMS), means that few of them can be identified using GC–MS. Only one volatile thiol was detected in Baijiu by HS-SPME–GC–MS (18). Although pulsed flame photometric detection (PFPD) and sulfur chemiluminescence detection (SCD) are highly selective and sensitive for the quantification of volatile thiols, they provide little identification information, except for their chromatographic retention time. As a result, additional and cumbersome identification procedures are required. Only three volatile thiols were identified by HS-SPME–GC–PFPD, and four volatile thiols were identified by GC × GC–SCD in Baijiu (13, 16). In addition, current GC-based methods for Baijiu volatiles are in general, laborious and time consuming, and some of them involve multiple sample manipulation steps, during which volatile thiols can be lost, or degraded. These limitations have led to the need for a simple, rapid method that enables the identification and quantification of Baijiu volatile thiols.
To improve the sensitivity and selectivity of the measurement method, and stabilize the sulfanyl, thiols from wine and coffee are usually derivatized prior to separation and analysis using liquid chromatography-electrospray ionization mass spectrometry (LC–ESI-MS) (19, 20). 4,4’-Dithiodipyridine (DTDP) is one of the available derivatization reagents. Thiols derivatized with DTDP show increased hydrophobicity, decreased polarity volatility, and stronger affinity for protonation, resulting in an enhanced LC signal for separation and positive-mode ESI-MS detection, which enables the quantification of thiols at ng/L levels in wine (21, 22).
As a result of the low odor thresholds of most volatile thiols, in combination with their low concentrations, it is likely that some potent volatile thiols remain to be discovered in Baijiu. Therefore, we developed a method to identify the volatile thiols by UPLC–MS/MS and UPLC–Q-TOF–MS, rather than adopting a conventional GC approach. The UPLC–MS/MS method was applied to a range of Baijiu samples to investigate the volatile thiol profile.
Materials and methods
Samples
Four aroma-types Baijiu samples were under investigation: 8 SSAB samples, 6 SAB samples, 6 RSAB samples, and 7 LAB samples. The detailed information is given in Supplementary Table 1. The samples were stored at room temperature and without light before analysis.
Chemicals and materials
The thiols studied were methanethiol, ethanethiol, ethyl 2-mercaptoacetate, 2-furfurylthiol, 2-sulfanylethanol, 2-methyl-3-furanthiol, benzenemethanethiol, 3-mercaptohexyl acetate, ethyl 2-mercaptopropionate, 1-butanethiol, 1-pentanethiol. The internal standard was 2-phenylethanethiol. All analytes were provided commercially at high-purity grade (>°96%) by Sigma-Aldrich (Shanghai, China). Ethylenediaminetetraacetic acid disodium salt (EDTA-Na2), 4,4’-Dithiodipyridine (DTDP, 97%), acetaldehyde (99%), and formic acid (99%) were bought from J&K Chemical Corp., (Beijing, China). C18 solid-phase extraction cartridges (6 mL, 500 mg) were purchased from ANPEL (Shanghai, China). LC–MS grade acetonitrile were purchased from Merck (Sigma-Aldrich, Shanghai, China).
Sample preparation and derivatization
A modified derivatization method for the analysis of thiols in Baijiu was used according to a previously described procedure (21). A solution of the Baijiu sample (20 mL) was spiked with 10 μL of 2-phenylethanethiol (6 mg/L) and used as an internal standard solution. The sample was diluted with water (20 mL, Millipore, USA) to a final concentration of about 25% ethanol by volume. EDTA-Na2 (40 mg), 50% acetaldehyde (160 μL), and freshly thawed DTDP (10 mM, 400 μL) was then added to the resulting solution. The mixture was vortex-assisted stirred for 5 min and rested for 25 min at room temperature. The sample was loaded onto a SPE cartridge, which was previously pretreated with 6 mL of methanol, followed by 6 mL of water. The column was washed with 50% methanol (12 mL). The analytes retained by SPE were eluted with methanol (3 mL) and concentrated to a final volume of 400 μL under nitrogen. The solution was filtered (0.22 μm) and stored at 4°C.
Mass spectrometry method development
A triple-quadrupole mass spectrometer (Xevo TQ-S, Waters, Milford, USA) was performed with positive ionization mode. The multiple-reaction-monitoring (MRM) conditions were optimized with infusion of derivatized thiols at 10 μL/min. Based on the mass spectra (Supplementary Figure 1) with the MassLynx software using the sample tune and develop method, two ion transitions were chosen for the quantification (quantifier) and the confirmation (qualifier) (23). Table 1 lists the best MRM parameters for each derived thiol with the following instrument settings: capillary voltage, 3 kV; desolvation temperature, 500°C; gas, 800 L/h.
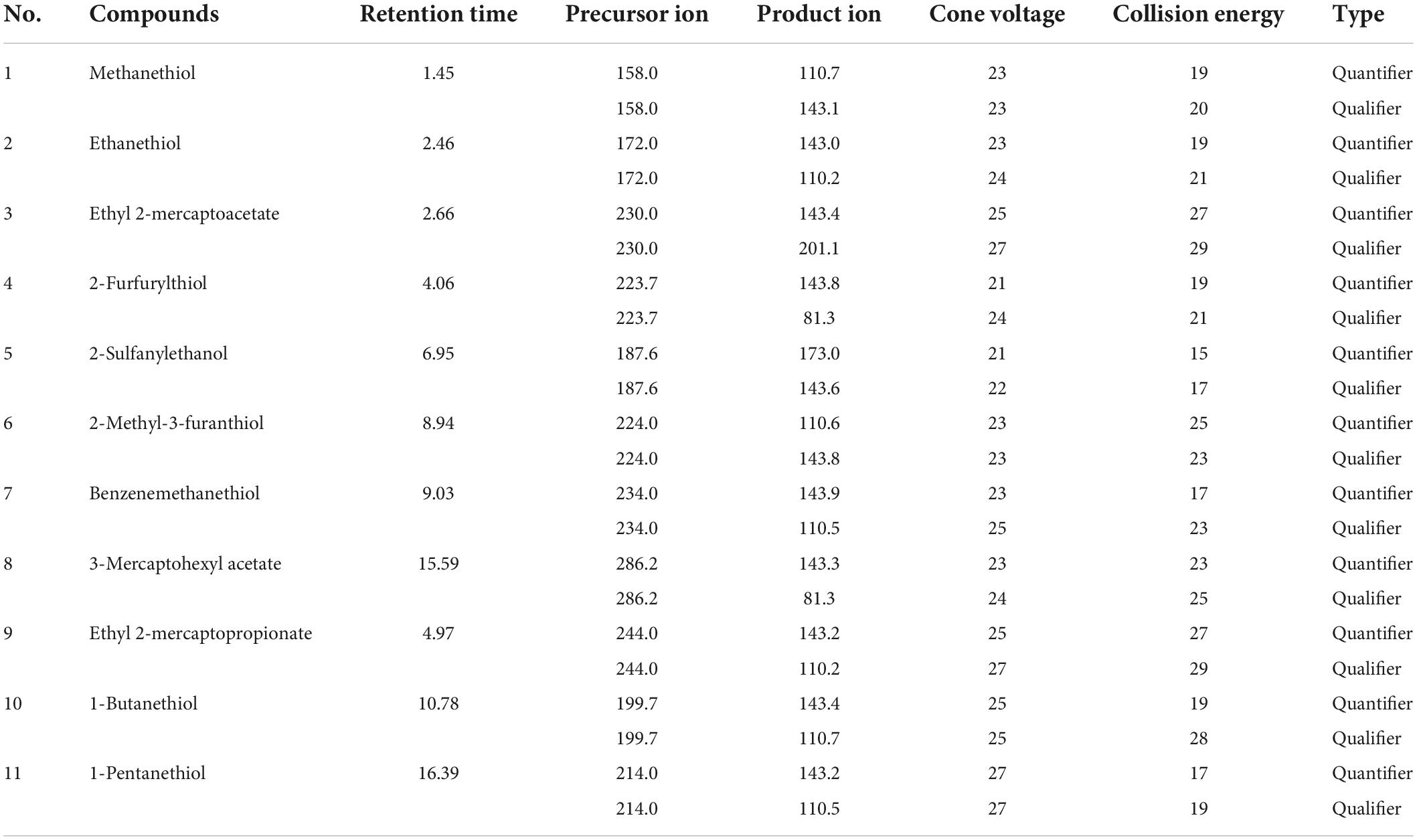
Table 1. Optimized multiple-reaction-monitoring (MRM) parameters for the derivatizeds thiol in Baijiu.
Ultraperformance liquid chromatography–mass spectrometry instrumentation and conditions
The thiols were separated by using an UPLC system (Waters, Milford, USA) equipped with a vacuum degasser, a binary solvent manager, and an autosampler. As stationary phase an analytical column (waters BEH C18, 100 × 2.1 mm, 1.7 μm) was used. Flow-rate was 0.3 mL/min and the composition of eluents was: solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). The linear gradient for solvent B was as follows: 0 min, 15%; 13 min, 22%; 14 min, 30%; 18 min, 35%; 18.5 min, 100%; 21.5 min, 100%; and 22 min, 15%. The injection volume was set at 10 μL.
The derivatization Baijiu sample was analyzed by means of UPLC–MS/MS using precursor ion scan screening for compounds releasing the diagnostic ion (m/z X→143.5) (24). In the source, cone voltage of 23 V and collision energy of 20 eV was applied. The eleven derivatives were quantified in MRM mode by monitoring their corresponding precursor ion, product ion, cone voltage, and collision energy, respectively (Table 1). Each Baijiu sample was tested in three different sessions to obtain an average value.
Thiols identification by ultraperformance liquid chromatography–quadrupole-time-of-flight mass spectrometry
The derivatized Baijiu sample was carried out by UPLC system (Waters, Milford, USA), coupled to a SYNAPT Q–TOF mass spectrometer (Waters, Milford, USA), using the following operation parameters: capillary voltage: 3,500 V; cone voltage: 20 V; collision energy: 20 eV; source temperature: 100°C; desolvation temperature: 400°C; desolvation gas flow: 700 L/h; cone gas flow: 50 L/h. The analytical column, mobile phase, and linear gradient were the same as those in the UPLC–MS/MS. The precursor ions were selected from UPLC–MS/MS spectra, which were produced by precursor ion scan mode. Then, the target ions were fragmented by collision-induced dissociation.
Method validation
Thiols standard solutions at different concentrations were obtained by diluting their corresponding stock solutions using a 50% ethanol-water solution and derivatized via the workflow developed in this study. The linearity of the derivatized thiol was evaluated according to the relative peak area versus the concentration and expressed using the correlation coefficient (R2). Relative to the calibration curve, thiol standards were added to the Baijiu samples at low, medium and high concentration levels. The recovery was calculated based on the concentrations of thiols measured in the spiked and non-spiked Baijiu samples. The intra-day precision was evaluated using the repeated analysis of 11 thiols found in the same Baijiu sample five times on the same day and the inter-day precision was determined on five consecutive days. The limits of detection (LOD) and limits of quantitation (LOQ) of the thiols were determined at their concentrations when the signal/noise (S/N) ratio was 3 and 10, respectively (25).
Determination of odor thresholds
Using a previously proposed method (26), the thiol odor thresholds were detected in a 46% ethanol-water solution using three-alternative forced choice tests. A sensory panel consisting of 20 panelists, 10 males, and 10 females, with an average age of 25 years. The odor activity value (OAV) was defined by dividing the concentration of the thiol to its odor threshold (27, 28).
Statistical analyses
The Masslynx software (waters) was used to process the data of UPLC–MS/MS and UPLC–Q-TOF–MS. Statistical analyses were carried out by using the Microsoft Excel 2010. Principal component analysis (PCA) of the concentrations of thiols in four different aroma-types Baijiu by XLSTAT 2018 software. Variable importance for projection (VIP) values were carried out using the SIMCA software.
Results and discussion
Identification of new thiols in Baijiu by ultraperformance liquid chromatography–mass spectrometry and ultraperformance liquid chromatography–quadrupole-time-of-flight mass spectrometry
Ultraperformance liquid chromatography–mass spectrometry analysis of derivatized thiols using multiple-reaction-monitoring
For the development of the MRM method, each commercial thiol standard derivative (1–8) was directly infused into the MS ion source in positive mode. The cone voltage (1–50 V) and collision energy (1–35 eV) were optimized to obtain the precursor ion and product ion with maximum fragment ion abundance. Table 1 lists the optimal MRM parameters for each thiol derivative based on their response and ion fragmentation (Supplementary Figure 1). All the derivatized thiols have a diagnostic ion observed between m/z 143 and 144 corresponding to a disulfanylpyridine group arising from the derivatized portion of the precursor ion. The fragmentation pathways and ions were similar to those previously reported (21); the fragment ion observed at m/z 143.5 in this study was used as the diagnostic ion for identification of the derivatized thiols (Figure 1A). To obtain a good separation of the derivatized thiols, several parameters in the chromatography method were systematically varied, allowing the derivatized thiols to be separated within 17 min, using the optimized gradient elution procedure (Figure 1B).
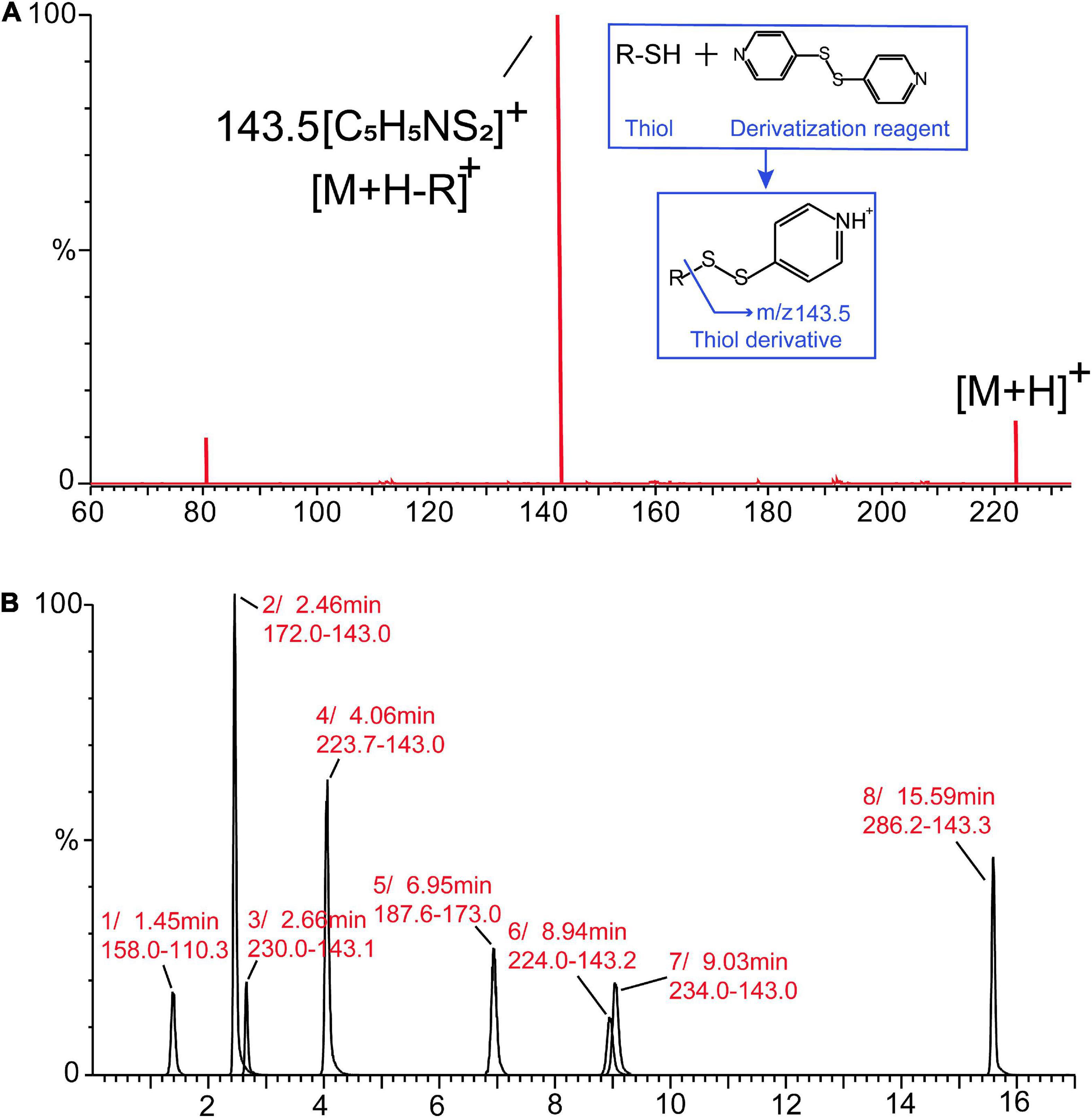
Figure 1. Mass spectrometry (MS/MS) spectrum of the derivatized thiols (A). Multiple-reaction-monitoring (MRM) analysis of the derivatized thiol standards (1–8) in positive mode by ultraperformance liquid chromatography (UPLC)–mass spectrometry (MS/MS) (B).
Precursor ion scan of m/z 143.5 in Baijiu by ultraperformance liquid chromatography–mass spectrometry
Based on the fragmentation behavior mentioned above, the m/z 143.5 peak was chosen as the diagnostic ion for the derivatized thiols. To improve the detection sensitivity of the constituent of interest, the volume of the SSAB sample was increased to 120 mL, to obtained a higher concentration of derivatized thiols. Based on the cone voltage and collision energy of the derivatized thiols (1–8), a cone voltage of 23 V and collision energy of 20 eV, were found to maximize the fragment ion abundance of the peak between m/z 143 and 144, for most of the derivatized thiols. Under these conditions, precursor ion scanning of m/z 143.5 was applied, to produce a single-ion chromatogram (Figure 2A). Following the diagnostic ion screening (m/z X→143.5), the peaks corresponding to the derivatized thiol candidates were exposed from the total ion chromatogram. Eight peaks (1–8) were identified as known thiols in Baijiu, by comparing the chromatographic retention time (Figure 1B) and MS fragmentation, with the authentic standards. Other than the eight known peaks, Figure 2A also shows three new peaks, unknown #1 (5.06 min), unknown #2 (10.83 min), and unknown #3 (16.46 min) in the chromatogram; three precursor ions at m/z 244, 200 and 214 were extracted from the three peaks, respectively (Figures 2B–D). The three peaks appeared to be potential new thiols, so their structures were identified using the following UPLC–Q-TOF–MS method.

Figure 2. Analysis of the derivatized Baijiu sample using precursor mode and ultraperformance liquid chromatography (UPLC)–mass spectrometry (MS/MS) screening for compounds releasing a disulfanylpyridin ion (m/z X→143.5) (A). MS spectra of the three unknown peaks by UPLC–MS/MS (B–D). MS peaks observed at 5.06 (unknown #1), 10.83 (unknown #2), and 16.46 min (unknown #3) using ultraperformance liquid chromatography quadrupole-time-of-flight mass spectrometry (UPLC–Q-TOF–MS) and their corresponding elemental composition (E–G).
New thiols in Baijiu
Quadrupole-time-of-flight mass spectrometry (Q-TOF–MS) was chosen because it can measure accurate masses with high resolution and provides the best information on the molecular composition of the compound of interest, so that its molecular formula can be confirmed, or a preliminary determination made (29, 30). UPLC–Q-TOF–MS analysis revealed the mass of unknown #1 to be m/z 244.0498 [M + H]+, corresponding to a molecular formula of C10H13NO2S2 (Figure 2E). Its main MS/MS fragments were m/z 142.9849 [M + H]+ (C5H5NS2+), 111.0135 [M + H]+ (C5H5NS+), and 79.0436 [M + H]+ (C5H5N+). The corresponding neutral losses of 101.0649, 31.9714, and 31.9699 appear to be losses of C5H9O2, S, and S, respectively, which appear to be fragments of the thiol after derivatization.
The derivatization reagent accounts for C5H5NS, so the molecular formula of unknown #1 was C5H10O2S. The candidate thiols were screened and identified using the database of flavor molecules (1,2 accessed in May 2022), then verified by comparison with authentic standard. Unknown #1 was identified as ethyl 2-mercaptopropionate. UPLC–Q-TOF–MS analysis revealed the accurate mass of unknown #2 to be m/z 200.0579 [M + H]+, corresponding to a molecular formula of C9H14NS2 (Figure 2F). The retention time of unknown #3 was 16.46 min and its molecular ion mass was 214.0736 [M + H]+, corresponding to a molecular formula of C10H16NS2 (Figure 2G). Subtracting the formula of the derivatizing reagent gave the molecular formula of unknown #2 and #3 as C4H10S and C5H12S, respectively. The database of flavor molecules and comparison with authentic standards unambiguously identified these compounds as 1-butanethiol and 1-pentanethiol, respectively. To our knowledge, this is the first time ethyl 2-mercaptopropionate, 1-butanethiol, and 1-pentanethiol have been detected in Baijiu. Ethyl 2-mercaptopropionate has been reported to be an important odorant, which correlates with age in wine (31). 1-Butanethiol and 1-pentanethiol have burned and sulfuryl odor qualities, which are the odorants of fruit brandy and coffee (32, 33).
Analytical characteristics of the ultraperformance liquid chromatography–mass spectrometry method
To check the performance and reliability of the newly developed method, quality parameters such as linear range, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy were determined.
Linear range, limits of detection, and limits of quantitation
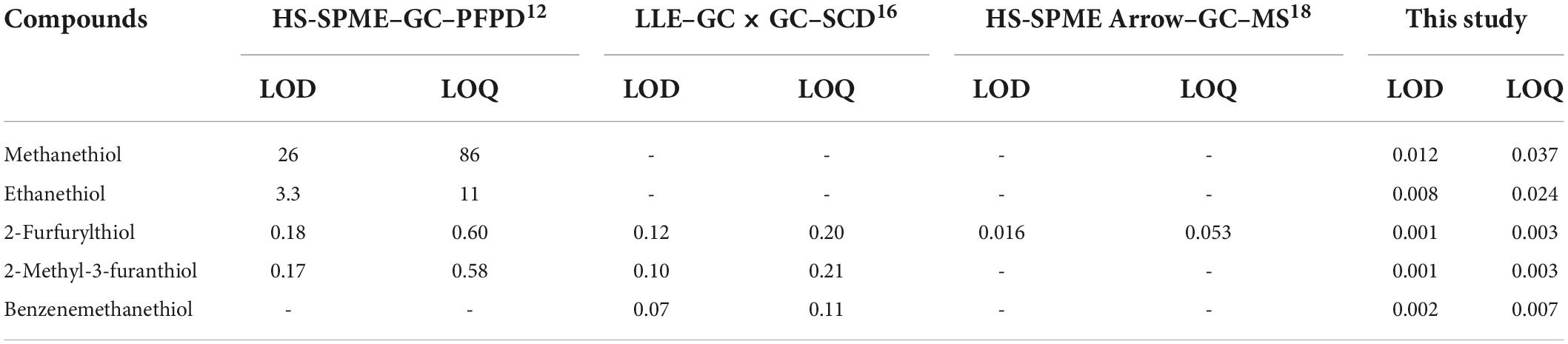
Linearity was evaluated for each thiol over at least seven different concentrations. The experimentally-determined linear ranges covered a wide concentration range (up to 819 μg/L). The correlation coefficients (R2) for the thiol derivatives were in the range 0.9911–0.9978. The LOD and LOQ obtained for the thiol derivatives were in the ranges 0.001–0.012 μg/L and 0.003–0.037 μg/L, respectively (Table 2). All LOQs of the thiol derivatives were below their respective odor thresholds (Table 3), making this method suitable for combined chemical analysis and sensory experiments. The UPLC–MS/MS method also performed better than GC for quantification of the volatile thiols (Table 4). For example, the LOQ of 2-furfurylthiol was 0.003 μg/L by UPLC–MS/MS, 200 times lower than by HS-SPME–GC–PFPD (0.60 μg/L) (13). In addition, the UPLC–MS/MS yielded an LOD of 0.001 μg/L for 2-methyl-3-furanthiol, 170 times lower than the 0.17 μg/L achieved by HS-SPME–GC–PFPD (34). Many methods have been proposed for the analysis of thiols in Baijiu, with common techniques involving GC. Several methods such as HS-SPME, SBSE, HS-SPME arrow, and direct injection (DI) coupled with GC are widely used for extraction of thiols in Baijiu. The PFPD, SCD, and TOFMS are highly selective and sensitive for sulfur determination. Compared with previous GC analytical methods for the number of thiols analyzed in Baijiu (Supplementary Figure 2), 11 thiols were studied in the present study [11 vs. 4 with GC × GC–SCD (16), 3 with GC–PFPD (13), 1 with HS-SPME Arrow–GC–MS (18), 0 with LLE–GC × GC–TOFMS (35), 0 with HS-SPME–GC–MS (36), 0 with SBSE–GC–MS (37), and 0 with DI–GC–MS (38)].
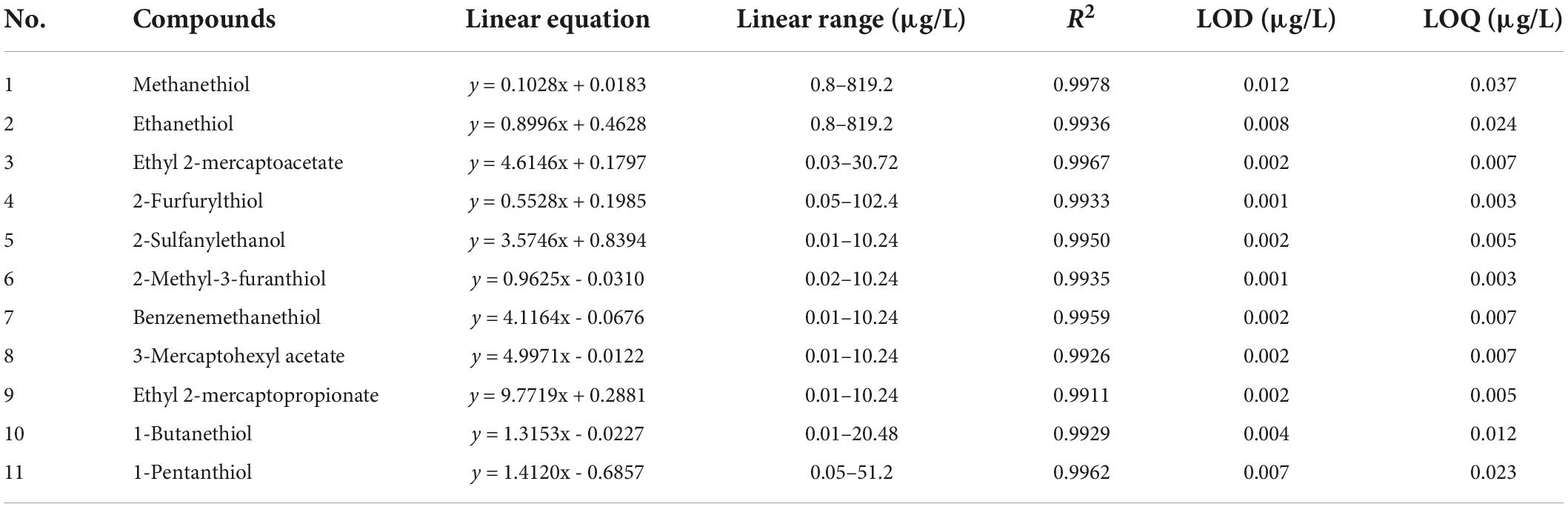
Table 2. Linear range, correlation coefficient (R2), limits of detection (LOD), and limits of quantitation (LOQ) of the established method for derivatized thiols.
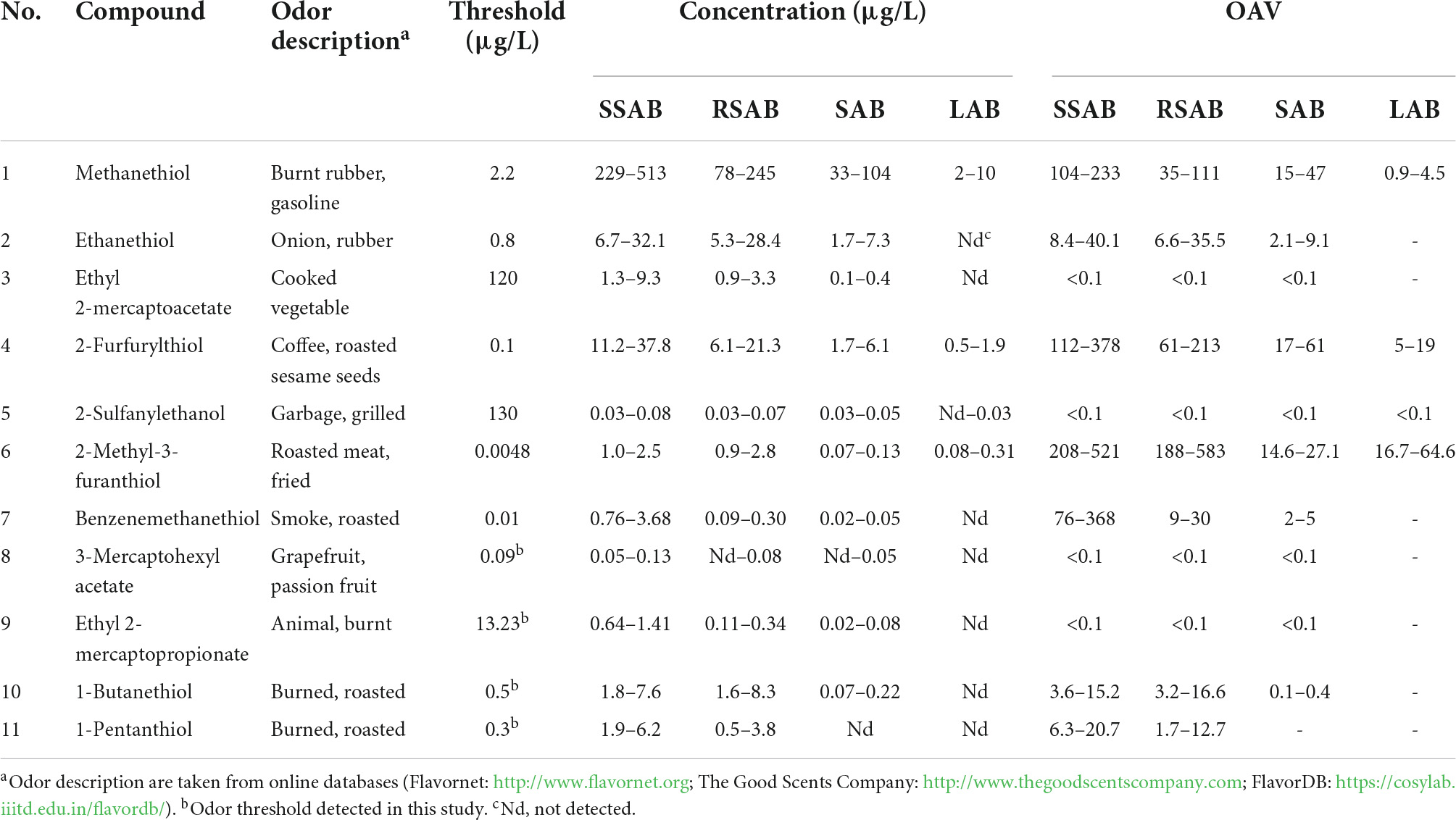
Table 3. The thiol concentrations in different aroma types Baijiu and their corresponding odor activity values (OAVs) range.
Precision and accuracy
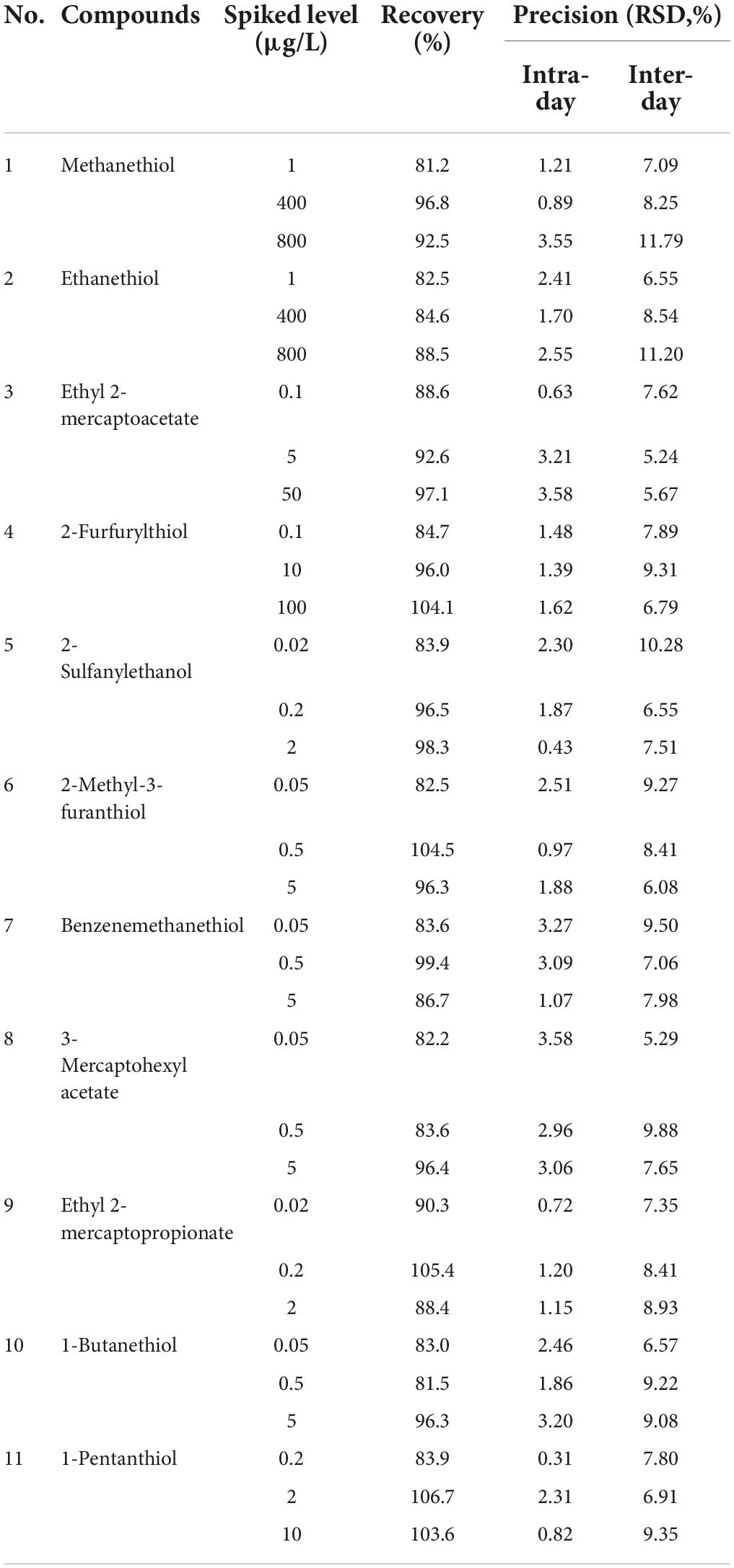
The method for precision measurement was based on stable instrument status, and the results were recorded as the intra- and inter-day precision with a number of replicates (n = 5) (Table 5). The RSDs of the intra-day measurements varied between 0.31% (1-pentanthiol) and 3.58% (ethyl 2-mercaptoacetate), and the inter-day RSDs varied between 5.24% (ethyl 2-mercaptoacetate) and 11.79% (methanethiol). The accuracy of the method was determined based on the recoveries. The recoveries of the thiol derivatives were all between 81.2% (methanethiol) and 106.7% (1-pentanthiol) at the three different concentration levels studied (low, medium, and high concentrations in the calibration graphs).
Analysis of practical samples
Quantitation of thiols in Baijiu samples and odor activity value analysis
To determine the practical utility of the new method, it was applied to a variety of typical aroma-type Baijiu samples obtained from diverse regions. The determined concentrations of 11 thiols are shown in Supplementary Table 2. The OAV can be used to evaluate the sensory contribution of an aroma compound, because it is obtained by dividing the concentration of the thiol by its odor threshold. An OAV much greater than 1 means that the compound may contribute to the odor of the Baijiu (39, 40).
The thiol concentrations in different Baijiu aroma types and their corresponding OAV ranges were determined (Table 3). When compared with the other samples, SSAB was characterized by a higher total thiol concentration. The concentrations of seven thiols in SSAB were higher than their corresponding odor thresholds. Methanethiol (229–513 μg/kg, OAVs 104–233), 2-furfurylthiol (11.2–37.8 μg/kg, OAVs 112–378), and 2-methyl-3-furanthiol (1.0–2.5 μg/kg, OAVs 208–521), which have been identified as important aroma compounds for SSAB in previous reports, were also confirmed in this study (34, 41). Two notable thiols, 1-butanethiol (1.8–7.6 μg/L, OAVs 4–15), and 1-pentanethiol (1.9–6.2 μg/L, OAVs 6–21), were quantified in Baijiu for the first time, in trace amounts, but their low odor thresholds still result in significant OAVs.
A total of 7 thiols with OAVs > 1 was found in RSAB. The highest OAV was found for 2-methyl-3-furanthiol (0.9–2.8 μg/kg, OAVs 188–583), which has a roasted meat odor and was identified in RSAB for the first time in this study. 2-Furfurylthiol (6.1–21.3 μg/kg, OAVs 61–213) was found at a concentration much higher than its odor threshold, and determined to be a typical potent odorant of RSAB (12). In the process of RSAB fermentation, Saccharomyces cerevisiae generates 2-furfurylthiol using furfural and L-cysteine as precursors (42). The OAVs of ethanethiol (6.6–35.5), benzenemethanethiol (9–30), 1-butanethiol (3.2–16.6), and 1-pentanethiol (1.7–12.7) exceeded their odor thresholds, indicating that they may contribute significantly to the overall aroma profile.
Five thiols were found at concentrations higher than their odor thresholds in SAB. Of these thiols, methanethiol was present at the highest concentration (33–104 μg/kg, OAVs 15–47), followed by ethanethiol (1.7–7.3 μg/kg, OAVs 2–9) and 2-furfurylthiol (1.7–6.1 μg/kg, OAVs 17–61). Lower concentrations were observed for 2-methyl-3-furanthiol (0.07–0.13 μg/kg, OAVs 15–27) and benzenemethanethiol (0.02–0.05 μg/kg, OAVs 2–5), which was present at concentrations below 1 μg/L in all samples. Only four thiols were found in LAB. Among them, methanethiol (2–10 μg/L, OAVs 1–5), 2-furfurylthiol (0.5–1.9 μg/L, OAVs 5–19) and 2-methyl-3-furanthiol (0.08–0.31 μg/L, OAVs 17–65) had OAVs > 1. These findings will facilitate further study of the contribution of thiols to the aroma of Baijiu.
Statistical analysis of thiols
Principal components analysis (PCA) is an unsupervised method for expressing the similarities and differences of groups of samples (43, 44). PCA analysis was performed on the thiol concentrations (detailed data in Supplementary Table 2) to visualize graphically, the thiol concentrations found in the different Baijiu aroma-types. The PCA scores plot (Figure 3A) clearly distinguished the four different Baijiu aroma-types. The first two principal components PCA (PC1 and PC2) accounted for 74.47% of the variation, indicating that the thiols significantly affect the flavor characteristics of Baijiu (45). Moreover, heat map and hierarchical cluster analysis were also visualized differences within the different Baijiu aroma-types (Figure 3B). The SSAB samples were characterized by the highest thiol concentrations and were all grouped on the far right of the PCA bi-plot. In contrast, the LAB samples with lowest thiol concentrations were on the far left.
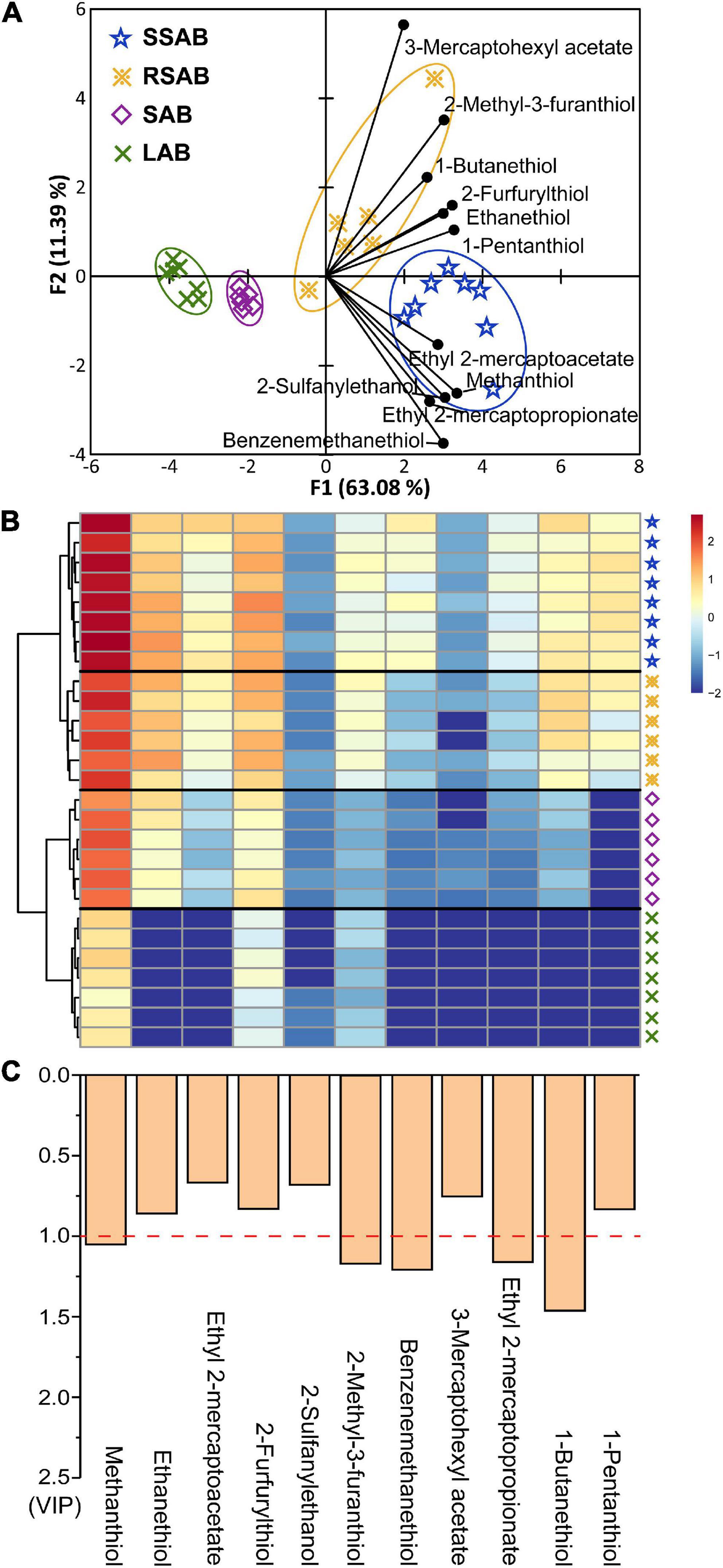
Figure 3. Principal component analysis (PCA) of the thiols in four aroma-types Baijiu samples (A). Heat map of thiols (B). The histogram shows the value of variable importance for projection (VIP) (C).
To identify the most discriminative thiols contributing to different Baijiu aroma-types, important compounds were calculated (46). Generally, variable importance for projection (VIP) values are considered a significant contributor to different samples. Thiols with VIP > 1.0 are the most relevant for explaining the different Baijiu aroma-types (47). Five thiols (methanethiol, 2-methyl-3-furanthiol, benzenemethanethiol, ethyl 2-mercaptopropionate, and 1-butanethiol) could be considered as being responsible for the differences in the aroma characteristics between Baijiu aroma-types (Figure 3C).
Conclusion
In this study, a novel strategy using UPLC–MS/MS and UPLC–Q-TOF–MS, was developed to identify thiols in Baijiu. The unique and consistent MS/MS fragmentation pathway of the DTDP-thiol derivatives provides a powerful way to expand the number of quantifiable thiols, and identify unknown thiols by targeting a diagnostic ion (m/z 143.5). In addition to the eight known thiols detected in the Baijiu samples, three new thiols were detected and identified (ethyl 2-mercaptopropionate, 1-butanethiol, and 1-pentanethiol) using the strategy developed in this study. In addition, a method using UPLC–MS/MS has been developed and validated to quantify thiols in Baijiu. Seven thiols were suggested as important aroma contributors for Baijiu based on OAVs. Five thiols could be used as markers for different Baijiu aroma-types.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YY: conceptualization, methodology, investigation, and writing—original draft. JL, YN, and CL: methodology and resources. SC: conceptualization, writing—review and editing, project administration, and funding acquisition. YX: resources, funding acquisition, and writing—review and editing. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32172331) and Guizhou Science and Technology Program [(2021) General project 137].
Conflict of interest
Author JL and CL were employed by Guizhou Guotai Liquor Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1022600/full#supplementary-material
Footnotes
References
1. Jin GY, Zhu Y, Xu Y. Mystery behind Chinese liquor fermentation. Trends Food Sci Technol. (2017) 63:18–28. doi: 10.1016/j.tifs.2017.02.016
2. Ye H, Wang J, Shi J, Du J, Zhou Y, Huang M, et al. Automatic and intelligent technologies of solid-state fermentation process of Baijiu production: applications, challenges, and prospects. Foods. (2021) 10:680. doi: 10.3390/foods10030680
3. Wu Q, Zhu Y, Fang C, Wijffels RH, Xu Y. Can we control microbiota in spontaneous food fermentation?-Chinese liquor as a case example. Trends Food Sci Technol. (2021) 110:321–31. doi: 10.1016/j.tifs.2021.02.011
4. Liu H, Sun B. Effect of fermentation processing on the flavor of baijiu. J Agric Food Chem. (2018) 66:5425–32. doi: 10.1021/acs.jafc.8b00692
5. Yang L, Fan W, Xu Y. GC × GC-TOF/MS and UPLC-Q-TOF/MS based untargeted metabolomics coupled with physicochemical properties to reveal the characteristics of different type daqus for making soy sauce aroma and flavor type baijiu. LWT-Food Sci Technol. (2021) 146:111416. doi: 10.1016/j.lwt.2021.111416
6. Yan Y, Chen S, Nie Y, Xu Y. Quantitative analysis of pyrazines and their perceptual interactions in soy sauce aroma type Baijiu. Foods. (2021) 10:441. doi: 10.3390/foods10020441
7. Qian Y, Zhang L, Sun Y, Tang Y, Li D, Zhang H, et al. Differentiation and classification of Chinese Luzhou-flavor liquors with different geographical origins based on fingerprint and chemometric analysis. J Food Sci. (2021) 86:1861–77. doi: 10.1111/1750-3841.15692
8. Gao J, Liu G, Li A, Liang C, Ren C, Xu Y. Domination of pit mud microbes in the formation of diverse flavour compounds during Chinese strong aroma-type Baijiu fermentation. LWT-Food Sci Technol. (2021) 137:110442. doi: 10.1016/j.lwt.2020.110442
9. Niu Y, Yao Z, Xiao Z, Zhu G, Zhu J, Chen J. Sensory evaluation of the synergism among ester odorants in light aroma type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res Int. (2018) 113:102–14. doi: 10.1016/j.foodres.2018.01.018
10. Wang Z, Wang Y, Zhu T, Wang J, Huang M, Wei J, et al. Characterization of the key odorants and their content variation in Niulanshan Baijiu with different storage years using flavor sensory omics analysis. Food Chem. (2022) 376:131851. doi: 10.1016/j.foodchem.2021.131851
11. Shen T, Liu J, Wu Q, Xu Y. Increasing 2-furfurylthiol content in Chinese sesame-flavored Baijiu via inoculating the producer of precursor l-cysteine in Baijiu fermentation. Food Res Int. (2020) 138:109757. doi: 10.1016/j.foodres.2020.109757
12. Sha S, Chen S, Qian M, Wang C, Xu Y. Characterization of the typical potent odorants in Chinese roasted sesame-like flavor type liquor by headspace solid phase microextraction-aroma extract dilution analysis, with special emphasis on sulfur-containing odorants. J Agric Food Chem. (2017) 65:123–31. doi: 10.1021/acs.jafc.6b04242
13. Chen S, Sha S, Qian M, Xu Y. Characterization of volatile sulfur compounds in Moutai liquors by headspace solid-phase microextraction gas chromatography-pulsed flame photometric detection and odor activity value. J Food Sci. (2017) 82:2816–22. doi: 10.1111/1750-3841.13969
14. Sun J, Wang Z, Sun B. Low quantity but critical contribution to flavor: review of the current understanding of volatile sulfur-containing compounds in Baijiu. J Food Compos Anal. (2021) 103:104079. doi: 10.1016/j.jfca.2021.104079
15. Chen L, Capone DL, Jeffery DW. Analysis of potent odour-active volatile thiols in foods and beverages with a focus on wine. Molecules. (2019) 24:2472. doi: 10.3390/molecules24132472
16. Song X, Zhu L, Wang X, Zheng F, Zhao M, Liu Y, et al. Characterization of key aroma-active sulfur-containing compounds in Chinese Laobaigan Baijiu by gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography coupled with sulfur chemiluminescence detection. Food Chem. (2019) 297:124959. doi: 10.1016/j.foodchem.2019.124959
17. Niu Y, Yao Z, Xiao Q, Xiao Z, Ma N, Zhu J. Characterization of the key aroma compounds in different light aroma type Chinese liquors by GC-olfactometry, GC-FPD, quantitative measurements, and aroma recombination. Food Chem. (2017) 233:204–15. doi: 10.1016/j.foodchem.2017.04.103
18. Zhang X, Wang C, Wang L, Chen S, Xu Y. Optimization and validation of a head space solid-phase microextraction-arrow gas chromatography-mass spectrometry method using central composite design for determination of aroma compounds in Chinese liquor (Baijiu). J Chromatogr A. (2019) 1610:460584. doi: 10.1016/j.chroma.2019.460584
19. Chen L, Capone DL, Jeffery DW. Identification and quantitative analysis of 2-methyl-4-propyl-1,3-oxathiane in wine. J Agric Food Chem. (2018) 66:10808–15. doi: 10.1021/acs.jafc.8b04027
20. Quintanilla-Casas B, Dulsat-Serra N, Cortes-Francisco N, Caixach J, Vichi S. Thiols in brewed coffee: assessment by fast derivatization and liquid chromatography-high resolution mass spectrometry. LWT-Food SciTechnol. (2015) 64:1085–90. doi: 10.1016/j.lwt.2015.07.010
21. Capone DL, Ristic R, Pardon KH, Jeffery DW. Simple quantitative determination of potent thiols at ultratrace levels in wine by derivatization and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis. Anal Chem. (2015) 87:1226–31. doi: 10.1021/ac503883s
22. Mafata M, Stander MA, Thomachot B, Buica A. Measuring thiols in single cultivar south african red wines using 4,4-dithiodipyridine (DTDP) derivatization and ultraperformance convergence chromatography-tandem mass spectrometry. Foods. (2018) 7:138. doi: 10.3390/foods7090138
23. Danek M, Fang XY, Tang J, Plonka J, Barchanska H. Simultaneous determination of pesticides and their degradation products in potatoes by MSPD-LC-MS/MS. J Food Compos Anal. (2021) 104:104129. doi: 10.1016/j.jfca.2021.104129
24. Meyer S, Dunkel A, Hofmann T. Sensomics-assisted elucidation of the tastant code of cooked crustaceans and taste reconstruction experiments. J Agric Food Chem. (2016) 64:1164–75. doi: 10.1021/acs.jafc.5b06069
25. Venisse N, Cambien G, Robin J, Rouillon S, Nadeau C, Charles T, et al. Development and validation of an LC-MS/MS method for the simultaneous determination of bisphenol A and its chlorinated derivatives in adipose tissue. Talanta. (2019) 204:145–52. doi: 10.1016/j.talanta.2019.05.103
26. Dong W, Shi K, Liu M, Shen C, Li A, Sun X, et al. Characterization of 3-methylindole as a source of a “mud”-like off-odor in strong-aroma types of base Baijiu. J Agric Food Chem. (2018) 66:12765–72. doi: 10.1021/acs.jafc.8b04734
27. Wang J, Ming Y, Li Y, Huang M, Luo S, Li H, et al. Characterization and comparative study of the key odorants in Caoyuanwang mild-flavor style Baijiu using gas chromatography-olfactometry and sensory approaches. Food Chem. (2021) 347:129028. doi: 10.1016/j.foodchem.2021.129028
28. Bi S, Wang A, Lao F, Shen Q, Liao X, Zhang P, et al. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. (2021) 339:127878. doi: 10.1016/j.foodchem.2020.127878
29. Muehlwald S, Buchner N, Kroh LW. Investigating the causes of low detectability of pesticides in fruits and vegetables analysed by high-performance liquid chromatography-Time-of-flight. J Chromatogr A. (2018) 1542:37–49. doi: 10.1016/j.chroma.2018.02.011
30. Feng T, Wu Y, Zhang Z, Song S, Zhuang H, Xu Z, et al. Purification, identification, and sensory evaluation of kokumi peptides from agaricus bisporus mushroom. Foods. (2019) 8:43. doi: 10.3390/foods8020043
31. Picard M, Thibon C, Redon P, Darriet P, de Revel G, Marchand S. Involvement of dimethyl sulfide and several polyfunctional thiols in the aromatic expression of the aging bouquet of red bordeaux wines. J Agric Food Chem. (2015) 63:8879–89. doi: 10.1021/acs.jafc.5b03977
32. Dziekonska-Kubczak U, Pielech-Przybylska K, Patelski P, Balcerek M. Development of the method for determination of volatile sulfur compounds (VSCs) in fruit brandy with the use of HS-SPME/GC-MS. Molecules. (2020) 25:1232. doi: 10.3390/molecules25051232
33. Charles-Bernard M, Roberts DD, Kraehenbuehl K. Interactions between volatile and nonvolatile coffee components. 2. Mechanistic study focused on volatile thiols. J Agric Food Chem. (2005) 53:4426–33. doi: 10.1021/jf048020y
34. Wang L, Fan S, Yan Y, Yang L, Chen S, Xu Y. Characterization of potent odorants causing a pickle-like off-odor in Moutai-aroma type Baijiu by comparative aroma extract dilution analysis, quantitative measurements, aroma addition, and omission studies. J Agric Food Chem. (2020) 68:1666–77. doi: 10.1021/acs.jafc.9b07238
35. Zhu S, Lu X, Ji K, Guo K, Li Y, Wu C, et al. Characterization of flavor compounds in Chinese liquor Moutai by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal Chim Acta. (2007) 597:340–8. doi: 10.1016/j.aca.2007.07.007
36. Ding X, Wu C, Huang J, Zhou R. Characterization of interphase volatile compounds in Chinese Luzhou-flavor liquor fermentation cellar analyzed by head space-solid phase micro extraction coupled with gas chromatography mass spectrometry (HS-SPME/GC/MS). LWT-Food Sci Technol. (2016) 66:124–33. doi: 10.1016/j.lwt.2015.10.024
37. Fan W, Shen H, Xu Y. Quantification of volatile compounds in Chinese soy sauce aroma type liquor by stir bar sorptive extraction and gas chromatography-mass spectrometry. J Sci Food Agric. (2011) 91:1187–98. doi: 10.1002/jsfa.4294
38. Sun J, Zhao D, Zhang F, Sun B, Zheng F, Huang M, et al. Joint direct injection and GC–MS chemometric approach for chemical profile and sulfur compounds of sesame-flavor Chinese Baijiu (Chinese liquor). Eur Food Res Technol. (2017) 244:145–60. doi: 10.1007/s00217-017-2938-7
39. Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, et al. Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed Engl. (2014) 53:7124–43. doi: 10.1002/anie.201309508
40. Zhu W, Cadwallader KR. Streamlined approach for careful and exhaustive aroma characterization of aged distilled liquors. Food Chem X. (2019) 3:100038. doi: 10.1016/j.fochx.2019.100038
41. Yan Y, Chen S, Nie Y, Xu Y. Characterization of volatile sulfur compounds in soy sauce aroma type Baijiu and changes during fermentation by GC × GC-TOFMS, organoleptic impact evaluation, and multivariate data analysis. Food Res Int. (2020) 131:109043. doi: 10.1016/j.foodres.2020.109043
42. Zha M, Yin S, Sun B, Wang X, Wang C. STR3 and CYS3 contribute to 2-furfurylthiol biosynthesis in Chinese sesame-flavored Baijiu yeast. J Agric Food Chem. (2017) 65:5503–11. doi: 10.1021/acs.jafc.7b01359
43. Cserhati T. Data evaluation in chromatography by principal component analysis. Biomed Chromatogr. (2010) 24:20–8. doi: 10.1002/bmc.1294
44. Lieb VM, Esquivel P, Cubero Castillo E, Carle R, Steingass CB. GC-MS profiling, descriptive sensory analysis, and consumer acceptance of Costa Rican papaya (Carica papaya L.) fruit purees. Food Chem. (2018) 248:238–46. doi: 10.1016/j.foodchem.2017.12.027
45. He F, Duan J, Zhao J, Li H, Sun J, Huang M, et al. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. (2021) 365:130430. doi: 10.1016/j.foodchem.2021.130430
46. He Y, Liu Z, Qian M, Yu X, Xu Y, Chen S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. (2020) 331:127335. doi: 10.1016/j.foodchem.2020.127335
Keywords: thiols, Baijiu, UPLC-MS/MS, quantification, odor activity value
Citation: Yan Y, Lu J, Nie Y, Li C, Chen S and Xu Y (2022) Characterization of volatile thiols in Chinese liquor (Baijiu) by ultraperformance liquid chromatography–mass spectrometry and ultraperformance liquid chromatography–quadrupole-time-of-flight mass spectrometry. Front. Nutr. 9:1022600. doi: 10.3389/fnut.2022.1022600
Received: 18 August 2022; Accepted: 08 September 2022;
Published: 03 October 2022.
Edited by:
Mingquan Huang, Beijing Technology and Business University, ChinaReviewed by:
Guangsen Fan, Beijing Technology and Business University, ChinaLiang Yang, Moutai Institute, China
Copyright © 2022 Yan, Lu, Nie, Li, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Chen, c2h1YW5nY2hlbkBqaWFuZ25hbi5lZHUuY24=
 Yan Yan
Yan Yan Jun Lu3
Jun Lu3 Yao Nie
Yao Nie Shuang Chen
Shuang Chen Yan Xu
Yan Xu