
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 05 October 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1018534
This article is part of the Research TopicFlavor Chemistry of Food: Mechanism, Interaction, New AdvancesView all 24 articles
Tomato is one of the most widely cultivated horticultural plants in the world, while the key volatile compounds of tomato fruits generally derive from fatty acid, carotenoid, phenylalanine, and branched-chain amino acid pathways. As an important endogenous signal molecule, methyl salicylate (MeSA) plays a crucial role in the fruit ripening process of plant. Recently, it has been demonstrated that MeSA can maintain the flavor quality of full ripe tomatoes after cold-storage preservation. However, few research teams attempted to investigate the effects of MeSA plus low temperature treatment on the different volatile biosynthetic pathways of tomatoes previously. Therefore, in this study, the effects of methyl salicylate pre-treatment (0.05 mM MeSA, 24 h) on the volatile profile and flavor-related key gene expressions of tomato fruits stored at 10°C were evaluated for the first time. Our results showed that the loss of volatile compounds in low temperature-treated tomato fruits could be effectively alleviated by MeSA pre-treatment. Although MeSA had no remarkable effect on the formation of carotenoid pathway- and branched-chain amino acid pathway-related volatiles in tomatoes subjected to low temperature, the content of fatty acid pathway-related volatiles (including cis-3-hexenal, hexanal, and trans-2-hexenal) in full red fruits of 10°C MeSA group was remarkably higher than that of 10°C control group. Furthermore, MeSA pre-treatment significantly up-regulated the expression of LOXC or LOXD gene in low temperature-treated fruits at breaker or full red stage, respectively. In conclusion, pre-treatment with MeSA might avoid the loss of aromatic compounds in tomato fruits stored at low temperature by activating the fatty acid pathway.
Tomato is one of the most widely cultivated horticultural plants in the world, which accounts for 23% of total output in the whole fruit and vegetable market (1, 2). It has been demonstrated that tomato fruits are rich in vitamins, flavonoids, carotenoids, and other bioactive compounds (3). As an important sensory quality parameter for tomato fruit, the aroma is closely associated with the consumers’ acceptance of tomatoes. Nowadays, over 400 volatile compounds have been identified in tomatoes, including primary aromatic compounds and a series of secondary aromatic compounds (4). Among them, aldehydes, alcohols, ketones, esters, phenols, and sulfurs play important roles in the flavor of tomatoes.
The key volatile compounds of tomato fruits usually derive from fatty acid, carotenoid, phenylalanine, and branched-chain amino acid pathways (5). It is reported that C6-aroma compounds can be synthesized from fatty acid pathway in tomato fruits, which are a large group of flavor substances with grassy and green odors, including hexanal, cis-3-hexenal, and trans-2-hexenal (6–8). Specifically, linolenic acid or linoleic acid is catalyzed by lipoxygenases (LOXs) and hydroperoxide lyase (HPL) to form hexanal or cis-3-hexenal, respectively. Under the action of aldehyde dehydrogenase 2 (ADH2), these compounds can be reduced to the corresponding alcohols (4). Furthermore, the aromatic compounds with fruity flavors such as neral, geranial, geranylacetone, 6-methyl-5-hepten-2-one, β-ionone, are derived from β-carotene precursors via carotenoid pathway in tomatoes. Carotenoids are able to be degraded by the carotenoid cleavage dioxygenase (LeCCDs) to form primary oxidation products, which further transform into volatile compounds under the actions of enzyme catalysis and acid hydrolysis (9).
Also, the volatiles synthesized from phenylalanine pathway have significant effects on the overall flavor of tomato fruits, containing 2-phenylacetaldehyde, 2-phenylethanol, methyl salicylate, guaiacol, eugenol, catechol, 1-nitro-2-ethylbenzene, and 2-phenylacetonitrile (10). On the one hand, phenylalanine can be catalyzed by phenylalanine ammonia lyase (PAL) to form trans-cinnamic acid, which further passes through different metabolic branches to form salicylic acid. One the other hand, amino acid decarboxylase (LeAADC) is able to promote the transformation of phenylalanine into phenylethylamine (11). Alternatively, the formation of aromatic compounds with floral smell (e.g., 2-phenylacetaldehyde and 2-phenylethanol) are related with the effects of LeAADC and phenethylamine reductase (PAR) (12). Regarding the branched-chain amino acid pathway, isoleucine and leucine are the representative precursors of aromatic components with caramel flavor (e.g., 2-methylbutyraldehyde, 3-methylbutyraldehyde, 2-methylbutanol, and 3-methylbutanol), fruity and spicy flavor (e.g., isovaleronitrile and isobutyl acetate) as well as green and fruity flavor (e.g., 2-isobutylthiazole). Notably, branched-chain amino acid aminotransferases (SlBCATs) are essential for the synthesis of these components (13).
Based on previous studies, the flavor of tomato fruits can be significantly influenced by their varieties and maturity stages as well as different postharvest treatments (including ethylene, low temperature, and hormone treatments) (14). Cold storage is shown to be one of the most effective postharvest treatment methods to prolong the shelf life of horticultural products (15). However, it may inhibit the flavor formation of tomato fruits. For instance, Wang et al. (6) investigated the changes of volatile compounds in tomatoes at the green ripening stage under low temperature (5°C) (6). The results showed that the level of twelve important compounds (including aldehydes and alcohols) in tomato fruits significantly decreased after treating under low temperature. In addition, the ripening process, ethylene release, and the respiratory rate of tomatoes were also inhibited by low temperature treatment although no obvious mechanical injury was observed. It was worth mentioning that the volatile content of fruits stored at 5°C was at a very low level before the color-breaking stage. In the research of Ponce-Valadez et al. (16), they observed that storing at 12.5°C over 9 days could lead to a decrease of total aroma volatiles in tomatoes, especially for hexanal, hexanol and cis-3-hexenol (16). Nevertheless, there was no significant difference in consumer’s flavor perception between the fruits treated at 12.5 and 20°C.
Methyl salicylate (MeSA), a plant volatile organic compound, is synthesized from salicylate acid (SA) and essential for the growth, development as well as functions of plants (17). As an important endogenous signal molecule, MeSA plays a crucial role in defense mechanism activation, responses against several abiotic and biotic stresses as well as the fruit ripening process (18). In recent years, postharvest treatment with MeSA has drawn increasing interest due to its effects on extending the shelf-life of fresh agricultural products and reducing their low-temperature susceptibility (19). For instance, the chilling injury symptoms of tomatoes exposed to 0.01 mM of MeSA for hours at room temperature were significantly less severe than those of control (20). Interestingly, in a previous investigation, it was found that MeSA pre-treatment could effectively improve the flavor quality of full ripe “FL 47” tomatoes after cold storage as well (6). To be specific, MeSA remarkably suppressed the loss of some key aroma volatiles (including geranylacetone, geranial, and MeSA) in tomato fruits subjected to chilling temperature. However, little information about the effects of MeSA plus low temperature treatment on the volatile biosynthetic pathways (fatty acid, carotenoid, phenylalanine, and branched-chain amino acid pathways) of tomato fruits was provided. Consequently, we aimed to explore the roles of MeSA pre-treatment in the flavor components and flavor-related key gene expressions of tomatoes stored at low temperature in the present study.
Mature green (G) “FL 47” tomatoes were harvested from a commercial field in Fort Pierce, FL. In the current research, fruits with moderate size and no obvious mechanical damage were selected and used in the following experiment.
One hundred and forty-four uniform and defect-free fruits with an average weight of 270 g were divided into two groups. Half of them were treated with MeSA at 20°C for 24 h, while the left fruits were stored in air. In terms of the MeSA treated group, the fruits were placed in a 45 L airtight glass container, from the top of which a 7 cm diameter filter paper disc soaked in 222.9 μL of MeSA was suspended. The final chemical vapor concentration in the container was 0.05 mM. After fumigating for 24 h at 20°C, the container was opened, and ventilated for 12 h. Subsequently, all fruits were further classified into four groups (36 fruits each group), respectively. To be specific, 72 fruits with and without MeSA pre-treatment were stored at 20°C for ripening, while another half were transferred to 10°C for 10 days before ripening at 20°C. The samples at G stage (0 day) and other three maturity stages [full red (R), pink (P), and breaker (BR) stages] were collected in this study. For 20°C control group, tomato fruits reached to BR, P or R stage on the 4th, 10th, or 14th day after MeSA pre-treatment, respectively. By contrast, it took 5, 11, or 14 days for fruits in the 20°C MeSA group to reach to BR, P or R stage after MeSA pre-treatment, respectively. The ripening of fruits in 10°C control and 10°C MeSA groups was significantly delayed by low temperature treatment. Particularly, tomatoes in 10°C control group reached to BR, P, or R stage on the 13th, 22nd, or 28th day after MeSA pre-treatment, respectively, whilst it took 13, 21, or 27 days for the fruits of 10°C MeSA group to reach the corresponding stage after treating with MeSA.
Volatile compound analysis was performed using gas chromatography-olfactometry-mass spectrometry (GC-O-MS) according to the method of Li et al. (21), with some modifications (21). Briefly, a sharp stainless steel knife was used to remove the peels from three tomato fruits at the same maturation stage per replicate, then the pulp tissues was squeezed into juice (Baijie, S-308, China). Subsequently, 10.0 g of juice was mixed with 1.0 g of NaCl solution and immediately transferred to a 40 mL headspace bottle, which was incubated in a 60°C thermostatic water bath for 10 min to activate volatile compounds. Volatiles compounds were separated by a DB-WAX capillary column (30 m × 0.25 mm × 0.25 μm) and further identified by 7890B Agilent GC coupled to 5977A mass spectrometer detector (Agilent Technologies, USA). The temperature programming was as follows: The initial temperature (35°C) was held for 0 min before increasing to 180°C at a rate of 3°C/min, then it ramped to 230°C at a rate of 15°C/min and maintained for 2 min. High purity helium was utilized as the carrier gas at a flow rate of 1.3 mL/min, while electron impact ionization at electron energy of 70 eV and a mass range of m/z 50–500 amu were used for MS.
Three assessors trained over 2 weeks were employed to conduct GC-O-MS analysis. The panel members determined aroma intensities using a ten-point intensity scale, wherein 1, 5, and 10 corresponded to weak intensity, moderate intensity, and extreme intensity, respectively. Each sample was sniffed by assessor panelists twice, and the parameters such as aroma descriptions, retention time and intensity value were recorded. The peak was considered the active aroma only if at least two experimenters found similar odor at the same retention time.
The total RNA was isolated from the pulp of tomatoes based on the method described by Singh et al. (22). Subsequently, the reversed transcription of RNA to cDNA was performed using the Revert Aid First-Strand cDNA Synthesis Kit (Fermentas, Madison, WI, USA) in accordance with the manufacturer’s instructions. To verify the expression patterns revealed by the RNA-seq technology, quantitative real-time PCR (qRT-PCR) analysis was conducted in our research. Genes tested included LOXs, ADH2, HPL, LeCCDs, and SlBCATs. Gene specific primers for selected genes were designed by online software,1 while the melt curve was used to analyze the amplification curve specificity. Besides, qRT-PCR analysis was performed using Fast SYBR Mixture on a Bio-Rad CFX connected with real-time PCR detection system, and the incubation conditions of test was based on a two-step method: 95°C for first 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Relative expression level was calculated based on the 2–Δ Δ Ct method using actin as an internal reference gene. Three biological replicates were used for all qRT-PCR experiments and average data from three was plotted.
The volatile compounds were identified by comparing their retention indexes (RI) and mass−fragmented patterns with standards, mass spectra in the NIST Database, and/or previously published studies (23). All the experiments were performed by triplicate assays, and the results were expressed as the means ± standard deviation (SD) using SPSS 19.0 (USA) for windows. The statistical significances of data were determined using one-way analysis of variance (ANOVA) followed by the comparison of Duncan’s Multiple Range Test (DMRT), p-values < 0.05 were regarded as significant.
As shown in Table 1, a total of 37 volatile compounds in “FL 47” tomato fruits were identified by GC-O-MS analysis, containing 16 aldehydes, five alcohols, five ketones, two esters, four hydrocarbons, three oxygen-containing heterocyclic compounds, as well as two sulfur and nitrogen-containing heterocyclic compounds. The findings were similar to those of Wang et al. (6), they identified 42 volatile compounds in full ripe “FL 47” tomatoes by headspace-solid-phase micro-extraction-GC-MS (HS-SPME-GC-MS) analysis (6). It was shown in Figure 1 that the content of aldehydes was the highest in volatile compounds of most groups and their flavors were described as green. As the tomato fruits ripened, the aldehyde content significantly increased. In line with Xi et al. (24), cis-3-hexenal was the most predominant compound among 16 aldehydes (24), while its percentage in total volatiles of tomatoes at P or R stage without any treatment was 38.48 or 69.89%, respectively (Table 2).
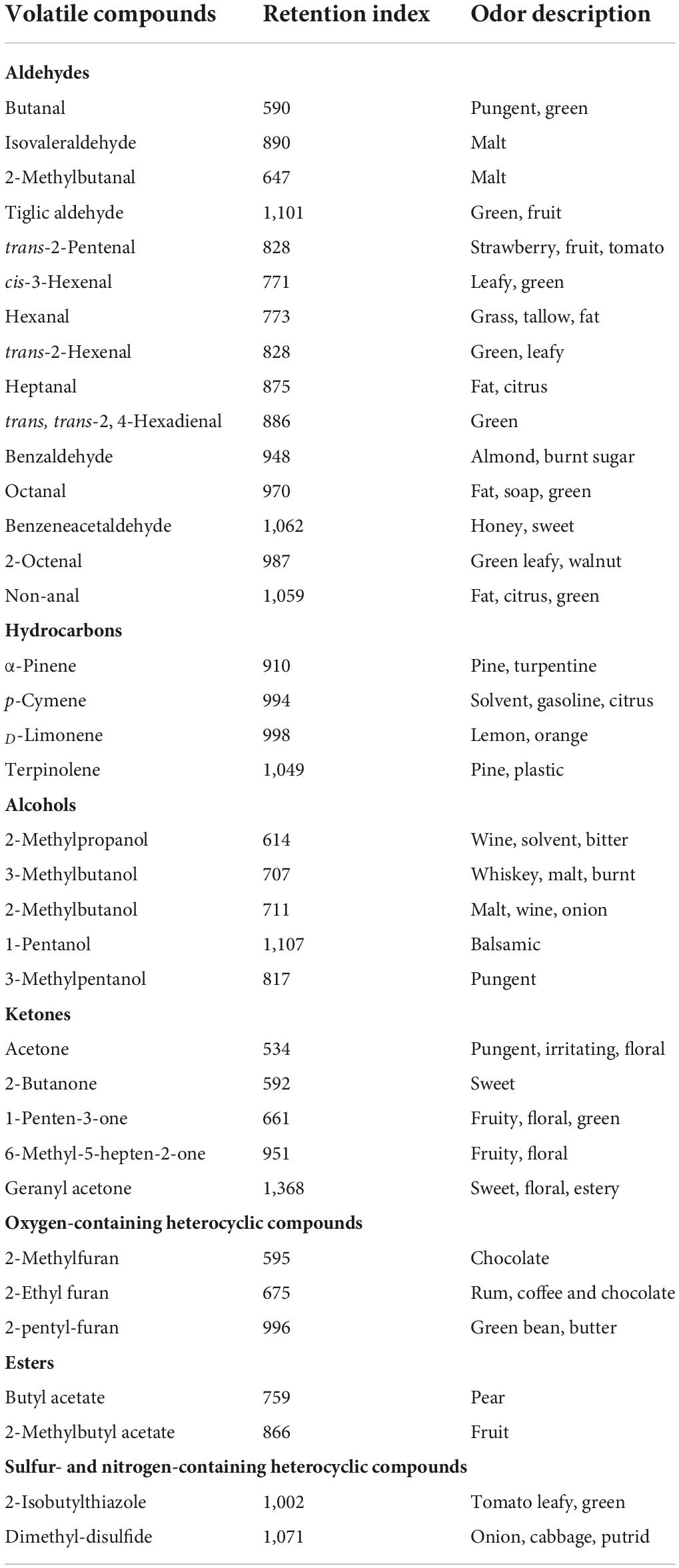
Table 1. The retention index and odor descriptions of the volatile compounds identified by the GC-MS analysis.
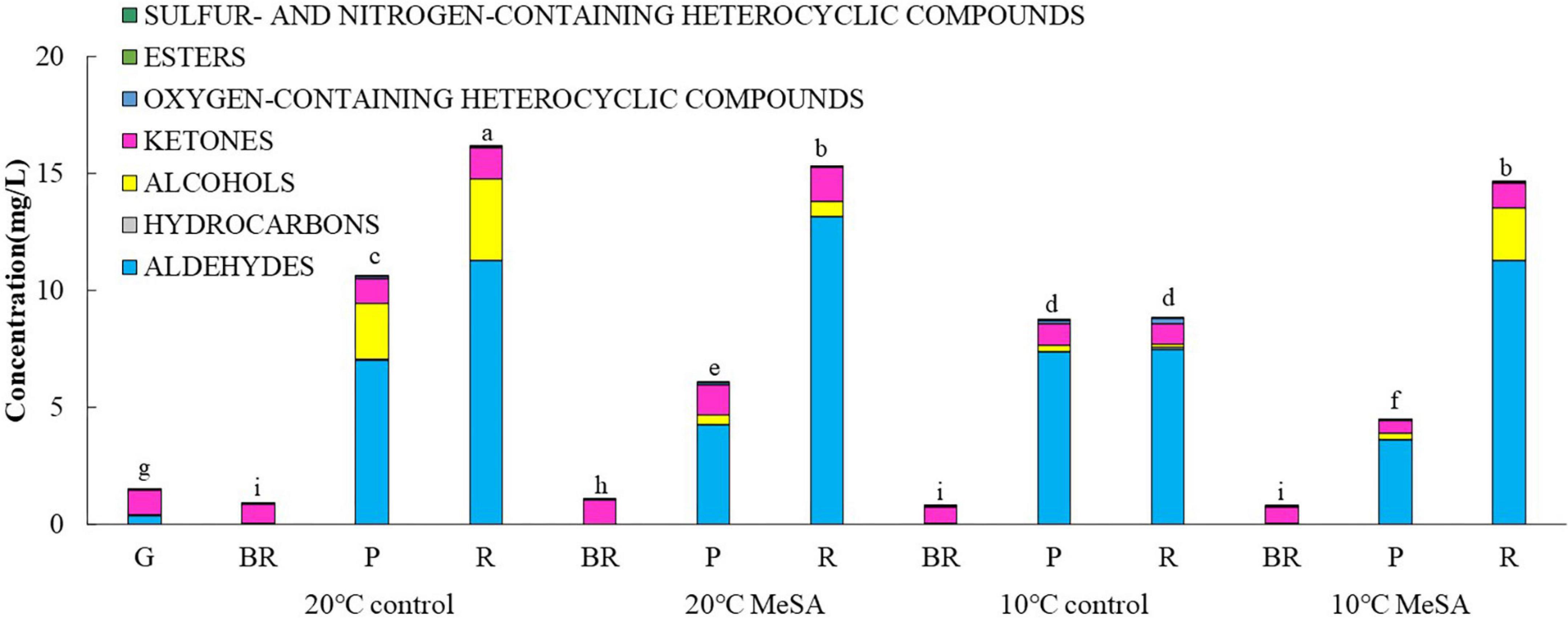
Figure 1. Effects of methyl salicylate (MeSA) on the contents of different types of volatile compounds in low temperature-treated tomato fruits. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by Duncan’s multiple range test (DMRT). Data marked with the same letter were no significant difference at p < 0.05.
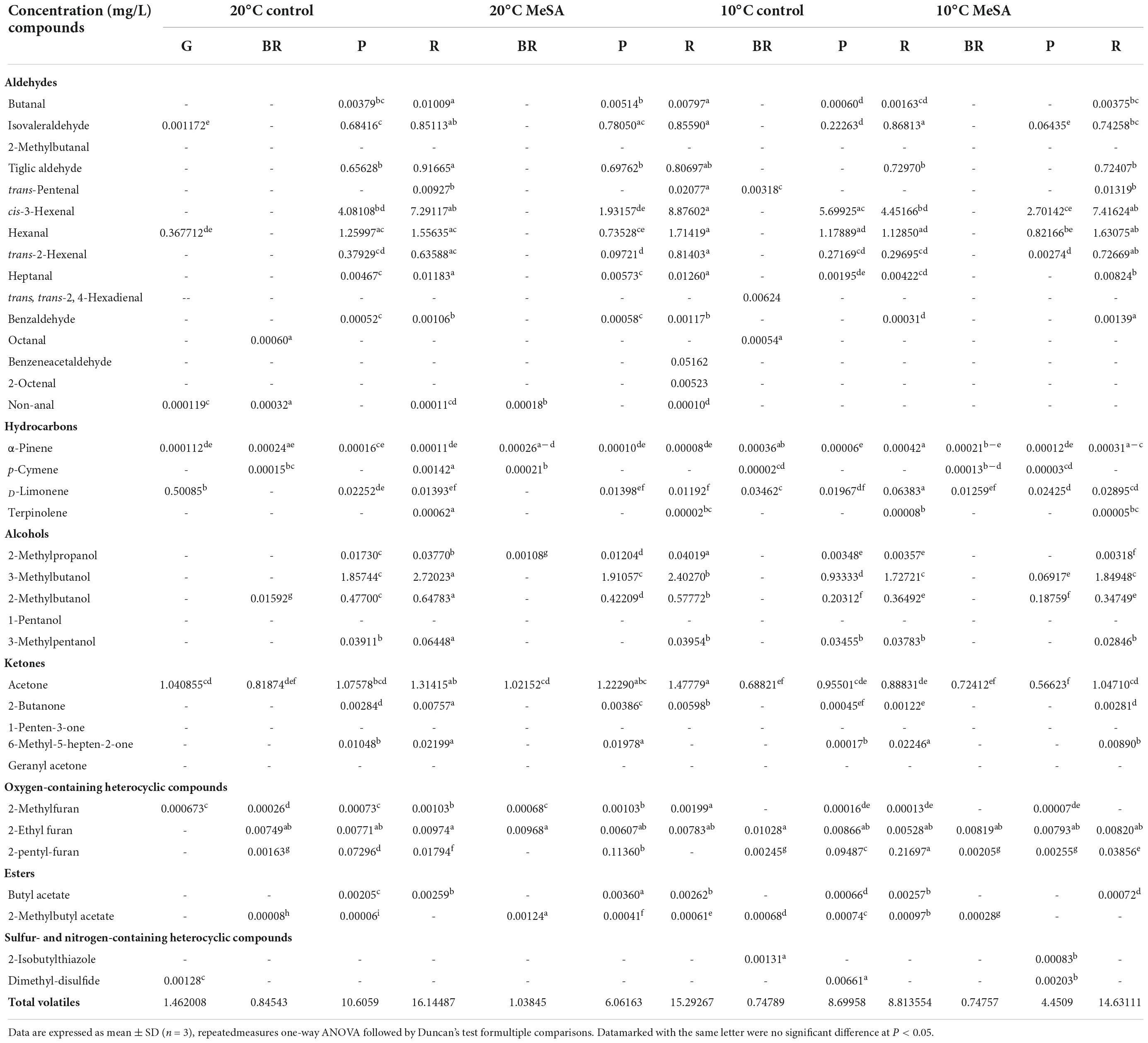
Table 2. Effects of methyl salicylate (MeSA) pre-treament on the volatile profiles of tomato fruits stored at low temperature.
Furthermore, the alcohols or ketones were the second most abundant volatile compounds, the odor of which was described as malt or flora, respectively. However, the variation trend of alcohol and ketone contents with maturity was different from that of aldehyde content. The alcohol level in flavor components of fruits without any treatment continuously increased prior to the P stage, while decreased at R stage. By contrast, the concentration of ketones at BR, or P stage was relatively lower than those at G and R stage. It might be attributed to the difference in the biosynthetic pathways of these aromatic compounds (22). It was worthwhile to note that 3-methylbutanol or acetone was the major alcohol or ketone compound in volatiles of the control, respectively. When the fruit was at R stage, the level of 3-methylbutanol or acetone could reach to 16.85 or 8.14%. On the basis of the data of volatile composition, green and malt might be the main flavors in the whole odor description of tomato fruits (Table 1).
To investigate the effects of postharvest treatments on the volatile biosynthetic pathways of tomatoes, the volatile compounds were divided into four groups in the current research as well, including fatty acid pathway- (cis-3-hexenal, hexanal, trans-2-hexenal, 1-pentanol, and 1-penten-3-one), carotenoid pathway- (6-methyl-5-hepten-2-one and geranyl acetone), phenylalanine pathway- (MeSA) and branched-chain amino acid pathway-related volatiles (2-methylbutanal, 3-methylbutanal, 2-methylbutanol, and 2-isobutylthiazole) (25). It could be found in Figure 2 that no MeSA was detected in the volatiles of tomato fruits, possibly due to the difference in the analytic method utilized in the present study. In addition, fatty acid pathway- and branched-chain amino acid pathway-related volatiles played more important roles in the aromatic compounds of tomato fruits in 20°C control group than carotenoid-related volatiles, while their levels gradually increased with the ripening of fruits. The contents of fatty acid pathway-related volatiles in fruits at P and R stages were significantly higher than those of other volatiles, while branched-chain amino acid pathway-related volatiles were the main components of aromatic compounds in tomatoes at BR stage. Additionally, the concentration of fatty acid pathway-related volatiles in fruits of 20°C control group at R stage was approximately 2.78-fold that of branched-chain amino acid pathway-related volatiles. Hence, it seemed that the fatty acid and branched-chain amino acid pathways could be greatly activated to promote the formation of relevant flavor components when the tomatoes ripened.
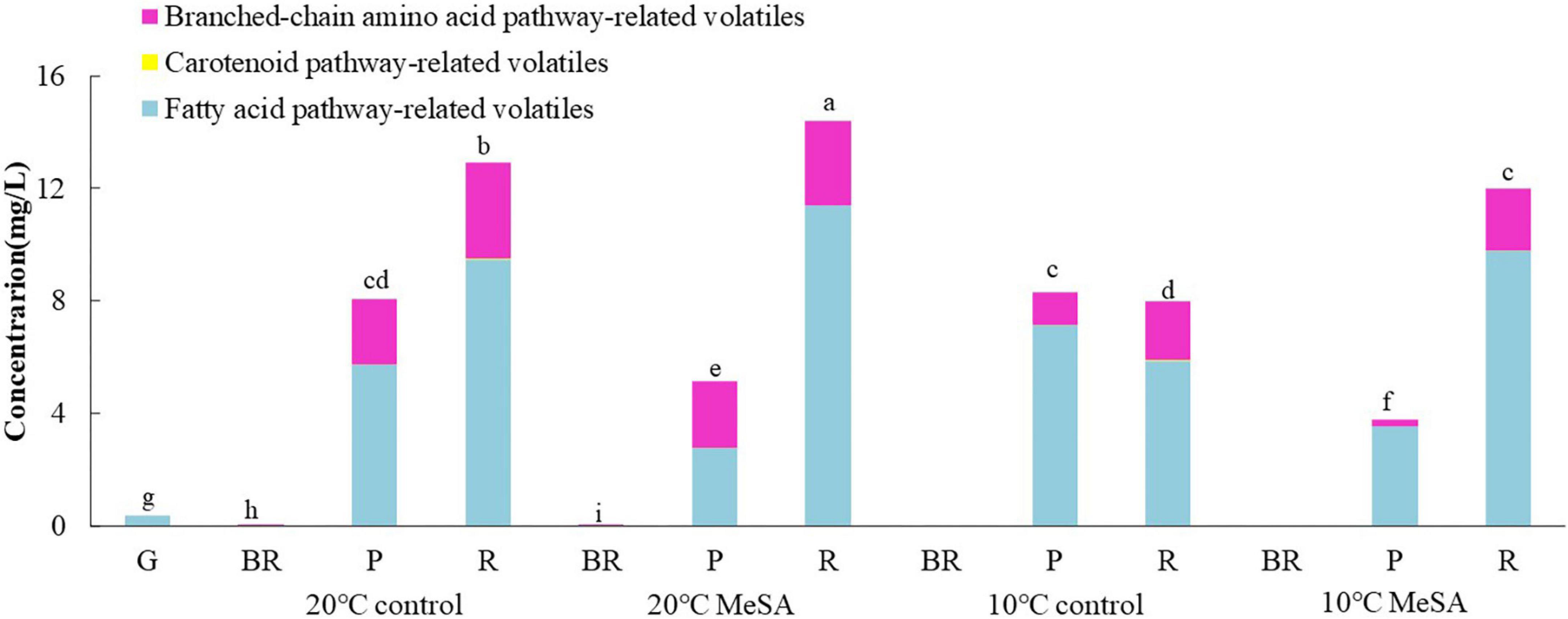
Figure 2. Effects of methyl salicylate (MeSA) pre-treatment on the levels of fatty acid pathway-, carotenoid pathway-, and branched-chain amino acid pathway-related volatiles in tomato fruits stored at low temperature. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by Duncan’s multiple range test (DMRT). Data marked with the same letter were no significant difference at p < 0.05.
A series of key genes were selected to further verified the data obtained in volatile analysis, such as LOXs, ADH2, HPL, LeCCDs, and SIBCATs. LOXs (LOXA, LOXB, LOXC, LOXD, and LOXE), ADH2 and HPL are one of the key enzymes involving the synthesis fatty acid pathway-related volatiles in tomato fruits (26). As shown in Figure 3, there was no significant difference in the relative expression of LOXA, LOXB and LOXE genes among the fruits at different maturity stages stored at 20°C, while the relative expressions of LOXC and LOXD genes in tomatoes at P and R stages were remarkably higher compared with those at G and BR stages. When the fruits were at R stage, the relative expression of LOXC and LOXD genes was 882.4 or 241.4% higher than that of fruits at G stage. According to Chen et al. (27), LOXC was chloroplast-targeted and generated volatile C6 flavor compounds from both linoleic and linolenic acid, whilst LOXD could participate in the biosynthesis of jasmonic acid (27). Although no significant difference was observed in the ADH2 gene expression of tomato fruits at four maturity stages without any treatments, the relative expression of HPL gene gradually reduced with the ripening of tomatoes (Figure 4). The expression level of HPL gene in fruits at R stage was on 41.2% that in those at G stage. Our data was similar to those obtained by Chen et al. (28) and Zhang et al. (29), they believed that the production of C6 aldehydes led to the changes in HPL gene expression (28, 29).
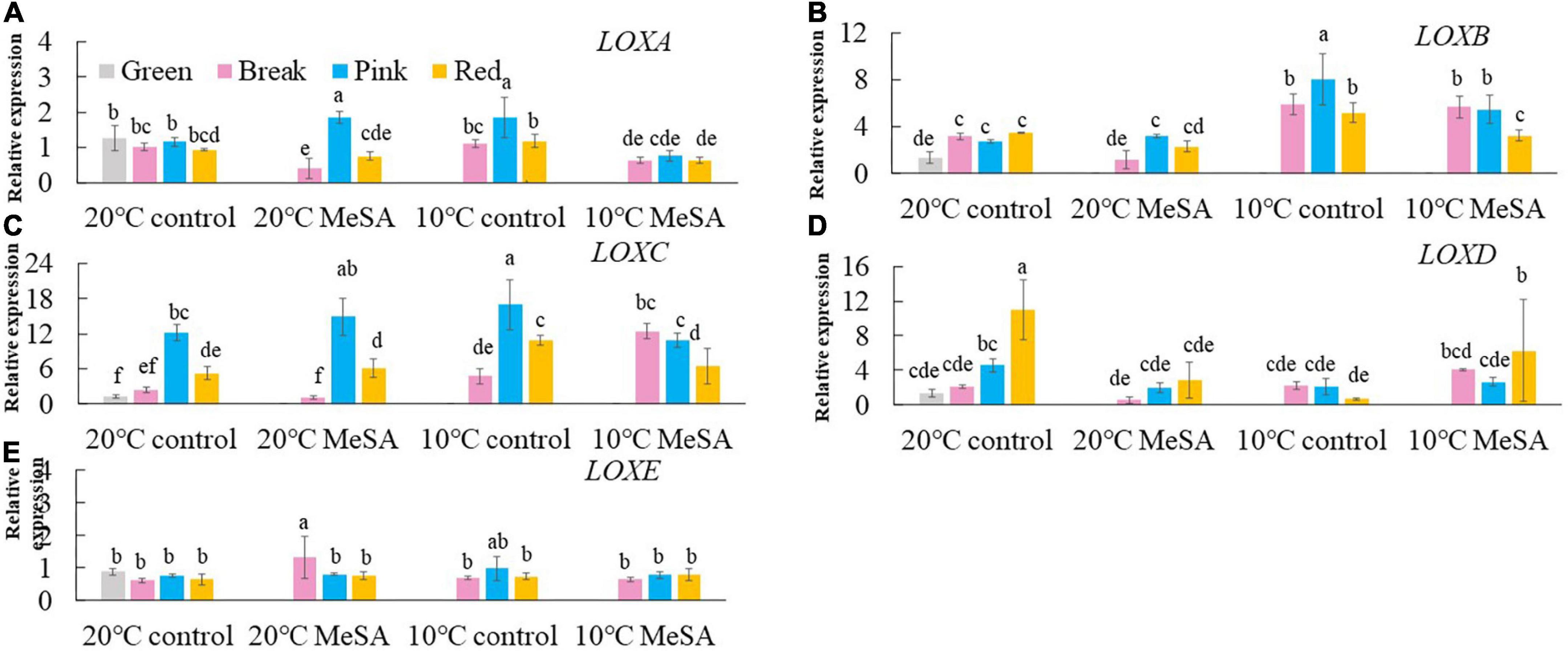
Figure 3. Effects of methyl salicylate (MeSA) pre-treatment on the relative expressions of LOXA (A), LOXB (B), LOXC (C), LOXD (D), and LOXE (E) genes in tomato fruits stored at low temperature. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by DMRT. Data marked with the same letter were no significant difference at p < 0.05.
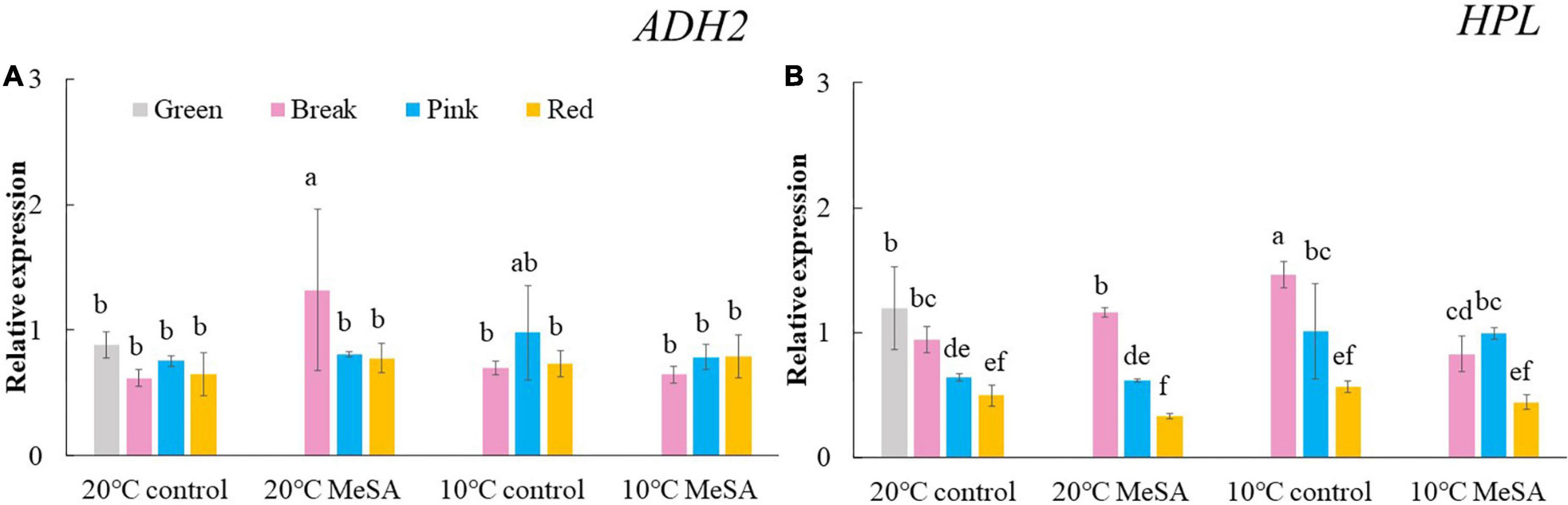
Figure 4. Effects of methyl salicylate (MeSA) pre-treatment on the relative expressions of ADH2 (A) and HPL (B) genes in tomato fruits stored at low temperature. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by DMRT. Data marked with the same letter were no significant difference at p < 0.05.
Carotenoid cleavage can occur at any conjugated double bonds by LeCCDs to form an aldehyde or ketone (30). Two genes (LeCCD1A-N and LeCCD1B) were chosen in the present study, it was observed that the relative expression of LeCCD genes in tomato fruits decreased before the BK stage and then slowly increased (Figure 5). There was no significant difference in the LeCCD gene expression levels among the fruits at different maturity stages stored at 20°C, which was in accordance with the findings of Jing et al. (31). In terms of branched-chain amino acid pathway, slBCATs are able to catalyze the initial step of degradation of leucine, isoleucine and valine to synthesize various flavor components (32). Figure 6 showed that the relative expressions of slBCAT1 and slBCAT2 genes in tomatoes of 20°C control group remarkably decreased with the ripening of fruits, which was probably brought about by the volatile production at different maturity stages (33). The slBCAT1 or slBCAT2 gene expression level in fruits at R stage stored at 20°C was 26.5 or 80.7% lower in comparison to that in those at G stage, respectively. In short, it seemed that the production of volatile compounds in tomato fruits was mainly influenced by their maturity through regulating fatty acid and branched-chain amino acid pathways.
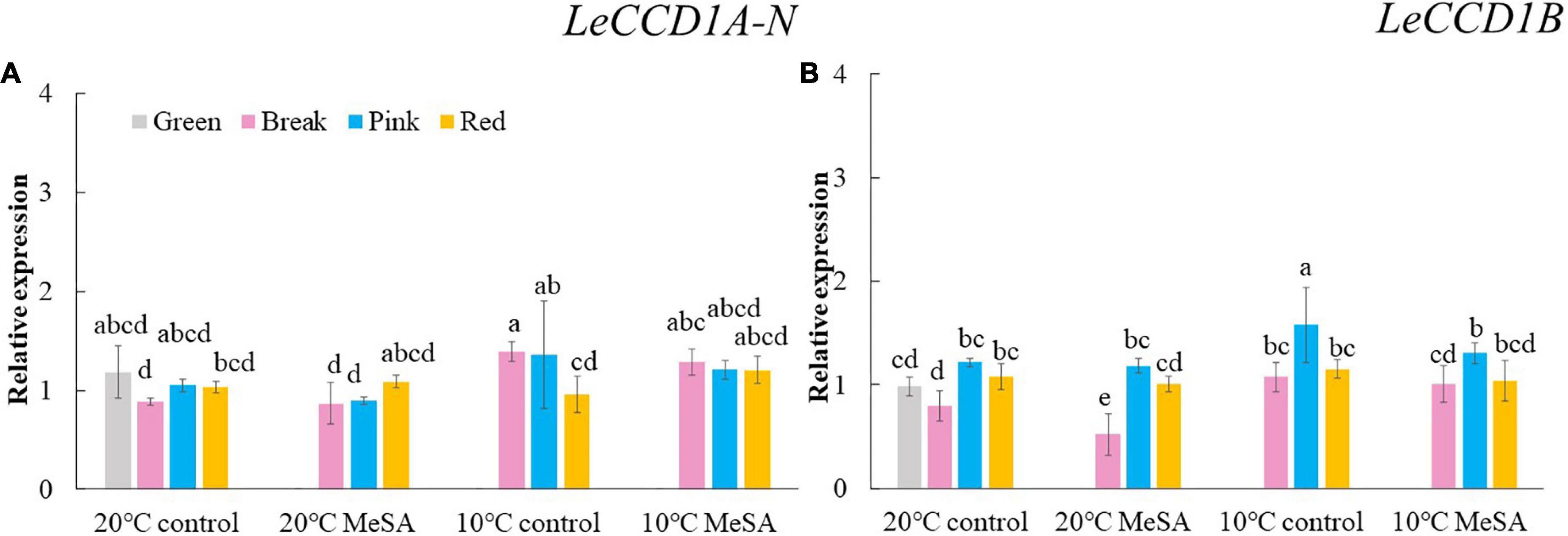
Figure 5. Effects of methyl salicylate (MeSA) pre-treatment on the relative expressions of LeCCD1A-N (A) and LeCCD1B (B) genes in tomato fruits stored at low temperature. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by DMRT. Data marked with the same letter were no significant difference at p < 0.05.
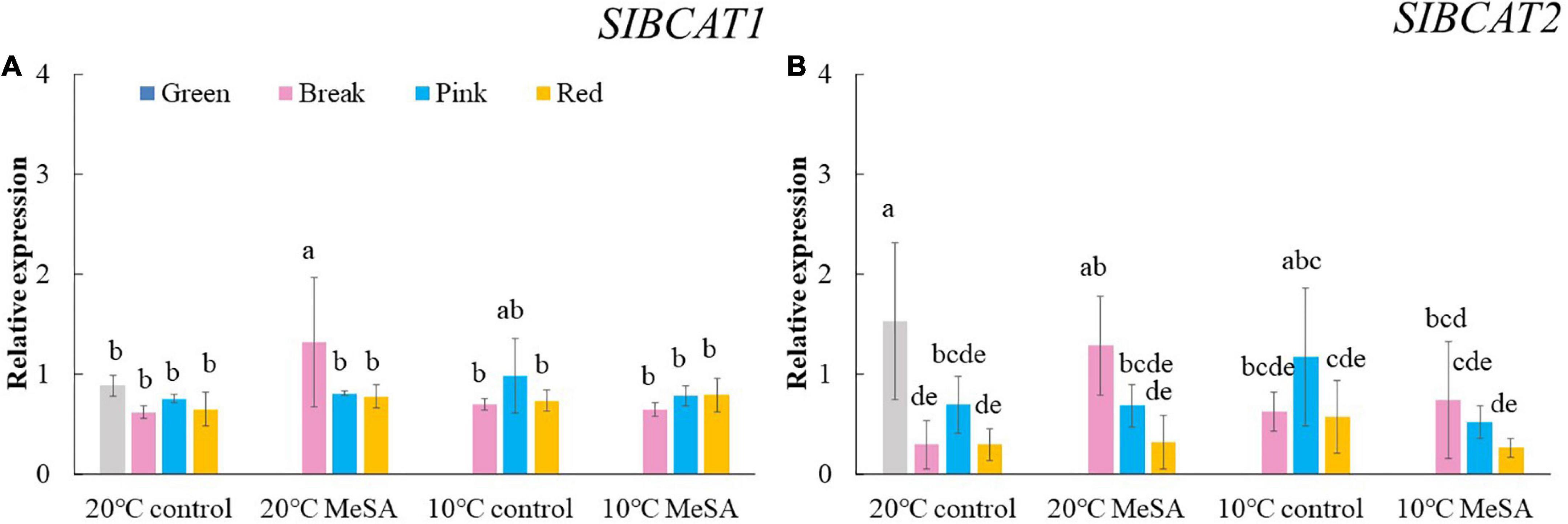
Figure 6. Effects of methyl salicylate (MeSA) pre-treatment on the relative expressions of slBCAT1 (A) and slBCAT2 (B) genes in tomato fruits stored at low temperature. Data are expressed as mean ± SD (n = 3), repeated measures one-way ANOVA followed by DMRT. Data marked with the same letter were no significant difference at p < 0.05.
It was shown in Table 2 that the total volatile contents in tomato fruits of 10°C control group were much lower compared to those of 20°C control group. When the fruits were at R stage, the total volatile content in fruits of 10°C control group was only 54.6% that a of 20°C control group. In line with previous studies (24), the formation of all types of aromatic compounds in tomatoes were considerably inhibited by low temperature treatment as well (Figure 1). The aldehyde, alcohol or ketone level in fruits at R stage stored at 20°C was 1.51-, 25.99-, or 1.47-fold that at 10°C, respectively.
Although there was no significant difference in the level of carotenoid pathway-related volatiles in fruits between 10°C control and 20°C control groups, the formation fatty acid pathway- and branched-chain amino acid pathway-related volatiles in tomatoes were suppressed by low temperature treatment (Figure 2). In fatty acid, carotenoid or branched-chain amino acid pathway, no significant difference was observed in the relative expressions of LOXA, LOXE, ADH2, HPL, LeCCD1A-N, LeCCD1B, SlBCAT1, and SlBCAT2 genes in fruits at R stage between 20°C control group and 10°C control group (Figures 3–6). Whereas, low temperature treatment remarkably enhanced the expression levels of LOXB and LOXC genes as well as inhibited the LOXD gene expression level. At R stage, the relative expression of LOXB, LOXC, or LOXD gene in fruits of 10°C control group was 149.6, 209.5, or 6.2% that of 20°C control group, respectively. It seemed that low temperature treatment could greatly reduce the formation of fatty acid pathway-related volatiles through inhibiting the LOXD activity in tomatoes.
Methyl salicylate pre-treatment is a common postharvest technique to reduce the chilling injury of tomato fruits (34). Table 2 showed that the total volatile content of tomatoes in 20°C MeSA group was a little lower as compared to that in 20°C control group, which was consistent with the results of Wang et al. (6). At P stage, the aldehyde or alcohol level in fruits of 20°C MeSA group was 60.6 or 17.7% that of 20°C control group, respectively (Figure 1). In addition, the decrease of volatile compounds in low-temperature treated tomato fruits was effectively alleviated by MeSA pre-treatment. The total volatile content of fruits at R stage in 10°C MeSA group was 66.0% higher in comparison with that in 10°C control group, while the level of aldehydes, alcohols, or ketones in tomatoes at R stage in 10°C MeSA group was 1.51-, 16.69-, or 1.16-fold that in 10°C control group, respectively. Notably, MeSA pre-treatment significantly increased the cis-3-hexenal, hexanal and trans-2-hexenal levels in fruits subjected to low temperature. The enhancement of these aroma compounds was possibly associated with the direct action to LOX and β-oxidation pathways by regulating activities and expressions of LOX, ADH, HPL, and a series of other enzymes (35). Thereby, the effects of MeSA on the volatiles derived from different pathways and key gene expressions in low temperature-treated tomato fruits were further estimated in our research.
As shown in Figure 2, on the one hand, no significant difference was found in the levels of carotenoid pathway- and branched-chain amino acid pathway-related aroma compounds in fruits at R stage between 10°C MeSA group and 10°C control group. On the other hand, the biosynthesis of fatty acid pathway-related aroma compounds in tomatoes at P stage under 20°C was suppressed by MeSA pre-treatment. Whereas, the content of these components in tomatoes treated by MeSA greatly increased when the fruits turned red. The phenomenon was observed by Liu et al. (36) as well, they put forward that a high concentration of exogenous MeSA would increase endogenous salicylic acid concentration and induce cell damage in plants, which could subsequently result in the increased emission of fatty acid-derived compounds. Naturally, it was easily understood that the administration of MeSA in tomatoes stored at 10°C considerably inhibited the decrease of fatty acid pathway-related volatiles.
LOX pathway contributes to the formation of fruity note flavors in various fruits, including pears, kiwifruits and peaches (35, 37, 38). It was shown in Figure 3 that there was no significant difference in the expression levels of LOXA, LOXB, LOXC, and LOXE genes in fruits at R stage between 20°C MeSA group and 20°C control group. However, MeSA pre-treatment remarkably down-regulated the expression of LOXD gene in full red tomatoes stored under 20°C, which might be brought about by the protective effects of MeSA against the reduced membrane integrity in fruits (39). Regarding low temperature-treated tomato fruits at different maturity stages, MeSA pre-treatment had no significant effect on the expression of LOXE gene. In response to chilling injury, the relative expressions of LOXA and LOXB genes in low temperature-treated fruits at R stage were down-regulated by MeSA pre-treatment. Nevertheless, treating fruits with MeSA and low temperature greatly up-regulated the LOXC gene expression at BK stage as well as LOXD gene expression at R stage (Figures 3C,D). Specifically, the LOX gene expression level in fruits (BK stage) or LOXD gene expression level in fruits (R stage) of 10°C MeSA group was 160.9% or 80.8% higher compared to that of 10°C control group, respectively. A higher expression of LOX genes could promote the formation of straight-chain alcohols, esters and lactones (34). Thus, the results obtained in volatile analysis were confirmed by those of qRT-PCR experiment.
ADH2 plays crucial roles in the reaction of aldehydes into alcohols (40), while HPL is essential for the green note flavors on account of its effects on the biosynthesis of aldehydes and oxoacids (41). As shown in Figure 4, MeSA pre-treatment had no remarkable impact on the ADH2 and HPL expression levels in low temperature-treated tomatoes at P and R stages. We hypothesized that the increase of alcohols and esters in volatile compounds of tomatoes at R stage in 10°C MeSA group was probably caused by the up-regulation effects of MeSA on the relative expression of alcohol o-acyltransferase rather than ADH2 (42). LeCCDs and slBCATs are involved in the metabolism carotenoid pathway- and branched-chain amino acid pathway-related volatiles. Figures 5, 6 showed that there was no significant difference in the relative expressions of LeCCD1A-N, LeCCD1B, slBCAT1 and slBCAT2 genes between 20°C MeSA group and 20°C control group, which was matched with the data of volatile analysis. Overall, MeSA pre-treatment might avoid the loss of volatiles compounds in tomato fruits stored at low temperature through activating the fatty acid pathway.
In the present investigation, the effects of MeSA pre-treatment on the volatile profile and volatile biosynthesis pathways of low temperature-treated tomatoes were studied. Our results indicated that low temperature treatment could inhibit the biosynthesis of aromatic components in tomato fruits at P and R stages. Whereas, MeSA pre-treatment was able to effectively alleviate the loss of volatile compounds in tomato fruits stored at 10°C, especially aldehydes, alcohols and ketones. Notably, the level of fatty acid pathway-related volatiles (including cis-3-hexenal, hexanal and trans-2-hexenal) in full red fruits of 10°C MeSA group was significantly higher as compared to that of 10°C control group, although MeSA pre-treatment had no remarkable effect on the formation of carotenoid pathway- and branched-chain amino acid pathway-related flavor components in tomatoes treated by low temperature. Based on the data of qRT-PCR analysis, MeSA plus low temperature treatment remarkably up-regulated the LOXC or LOXD gene expression in fruits at BK or R stage compared to low temperature treatment, while there was no significant difference in the relative expressions of ADH2, HPL, LeCCD, and slBCAT genes between 20°C MeSA group and 20°C control group. In a nutshell, the loss of aromatic compounds in low temperature-treated tomato fruits could be suppressed by MeSA pre-treatment through activating the fatty acid pathway.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
XZ: resources (lead), writing—original draft (lead), and funding acquisition (lead). LW and JHZ: supervision (supporting). YF, YL, JLZ, and RC: writing—review and editing (supporting). JL: conceptualization (lead), funding acquisition (lead), supervision (lead), and writing—review and editing (lead). All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No: 31772038) and the Research Foundation for Youth Scholars of Beijing Technology and Business University (Project No: QNJJ2022-10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zou J, Chen J, Tang N, Gao Y, Hong M, Wei W. Transcriptome analysis of aroma volatile metabolism change in tomato (Solanum lycopersicum) fruit under different storage temperatures and 1-MCP treatment. Postharvest Biol Technol. (2018) 135:57–67. doi: 10.1016/j.postharvbio.2017.08.017
2. Fritz EL, Rosenberg BR, Lay K, Mihailovi A, Papavasiliou FN. A comprehensive analysis of aid’s effects on the transcriptome and methylome of activated b cells. Nat Immunol. (2013) 14:749–55. doi: 10.1038/ni.2616
3. Garbowicz K, Liu Z, Alseekh S, Tieman D, Taylor M, Kuhalskaya A, et al. Quantitative trait loci analysis identifies a prominent gene involved in the production of fatty acid-derived flavor volatiles in tomato. Mol Plant. (2018) 11:1147–65. doi: 10.1016/j.molp.2018.06.003
4. Wang L, Baldwin EA, Bai J. Recent advance in aromatic volatile research in tomato fruit: the metabolisms and regulations. Food Bioprocess Tech. (2016) 9:203–16. doi: 10.1007/s11947-015-1638-1
5. Mathieu S, Cin VD, Fei Z, Li H, Bliss P, Taylor MG. Flavour compounds in tomato fruits: identification of loci and potential pathways affecting volatile composition. J Exp Bot. (2009) 60:325–37. doi: 10.1093/jxb/ern294
6. Wang L, Baldwin EA, Plotto A, Luo W, Raithore S, Yu Z, et al. Effect of methyl salicylate and methyl jasmonate pre-treatment on the volatile profile in tomato fruit subjected to chilling temperature. Postharvest Biol Technol. (2015) 108:28–38. doi: 10.1016/j.postharvbio.2015.05.005
7. Zeng X, Jiang W, Du Z, Kokini JL. Encapsulation of tannins and tannin-rich plant extracts by complex coacervation to improve their physicochemical properties and biological activities: a review. Crit Rev Food Sci. (2022). [Epub ahead of print]. doi: 10.1080/10408398.2022.2075313
8. Zeng X, Li H, Jiang W, Li Q, Xi Y, Wang X, et al. Phytochemical compositions, health-promoting properties and food applications of crabapples: a review. Food Chem. (2022) 386:132789. doi: 10.1016/j.foodchem.2022.132789
9. da Silva Souza MA, Peres LE, Freschi JR, Purgatto E, Lajolo FM, Hassimotto NM. Changes in flavonoid and carotenoid profiles alter volatile organic compounds in purple and orange cherry tomatoes obtained by allele introgression. J Sci Food Agric. (2020) 100:1662–70. doi: 10.1002/jsfa.10180
10. Gonda I, Davidovich-Rikanati R, Bar E, Lev S, Jhirad P, Meshulam Y, et al. Differential metabolism of L–phenylalanine in the formation of aromatic volatiles in melon (Cucumis melo L.) fruit. Phytochemistry. (2018) 148:122–31. doi: 10.1016/j.phytochem.2017.12.018
11. Espino-Díaz M, Sepúlveda DR, González-Aguilar G, Olivas GI. Biochemistry of apple aroma: a review. Food Technol Biotech. (2016) 54:375–94. doi: 10.17113/ftb.54.04.16.4248
12. Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci USA. (2006) 103:8287–92. doi: 10.1073/pnas.0602469103
13. Matysik S, Herbarth O, Mueller A. Determination of microbial volatile organic compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere. (2009) 76:114–9. doi: 10.1016/j.chemosphere.2009.02.010
14. Li J, Di T, Bai J. Distribution of volatile compounds in different fruit structures in four tomato cultivars. Molecules. (2019) 24:2594. doi: 10.3390/molecules24142594
15. Gomes BL, Fabi JP, Purgatto E. Cold storage affects the volatile profile and expression of a putative linalool synthase of papaya fruit. Food Res Int. (2016) 89:654–60. doi: 10.1016/j.foodres.2016.09.025
16. Ponce-Valadez M, Escalona-Buendía HB, Villa-Hernández JM, de León-Sánchez FD, Rivera-Cabrera F, Alia-Tejacal I, et al. Effect of refrigerated storage (12.5 °C) on tomato (Solanum lycopersicum) fruit flavor: a biochemical and sensory analysis. Postharvest Biol Technol. (2016) 111:6–14. doi: 10.1016/j.postharvbio.2015.07.010
17. Li H, Suo J, Han Y, Liang C, Jin M, Zhang Z, et al. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’kiwifruit during cold storage. Postharvest Biol Technol. (2017) 124:107–18. doi: 10.1016/j.postharvbio.2016.10.003
18. Min D, Li F, Zhang X, Shu P, Cui X, Dong L, et al. Effect of methyl salicylate in combination with 1−methylcyclopropene on postharvest quality and decay caused by Botrytis cinerea in tomato fruit. J Sci Food Agric. (2018) 98:3815–22. doi: 10.1002/jsfa.8895
19. Giménez MJ, Valverde JM, Valero D, Zapata PJ, Castillo S, Serrano M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘early lory’ sweet cherry. Postharvest Biol Technol. (2016) 117:102–9. doi: 10.1016/j.postharvbio.2016.02.006
20. Ding CK, Wang CY, Gross KC, Smith DL. Reduction of chilling injury and transcript accumulation of heat shock proteins in tomato fruit by methyl jasmonate and methyl salicylate. Plant Sci. (2001) 161:1153–9. doi: 10.1016/S0168-9452(01)00521-0
21. Li J, Fu Y, Bao X, Li H, Zuo J, Zhang M, et al. Comparison and analysis of tomato flavor compounds using different extraction methods. J Food Meas Charact. (2020) 14:465–75. doi: 10.1007/s11694-019-00102-x
22. Singh RK, Srivastava S, Chidley HG, Nath P, Sane VA. Overexpression of mango alcohol dehydrogenase (MiADH1) mimics hypoxia in transgenic tomato and alters fruit flavor components. Agri Gene. (2018) 7:23–33. doi: 10.1016/j.aggene.2017.10.003
23. Li J, Fu Y, Bao X, Li H, Zuo J. Optimization of solid phase microextraction combined with gas chromatography−mass spectrometry (GC−MS) to analyze aromatic compounds in fresh tomatoes. J Food Biochem. (2019) 43:e12858. doi: 10.1111/jfbc.12858
24. Xi Y, Li Q, Yan J, Baldwin E, Plotto A, Rosskopf E, et al. Effects of harvest maturity, refrigeration and blanching treatments on the volatile profiles of ripe “Tasti-Lee” Tomatoes. Foods. (2021) 10:1727. doi: 10.3390/foods10081727
25. Tieman D, Zhu G, Resende MF Jr., Lin T, Nguyen C, Bies D. A chemical genetic roadmap to improved tomato flavor. Science. (2017) 355:391–4. doi: 10.1126/science.aal1556
26. Upadhyay RK, Mattoo AK. Genome-wide identification of tomato (Solanum lycopersicum L.) lipoxygenases coupled with expression profiles during plant development and in response to methyl-jasmonate and wounding. J Plant Physiol. (2018) 231:318–28. doi: 10.1016/j.jplph.2018.10.001
27. Chen Z, Chen X, Yan H, Li W, Li Y, Cai R, et al. The lipoxygenase gene family in poplar: identification, classification, and expression in response to MeJA treatment. PLoS One. (2015) 10:e0125526. doi: 10.1371/journal.pone.0125526
28. Chen G, Hackett R, Walker D, Taylor A, Lin Z, Grierson D. Identification of a specific isoform of tomato lipoxygenase (TomloxC) involved in the generation of fatty acid-derived flavor compounds. Plant Physiol. (2004) 136:2641–51. doi: 10.1104/pp.104.041608
29. Zhang B, Shen JY, Wei WW, Xi WP, Xu CJ, Ferguson I, et al. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J Agric Food Chem. (2010) 58:6157–65. doi: 10.1021/jf100172e
30. Vogel JT, Tan BC, McCarty DR, Klee HJ. The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem. (2008) 283:11364–73. doi: 10.1074/jbc.M710106200
31. Jing G, Li T, Qu H, Yun Z, Jia Y, Zheng X, et al. Carotenoids and volatile profiles of yellow-and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biol Technol. (2015) 109:114–9. doi: 10.1016/j.postharvbio.2015.06.006
32. Kochevenko A, Araújo WL, Maloney GS, Tieman DM, Do PT, Taylor MG, et al. Catabolism of branched chain amino acids supports respiration but not volatile synthesis in tomato fruits. Mol Plant. (2012) 5:366–75. doi: 10.1093/mp/ssr108
33. Yang X, Song J, Fillmore S, Pang X, Zhang Z. Effect of high temperature on color, chlorophyll fluorescence and volatile biosynthesis in green-ripe banana fruit. Postharvest Biol Technol. (2011) 62:246–57. doi: 10.1016/j.postharvbio.2011.06.011
34. Zhang X, Shen L, Li F, Meng D, Sheng J. Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J Agr Food Chem. (2011) 59:9351–7. doi: 10.1021/jf201812r
35. Zhou D, Sun Y, Li M, Zhu T, Tu K. Postharvest hot air and UV-C treatments enhance aroma-related volatiles by simulating the lipoxygenase pathway in peaches during cold storage. Food Chem. (2019) 292:294–303. doi: 10.1016/j.foodchem.2019.04.049
36. Liu B, Kaurilind E, Jiang Y, Niinemets Ü. Methyl salicylate differently affects benzenoid and terpenoid volatile emissions in Betula pendula. Tree Physiol. (2018) 38:1513–25. doi: 10.1093/treephys/tpy050
37. Li G, Jia H, Li J, Li H, Teng Y. Effects of 1-MCP on volatile production and transcription of ester biosynthesis related genes under cold storage in ‘Ruanerli’pear fruit (Pyrus ussuriensis Maxim.). Postharvest Biol Technol. (2016) 111:168–74. doi: 10.1016/j.postharvbio.2015.08.011
38. Yin XR, Zhang Y, Zhang B, Yang SL, Shi YN, Ferguson IB, et al. Effects of acetylsalicylic acid on kiwifruit ethylene biosynthesis and signaling components. Postharvest Biol Technol. (2013) 83:27–33. doi: 10.1016/j.postharvbio.2013.03.012
39. Glowacz M, Bill M, Tinyane PP, Sivakumar D. Maintaining postharvest quality of cold stored ‘Hass’ avocados by altering the fatty acids content and composition with the use of natural volatile compounds–methyl jasmonate and methyl salicylate. J Sci Food Agric. (2017) 97:5186–93. doi: 10.1002/jsfa.8400
40. Vancanneyt G, Sanz C, Farmaki T, Paneque M, Ortego F, Castañera P. Hydroperoxide lyase depletion in transgenic potato plants leads to an increase in aphid performance. Proc Natl Acad Sci USA. (2001) 98:8139–44. doi: 10.1073/pnas.141079498
41. Cano-Salazar J, López ML, Crisosto CH, Echeverría G. Volatile compound emissions and sensory attributes of ‘Big Top’nectarine and ‘Early Rich’peach fruit in response to a pre-storage treatment before cold storage and subsequent shelf-life. Postharvest Biol Technol. (2013) 76:152–62. doi: 10.1016/j.postharvbio.2012.10.001
Keywords: methyl salicylate, low temperature, tomato, volatile biosynthetic pathways, flavor compounds
Citation: Zeng X, Wang L, Fu Y, Zuo J, Li Y, Zhao J, Cao R and Li J (2022) Effects of methyl salicylate pre-treatment on the volatile profiles and key gene expressions in tomatoes stored at low temperature. Front. Nutr. 9:1018534. doi: 10.3389/fnut.2022.1018534
Received: 13 August 2022; Accepted: 08 September 2022;
Published: 05 October 2022.
Edited by:
Yanyan Zhang, University of Hohenheim, GermanyCopyright © 2022 Zeng, Wang, Fu, Zuo, Li, Zhao, Cao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Li, bGlqaWFuQHRoLmJ0YnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.