
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 14 November 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1013960
 Wan-jun Yin1,2,3,4†
Wan-jun Yin1,2,3,4† Li-jun Yu1,2,3,4†
Li-jun Yu1,2,3,4† Peng Wang1,2,3,4†
Peng Wang1,2,3,4† Rui-xue Tao5
Rui-xue Tao5 Xiao-min Jiang6
Xiao-min Jiang6 Ying Zhang7
Ying Zhang7 Dao-min Zhu8,9,10*
Dao-min Zhu8,9,10* Peng Zhu1,2,3,4*
Peng Zhu1,2,3,4*Background: The relationship between vitamin D status and gestational cardiovascular health (CVH) is inconsistent in previous studies. Emerging evidence shows that sleep behaviors are related to vitamin D metabolism. However, no studies evaluate the interaction of vitamin D and sleep behaviors on gestational CVH.
Objective: We aimed to estimate the relationship between 25-hydroxyvitamin D [25(OH)D] concentrations and gestational CVH, and whether the relationship was modified by sleep behaviors.
Methods: The data of this study was from a multicenter birth cohort study. A total of 9,209 pregnant women at 16–23 weeks of gestation were included. 25(OH)D concentrations were measured from collected blood. Sleep patterns consisted of major sleep behaviors including duration, chronotype, insomnia, snoring, and excessive daytime sleepiness. Data on poor CVH was based on four “clinical” CVH metrics, including body mass index, blood pressure, total cholesterol, and glucose levels.
Results: The proportion of women with poor CVH was 25.0%. The relative risk (RR) (95%CI) of poor CVH was 0.67 (0.58–0.76) in women with 25(OH)D ≥ 50 nmol/L after multivariate adjustments. Lower 25(OH)D concentrations were significantly associated with poor CVH. Such association was also evident in subgroups analysis. We found a significant interaction of 25(OH)D (P for interaction = 0.01) with sleep patterns on the risk of poor CVH. A negative dose-response relation was observed between 25(OH)D concentrations and poor CVH risk in healthy or intermediate sleep, not poor sleep. 25(OH)D concentrations were lower and the risk of poor CVH was higher in pregnant women with poor sleep patterns (P < 0.05).
Conclusion: Our study suggests that sleep patterns modify the association of 25(OH)D concentrations with the CVH among pregnant women.
Pregnancy is increasingly recognized as a critical period to address female lifetime cardiovascular health (CVH) (1). Exposure to cardiovascular risk factors (obesity, hyperglycemia, hypertension, hyperlipidemia) during pregnancy may lead to a higher risk trajectory for cardiovascular disease (CVD) in females (2–4). Thus, primary preventive CVD interventions applicable to broad pregnant women are needed, and increasing 25-hydroxyvitamin D [25(OH)D] concentrations may be an attractive strategy.
Within the last 1 or 2 decades, there is growing recognition that vitamin D plays an important role in CVD prevention and treatment. Several studies have explored the association of vitamin D with CVD (5–9). However, the impacts of vitamin D supplementation on CVH were mixed across interventions (5, 6, 10). In addition, a meta-analysis (11) of prospective studies amassed that an increased risk of CVD is associated with low serum 25(OH)D concentrations, yet significant heterogeneity was detected in these studies. Inconsistent results could partly be attributed to lifestyle variations, such as sleep behaviors, related to CVD and vitamin D status across the evidence.
Significant evidence has indicated the association between various sleep behaviors and vitamin D metabolism (12, 13). Several observational meta-analyses (14, 15) showed obstructive sleep apnea (OSA) and sleep disorders were associated with lower 25(OH)D concentrations. There is evidence in showing there are vitamin D receptors in the brain involved in the sleep-wake cycle (16), providing a mechanistic explanation for how vitamin D deficiency can contribute to sleep disturbances. Notably, emerging evidence suggests that poor sleep behaviors were also associated with an increased risk of CVD (17–19). Therefore, we hypothesized that sleep patterns modify the association of 25(OH)D concentrations with the CVH among pregnant women.
We conducted a prospective birth cohort study to investigate the association between serum 25(OH)D and CVH in pregnant women and particularly assessed the potential modifying effect of a combination of major sleep behaviors (duration, chronotype, insomnia, snoring, and excessive daytime sleepiness), which was characterized by a healthy sleep score. We also aimed to estimate the modifying effect of the healthy sleep score on vitamin D supplementation behavior and CVH of these women.
This study was conducted based on the Maternal and Infant Health cohort study in Hefei (MIH-Hefei) (20) comprising 9,886 pregnant women in Hefei (32°N latitude), China. Participants aged 18–45 years were recruited from March 2015 to October 2021 across Anhui Women and Child Health Care Hospital, the First People’s Hospital of Hefei City, and the First Affiliated Hospital of Anhui Medical University. The inclusion criteria of this study included 16–23 weeks of gestation, no communication problems, no assisted conception, delivered at the recruited hospital, and singleton pregnancy.
At recruitment, A face-to-face interview or medical records were used to collect data on sociodemographic characteristics, perinatal health status, lifestyle, dietary habits, vitamin D supplementation status, and sleep behaviors. After that, trained staff collected venous blood.
The exclusion criteria were the following: serious pregnancy complications (e.g., heart failure, severe anemia); abnormal liver, renal, or thyroid function; missing blood samples, and unavailable gestational CVH data. Given that blood pressure (BP) is a component of CVH, women with eclampsia or preeclampsia were not excluded. Finally, full data were obtained for 9 209 pregnant women (Supplementary Figure 1). The study was approved by the Ethics Committee of Anhui Medical University (NO. 2015002). The study was conducted according to all relevant guidelines and regulations, and each participant provided written informed consent.
Venous blood was collected at 16–23 weeks of gestation. The centrifuged serum sample was promptly refrigerated at 4°C, then placed in an −80°C freezer within 8 h. Single measurements were performed for serum 25(OH)D by well-trained staff at the Ministry of Education Key Laboratory of Population Health Across Life Cycle using a commercial chemiluminescence immunoassay (DiaSorin Stillwater, MN, USA). The intra-assay and inter-assay coefficients of variation were 8.8 and 11.1%, respectively. In addition, 25(OH)D concentrations were divided into five groups by the quintile (Q1 < P20, P20 ≤ Q2 < P40, P40 ≤ Q3 < P60, P60 ≤ Q4 < P80, Q5 ≥ P80).
At recruitment (16–23 weeks of gestation), sleep habits of 9,209 pregnant women over the past month were collected with a single shot by a standard questionnaire. The sleep patterns were derived from self-reported records and included five sleep behaviors (duration, chronotype, insomnia, snoring, and excessive daytime sleepiness)(17). The following are the definitions of healthy sleep behaviors during pregnancy: 7–8 h of sleep per day, no excessive daytime sleepiness (the score of Epworth Sleeping Scale ≤ 10 points) (21), never or rarely insomnia, no snoring, and early chronotype (morning or morning then evening). For each sleep factor, healthy behaviors were coded as 1, and the others were coded as 0. The healthy sleep score was composed of those five sleep factors varying from 0 to 5. The overall sleep patterns were defined as “poor sleep” (scores < 3), “intermediate sleep” (scores of 3 or 4), and “healthy sleep” (scores of 5).
Four “clinical” CVH metrics were used to assess gestational CVH [body mass index (BMI), BP, total cholesterol (TC) level, and, glucose level] (22). Each CVH metric was rated as ideal (2 points), intermediate (1 point), or poor (0 points). The following are the detailed classification criteria. Ideal BMI: < 28.4 kg/m2, intermediate BMI: 28.5–32.9 kg/m2, poor BMI: ≥ 33 kg/m2. Ideal BP: systolic blood pressure (SBP) < 120 mm Hg and diastolic blood pressure (DBP) < 80 mm Hg; intermediate BP: SBP 120–139 mm Hg or DBP 80–89 mm Hg; poor BP: SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg. Ideal TC: < 260 mg/dL, intermediate TG: 260–299 mg/dL, and poor TG: ≥ 300 mg/dL. glucose: ideal: non-gestational diabetes mellitus (GDM), poor: diagnosis of GDM. (22) At 24–28 gestational weeks, we obtained the results of four “clinical” CVH metrics in the hospitals (BMI, BP, TC, and blood glucose). Poor CVH was defined as having one or more of these indicators as poor.
In this study, the potential confounder was age (<30 and ≥ 30 years), prepregnancy BMI (< 24 and ≥ 24 kg/m2), gestational weight gain (GWG) rate (< P75 and ≥ P75, kg/w), family history of diabetes, hypertension, CVD (including myocardial infarction, coronary heart disease, stroke), daily outdoor time, sun-bathing, vitamin D supplementation frequency, the season of blood collection, and dietary vitamin D intake habits (sea-fish, egg, milk, fungi, red meat, and white meat intake) (23, 24) which have previously been linked to CVH or 25(OH)D concentrations in pregnant women. The exact dose of vitamin D supplementation was not available. According to reports of pregnant women on vitamin D supplementation, the dose ranged from 400 IU to 600 IU daily. The dietary vitamin D intake habits was from food frequency questionnaires. GWG rate was the following: (current weight- self-reported prepregnancy weight)/current gestational week. Depression in pregnant women was screened using the Edinburgh Postpartum Depression Scale (points ≥ 10). Other potential confounders included education (12 and > 12 years of completed schooling), household income (less than 6,000 RMB or more than 6,000 RMB), parity (primipara or multipara), residence (urban or non-urban), physical activity, and husband smoking during pregnancy. Often activity was defined as physical activity (including table tennis, badminton, and vigorous walking) for at least 30 min per day.
A t-test was used to compare the difference in 25(OH)D concentrations among all groups of characteristics. The relationship between 25(OH)D concentrations and poor CVH across the different healthy sleep scores was fitted in logistics regression models. Models were adjusted for age, education, household income, residence, the season of blood collection, prepregnancy BMI, GWG rate, parity, depression, family history of diabetes, hypertension, and CVD, smoking, husband’s smoking, daily outdoor time, sun exposure, activities, dietary vitamin d intake habits (sea-fish, egg, milk, fungi, red meat, and white meat intake), vitamin D supplementation.
Stratified analyses were used to estimate the association of 25(OH)D concentrations in quintiles with poor CVH or each individual “clinic index” according to healthy sleep patterns. The likelihood ratio test comparing models was used to estimate the interaction test between 25(OH)D concentrations and sleep patterns. All statistical analyses were conducted in SPSS 23.0 (IBM Corp., Armonk, NY, USA).
The distributions of sociodemographic characteristics, perinatal health status, lifestyle, and dietary vitamin D intake habits of participants did not differ from those who withdrew, according to attrition analyses. The average participant age was 28.5 years [standard deviation (SD) = 3.8], the mean prepregnancy BMI was 21.3 [SD = 2.9]) kg/m2, and the mean concentrations of 25(OH)D were 40 [SD = 18]) nmol/L. The proportion of women with poor CVH was 25.0%.
Table 1 shows the characteristics of the study population across the differences in 25(OH)D concentrations. Participants with higher 25(OH)D were higher educational attainment, blood collection in summer or fall, lower prepregnancy BMI, lower GWG rate, more outdoor time, frequent sunbathing, frequent vitamin D supplementation, and more frequent egg, milk, or white meat consumption.
Lower 25(OH)D concentrations were found to be significantly associated with poor CVH risk in the multivariate-adjusted models (Table 2). A negative dose-response relationship was observed between 25(OH)D concentrations and poor CVH risk. Such association was also evident in subgroups subgroup analysis. The RR (95% CI) of poor CVH was 0.67 (0.58–0.76) in women with 25(OH)D ≥ 50 nmol/L, and the highest quintile of 25(OH)D had a 55% lower poor CVH risk than the lowest quintile.
The stratified analysis showed whether sleep patterns modified the association between 25(OH)D and the risk of poor CVH according to the healthy sleep scores (Figure 1). The interaction between 25(OH)D and sleep patterns on the risk of poor CVH was significant (P for interaction = 0.01). In healthy or intermediate sleep, 25(OH)D was inversely related to the risk of poor CVH in a dose-response fashion. There was no significant negative correlation of poor CVH for quintile 2–4 groups compared with quintile 1 group of 25(OH)D among poor sleep. The association between 25(OH)D and GDM, high BP, high total cholesterol, or overweight was similar to that of poor CVH (Supplementary Figure 2).
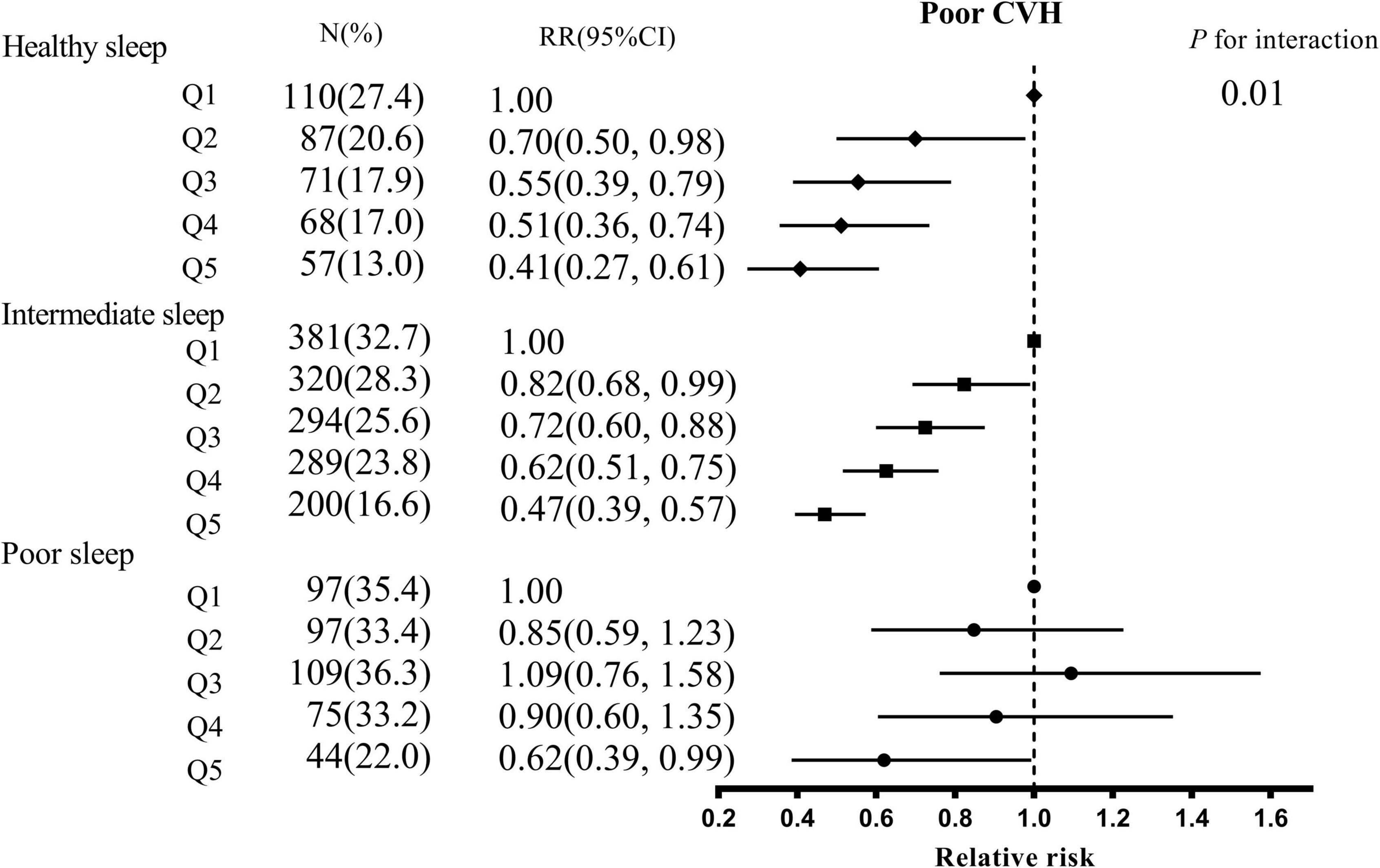
Figure 1. The association between serum 25(OH)D concentrations and poor CVH stratified by overall sleep patterns. CVH, cardiovascular health; CI, confidence interval; Q, quintile. Adjusted for age, education, household income, residence, the season of blood collection, prepregnancy BMI, GWG rate, parity, depression, family history of diabetes, hypertension, and CVD, smoking, husband’s smoking, daily outdoor time, sun exposure, activities, dietary vitamin d intake habits (sea-fish, egg, milk, fungi, red meat, and white meat intake), vitamin D supplementation.
Table 3 shows the association of sleep patterns with serum 25(OH)D concentrations and poor CVH. We found that 25(OH)D concentrations were lower and the risk of poor CVH was higher in pregnant women with poor sleep patterns (P < 0.05). We also found similar results in the association of sleep behaviors (sleep duration, daytime sleepiness, insomnia, snoring) with serum 25(OH)D concentrations and poor CVH (Supplementary Table 1).
To our knowledge, this is the first study to assess the effect of sleep behaviors on the association between 25(OH)D and gestational CVH. A negative dose-response association was observed between 25(OH)D concentrations and poor CVH risk, independent of traditional risk factors. In addition, we also discovered that sleep patterns might significantly modify such association, while the decreased poor CVH risk associated with high serum 25(OH)D concentrations appeared to be attenuated among participants with poor sleep patterns.
Consistent with our findings, several observational studies (8, 9, 25) showed that serum 25(OH)D were inversely associated with poor CVH risk, and previous randomized controlled trials (RCTs) also observed that vitamin D supplementation improved some CVH markers. (10, 26) Although the mechanisms underlying the observed inverse relationship between serum 25(OH)D and CVD risk remain unknown, several potential explanations exist. For example, vitamin D deficiency might activate the renin-angiotensin-aldosterone system and increase the CVD risk (27). A recent RCT also suggested that supplementation with high-dose vitamin D3 in patients with grades I-II essential hypertension reduces BP (28). In addition, a previous animal study showed that adequate 25(OH)D may decrease CVD risk through anti-inflammatory processes (29). 25(OH)D may control immunological and inflammatory cell proliferation, differentiation, and function, increasing anti-inflammatory pathways and decreasing the activation of these cells (30).
For the first time, we observed that sleep patterns could modify the association of 25(OH)D with gestational CVH. We also discovered that healthy sleep patterns, such as sleep 7–8 h per day, no excessive daytime sleepiness, never or rarely insomnia, no snoring, and early chronotype were associated with a decreased risk of poor gestational CVH, including the risk of obesity, hyperglycemia, hypertension, hyperlipidemia. Notably, previous meta-analyses found significant heterogeneity in the associations of 25(OH)D with CVD across studies (11). RCTs (10, 31) also yielded inconclusive results regarding whether vitamin D supplementation may reduce the risk of CVD. Moreover, the evidence from the Mendelian randomization study (32) has shown that population-wide correction of vitamin D deficiency could reduce the burden of CVD. Our findings indicate that variable sleep behaviors may account for some of this heterogeneity, and healthy sleep patterns may reinforce the validity of the association of 25(OH)D with poor CVH in pregnant women.
Poor sleep patterns may modify the association between vitamin D and CVH through inflammatory pathways. Prior studies (33) have noted the association between poor sleep and increased inflammation, and chronic inflammation was a potential mechanism of CVD. The data (34) from the National Health and Nutrition Examination Survey have shown the reduction of inflammatory diet may attenuate the adverse effects of poor sleep on CVD. Our previous study (35) has found that adequate 25(OH)D plays an essential anti-inflammatory role in gestational CVH. So, poor sleep patterns may attenuate the positive effects of adequate 25(OH)D on CVH by increasing inflammation.
Our results of the interactions between 25(OH)D and sleep behaviors were biologically plausible. The main pathway generating 25(OH)D is endogenously synthesized via skin exposure to sunlight (36). Unhealthy sleep behaviors may have an impact on the amount of skin exposed to sunlight, leading to vitamin D deficiency (37). For example, daytime sleepiness may be associated with reduced outside activities and sun exposure, which reduced circulating 25(OH)D. Animal studies also showed that chronic sleep deficiency in rats significantly reduced circulating 25(OH)D concentrations (38). Furthermore, vitamin D deficiency may influence sleep patterns. Previous observational study found a relationship between 25(OH)D concentrations and daytime sleepiness. (39) An RCT (40) on US veterans with 25(OH)D concentrations < 75 nmol/L and short sleep duration (slightly over 4.5 h) showed approximately 0.75 h longer sleep duration after supplementation with vitamin D 1200 IU daily or 50 000 IU weekly for 3 months. Another RCT of vitamin D supplementation in patients with abnormal sleep suggests direct central effects of vitamin D on sleep (41). Moreover, the inverse associations between sleep behaviors including unhealthy sleep duration, or excessive daytime sleepiness, and 25(OH)D were also detected. An updated meta-analysis study (42) showed that OSA, as manifested by snoring symptoms, was a partial contributor to low serum 25(OH)D concentrations. The previous study (43) also suggested that patients with more severe OSA indices had lower 25(OH)D concentrations associated with abnormal glucose metabolism.
To our knowledge, this is the first study to evaluate weather sleep behaviors modify the association of 25(OH)D with gestational CVH. The large sample size and prospective design are two of this study’s major strengths. More importantly, a wide range of covariates was collected, including lifestyle, dietary habits, and sun exposure, allowing for rigorous confounding adjustment. However, a few potential restrictions need to be addressed. First, as we used self-reported sleep data in this investigation, exposures may have been incorrectly classified. Second, the healthy sleep score did not account for other sleep behavior such restless legs syndrome, which may interact with vitamin D on the gestational CVH. In addition, a note of caution should be exercised while interpreting our study. According to multiple literature data (44, 45), other components of the CVH score in this study (BP, BMI, TC, and glucose levels) are in inverse correlation with 25(OH)D concentrations. Therefore, higher CVH score will be automatically associated with higher 25(OH)D concentrations. Third, because this investigation was observational in nature, causality could not be established. Randomized clinical trials are required to validate our findings. Fourth, even though we rigorously eliminated a number of possible confounders from the analyses, such as lifestyle, sun exposure, and diet factors, residual confounding may still exist. Finally, because the current study was based on data from just one city, it is important to use caution when extrapolating the findings to other communities.
In conclusion, our study observes that lower 25(OH)D concentrations are related to poor gestational CVH, and such associations are modified by sleep patterns. Our findings suggest that future RCTs of vitamin D supplementation need to consider the importance of lifestyle factors such as sleep behaviors in the prevention of cardiovascular diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Anhui Medical University (No. 2015002). The patients/participants provided their written informed consent to participate in this study.
W-JY performed the experiments and was responsible for the collection and compilation of data, analysis of data, and wrote the manuscript. PW contributed to the compilation of the data and helped write the manuscript. L-JY was responsible for the collection of clinical data and contributed to clinical assessments. X-MJ and YZ designed the study and assisted with data collection. PZ and D-MZ were the guarantor of this work designed and supervised the study, and revised the manuscript. All authors read and approved the final manuscript.
This research was supported by the National Natural Science Foundation of China (81872631 and 82173531), the Foundation for Scientific Research Improvement of Anhui Medical University (2021xkjT009), and the National Key R&D Program of China (2022YFC2702901).
We thank the doctors and nurses of Obstetrics and Gynecology for helping with the recruitment of subjects.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1013960/full#supplementary-material
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; CVD, cardiovascular disease; CVH, cardiovascular health; DBP, diastolic blood pressure; GDM, gestational diabetes mellitus; GWG, gestational weight gain; OGTT, oral glucose tolerance test; OSA, obstructive sleep apnea; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol.
1. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd-Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. (2020) 9:e15123. doi: 10.1161/JAHA.119.015123
2. Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. (2013) 347:f4539. doi: 10.1136/bmj.f4539
3. Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sorensen HT, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. (2019) 367:l6398. doi: 10.1136/bmj.l6398
4. Hammad IA, Meeks H, Fraser A, Theilen LH, Esplin MS, Smith KR, et al. Risks of cause-specific mortality in offspring of pregnancies complicated by hypertensive disease of pregnancy. Am J Obstet Gynecol. (2020) 222:71–5. doi: 10.1016/j.ajog.2019.07.024
5. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. (2019) 380:33–44. doi: 10.1056/NEJMoa1809944
6. Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. (2013) 98:1425–32. doi: 10.3945/ajcn.113.072785
7. Ohlund I, Lind T, Hernell O, Silfverdal SA, Liv P, Karlsland AP. Vitamin D status and cardiometabolic risk markers in young swedish children: a double-blind randomized clinical trial comparing different doses of vitamin D supplements. Am J Clin Nutr. (2020) 111:779–86. doi: 10.1093/ajcn/nqaa031
8. Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliovaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. (2009) 170:1032–9. doi: 10.1093/aje/kwp227
9. Gholami F, Moradi G, Zareei B, Rasouli MA, Nikkhoo B, Roshani D, et al. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. (2019) 19:248. doi: 10.1186/s12872-019-1236-7
10. Rajakumar K, Moore CG, Khalid AT, Vallejo AN, Virji MA, Holick MF, et al. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: a randomized clinical trial. Am J Clin Nutr. (2020) 111:757–68. doi: 10.1093/ajcn/nqz340
11. Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. (2012) 5:819–29. doi: 10.1161/CIRCOUTCOMES.112.967604
12. McCarty DE, Chesson AJ, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. (2014) 18:311–9. doi: 10.1016/j.smrv.2013.07.001
13. Hejazian SM, Ahmadian E, Zununi VS., Faraji GL, Farnood F. The association of sleep quality and vitamin D levels in hemodialysis patients. Biomed Res Int. (2021) 2021:4612091. doi: 10.1155/2021/4612091
14. Yan S, Tian Z, Zhao H, Wang C, Pan Y, Yao N, et al. A meta-analysis: does vitamin D play a promising role in sleep disorders? Food Sci Nutr. (2020) 8:5696–709. doi: 10.1002/fsn3.1867
15. Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between vitamin D deficiency and sleep disorders: a systematic review and meta-analysis. Nutrients. (2018) 10:1395. doi: 10.3390/nu10101395
16. Muscogiuri G, Barrea L, Scannapieco M, Di Somma C, Scacchi M, Aimaretti G, et al. The lullaby of the sun: the role of vitamin D in sleep disturbance. Sleep Med. (2019) 54:262–5. doi: 10.1016/j.sleep.2018.10.033
17. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. (2020) 41:1182–9. doi: 10.1093/eurheartj/ehz849
18. Dominguez JE, Habib AS, Krystal AD. A review of the associations between obstructive sleep apnea and hypertensive disorders of pregnancy and possible mechanisms of disease. Sleep Med Rev. (2018) 42:37–46. doi: 10.1016/j.smrv.2018.05.004
19. Laposky AD, Pemberton VL. Sleep-disordered breathing and pregnancy-related cardiovascular disease. J Womens Health. (2021) 30:194–8. doi: 10.1089/jwh.2020.8869
20. Yin WJ, Tao RX, Hu HL, Zhang Y, Jiang XM, Zhang MX, et al. The association of vitamin D status and supplementation during pregnancy with gestational diabetes mellitus: a Chinese prospective birth cohort study. Am J Clin Nutr. (2020) 111:122–30. doi: 10.1093/ajcn/nqz260
21. Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. (1991) 14:540. doi: 10.1093/sleep/14.6.540
22. Perak AM, Lancki N, Kuang A, Labarthe DR, Allen NB, Shah SH, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. (2021) 325:658. doi: 10.1001/jama.2021.0247
23. Mileševiæ J, Samaniego L, Kiely M, Glibetiæ M, Roe M, Finglas P. Specialized food composition dataset for vitamin D content in foods based on european standards: application to dietary intake assessment. Food Chem. (2018) 240:544–9. doi: 10.1016/j.foodchem.2017.07.135
24. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the epic-oxford study. Public Health Nutr. (2011) 14:340–6. doi: 10.1017/S1368980010002454
25. Dziedzic EA, Smyk W, Sowinska I, Dabrowski M, Jankowski P. Serum level of vitamin D is associated with severity of coronary atherosclerosis in postmenopausal women. Biology. (2021) 10:1139. doi: 10.3390/biology10111139
26. Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clin Nutr. (2019) 38:2098–105. doi: 10.1016/j.clnu.2018.10.028
27. Al-Ishaq RK, Kubatka P, Brozmanova M, Gazdikova K, Caprnda M, Busselberg D. Health implication of vitamin D on the cardiovascular and the renal system. Arch Physiol Biochem. (2021) 127:195–209. doi: 10.1080/13813455.2019.1628064
28. Chen WR, Liu ZY, Shi Y, Yin DW, Wang H, Sha Y, et al. Vitamin D and nifedipine in the treatment of chinese patients with grades i-ii essential hypertension: a randomized placebo-controlled trial. Atherosclerosis. (2014) 235:102–9. doi: 10.1016/j.atherosclerosis.2014.04.011
29. Chen S, Swier VJ, Boosani CS, Radwan MM, Agrawal DK. Vitamin D deficiency accelerates coronary artery disease progression in Swine. Arterioscler Thromb Vasc Biol. (2016) 36:1651–9. doi: 10.1161/ATVBAHA.116.307586
30. Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. (2017) 85:78–97. doi: 10.1016/j.jaut.2017.07.007
31. Scragg R, Stewart AW, Waayer D, Lawes C, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. Jama Cardiol. (2017) 2:608–16. doi: 10.1001/jamacardio.2017.0175
32. Zhou A, Selvanayagam JB, Hypponen E. Non-linear mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur Heart J. (2022) 43:1731–9. doi: 10.1093/eurheartj/ehab809
33. Blair LM, Porter K, Leblebicioglu B, Christian LM. Poor sleep quality and associated inflammation predict preterm birth: heightened risk among African Americans. Sleep. (2015) 38:1259–67. doi: 10.5665/sleep.4904
34. Wang L, Sun M, Guo Y, Yan S, Li X, Wang X, et al. The role of dietary inflammatory index on the association between sleep quality and long-term cardiovascular risk: a mediation analysis based on nhanes (2005-2008). Nat Sci Sleep. (2022) 14:483–92. doi: 10.2147/NSS.S357848
35. Yin WJ, Yu LJ, Wu L, Zhang L, Li Q, Dai FC, et al. Adequate 25(OH)D moderates the relationship between dietary inflammatory potential and cardiovascular health risk during the second trimester of pregnancy. Front Nutr. (2022) 9:952652. doi: 10.3389/fnut.2022.952652
37. Mosavat M, Smyth A, Arabiat D, Whitehead L. Vitamin D and sleep duration: is there a bidirectional relationship? Horm Mol Biol Clin Investig. (2020) 40:1–9. doi: 10.1515/hmbci-2020-0025
38. Xu X, Wang L, Chen L, Su T, Zhang Y, Wang T, et al. Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J Orthop Surg Res. (2016) 11:87. doi: 10.1186/s13018-016-0418-6
39. McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. (2012) 8:693–7. doi: 10.5664/jcsm.2266
40. Huang W, Shah S, Long Q, Crankshaw AK, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. (2013) 29:341–7. doi: 10.1097/AJP.0b013e318255655d
41. Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. (2012) 79:132–5. doi: 10.1016/j.mehy.2012.03.031
42. Li X, He J, Yun J. The association between serum vitamin D and obstructive sleep apnea: an updated meta-analysis. Respir Res. (2020) 21:294. doi: 10.1186/s12931-020-01554-2
43. Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T. The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine. (2012) 41:518–25.
44. Mirhosseini N, Vatanparast H, Kimball SM. The association between serum 25 (OH)D status and blood pressure in participants of a community-based program taking vitamin D supplements. Nutrients. (2017) 9:1244. doi: 10.3390/nu9111244
Keywords: vitamin D, sleep patterns, cardiovascular health, pregnant women, birth cohort
Citation: Yin W-j, Yu L-j, Wang P, Tao R-x, Jiang X-m, Zhang Y, Zhu D-m and Zhu P (2022) Sleep patterns modify the association of 25(OH)D with poor cardiovascular health in pregnant women. Front. Nutr. 9:1013960. doi: 10.3389/fnut.2022.1013960
Received: 08 August 2022; Accepted: 01 November 2022;
Published: 14 November 2022.
Edited by:
Vanessa Fuchs-Tarlovsky, General Hospital of Mexico, MexicoReviewed by:
Maria Morales Suare-Varela, University of Valencia, SpainCopyright © 2022 Yin, Yu, Wang, Tao, Jiang, Zhang, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dao-min Zhu, aGZzeXpkbTc3NzhAMTYzLmNvbQ==; Peng Zhu, enBwb3N0QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.