
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 18 November 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1013643
This article is part of the Research Topic Clinical Nutrition and Oncologic Outcomes, volume II View all 28 articles
 Linglong Peng1†
Linglong Peng1† Ling Xiang2†
Ling Xiang2† Zhiquan Xu1†
Zhiquan Xu1† Haitao Gu1
Haitao Gu1 Zhiyong Zhu1
Zhiyong Zhu1 Yunhao Tang1
Yunhao Tang1 Yahui Jiang1
Yahui Jiang1 Hongmei He1
Hongmei He1 Yaxu Wang1*
Yaxu Wang1* Xiaodong Zhao3*
Xiaodong Zhao3*Background: Low-fat diet reduces the risk of chronic metabolic diseases such as obesity and diabetes, which exhibit overlapping mechanisms with liver cancer. However, the association between low-fat diet and liver cancer risk remains unclear.
Aim: To investigate whether adherence to low-fat diet is associated with a reduced risk of liver cancer in a prospective study.
Materials and methods: Data of participants in this study were collected from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. A low-fat diet score was calculated to reflect adherence to low-fat dietary pattern, with higher scores indicating greater adherence. Cox regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for liver cancer incidence with adjustment for potential covariates. Restricted cubic spline model was used to characterize liver cancer risk across the full range of the low-fat diet score. Prespecified subgroup analyses were used to identify potential impact modifiers. Sensitivity analyses were performed to test the robustness of this association.
Results: A total of 98,455 participants were included in the present analysis. The mean (standard deviation) age, low-fat diet score, and follow-up time were 65.52 (5.73) years, 14.99 (6.27) points, and 8.86 (1.90) years, respectively. During 872639.5 person-years of follow-up, 91 liver cancers occurred, with an overall incidence rate of 0.01 cases per 100 person-years. In the fully adjusted Cox model, the highest versus the lowest quartile of low-fat diet score was found to be associated with a reduced risk of liver cancer (HRQ4 vs. Q1: 0.458; 95% CI: 0.218, 0.964; P = 0.035 for trend), which remained associated through a series of sensitivity analyses. The restricted cubic spline model showed a linear dose–response association between low-fat diet score and liver cancer incidence (p = 0.482 for non-linear). Subgroup analyses did not show significant interaction between low-fat diet score and potential impact modifiers in the incidence of liver cancer.
Conclusion: In this study, low-fat diet score is associated with reduced liver cancer risk in the US population, indicating that adherence to low-fat diet may be helpful for liver cancer prevention. Future studies should validate our findings in other populations.
Primary liver cancer is one of the seven most common cancers and the second leading cause of cancer deaths worldwide. In 2020, approximately 905,677 cases were newly diagnosed with liver cancer, and an estimated 830,180 individuals died from liver cancer (1). It is well-established that hepatitis B/C virus infection and alcohol consumption are the main risk factors for liver cancer (2). However, a proportion of cases cannot be explained by traditional risk factors. Emerging evidence concerning diet, including single nutrients and dietary patterns has confirmed a close association between liver cancer risk and diet, and certain dietary patterns are advised for liver cancer prevention (3). For example, in a US population study with average follow-up time of 32 years, the incidence of liver cancer was reduced by a maximum of 39% in participants with a high healthy eating index (4). In a study integrating two Chinese cohorts, a total of 132,837 participants were divided into quartiles based on a vegetable-based dietary pattern, and the risk of liver cancer for participants in the highest quartile was reduced by 42% compared with the lowest quartile (5). Although both dietary patterns were not specifically developed to prevent cancer, they were related to each other and share low-fat dietary components. Low-fat diet is a dietary pattern designed to reduce total fat and calorie intake, which has been shown to be beneficial in reducing the risk of chronic metabolic diseases such as obesity and diabetes (6–8). Additionally, human and animal studies also suggest that low-fat diet has the potential to reduce the secretion of inflammatory cytokines and mediators, including interleukins, tumor necrosis factor-α, Toll-like receptors and complements, and the activity of the transcription factor NF-kB, which was demonstrated to be closely related to increased cancer risk including liver cancer (9–11). However, there are currently large knowledge gaps regarding the association between low-fat diet and liver cancer risk. Thus, the aim of this study was to investigate the association of low-fat diet with the risk of liver cancer in a large population.
All data included in this study were from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial administered by the National Cancer Institute (NCI). The PLCO trial is a large randomized controlled study involving 154,887 participants in ten United States centers from 1993 to 2001, and its main purpose is to determine whether mortality from prostate, lung, colorectal, and ovarian cancers can be reduced using related screening methods in people between the ages of 55 and 74 years (12). According to the design of the PLCO trial, participants were randomly assigned to control or intervention groups in equal proportions after providing informed consent; usual care was received in the control group and screening exams were performed in the intervention group. Several questionnaires, including Baseline Questionnaire (BQ), Diet History Questionnaire (DHQ), and Supplemental Questionnaire (SQX), were completed by the participants in a self-reported manner. The BQ was used to collect the baseline risk factors, such as demographics and medical history, at the time of participant randomization. The DHQ was used to collect the dietary information of participants based on the 137-item Food Frequency Questionnaire (FFQ), and multiple studies have confirmed that the FFQ is a good nutrient assessment pattern (13, 14). The SQX was used to supplement some data not collected by the BQ. Detailed information on the PLCO trial is provided in the literature (12). Our present study was approved by the NCI (Project ID: PLCO-987).
Participants for this study were excluded using the following exclusion criteria: (I) participants who did not return the BQ (n = 4,918); (II) participants with an invalid DHQ, identified as participants who lacked 8 + frequency responses on the DHQ, whose calorie intake was extreme (the first and last percentile) for each gender as assessed by the DHQ, and the DHQ completion date was available and prior to the date of death (n = 38,462); (n = 38,462); (III) participants with a personal history of any cancer prior to DHQ analysis (n = 9,684); (IV) participants with an outcome event between randomization and DHQ completion, which for the present study were defined as those participants who developed liver cancer, died, or were lost to follow-up (n = 72); and (V) participants with potentially unreliable dietary intake, defined as very low or high caloric intake (< 600 or > 3,500 kcal/day for female and < 800 or > 4,200 kcal/day for male) (15) (n = 3,296). Finally, a total of 98,455 participants were eligible for inclusion (Figure 1).
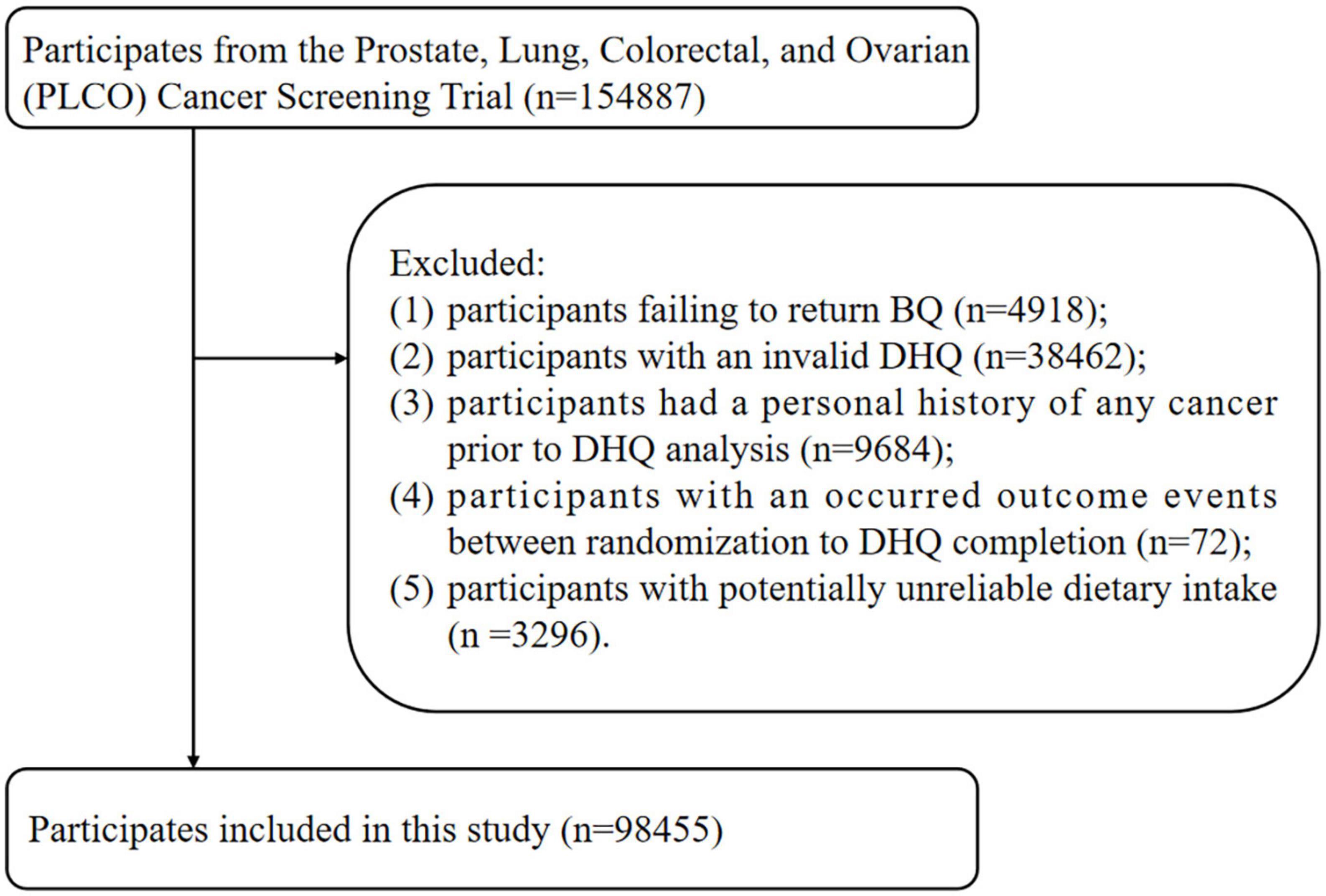
Figure 1. The study flow chart of identifying eligible participants. PLCO, Prostate, Lung, Colorectal, and Ovarian; BQ, baseline questionnaire; DHQ, diet history questionnaire.
The low-fat diet score was calculated according to the criteria reported by Shan et al. (15). Specifically, individuals were classified into 11 strata based on each of percentage of energy from carbohydrate, protein, and fat (Supplementary Table 1). For carbohydrate and protein, individuals in the lowest stratum received 0 points and those in the highest stratum received 10 points. For fat, the order of the strata was reversed. Then, the points for the three macronutrients were summed to calculate each participant’s low-fat diet score, which ranged from 0 to 30. Thus, the higher the score, the more closely the participant’s diet followed the pattern of a low-fat diet. In the present study, nutrient variables used for computing the low-fat diet score were extracted from the above-mentioned DHQ. DHQ nutrient variables are calculated from the questionnaire responses by the DietCalc software, which takes into account serving size, food frequency, and other responses, and uses these in conjunction with CSFII nutrient databases to calculate the daily intake of all nutrients (16). Of note, although the dietary information collected through the DHQ was a one-time inquiry of participants’ dietary status over the past 12 months and was not cumulatively updated during follow-up, the reproducibility and validity of the DHQ have been demonstrated elsewhere (17).
In this study, demographic, lifestyle, and medical information, including sex, race, educational level, arm (intervention or control group), body mass index (BMI), smoking status, pack-years of smoking, history of diabetes, history of liver comorbidities (hepatitis or cirrhosis), and aspirin use, were assessed with the BQ. BMI was calculated as weight (kg) divided by height squared (m2). Age at DHQ completion, drinking status, alcohol consumption, and macronutrients intake were assessed with the DHQ. Physical activity level was collected with the SQX and defined as the summarized minutes of self-reported moderate to vigorous activity per week.
Individuals diagnosed with primary liver cancer were collected through annual reporting methods including but not limited to self-reports, family reports, and death certificates. Cancer reports were tracked and ascertained by extracting any available medical records. In this study, the end point was the incidence of primary liver cancer which included hepatocellular carcinoma (ICD-O-2, C220) and intrahepatic bile duct cancer (ICD-O-2, C221).
For categorical and continuous covariates with < 5% missing values, modal and median values were used to impute missing data, respectively. The covariate “physical activity level” was imputed by the multiple imputation method as up to 25.3% of the values were missing (18). More detail information of imputation data was shown in Supplementary Table 2, and statistical analyses were performed using the imputed datasets.
A Cox proportional hazards regression model was used to assess the association between low-fat diet and liver cancer risk, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with follow-up time as the time metric. It is worth noting that the follow-up time in this study refers to the date from DHQ completion to the occurrence of liver cancer, death, loss to follow-up, or the end of follow-up (i.e., December 31, 2009), whichever came first (Figure 2). In this model, the low-fat diet scores were divided into quartiles, and the person-years of each quartile were calculated based on the length of the follow-up period. A trend test across quartiles of low-fat diet score for the liver cancer risk estimation was also performed in the Cox regression model by treating the quartiles as a continuous variable with the lowest quartile as the reference group. In multivariate analyses, Model 1 was adjusted for age, sex, and race. Model 2 was further adjusted for education level, arm, BMI, smoking status, smoking pack-years, drinking status, alcohol consumption, aspirin use, history of liver comorbidity, history of diabetes, physical activity level and energy intake from diet. To further characterize liver cancer risk across the full range of the low-fat diet score, a restricted cubic spline model was employed in this study. In addition, we further analyzed the effect of poly-unsaturated fatty acids (PUFA), mono-unsaturated fatty acids (MUFA), and saturated fatty acids (SFA) on the risk of liver cancer using the above-mentioned methods. Specially, PUFA, MUFA, and SFA intakes were derived from DHQ and divided into quartiles, with the lowest quartile as the referent.
A series of subgroup analyses were conducted after stratifying for age (> 65 versus ≤ 65 years), sex (male versus female), BMI (≤ 25 versus > 25 kg/m2), smoking status (never versus current/former), drinking status (no versus yes), alcohol consumption (≤ medium versus > medium), history of liver comorbidity (no versus yes), history of diabetes (no versus yes), physical activity (≤ medium versus > medium), and energy intake from diet (≤ medium versus > medium). A Pinteraction was computed by comparing models with and without multiplicative interaction terms before performing the above subgroup analyses to avert spurious subgroup effects.
We conducted several sensitivity analyses to test the robustness of our findings. (I) we repeated the primary analysis in participants with non-missing data; (II) we excluded participants with a history of diabetes, as these participants may be prone to a high-fat diet; (III) we excluded participants with a history of liver comorbidity, as these participants may be more likely to develop liver cancer; and (IV) we excluded cases observed within the first 2 and 4 years of follow-up to address the concern of reverse causality.
The statistical analyses were conducted using R 4.1.1 software. A two-tailed P value less than 0.05 was considered significant.
In the 98,455 included participants, the mean (standard deviation) for low-fat diet score was 14.99 (6.27). Based on the score, we divided participants into quartiles [Quartile 1 (LFD score ≤ 10), n = 26,718; Quartile 2 (LFD score 11–15), n = 26,149; Quartile 3 (LFD score 16–20), n = 24,762; Quartile 4 (LFD score ≥ 21), n = 20,826]. The higher the quartile, the more likely the participants were to follow a low-fat dietary pattern. Compared with participants in the lowest quartile group (Quartile 1), participants in the highest quartile group (Quartile 4) were more likely to be older and female and to have a higher educational level, and total carbohydrate and protein intake; but were less likely to have a higher BMI, physical activity level, diet energy and total fat intake. There were more non-smokers and non-drinkers in the highest quartile than in the lowest quartile, and fewer pack-years of current or former smokers and less alcohol consumption by drinkers were observed in the highest quartile. The detailed baseline characteristics of the study population according to quartiles of low-fat diet scores are shown in Table 1.
During 872639.5 person-years of follow-up, we documented a total of 91 liver cancer cases, with an overall incidence rate of 0.01 cases per 100 person-years. The mean (standard deviation) follow-up length was 8.862 (1.897) years. In the unadjusted model, participants in the highest quartile had a significantly lower risk of liver cancer than those in the lowest quartile (HRQ4 vs. Q1: 0.369; 95% CI: 0.182, 0.749; P = 0.003 for trend) (Table 2). After full adjustment for potential confounders, the inverse association of the low-fat diet score with the risk of liver cancer was also observed (HRQ4 vs. Q1: 0.458; 95% CI: 0.218, 0.964; P = 0.035 for trend) (Table 2). Notably, this inverse relationship was not altered when repeated analysis was performed using non-missing data (HRQ4 vs. Q1: 0.277; 95% CI: 0.076, 1.010; P = 0.032 for trend) (Table 3). For fat components, liver cancer risk was not significantly associated with PUFA (Supplementary Table 3), MUFA (Supplementary Table 4), and SFA (Supplementary Table 5) in the full adjusted model.
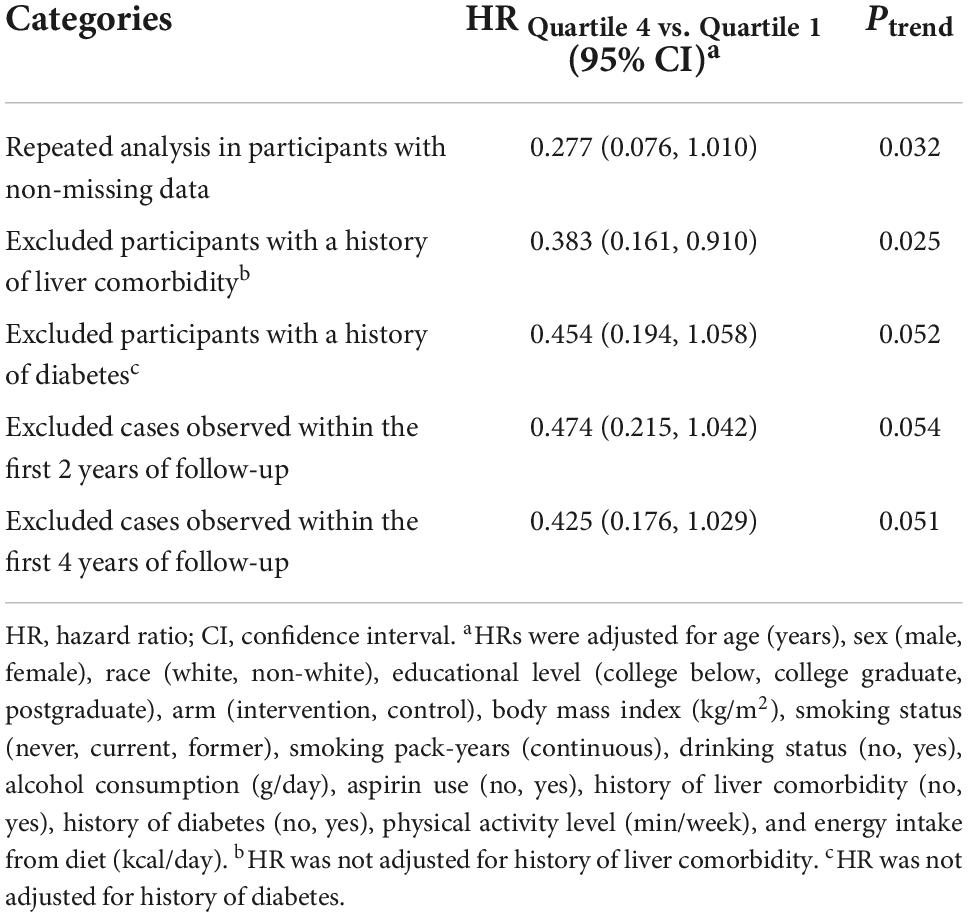
Table 3. Sensitivity analyses on the association of low-fat diet scores with the risk of liver cancer.
We employed a restricted cubic spline model to describe the liver cancer risk across the range of low-fat diet scores, and the results showed that the low-fat diet score was inversely associated with the risk of liver cancer in a linear dose–response manner (P = 0.482 for non-linear) (Figure 3). In subgroup analyses, we did not observe a significant interaction between low-fat diet score and age, sex, BMI, smoking status, drinking status, alcohol consumption, history of liver comorbidity, history of diabetes, physical activity level or energy intake from diet in the incidence of liver cancer (all P > 0.05 for interaction) (Table 4). In sensitivity analyses, the associations remained similar when we further excluded participants with a history of liver comorbidity or diabetes and excluded cases observed within the first 2 years or 4 years of follow-up, indicating a good robustness of the inverse association of low-fat diet score with liver cancer risk (Table 3).
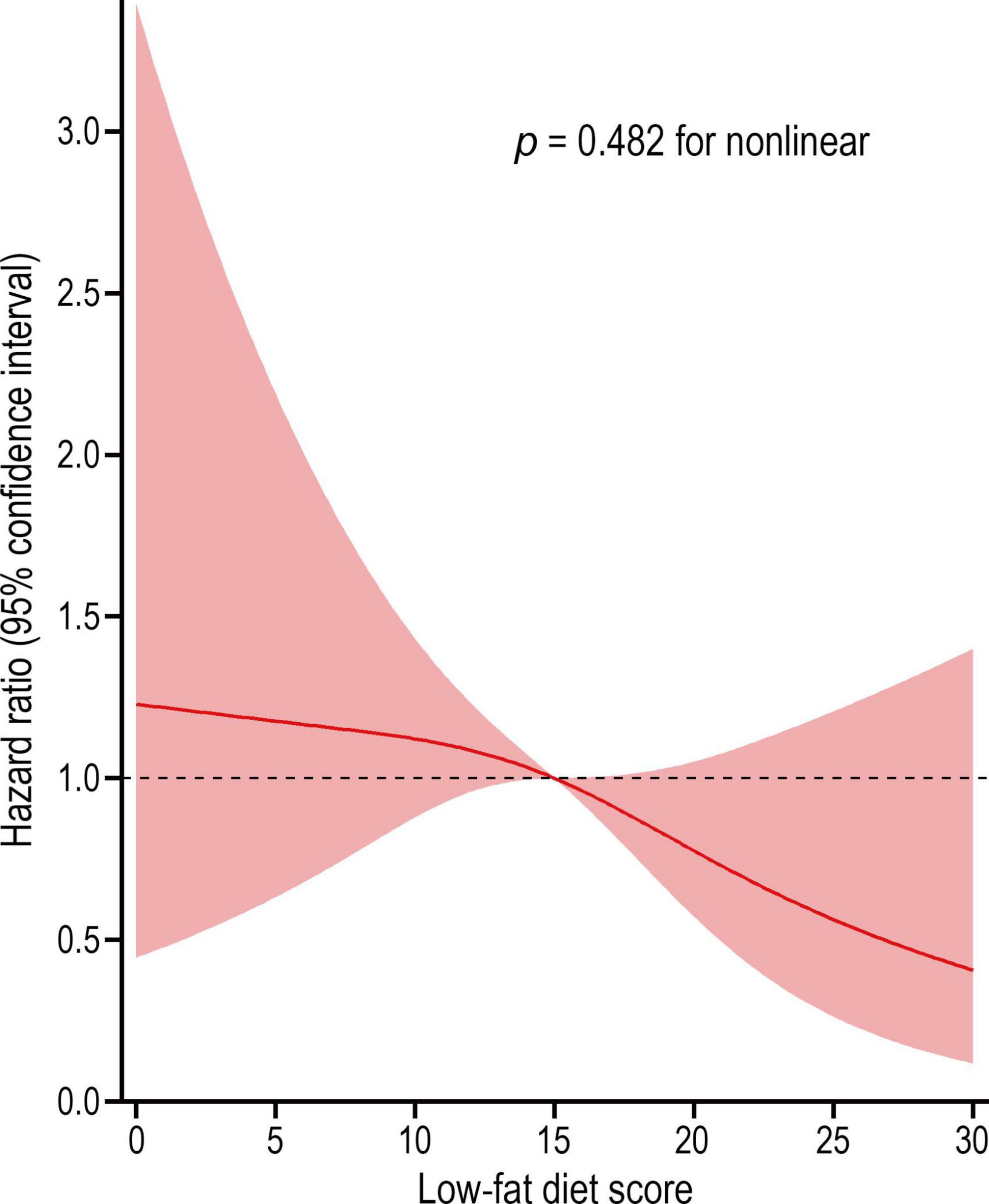
Figure 3. Dose-response association between low-fat diet score and the risk of liver cancer. Hazard ratio was adjusted for age, sex, race, educational level, arm, body mass index, smoking status, smoking pack-years, drinking status, alcohol consumption, aspirin use, history of liver comorbidity, history of diabetes, physical activity level, and energy intake from diet (p = 0.482 for non-linear).
In this study, we explored whether adherence to low-fat diet is associated with a reduced risk of liver cancer in a large prospective multicenter study. Our results showed a significant inverse association between low-fat diet score and the occurrence of liver cancer, regardless of adjustment for suspected and established confounders. The restricted cubic spline model revealed that this correlation is a non-linear dose-dependent relationship, which means that people who followed a low-fat dietary pattern had a lower risk of liver cancer. In addition, this inverse association remained unchanged even after we excluded several confounding factors through multiple sensitivity analyses.
For decades, the focus on low-fat diet mainly stemmed from the established evidence that low-fat diet can prevent the risk of obesity and diabetes (6, 7). Although dietary recommendations suggest that low-fat diet may be beneficial for cancer prevention, relevant studies are incomplete and controversial due to the wide variety of cancers and conflicting results (19). For example, in a study with a median follow-up time of 8.1 years, the low-fat diet group had a 36% reduced risk in ER-positive and PR-negative breast cancers (20). However, in another cohort study with a mean follow-up of 10 years, the risk of invasive breast cancer was not affected by intervention with low-fat diet in a high-risk population (21). Moreover, the risk for relapse and death was not reduced by the low-fat diet in a population with a very low-risk of breast cancer during a 7.3-year follow-up period (22). In addition to breast cancer, published studies have also linked low-fat diet to pancreatic cancer and skin cancer. In these studies, a reduced risk of pancreatic cancer was observed in women with overweight (BMI ≥ 25 kg/m2) among 46,200 participants followed up to 14.7 years (23), but low-fat diet did not decrease the risk of non-melanoma skin cancer (24). One possible reason for these contradictory results is that the definition of low-fat diet was inconsistent among these studies.
To our knowledge, no published data have investigated the association of low-fat diet with liver cancer risk in large populations. The low-fat diet score used in our study has been proven to be very reliable for assessing a low-fat dietary pattern that comprehensively consider the effects of fats, carbohydrates, proteins and energy (15), not just the percentage of fat in total energy (usually < 30% energy) (25). In our study of 98,455 participants with a mean follow-up length of 8.9 years, the incidence of liver cancer was reduced by 55% in participants in the highest quartile of low-fat diet scores compared with the lowest quartile. The risk of liver cancer decreased linearly with increasing low-fat diet score, as an inverse linear association was observed in the restricted cubic spline model (p for non-linear = 0.482). For fat components, we did not find a significantly association between liver cancer risk and the intakes of PUFA, MUFA, and SFA in our analyses. However, multiple studies involving fat intake-related dietary patterns, rather than low-fat diet, have obtained contradictory results in relation to liver cancer risk. Polesel et al. reported that the risk of hepatocellular carcinoma can be decreased by 40% in participants with a higher intake of PUFA (26). A significant inverse association between hepatocellular carcinoma risk and total fat intake (HR = 0.80) and MUFA (HR = 0.71) was also observed in a prospective study from Europe (27).
The observed association between low-fat diet and liver cancer risk may be explained by the following mechanisms. Physically, the liver connects to the gut through the bile duct, and the portal vein transports the products of the gut microbiota to the liver (28). Therefore, the crosstalk of gut microbiota between the liver and gut (gut-liver axis) can integrate signals into an interconnected system (29). Dietary patterns alter the gut microbiome balance and subsequently change the immune and inflammatory metabolism landscapes, eventually leading to tumor occurrence and progression (30, 31). For example, it was previously shown that gut microbiota dysbiosis induced by fiber-enriched foods (as inulin-enriched high-fat diet) prone to dysbiosis leads to inflammation, cholestasis, and hepatocellular carcinoma in mice (32). Excessive intake of high-fat diet stimulates the liver to synthesize bile acids, thereby producing large amounts of secondary bile acids in the gut (33), which have been shown to be messengers for microbiota–gut–liver interactions contributing to cancer risk (30, 34), although the underlying mechanisms are unclear. Furthermore, animal and human studies have reported that prolonged consumption of high-fat diet may produce adverse metabolic effects and up-regulate inflammatory mediators, putting the body in a state of chronic inflammation and high postprandial blood glucose and insulin response (11, 35), which is not only involved in the pathogenesis of type 2 diabetes and obesity but also closely related to hepatocarcinogenesis, as liver cancer risk consistently increases with obesity and diabetes (36–38). Conversely, low-fat diet can reduce the secretion of inflammatory mediators and inhibit the activation of tumor-related signaling pathways, ultimately preventing tumor development (39).
This study has significant strengths. This prospective analysis showed for the first time that low-fat diet reduces the incidence of liver cancer in a large population. In addition, good robustness for this inverse association was obtained by multiple sensitivity analyses. For instance, the influence of reverse causation was decreased when excluding cases that occurred in the first 2 years and 4 years of follow-up. In this study, the follow-up time was calculated based on the date of DHQ completion rather than BQ completion, thus ensuring the reliability of low-fat diet score acquisition, and a follow-up time of up to 8 years was sufficient to ensure the occurrence of end-point events. Moreover, the results of this study were extensively adjusted for potential confounders including demographic, lifestyle, medical and dietary factors, thereby minimizing the influence of residual confounders on observed events.
However, several limitations existed in this study. First, we did not find a significant interaction on the incidence of liver cancer between low-fat diet score and potential impact modifiers in a series of subgroup analyses, although the reason may be due to the limited liver cancer cases in each subgroup, resulting in insufficient statistical power for the interaction test. Thus, we cannot provide dietary guidance for specific subgroups based on the results of this study. Second, the low-fat diet score was assessed using a one-time questionnaire without considering that the dietary habits of the participants may change during the follow-up period, which may result in non-differential bias. However, studies have reported that using cumulative averages to assess dietary patterns generally leads to a similar statistical association for disease risk analysis (40), and it is always assumed that the dietary habits of adults generally do not change in nutritional epidemiological studies (41). Third, as the population of our study was American adults aged 55–74 years, we cannot guarantee that the inverse association of low-fat diet with liver cancer risk is applicable for other age groups and non-American populations.
In conclusion, low-fat diet is associated with a reduced risk of liver cancer in the US population. These findings suggest that adherence to a low-fat diet is helpful for the prevention of liver cancer. Future studies should validate our findings in other populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Cancer Institute and each screening center. Written informed consent was obtained from all individuals. The patients/participants provided their written informed consent to participate in this study.
XZ, YW, and LP designed the study. LP, LX, and ZX collected and organized the original data. LP, ZX, and LX analyzed the data. YW, HG, and YT assisted with statistical analysis. LP, ZX, LX, HG, YT, ZZ, YJ, and HH assisted in the interpretation of the results. LP, LX, and XZ drafted the manuscript. All authors contributed to the article and approved the submitted manuscript.
This work was supported by the General Project of Chongqing Natural Science Foundation, Chongqing Science and Technology Commission, China [grant numbers: cstc2021jcyj-msxmX0153 (LP) and cstc2021jcyj-msxmX0112 (YW)].
We thank the NIH PLCO study group and the National Cancer Institute (NCI) for access to NCI’s data collected by the PLCO Cancer Screening Trial. This study has been approved by the NCI (approval number: PLCO-987).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1013643/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. (2013) 47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29
3. Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, et al. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. (2020) 124:330–40. doi: 10.1017/s0007114520001208
4. Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, et al. Dietary patterns and risk of hepatocellular carcinoma among U.S. Men and women. Hepatology. (2019) 70:577–86. doi: 10.1002/hep.30362
5. Zhang W, Xiang YB, Li HL, Yang G, Cai H, Ji BT, et al. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women’s and men’s health studies. Cancer Sci. (2013) 104:1353–61. doi: 10.1111/cas.12231
6. Astrup A, Grunwald GK, Melanson EL, Saris WH, Hill JO. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord. (2000) 24:1545–52. doi: 10.1038/sj.ijo.0801453
7. Lindström J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, et al. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia. (2006) 49:912–20. doi: 10.1007/s00125-006-0198-3
8. Prentice RL, Aragaki AK, Howard BV, Chlebowski RT, Thomson CA, Van Horn L, et al. Low-fat dietary pattern among postmenopausal women influences long-term cancer, cardiovascular disease, and diabetes outcomes. J Nutr. (2019) 149:1565–74. doi: 10.1093/jn/nxz107
9. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
10. Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. (2013) 191:4337–47. doi: 10.4049/jimmunol.1300298
11. Doerner SK, Reis ES, Leung ES, Ko JS, Heaney JD, Berger NA, et al. High-fat diet-induced complement activation mediates intestinal inflammation and neoplasia, independent of obesity. Mol Cancer Res. (2016) 14:953–65. doi: 10.1158/1541-7786.Mcr-16-0153
12. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. (2000) 21:273s–309s. doi: 10.1016/s0197-2456(00)00098-2
13. Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. (2008) 11:183–95. doi: 10.1017/s1368980007000419
14. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. (2001) 154:1089–99. doi: 10.1093/aje/154.12.1089
15. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. (2020) 180:513–23. doi: 10.1001/jamainternmed.2019.6980
16. Dwyer J, Picciano MF, Raiten DJ. Future directions for the integrated CSFII-NHANES: what we eat in America-NHANES. J Nutr. (2003) 133:576s–81s. doi: 10.1093/jn/133.2.576S
17. Csizmadi I, Boucher BA, Lo Siou G, Massarelli I, Rondeau I, Garriguet D, et al. Using national dietary intake data to evaluate and adapt the US Diet History Questionnaire: the stepwise tailoring of an FFQ for Canadian use. Public Health Nutr. (2016) 19:3247–55. doi: 10.1017/s1368980016001506
18. Brancato G, Pezzotti P, Rapiti E, Perucci CA, Abeni D, Babbalacchio A, et al. Multiple imputation method for estimating incidence of HIV infection. The Multicenter Prospective HIV Study. Int J Epidemiol. (1997) 26:1107–14. doi: 10.1093/ije/26.5.1107
19. Ludwig DS, Willett WC, Volek JS, Neuhouser ML. Dietary fat: from foe to friend? Science. (2018) 362:764–70. doi: 10.1126/science.aau2096
20. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. Jama. (2006) 295:629–42. doi: 10.1001/jama.295.6.629
21. Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. (2011) 71:123–33. doi: 10.1158/0008-5472.Can-10-1436
22. Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. (2007) 298:289–98. doi: 10.1001/jama.298.3.289
23. Jiao L, Chen L, White DL, Tinker L, Chlebowski RT, Van Horn LV, et al. Low-fat dietary pattern and pancreatic cancer risk in the women’s health initiative dietary modification randomized controlled trial. J Natl Cancer Inst. (2018) 110:49–56. doi: 10.1093/jnci/djx117
24. Black HS, Thornby JI, Wolf JE Jr., Goldberg LH, Herd JA, Rosen T, et al. Evidence that a low-fat diet reduces the occurrence of non-melanoma skin cancer. Int J Cancer. (1995) 62:165–9. doi: 10.1002/ijc.2910620210
25. Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971-2006. Am J Clin Nutr. (2011) 93:836–43. doi: 10.3945/ajcn.110.000141
26. Polesel J, Talamini R, Montella M, Maso LD, Crovatto M, Parpinel M, et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur J Cancer. (2007) 43:2381–7. doi: 10.1016/j.ejca.2007.07.012
27. Duarte-Salles T, Fedirko V, Stepien M, Aleksandrova K, Bamia C, Lagiou P, et al. Dietary fat, fat subtypes and hepatocellular carcinoma in a large European cohort. Int J Cancer. (2015) 137:2715–28. doi: 10.1002/ijc.29643
28. Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. (2018) 15:397–411. doi: 10.1038/s41575-018-0011-z
29. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
30. Silveira MAD, Bilodeau S, Greten TF, Wang XW, Trinchieri G. The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer. (2022) 8:583–97. doi: 10.1016/j.trecan.2022.02.009
31. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505:559–63. doi: 10.1038/nature12820
32. Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. (2018) 175:679–94.e22. doi: 10.1016/j.cell.2018.09.004
33. Trefflich I, Marschall HU, Giuseppe RD, Ståhlman M, Michalsen A, Lampen A, et al. Associations between dietary patterns and bile acids-results from a cross-sectional study in vegans and omnivores. Nutrients. (2019) 12:47. doi: 10.3390/nu12010047
34. Ocvirk S, O’Keefe SJ. Influence of bile acids on colorectal cancer risk: potential mechanisms mediated by diet - gut microbiota interactions. Curr Nutr Rep. (2017) 6:315–22. doi: 10.1007/s13668-017-0219-5
35. Abdulmalek SA, Fessal M, El-Sayed M. Effective amelioration of hepatic inflammation and insulin response in high fat diet-fed rats via regulating AKT/mTOR signaling: role of Lepidium sativum seed extracts. J Ethnopharmacol. (2021) 266:113439. doi: 10.1016/j.jep.2020.113439
36. Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane Freeman LE, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. Adults. Cancer Res. (2016) 76:6076–83. doi: 10.1158/0008-5472.Can-16-0787
37. Petrick JL, Freedman ND, Demuth J, Yang B, Van Den Eeden SK, Engel LS, et al. Obesity, diabetes, serum glucose, and risk of primary liver cancer by birth cohort, race/ethnicity, and sex: multiphasic health checkup study. Cancer Epidemiol. (2016) 42:140–6. doi: 10.1016/j.canep.2016.04.009
38. Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. (2017) 60:1022–32. doi: 10.1007/s00125-017-4229-z
39. Heymach JV, Shackleford TJ, Tran HT, Yoo SY, Do KA, Wergin M, et al. Effect of low-fat diets on plasma levels of NF-κB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev Res. (2011) 4:1590–8. doi: 10.1158/1940-6207.Capr-10-0136
40. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. (1999) 149:531–40. doi: 10.1093/oxfordjournals.aje.a009849
Keywords: low-fat diet, liver cancer, prevention, prostate, lung, colorectal, ovarian cancer screening trial, cox regression analysis
Citation: Peng L, Xiang L, Xu Z, Gu H, Zhu Z, Tang Y, Jiang Y, He H, Wang Y and Zhao X (2022) Association between low-fat diet and liver cancer risk in 98,455 participants: Results from a prospective study. Front. Nutr. 9:1013643. doi: 10.3389/fnut.2022.1013643
Received: 07 August 2022; Accepted: 26 October 2022;
Published: 18 November 2022.
Edited by:
Kalliopi-Anna Poulia, Agricultural University of Athens, GreeceReviewed by:
Anthony Bernard Miller, University of Toronto, CanadaCopyright © 2022 Peng, Xiang, Xu, Gu, Zhu, Tang, Jiang, He, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaxu Wang, MzAwODk3QGhvc3BpdGFsLmNxbXUuZWR1LmNu; Xiaodong Zhao, emhhb3hkNTMwQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.