
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 08 December 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1012023
This article is part of the Research TopicBioaccessibility and Bioavailability Studies and Their Importance in the Evaluation of Health-Promoting Properties of Bioactive CompoundsView all 5 articles
 Christiana Oluwatoyin Ajanaku1
Christiana Oluwatoyin Ajanaku1 Olabisi Theresa Ademosun1
Olabisi Theresa Ademosun1 Prudence Osahenomanse Atohengbe1
Prudence Osahenomanse Atohengbe1 Samuel Oluwakayode Ajayi1
Samuel Oluwakayode Ajayi1 Yemisi Dorcas Obafemi2
Yemisi Dorcas Obafemi2 Olayinka Ayotunde Owolabi3
Olayinka Ayotunde Owolabi3 Paul Akinniyi Akinduti2
Paul Akinniyi Akinduti2 Kolawole Oluseyi Ajanaku1*
Kolawole Oluseyi Ajanaku1*Nutrition plays a very important role in the health promotion of individuals and brought about a global paradigm shift from pharmaceuticals to nutraceuticals. This is due to the high cost, non-availability, and side effects associated with the unregulated consumption of pharmaceuticals. Over the ages, nutraceuticals from food products were reported to contain bioactive compounds with great health and physiological benefits. This report reviews bioactive compounds in selected foods namely ginger (Zingiber officinale), turmeric (Curcuma longa), and garlic (Allium sativum) as potential natural therapeutics for ailments of cancer and heart-related diseases. Analytical profiles, functional activities, and characterization of these compounds were discussed with possible recommendations for the prospective treatment of diseases using these nutraceuticals.
Nutraceuticals are nutritional products that help to improve health and prevent disease. They include a large number of products, dietary supplements, nutrients, herbal products, and specific processed food and beverages (1). These products are mostly plant-based and some animal-based foods, and omega fatty acids. Bioactive compounds obtained from herbs, fruits, foods, and spices were used for culinary purposes and consumption (2). Fortified foods are categorized chemically as: carbohydrate derivatives (oligosaccharides, non-starch polysaccharides), fatty acids, structural lipids (mono and polyunsaturated fatty acids), derivatives of isoprenoid (terpenoids, saponins, carotenoids, terpenes and all forms of vitamin E such as tocopherols, tocotrienols), phenolic compounds and vitamin C, for the benefit of humankind and nature (3). Bioactive compounds present in natural products, foods, and fruits exert pharmacological effects and therefore add to the functionality of foods (4). This review detailed the potential health-associated benefits of bioactive compounds obtained from selected plant-based food products including ginger, turmeric, and garlic were presented in Figure 1.
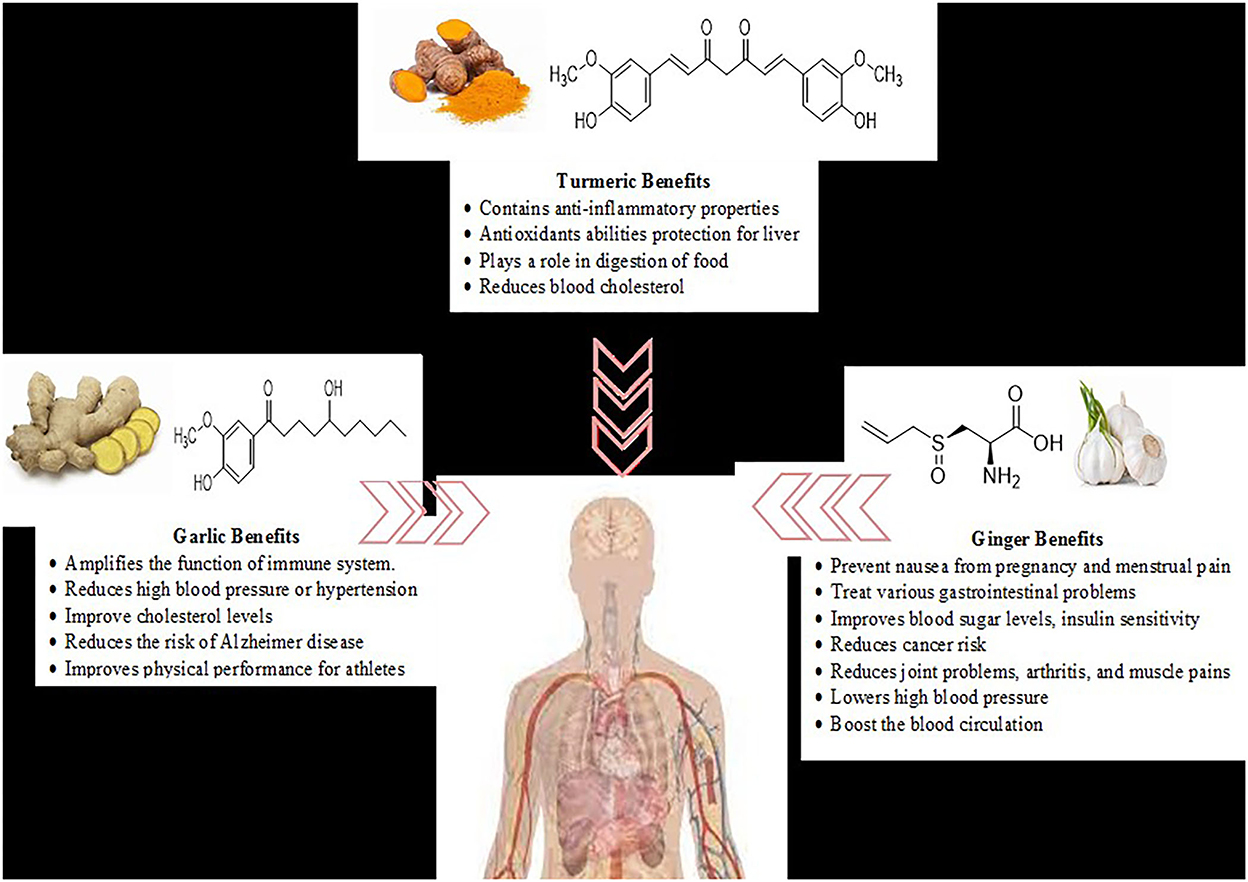
Figure 1. Health-associated benefits of ginger (Zingiber officinale), turmeric (Curcuma longa), and garlic (Allium sativum) as potential nutraceuticals.
Ginger (Zingiber officinale) is a tropical flowering plant that was originally cultivated in southeastern Asia but has now been widely planted and distributed around the world. It is classified as a member of the Zingiberaceae family that produces a cluster of greenish-purple flowers about three feet tall. The root is called a rhizome and has been used partly as a flavor. The ginger flowering plants contain a lot of varieties of around 47 genera, ≥1,000 species with different floral arrangements, and sizes of the rhizome. Some of the varieties of ginger include Crepe Ginger (Costus speciosus), Kahili Ginger (Hedychium gardnerianum), Pineapple Ginger (Tapeinochilos ananassae), Torch Ginger (Etlingera elatior), and White Ginger (Hedychium coronarium) (5, 6).
The annual production of ginger was reported to be about 3.3 million tons in India which accounted for 34% of the global garlic production as shown in Table 1 (6). The ginger rhizome contains 60–70% carbohydrates, 3–8% crude fiber, 9% protein, 8% ash, 3–6% fatty oil, and 2–3% volatile oil, phenolic compounds, and terpenes (7, 8). The terpene components in ginger include zingiberene, beta-bisabolene, alpha-farnesene, beta-sesquiphellandrene, and alpha-curcumene, while phenolic compounds include gingerol, paradol, and shogaol. These gingerols and shogaols are of higher concentration than other compounds (9).
The locals in Nigeria have used ginger over the ages to prevent nausea resulting from pregnancy and menstrual pain (10). It is used to treat various gastrointestinal problems such as upset stomach, diarrhea, dyspepsia (discomfort after eating), morning sickness, bloating, heartburn, and loss of appetite (11). Ginger helps to improve blood sugar levels and insulin sensitivity and reduces cancer risk (12). Its fresh juice is also used locally as an anti-inflammatory agent that helps to reduce joint problems, osteoarthritis, rheumatoid, arthritis, and muscle pains (13). Fresh juice from ginger was also used to treat skin burns (14). The active component of ginger was used as an antacid medication and a laxative. It lowers high blood pressure and boosts the circulation of blood by warming the body (15). This warming effect enables ginger to act as an antiviral for treating flu and cold (16). Chronic inflammation and oxidative stress are key drivers of Alzheimer's disease and the cognitive decline that accompanies age (17). There are suggestions that the bioactive compounds and antioxidants present in ginger help to inhibit inflammatory responses that occur in the brain and have ameliorative effects in folk medicine for the management of various ailments (18).
Zingerone (4(4-hydroxy-3-methoxyphenyl)-2-butanone) is a volatile bioactive compound produced by the degradation of shogaols and gingerols. It consists of a phenolic ring with a methoxy group attached to a benzene ring. This compound exhibits a wide range of pharmacological activities and is relatively non-toxic (19). Zingerone is present in dried ginger and can also be obtained from gingerol through retro aldol reaction (20). Reports from the pharmacokinetic study of zingerone revealed oxidation of side chains in all available sites upon ingestion of zingerone orally or intraperitoneally. Its pharmacological activities consist of anti-cancer, antioxidant, and antimicrobial (21). Fresh ginger was reported to contain some contents of 6-gingerol, 8-gingerol, and 10-gingerol using high-profile liquid chromatography with an increased amount of zingerone upon drying or roasting (22).
Zingiberene was generated through the isoprenoid pathway from farnesyl pyrophosphate (FFP) which was rearranged to form nerolidyl diphosphate. After the pyrophosphate has been removed, the ring is closed, leaving only the tertiary carbon carbocation that is attached to the ring. A more stable allylic carbocation is then generated by the occurrence of a 1,3-hydride shift. The final step involves the removal of the cyclic allylic proton and the subsequent double bond formation. The enzyme responsible for catalyzing this reaction is known as zingiberene synthase and is used in forming other mono- and sesquiterpenes (23).
Turmeric (Curcuma longa) is a flowering plant used as a condiment that belongs to the family Zingiberaceae. The plant grows in Asia, especially in India and Central America. A source of red spice originating from plants which enables it to act as a coloring agent, flavoring agent, and as ingredient for curry powder. Turmeric is of different varieties including Suvana, Sudharsana, Prabha, Suguna, Pragati, Kedaram, and Prathibha. Turmeric is added as a supplement to teas, powder-containing capsules, and extracts (24).
The United State Department of Agriculture (USDA) National Nutrient Database stated that one tablespoon of turmeric powder contains: 29 calories, protein (0.91 g), fat (0.31 g), carbohydrate (6.31 g), fiber (2.1 g), sugar (0.3 g), iron (16%), potassium (5%), vitamin C (3%), and manganese (26%) based on daily human requirements (25). Turmeric contains phytochemical components including diarylheptanoids and curcuminoids such as curcumin, dimethoxycurcumin, and bisdimethoxycurcumin (26, 27). The compounds Turmerone, germacrone, zingiberene, and atlantone generate the essential oils present in turmeric (28).
Turmeric was used in Ayurvedic medicine for different health issues (29). Turmeric containing anti-inflammatory properties helps to reduce the pain of people who are suffering from arthritis. Turmeric has antioxidant abilities that help to protect the liver from being damaged by toxins (30). It adds flavor to food and spice and plays a role in the digestion of food (31). It is an effective aid in reducing blood cholesterol (32). Other possible uses include treatment for cancer, pre-diabetes, tuberculosis, and Alzheimer's disease (33). However, pregnant women should avoid taking turmeric supplements because of their blood-thinning effects (34). The stomach produces more gastric acid due to stimulations from turmeric. It could positively affect the digestive system of some people while others could be negatively affected (35, 36).
Curcumin is the principal curcuminoid found in turmeric. Curcuminoids are diarylheptanoids that consist of curcumin and its various derivatives such as demethoxycurcumin, bis-curcumin, and cyclic curcumin (37). Chemical groups are added to them to make curcumins more soluble which enhances their suitability for drug use. It is a symmetric molecule also called diferuloylmethane (38). The IUPAC name of curcumin is (1E, 6E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione with chemical formula C21H20O6. It consists of three different chemical entities in its structure and they include two aromatic ring systems containing o-methoxy phenolic groups, connected by a seven-carbon linker that consists of an alpha,beta-unsaturated beta-diketone moiety (39).
The diketone group can form stable enols and readily undergo keto-enol tautomerism, which depending on the environment can exist as different types of conformers (40). It exists in a cis-enol configuration at its crystal state which is stabilized by resonance-assisted hydrogen bonding and the structure consists of three substituted planar groups interconnected through two double bonds. Depending on the nature of the solvent, which is mainly polar or non-polar, the enol form is generally more stabilized than the keto form. A pi electron cloud surrounds the molecule because of extended conjugation. Curcumin exists as cis-trans isomers when present in a solution form. In its trans-form, it contains two phenolic methoxy groups on the opposite side of its backbone and is more stabilized than the cis form where the phenolic methoxy groups are on the same side up the backbone. It is sparingly soluble in water and hydrocarbon solvents such as hexane but dissolves readily in polar solvents such as chloroform, acetonitrile, ethyl acetate, ethanol, and methanol (41).
Curcumin has three highly reactive functional groups namely one diketone moiety and two phenolic groups (42). It exhibits various chemical reactions that enhance its bioactivity and they include nucleophilic addition reactions that could be either reversible or irreversible, enzymatic reactions, and hydrogen transfer which leads to the oxidation of curcumin. Curcumin also forms stable complexes with metals and non-metals. It is a monobasic bidentate ligand and acts as an excellent chelating agent (43).
Garlic (Allium sativum) is an important plant from the family Amaryllidaceae. It is a specie in the onion genus Allium. It is grown for its flavorful bulb as shown in Figure 1. It is mainly cultivated in Asia but is also found in several places across Europe. Raw garlic has a pungent taste and its bulb releases a powerful onion-like aroma. It is consumed either as a vegetable raw material or after processing in the form of oil, extract, and even powder, with these different garlic formulations, a discrepancy in the chemical composition and consequently the bioactive content of compounds is observed (44). Garlic varieties are of two major types namely: softneck (Alium sativum) and hardneck (Allium ophioscorodon). Examples of softneck varieties include “applegate,” “Italian purple,” “California late,” “Polish Red,” “Red toch,” “California Early,” “Inchelium red,” “Galiano,” “Polish white,” and “kettle River grant,”. Examples of hardneck varieties include “Chesnok red,” “German white,” “Porcelain,” “Persian star,” and “Purple stripe” (45). The estimated global production for garlic (Allium sativum) was recorded at 26.6 million tons with China accounting for 80% of total production as shown in Table 2 (6).
Garlic is an annual crop and is propagated by planting cloves or top bulbils. The main quality feature of garlic products is the distinct flavor of cloves, because of complex biochemical reactions (46). It comes in different forms such as paste, powder, and extracts. The chemical constituents present in garlic are mainly sulfur-containing, non-volatile amino acids (thiosulfinates), which include alliin or S-allyl-cysteine, ajoene, diallyl polysulfides, vinyldithins, Alliinase, and saponins. A clove (3 g) of raw garlic contains manganese (2%), vitamin B6 (2%), vitamin C (2%), selenium (1%), fiber (0.06 g), and trace amounts of iron, vitamin B1, potassium, calcium, phosphorus, and calcium based on the human daily requirement (25). Diallyl disulfide, diallyl trisulfide, and allyl propyl sulfide are principal components of essential added to food to complement it and have medicinal properties (47). Garlic has a wide range of health benefits to the body system. It is known to amplify the function of the immune system. The active compounds in garlic help to reduce high blood pressure or hypertension and improve cholesterol levels. The antioxidants in garlic support the mechanism provided in the body to prevent health issues because of oxidative stress, Alzheimer's disease, itching, ringworm, and athlete's foot (48, 49).
Organosulfur compounds are the major bioactive compounds found in garlic and they are mainly allicin, allin, and ajoene. Allicin is an oily yellow liquid that provides the unique smell of garlic. Allicin's significance as a biologically active compound is due to its high reactivity with a low and high molecular weight of thiol-containing protein, antioxidant activity, and accessibility due to its high permeability (50). Allicin is formed by enzymatic reactions by the enzyme allinase on allin. It is activated when the bulb of garlic is crushed. The reaction begins with the conversion of cysteine into S-allyl-L-cysteine. This thioether is then oxidized to form a sulfoxide known as allin. Allinase enzyme with the aid of pyridoxal phosphate cleaves allin, and the dehydration of allin occurs. Allin is then converted into allyl sulfenic acid, pyruvic acid, and ammonia. Allyl sulfenic acid is highly reactive at room temperature and the elimination of water occurs. At room temperature, two molecules of allyl sulfenic acid condense and forms allicin (51). Allicin exists mainly as a racemate, although it is chiral. It is unstable and breaks down within 16 h at 23°C. Allicin is decomposed into diallyl disulfide, diallyl trisulfide, vinyldithins, and ajoene. These pungent compounds are metabolized to form allyl methyl sulfide. This allyl methyl sulfide takes several hours to be released to the lungs and the skin; hence, the effect of eating food-containing garlic is felt for a long time. Allicin stability and biological activity is enhanced by the formation of an inclusion complex with cyclodextrins. Ajoene which has a chemical formula C9H14OS3 and an IUPAC name (E)-1-(prop-2-enyldisulfanyl)-3-prop2-enylsulfainylprop-1-ene is formed from allicin decomposition and can be used for a large range of biological activities such as anticancer activity. It consists of E and Z isomers and contains sulfoxide and disulfide functional groups (52).
Bioactive compounds have played a crucial role in improving health and chronic disease prevention. These phenolic compounds including flavonoids, terpenes, curcumin, organosulfur compounds, and various other bioactive compounds present in food serve as antioxidants, anti-inflammatories, and anticancer. The phenolic compounds such as flavonoids present in small quantities in garlic suppress the synthesis of reactive oxygen species, inhibit enzymatic action, and act as chelating agents to prevent the production of free radicals. It was also used to improve antioxidant defenses and the scavenging of reacting oxygen species. The antioxidant activity of these flavonoids is due to their functional group conformational disposition and it involves their configuration, substitution, and the total number of hydroxyl group present (53).
Ginger was discovered to contain active ingredients that combat degenerative, allergic, metabolic, and cardiovascular disorders. Ginger has also been suggested as an anti-diabetic agent; however, it is required that its active constituent's anti-diabetic, anti-hyperlipidemic, and anti-hyperglycemic effects should be assessed. Gingerols present in ginger enhanced the ingestion of glucose in adipocytes of the skeletal muscle (54). This uptake was attained by the rise of GLUT4 expression on the cell membrane and the AMP-activated protein kinase (AMPK) pathway becoming activated leading to the reversion of hyperglycemia and other abnormal metabolic condition from diabetes. The bioactive compounds including the gingerols, shogaols, and paradols showed greater anti-hypoglycemic activity due to the presence of unbranched alkyl chains, unlike zingerone which showed limited activity with various functional pathways affected by these bioactive compounds. In addition, shorter side chains shogaols enhance glucose utilization, and longer chain shogaols impede the accumulation of lipids in adipocytes (55). Paradols are reported to have anti-obesity activities that may be enhanced with increasing chain length through thermogenesis activation in brown adipose tissue in the body (55).
Zingerone has the ability to scavenge reactive oxygen species, free radicals, peroxides, and other oxidants. This allows for high-potential therapeutic candidates for the treatment of various diseases such as Alzheimer's disease, atherosclerosis, and Parkinson's disease (56). Xanthine oxidase functions by producing free radical that causes oxidative damage and zingerone has been discovered to protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. (56). Zingerone also acts as an antidiarrheal by modification of bacteria and the various metabolism in the cell. Ginger supplements aided in the prevention of nausea generated due to chemotherapy (56, 57).
Curcumin extracted from turmeric was reported to possess scavenging activity on reactive oxygen species through the transfer of electrons and protons from the phenolic group. The extended conjugation of curcumin stabilizes the phenoxyl radicals generated by this reaction. The pleiotropic activity of curcumin enables it to interact with the various molecules in the cell, which include DNA, metals, metalloproteins, proteins, and lipids. The phenolic moiety, the keto-enol tautomeric group, and the carbon linkage functional group of curcumin are all involved in various covalent and non-covalent interactions of curcumin with biomolecules. This carbon linkage helps curcumin become more flexible for more productive hydrophobic interaction (58). Curcumin acts as an anti-tumor through its interactions with nucleic acids. From reviews, using yeast RNA and calf thymus DNA, through hydrophobic interactions or hydrogen bonding, curcumin and its derivatives such as dimethoxy curcumin and diacetyl curcumin could bind DNA and RNA. Over the years, there have been many reviews on curcumin metal complexes (58, 59). Curcumin forms stable complexes with most metals and non-metals and as such acts as a chelating agent. The enolic group forms this complex as the proton is substituted by the metal ion. The stability and reactivity of the complexes depend on the metal ion nature as well as the stoichiometric conditions of the reactions and these determine the structure and physical properties of the complexes. Curcumin metal complexes decrease toxicity and they affect the biological reactivity of metals, thereby creating a form of metal-based antioxidants. Alzheimer's disease was found to be greatly curtailed by the regular consumption of curcumin. This was observed by curcumin's lipophilic ability that enabled it to permeate the brain barrier and decrease toxicity in the neurons by chelating toxic metal ions present (59).
Reports indicated that curcumin exhibits anti-inflammatory properties through different mechanisms that involve the reduction of inflammatory transcription factors, redox status, and protein kinases in the nucleus. Curcumin was also reported to help in reducing knee and joint pains for patients of osteoarthritis resulting in the use of curcumin to reduce the circulation of cytokines in the body such as interleukin-6 that play a role in inflammatory pain (60). Curcumin-containing nutraceuticals were also reportedly given to patients with metabolic diseases and were discovered to improve the serum lipid levels of these patients. Curcumin-containing nutraceuticals lower the circulation of C-reactive protein that serves as a biomarker for predicting cardiovascular disease (61). Curcumin and various curcuminoids help to treat various skin diseases with their antimicrobial, antioxidant, anti-inflammatory, and various other wound healing activities (61).
Organosulfur compounds are abundant major bioactive compounds present in garlic. These sulfur-containing compounds such as allin, allicin, ajoene, vinyldithins, and ally sulfides are responsible for its flavor, but they also provide garlic with medicinal properties such as anticancer, antioxidant, antimicrobial, anti-inflammatory, cardioprotective, immunomodulatory, and antidiabetic activities (62). Garlic generally possesses strong defensive mechanisms against various pests and pathogens due to the volatile nature of the bioactive compounds. There were reports on the bioactive properties of organosulfur compounds: Ajoene and ally sulfides contained anticancer compounds along with allicin that obstructs the release of cytokines C by mitochondria, inhibit nitrosamines bioactivation, and serve as a blockage for the proliferation of cancer cells (62). Organosulfur compounds contained antioxidant properties involved in activating radicals, generating interactions with thiol-containing proteins, scavenging hydroxyl radicals, modification of SH-dependent activities, and obstructing the generation of superoxide and NO radicals. Cardio-protective properties were found in ajoene and allicin involving platelet aggregation obstruction, lowering of blood pressure, lipid profile alteration, vasodilatation enhancement, and inhibition of cholesterol biosynthesis (63).
Over the years, ginger was extracted from its rhizome and various active components have been obtained by various methods. The reported extraction methods that gave insights into the physicochemical properties, molecular weight, and antioxidant activity of ginger include ultrasonic-assisted extraction and hot water extraction (64). For example, ginger pomace polysaccharides were characterized by using High-performance Gel Filtration Chromatography (HPGFC), High-Pressure Liquid Chromatography (HPLC), ultraviolet spectra, and infrared spectra purposes (65). The Kjeldahl method, dinitrosalicylic acid colorimetric method, ambient pressure drying, and soxhlet extraction methods were used to obtain protein content, total sugar content, water, and lipid content, respectively. The Folin–Ciocalteu method was used to determine the phenolic compounds while the aluminum chloride colorimetric method was used for flavonoid compounds. The main phenolic concentrations such as 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol were obtained by HPLC. The mobile phase used in this separation involved two solvents that were water and acetonitrile at column temperature maintained at 30°C and 280 nm wavelength as well as HPLC, NMR, or GC/MS method. This HSCCC method generated an extremely pure concentration of 6-gingerol, 8-gingerol, and 10-gingerol and saved less time compared to conventional methods of extraction (66).
Ginger oleoresin and volatile oils were extracted by solvent extraction and steam distillation. The techniques of extraction required multiple unit operations and involved high temperatures, which leads to degradation in its active compounds and yield. Supercritical CO2 can extract compounds without introducing harmful chemicals and there is no form of degradation by heat. Supercritical CO2 can be used to isolate and characterize the various volatile oils present in ginger by fractional separation. Extraction and characterization of 6, 8, 10 gingerols, and 6-shogaols, using supercritical CO2 and HPLC revealed a higher yield of gingerols, zingerones, and alpha-zingiberene active compounds and quality oleoresin in comparison to conventional soxhlet extraction methods (19). Reflux extraction of various active compounds from ginger has been carried out through the process of agitated extraction, ultrasonic and microwave micellar extraction of Zingerone, gingerol, paradol and shogaol coupled with Ultra-High Performance Liquid Chromatography-UV as detector (UHPLC-UV) (67). Comparison between the microwave-assisted micellar extraction with ultrasonic-assisted extraction, soxhlet extraction and other methods revealed that the microwave-assisted micellular extraction coupled with UHPLC-UV detector had a short extraction time and the use of deoxycholic acid sodium salt in aqueous solution is environmentally friendly when compared with organic solvents.
Curcumin, the major bioactive compound generated from turmeric, has been extracted by various means from dried turmeric rhizome (68). The solvent then passes through distillation where oleoresin containing 25–35% coloring matter is yielded with volatile oil and other resinous extracts. The oleoresin is further washed by selective solvents that extract the curcumin pigment from the oleoresin and produces 90% coloring matter content along with a little volatile matter and other dry matter. Common solvents used include isopropanol, ethyl acetate, ethanol, methanol, acetone, and carbon dioxide (69).
The extraction of curcumin oil from turmeric is by distilling fresh rhizomes and leaves with Clevenger-type apparatus for 4 h; the oil obtained is passed through anhydrous sodium sulfate to ensure the oil is dried. Analysis of these oils was either carried out by Gas Chromatography at a temperature range of 70–240°C using helium, nitrogen, or carbon dioxide for separation and with a flame ionization detector (FID) for detection (70). The volatile components were detected by comparison of their retention indices. Chlorinated solvents cannot be used for the extraction of turmeric, as they are deemed harmful to food. Other methods of extracting curcumin such as dipping methods, zone-refining methods, microwave extractions, soxhlet, and ultrasonic extraction. It was concluded that soxhlet, ultrasonic, and microwave extractions are the generally preferred methods for extraction with ultrasonic- and microwave-assisted extraction discovered to show greater improvements than continuous methods (71). Column chromatography is used for the separation of curcumin from the derivatives of curcuminoids such as demethoxycurcumin and bisdimethoxycurcumin. This is done through the adsorption of the mixture by silica gel (stationary phase) with various solvent mixtures such as methanol/chloroform or chloroform/dichloroform acting as the mobile phase. Methods for detecting curcumin include UV absorption detectors, Liquid Chromatography/Mass Spectrometry, and HPLC. Metabolism, biodistribution, and pharmacokinetics have been assessed by detecting curcumin in biofluids using LC/MS and HPLC.
Ultrasonic extraction can generate a high product yield with a reduction in processing time and less solvent consumption. This increase in the product is due to sound waves being propagated in the solvent and the creation of microcavities, which resulted in the reduction of particle size. Bubbles from the cavities are imploded and this leads to high-velocity liquid circulation, collisions of inter-particle, and micro-turbulence formation that lead to an acceleration in diffusion. Curcumin is easily extracted into the extraction medium by the rupturing of the cell wall due to the penetration of ultrasound (72). The ultrasound extraction of natural products indicated that the increase in the yield through the process could be achieved when ultrasound is used in conjunction with other extraction technologies such as microwave and super-crystal extraction, and solid phase extraction is achieved by combining Molecular Imprinted Polymers (MIP) with ultrasound. Molecular Imprinted Polymers is a synthetic sorbent material that is highly porous and contains cavities that selectively bind these molecules and are used for the identification of a single molecule or a group of related molecules (73).
Garlic contains a large number of bioactive compounds that have been reported for medical benefits. Conventional extraction of bioactive compounds from garlic involved the use of polar or non-polar solvents. Bioactive compounds including allicin and allin are major organosulfur compounds that could be extracted using ethanol or water (74).
Extraction of organosulfur compounds from garlic involved incubation of a powdered mixture containing de-ionized water at 25°C, thereby leading to the formation of organosulfur compounds by enzyme-modulated reactions. Another method of extraction of organosulfur compounds is solid-phase micro-extraction coupled with HPLC or GC which involves the partition of the sample matrix bulk contained in a sealed vial and the stationary phase which is a sorption polymer. The ultrasonic-assisted method for extraction of various organosulfur compounds generated a change in the physical and chemical properties of plant materials through the cavitation effect that lead to plant cell disruption, enhancing the release of extractable compounds and mass transport for easy penetration into the sample matrix, thereby enabling solid phase and liquid phase to maintain a high surface area contact. This method was effective in extracting organosulfur compounds, total phenols, and flavonoids, with all compounds except flavonoids dependent on temperature (75).
Another method for extraction of organosulfur compounds is Dispersive Liquid-Liquid Micro-Extraction (DLLME). This method was applied in response to the trends of using more miniature and greener extraction techniques. Solutes which are hydrophobic in nature are present in large quantities in the extraction solvent and they are dispersed into the bulk aqueous solution. Acetonitrile was used as the dispersive solvent because it is miscible in both aqueous and organic phases while chloroform was used as the extractive solvent due to its higher density than water, and after extraction, the HPLC-UV detector analyzes it. This method reduced analysis time, was more friendly to the environment, and was able to extract higher quality organosulfur compounds from garlic (76). The extraction and analysis of bioactive compounds of garlic using supercritical CO2 extraction coupled with supercritical fluid chromatography and mass spectrometry have been carried out (77). This method was generated due to the high lipophilicity of supercritical CO2, which inhibited polar compounds' dissolution. The supercritical CO2 served as the mobile phase in the chromatographic setup. Methanol was added to the supercritical CO2 to enhance the elution capacity of the mobile phase. This method of extraction achieved better separation in less time and bioactive compounds showed acceptable recovery, precision, and sensitivity (78, 79).
The use of the High-performance liquid chromatographic (HPLC) method has been reported as the usual method of analysis for 6-gingerol, 6-shogaol, 8-gingerol, and 10-gingerol extraction and quantification (80). A recent study shows an alcohol-based deep eutectic solvent of 75% v/v composition, ethanol, and water as a suitable method of extraction with high yields of gingerols in water (81) and indicated that gingerol contents were more from the diluted deep eutectic solvents (DES) than the traditional solvents of ethanol and water.
The different extraction techniques for curcuminoids from turmeric indicated a higher yield from microwave-assisted extraction than the conventional soxhlet extraction (82). Different extraction, isolation, and quantification methods of curcumin and its potential application in respiratory diseases such as COVID-19 were considered. Several methods were found for extraction, isolation, and quantification. But the subcritical water extraction method and HPLC are the most effective method with a higher extraction yield (83).
In the extraction of allicin from garlic (84), studied the three methods of immersion, boiling, and ultrasound using water/ethanol solvents for garlic extraction and compared each other in terms of the extraction speed and time, the antioxidant property of the extract, and the quantity of the heat-sensitive active ingredient. The outcome of the study revealed that ultrasonic extraction is a good alternative to traditional extraction methods. The overview of phytochemicals and their respective bioactive components in ginger, turmeric, and garlic are shown in Table 3.
Most of the bioactive compounds in the selected plants have antioxidants and anti-inflammatory properties and as such have a good medicinal ability which tends to protect the human body from free radicals by properly neutralizing the radicals (85). They act by protecting against oxidative stress by reducing the reactive oxygen species which are responsible for lipid peroxidation. It is believed that these are the mechanisms of aging because the reactive oxygen species react with organic substances present in the human body (86).
The anti-inflammatory ability of bioactive compounds is via suppression of major molecules that are known to play a vital role in cell inflation. Bioactive compounds tend to stop this inflammation, and thereby prevent, inhibit, and downregulate the signal pathway in the cancer model, thereby controlling cancer cells' growth and spread (87).
The prevalence of existing diseases and the rising cost of treatment/management demands a more sustainable solution with nutraceuticals in view. The bioactive compounds in ginger, garlic, and turmeric such as polyphenolic compounds, organosulfur compounds, vitamins, carotenes, curcumin, and lycopene provided many natural therapeutic benefits. However, the mode of action and effective strategy employed by these compounds are yet to be properly documented. Furthermore, in-silico studies and clinical trials involving the use of purified bioactive compounds for therapeutic purposes are important to properly situate their usage as nutraceuticals for biomedical applications.
CA, OA, PAt, and PAk: manuscript preparation and editing. SA, YO, PAk, and OO: manuscript reviewing. KA: supervision. All authors read and approved the final manuscript.
The authors hereby appreciate the Covenant University Centre for Research Innovation and Development (CUCRID), Ota, Ogun State, Nigeria for the publication cost.
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sachdeva V, Roy A, Bharadvaja N. Current prospects of nutraceuticals: a review. Curr Pharm Biotechnol. (2020) 21:884–96. doi: 10.2174/1389201021666200130113441
2. Khanal A, Devkota HP, Kaundinnyayana S, Gyawali P, Ananda R, Adhikari R. Culinary herbs and spices in Nepal: a review of their traditional uses, chemical constituents, and pharmacological activities. Ethnobotany Res Appl. (2021) 21:1–18. doi: 10.32859/era.21.40.1-18
3. Prakash D, Gupta C. Phytopharmaceutical applications of nutraceutical and functional foods. In: Complementary and Alternative Medicine: Breakthroughs in Research and Practice. Hershey: IGI Global (2019). p. 182–204. doi: 10.4018/978-1-5225-7039-4.ch008
4. Olaiya CO, Soetan KO, Esan AM. The role of nutraceuticals, functional foods and value-added food products in the prevention and treatment of chronic diseases. Afr J Food Sci. (2016) 10:185–93. doi: 10.5897/AJFS2015.1402
5. Li C, Li J, Jiang F, Tzvetkov NT, Horbanczuk JO Li Y, et al. Vasculoprotective effects of ginger (Zingiber officinale Roscoe) and underlying molecular mechanisms. Food Funct. (2021) 12:1897–913. doi: 10.1039/D0FO02210A
6. FAOSTAT. Production/Crops/World for 2016. Rome: Food and Agricultural Organization of the United Nations Statistics Division (FAOSTAT) (2016).
7. Wannaprasert P, Choenkwan S. Impacts of the COVID-19 pandemic on ginger production: supply chains, labor, and food security in Northeast Thailand. For Soc. (2021) 1:120–35. doi: 10.24259/fs.v5i1.11897
8. Singh R, Singh K. Zingiber officinale: a spice with multiple roles. Res J Life Sci Bioinformat Pharm Chem Sci. (2019) 5:113–25. doi: 10.26479/2019.0502.09
9. Aleem M, Khan MI, Shakshaz FA, Akbari N, Anwar D. Botany, phytochemistry and antimicrobial activity of ginger (Zingiber officinale): a review. Int J Herbal Med. (2020) 8:36–49. doi: 10.22271/flora.2020.v8.i6a.705
10. Dafam DG, Denou A, Idoko A, Jimam NS, Okwori VA, Ohemu TL, et al. Use of herbal medicine during pregnancy and attitudes of pregnant women in Jos, Nigeria. J Pharm Bioresour. (2021) 18:77–86. doi: 10.4314/jpb.v18i1.9
11. Islam MM, Manik MIN, Zobayed A, Noor F. A review on medicinal properties of some commonly used culinary agents of Bangladesh. J Med Plants. (2021) 9:111–7.
12. Anh NH, Kim SJ, Long NP, Min JE, Yoon YC, Lee EG, et al. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients. (2020) 12:157. doi: 10.3390/nu12010157
13. Razavi BM, Ghasemzadeh RM, Hosseinzadeh H, A. review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytotherapy Res. (2021) 35:6489–513. doi: 10.1002/ptr.7224
14. Jahan F, Happy AA, Chowdhury MH, Hossain MA. Natural herbs and spices: A great resource for skin care cosmetics. J Plant Sci. (2019) 7:86–99. doi: 10.11648/j.jps.20190704.13
15. Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. (2006) 185:S1–24. doi: 10.5694/j.1326-5377.2006.tb00548.x
16. Farzaei MH, Bahramsoltani R, Abbasabadi Z, Braidy N, Nabavi SM. Role of green tea catechins in prevention of age-related cognitive decline: pharmacological targets and clinical perspective. J Cell Physiol. (2019) 234:2447–59. doi: 10.1002/jcp.27289
17. Kiyama R. Nutritional implications of ginger: chemistry, biological activities, and signaling pathways. J Nutr Biochem. (2020) 86:108486. doi: 10.1016/j.jnutbio.2020.108486
18. Okesola MA, Ogunlana, O, Afolabi I, Onasanya A. Ameliorative Effect of Zingiber officinale on Chemical Induced DNA Damage in Rats Using PCR Analysis. Biointerface Res Appl Chem. (2021) 11:11135–144. doi: 10.33263/BRIAC114.1113511144
19. Dalsasso RR, Valencia GA, Monteiro AR. Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res Int. (2022) 154:111043. doi: 10.1016/j.foodres.2022.111043
20. Geng X, Liu H, Yuwen Q, Wang J, Zhang S, Zhang X, et al. Protective effects of zingerone on high cholesterol diet-induced atherosclerosis through lipid regulatory signaling pathway. Hum Exp Toxicol. (2021) 40:1732–745. doi: 10.1177/09603271211006170
21. Xue G, Su S, Yan P, Shang J, Wang J, Yan C, et al. Quality control of Zingiberis rhizoma and its processed products by UHPLC-Q-TOF/MS-based non-targeted metabonomics combining with SIBDV method. Food Res Int. (2022) 154:111021. doi: 10.1016/j.foodres.2022.111021
22. Durairaj J, Di Girolamo A, Bouwmeester HJ, de Ridder D, Beekwilder J, van Dijk AD. An analysis of characterized plant sesquiterpene synthases. Phytochemistry. (2019) 158:157–65. doi: 10.1016/j.phytochem.2018.10.020
23. Fordjour E, Mensah EO, Hao Y, Yang Y, Liu X, Li Y, et al. Toward improved terpenoids biosynthesis: strategies to enhance the capabilities of cell factories. Bioresour Bioprocess. (2022) 9:1–33. doi: 10.1186/s40643-022-00493-8
24. Idowu-Adebayo F, Toohey MJ, Fogliano V, Linnemann AR. Enriching street-vended zobo (Hibiscus sabdariffa) drink with turmeric (Curcuma longa) to increase its health-supporting properties. Food Funct. (2021) 12:761–70. doi: 10.1039/D0FO02888F
25. Restrepo-Osorio J, Nobile-Correa DP, Zuñiga, O, Sánchez-Andica RA. Determination of nutritional value of turmeric flour and the antioxidant activity of Curcuma longa rhizome extracts from agroecological and conventional crops of Valle del Cauca-Colombia. Rev Colomb Química. (2020) 49:26–32. doi: 10.15446/rev.colomb.quim.v1n49.79334
26. Pagano E, Souto EB, Durazzo A, Sharifi-Rad J, Lucarini M, Souto SB, et al. Ginger (Zingiber officinale Roscoe) as a nutraceutical: Focus on the metabolic, analgesic, and anti-inflammatoryanti-inflammatory effects. Phytotherapy Res. (2021) 35:2403–17. doi: 10.1002/ptr.6964
27. Venkatakrishnan K, Chiu HF, Wang CK. Extensive review of popular functional foods and nutraceuticals against obesity and its related complications with a special focus on randomized clinical trials. Food Funct. (2019) 10:2313–29. doi: 10.1039/C9FO00293F
28. Ray A, Mohanty S, Jena S, Sahoo A, Acharya L, Panda PC, et al. Drying methods affects physicochemical characteristics, essential oil yield and volatile composition of turmeric (Curcuma longa L). J Appl Res Med AromatPlants. (2022) 26:100357. doi: 10.1016/j.jarmap.2021.100357
29. Dosoky NS, Setzer WN. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients. (2018) 10:1196. doi: 10.3390/nu10091196
30. Memarzia A, Khazdair MR, Behrouz S, Gholamnezhad Z, Jafarnezhad M, Saadat S, et al. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. Biofactors. (2021) 47:311–50. doi: 10.1002/biof.1716
31. Shah M, Murad W, Mubin S, Ullah O, Rehman NU, Rahman M, et al. Multiple health benefits of curcumin and its therapeutic potential. Environ Sci Pollut Res. (2022) 29, 43732–43744. doi: 10.1007/s11356-022-20137-w
32. Rehman U, Syed QA, Asghar HA, Arshad MK, Sultan G, Asghar, et al. Alimentary and recuperative prospective of curcuma longa (Turmeric) Scholars. Int J Biochem. (2022) 5:67–75. doi: 10.36348/sijb.2022.v05i05.001
33. Bhatt T, Patel K. Carotenoids: potent to prevent diseases review. Nat Products Bioprospect. (2020) 10:109–17. doi: 10.1007/s13659-020-00244-2
34. Timba PP, Giri SG, Panchal RV. Health benefits and possible risks of turmeric, garlic and ginger: a short. Health. (2019) 6:4656–9. doi: 10.22270/jddt.v12i3-s.5496
35. Kwiecien S, Magierowski M, Majka J, Ptak-Belowska A, Wojcik D, Sliwowski Z, et al. Curcumin: a potent protectant against esophageal and gastric disorders. International Journal of Molecular Sciences. (2019) 20:1477–91. doi: 10.3390/ijms20061477
36. Edwards RL, Luis PB, Nakashima F, Kunihiro AG, Presley SH, Funk JL, et al. Mechanistic differences in the inhibition of NF-κB by turmeric and its curcuminoid constituents. J Agric Food Chem. (2020) 68:6154–60. doi: 10.1021/acs.jafc.0c02607
37. Lim J, Nguyen TH, Pal K, Kang CG, Park C, Kim SW, et al. Phytochemical properties and functional characteristics of wild turmeric (Curcuma aromatica) fermented with Rhizopus oligosporus. Food Chem X. (2022) 13:100198. doi: 10.1016/j.fochx.2021.100198
38. Noureddin SA, El-Shishtawy RM, Al-Footy KO. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur J Med Chem. (2019) 182:111631. doi: 10.1016/j.ejmech.2019.111631
39. Abadeh ZA, Saviano G, Ballirano P, Santonicola, MG. Curcumin-loaded zeolite as anticancer drug carrier: effect of curcumin adsorption on zeolite structure. Pure Appl Chem. (2020) 92:461–71. doi: 10.1515/pac-2018-1213
40. Attia S, Schmidt MC, Schröder C, Weber J, Baumann AK, Schauermann S. Keto–Enol Tautomerization as a First step in hydrogenation of carbonyl compounds. J Phys Chem C. (2019) 123:29271–77. doi: 10.1021/acs.jpcc.9b10181
41. Yang D, Liu M, Xiao X, Tao Z, Redshaw C. Polymeric self-assembled cucurbit[n]urils: synthesis, structures and applications. Coord Chem Rev. (2021) 434:213733. doi: 10.1016/j.ccr.2020.213733
42. Jiang T, Liao W, Charcosset C. Recent advances in encapsulation of curcumin in nanoemulsions: a review of encapsulation technologies, bioaccessibility and applications. Food Res Int. (2020) 132:109035. doi: 10.1016/j.foodres.2020.109035
43. Tijani K, Alfa A, Sezor A. Studies on phytochemical, nutraceutical profiles and potential medicinal values of Allium sativum Linn (Liliaceae) on bacterial meningitis. Int Neuropsych Dis J. (2019) 13:1–15. doi: 10.9734/indj/2019/v13i230105
44. Izol E, Temel H, Yilmaz MA, Yener I, Olmez OT, Kaplaner E, et al. A detailed chemical and biological investigation of twelve Allium species from Eastern Anatolia with chemometric studies. Chem Biodivers. (2021) 18:e2000560. doi: 10.1002/cbdv.202000560
45. Ghoran SH, Rahimi H, Kazemi A, Scognamiglio M, Naderian M, Iraji A, et al. Allium hooshidaryae (Alliaceae); Chemical compositions, biological and ethnomedicine uses. J Ethnopharmacol. (2021) 274:113918. doi: 10.1016/j.jep.2021.113918
46. Aghajanzadeh TA, Reich M, Hawkesford MJ, Burow M. Sulfur metabolism in Allium cepa is hardly affected by chloride and sulfate salinity. Arch Agron Soil Sci. (2019) 65:945–56. doi: 10.1080/03650340.2018.1540037
47. Ezeorba TPC, Chukwudozie KI, Ezema CA, Anaduaka EG, Nweze EJ, Okeke ES. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol Res Mod Chin Med. (2022) 3, 100075. doi: 10.1016/j.prmcm.2022.100075
48. Saif S, Hanif MA, Rehman R, Riaz M. Garlic. In: Medicinal Plants of South Asia. Amsterdam: Elsevier (2020). p. 301–15. doi: 10.1016/B978-0-08-102659-5.00023-9
49. Dokunmu TM, Yakubu OF, Adebayo AH, Olasehinde GI, Chinedu SN. Cardiovascular risk factors in a suburban community in Nigeria. Int J Hypertens. (2018) 1, 6. doi: 10.1155/2018/6898527
50. Leontiev R, Hohaus N, Jacob C, Gruhlke MC, Slusarenko AJ, A. comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci Rep. (2018) 8:1–19. doi: 10.1038/s41598-018-25154-9
51. Catanzaro E, Canistro D, Pellicioni V, Vivarelli F, Fimognari C. Anticancer potential of allicin: a review. Pharmacol Res. (2022) 177, 106118. doi: 10.1016/j.phrs.2022.106118
52. Zhou Y, Feng J, Peng H, Guo T, Xiao J, Zhu W, et al. Allicin inclusions with α-cyclodextrin effectively masking its odor: Preparation, characterisation, and olfactory and gustatory evaluation. J Food Sci. (2021) 86:4026–36. doi: 10.1111/1750-3841.15882
53. Dhalaria R, Verma R, Kumar D, Puri S, Tapwal A, Kumar V, et al. Bioactive compounds of edible fruits with their anti-aging properties: a comprehensive review to prolong human life. Antioxidants. (2020) 9:1123. doi: 10.3390/antiox9111123
54. Azeez TB, Lunghar J. Anti-inflammatory effects of turmeric (Curcuma longa) and ginger (Zingiber officinale). Inflamm Nat Prod. (2021) 127-146. doi: 10.1016/B978-0-12-819218-4.00011-0
55. Tramontin NDS, Luciano TF, Marques SDO, de Souza CT, Muller AP. Ginger and avocado as nutraceuticals for obesity and its comorbidities. Phytotherapy Res. (2020) 34:1282–290. doi: 10.1002/ptr.6619
56. Wei CK, Tsai YH, Korinek M, Hung PH, El-Shazly M, Cheng YB, et al. 6-paradol and 6-shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-paradol reduces blood glucose in high-fat diet-fed mice. Int J Mol Sci. (2017) 18:168–86. doi: 10.3390/ijms18010168
57. Ma RH Ni ZJ, Zhu YY, Thakur K, Zhang F, Zhang YY, et al. A recent update on the multifaceted health benefits associated with ginger and its bioactive components. Food Funct. (2021) 12:519–42. doi: 10.1039/D0FO02834G
58. Wu B, Liu X, Shi X, Han W, Wang C, Jiang L. Highly photoluminescent and temperature-sensitive P, N, B-co-doped carbon quantum dots and their highly sensitive recognition for curcumin. RSC Adv. (2019) 9:8340–9. doi: 10.1039/C9RA00183B
59. Amadi CN, Offor SJ, Frazzoli C, Orisakwe OE. Natural antidotes and management of metal toxicity. Environ Sci Pollut Res. (2019) 26:18032–52. doi: 10.1007/s11356-019-05104-2
60. Abdel-Rahman RF, Abd-Elsalam RM, Amer MS, El-Desoky AM, Mohamed SO. Manjarix attenuated pain and joint swelling in a rat model of monosodium iodoacetate-induced osteoarthritis. Food Funct. (2020) 11:7960–72. doi: 10.1039/D0FO01297A
61. Mirsafaei L, Reiner Ž, Shafabakhsh R, Asemi Z. Molecular and biological functions of quercetin as a natural solution for cardiovascular disease prevention and treatment. Plant Foods Hum Nutr. (2020) 75:307–15. doi: 10.1007/s11130-020-00832-0
62. Ribeiro M, Alvarenga L, Cardozo LF, Chermut TR, Sequeira J, Moreira LD, et al. From the distinctive smell to therapeutic effects: garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin Nutr. (2021) 40:4807–19. doi: 10.1016/j.clnu.2021.03.005
63. Walag AMP, Ahmed O, Jeevanandam J, Akram M, Ephraim-Emmanuel BC, Egbuna C, et al. Health benefits of organosulfur compounds. In: Functional Foods and Nutraceuticals. New York, NY; Cham: Springer (2020). p. 445–72.
64. Imran A, Quispe C, Zeeshan A, Imran M, Nadeem M, Gilani SA, et al. Development and antioxidant characterisation of ginger-mint drink prepared through different extraction techniques. J Food Meas Character. (2021) 15:2576–90. doi: 10.1007/s11694-021-00843-8
65. Wu Q, Li F, Zhu X, Ahn Y, Zhu Y. Isolation and characterisation of cyromazine degrading Acinetobacter sp. ZX01 from a Chinese ginger cultivated soil. Environ Sci Pollut Res. (2022) 29, 1–11. doi: 10.1007/s11356-022-20538-x
66. Chiriac ER, Chitescu CL, Geană EI, Gird CE, Socoteanu RP, Boscencu R. Advanced analytical approaches for the analysis of polyphenols in plants matrices—a review. Separations. (2021) 8:65. doi: 10.3390/separations8050065
67. Wang CX, Wang LX Li CY, Hu C, Zhao SH. Anti-proliferation activities of three bioactive components purified by high-speed counter-current chromatography in essential oil from ginger. Eur Food Res Technol. (2020) 246:795–805. doi: 10.1007/s00217-020-03446-7
68. Goëlo V, Chaumun M, Gonçalves A, Estevinho BN, Rocha F. Polysaccharide-based delivery systems for curcumin and turmeric powder encapsulation using a spray-drying process. Powder Technol. (2020) 370:137–46. doi: 10.1016/j.powtec.2020.05.016
69. Rahim AF, Jamaluddin N, Kadri A, Idris SS, Abd Rahman N. Microwave-assisted hydrodistillation of Aquilaria subintegra. J Adv Res Fluid Mech Therm Sci. (2020) 72:1–12. doi: 10.37934/arfmts.72.2.112
70. Obode CO, Adebayo AH, Li C. Gas chromatography-mass spectrometry analysis and in vitro inhibitory effects of Phoenix dactylifera L. on key enzymes implicated in hypertension. J Pharm Pharmacogn Res. (2020) 8:475–90. Available online at: https://jppres.com/jppres/pdf/vol8/jppres20.838_8.5.475
71. da Rosa GS, Vanga SK, Gariepy Y, Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L). Innovat Food Sci Emerg Technol. (2019) 58:102234. doi: 10.1016/j.ifset.2019.102234
72. Bachtler S, Bart HJ. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave-assisted extraction technologies. Food Bioprod Process. (2021) 125:1–13. doi: 10.1016/j.fbp.2020.10.009
73. Singla M, Sit N. Application of ultrasound in combination with other technologies in food processing: a review. Ultrason Sonochem. (2021) 73:105506. doi: 10.1016/j.ultsonch.2021.105506
74. Putnik P, Gabrić D, Roohinejad S, Barba FJ, Granato D, Mallikarjunan K, et al. An overview of organosulfur compounds from Allium spp: from processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. (2019) 276:680–91. doi: 10.1016/j.foodchem.2018.10.068
75. Kumar RB, Varma RK, Sen S, Oruganti S. “Head-space miniaturization techniques,” in Emerging Freshwater Pollutants. Amsterdam: Elsevier (2022). p. 95–116. doi: 10.1016/B978-0-12-822850-0.00018-1
76. Li J, Dadmohammadi Y, Abbaspourrad A. Flavor components, precursors, formation mechanisms, production and characterisation methods: garlic, onion, and chili pepper flavors. Crit Rev Food Sci Nutr. (2021) 62, 1–23. doi: 10.1080/10408398.2021.1926906
77. Essien SO, Young B, Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci Technol. (2020) 97:156–69. doi: 10.1016/j.tifs.2020.01.014
78. Usman I, Hussain M, Imran A, Afzaal M, Saeed F, Javed M, et al. Traditional and innovative approaches for the extraction of bioactive compounds. Int J Food Prop. (2022) 25:1215–33. doi: 10.1080/10942912.2022.2074030
79. Lesellier E, West C. Supercritical fluid chromatography for the analysis of natural dyes: from carotenoids to flavonoids. J Sep Sci. (2022) 45:382–93. doi: 10.1002/jssc.202100567
80. Promdam N, Panichayupakaranant P. Quantitative HPLC method and alternative green solvents for extraction of [6]-gingerol from ginger. Pak J Pharm Sci. (2022) 35:851–7.
81. Hsieh YH Li Y, Pan Z, Chen Z, Lu J, Yuan J, et al. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason Sonochem. (2020) 63:104915. doi: 10.1016/j.ultsonch.2019.104915
82. Zielińska A, Alves H, Marques V, Durazzo A, Lucarini M, Alves TF, et al. Properties, extraction methods, and delivery systems for curcumin as a natural source of beneficial health effects. Medicina. (2020) 56:336. doi: 10.3390/medicina56070336
83. Tripathy S, Verma DK, Thakur M, Patel AR, Srivastav PP, Singh S, et al. Curcumin extraction, isolation, quantification and its application in functional foods: a review with a focus on immune enhancement activities and COVID-19. Front Nutr. (2021) 8:747956. doi: 10.3389/fnut.2021.747956
84. Loghmanifar S, Nasiraie LR, Nouri H, Jafarian S. Effects of different extraction methods on antioxidant properties and Allicin content of garlic. J Food Sci Hyg. (2020) 1:16–25.
85. Yu C, Yang B, Najafi M. Targeting of cancer cell death mechanisms by curcumin: implications to cancer therapy. Basic Clin Pharmacol Toxicol. (2021) 129:397–415. doi: 10.1111/bcpt.13648
86. Wang L, Shen J, Li N, Zhang Y, Hu F, Dai H, et al. Curcumin affects Parkinson protein 7 (PARK7; DJ-1) expression and regulates proliferation and apoptosis of breast cancer cells by up-regulating miR-203. J Biomater Tissue Eng. (2021) 11:2484–90. doi: 10.1166/jbt.2021.2851
87. Mathur S, Pareek S, Verma R, Shrivastava D, Bisen PS. Therapeutic potential of ginger bio-active compounds in gastrointestinal cancer therapy: the molecular mechanism. Nutrire. (2022) 47:1–17. doi: 10.1186/s41110-022-00166-8
88. Stoner GD. Ginger: is it ready for prime time? Cancer Prev Res. (2013) 6:257–62. doi: 10.1158/1940-6207.CAPR-13-0055
89. Ji K, Fang L, Zhao H, Li Q, Shi Y, Xu C, et al. Ginger oleoresin alleviated γ-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human mesenchymal stem cells. Oxid Med Cell Longevity. (2017) 1480294. doi: 10.1155/2017/1480294
Keywords: ginger, garlic, nutraceuticals, nutrition, therapeutics, turmeric
Citation: Ajanaku CO, Ademosun OT, Atohengbe PO, Ajayi SO, Obafemi YD, Owolabi OA, Akinduti PA and Ajanaku KO (2022) Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 9:1012023. doi: 10.3389/fnut.2022.1012023
Received: 04 August 2022; Accepted: 08 November 2022;
Published: 08 December 2022.
Edited by:
Marilena Antunes-Ricardo, Tecnologico de Monterrey, MexicoReviewed by:
J. Fernando Ayala-Zavala, Consejo Nacional de Ciencia y Tecnología (CONACYT), MexicoCopyright © 2022 Ajanaku, Ademosun, Atohengbe, Ajayi, Obafemi, Owolabi, Akinduti and Ajanaku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kolawole Oluseyi Ajanaku, a29sYS5hamFuYWt1QGNvdmVuYW50dW5pdmVyc2l0eS5lZHUubmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.