
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 13 December 2022
Sec. Nutrition and Sustainable Diets
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1009807
This article is part of the Research Topic Dietary Change Strategies for Sustainable Diets and their Impact on Human Health - Volume 1 View all 44 articles
 Anwar Ali1
Anwar Ali1 Liang Yu2*
Liang Yu2* Safura Kousar3
Safura Kousar3 Waseem Khalid3
Waseem Khalid3 Zahra Maqbool3
Zahra Maqbool3 Afifa Aziz3
Afifa Aziz3 Muhammad Sajid Arshad3
Muhammad Sajid Arshad3 Rana Muhammad Aadil4
Rana Muhammad Aadil4 Monica Trif5
Monica Trif5 Sakhawat Riaz6,7
Sakhawat Riaz6,7 Horia Shaukat1
Horia Shaukat1 Muhammad Faisal Manzoor8,9*
Muhammad Faisal Manzoor8,9* Hong Qin1*
Hong Qin1*Crocin is a bioactive compound that naturally occurs in some medicinal plants, especially saffron and gardenia fruit. Different conventional and novel methods are used for its extraction. Due to some control conditions, recent methods such as ultrasonic extraction, supercritical fluid extraction, enzyme-associated extraction, microwave extraction, and pulsed electric field extraction are widely used because these methods give more yield and efficiency. Crocin is incorporated into different food products to make functional foods. However, it can also aid in the stability of food products. Due to its ability to protect against brain diseases, the demand for crocin has been rising in the pharmaceutical industry. It also contain antioxidant, anti-inflammatory, anticancer and antidepressant qualities. This review aims to describe crocin and its role in developing functional food, extraction, and bioavailability in various brain-related diseases. The results of the literature strongly support the importance of crocin against various diseases and its use in making different functional foods.
Crocins, a series of polyene dicarboxylic acid, mono and di-glucosyl esters of crocetin, are the major colors causing compounds of saffron and gardenia. In China, the contents of gardenia fruits are used as herbal remedies and natural colors (1). Other plants, such as Buddleja spp., also generate crocins, but because of their low concentration, they are not commercially utilized (2). Crocin is a chemical diester composed of the dicarboxylic acid crocetin and disaccharide gentiobiose (3). Crocins are crocetin glycosyl esters generated via the esterification of crocetin with various glycosides, including geometric isomers (3). The activity of glucosyltransferases causes the transfer of crocetin molecules, which add varying amounts of glycosidic to yield crocins, a primary component of saffron that confers water solubility (4). Crocins are responsible for many of this valuable plant's pharmacologic effects (5). Crocins, unlike other carotenoids, have 20 carbons and several glycosides. Several earlier studies demonstrated that crocins, particularly alpha crocin, had radical quenching, antioxidant, and anti-inflammatory properties (6).
Crocin (mono- or di-glycosyl polyene esters) is a key bioactive component in saffron that dissolves easily in water and produces a distinctive red color, making saffron a natural coloring compound (7). Trans-crocetin di-(-D-gentiobiosyl) ester, crocin1 is the most prevalent crocin, with a golden-yellow tint. Crocin has the highest recorded absorbance at 440 nm (8). Because of its limited stability, crocins lose its functionality when exposed to light, heat, and acidic nature (9). Also have low immersion and bioavailability, as it is un-absorbed if taken orally till hydrolyzed to produce deglycosylated trans-crocetin in the intestinal tract by enzymatic conditioning in the intestinal epithelial cells and by the fecal bacteria (9).
Crocin has several pharmacological actions, including anti-inflammatory and cancer cell growth inhibitor properties (10–12). Under various experimental settings, crocin has also been shown to protect against oxidative damage to brain vasculature, renal tissues, the heart, and the retina (3). In addition to their anti-hypertensive, anti-platelet aggregation, nephron-protective effects, anti-depressant, and anti-atherosclerotic, these phytoconstituents are radical scavengers, particularly against superoxide anions (13). Many people are afflicted with neurodegenerative disorders such as epilepsy, Parkinson's, and Alzheimer's, with increasing age being the primary risk factor (14). The naturally occurring carotenoid molecule, crocin, has been shown to offer therapeutic potential in treating neurological disorders (15). Crocin also increases dopamine in the brain during Parkinson's disease. As a result, this chemical has been demonstrated to be a promising treatment option for neurodegenerative diseases (16). According to randomized, double-blind, placebo-controlled experiments, crocin medicinal levels do not harm the body. In a double blind randomized clinical trial it was found that crocin (20 mg/day) is safe in healthy volunteers, with no notable changes in hematological, hormonal, biochemical, or urine markers (17). This review aims to describe the role of crocin in developing functional food, extraction and critically evaluate prior and current findings on the biological/pharmacological actions of crocin against various brain related diseases.
The crocins are a class of hydrophilic carotenoids that are either mono- or di-glycosyl polyene esters of crocetin in which D-gentiobiose and/or D-glucose appear as carbohydrate residues (18). A carotenoid with a 20-carbon dicarboxylic structure. Saffron contains a variety of carotenoid chemicals, containing trace levels of zeaxanthin, alpha and beta carotene, and lycopene (19).
Six different forms of the crocin family's glycosyl esters have been found in saffron. Trans-crocins 3 and 4 are the most prevalent of the crocin analogs, which include crocins 1–4 and are virtually glycosides of trans-crocetin in saffron. These crocins range in concentration in Spanish saffron between 0.01 and 9.44 percent and 0.46 and 12.12 percent, respectively, whereas cis-crocetin and its glycosides are minor constituents (20). Except for crocin-1, all crocin derivatives are said to exist as pairs of cis-trans isomers. Trans-crocins undergo photoisomerization events and change to cis-crocins, according to a research by (21); this process is reliant on the agro-ecological circumstances in the region of the plant's origin.
Due to its high water solubility, crocin, also known as alltrans crocetin di-b-D-gentiobiosyl ester, has the best coloring capacity when compared to the other carotenoides of saffron. Crocin, which is also soluble in methanol and ethanol, is considered as the preferred water-soluble food additive because of its ability to quench free radicals and possess tumor-fighting characteristics. Structure of crocetin and its glycosides are presented in (Figure 1).
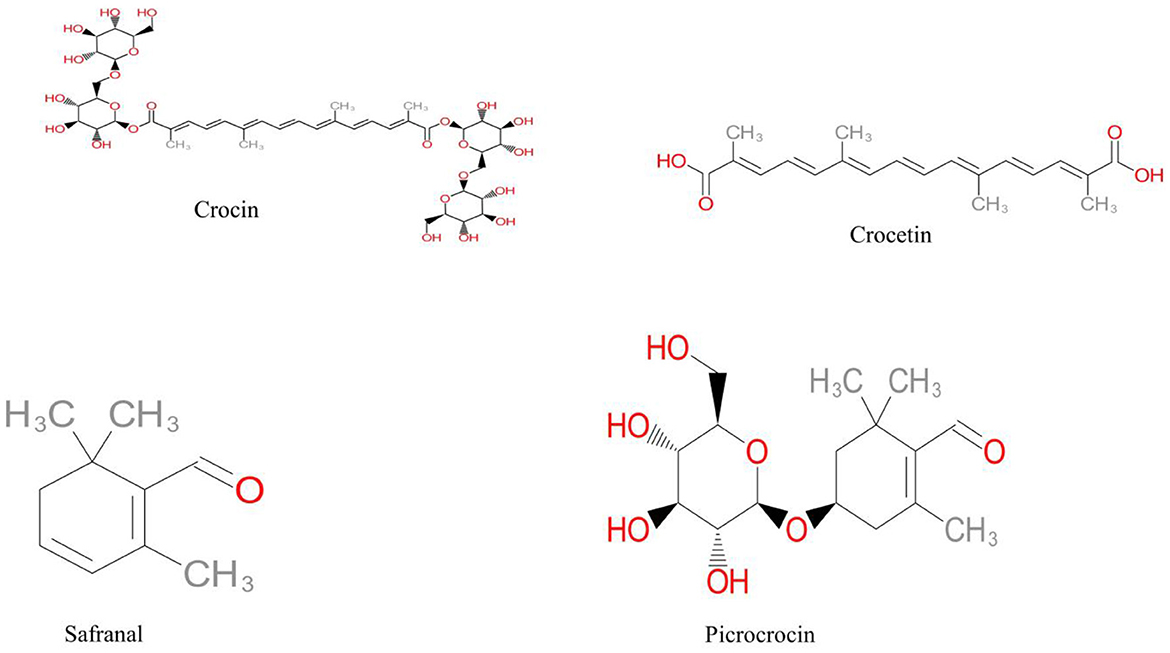
Figure 1. Chemical structure of crocin, dicarboxylic acid (crocetin), monoterpen aldehyde (safranal) and glycoside (picrocrocin).
Crocin is the pigment that gives saffron its color (22). It is also taken from the fruits of the gardenia (23). It occurs as a red powder as a solid, yet it gives a yellow color to dishes (24). crocin as a spice produced by the s Crocus Sativus L. is produced primarily in Western Asia, with Iran being the world's largest producer, but it is also economically significant in huge sections of Mediterranean Europe (25). The bitter flavor of a saffron spice is attributed to the monoterpene glycoside picrocrocin, whereas the scent is attributed to the aglycone safranal (26). The closed flowers of saffron are hand-picked in the early morning to ensure greater resistance to the degenerative processes of the floral organs and obtain a spice with high qualitative traits (25). A mechanical system can also do harvesting with two primary parts: the first detaches the corolla from the stem, and the second gathers the removed flower using a vacuum collector (25). It takes 370–470 h to make 1 kg of dried saffron through manual plucking (27). Using high-performance liquid chromatography (HPLC), the principal features of the saffron extract related to crocins and safranal concentration were identified (28).
Gardenia is an evergreen shrub commonly used in landscaping with characteristics like sweet, softly aromatic blossoms (29). It is a well-known fruit for ayurvedic purposes in China due to its chilly and bitter characteristics (30). Therefore, medicinal importance of this shrub includes curing stomach aches and hepatic, as well as treat diuretic, anti-phlogistic, choleretic, laxative, and homeostatic qualities (30). Also, it is used to obtain yellow color since it contains crocin and crocetin, primary plant carotenoid constituents (30).
The derivatives derived from G. jasminoides are less poisonous, less allergenic, and more environmentally friendly than saffron (31). A homogenate extraction procedure was used to extract crocin from G. jasminoides (32, 33). Another work used ethanol/water cold percolation to extract crocin from G. jasminoides without affecting percolation (34). The Microwave-assisted extraction (MAE) method boosted the extraction yield of crocin from G. jasminoides' edible yellow pigment by 50% over the usual extraction method (35).
Crocin microbial production has attracted significant interest recently, but its implementation is limited as per the literature studies (36). Carotenoids such as astaxanthin, lycopene, and carotene have been genetically engineered into Escherichia coli (E. coli). As a result, experiments were done to develop E. coli cell factories that could produce crocin. E. coli has a distinctive genetic make-up, expands quickly under simple culture conditions, and is capable of a variety of well-known large-scale fermentation processes. The YL4 and YL5 strains are the ones that start crocin synthesis. Finally, the researchers were able to establish strains that produced crocetin and crocin-5 by integrating and optimizing the expression of the heterologous genes (37).
Extracting bioactive components from saffron necessitates ongoing research into environmentally and economically viable extraction strategies (38, 39). Traditional extraction procedures are time-consuming and necessitate a large amount of solvent (40). As a result, novel extraction strategies for extracting bioactive components from saffron have been devised, reducing extraction time and solvent usage while improving extract extraction yield and quality (38). Several approaches have been developed to extract bioactive components from saffron with maximum extraction and purity efficiency (38). It is confirmed that, compared to traditional procedures, the targeted bioactive components can be extracted more efficiently in terms of solvent volume and extraction time by employing the right extraction method (41–43). According to El Asbahani et al. (44), the extraction method should be chosen based on the desired bioactive component, heat sensitivity, tissue complexity, etc. Conventional extraction processes (soxhlet extraction, maceration, solvent extraction vapor, or hydro-distillation) are generally non-selective, require a high volume of organic solvents, and require longer extraction times in certain cases, damage heat-sensitive bioactive chemicals (16, 45). These current extraction processes, known as “green methods,” are environmentally friendly, safer, faster, more efficient, and more precise (46). Green approaches include several extraction techniques, including membrane and emulsion liquid ultrasonic extraction, supercritical fluid extraction, enzyme-associated extraction, microwave extraction, and pulsed electric field extraction (41, 47). These approaches can efficiently extract saffron bioactive components. In general, the efficiency of extraction procedures is primarily determined by the selection of appropriate solvents, taking into account solvent-solute affinity and the employment of coextraction techniques (48). To get saffron bioactive components such as crocin, picrocrocin, and safranal, a wide range of solvents, such as water and organic solvents, and their combinations have been utilized (49). In general, fewer polar chemicals (safranal) are extracted with carbon dioxide, whereas initially, polar substances (crocin, crocetin) are extracted with organic solvents (e.g., ethanol) (50). Mohajeri et al. (51) demonstrated the extraction of crocin from saffron using molecularly imprinted polymer methods. Recent research on saffron used MAE to extract several bioactive components (picrocrocin, safranal, and crocin). The components' contents were determined spectrophotometrically at 257, 330, and 440 nm (the peak absorbance values of picrocrocin, safranal and crocin), respectively (52). The extraction and purification techniques depend on obtaining any important elements such as bioactive chemicals (crocins, crocetin, safranal, and picrocrocins) naturally found in plants. An effective bioactive extraction process should fulfill green chemistry standards such as safety, environmental friendliness, low or no contaminants, efficiency, and economics (41, 53). Saffron's key bioactive components—picrocrocin, safranal, and crocin—were extracted using subcritical water. The response surface approach was used to study the effect of extraction time (5–15 min) and temperature (105–125°C) on process efficiency. The crystallization process was used to recover complete crocin from saffron stigmas. The optimal extraction solvent was determined to be 80% ethanol. At different temperatures, the crystallization process was carried out in one and two. As a crystallization media, 80% ethanol was employed. Crocin crystals were obtained with low purity from 1st crystallization as compared to 2nd crystallization produced pure crystals at a low temperature (−5°C) quantified using UV-visible spectrophotometer and HPLC followed by Fluka product and saffron extraction using methanol (54, 55). The results showed that its purity was ~13 times greater than the Fluka products. Despite our expectations, the Fluka product was not a pure alpha-crocin sample; its chromatogram revealed five forms of crocins and an unknown impurity (54). The purity of total crystalline crocin was >97% (54). Crocin can only be split into seven fractions using this approach. The approach also necessitates time-consuming multiple treatments before pure crocin can be extracted. Table 1 shows the conventional and novel extraction methods of crocin.
Saffron is a major source of various bioactive compounds, including crocins, picrocrocin, and safranal (49). In various food products, such as bakeries and beverages, the stigma of saffron is widely used as a coloring agent and an aroma (73). Studies also show that different parts of the saffron plant are used in the development of functional food products (74). The development of functional cookies enriched with the stigma of saffron showed distinct attributes like good sensory scores, increased shelf life, and high antioxidant activities (75). Different beverages enriched with saffron petals demonstrate increased antioxidant properties after fermentation (76). Various products are available in the market encapsulated with bioactive compounds commonly found in the stigma and petals of saffron (41). The enrichment of saffron bioactive ingredients is done on a large scale. The saffron-enriched pasta products exhibit a low glycemic index due to resistant starch digestibility (77). It was also observed that the crocin-encapsulated tablet decreased the glycemic index due to reduce amylase activity in healthy people (78). Saffron carotenoids and crocin are used as coloring agents. Various food dishes are prepared with crocin, like the low kebab in Iran, pulao in India, and paella rice in Spain (Table 2).
The bioactive compounds of saffron are commonly used to develop various functional food products, including dairy products (88). Moreover, in dairy products, various types of cheese are developed that are enriched with saffron (89). Various cheeses, including Luneberg cheese, Pecorino allo Zafferano and Piacentinu Ennese cheese, are obtained from different animal milk sources like Austrian cow's milk, Italian sheep's milk, and Sicily sheep's milk. Enriching saffron ingredients like crocin in sheep's milk influenced physicochemical properties (90). It was evident from studies that cheese enriched with saffron showed more distinct properties than others (91). This type of cheese is yellowish in color having good elasticity and microbiologically more stable (134). A group of researchers examined the effect of saffron-rich cheese in different aspects, including physicochemical properties, sensory attributes, color, and antioxidant activities. No significant change occurred in all properties, but the saffron-enriched product's antioxidant activity and antimicrobial capacity increased (91).
In dessert products, two types of dessert products, cheesecake incorporated with saffron and orange jam; the other white chocolate soup incorporated with saffron and yogurt, were evaluated in various physicochemical properties (92). A standardized level of crocin was incorporated in both types of desserts. It was concluded that the dessert incorporated with crocin had increased consumer acceptability and precise control dosage compared to dessert incorporated with saffron stigma (135). Several factors like easily water-soluble, undesirable fibers, and the small size of powder saffron do not affect the properties of the desired product. The saffron extract is considered more valuable due to its uniform color intensity and no need for preheating treatments (9).
The applications of saffron in cereal products are very effective in reducing disease risk and improving the health status of the modern-age population (93). The incorporation of saffron bioactive compounds, especially crocin, in cereal products is examined in several aspects like physicochemical properties, color, texture, and sensory attributes (94). The pasta incorporated with saffron showed distinct variations in different properties, including aroma, taste, color, gumminess, hardness, chewiness, and overall acceptability (95). It was evident from the DPPH and ABTS assays that saffron-incorporated pasta showed high antioxidant properties (95). Several studies showed that the water uptake during the cooking process of pasta incorporated with a high amount of saffron is reduced due to non-soluble compounds found in saffron that are responsible for inhibiting the diffusion of water in the gluten matrix (96).
Saffron extract is widely used in the beverage industry to prepare alcoholic and non-alcoholic beverages, herbal teas, vermouth, and several bitter drinks (97). In beverages, the bitterness due to saffron extract is a limited aspect of consumers' acceptance (98). It was observed that the phenolic content in the herbal tea blend improves the bioaccessibility of crocin by reducing the crocin oxidation during the digestion process. The previous studies showed the bioavailability and bioaccessibility of saffron carotenoids in beverage industries (99).
Crocin is a natural neuroprotective molecule with anti-depressant properties and potential use in treating neuropsychological problems (100). Crocin has been discovered to reduce beta-amyloid aggregation, a key step in Alzheimer's disease pathogenesis. Crocin helps those with chronic obstructive pulmonary disease and lipopolysaccharide who are depressed. Crocin can also act as an anti-inflammatory agent (101). Figure 2 presents the biological activity of crocin in different brain-related disorders. The pharmacological potential of crocin in brain-related diseases is shown in Table 3.
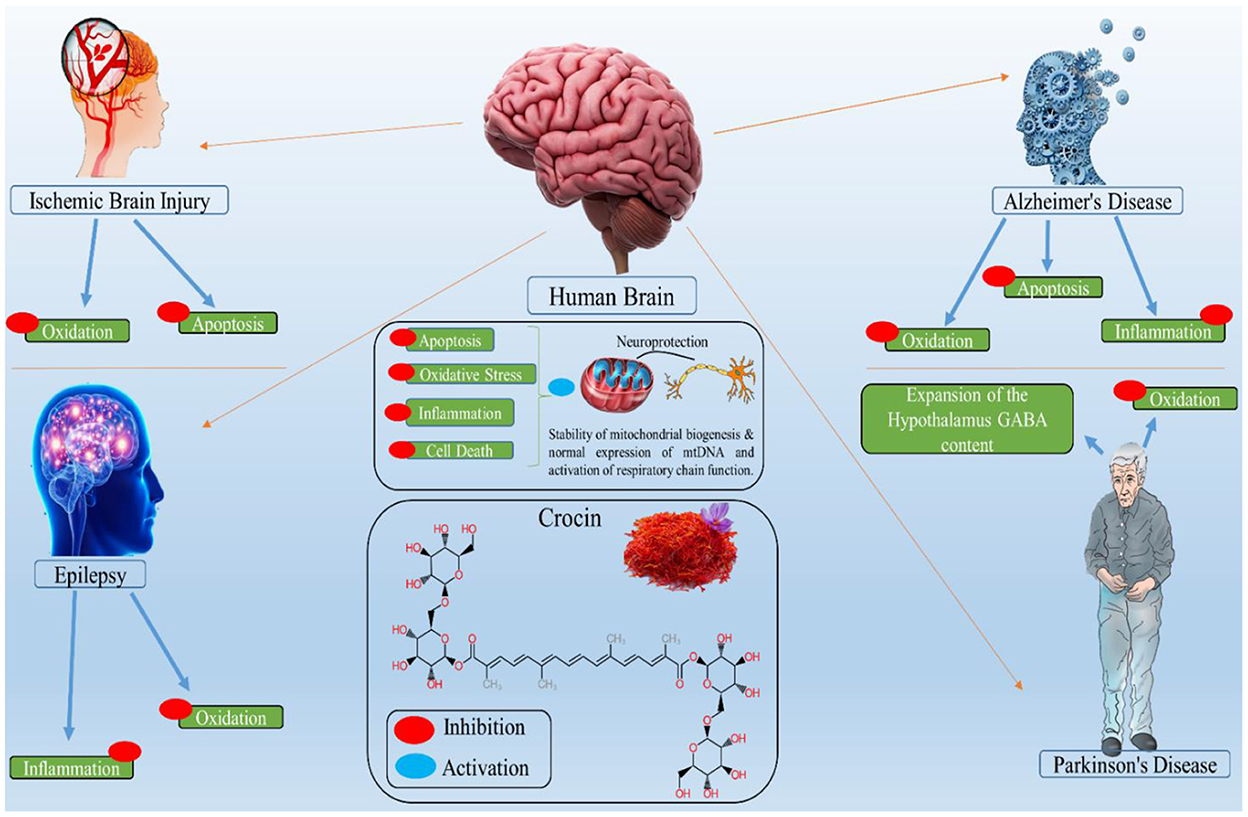
Figure 2. Biological activity of crocin in different brain related disorders, where the red circle shows the inhibitory property of crocin and blue circle shows the activation.
Alzheimer's is a neurodegenerative disease that causes mental capacity development and disrupts neurocognitive functions (109). Neurodegeneration, neuronal loss, and the formation of neurofibrillary tangles and Ab plaques are all signs and symptoms of this neuropathological disorder (109). Alzheimer's disease is the major cause of dementia in people over 60. Alzheimer's disease affects between 50 and 75% of patients with dementia (109). There is a lack of a logical chronological order of events in Alzheimer's disease and acceptable and effective treatment (110). The interaction of Ab protein oligomers with glial cells and neurons causes a variety of pathological and physiological abnormalities, including mitochondrial dysfunction, pro-inflammatory cascade stimulation, increased tau phosphorylation and oxidative stress, calcium metabolism deregulation, increased glycogen synthase kinase (GSK)-3b activity, cell death stimulation, and neuronal apoptosis (111, 112).
Alzheimer's disease is a difficult illness to prevent and treat because of its complex pathophysiology (113). Herbal compounds have been proposed as potential anti-Alzheimer's agents (114). Crocin, the primary component extracted from Crocus sativus L, has various pharmacological effects, including anti-apoptotic activity. Crocin, the primary component of Crocus sativus L. extract, is a yellow carotenoid with anti-inflammatory, anti-depressant, and memory-improving qualities, as well as various pharmacological activities, including anti-apoptotic capabilities (115).
Crocin's neuroprotective actions boost memory by scavenging free radicals, reducing the synthesis of peroxidized membrane lipids, and reestablishing SOD activity, reducing ROS and AGEs while lowering MDA levels and increasing GPx activity. Crocin's antioxidant effect effectively protects cerebrocortical and hippocampal neurons from ischemia, improving spatial cognitive abilities. Crocin modulated Mitogen-Activated Protein Kinase, which suppressed A concentration (MAPK) and tau phosphorylation, reducing acrolein-induced oxidative pressure. Acrolein has been linked to the development of Alzheimer's disease. In rats, acrolein at 3 mg/kg/day p.o. lowers Glutathione (GSH) levels, increases MDA, A, and Pt in the brain, and activates MAPK signaling pathways (16).
Parkinson's disease (PD) is the most prevalent neurological disorder. It is a progressive neurological disease that primarily affects the elderly (116). Anxiety, depression, sleep problems, and cognitive modifiability are common neuropsychiatric diseases in people with Parkinson's disease. For people with Parkinson's disease, these disturbances often create greater difficulty and distress than the disease's motor symptoms. Depression is the most frequent neuropsychiatric symptom in Parkinson's disease, with up to 50% of PD patients suffering from this psychiatric illness. The loss of nigrostriatal dopamine (DA) neurons is a characteristic symptom of Parkinson's disease (101). Development of filamentous, cytoplasmic inclusions, primary aggregations of synuclein as Lewy bodies (LB) or neurites is a pathological characteristic of PD. Fibrillization and Synuclein phosphorylation lead to the development of LB and neuron death. In Parkinson's patients, synuclein aggregates have been observed in the dorsal motor nucleus (DMN), vagus nerve, spinal sympathetic epicardium nerves, and preganglionic neurons. In 60% of PD patients with bladder hypersensitivity, urinary tract abnormalities are identified, resulting in voiding urgency, incontinence, and frequency (117).
Furthermore, it has been demonstrated that crocin treatment can inhibit AChE activation from increasing. As a result of these qualities, we chose to test crocin's neuroprotective effects against dopaminergic neuron damage and PD consequences in a model (mouse model) of this disease through 1-methyl4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Crocin also lowers depressive-like symptoms such as anxiety in adult male rats exposed to teen stress and dendritic remodeling in the PFC (prefrontal cortex). Crocin has been demonstrated as effective for disease in several investigations. Crocin also enhanced aversive memory in a Parkinson's patient model based on 6-hydroxydopamine. These findings show that crocin could be a novel contender for Parkinson's disease and depression treatment (101).
Crocin has also been shown to lower the amount of -synuclein in rats with rotenone-induced Parkinson's disease. As a result, the protective impact of crocin (10, 20, 40 mg/kg, 28 days, i.p.) on oxidative stress, malathion-induced Parkinson's disease, and inflammation in the rat striatum were examined (118).
In another animal model of disease caused due to MPTP, crocin treatment was found to reduce motor deficits and preserve dopaminergic neurons and by blocking the opening of the mitochondrial permeability transition pore (mPTP) protect against mitochondrial dysfunction (119).
Hypoxic-ischemic brain damage could lead to morbidity and mortality among all age groups. One of the most common causes of infant brain damage is hypoxic-ischemic (HI) injury. In the United States, 1–4 neonatal HI brain injury occurrences occur per 1,000 live births, accounting for around one-fourth of all neonatal deaths globally. Intrauterine hypoxia related to circulation issues, such as placental abruption, placental artery clotting, and inflammatory processes, is the most common cause of neonatal hypoxic brain injury (120). Using these models, researchers have discovered numerous distinct characteristics of neonatal HI brain injury, which may be related to the nervous system's immaturity. Around birth, antioxidant enzymes (e.g., copper-zinc superoxide dismutase and glutathione peroxidase) have a restricted action in the young brain. As a result, oxidative damage induced by HI injury is more likely in the infant's brain (121).
Crocin is an active ingredient isolated from saffron with anti-inflammatory, antioxidant, and neuroprotective effects. According to a previous study, crocin pretreatment reduced cerebral edema and enhanced functional outcomes in a mouse model of traumatic brain injury. Crocin was also reported to reduce brain infarct volume in the rat ischemia-reperfusion paradigm. It is unknown whether crocin has a neuroprotective effect on HIE (122).
Crocin's therapeutic impact in reducing blood brain barrier (BBB) disruption was investigated. Twenty-four-month-old rats were administered either vehicle (controls) or crocin (10, 20, 40, or 60 mg/kg) every other day for 2 months before ischemia induction. In the presence of cerebral ischemia, Crocin preserved BBB function. In addition, the crocin-treated group had increased NADPH oxidase. The antioxidant ability of crocin was shown in these experiments to help minimize the damage induced by ischemia (123).
Epilepsy, a neurological disorder marked by recurring seizures, is frequently linked to earlier nervous system abnormalities. Epilepsy is caused by a disruption in the regulation of inflammatory cell activation and resolution in injured neuronal tissue. However, this imbalanced inflammatory regulation that contributes to epilepsy is still unknown (124). Epileptic convulsions are due to disruptions in the physiology of the brain. Abnormalities in the membrane properties of neurons, decreased inhibition of neurotransmission (by gamma-aminobutyric acid, GABA), changes in the ionic microenvironment surrounding the neuron, or increased excitatory neurotransmission (by the acidic amino acid glutamate) are all causes of epileptic seizures. In all ionotropic glutamate receptors, the intake of sodium and the outflow of potassium ions by the channels can depolarize the membrane by forming the action potential. In the resting state, Mg++ ions block the Ca++ channel in NMDA receptors, depolarizing the local membrane; channels change to permeable for calcium ions with the shifts of Mg++. In high neuronal activation (e.g., status epilepticus), Ca++ inflow can cause cell depolarization to play a part in Ca++-mediated neuronal damage, leading to cell death (125).
Because epilepsy involves complicated neural pathogenic pathways, multi-targeted pharmacological treatments have been proposed for its complete care. It is been studied extensively in animal models of neurological disorders, including depression, epilepsy, anxiety, and memory. Crocin's efficacy in neurological diseases characterized by aberrant central excitatory and inhibitory nature shows that it interacts with various neuronal pathophysiological pathways (126).
Crocin has an unsettling effect on the cell reinforcement framework, resulting in increased ROS production and subsequent ROS-induced mitochondrial malfunction, frequently found following seizures and throughout epileptogenesis. Its antioxidant properties also aid crocin's antiepileptic properties. Antiepileptic medicines improve GABA-mediated inhibition and raise GABA levels in the brain (16). Crocin infusions in penicillin-activated epileptiform action in rats resulted in antiepileptic effects. Crocin's antiepileptic effect is due to its ability to increase the tone of inhibitory neurotransmitters by increasing the working of GABA (A)-benzodiazepine. Crocin stimulates glutamic acid decarboxylase, an important enzyme for GABA synthesis, greatly increasing the hypothalamic GABA concentration in rats (127).
The present study is deliberate to measure the effects of crocin extract in functional foods and its pharmacological properties against various disorders. Crocin is a biologically active substance in the stigma of saffron and fruit of guardian. These bioactive substance can be extracted utilizing conventional (solvent extraction, soxhlet extraction, vapor or hydro distillation, and maceration), and novel techniques (supercritical fluid extraction, microwave-assisted extraction, ultrasound-assisted extraction, pulsed electric field extraction, emulsion liquid membrane extraction and enzyme-associated extraction). In various food products, such as bakeries and beverages, the stigma of saffron is widely used as a coloring agent and an aroma. The antioxidant profile of crocin, may inhibit the oxidation process in different foods. The development of baking products, beverages, dairy products, dessert and cereal products are enriched with the stigma of saffron showed distinct attributes (sensory scores, good shelf life, antioxidant activity). These products having saffron in them have very effective in reducing disease risk and improving the health status of the population. Pharmacologically, crocin may be helpful in different brain-related disorders, including Alzheimer's, Parkinson's, Ischemic brain injury, and Epilepsy.
AAl, SK, WK, and MM: conceptualization. AAl, SK, WK, ZM, AAz, and MA: writing-original draft preparation. RA, MT, LY, HQ, SR, HS, and MM: writing-review and editing. LY, HQ, and MM: supervision. All authors have read and agreed to the published version of the manuscript.
This work was funded by Hunan Provincial Natural Science Foundation of China (2022JJ30192).
The authors pay special thanks to Food and Nutrition Society Gilgit Baltistan Pakistan for giving help to access different journals.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pan Y, Zhao X, Wang Y, Tan J, Chen D-X. Metabolomics integrated with transcriptomics reveals the distribution of iridoid and crocin metabolic flux in Gardenia jasminoides Ellis. PLoS ONE. (2021) 16:e0256802. doi: 10.1371/journal.pone.0256802
2. Martí M, Diretto G, Aragonés V, Frusciante S, Ahrazem O, Gómez-Gómez L, et al. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab Eng. (2020) 61:238–50. doi: 10.1016/j.ymben.2020.06.009
3. Cerdá-Bernad D, Valero-Cases E, Pastor J-J, Frutos MJ. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit Rev Food Sci Nutr. (2022) 62:3232–49. doi: 10.1080/10408398.2020.1864279
4. Moratalla-López N, Bagur MJ, Lorenzo C, Martínez-Navarro M, Salinas MR, Alonso GL. Bioactivity and bioavailability of the major metabolites of crocus sativus L. Flower Molecules. (2019) 24:2827. doi: 10.3390/molecules24152827
5. Dhiman N, Kharkwal H. Biosynthesis and Derivatization of the Major Phytoconstituents of Saffron. Saffron: Elsevier (2020). p. 83–92.
6. Abedimanesh N, Motlagh B, Abedimanesh S, Bathaie SZ, Separham A, Ostadrahimi A. Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF-κB, and MCP-1 in patients with coronary artery disease: a randomized placebo-controlled clinical trial. Phytother Res. (2020) 34:1114–22. doi: 10.1002/ptr.6580
7. Cid-Pérez TS, Nevárez-Moorillón GV, Ochoa-Velasco CE, Navarro-Cruz AR, Hernández-Carranza P, Avila-Sosa R. The relation between drying conditions and the development of volatile compounds in saffron (Crocus sativus). Molecules. (2021) 26:6954. doi: 10.3390/molecules26226954
8. Mirhadi E, Nassirli H, Malaekeh-Nikouei B. An updated review on therapeutic effects of nanoparticle-based formulations of saffron components (safranal, crocin, and crocetin). J Pharmaceutical Invest. (2020) 50:47–58. doi: 10.1007/s40005-019-00435-1
9. Allahdad Z, Khammari A, Karami L, Ghasemi A, Sirotkin VA, Haertlé T, et al. Binding studies of crocin to β-Lactoglobulin and its impacts on both components. Food Hydrocoll. (2020) 108:106003. doi: 10.1016/j.foodhyd.2020.106003
10. Ali A, Mughal H, Ahmad N, Babar Q, Saeed A, Khalid W, et al. Novel therapeutic drug strategies to tackle immune-oncological challenges faced by cancer patients during COVID-19. Exp Rev Anticancer Ther. (2021) 21:1371–83. doi: 10.1080/14737140.2021.1991317
11. Ali A, Manzoor MF, Ahmad N, Aadil RM, Qin H, Siddique R, et al. The burden of cancer, government strategic policies, and challenges in pakistan: a comprehensive review. Front Nutr. (2022) 9:1–17. doi: 10.3389/fnut.2022.940514
12. Bastani S, Vahedian V, Rashidi M, Mir A, Mirzaei S, Alipourfard I, et al. An evaluation on potential anti-oxidant and anti-inflammatory effects of crocin. Biomed Pharmacother. (2022) 153:113297. doi: 10.1016/j.biopha.2022.113297
13. Shahidani S, Rajaei Z, Alaei H. Pretreatment with crocin along with treadmill exercise ameliorates motor and memory deficits in hemiparkinsonian rats by anti-inflammatory and antioxidant mechanisms. Metab Brain Dis. (2019) 34:459–68. doi: 10.1007/s11011-018-0379-z
14. Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. (2020) 19:255–65. doi: 10.1016/S1474-4422(19)30411-9
15. Cho KS, Shin M, Kim S, Lee SB. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Med Cell Long. (2018) 2018:1–13. doi: 10.1155/2018/4120458
16. Ahmed S, Hasan MM, Heydari M, Rauf A, Bawazeer S, Abu-Izneid T, et al. Therapeutic potentials of crocin in medication of neurological disorders. Food Chem Toxicol. (2020) 145:111739. doi: 10.1016/j.fct.2020.111739
17. Kazemi F, Vosough I, Sepahi S, Mohajeri SA. Effect of crocin versus fluoxetine in treatment of mild to moderate obsessive-compulsive disorder: a double blind randomized clinical trial. Human Psychopharmacol. (2021) 36:e2780. doi: 10.1002/hup.2780
18. Amanpour A, Kelebek H, Selli S. GLC/HPLC methods for Saffron (L.). Bioactive Mol Food. (2019) 1987–2035. doi: 10.1007/978-3-319-78030-6_42
19. Bathaie SZ, Farajzade A, Hoshyar R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotechnic Histochem. (2014) 89:401–11. doi: 10.3109/10520295.2014.890741
20. Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. (2014) 64:65–80. doi: 10.1016/j.fct.2013.11.016
21. Ni Y, Li L, Zhang W, Lu D, Zang C, Zhang D, et al. Discovery and LC-MS characterization of new crocins in gardeniae fructus and their neuroprotective potential. J Agric Food Chem. (2017) 65:2936–46. doi: 10.1021/acs.jafc.6b03866
22. Hussein RA, Salih NA, Eman Thabit N. Bioactivity of crocin pigment of saffron plant. Plant Arch. (2018) 18:357–64. Available online at: http://plantarchives.org/PDF%20181/357-364__PA3_4074_.pdf
23. Huang H, Zhu Y, Fu X, Zou Y, Li Q, Luo Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. (2022) 380:132216. doi: 10.1016/j.foodchem.2022.132216
24. Singla RK, Bhat G. Crocin: an overview. Indo Global J Pharmaceut Sci. (2011) 1:281–6. doi: 10.35652/IGJPS.2011.27
25. Zeka K, Ruparelia KC, Continenza MA, Stagos D, Vegliò F, Arroo RR. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia. (2015) 107:128–34. doi: 10.1016/j.fitote.2015.05.014
26. Catinella G, Borgonovo G, Dallavalle S, Contente ML, Pinto A. From saffron residues to natural safranal: valorization of waste through a β-glucosidase. Food Bioprod Proc. (2022) 131:144–8. doi: 10.1016/j.fbp.2021.11.002
27. Cardone L, Castronuovo D, Perniola M, Cicco N, Candido V. Saffron (Crocus sativus L.). the king of spices: an overview. Sci Horticult. (2020) 272:109560. doi: 10.1016/j.scienta.2020.109560
28. Mashmoul M, Azlan A, Yusof BNM, Khaza'ai H, Mohtarrudin N, Boroushaki MT. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J Funct Foods. (2014) 8:180–7. doi: 10.1016/j.jff.2014.03.017
29. Garrett H. Shrubs and “Sort of” Shrubs. Plants for Houston and the Gulf Coast. Austin, TX: University of Texas Press (2021). p. 75–100.
30. Defilipps RA, Krupnick GA. The medicinal plants of Myanmar. PhytoKeys. (2018) 1:1–341. doi: 10.3897/phytokeys.102.24380
31. Hashemi M, Hosseinzadeh H. A comprehensive review on biological activities and toxicology of crocetin. Food Chem Toxicol. (2019) 130:44–60. doi: 10.1016/j.fct.2019.05.017
32. Liqin T, Haocheng L, Jing W, Yujuan X, Wenni T, Lu L, et al. Study on ultrahigh-pressure extraction technology on properties of yellow extract from gardenia fruit. J Food Composit Analysis. (2021) 104:104186. doi: 10.1016/j.jfca.2021.104186
33. Ali A, Riaz S, Sameen A, Naumovski N, Iqbal MW, Rehman A, et al. The disposition of bioactive compounds from fruit waste, their extraction, and analysis using novel technologies: a review. Processes. (2022) 10:2014. doi: 10.3390/pr10102014
34. Prado JM, Veggi PC, Náthia-Neves G, Meireles MA. Extraction methods for obtaining natural blue colorants. Curr Analyt Chem. (2020) 16:504–32. doi: 10.2174/1573411014666181115125740
35. Qi Y, Zhang H, Liang S, Chen J, Yan X, Duan Z, et al. Ispitivanje antidepresivnog učinka funkcionalnog napitka s aktivnim peptidima, mentolom i eleuterozidom, te mehanizmi njegovog djelovanja u mišjem modelu. Food Technol Biotechnol. (2020) 58:295–302. doi: 10.17113/ftb.58.03.20.6568
36. Kupnik K, PrimoŽič M, Kokol V, Leitgeb M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers. (2020) 12:2825. doi: 10.3390/polym12122825
37. Wang W, He P, Zhao D, Ye L, Dai L, Zhang X, et al. Construction of Escherichia coli cell factories for crocin biosynthesis. Microb Cell Fact. (2019) 18:1–11. doi: 10.1186/s12934-019-1166-1
38. Rahaman A, Kumari A, Farooq MA, Zeng X-A, Hassan S, Khalifa I, et al. Novel extraction techniques: an effective way to retrieve the bioactive compounds from saffron (Crocus Sativus). Food Rev Int. (2021) 10:1–29. doi: 10.1080/87559129.2021.1967377
39. Manzoor MF, Hussain A, Naumovski N, Ranjha MM, Ahmad N, Karrar E, et al. A narrative review of recent advances in rapid assessment of anthocyanins in agricultural and food products. Front Nutr. (2022) 9:1–14. doi: 10.3389/fnut.2022.901342
40. Garcia-Vaquero M, Rajauria G, Tiwari B. Conventional extraction techniques: solvent extraction. Sust Seaweed Technol. (2020) 171–89. doi: 10.1016/B978-0-12-817943-7.00006-8
41. Garavand F, Rahaee S, Vahedikia N, Jafari SM. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci Technol. (2019) 89:26–44. doi: 10.1016/j.tifs.2019.05.005
42. Vernès L, Vian M, Chemat F. Ultrasound and microwave as green tools for solid-liquid extraction. Liquid Phase Extract. (2020) 355–74. doi: 10.1016/B978-0-12-816911-7.00012-8
43. Manzoor MF, Hussain A, Sameen A, Sahar A, Khan S, Siddique R, et al. Novel extraction, rapid assessment and bioavailability improvement of quercetin: a review. Ultrason Sonochem. (2021) 78:105686. doi: 10.1016/j.ultsonch.2021.105686
44. Asbahani A, Miladi K, Badri W, Sala M, Addi EA, Casabianca H, et al. Essential oils: From extraction to encapsulation. Int J Pharm. (2015) 483:220. doi: 10.1016/j.ijpharm.2014.12.069
45. Manzoor MF, Ahmad N, Ahmed Z, Siddique R, Zeng XA, Rahaman A, et al. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J Food Biochem. (2019) 43:e12974. doi: 10.1111/jfbc.12974
46. Lee KX, Shameli K, Yew YP, Teow S-Y, Jahangirian H, Rafiee-Moghaddam R, et al. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int J Nanomed. (2020) 15:275. doi: 10.2147/IJN.S233789
47. Manzoor MF, Zeng X-A, Rahaman A, Siddeeg A, Aadil RM, Ahmed Z, et al. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J Food Sci Technol. (2019) 56:2355–64. doi: 10.1007/s13197-019-03627-7
48. O'sullivan A, O'callaghan Y, O'grady M, Hayes, M, Kerry J, O'brien N. The effect of solvents on the antioxidant activity in Caco-2 cells of Irish brown seaweed extracts prepared using accelerated solvent extraction (ASE®). J Funct Foods. (2013) 5:940–8. doi: 10.1016/j.jff.2013.02.007
49. Sarfarazi M, Jafari SM, Rajabzadeh G. Extraction optimization of saffron nutraceuticals through response surface methodology. Food Anal Methods. (2015) 8:2273–85. doi: 10.1007/s12161-014-9995-3
50. Goleroudbary MG, Ghoreishi S. Response surface optimization of safranal and crocin extraction from crocus sativus L. via supercritical fluid technology. J Supercrit Fluids. (2016) 108:136–44. doi: 10.1016/j.supflu.2015.10.024
51. Mohajeri SA, Hosseinzadeh H, Keyhanfar F, Aghamohammadian J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J Sep Sci. (2010) 33:2302–9. doi: 10.1002/jssc.201000183
52. Sarfarazi M, Jafari SM, Rajabzadeh G, Galanakis CM. Evaluation of microwave-assisted extraction technology for separation of bioactive components of saffron (Crocus sativus L.). Industrial Crops Prod. (2020) 145:111978. doi: 10.1016/j.indcrop.2019.111978
53. Manzoor MF, Hussain A, Tazeddinova D, Abylgazinova A, Xu B. Assessing the nutritional-value-based therapeutic potentials and non-destructive approaches for mulberry fruit assessment: an overview computational. Intellig Neurosci. (2022) 2022:1–16. doi: 10.1155/2022/6531483
54. Hadizadeh F, Mohajeri S, Seifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pakistan J Biol Sci. (2010) 13:691–8. doi: 10.3923/pjbs.2010.691.698
55. Manzoor MF, Xu B, Khan S, Shukat R, Ahmad N, Imran M, et al. Impact of high-intensity thermosonication treatment on spinach juice: bioactive compounds, rheological, microbial, and enzymatic activities. Ultrason Sonochem. (2021) 78:105740. doi: 10.1016/j.ultsonch.2021.105740
56. Pham TQ, Cormier F, Farnworth E, Tong VH, Van Calsteren M-R. Antioxidant properties of crocin from Gardenia jasminoides ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J Agric Food Chem. (2000) 48:1455–61. doi: 10.1021/jf991263j
57. Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. (2007) 100:1126–31. doi: 10.1016/j.foodchem.2005.11.020
58. Bakshi H, Sam S, Rozati R, Sultan P, Islam T, Rathore B, et al. DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev. (2010) 11:675–9. Available online at: http://journal.waocp.org/article_25263.html
59. Loskutov AV, Hong W-P, Sink KC. Biotechnology for the production of crocin in callus cultures of Gardenia jasminoides Ellis. Plant Biol. (2000) 2:161–5. Available online at: https://www.researchgate.net/profile/Andrey-Loskutov/publication/232318146_Biotechnology_for_the_production_of_crocin_in_callus_cultures_of_Gardenia_jasminoides_Ellis/links/0fcfd5081ed3a1232c000000/Biotechnology-for-the-production-of-crocin-in-callus-cultures-of-Gardenia-jasminoides-Ellis.pdf
60. Yang B, Liu X, Gao Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innovat Food Sci Emerg Technol. (2009) 10:610–5. doi: 10.1016/j.ifset.2009.03.003
61. Iborra JOL, Castellar MR, Cánovas MA, Manjón AR. TLC preparative purification of picrocrocin, HTCC and crocin from saffron. J Food Sci. (1992) 57:714–6. doi: 10.1111/j.1365-2621.1992.tb08079.x
62. Bortolomeazzi R, Sebastianutto N, Toniolo R, Pizzariello A. Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. (2007) 100:1481–9. doi: 10.1016/j.foodchem.2005.11.039
63. Yousefi-Nejad S, Heidarbeigi K, Roushani M. Electronic tongue as innovative instrument for detection of crocin concentration in saffron (Crocus sativus L.). J Food Sci Technol. (2022) 59:1–9. doi: 10.1007/s13197-021-05349-1
64. Zhang A, Shen Y, Cen M, Hong X, Shao Q, Chen Y, et al. Polysaccharide and crocin contents, and antioxidant activity of saffron from different origins. Ind Crops Prod. (2019) 133:111–7. doi: 10.1016/j.indcrop.2019.03.009
65. Heydari S, Haghayegh GH. Extraction and microextraction techniques for the determination of compounds from saffron. Can Chem Trans. (2014) 2:221–47. doi: 10.13179/canchemtrans.2014.02.02.0097
66. Kadkhodaee R, Hemmati-Kakhki A. Ultrasonic extraction of active compounds from saffron. Int Symp Saffron Biol Technol. (2006) 739:417–25. doi: 10.17660/ActaHortic.2007.739.55
67. Tong Y, Jiang Y, Guo D, Yan Y, Jiang S, Lu Y, et al. Homogenate extraction of crocins from saffron optimized by response surface methodology. J Chem. (2018) 2018:1–6. doi: 10.1155/2018/9649062
68. Sarfarazi M, Rajabzadeh Q, Tavakoli R, Ibrahim SA, Jafari SM. Ultrasound-assisted extraction of saffron bioactive compounds; separation of crocins, picrocrocin, and safranal optimized by artificial bee colony. Ultrason Sonochem. (2022) 86:105971. doi: 10.1016/j.ultsonch.2022.105971
69. Kyriakoudi A, Chrysanthou A, Mantzouridou F, Tsimidou MZ. Revisiting extraction of bioactive apocarotenoids from Crocus sativus L. dry stigmas (saffron). Anal Chim Acta. (2012) 755:77–85. doi: 10.1016/j.aca.2012.10.016
70. Karasu S, Bayram Y, Ozkan K, Sagdic O. Extraction optimization crocin pigments of saffron (Crocus sativus) using response surface methodology and determination stability of crocin microcapsules. J Food Measur Characteriz. (2019) 13:1515–23. doi: 10.1007/s11694-019-00067-x
71. Sobolev AP, Carradori S, Capitani D, Vista S, Trella A, Marini F, et al. Saffron samples of different origin: an NMR study of microwave-assisted extracts. Foods. (2014) 3:403–19. doi: 10.3390/foods3030403
72. Manna L, Bugnone CA, Banchero M. Valorization of hazelnut, coffee and grape wastes through supercritical fluid extraction of triglycerides and polyphenols. J Supercrit Fluids. (2015) 104:204–11. doi: 10.1016/j.supflu.2015.06.012
73. Alehosseini A, Gómez-Mascaraque LG, Ghorani B, Lopez-Rubio A. Stabilization of a saffron extract through its encapsulation within electrospun/electrosprayed zein structures. Lwt. (2019) 113:108280. doi: 10.1016/j.lwt.2019.108280
74. De Monte C, Cesa S. Use of Saffron as a Functional Food and Saffron Nutraceuticals. Saffron: Elsevier (2021). p. 241–73.
75. Bhat NA, Wani IA, Hamdani AM, Gani A. Development of functional cakes rich in bioactive compounds extracted from saffron and tomatoes. J Food Sci Technol. (2022) 59:2479–91. doi: 10.1007/s13197-021-05267-2
76. Dabbagh Moghaddam A, Garavand F, Razavi SH, Dini Talatappe H. Production of saffron-based probiotic beverage by lactic acid bacteria. J Food Measurem Characteriz. (2018) 12:2708–17. doi: 10.1007/s11694-018-9888-z
77. Armellini R, Peinado I, Pittia P, Scampicchio M, Heredia A, Andres A. Effect of saffron (Crocus sativus L.) enrichment on antioxidant and sensorial properties of wheat flour pasta. Food Chem. (2018) 254:55–63. doi: 10.1016/j.foodchem.2018.01.174
78. Bakshi RA, Sodhi NS, Wani IA, Khan ZS, Dhillon B, Gani A. Bioactive constituents of saffron plant: extraction, encapsulation and their food and pharmaceutical applications. Appl Food Res. (2022) 100076:1–15. doi: 10.1016/j.afres.2022.100076
79. Mehrnia M-A, Jafari S-M, Makhmal-Zadeh BS, Maghsoudlou Y. Crocin loaded nano-emulsions: Factors affecting emulsion properties in spontaneous emulsification. Int J Biol Macromol. (2016) 84:261–7. doi: 10.1016/j.ijbiomac.2015.12.029
80. Rahaiee S, Shojaosadati SA, Hashemi M, Moini S, Razavi SH. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int J Biol Macromol. (2015) 79:423–32. doi: 10.1016/j.ijbiomac.2015.04.041
81. Mehrnia M-A, Jafari S-M, Makhmal-Zadeh BS, Maghsoudlou Y. Rheological and release properties of double nano-emulsions containing crocin prepared with angum gum, arabic gum and whey protein. Food Hydrocoll. (2017) 66:259–67. doi: 10.1016/j.foodhyd.2016.11.033
82. Rahaiee S, Hashemi M, Shojaosadati SA, Moini S, Razavi SH. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: antioxidant activities, bioavailability and anticancer properties. Int J Biol Macromol. (2017) 99:401–8. doi: 10.1016/j.ijbiomac.2017.02.095
83. Armellini R, Peinado I, Asensio-Grau A, Pittia P, Scampicchio M, Heredia A, et al. In vitro starch digestibility and fate of crocins in pasta enriched with saffron extract. Food Chem. (2019) 283:155–63. doi: 10.1016/j.foodchem.2019.01.041
84. Li D, Wu G, Zhang H, Qi X. Preparation of crocin nanocomplex in order to increase its physical stability. Food Hydrocoll. (2021) 120:106415. doi: 10.1016/j.foodhyd.2020.106415
85. Chalatashvili A, Khositashvili M, Khositashvili T, Gorgiladze M, Buishvili G. Processing of tincture production technology from various plant raw materials containing crocin. Winemaking Theory Pract. (2018) 3:3–7. doi: 10.13187/winem.2018.1.3
86. Fan M, Li N, Li Q, Li Y, Qian H, Zhang H, Rao Z, Wang L. Development of steamed bread fortified with gardenia fruit pomace: an evaluation of its bioactive compounds and quality characteristics. (2022) 1–26. doi: 10.2139/ssrn.4074755
87. Bajerska J, Mildner-Szkudlarz S, Podgórski T, Oszmatek-Pruszyńska E. Saffron (Crocus sativus L.) powder as an ingredient of rye bread: an anti-diabetic evaluation. J Med Food. (2013) 16:847–56. doi: 10.1089/jmf.2012.0168
88. Shvachko NA, Loskutov IG, Semilet TV, Popov VS, Kovaleva ON, Konarev AV. Bioactive components in oat and barley grain as a promising breeding trend for functional food production. Molecules. (2021) 26:2260. doi: 10.3390/molecules26082260
89. Ritota M, Comitato R, Manzi P. Cow and ewe cheeses made with saffron: characterization of bioactive compounds and their antiproliferative effect in cervical adenocarcinoma (HeLa) and breast cancer (MDA-MB-231) cells. Molecules. (2022) 27:1995. doi: 10.3390/molecules27061995
90. Licón C, Carmona M, Molina A, Berruga M. Chemical microbiological, textural, color, and sensory characteristics of pressed ewe milk cheeses with saffron (Crocus sativus L) during ripening. J Dairy Sci. (2012) 95:4263–74. doi: 10.3168/jds.2012-5389
91. Aktypis A, Christodoulou ED, Manolopoulou E, Georgala A, Daferera D, Polysiou M. Fresh ovine cheese supplemented with saffron (Crocus sativus L.): impact on microbiological physicochemical, antioxidant, color and sensory characteristics during storage. Small Ruminant Res. (2018) 167:32–8. doi: 10.1016/j.smallrumres.2018.07.016
92. Almodóvar P, Prodanov M, Arruñada O, Inarejos-García AM Affron® eye. a natural extract of saffron (Crocus sativus L) with colorant properties as novel replacer of saffron stigmas in culinary and food applications. Int J Gastronomy Food Sci. (2018) 12:1–5. doi: 10.1016/j.ijgfs.2018.03.001
93. Arnold M, Rajagukguk YV, Gramza-Michałowska A. Functional food for elderly high in antioxidant and chicken eggshell calcium to reduce the risk of osteoporosis—a narrative review. Foods. (2021) 10:656. doi: 10.3390/foods10030656
94. Gani A, Jan R, Ashwar BA, Ul Ashraf Z, Shah A, Gani A. Encapsulation of saffron and sea buckthorn bioactives: Its utilization for development of low glycemic baked product for growing diabetic population of the world. LWT. (2021) 142:111035. doi: 10.1016/j.lwt.2021.111035
95. Delfanian M, Sahari MA. Improving functionality, bioavailability, nutraceutical and sensory attributes of fortified foods using phenolics-loaded nanocarriers as natural ingredients. Food Res Int. (2020) 137:109555. doi: 10.1016/j.foodres.2020.109555
96. Mzabri I, Addi M, Berrichi A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics. (2019) 6:63. doi: 10.3390/cosmetics6040063
97. Arora P, Ansari S, Arora S. Nutritional beverages. Am J Pharmatech Res. (2019) 9:1–28. doi: 10.46624/ajptr.2020.v10.i3.015
98. Chrysanthou A, Pouliou E, Kyriakoudi A, Tsimidou MZ. Sensory threshold studies of picrocrocin, the major bitter compound of saffron. J Food Sci. (2016) 81:S189–98. doi: 10.1111/1750-3841.13152
99. Kyriakoudi A, Pouliou E, Ordoudi SA, Tsimidou S. Sensorial and functional attributes of herbal infusions containing saffron. 2nd Imeko. Foods. (2016) 304–9. Available online at: https://www.imeko.org/publications/tc23-2016/IMEKO-TC23-2016-061.pdf
100. Noori T, Sureda A, Sobarzo-Sánchez E, Shirooie S. The role of natural products in treatment of depressive disorder. Curr Neuropharmacol. (2022) 20:929–49. doi: 10.2174/1570159X20666220103140834
101. Tang J, Lu L, Wang Q, Liu H, Xue W, Zhou T, et al. Crocin reverses depression-like behavior in Parkinson disease mice via VTA-mPFC pathway. Mol Neurobiol. (2020) 57:3158–70. doi: 10.1007/s12035-020-01941-2
102. Abbaszadeh-Mashkani S, Hoque SS, Banafshe HR, Ghaderi A. The effect of crocin (the main active saffron constituent) on the cognitive functions, craving, and withdrawal syndrome in opioid patients under methadone maintenance treatment. Phytother Res. (2021) 35:1486–94. doi: 10.1002/ptr.6913
103. Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent. crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. (2012) 26:381–6. doi: 10.1002/ptr.3566
104. Duan X, Liu F, Kwon H, Byun Y, Minn I, Cai X, et al. (S)-3-(Carboxyformamido)-2-(3-(carboxymethyl) ureido) propanoic acid as a novel PSMA targeting scaffold for prostate cancer imaging. J Med Chem. (2020) 63:3563–76. doi: 10.1021/acs.jmedchem.9b02031
105. Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. (2011) 219:197–204. doi: 10.1016/j.bbr.2011.01.007
106. Gol S, Pena RN, Rothschild MF, Tor M, Estany J. A polymorphism in the fatty acid desaturase-2 gene is associated with the arachidonic acid metabolism in pigs. Sci Rep. (2018) 8:1–9. doi: 10.1038/s41598-018-32710-w
107. Nam KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. (2010) 648:110–6. doi: 10.1016/j.ejphar.2010.09.003
108. Naghizadeh B, Mansouri M, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. (2013) 20:537–42. doi: 10.1016/j.phymed.2012.12.019
109. Srivastava S, Ahmad R, Khare SK. Alzheimer's disease and its treatment by different approaches: a review. Eur J Med Chem. (2021) 216:113320. doi: 10.1016/j.ejmech.2021.113320
110. Cenini G, Voos W. Mitochondria as potential targets in Alzheimer disease therapy: an update. Front Pharmacol. (2019) 10:902. doi: 10.3389/fphar.2019.00902
111. Ghoweri AO, Gagolewicz P, Frazier HN, Gant JC, Andrew RD, Bennett BM, et al. Neuronal calcium imaging, excitability, and plasticity changes in the Aldh2–/–mouse model of sporadic Alzheimer's disease. J Alzheimer's Dis. (2020) 77:1623–37. doi: 10.3233/JAD-200617
112. Ali A, Ain Q, Saeed A, Khalid W, Ahmed M, Bostani A. Biomolecular Characteristics of Whey Proteins with Relation to Inflammation. IntechOpen. (2021). p. 1–18. doi: 10.5772/intechopen.99220
113. Saito T, Saido TC. Neuroinflammation in mouse models of Alzheimer's disease. Clin Exp Neuroimmunol. (2018) 9:211–8. doi: 10.1111/cen3.12475
114. Latif A, Bibi S, Ali S, Ammara A, Ahmad M, Khan A, et al. New multitarget directed benzimidazole-2-thiol-based heterocycles as prospective anti-radical and anti-Alzheimer's agents. Drug Dev Res. (2021) 82:207–16. doi: 10.1002/ddr.21740
115. Wang C, Cai X, Hu W, Li Z, Kong F, Chen X, et al. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer's disease. Int J Mol Med. (2019) 43:956–66. doi: 10.3892/ijmm.2018.4032
116. Li J-Y, Li N-N, Wang L, Peng J-X, Duan L-R, Chen C-L, et al. A compound heterozygous PINK1-associated juvenile Parkinson's disease with pregnancy in Chinese. J Neurol. (2021) 268:2223–7. doi: 10.1007/s00415-021-10405-z
117. Raza C, Anjum R. Parkinson's disease: mechanisms, translational models and management strategies. Life Sci. (2019) 226:77–90. doi: 10.1016/j.lfs.2019.03.057
118. Mohammadzadeh L, Rahbardar MG, Razavi BM, Hosseinzadeh H. Crocin protects malathion-induced parkinson-like disease by inhibiting apoptosis and α-synuclein accumulation in rats'. Striatum. (2021) 1–21. doi: 10.21203/rs.3.rs-1028580/v1
119. El Midaoui A, Ghzaiel I, Vervandier-Fasseur D, Ksila M, Zarrouk A, Nury T, et al. Saffron (Crocus sativus L.): a source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients. (2022) 14:597. doi: 10.3390/nu14030597
120. Pekna M, Stokowska A, Pekny M. Targeting complement C3a receptor to improve outcome after ischemic brain injury. Neurochem Res. (2021) 46:2626–37. doi: 10.1007/s11064-021-03419-6
121. Povroznik JM, Engler-Chiurazzi EB, Nanavati T, Pergami P. Absolute lymphocyte and neutrophil counts in neonatal ischemic brain injury. SAGE Open Medicine. (2018) 6:2050312117752613. doi: 10.1177/2050312117752613
122. Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. (2019) 173:1–17. doi: 10.1016/j.pneurobio.2018.05.004
123. Azami S, Shahriari Z, Asgharzade S, Farkhondeh T, Sadeghi M, Ahmadi F, et al. Therapeutic potential of saffron (Crocus sativus L) in ischemia stroke. Evid Based Complem Alternat Med. (2021) 2021:1–8. doi: 10.1155/2021/6643950
124. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
125. Motaghinejad M, Safari S, Feizipour S, Sadr S. Crocin may be useful to prevent or treatment of alcohol induced neurodegeneration and neurobehavioral sequels via modulation of CREB/BDNF and Akt/GSK signaling pathway. Med Hypotheses. (2019) 124:21–5. doi: 10.1016/j.mehy.2019.01.017
126. Mazumder AG, Sharma P, Patial V, Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-κB activation. Basic Clin Pharmacol Toxicol. (2017) 120:426–33. doi: 10.1111/bcpt.12694
Keywords: crocin, extraction techniques, food applications, medicinal, brain disorders
Citation: Ali A, Yu L, Kousar S, Khalid W, Maqbool Z, Aziz A, Arshad MS, Aadil RM, Trif M, Riaz S, Shaukat H, Manzoor MF and Qin H (2022) Crocin: Functional characteristics, extraction, food applications and efficacy against brain related disorders. Front. Nutr. 9:1009807. doi: 10.3389/fnut.2022.1009807
Received: 02 August 2022; Accepted: 21 November 2022;
Published: 13 December 2022.
Edited by:
Fatih Ozogul, Çukurova University, TurkeyReviewed by:
Gulzar Ahmad Nayik, Govt. Degree College, IndiaCopyright © 2022 Ali, Yu, Kousar, Khalid, Maqbool, Aziz, Arshad, Aadil, Trif, Riaz, Shaukat, Manzoor and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Yu, a3ljeXVsaWFuZ0BobmZudS5lZHUuY24=; Hong Qin, cWluaG9uZ0Bjc3UuZWR1LmNu; Muhammad Faisal Manzoor, ZmFpc2FsdW9zMjZAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.