- 1Guangxi Key Laboratory of Environmental Exposomics and Entire Lifecycle Heath, Department of Environmental Health and Occupational Medicine, School of Public Health, Guilin Medical University, Guilin, China
- 2Department of Political Science and Administration, School of International Affairs and Public Administration, Ocean University of China, Qingdao, China
- 3Department of Environmental and Occupational Health, School of Public Health, Guangxi Medical University, Nanning, China
- 4Department of Food Science, Faculty of Veterinary and Agricultural, The University of Melbourne, Parkville, VIC, Australia
Objective: This study aimed to assess the relationship between the dietary intake of saturated fatty acids (SFAs) and its subtypes (C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0) and hypertension.
Design, participants, and methods: Adults aged 20 years and older based used the U.S. Health and Nutrition Survey (1999–2018) were used as participants. Two averages of 24 h dietary recall data were obtained for weight-adjusted continuous cross-sectional analysis. Two 24-h recall interview data means were obtained for weight-adjusted continuous cross-sectional analysis. A logistic regression model was used to estimate the weighted odds ratio (OR) and its 95% confidence interval (CI) for hypertension.
Results: The study included 7,222 respondents over 20 years of age with a hypertension prevalence of 23.2% and a significant difference in the dietary intake of carbohydrates among patients with hypertension. Dietary intake of nutrients was more in men than in women with hypertension. After adjusting for confounders, adjusting for nutrients, and reducing covariance among nutrients, the OR (95% CI) for women’s dietary intake of SFAs, C14:0, C16:0, C18:0 fourth quartile, and C14:0 third quartile were 0.57 (0.34, 0.95), 0.57 (0.34, 0.95), 0.57 (0.34, 0.95), 0.57 (0.34, 0.95), and 0.57 (0.34, 0.95), respectively, which may be a risk factor for hypertension. In older (≥65, years) respondents, the OR (95% CI) for dietary intake of SFAs, C4:0, C14:0, C16:0 fourth quartile, and C12:0 third quartile were 0.42 (0.21, 0.86), 0.46 (0.22, 0.95), 0.39 (0.18, 0.85), 0.38 (0.17, 0.84), and 0.45 (0.20, 0.99), respectively, which may be a protective factor for hypertension.
Conclusion: The study was based on the American Health and Nutrition Examination Survey, and a strong correlation was found between dietary intake of SFAs, C14:0, C16:0, and C18:0 and hypertension in women (dietary intake of SFAs, C4:0, C12:0, C14:0, and C16:0) and middle-aged and older adults (dietary intake of SFAs, C4:0, C12:0, C14:0, and C16:0). In addition, dietary nutrient intake should be carefully selected for the rational prevention of hypertension.
Introduction
According to the World Health Organization, 17 million people die each year from cardiovascular diseases. Hypertension accounts for 45% of all heart disease-related mortality. Approximately 40% of adults aged 25 years and older are diagnosed with hypertension worldwide, and the prevalence of hypertension has reached 35% among adults in the United States (1, 2). Hypertension has become a global public health problem that cannot be ignored.
Common factors influencing hypertension include dietary, psychosocial, socioeconomic status, and genetic (3). Diet plays an important role in cardiovascular disease risk, and adherence to a scientific diet is an essential strategy to reduce the incidence of hypertension. The protective effect of nutrients on hypertension is not negligible (4, 5). Among them, saturated fatty acids (SFAs), polyunsaturated fatty acids, and monounsaturated fatty acids are closely related to hypertension (6, 7). Common sources of SFA are coconut oil, rice bran oil, red meat, high-fat dairy products, and human breast milk. In general, SFAs are considered to be a risk factor for hypertension (8). The Dietary Guidelines for Americans recommend reducing SFA to less than 7% of energy to reduce the incidence of cardiovascular diseases (9). However, one study has found that SFA may improve cardiovascular health (7).
Studies have reported that the smaller the chain length, the stronger the effect of raising HDL (10). Gender (11) and age have a significant effect on patients with hypertension. High levels of SFA in serum correlate with hypertension in men (12). Dietary intake of SFAs, C12:0, C14:0, and C16:0 in the elderly is beneficial to health (7). A study found that the effect of SFA intake on hypertension was not significant in young and middle-aged adults compared with older adults. Therefore, the present study investigated the relationship between SFAs and their subtypes and hypertension, which may provide some reference for the dietary intake of SFAs and subtypes to reduce the occurrence of hypertension.
Materials and methods
Study design and population
Based on the publicly available data from the National Health and Nutrition Examination Survey (NHANES) (13, 14), trained professional investigators conducted face-to-face, telephone interviews, and laboratory tests to collect data. The National Center for Health Statistics Institutional Review Board/Ethics Review Board approved this survey (NHANES, 1999–2016)1 to obtain informed consent from participants and continue the study through home interviews and examinations. This study used a case-control study to obtain 10 cross-sectional survey cycles of NHANES database respondents (n = 7,222) between 1999 and 2018. The study population was estimated to be representative of the United States (n = 38,123,806), the hypertension group (n = 5,550), and the control group (n = 1,672). Complete questionnaire data, laboratory test data, reliable data of two 24 h recalled dietary intakes, and accurate calculation of nutrient intake were obtained. Exclusion criteria were as follows: (1) respondents aged < 20 years; (2) excluding energy intake (male: <500 or >8,000 kcal/day, female: <500 or >5,000 kcal/day); (3) respondents with diabetes, hyperlipidemia; (4) repeat respondents; (5) respondents with missing data (Figure 1).
Assessment of nutrients
Dietary sources of energy, protein, carbohydrate, dietary fiber, total fat, dietary monounsaturated fatty acids, polyunsaturated fatty acids, SFAs, and their subtypes (C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0) were obtained by two 24-h recall interviews. Nutrient types were selected and calculated with reference to NHANES support material2. The instrument for measuring the dietary intake of respondents was described in detail in the measurement guide (15). Dietary nutrient intakes were calculated using the Food and Nutrition Database for Dietary Studies 5.0 (FNDDS 5.0) standard of the Ministry of Agriculture (16, 17).
Definition of hypertension
Participants’ blood pressure measurements were taken according to the NHANES description (18), with systolic blood pressure (SBP) and diastolic blood pressure (DBP) taken as the average of three or more measurements. Hypertension is defined as (1) taking hypertensive medication; (2) being informed by a licensed physician of hypertension or stating in the questionnaire to take prescribed medication for hypertension; and (3) measuring the participant’s SBP mean ≥ 140 mmHg and/or DBP ≥ 90 mmHg (mean of three times) (19). Any one of the above three conditions can be diagnosed as hypertension (20).
Measurements of covariates
Demographic information was collected by questionnaire, including age (20–44, 45–64, 65, and older), gender, race (non-Hispanic white, non-Hispanic black, Hispanic, and others), smoking status (never smoked [less than 100 cigarettes in a lifetime], formerly smoked [smoked more than 100 cigarettes in lifetime, now not smoking at all], and currently smoking [smoked more than 100 cigarettes in lifetime, some days, or every day]), education (less than high school, high school graduate/general education, some college, or above), and family income to poverty ratio (less than 1.30, 1.30 to <3.00, 3.00 to <5.00, 5.00 and above) represents the ratio of family income to the federal poverty threshold, adjusting for household size. A higher ratio indicates higher levels of income, marital status (marriage, divorce, unmarried), and drinking status (current heavy drinker [women drink ≥ 3 drinks per day/men drink ≥ 4 drinks per day/binge drinking 5 days or more]; current moderate drinkers [women drink ≥ 2 drinks per day/men drink ≥ 3 drinks per day/binge drinking ≥ 2 days per month]; current light alcohol users [not the above two categories] (6); and no alcohol consumption). Physical activity (using total Mets for the week: <600, Mets, min/week, when exercise ≥ 600, Mets, min/week. Exercise also has significant physical benefits (21). The diagnosis of diabetes consists of the following components, any of which can be met to be diagnosed with diabetes: (1) the individual has been diagnosed with diabetes by a doctor; (2) glycosylated hemoglobin (HbA1c) (%) > 6.5; (3) fasting blood glucose (DM, mmol/l) ≥ 7.0; (4) random blood glucose (mmol/L) ≥ 11.1; (5) 2 h OGTT blood glucose (mmol/l) ≥ 11.1; and (6) use of diabetes medication or insulin. Body mass index (BMI) was as follows: <25 kg/m2, 25–30 kg/m2, and ≥30 kg/m2. Hyperlipidemia was diagnosed by meeting any of the following criteria: hypertriglyceridemia (TG ≥ 150 mg/dL) and/or hypercholesterolemia {TG 200 mg/dL [5.18 mmol/L], LDL 130 mg/dL [3.37 mmol/L], HDL < 40 mg/dL [1.04 mmol/L] (male), 50 mg/L [1.30mmol/L] (female)}.
Statistical analysis
Continuous variables were described by mean ± SD, and continuous variables were compared using Student’s t-test. Categorical variables were described by n (%), and they were compared between groups using a chi-squared test. Nutrient intake was corrected using the residual method. Nutrient intakes were grouped into quartiles and analyzed for the inter-quartile group trend. The lowest quartile (first quartile) was defined as the reference group in each model. The results were expressed as weighted (OR [95% CI]). Nutrients entered into the model using a variance inflation factor (<10) (22) (energy, kcal: 42.763; protein, g: 3.127; carbohydrate, g: 13.381; dietary fiber, g: 1.977, total fat, g: 44.032; total mfat, g: 17.812; total pfat, g: 6.083). Logistic regression models were used to analyze the association between SFAs and their subtypes (C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0) and hypertension. Stratified analyses and interactions were performed in accordance with sex, age, and BMI to explore the effects of SFAs and their subtypes on hypertension in different exposure settings. All analyses were performed in R (4.1.3) language, using NHANESR (version 0.9.2.8) package, survey (version 4.1-1) package compareGroups (version 4.5.2) package, forest plot (version 2.0.1) package, etc. for statistical analysis. A two-tailed P-value of < 0.05 was considered statistically significant.
Results
Table 1 shows the number of respondents and the weighted percentages. In the total population with 10 survey periods, a significant difference in age and BMI (P-value < 0.001) was observed between the hypertension group and control group, with the highest prevalence of hypertension (45–64, years). The prevalence was higher in men than in women in the total population. Gender and age are recognized risk factors for hypertension, which will be explored in a stratified analysis with interaction.
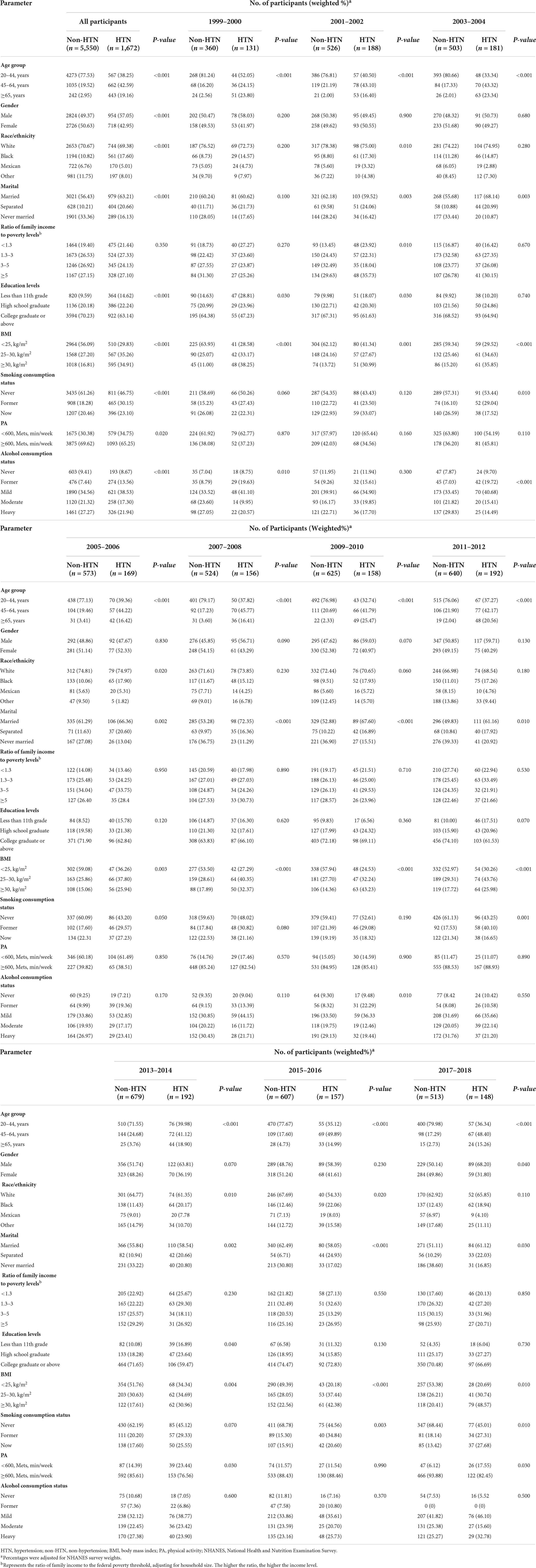
Table 1. Socio-demographic characteristics of US adults with hypertension by NHANES survey cycle, 1999–2018 (N = 7,222).
Tables 2, 3 shows the description of demographic characteristics and nutrient intake. The ratio of family income to poverty levels did not differ significantly between genders. In the hypertension group, no significant differences in race and level of education were observed between genders. In the control group, age and PA level did not differ significantly between genders. All other demographic indicators showed statistically significant differences. Carbohydrate versus C10:0 in the total population was significantly different in the hypertensive group versus the control group. Nutrient intake was lower in women than in men.
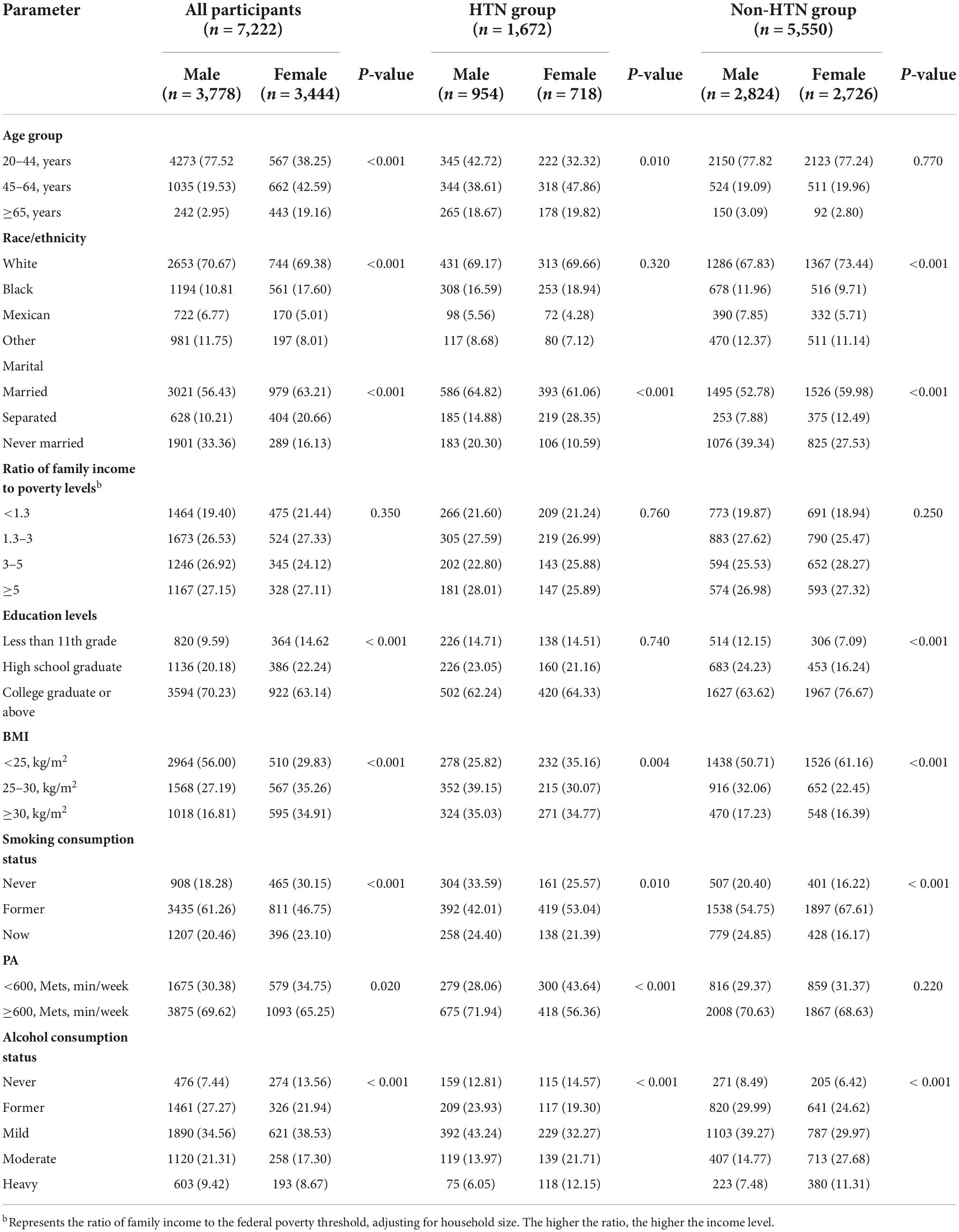
Table 2. Participant characteristics by hypertension status with sex differences: a cross-sectional study using NHANES data from 1999 to 2018.

Table 3. Characteristics of the weighted dietary intake of nutrients in the hypertensive and non-hypertensive groups among the participantsa.
Table 4 demonstrates the relationship between SFAs and their subtypes and hypertension. No significant correlation was found between SFAs and their subtypes and hypertension.
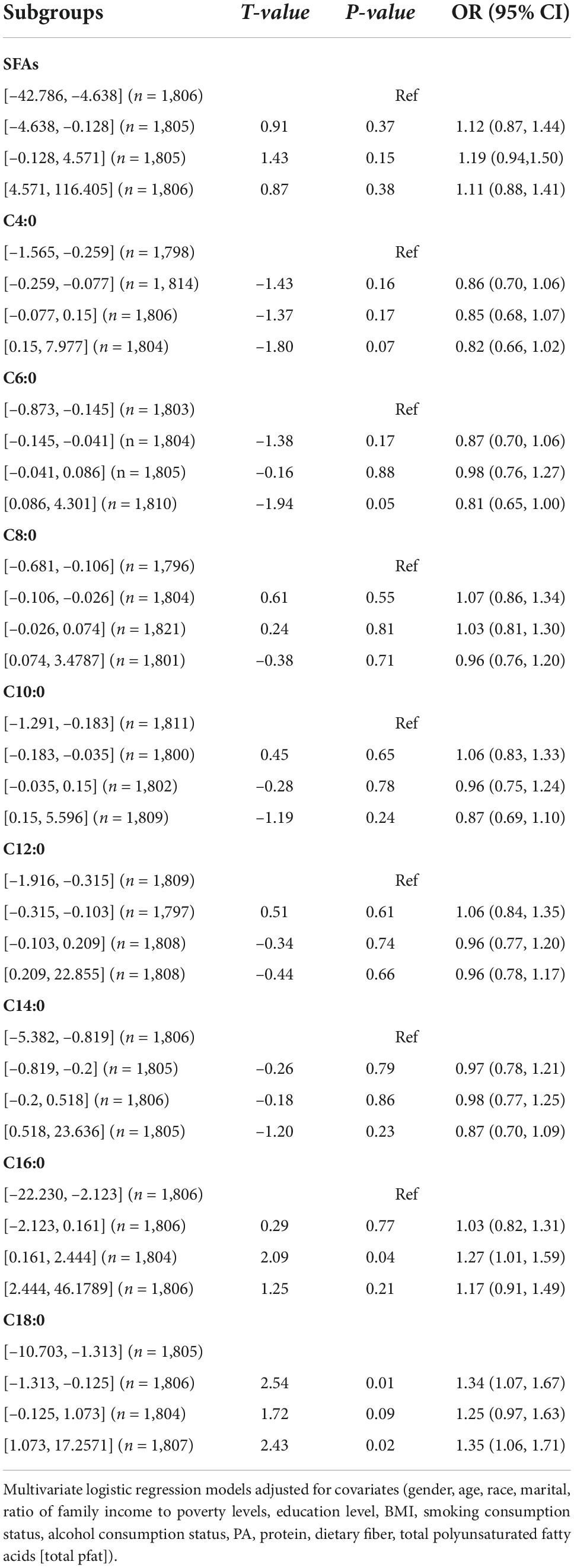
Table 4. Univariate logistic regression results of the relationship between saturated fatty acids and their subtypes and hypertension.
In Table 5, no association was found between the dietary intake of SFAs and their subtypes and hypertension by gender. In Table 5, dietary intake of SFAs and their subtypes were not found to be associated with hypertension in different age groups. Inter-quartile trend analysis indicated a linear relationship between the C18:0 and C4:0 quartile subgroups of dietary intake.
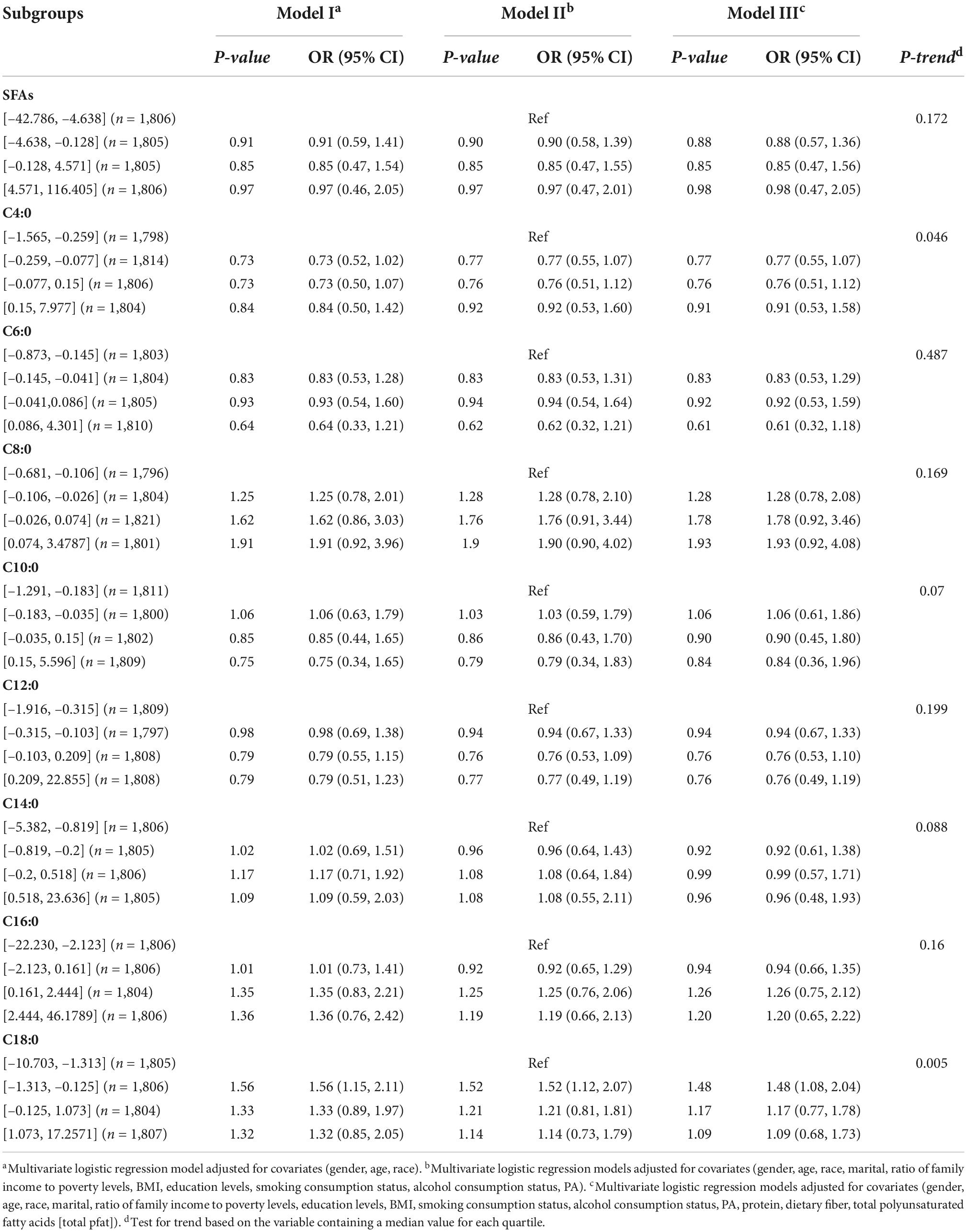
Table 5. Weighted odds ratio sum (95% CI) of quartiles of hypertension and adjusted dietary saturated fatty acid intake: a cross-sectional study using NHANES 1999–2018 data (N = 7,222).
In Tables 6, 7, female dietary intake of SFAs, C14: 0, C16: 0, C18: 0 fourth quartile, C14: 0 third quartile (OR [95% CI]) was as follows: (0.57 [0.34, 0.95]), (0.57 [0.34, 0.95]), (0.57 [0.34, 0.95]), (0.57 [0.34, 0.95]), (0.57 [0.34,0.95]), and (0.57 [0.34, 0.95]).
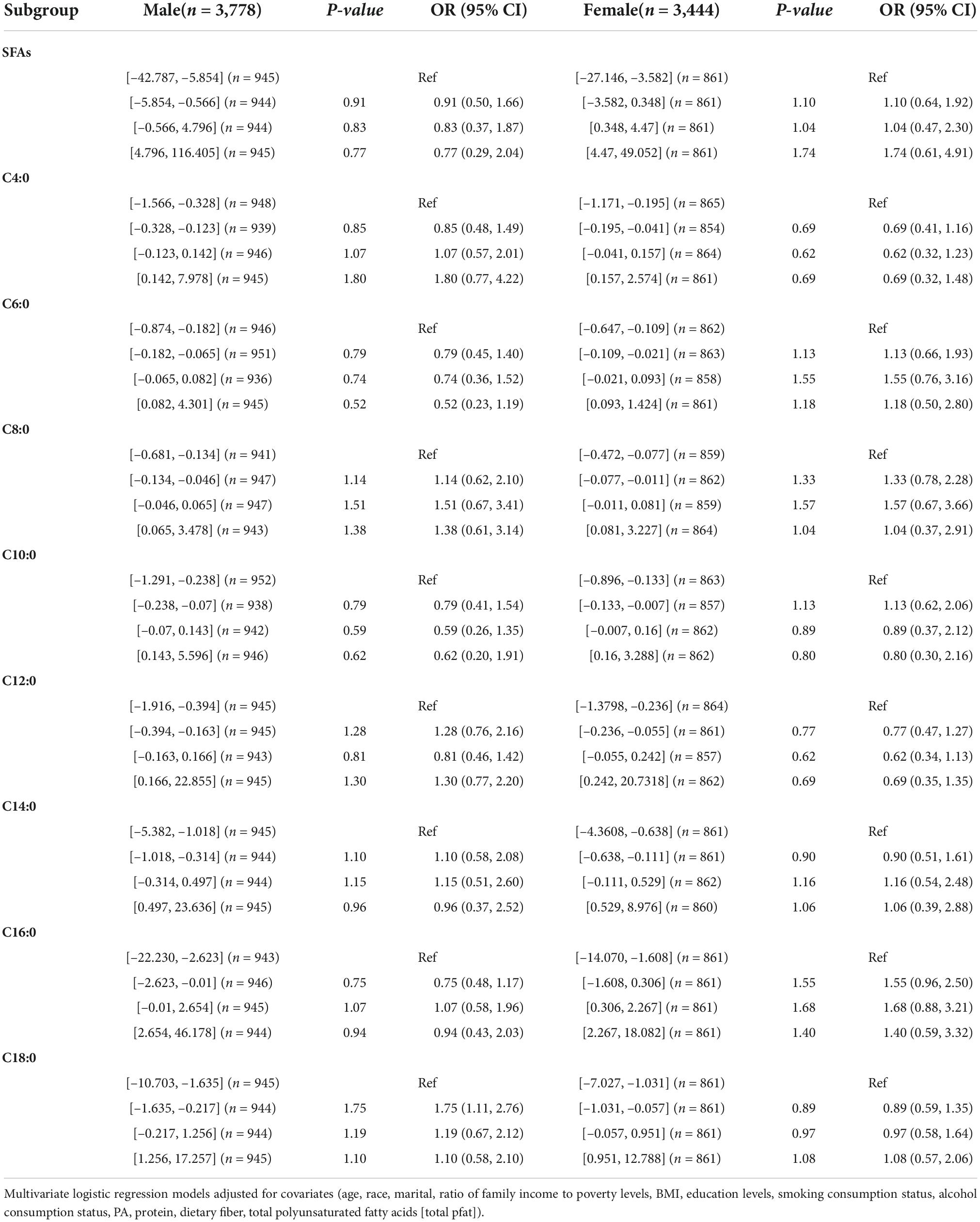
Table 6. Weighted OR (95% CI) for hypertension in quartiles of adjusted dietary saturated fatty acid intake stratified by gender in NHANES, 1999–2018.
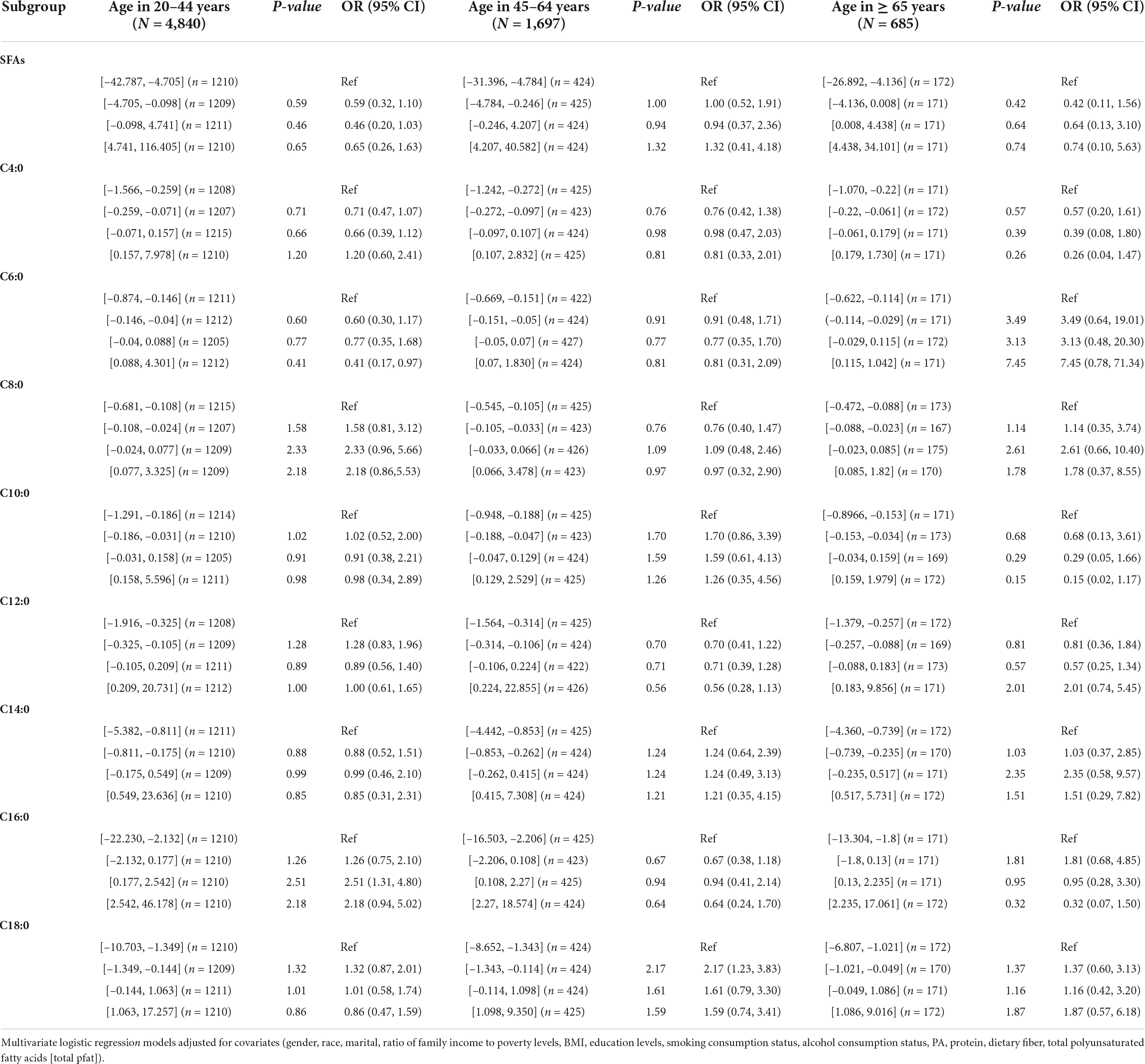
Table 7. Weighted OR (95% CI) for hypertension in quartiles of adjusted dietary saturated fatty acid intake stratified by age in NHANES, 1999–2018.
In Tables 8, 9, among (45–64, years) respondents, the (OR [95% CI]) of the second quartile of C6:0 and C14:0 were (1.67 [1.00, 2.78]) and (1.77 [1.05, 3.00]), respectively. Among (≥65, years) respondents with dietary intake of SFAs, the (OR [95% CI]) of the C4:0, C14:0, C16:0 fourth quartile, and C12:0 third quartile were (0.42 [0.21, 0.86]), (0.46 [0.22, 0.95]), (0.39 [0.18, 0.85]), (0.38 [0.17, 0.84]), and (0.45 [0.20, 0.99]), respectively.
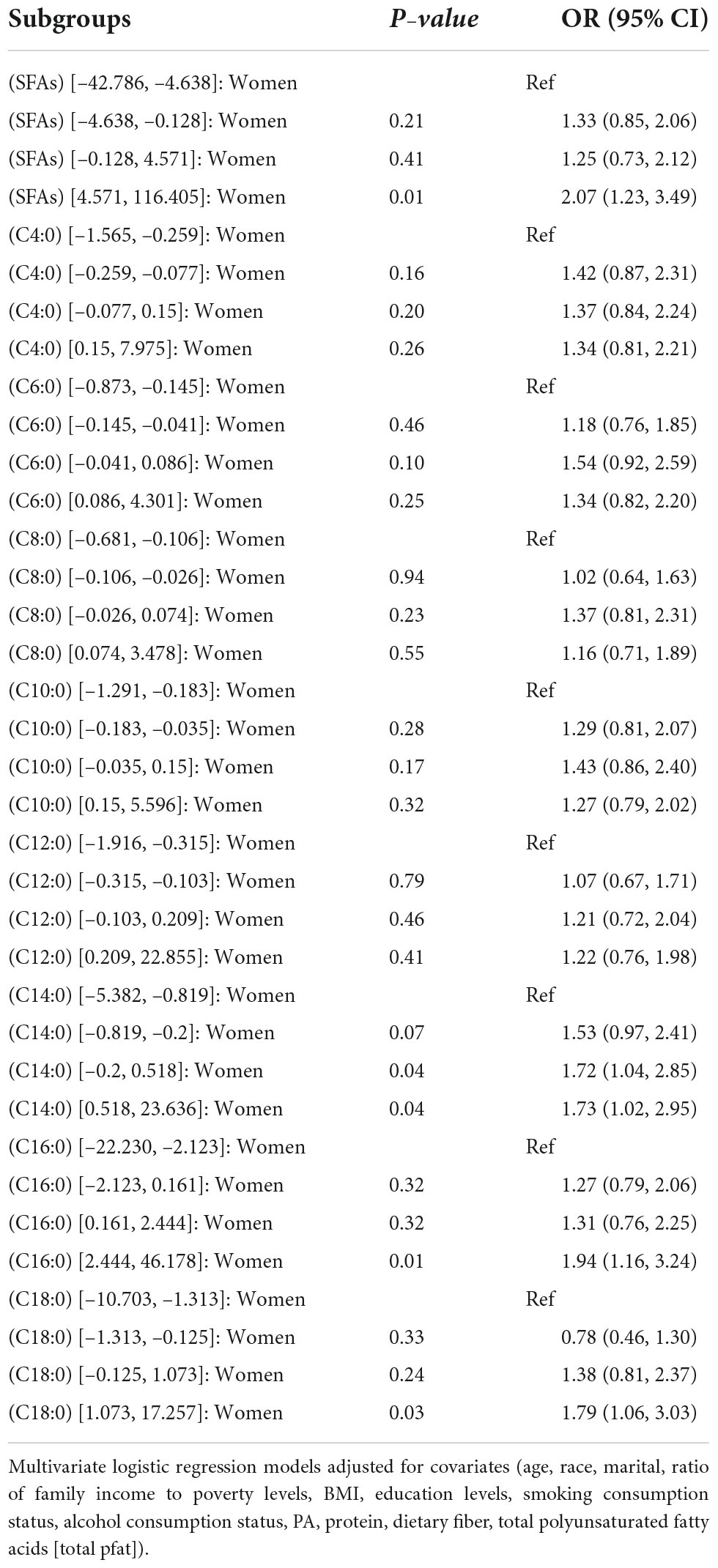
Table 8. OR (95% CI) for hypertension in quartiles of adjusted intake of dietary saturated fatty acids and their subtypes interacted with sex in NHANES, 1999–2018.
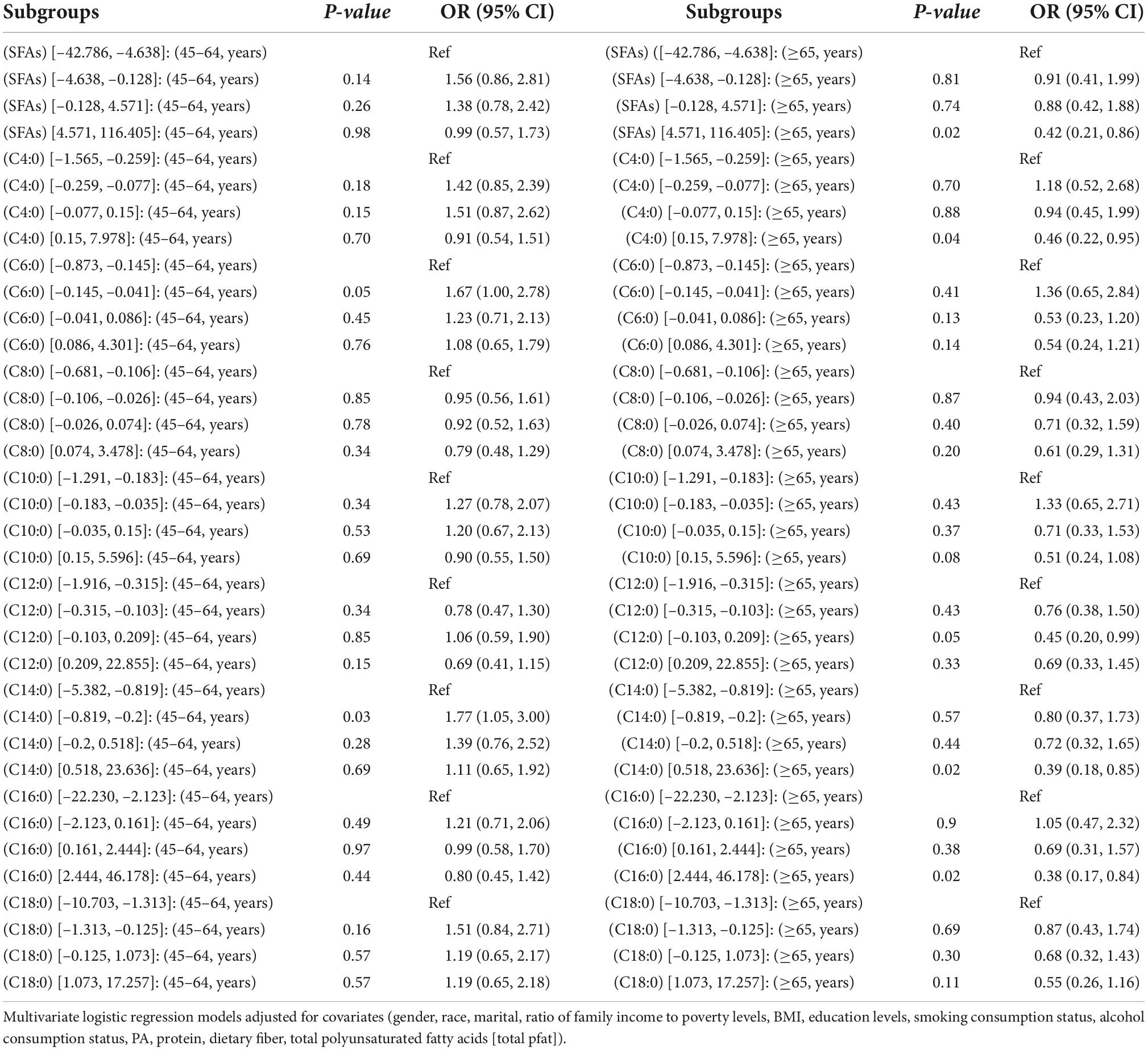
Table 9. OR (95% CI) for hypertension in quartiles of adjusted intake of dietary saturated fatty acids and their subtypes interacted with age in NHANES, 1999–2018.
Discussion
The aim of this study was to investigate the relationship between SFAs and their subtypes and hypertension. The main findings were that there was no significant correlation between dietary intake of SFAs and their subtypes on hypertension in the logistic regression models (I, II, and III). Hypertension may be more likely to occur when the dietary intake of female respondents is in the fourth quartile of SFAs, the third and fourth quartiles of C14:0, the fourth quartile of C16:0, and the fourth quartile of C18:0. Hypertension may be more likely to occur when dietary intake in middle-aged adults (45–64, years) is in the second quartile of C6:0 and C14:0. Hypertension may be more likely to occur when dietary intake in older adults (≥65, years) is in the fourth quartile of SFAs, fourth quartile of C4:0, third and fourth quartiles of C12:0, fourth quartile of C14:0, and fourth quartile of C16:0.
The study found no significant association between dietary intake of fatty acids and their subtypes and hypertension in overall respondents, and the results of a community cohort study (23) and a meta-analysis (24) were similar to our results. Dietary intake of SFAs is considered a risk factor in the cardiovascular field, particularly high intakes of red meat and high-fat dairy products, which are the major sources of SFAs in the diet. The American Heart Association recommends and limits a daily intake of < 7% SFAs (8). However, some studies have found that intake of SFAs can lead to a lower risk of hypertension (25). Short- to medium-chain SFAs (C4:0–C10:0) were not significantly associated with the risk of coronary heart disease (26), and high intakes of long-chain fatty acids C14:0 and C16:0 were negatively associated with the risk of developing hypertension in older adults. Moreover, differences in chain length account for their differential effects on the risk of coronary heart disease (7), prompting further discussion of the effect of SFA subtypes on hypertension effects.
In female respondents, we found an interaction between SFAs, C14:0, C16:0, and C18:0 and hypertension. When dietary intake was high, SFAs may be a risk factor for hypertension. The correlation between dietary intake of SFAs and their subtypes and hypertension has been less studied in previous studies. However, gender differences in hypertension are common in the cardiovascular field (11). A review reported that high levels of serum SFA were associated with hypertension in men but not in women; and the high prevalence of hypertension in older women was partly due to decreased ovarian estrogen production caused by elevated blood pressure after menopause (27). However, more evidence is needed to determine whether this result is associated with a dietary intake of SFAs. A previous prospective cohort study in women noted that C4:0, C6:0, C8:0, and C10:0 were not associated with the risk of developing coronary heart disease, but C14:0, C16:0, and C18:0 were associated with the risk of coronary heart disease (26). Moreover, the serum cholesterol elevating effect of dietary SFAs may be related to C14:0, C16:0, and C18:0 (28, 29), which is similar to our findings.
We found that age interacted with SFAs, C4:0, C6:0, C12:0, C14:0, and C16:0. Age is considered an important influencing factor for hypertension. In the middle-aged population, dietary intake of C6:0 and C14:0 may be a risk factor for hypertension. SFAs were strongly associated with hypertension in the elderly, and total SFA, C4:0, C12:0, C14:0, and C16:0 may be protective factors for hypertension. Our findings were consistent with those obtained by a study in Japan (7). That is, C12:0 increases the activity of superoxide dismutase, catalase, and glutathione peroxidase, improving antioxidant activity (30). C12:0 improves vascular endothelial function and delays vascular aging, which is associated with impaired pressure-sensing reflex sensitivity and the development of hypertension (31). Reactive oxygen species contribute to the reduction of pressure reflex sensitivity (32, 33). Therefore, the observed association between SFAs and their isoforms and hypertension may be attributed to their antioxidant activity, which ultimately exhibits a protective effect. The fatty acid composition of plasma cholesteryl esters was positively associated with the 6-year incidence of hypertension in the ARIC study, but the mean age of the respondents was <65 years (23), which may indicate that age differences in distribution led to differences in results.
Considering that the underlying mechanisms were not examined in this study, the differences in the results might be due to several possible reasons: first, there may be internal mechanisms for SFAs and their subtypes, and differences in the confounding factors adjusted by regression analysis may lead to differences in the results. Second, SFA intake was measured by using the FFQ, a self-reporting-dependent tool that may lead to errors. Third, the possibility of reverse causation cannot be ruled out because of observational studies. Fourth, Neyman bias may exist during the NHANES survey. Fifth, only two measurements of dietary intake were averaged. Although diabetes and hyperlipidemia were excluded, long-term dietary habits and whether dietary habits changed after disease diagnosis could not be detected.
Conclusion
This study was based on the National Health and Nutrition Examination Survey of the US population, and no significant effect of total SFAs was found in the total respondents. Female respondents may need to be aware that higher dietary intake of saturated fatty acids (SFAs, C14:0, C16:0, C18:0) may be a risk factor for hypertension. Dietary intake of SFAs, C4:0, C6:0, C12:0, C14:0, and C16:0 may be a protective factor for hypertension when higher in older adults. Women and elderly respondents in the United States should be cautious in their intake of SFAs to actively prevent the development of hypertension.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RG, YG, and JQ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. RG and YG drafted the manuscript. QG, TLu, ZC, YL, KH, SX, RL, TLi, JX, and YC critically revised the manuscript for important intellectual content. RG contributed to the statistical analysis. ZZ obtained the funding. ZZ and YL supervised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81960583), Guangxi Graduate Education Innovation Project (Grant/Award No. GYYK2021001), and the Guangxi Science and Technology Major Special Project (Grant No. Gui Ke AA22096026).
Acknowledgments
We thank the Longevity Group of Guilin Medical University and second, the Guangxi Key Laboratory of Environmental Exposomics and Entire Lifecycle Heath of Guilin Medical University. We also thank Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, the nhanesR package and webpage, makes it easier for us to explore the NHANES database. We are grateful to the editor and reviewers for their careful work and thoughtful comments, which have significantly improved this article substantially.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://www.cdc.gov/nchs/nhanes/irba98.htm
- ^ https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DSQTOT_J.htm
References
1. Pius ALB, Dewi I, Akhir Yani SH. Hypertension: a global health crisis. Ann Clin Hypertens. (2021) 5:8–11. doi: 10.29328/journal.ach.1001027
2. World Health Organization. A Global Brief on Hypertension: silent Killer, Global Public Health Crisis: world Health Day 2013. Geneva: World Health Organization (2013).
3. Cuevas AG, Williams DR, Albert MA. Psychosocial factors and hypertension: a review of the literature. Cardiol Clin. (2017) 35:223–30. doi: 10.1016/j.ccl.2016.12.004
4. Cohen J. Health metrics. A controversial close-up of humanity’s health. Science. (2012) 338:1414–6. doi: 10.1126/science.338.6113.1414
5. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380: 2224–60.
6. Ni S, Zhong Z, Wei J, Zhou J, Cai L, Yang M, et al. Association between dietary intake of polyunsaturated fatty acid and prevalence of hypertension in U.S. Adults: a cross-sectional study using data from NHANES 2009–2016. Hypertens Res. (2022) 516:526. doi: 10.1038/s41440-021-00849-1
7. Nakamura H, Tsujiguchi H, Kambayashi Y, Hara A, Nakamura H. Relationship between saturated fatty acid intake and hypertension and oxidative stress. Nutrition. (2018) 61:8–15. doi: 10.1016/j.nut.2018.10.020
8. Lichtenstein AH. Diet and lifestyle recommendations revision 2006: a scientific statement from the American heart association nutrition committee. Circulation. (2006) 114:82. doi: 10.1161/CIRCULATIONAHA.106.176158
9. United States Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: United States Department of Health and Human Services (2010).
10. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. (2010) 45:893–905. doi: 10.1007/s11745-010-3393-4
11. Song JJ, Ma Z, Wang J, Chen LX, Zhong JC. Gender differences in hypertension. J Cardiovasc Transl Res. (2019) 13:47–54. doi: 10.1007/s12265-019-09888-z
12. Grynberg A. Hypertension prevention: from nutrients to (fortified) foods to dietary patterns. Focus on fatty acids. J Hum Hypertens. (2005) 19 Suppl 3:S25. doi: 10.1038/sj.jhh.1001957
16. Montville JB, Ahuja J, Martin CL, Heendeniya KY, Omolewa-Tomobi G, Steinfeldt LC, et al. USDA food and nutrient database for dietary studies (FNDDS), 5.0. Procedia Food Sci. (2013) 2:99–112. doi: 10.1016/j.profoo.2013.04.016
17. Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of USDA’s dietary intake data system. J Food Compos. Anal. (2004) 17:545–55. doi: 10.1016/j.jfca.2004.02.013
19. O’Shea PM, Griffin TP, Fitzgibbon M. Hypertension: the role of biochemistry in the diagnosis and management. Clin Chim Acta. (2017) 465:131–43. doi: 10.1016/j.cca.2016.12.014
20. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. (2014) 311:507–20. doi: 10.1001/jama.2013.284427
21. Vilar-Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. (2022) 75:1491–506. doi: 10.1002/hep.32207
22. Kassambara A. Machine Learning Essentials: Practical Guide in R. Marseille: Bioinformatics R&D Scientist HalioDx (2018).
23. Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. (1999) 150:492–500. doi: 10.1093/oxfordjournals.aje.a010038
24. Geleijnse JM, Brouwer ID, Kromhout D. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. (2014) 161:458–9. doi: 10.7326/L14-5018-10
25. Praagman J, Beulens JW, Alssema M, Zock PL, Wanders AJ, Sluijs I, et al. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European prospective investigation into cancer and nutrition–Netherlands cohort. Am J Clin Nutr. (2016) 103:356–65. doi: 10.3945/ajcn.115.122671
26. Hu FB, Stampfer MJ, Manson JE, Alberto A, Colditz GA, Speizer FE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. (1999) 70:1001–8. doi: 10.1093/ajcn/70.6.1001
27. August P, Oparil S. Hypertension in women. J Clin Endocrinol Metab. (1999) 84:1862–6. doi: 10.1210/jcem.84.6.5724
28. Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet. Metabolism. (1965) 14:759–65. doi: 10.1016/0026-0495(65)90002-8
29. Hegsted DM, Mcgandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. (1965) 17:281–95. doi: 10.1093/ajcn/17.5.281
30. Milic VD, Stankov K, Injac R, Djordjevic A, Srdjenovic B, Govedarica B, et al. Activity of antioxidative enzymes in erythrocytes after a single dose administration of doxorubicin in rats pretreated with fullerenol C60(OH)24. Toxicol Mech Method. (2008) 19:24–8. doi: 10.1080/01612840802203098
31. Dauphinot V, Kossovsky MP, Gueyffier FO, Pichot V, Gosse P, Roche F, et al. Impaired baroreflex sensitivity and the risks of new-onset ambulatory hypertension, in an elderly population-based study. Int J Cardiol. (2013) 168:4010–4. doi: 10.1016/j.ijcard.2013.06.080
32. De Queiroz TM, Monteiro MMO, Braga VA. Angiotensin-II-derived reactive oxygen species on baroreflex sensitivity during hypertension: new perspectives. Front Physiol. (2013) 4:105. doi: 10.3389/fphys.2013.00105
Keywords: dietary saturated fatty acids, subtype, cardiovascular disease, dose-response, NHANES
Citation: Gou R, Gou Y, Qin J, Luo T, Gou Q, He K, Xiao S, Li R, Li T, Xiao J, Chen Z, Chen Y, Li Y and Zhang Z (2022) Association of dietary intake of saturated fatty acids with hypertension: 1999–2018 National Health and Nutrition Examination Survey. Front. Nutr. 9:1006247. doi: 10.3389/fnut.2022.1006247
Received: 29 July 2022; Accepted: 04 October 2022;
Published: 03 November 2022.
Edited by:
Hui Cai, Vanderbilt University Medical Center, United StatesReviewed by:
Jing Xiao, Nantong University, ChinaEmyr Reisha Isaura, Airlangga University, Indonesia
Qiaolan Liu, Sichuan University, China
Copyright © 2022 Gou, Gou, Qin, Luo, Gou, He, Xiao, Li, Li, Xiao, Chen, Chen, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Li, bGl5b3UxMjEzMDBAMTYzLmNvbQ==; Zhiyong Zhang, cnBhenpAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ruoyu Gou
Ruoyu Gou Yufan Gou2†
Yufan Gou2† Zhiyong Zhang
Zhiyong Zhang