- 1Neurology Department of Shenzhen Second People’s Hospital/First Affiliated Hospital of Shenzhen University Health Science Center, Shenzhen, China
- 2College of Food Science and Technology, Guangdong Ocean University, Zhanjiang, China
- 3Key Laboratory of Precision Nutrition and Food Quality, Ministry of Education, Department of Nutrition and Health, China Agricultural University, Beijing, China
- 4Guangdong Medical University, Guangzhou, China
- 5Shenzhen Evergreen Medical Institute, Shenzhen, China
- 6Graduate School at Shenzhen, Tsinghua University, Shenzhen, China
- 7Institute of Biomedicine, Anhui Medical University, Hefei, China
- 8AUSA Research Institute, Shenzhen AUSA Pharmed Co., Ltd., Shenzhen, China
- 9The First People’s Hospital of Lianyungang City/The First Affiliated Hospital of Kangda College of Nanjing Medical University, Lianyungang, China
- 10Division of Nephrology, Nanfang Hospital, Southern Medical University; National Clinical Research Center for Kidney Disease; State Key Laboratory of Organ Failure Research; Guangdong Provincial Institute of Nephrology, Guangdong Provincial Key Laboratory of Renal Failure Research, Guangzhou Regenerative Medicine and Health Guangdong Laboratory, Guangzhou, China
- 11Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Background: The prospective association between plasma Se and stroke risk remains inconclusive. The relationship between Se and ischemic stroke among a low circulating Se status population deserves more attention, especially for Chinese people who were a high-risk group for Se deficiency.
Objective: The relationship between plasma Se concentration and ischemic stroke risk in a large-scale Chinese community-based population and any potential effect modifiers were investigated.
Methods: A nested, case-control study, using data from the “China H-type Hypertension Registry Study” were conducted. A total of 1,904 first ischemic stroke cases and 1,904 controls matched for age, sex, and village were included in this study. The association between plasma Se and first ischemic stroke was evaluated by conditional logistic regression analyses.
Results: The median value of plasma Se was 65.8 μg/L among total participants. Overall, a significant inverse relationship between plasma Se and first ischemic stroke risk was found (per SD increment; adjusted OR: 0.87; 95% CI: 0.80 and 0.95). Accordingly, a significantly lower risk of first ischemic stroke was found in participants in quartile 3 (65.8−<77.8 μg/L) (adjusted OR: 0.78; 95% CI: 0.63 and 0.96) and quartile 4 (≥77.8 μg/L) (adjusted OR: 0.76; 95% CI: 0.59 and 0.96), compared with those in quartile 1 (<56.0 μg/L). Furthermore, a significantly lower ischemic stroke risk was found in those with lower low-density lipoprotein cholesterol (LDL-C) levels (<3.4 vs. ≥3.4 mmol/L; P for interaction = 0.015) or those with lower homocysteine levels (<12.1 (median) vs. ≥12.1 μmol/L; P for interaction = 0.027) at baseline.
Conclusion: Plasma Se was significantly inversely associated with the risk of first ischemic stroke among a large-scale Chinese community-based population (most adults with hypertension and elevated total homocysteine), especially among those with lower LDL-C and lower homocysteine levels.
Introduction
In 2019, there were nearly four million incident stroke cases in China, which is also a major cause of death and disability, leading substantially to the disease burden (1). In fact, ischemic stroke was the main type of stroke for Chinese with a proportion of more than 70% (2, 3). The adults with hypertension were considered to be at a high-risk group of stroke, and significantly higher risks of first stroke were found in those with elevated total homocysteine (4). More than 75% of adults with hypertension have been found with elevated total homocysteine in China (5). Thus, the identification and management of novel risk factors (6), are essential for stroke prevention among this high-risk group.
Selenium (Se) is a trace element that is a required cofactor for the synthesis of specific enzymes involved in anti-inflammatory processes, such as glutathione peroxidases (7). Relatively low circulating Se status in Chinese populations has been reported (8). Higher serum Se status had been found associated with a lower risk of ischemic heart disease in a Danish population, indicating Se may play a certain role in the prevention of CVD (9). However, in meta-analysis on coronary heart disease as well as some other CVDs, the association of Se and its beneficial effects against disease incidence presented as U-shaped (10). Overall, there were limited data regarding the prospective association between plasma Se status and stroke risk, and the current evidences is inconsistent (11). Serum Se concentration was not significantly associated with stroke in a 10-year follow-up study in Finland (12). Moreover, a cohort study in China indicated a significant reverse association in the relationship between Se and hemorrhagic stroke, but not for ischemic stroke (13). Recent results based on China Stroke Primary Prevention Trial (CSPPT) found between plasma Se was inversely associated with first stroke among Chinese adults with hypertension (14). Thus, the relationship between Se and ischemic stroke among a low circulating Se status population deserves more attention, especially for Chinese people who are a high-risk group in Se deficiency.
Therefore, the prospective association between plasma Se concentrations and first ischemic stroke and its potential modifiers were evaluated among a large-scale Chinese community-based population, through a nested, case-control study.
Materials and methods
Study design and population
Our present study is a subset of the China H-type Hypertension Registry Study (CHHRS; URL: Unique identifier: ChiCTR1800017274)1, which is an ongoing community-based, observational, multicenter, real-world registry study as previously reported (15). The H-type hypertension was defined as hypertension with elevated total homocysteine (tHcy ≥ 10 μmol/L) (16). Eligible participants were those aged over 18 years with essential hypertension, and hypertension was defined as baseline seated, systolic blood pressure (SBP) ≥140 mmHg and/or seated, diastolic blood pressure (DBP) ≥90 mmHg. The exclusion criteria included those who had psychological or nervous system impairment resulting in an inability to demonstrate informed consent or were unable to be followed-up according to the study protocol. The trial consisted of two stages: Screening and recruitment, and a long-term observation follow-up period. Physical examination and clinical outcomes were recorded during follow-ups every 3 months. We used a nested, case-control study design from the population of this community-based CHHRS study of Lianyungang.
Outcomes
The primary outcome of the current study included first ischemic stroke, first hemorrhagic stroke, and first uncertain type stroke. First stroke record information was obtained via the Centers for Disease Control and Prevention of Lianyungang and checked against the national health insurance system with electronic linkage to all hospitalizations or ascertained through active follow-up (15).
Statistical analysis
For continuous variables, data are presented as median (IQR) and differences in baseline characteristics between cases and controls were compared using rank sum tests. For categorical variables, data are presented as frequency (%) and differences in baseline characteristics were evaluated by chi-square tests. The association of plasma Se with ischemic stroke was evaluated as a continuous variable per SD increment and as a categorical variable using quartiles with quartile 1 (Q1) as the reference group. Conditional logistic regression analysis was performed to assess the odds ratios (OR) and 95% confidence intervals (CI) for the association between plasma Se and first ischemic stroke. Potential effect modifications of the association between plasma Se and ischemic stroke were also performed based on previous studies (17, 18). Heterogeneity among subgroups was examined by fitting unconditional logistic regressions and presented in forest plots. Likelihood ratio tests were performed to examine the interactions between subgroups and plasma Se in first ischemic stroke risk. The indirect effect of Se on first ischemic stroke mediated through other risk factors of stroke compared with the total effect of Se on first ischemic stroke was conducted by mediation analysis, using the product of coefficients method (19).
A 2-tailed P < 0.05 was statistically significant in all analyses. (R software version 3.6.1)2 and Empower version (R) (version 3.0; X&Y Solutions, Inc., Boston, MA, USA)3 were used for all statistical analyses.
Results
Study participants and characteristics
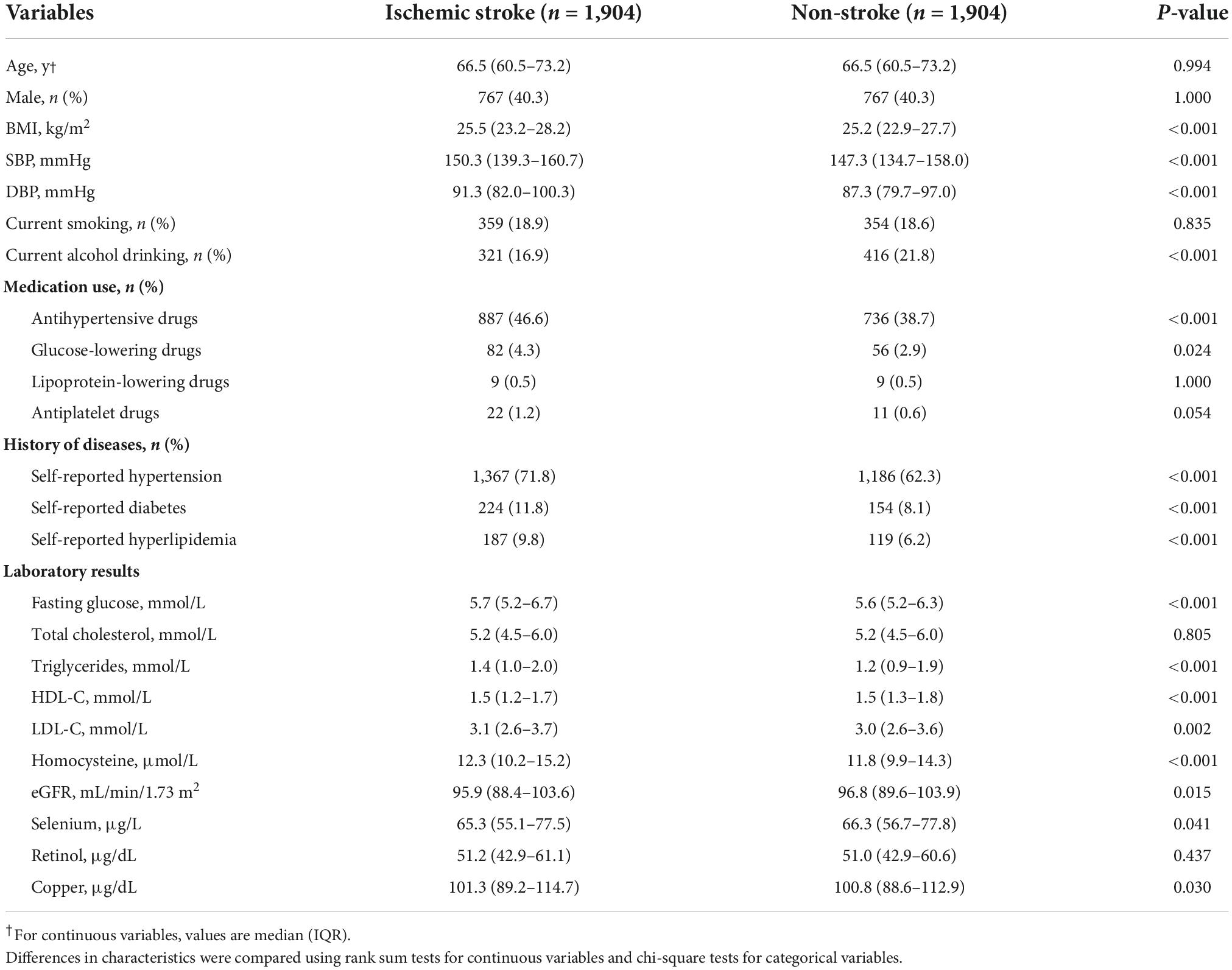
As shown in Figure 1, 1,904 ischemic stroke cases and 1,904 matched controls were included in the current study. Overall, the median of plasma Se was 65.8 μg/L among total participants. Baseline characteristics of the study participants by ischemic stroke cases and controls were presented in Table 1. Median values of plasma Se were lower in participants with stroke (65.3 μg/L) than in control participants (66.3 μg/L) and the difference was statistically significant (P = 0.041). Moreover, ischemic stroke cases had higher BMI, SBP, DBP, FBG, TG, LDL-C, homocysteine, and copper concentration at baseline, while HDL-C and eGFR were lower among ischemic stroke cases than that of controls. Additionally, a higher number of ischemic stroke cases had a history of hypertension, hyperlipidemia and diabetes, and use of glucose-lowering drugs and antihypertensive drugs than that of controls.
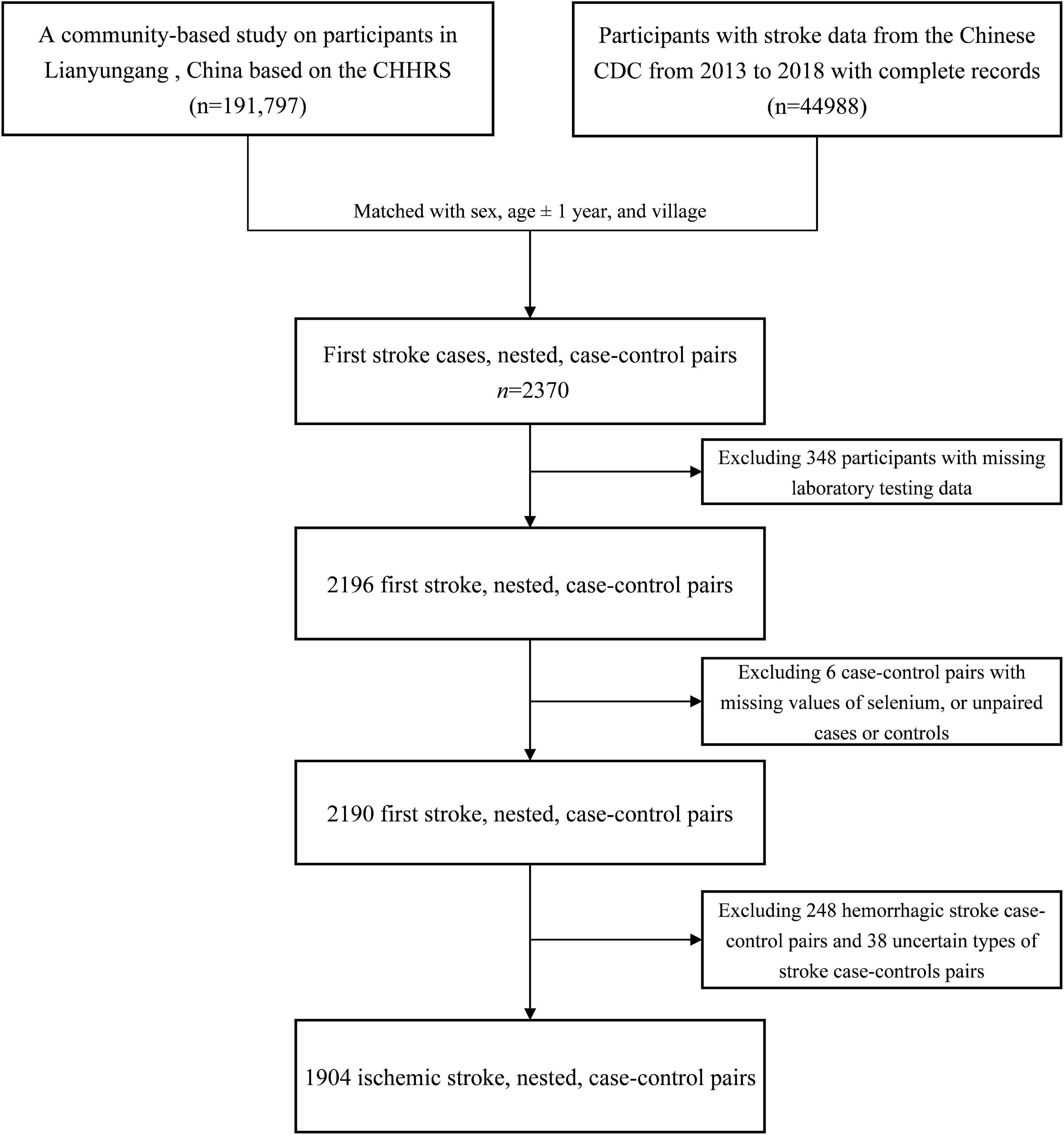
Figure 1. Flowchart of the study participants: A nested case-control design based on the CHHRS in Lianyungang. CHHRS, China H-type Hypertension Registry Study.
In addition, baseline characteristics among participants, stratified by Se concentration, are illustrated in Supplementary Table 2. Participants with higher plasma Se concentrations tended to have higher BMI, SBP, DBP, FBG, TC, HDL-C, LDL-C, retinol, and copper, whereas they tended to have lower homocysteine. Participants with higher Se concentrations have a higher proportion of current smokers and alcohol drinkers and have a history of hypertension, diabetes, and hyperlipidemia and a higher proportion of antihypertensive drugs usage than those with lower Se concentrations. Besides, the baseline characteristics stratified by sex were illustrated in Supplementary Table 1.
Association between selenium and first ischemic stroke
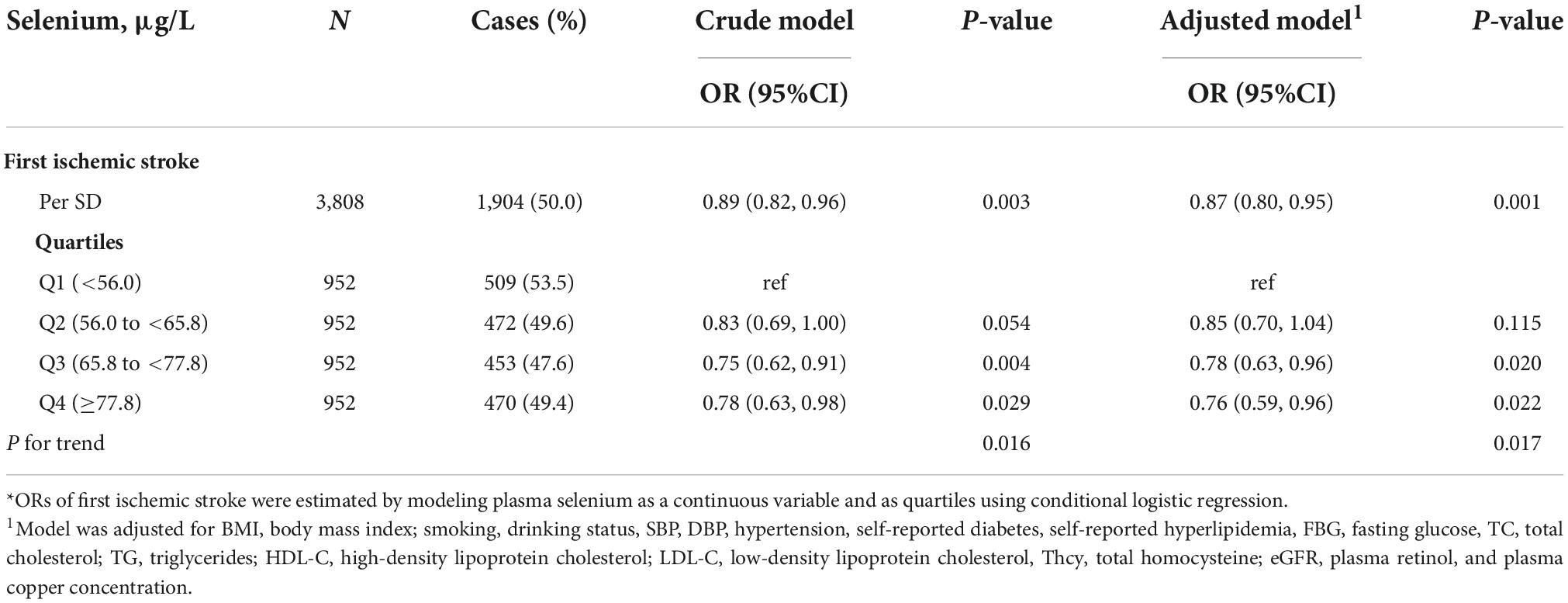
The association between plasma Se and ischemic stroke risk was shown in Table 2. Overall, a significant inverse association between plasma Se and the risk of first ischemic stroke (per SD increment; adjusted OR: 0.87; 95% CI: 0.80 and 0.95) was found, after adjustment for important confounders (Figure 2). Consistently, a significant, lower ischemic stroke risk was found among participants in quartile 3 (65. 8−<77.8 μg/L) and quartile 4 (≥77.8 μg/L). Specifically, the adjusted ORs of quartile 3 and quartile 4 were 0.78 (95% CI: 0.63 and 0.96) and 0.76 (95% CI: 0.59 and 0.96), respectively, when compared with those in quartile 1 (<56.0 μg/L) (P-trend = 0.017).
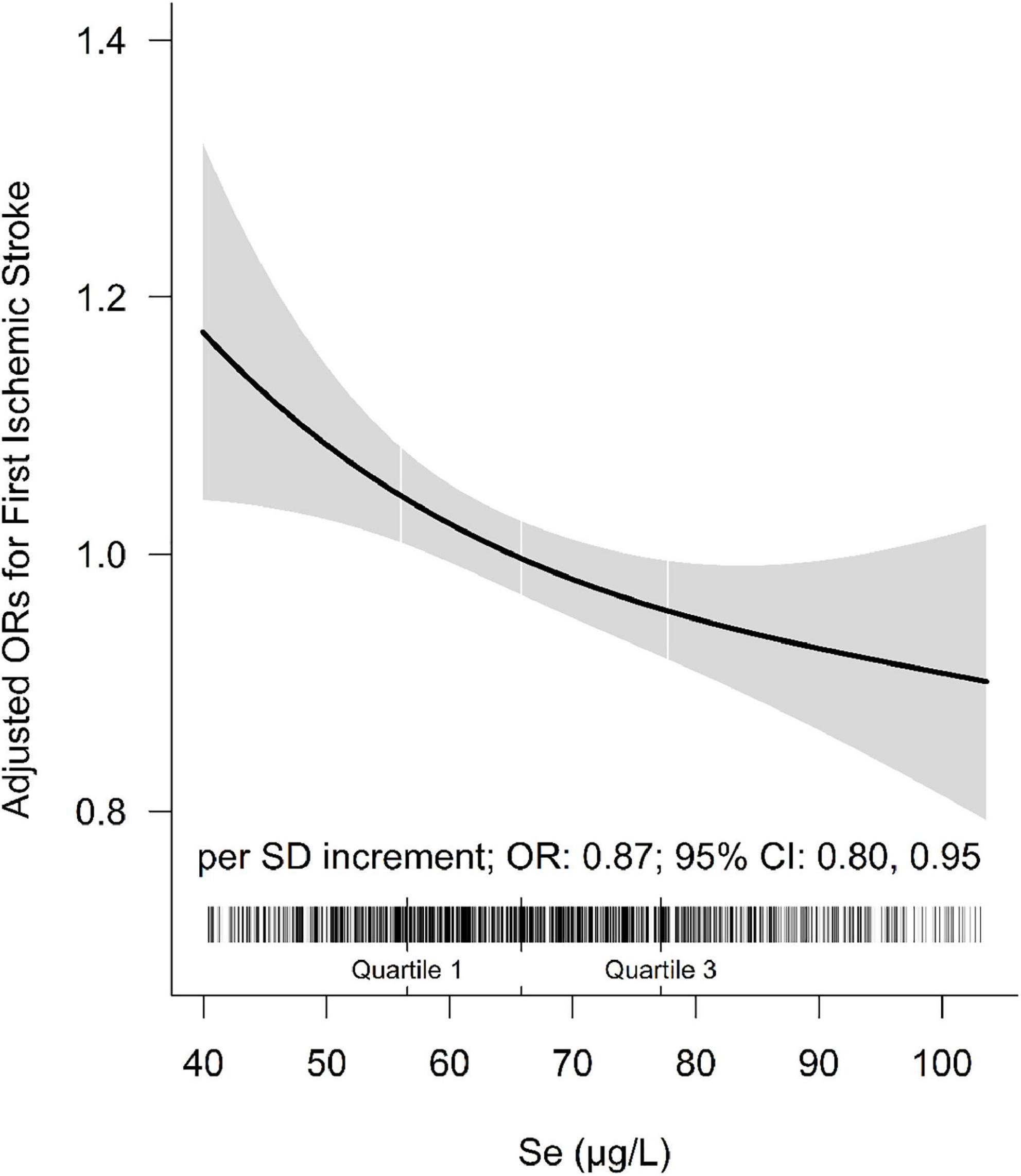
Figure 2. The association between baseline plasma selenium (Se) and risk of ischemic stroke. Adjusted for BMI, body mass index; smoking, drinking status, SBP, DBP, hypertension, self-reported diabetes, self-reported hyperlipidemia, fasting glucose, TC, total cholesterol; TG, triglycerides, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol, tHcy, total homocysteine, eGFR, plasma retinol, and plasma copper concentration.
However, as shown in Supplementary Table 3, further adjustment for history of cardiovascular diseases and the use of medications did not substantially change the significant inverse relationship between plasma Se and first ischemic stroke (per SD increment; adjusted OR: 0.86; 95% CI: 0.79 and 0.94). Moreover, only 3.94% (95% CI: 0.76 and 11.53; P = 0.022) and 4.52% (95% CI: 0.99 and 12.85; P = 0.012) of the observational relationship between Se and risk of first ischemic stroke was mediated through TC and DBP, respectively (Supplementary Table 4).
Subgroup analyses
The relationship of plasma Se concentration (per SD increment) with the risk of first ischemic stroke in various subgroups was examined to reveal possible modifiers (Figure 3 and Supplementary Figure 1).
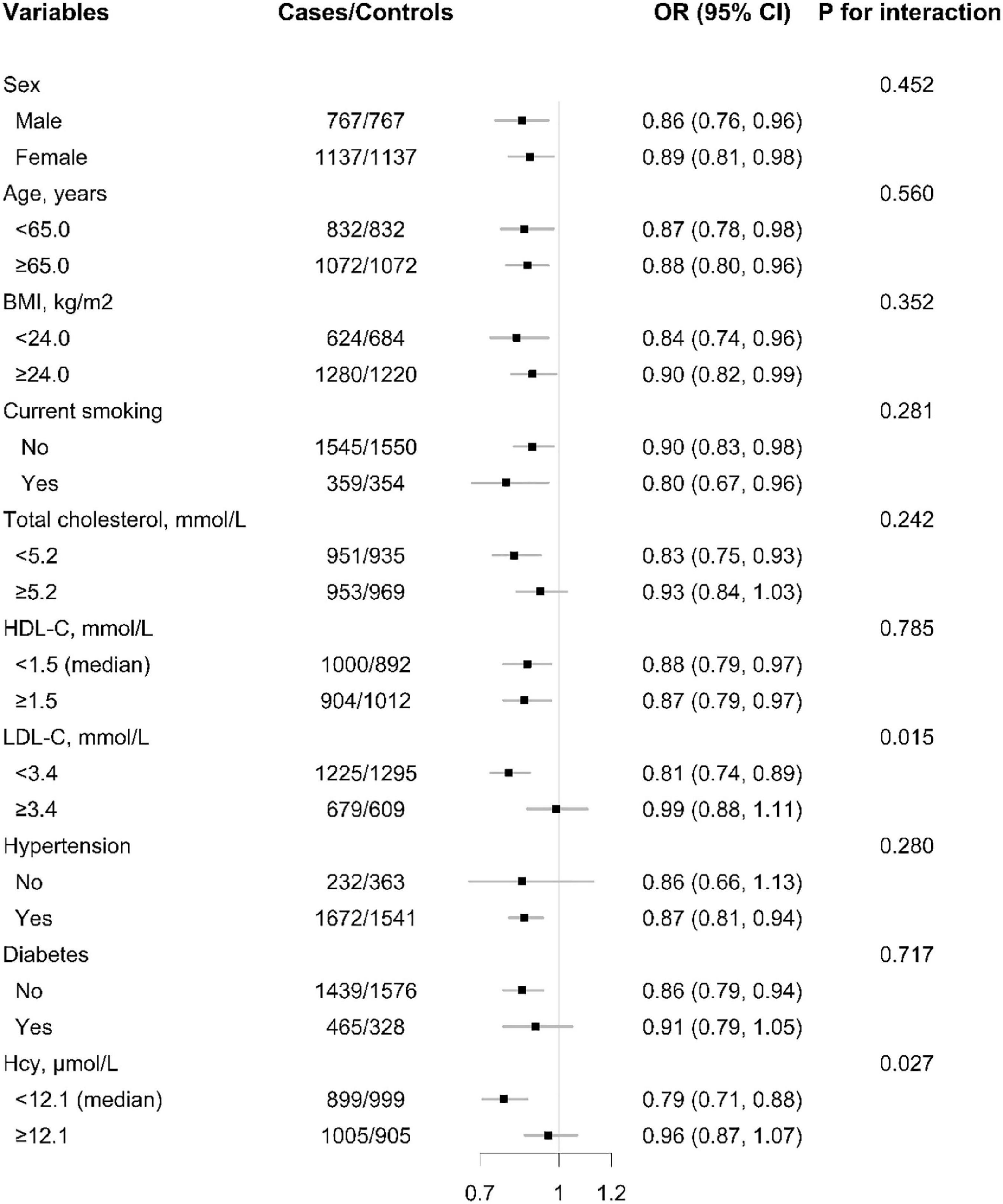
Figure 3. The association between baseline plasma selenium (Se) (per SD increment) concentration and risk of ischemic stroke among each subgroup. ORs of ischemic stroke in relation to plasma concentrations of selenium (Se) (per SD increment) were calculated using unconditional logistic regression models. P-value for interaction were calculated using log likelihood ratio tests. Each subgroup analysis adjusted, if not stratified for sex, age, BMI, body mass index; smoking, drinking status, SBP, DBP, hypertension, self-reported diabetes, self-reported hyperlipidemia, FBG, fasting glucose; TC, total cholesterol, TG, triglycerides, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Thcy, total homocysteine, eGFR, plasma retinol, and plasma copper concentration.
A significantly lower risk of first ischemic stroke was found in those with LDL-C < 3.4 mmol/L (adjusted OR: 0.81; 95% CI: 0.74 and 0.89; vs. ≥3.4 mmol/L; adjusted OR: 0.99; 95% CI: 0.88 and 1.11; P for interaction = 0.015). Moreover, the inverse association between plasma Se and first ischemic stroke risk was also modified by homocysteine levels and a significantly lower ischemic stroke risk was found in participants with tHcy < 12.1 μmol/L (adjusted OR: 0.79; 95% CI: 0.71 and 0.88; vs. ≥12.1 μmol/L; adjusted OR: 0.96; 95% CI: 0.87 and 1.07; P for interaction = 0.027) (Figure 3).
Discussion
The current study shows that, based on the largest nested case-control study in a Chinese population with relatively low plasma Se levels so far, a higher baseline Se concentration is associated with a lower risk of first ischemic stroke. Moreover, the inverse association of plasma Se with first ischemic stroke was more pronounced in participants with HDL-C < 3.4 mmol/L and those with tHcy < 12.1 μmol/L.
Overall, relatively limited longitudinal studies reported the association of circulating Se status with stroke risk, and their findings were still inconsistent. Based on the Chinese Dongfeng–Tongji cohort, it was found that Se was significantly negatively correlated with hemorrhagic stroke, but not with ischemic stroke (per IQR increment, adjusted OR:0.92; 95% CI: 0.82–1.05) (14). Another study found that plasma Se was significantly non-linear associated with first stroke in males (20). However, no significant association was found between serum Se and stroke in the Finnish elderly (12). It is worth noting that most previous studies have failed to exclude the traditional risk factors of stroke and the influence of other antioxidants as possible, so it may not be sufficient to fully reveal the relationship between Se and stroke risk. Especially in the Chinese population with relatively low status of Se and high-risk of Se deficiency, the evidence concerning the association between Se and stroke and its possible potential effect modifiers is still insufficient.
This study can comprehensively evaluate the dose relationship of plasma Se concentration with first ischemic stroke risk, among a large-scale Chinese community-based population with relatively low circulating Se status. Our study provides two new perspectives. First of all, this study is the largest one conducted in a Chinese community-based population with low circulating Se status so far, to evaluate the relationship between plasma Se concentration and first ischemic stroke. Plasma Se concentration was found to be inversely associated with the risk of first ischemic stroke in this study. This finding is consistent with that of a nested case-control study among a Chinese hypertensive population, which indicated first stroke and ischemic stroke risk were decreased when increasing plasma Se concentration (14). This condition might be because Se and selenoproteins are required as modulators in brain metabolism (21, 22). Glutathione peroxidase (GPX) is one of the most studied Se-dependent proteins (selenoproteins), which reduces brain damage and edema as well as inflammatory infiltration in animal models with ischemic stroke (23). Moreover, Se was considered as an antioxidant for humans, and its potential in scavenging free radicals and modulating inflammation may play a key role in lowering ischemic stroke risk (24–26). However, more research is needed to verify our findings and further examine the potential biological mechanisms.
Second, our results indicated that the relation of plasma Se with first ischemic stroke was modified by LDL-C and total homocysteine concentrations. The risk of first ischemic stroke was significantly lower among those participants with lower LDL-C concentrations (<3.4 mmol/L) and those with lower total homocysteine concentrations (<12.1 μmol/L). In our study, an increase in blood pressure, FBG levels, TC levels, and LDL-C levels corresponded to the increase of plasma Se concentrations, in keeping with the results of several previous large cross-sectional studies (27–29). Hyperlipidemia is a widely accepted risk factor of stroke, thereby Se and blood lipids may compete and inhibit each other in stroke prevention (1). Based on our data, the beneficial effect on first ischemic stroke associated with high concentrations of plasma Se may be attenuated by the adverse effect of higher LDL-C concentrations. Previous studies found interactions of serum copper, Se, and low-density lipoprotein cholesterol in atherogenesis (30). And another study indicated that dietary Se increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients (31). We also found that the relationship between plasma Se and first ischemic stroke was partially mediated by TC (Supplementary Table 4), which indicated the benefit of Se on ischemic stroke may be partially through the potential effect of Se on lowering TC. Overall, the interaction between plasma Se and blood lipids may play a certain role in the primary prevention of first ischemic stroke, and it still needs to be further confirmed. Additionally, the inverse relationship of plasma Se with first ischemic stroke risk was more prominent in participants with tHcy < 12.1 μmol/L in our study. This finding is consistent with the results of CSPPT (14, 32) in it was which reported that significantly lower first stroke risk was found among those with higher baseline folate concentration. Se may mediate endothelial protective effects and thus reduce the high-risk of stroke due to hypertension and high homocysteine. A relevant study found that Se inhibits homocysteine-induced endothelial dysfunction and apoptosis via activation of AKT (33). Thus, proper increment of dietary Se intake or Se supplementation (34–36), along with homocysteine lowering therapy (HLT) in individuals with hypertension and hyperhomocysteinaemia (Hhcy), appears to be a more acceptable strategy for stroke primary prevention based on the present study. In general, this study demonstrated that maintaining appropriate plasma Se, LDL-C and Hcy levels may be of great significance for primary prevention of stroke.
There were several limitations of this work. Firstly, although a wide range of covariates are included in the regression model, residual confounding effects of unmeasured factors cannot be excluded, so these findings should be interpreted with caution. Secondly, we only assessed baseline plasma Se concentrations for the enrolled patients. The relationship between plasma Se concentration and first ischemic stroke risk might be better illustrated if plasma Se concentration were tested at different time points during follow-up. Thirdly, since this was an analysis based on a subset study of the CHHRS, dietary information related to Se supplementation was not collected at baseline. However, dietary Se supplementation would only have a minimal impact on our population, since we enrolled people from the same study site (Lianyungang, China) who shared similar regional soil, water supply, and dietary habits. In future research, we plan to collect more detailed information on dietary Se supplementation and expand the population base to include the whole country. Fourthly, the generalization of our findings to the general population should be cautious. However, there were no differences in the relation of Se with first ischemic stroke among the normal subjects or those with hypertension in the subgroup analysis when excluding many potential confounders. Overall, our results should be considered as hypothesis generation. More research is needed to verify our results and reveal the biological mechanisms involved.
Conclusion
We indicated that plasma Se concentrations were inversely associated with first incident ischemic stroke among an Eastern Chinese community-based population, especially in those with lower plasma LDL-C and tHcy levels. A Chinese population, especially those with hypertension and elevated total homocysteine, with well-controlled lipid and tHcy levels should be the target for future investigations on Se supplementation for the primary prevention of stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZW and SH conceived and designed the study and wrote the manuscript. YS, LL, HZ, HG, BW, XQ, YS, and XX contributed to the study concept and design. LR designed and conceptualized the study. ZZ, YW, and TL acquired the data. ZW and LL performed the data management. BR, ZW, and SH interpreted the data. MH contributed to data cleaning and methodology rechecking. All authors critical review and revision of the manuscript for important intellectual content.
Funding
This study was supported by the Shenzhen Science and Technology Innovation Committee (GJHZ20200731095602009, GJHS20170314114526143, and JSGG20180703155802047), National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, and 2018ZX09301034003), Department of Science and Technology of Guangdong Province (2020B121202010), and Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390, and 201705051617070).
Acknowledgments
We acknowledge the contribution of all the staff who participated in this study as well as the study participants.
Conflict of interest
Author YS was employed by Shenzhen AUSA Pharmed Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1001922/full#supplementary-material
Footnotes
References
1. Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the global burden of disease study 2019. Lancet Public Health. (2021) 6:e897–906.
2. Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480?687 adults. Circulation. (2017) 135:759–71. doi: 10.1161/CIRCULATIONAHA.116.025250
3. GBD 2016 Lifetime Risk of Stroke Collaborators Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
4. Li J, Jiang S, Zhang Y, Tang G, Wang Y, Mao G, et al. H-type hypertension and risk of stroke in Chinese adults: a prospective, nested case-control study. J Transl Int Med. (2015) 3:171–8.
5. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. (2015) 313:1325–35.
6. Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. (2018) 362:k3310.
7. Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev. (2004) 45:164–78. doi: 10.1016/j.brainresrev.2004.03.004
8. Li N, Gao Z, Luo D, Tang X, Chen D, Hu Y. Selenium level in the environment and the population of Zhoukoudian area, Beijing, China. Sci Total Environ. (2007) 381:105–11. doi: 10.1016/j.scitotenv.2007.03.027
9. Suadicani P, Hein HO, Gyntelberg F. Serum selenium concentration and risk of ischaemic heart disease in a prospective cohort study of 3000 males. Atherosclerosis. (1992) 96:33–42. doi: 10.1016/0021-9150(92)90035-f
10. Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. (2006) 84:762–73.
11. Ding J, Zhang Y. Relationship between the circulating selenium level and stroke: a meta-analysis of observational studies. J Am Coll Nutr. (2022) 41:444–52.
12. Marniemi J, Alanen E, Impivaara O, Seppänen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. (2005) 15:188–97. doi: 10.1016/j.numecd.2005.01.001
13. Xiao Y, Yuan Y, Liu Y, Yu Y, Jia N, Zhou L, et al. Circulating multiple metals and incident stroke in chinese adults. Stroke. (2019) 50:1661–8.
14. Wang Z, Ma H, Song Y, Lin T, Liu L, Zhou Z, et al. Plasma selenium and the risk of first stroke in adults with hypertension: a secondary analysis of the China stroke primary prevention Trial. Am J Clin Nutr. (2022) 115:222–31.
15. Hu L, Bi C, Lin T, Liu L, Song Y, Wang P, et al. Association between plasma copper levels and first stroke: a community-based nested case-control study. Nutr Neurosci. (2022) 25:1524–33. doi: 10.1080/1028415X.2021.1875299
16. Liu LS. Writing group of 2010 Chinese guidelines for the management of hypertension. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. (2011) 39:579–615.
17. Yu Y, Zhang H, Song Y, Lin T, Zhou Z, Guo H, et al. Plasma retinol and the risk of first stroke in hypertensive adults: a nested case-control study [published correction appears in Am J Clin Nutr. 2019 Jun 1;109(6):1746]. Am J Clin Nutr. (2019) 109:449–56.
18. Zhang J, Cao J, Zhang H, Jiang C, Lin T, Zhou Z, et al. Plasma copper and the risk of first stroke in hypertensive patients: a nested case-control study. Am J Clin Nutr. (2019) 110:212–20.
19. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. (2010) 15:309–34.
20. Hu H, Bi C, Lin T, Liu L, Song Y, Wang B, et al. Sex difference in the association between plasma selenium and first stroke: a community-based nested case-control study. Biol Sex Differ. (2021) 12:39. doi: 10.1186/s13293-021-00383-2
21. Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. (2019) 177:1262–79.e25. doi: 10.1016/j.cell.2019.03.032
22. Mahadik SP, Makar TK, Murthy JN, Ortiz A, Wakade CG, Karpiak SE, et al. Temporal changes in superoxide dismutase, glutathione peroxidase, and catalase levels in primary and peri-ischemic tissue. Monosialoganglioside (GM1) treatment effects. Mol Chem Neuropathol. (1993) 18:1–14. doi: 10.1007/BF03160018
23. Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, et al. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2010) 91:923–31. doi: 10.3945/ajcn.2009.28169
24. Zhang C, Ge J, Lv M, Zhang Q, Talukder M, Li JL. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ Pollut. (2020) 260:113873. doi: 10.1016/j.envpol.2019.113873
25. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. (2012) 16:705–43. doi: 10.1089/ars.2011.4145
26. Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER III, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. (2008) 88:416–23.
27. Vinceti M, Chawla R, Filippini T, Dutt C, Cilloni S, Loomba R, et al. Blood pressure levels and hypertension prevalence in a high selenium environment: results from a cross-sectional study. Nutr Metab Cardiovasc Dis. (2019) 29:398–408. doi: 10.1016/j.numecd.2019.01.004
28. Li Z, Li X, Ju W, Wu G, Yang X, Fu X, et al. High serum selenium levels are associated with impaired fasting glucose and elevated fasting serum glucose in Linyi, China. J Trace Elem Med Biol. (2018) 45:64–9. doi: 10.1016/j.jtemb.2017.09.023
29. Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E. Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med. (2011) 154:656–65.
30. Salonen JT, Salonen R, Seppänen K, Kantola M, Suntioinen S, Korpela H. Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. BMJ. (1991) 302:756–60. doi: 10.1136/bmj.302.6779.756
31. Hussein O, Rosenblat M, Refael G, Aviram M. Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients. Transplantation. (1997) 63:679–85. doi: 10.1097/00007890-199703150-00012
32. Huang X, Li Y, Li P, Li J, Bao H, Zhang Y, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology. (2017) 89:2101–7. doi: 10.1212/WNL.0000000000004648
33. Ren H, Mu J, Ma J, Gong J, Li J, Wang J, et al. Selenium inhibits homocysteine-induced endothelial dysfunction and apoptosis via activation of AKT. Cell Physiol Biochem. (2016) 38:871–82. doi: 10.1159/000443041
34. D’Amato R, Regni L, Falcinelli B, Mattioli S, Benincasa P, Dal Bosco A, et al. Current knowledge on selenium biofortification to improve the nutraceutical profile of food: a comprehensive review. J Agric Food Chem. (2020) 68:4075–97. doi: 10.1021/acs.jafc.0c00172
35. Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. (2008) 400:115–41.
Keywords: first ischemic stroke, hypertension, LDL-C, homocysteine, selenium
Citation: Wang Z, Hu S, Song Y, Liu L, Huang Z, Zhou Z, Wei Y, Lin T, Huang M, Zhang H, Guo H, Sun Y, Wang B, Qin X, Xu X, Chi F, Ren B and Ren L (2022) Association between plasma selenium and risk of ischemic stroke: A community-based, nested, and case-control study. Front. Nutr. 9:1001922. doi: 10.3389/fnut.2022.1001922
Received: 24 July 2022; Accepted: 19 October 2022;
Published: 18 November 2022.
Edited by:
Diego Augusto Santos Silva, Federal University of Santa Catarina, BrazilReviewed by:
Fangrong Yan, China Pharmaceutical University, ChinaQun Wang, Chinese PLA General Hospital, China
Copyright © 2022 Wang, Hu, Song, Liu, Huang, Zhou, Wei, Lin, Huang, Zhang, Guo, Sun, Wang, Qin, Xu, Chi, Ren and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijie Ren, MTM2MzE2MDU5NjZAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhuo Wang
Zhuo Wang Shiyu Hu1†
Shiyu Hu1† Yun Song
Yun Song Lishun Liu
Lishun Liu Zhengzheng Huang
Zhengzheng Huang Yaping Wei
Yaping Wei Meiqing Huang
Meiqing Huang Huiyuan Guo
Huiyuan Guo Binyan Wang
Binyan Wang Xianhui Qin
Xianhui Qin Xiping Xu
Xiping Xu Lijie Ren
Lijie Ren