
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 20 January 2022
Sec. Nutrition and Food Science Technology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.788446
 Mohamed A. E. Gomaa1†
Mohamed A. E. Gomaa1† Marwa G. Allam1†
Marwa G. Allam1† Abdallah A. I. M. Haridi2,3†
Abdallah A. I. M. Haridi2,3† Alaa-Eldin M. Eliwa3†
Alaa-Eldin M. Eliwa3† Amira M. G. Darwish4*†
Amira M. G. Darwish4*†Upcoming developments are attracting attention to both high-protein and probiotics supplementation for the sports community to promote good health and exercise performance. This study aimed at the production of high-protein concentrated pro-yogurt (Pro-WPI) enriched with 10 and 20% whey protein isolate (WPI) and investigation of the response of daily consumption on anthropometric, hematology parameters, and athletic performance in parallel with safety consideration assessment. Twenty-four athletes (19.6 ± 1.45 years; 175.96 ± 5.24 cm; 73.16 ± 8.65 kg) were participated in a randomized placebo-control study. They consumed Pro-WPI products with 10 (T1) and 20% (T2) WPI for treatments G1 (Pro-WPI30) and G2 (Pro-WPI60), respectively, 3 times per day/5 days per week/9 weeks. The taste of Pro-WPI products was sour and cheesy, while mouthfeel was described as soft and thick because of the increased protein content in T1 and T2 (14.15 and 22.58%). The hemoglobin of the athletes increased significantly from a baseline of 12.69 g/dl to 16 and 16.66 g/dl in G1 and G2, respectively. Furthermore, the athletic performance was enhanced in vertical jump, long jump, sprinting velocity, half squats, and pushups, which reached 58.75 cm, 255 cm, 3.5 m/s, 218.75 counts, and 85 counts, respectively in G2. The healthy gut microbiome (probiotics) in parallel with increased iron bioavailability by mineral binding (whey bioactive peptides), influenced iron status and can represent a healthy practice to improve athletic anemia and performance. On the other hand, urinary albumin exceeded the border of reference range (<30 mg/g) and reached 38.25 and 44.13 mg/g in G1 and G2, while urine pH was in the normal range (4.5–8). Increased urinary albumin might be due to high rates of protein metabolism that follow high protein intake. This study provided preliminary information on metabolic responses to high protein concentrated yogurt intake in athletes who engaged in daily exercise. Further studies are needed to determine the recommended intensity of 10 and 20% Pro-WPI product consumption to achieve its benefits and avoid implications on kidney function.
Adequate protein is required to optimize the rate of muscle protein synthesis and achieve positive net muscle protein balance. A daily protein intake of 1.2–1.7 g protein/kg body weight (BW) has been suggested for athletes and bodybuilders to maintain muscle mass. Body proteins will be broken down to supply the energy needs of the body if dietary protein is insufficient (1). Additionally, high protein diets are increasingly popularized as a promising strategy for weight loss by providing the twin benefits of improving satiety and decreasing fat mass (2, 3). Anemia is a sign of malnutrition as red blood cells (RBCs) and Hb values are insufficient to maintain body health. Decreased Hb levels in the blood cause symptoms of fatigue, as Hb functions as an oxygen carrier (4). Athletes generally have lower hemoglobin concentrations than the general population that so-called “sports anemia”. Sports anemia is an iron deficiency caused by increased nutritional demands, dietary restrictions, decreased absorption and increased energy losses due to aerobic exercise. The anemia in athletes deserves a careful and multifactorial approach including iron bioavailability. Iron-depleted athletes improved their iron status and, possibly, physical performance, in addition to a healthy gut microbiome that also influences iron status (5).
Whey protein (WPs) are high-quality proteins that contain essential amino acids and can help in gaining muscle mass and improved performance. WP consumption has been popular, particularly among athletes (6, 7). Mineral binding properties of whey bioactive peptides and intestinal microbiota regulation increase mineral absorption in the digestive tract. Furthermore, WPs improve sensory characteristics of food, which are the result of their technological properties (8). Although dependent on the source of protein, debate on potential effects of excess protein supplement intake focused on implications on kidney function. Thus, albumin and urinary pH values are important parameters to be monitored in high-protein diet groups (2).
Probiotic microorganisms are increasingly applied to enhance the nutritional quality of cultured dairy products (9). Probiotics, such as Lactobacilli, obtain the ability to hydrolyze proteins present in their environment. This proteolytic activity not only generates the free amino acids needed by bacteria but also a large variety of peptides, some of which are endowed with biological activities (10). Lactobacillus casei is one of the most studied species in athletes and active individuals. Probiotics supplementation in athletes affects physiological changes in gut microbiota and immune function. Additionally, probiotic products contain energy and carbohydrates that are recommended to be part of overall nutrition plan of an athlete (11).
The term “functional food” is often used to refer to food products with demonstrated physiological benefits that are useful to the human body in some way (12). Functional foods designed for athletes have emerged as a novel sector of special purposes food products (13). Yogurt is considered a good vehicle for delivering innovative dairy products, in addition to the probiotic functional role. High-protein yogurts with high content of whey proteins could be beneficial in sports nutrition due to the ability of whey proteins to increase amino acids, trigger muscle protein synthesis and help in calorie-restricted diet (14, 15).
This study aimed at the production of high-protein concentrated pro-yogurt (Pro-WPI) enriched with 10 and 20% WP isolate, and investigation of the response of their daily consumption on anthropometric, hematology parameters, and athletic performance in parallel with safety consideration assessment.
Raw milk (containing 3% fat, 3.1% protein, and 12.25% total solid) of the cow was obtained from the Faculty of Agriculture Farm, Alexandria University, Alexandria, Egypt. Skimmed milk powder (SMP) was obtained from Dairy America, Inc. (CA, United States), and it was composed of 34% protein, 51% lactose, 1.2% fat, 8.2% minerals, and 4% moisture. Whey protein isolate (WPI) was obtained from Burt Lewis Ingredients, LLC (United States), and it was composed of 91% protein, 6% moisture, and 3% fat.
Commercial freeze-dried lactic acid starter culture for direct-to-vat set (DVS) (Actimel); containing Lactobacillus casei CNCM-1518 (LFMP) was obtained from Danone, Egypt. In addition, yogurt commercial starter culture (Yo-Mix 495) 100 Direct Culture Unit (DCU) containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus was obtained from Danisco (Egypt). The cultures were kept at −18°C.
The milk of cow (12.25% TS) was standardized to 14% total solids with SMP, pasteurized (82°C for 10 s, cooled to 38°C, and inoculated with Yo-Mix 495 (0.1 DCU/L w/v) for control plain yogurt preparation (16). In the case of Pro-WPI, WPI was reconstituted in the pasteurized cow milk at 4°C at ratios 10 and 20% WPI for treatments Pro-WPI30 and Pro-WPI60, respectively. The mixes were then blended using a kitchen machine at high speed for 3 min to homogenize, and aged at 4°C for 18 h. The homogenized milk was then heated at 82°C for10 s, and cooled to 38°C to be cultured with 109 CFU of the probiotic culture Lactobacillus casei CNCM-1,518 an hour before inoculation with Yo-Mix 495 (0.1 UDC/L). After distribution in 100-g cups, the products were incubated at 38°C for 3.5 h until coagulation, and subsequently stored at 4°C.
The chemical composition of control plain yogurt and Pro-WPI products was evaluated according to (17). pH was measured using a digital pH meter (AD3000; ADWA, Hungary).
Serial dilutions of Pro-WPI yogurt samples were prepared for microbial enumeration using a buffered sodium chloride peptone solution (pH 7) containing potassium dihydrogen phosphate 3.5 g/L, disodium hydrogen phosphate anhydrous 5.8 g/L, sodium chloride 4.3 g/L, and peptone (meat) 1 g/L. Total count enumeration was performed on MRS agar (Biolife, Italy) and incubated at 38°C/72 h (18). Colony-forming units were counted and expressed as Log10 CFU/g.
The sensory evaluation of Pro-WPI yogurt samples was assessed by a trained committee at The Faculty of Agriculture Saba Basha, Alexandria University, Egypt, on fresh products according to (19, 20), with some modifications. The 7-member sensory evaluation panel was asked to assess Pro-WPI30 and Pro-WPI60 in contrast with the control (Pro-WPI). The samples, which were stored at 4°C, were allowed to rest at room temperature (25°C) 10 min before evaluation. The samples were evaluated using a 5-point Hedonic scale (ISO, 2009). This scale consisted of the following parameters: odor (milky, yogurt, cheese, and sour milk), appearance (compact, bubbles, heterogeneous, soft, and thick), flavor (creamy, buttery, cheese, acid, sweet, bitter, and sour milk), and mouthfeel (light, thick, floury, sandy, and small lumps), accompanied by a scale of five categories as: 5 = extremely and 1= slightly.
Twenty-four athletes (19.6 ± 1.45 years; 175.96 ± 5.24 cm; 73.16 ± 8.65 kg) participated in this randomized double-blind placebo control study. For maintained and unified diet, lifestyle, and physical training, all the participants were accommodated at Student Housing Dormitories and studied at The Faculty of Physical Education for Men, Abu Qir, Alexandria University, Egypt. Eligibility testing (blood panel, eligibility for exercise, clinic checkup, and one-on-one interview) was finalized prior to the first exercise test. Inclusion criteria were: healthy, non-smokers, and no dietary or nutritional supplement use within 4 weeks prior to the first exercise test as described by the Austrian and German standards in sports medicine (21, 22). Standard hematology biochemical analyses were determined after overnight fast. Baseline characteristics of the participants before intervention are presented in Table 1.
This study was performed in accordance with the World Medical Association (WMA) Declaration of Helsinki–ethical principles for medical research involving human subjects and approved by the postgraduate and research council and the ethical committee of The Faculty of Physical Education for Men, Abu Qir, Alexandria University. The athletes were briefed about the procedures of this study before signing informed consent form; all information and consent were written in the mother tongue (Arabic).
The participants were instructed to maintain their habitual diet at Student Housing Dormitories, and their lifestyle and training regimen during the 9 weeks of study. Diets were proportionally equivalent in macro-and micronutrient quantity for all the participants, containing 100% of the recommended daily allowance (RDA) for all nutrients. The athletes were strictly monitored, and compliance with the study was assessed weekly by individual interview.
The twenty-four participants were equally randomized into three groups (eight participants each). During the 9 week experimental period, the students received the products 3 times for 5 days per week in parallel with their meals. The placebo group (C) received plain control yogurt; G1 received Pro-WPI 10% WPI-enriched fermented dairy product (Pro-WPI 30), delivering 30 g WPI daily dose; and G2 received Pro-WPI 20% WP-enriched fermented dairy product (Pro-WPI 60), delivering 60 g WPI daily dose. All the participants were checked by the physician before each exercise test.
The anthropometric measurements taken for the assessment of participants were height, body weight, body mass index (BMI), basal metabolic rate (BMR), and body composition with the bioelectrical impedance analysis (BIA) method that indirectly estimates fat-free mass (FFM) and total body water (TBW) according to (23). An anthropometric rod was used for measuring the height of the participants and was recorded in cm. Measurement was taken from the floor to the vertex of the head. The participants were asked to stand erect against a wall on an even surface with feet close to each other, with the hips, back, and heels touching the wall. The body weight of the participants was measured in kg with a portable weighing machine and minimum calibration of 0.5 kg. The accuracy of the machine was checked before the subjects were asked to stand still on the platform of the machine and the bodyweight of the subjects was recorded. Body mass index (BMI), or Quetelet's index, is a measure for human body shape based on the body weight and height (kg/m2) of a subject. Basal metabolic rate (BMR) was considered as a dependent variable. Fat percentage, fat mass, free mass, and total body water (TBW) were considered as independent variables. To obtain accurate results, the participants were instructed to go overnight fasting and not to conduct any heavy physical activity the previous day, and their normal hydration status was ensured. Data were represented as means of n = 8 for each group ± SD.
Blood samples were collected at 8.00 am on an empty stomach from each subject at the beginning and end of the experiment for complete blood count (CBC) analyses (RBCs, WBCs, Hb, platelets, MCV, and MCH) as anemia indicator. The participants were instructed to collect their urine (discarding the first urine) for determining urine color, turbidity, pH, specific gravity, glucose, bilirubin, pus cells, RBC, ketones, and albumin. All biochemical analyses were conducted using an automated chemistry analyzer (Hitachi, Tokyo, Japan) according to standard methods described in Tietz Textbook and Tietz Clinical Guide (24, 25). The data were represented as means of n = 8 for each group ± SD.
The participants were instructed not to perform physical training 3 days prior to any exercise test. The athletes completed a 20-min general standardized warm-up, including 15 min of general exercises (i.e., 10 min of running at a moderate pace followed by 5 min of lowerlimb active stretching) and, prior to each test, 5-min of test-specific exercises. For vertical jumping, the participants were instructed to execute a downward movement followed by complete extension of the legs with the hands fixed on the hips. The standing long jump test, also called the Broad Jump, is a common and easy to administer test of explosive leg power. An excellent result is >250 cm for men. A total of five attempts were allowed for both vertical and long jumps, interspersed with a 15-s interval. The best attempts were retained. Sprinting velocity (in 30 m) was measured in m/s, while pushups and half-squat exercises were counted. The data were represented as means of n = 8 for each group ± SD.
The data were presented as mean values ± standard deviation. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan's test. Differences were considered significant at (p < 0.05). The IBM SPSS Statistics 23 software program was used for statistical analyses [IBM Corp (2015) IBM SPSS Statistics for Windows, Version 23.0; IBM Corp, Armonk, NY, United States].
The chemical composition of the Pro-WPI yogurt products compared to that of the plain control is presented in Table 1. Comparing to plain control, the significant increase in protein, fat, ash and total solids content is expected due to concentrated protein content of applied WPI (91%), fat (3%) and its minerals content (2.3%). This enrichment achieved increase in protein content of up to 14.15 and 22.58 % in T1 and T2, respectively. On the other hand, T2 contained 2-folds of WPI as T1, which led to elevated nutrient content. These results may explain the elevated SNF % (0.22, 0.13, and 0.11) and decreased FDM % (11.4, 21.56, and 30.67) for C, T1, and T2, respectively. Based on the Codex standard definition of “concentrated fermented milk,” it is hereby proposed that “high-protein yoghrt” is a yoghrt containing a minimum of 5.6% protein and <15% fat (26).
During processing, starting from pH 6.6 for all the yogurt products, monitoring of pH showed that the high protein Pro-WPI yogurt products showed an insignificant decrease in pH after 180 min, 4.4 and 4.68 for T1 and T2, respectively, against 5.2 for the plain control yogurt, and the same pattern was observed during the storage period, 3.5 and 3.12 for T1 and T2, respectively, against 3.78 for the control on the 15th day of cold storage (Figure 1). The results obtained were consistent with acidity values of 1.27, 1.3, and 1.34 for C, T1, and T2 respectively (Table 1). Titratable acidity was reported to increase as milk protein content increases, as milk has a base acid content attributed to proteins, minerals, and dissolved gasses, in addition to bacterial activity. On the other hand, milk protein has a strong buffering capacity that resists changes in acid or alkali content, which may have affected this increase in acidity to be insignificant (27).
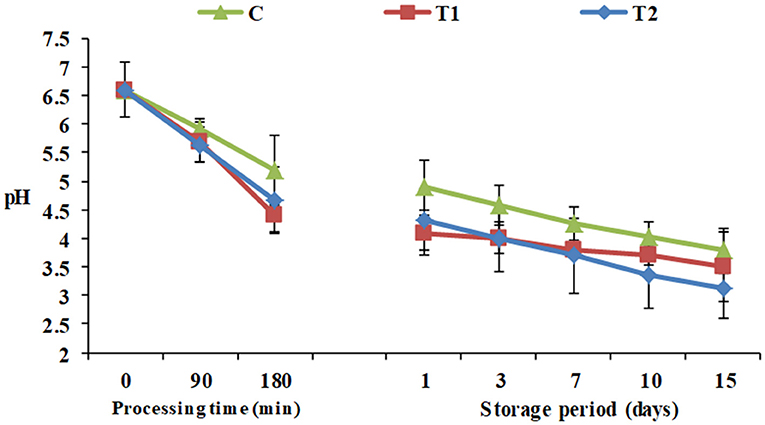
Figure 1. pH of yogurt products during processing and cold storage. C, control plain yogurt; T1, Pro-WPI 10% WPI-enriched concentrated yogurt; T2, Pro-WPI 20% WPI-enriched concentrated yogurt.
Total viable counts of yogurt products during processing and cold storage are exhibited in Figure 2. To receive health benefits associated with probiotics, their viability in foods is required (28). Viable counts showed a constant decline in all the products throughout the storage period. Additionally, the results showed that WPI enrichment significantly affected total viable counts in T1 and T2 (9.6 and 9.2 Log10 CFU/ g) compared to the control (10.2 Log10CFU/ g) when fresh, and along the cold storage period up to the 15th day of storage to reach 6.8 and 6.1 Log10 CFU/g, respectively, vs. 7.4 Log10 CFU/g for the control. The results were compatible with pH and acidity results (Figure 1). Similar observations were recorded by Dave and Shah (18). Nevertheless, for all the products until the 15th day of storage, viable counts were higher than 106 CFU/ g, the optimum viable count to have therapeutic merit according to Dias et al. (29).
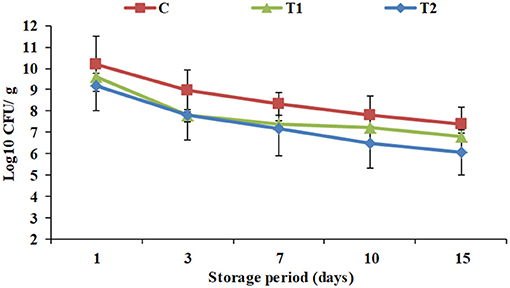
Figure 2. Total viable counts of yogurt products during processing and cold storage. C, control plain yogurt; T1, Pro-WPI 10% WPI-enriched concentrated yogurt; T2, Pro-WPI 20% WPI-enriched concentrated yogurt.
The effects of enrichment on the sensory properties of the two Pro-WPI yogurt treatments compared to the control plain yogurt when fresh are illustrated in Figures 3A–D. The odor of the Pro-WPI yogurt T1 and T2 tended to be milky, cheesy, and sour compared to the plain control yogurt (Figure 3A). The appearance of the enriched yogurt T1 and T2 was described as thick, compact, heterogeneous, and soft compared to the plain control yogurt that was soft (Figure 3B). Concerning flavor; the Pro-WPI products T1 and T2 tended to be creamy, buttery, cheesy, and sweet with acid flavor, while the acidic flavor of the plain control yogurt was the most significant (Figure 3C). The mouthfeel of the Pro-WPI yogurt products was thick compared to the light mouthfeel of the plain control yogurt (Figure 3D). The results showed a reflection of the chemical composition on the organoleptic properties of the enriched Pro-WPI products (Table 1). The obtained results came inconsistency with (14), who also stated that attributes, such as thickness, creaminess, and softness, are important drivers of liking high-protein yogurts.
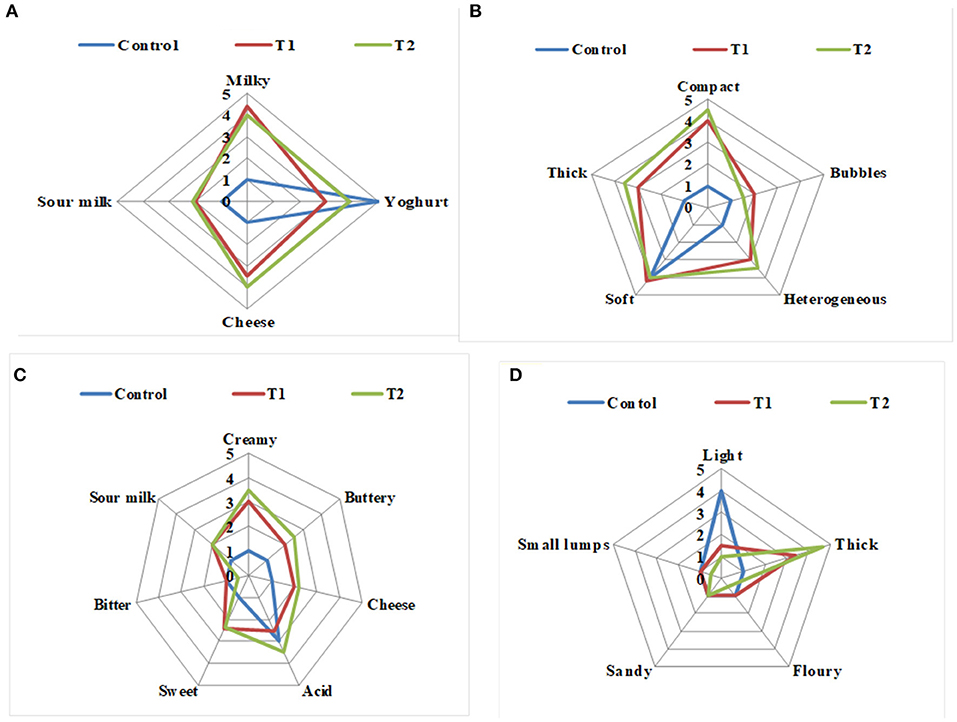
Figure 3. Sensory evaluation of probiotic WPI-enriched concentrated yogurt products. (A) Odor; (B) appearance; (C) flavor; (D) mouthfeel. Data represented are means of 27 processed patches (3 times per week/9 weeks). C, control plain yogurt; T1, Pro-WPI 10% WPI-enriched concentrated yogurt; T2, Pro-WPI 20% WPI-enriched concentrated yogurt.
Baseline characteristics, anthropometric measurements, and hematology biochemical parameters of the 24 athlete-participants before the intervention are summed in (Table 2). Due to unified conditions, low deviations were recorded in terms of age and BMI of the athlete-participants with mean values of 19.6 years and 23.54 kg/m2, respectively. The results showed that the mean hemoglobin level (12.69 g/dl) was less than the reference range (14–18 g/dl), and that the red blood cell (s) counts were on edge of the least reference range. The other hematology biochemical parameters were within the average range. The World Health Organization defines anemia as blood hemoglobin values of <13 g/dl (30).
The effect of PRO-WPI products on anthropometric measurements and hematology biochemical parameters are shown in (Table 3). BMR reflected that its value of G1 (1,854.25 Kcal/day) was significantly less than that of the placebo group (1,892.14 Kcal/day), and that the value of G2 was highest (2,040 Kcal/day). These results were reflected on weight and BMI of G1 (10% WPI) that were significantly less compared to those of the placebo group that ate plain yogurt. High-protein diets are increasingly popularized as a promising strategy for weight loss by providing the twin benefits of improving satiety and altering metabolism, leading to decreased fat mass (2, 3). Increased BMR due to increased fat content of Pro-WPI60 (20% WPI) (Table 1) may be a reason for significant elevation in weight, fat, fat mass, fat-free mass (FFM), and, consequently, BMI (79.64 kg, 13.09%, 10.42 kg, 69.23 kg, and 25.1 kg/m2, respectively) in G2 members compared to G1 (69.19 kg, 8.35%, 5.87 kg, 63.57 kg, and 22.59 kg/m2).
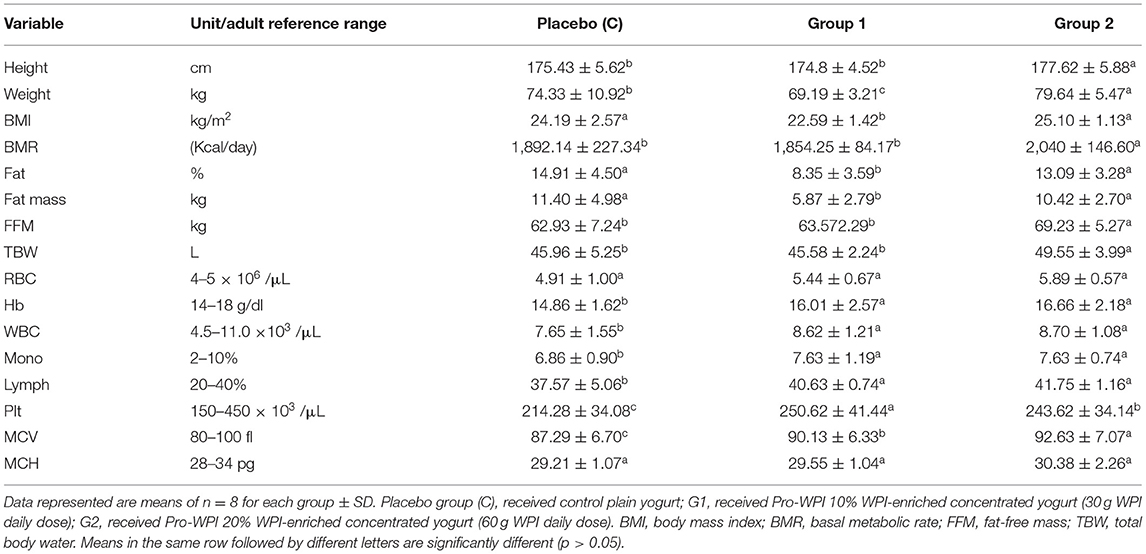
Table 3. Effect of Pro-WPI products on anthropometric measurements and hematology biochemical parameters.
Concerning hematology parameters; compared to Hb baseline (12.69 g/dl) (Table 2), all the fermented products (Pro-WPI, Pro-WPI 30, and Pro-WPI 60) in placebo, G1, and G2 achieved a significant increase in Hb to 14.86, 16, and 16.66 g/dl, respectively. Similarly, in RBC, a baseline was 3.94 × 106/μl, which was elevated to 4.91, 5.44, and 5.89 × 106/μl, respectively. Fermented milk contains lactic acid and other organic acids that are produced during fermentation, and it increases the absorption of iron especially when consumed at mealtimes (31). Lactobacilli sp. are featured with developed ability to hydrolyze proteins in their environment. This proteolytic activity not only generates free amino acids needed by bacteria but also a large variety of peptides, some of which are endowed with biological activities. The so-called “bioactive peptides” (BAPs) are interesting from nutrition and healthcare perspectives (10). Probiotic bacteria were reported to enhance iron absorption and utilization because of bioactive factors of fermented milk; hence they enhance and protect from iron-deficiency anemia (32–34). On the other hand, whey protein-derived bioactive peptides were reported to show considerable capacity for binding properties for cations, such as calcium, iron, and zinc (35), and the potential to regulate the intestinal microbiota that encourages mineral absorption by the digestive tract (8), which can explain the significant increase of Hb of G1 and G2 who ate the PRO-WPI products compared to placebo. The obtained results reflected that healthy gut microbiome (probiotics), in parallel with increased iron bioavailability by mineral binding (whey bioactive peptides), influences iron status and can represent a healthy practice to improve athletic anemia (5). Slight increases observed in WBC and lymph were previously reported because of immune modulation by starter culture and probiotic bacteria (36). Significant increases in platelets up to (250.62 and 243.62 × 103 /μl) in G1 and G2, respectively, comparing to placebo (214.28 × 103 /μl) came in accordance with Mazzuca et al. (37), who referred these results to the whey proteins ability to improve vitamin B12 and folate absorption that consequently could increase platelet count and reduce platelet depletion as well. No significant differences were obtained with respect to MCV and MCH.
Influences of the Pro-WPI products on athletic performance are exhibited in Table 4. Significant enhancements were recorded for most of the tested athletic performance parameters; vertical and long jumps, half squat, and pushup counts. Whey proteins were reported to increase protein synthesis and recovery of athletes after training and competition (8). Moreover, probiotic supplementation for athletes with the key term “probiotic athlete” was most commonly studied applying species such as; Lactobacillus casei, L. fermentum, L. acidophilus, and L. rhamnosus, to determine the effects of these probiotics on clinical measures and immune function in a placebo controlled design experiments (11). As the Pro-WPI products contain both whey proteins and probiotics, they can have a positive effect on athletic performance and can be recommended.
Urine biochemical assessment of the participants after consumption of the PRO-WPI products is exhibited in Table 5. Postures or exercises were reported to cause elevation, so experts recommend first-morning spot collection for screening purposes (38). Nevertheless, protein-rich diets are acidogenic because of the release of excessive non-carbonic acids, which are produced by the metabolism of protein (1), but pH and other examined urine parameters showed to be comparable to the placebo group except for significant differences recorded for albumin concentrations. The consumption of high amounts of proteins was reported to contribute to changes in urinary composition in high-protein dieted rats which vary according to the protein type either; dairy, non-dairy, animal, or vegetable protein (6). Microalbuminuria (MA) refers to increase in urine albumin excretion, and consumption of dietary protein is one of its reported causative factors (39). In the 1980s, McCarthy reported that daily protein intake of up to 2 g/kg was not detrimental to kidney function in male athletes (7, 40). As the daily intake of PRO-WPI products for the athletes participants (0.82 g/kg), caused elevated concentration of albumin (38.25 and 44.13 mg/g in G1 and G2, respectively) compared to placebo group (8.57 mg/g), exceeding the reference range (<30 mg/g), it is recommended to reduce the daily intake of PRO-WPI (10%) to be twice a day on daily basis. However, restriction of dietary protein intake (DPI) reduces urinary albumin levels (39).
High-protein concentrated pro-yoghrt (Pro-WPI) enriched with 10 and 20% WPI showed acidic flavor with soft thick texture because of increased protein content. When daily consumed by the 24 athletes, the products enhanced the hemoglobin significantly and consequently improved both athletic anemia and performance because of both whey proteins and probiotics. Urinary albumin exceeded the border of the reference range because of high rates of protein intake, while urine pH was normal. This study provided preliminary information on high-protein concentrated yogurt intake in athletes who engaged in daily exercise. Further studies are needed to determine the recommended intensity for Pro-WPI products consumption to achieve their benefits and avoid implications on kidney function.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Committee of Faculty of Physical Education, Alexandria University, Egypt. The patients/participants provided their written informed consent to participate in this study.
MG, A-EE, and AD were responsible for conceptualization, experimental design, methodology, and data curation and analysis. MG, MA, and AH performed formal analyses. MG, AH, and AD wrote the original draft of the manuscript. All authors revised, edited, and confirmed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kim H, Lee S, Choue R. Metabolic responses to high protein diet in Korean elite bodybuilders with high-intensity resistance exercise. J Int Soc Sports Nutr. (2011) 8:10. doi: 10.1186/1550-2783-8-10
2. Dominik P, Varman S. A high-protein diet for reducing body fat : mechanisms and possible caveats. Nutr Metab. (2014) 11:1–8. doi: 10.1186/1743-7075-11-53
3. French WW, Dridi S, Shouse SA, Wu H, Hawley A, Lee SO, et al. A high-protein diet reduces weight gain, decreases food intake, decreases liver fat deposition, and improves markers of muscle metabolism in obese Zucker rats. Nutrients. (2017) 9:1–19. doi: 10.3390/nu9060587
4. Andoyo R, Aini MN, Wira DW, Wilar G, Darwis RS, Huda S, et al. Pre-clinical study of the high protein food based on denaturized whey protein. Syst Rev Pharm. (2021) 12:759–70. doi: 10.31838/srp.2021.1.111
5. Damian M, Vulturar R, Login CC, Damian L, Chis A, Bojan A. Anemia in sports : a narrative review. Life. (2021) 11:1–12. doi: 10.3390/life11090987
6. Mithie C, Hans H, Tiselius G, Pfeferman I. Whey protein and albumin effects upon urinary risk factors for stone formation. Urolithiasis. (2017) 5:421–428. doi: 10.1007/s00240-017-0975-0
7. Poortmans JR, Dellalieux O. Do regular high protein diets have potential health risks on kidney function in athletes? Int J Sport Nutr Exerc Metab. (2000) 10:28–38. doi: 10.1123/ijsnem.10.1.28
8. Batista MA, Campos NCA, Silvestre MPC. Whey and protein derivatives: applications in food products development, technological properties and functional effects on child health. Cogent Food Agric. (2018) 4:1509687. doi: 10.1080/23311932.2018.1509687
9. Widyastuti Y, Febrisiantosa A, Tidona F. Health-promoting properties of lactobacilli in fermented dairy products. Front Microbiol. (2021) 12:1–8. doi: 10.3389/fmicb.2021.673890
10. Raveschot C, Cudennec B, Coutte F, Flahaut C, Fremont M, Drider D, et al. Production of bioactive peptides by lactobacillus species: from gene to application. Front Microbiol. (2018) 9:1–14. doi: 10.3389/fmicb.2018.02354
11. Pyne DB, West NP, Cox AJ, Cripps AW. Probiotics supplementation for athletes – clinical and physiological effects. Eur J Sport Sci. (2015) 15:63–72. doi: 10.1080/17461391.2014.971879
12. Yoha KS, Nida S, Dutta S, Moses JA, Anandharamakrishnan C. Targeted Delivery of Probiotics: Perspectives on Research and Commercialization. Probiotics Antimicrob Proteins. (2021) 27:1–34. doi: 10.1007/s12602-021-09791-7
13. Gomaa MAE, Khalaf F, Ayad E. Athletes nourishment: introducing novel l-glutamine fortified dairy products. J Food Dairy Sci. (2018) 9:97–102. doi: 10.21608/jfds.2018.35409
14. Jørgensen CE, Abrahamsen RK, Rukke EO, Hoffmann TK, Johansen AG, Skeie SB. Processing of high-protein yoghurt – a review. Int Dairy J. (2019) 88:42–59. doi: 10.1016/j.idairyj.2018.08.002
15. Gomaa MAE. Nutraceuticals impact on probiotics growth: a challenge in synbiotic-yoghurt production. J Food Dairy Sci. (2018) 9:41–9. doi: 10.21608/jfds.2018.35175
16. Tamime AY, Robenson RK. Tamime and Robenson's Yoghurt Science and Technology. 3rd ed. Cambridge, England: Woodhead Publishing Ltd and CRC Press LLC (2007).
17. AOAC International. Official methods of analysis. 20th ed. Rockville: Latimer AOAC International (2016).
18. Dave RI, Shah NP. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. (1998) 81:2804–16. doi: 10.3168/jds.S0022-0302(98)75839-4
19. Ozturk B, Argin S, Ozilgen M, McClements DJ. Nanoemulsion delivery systems for oil-soluble vitamins: influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. (2015) 187:499–506. doi: 10.1016/j.foodchem.2015.04.065
20. El-Kholy WM, Soliman TN, Darwish AMG. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE. (2019) 14:e0222789. doi: 10.1371/journal.pone.0222789
21. Caine DJ, Russell K, Lim L. Handbook of Sports Medicine and Science. Wiley Blackwell: A John Wiley & Sons, Ltd., Publication (2013).
22. DGSP. Commission of Experts of the German Society of Sports Medicine Prevention: Guidelines for Testing in Sports Medicine. Plane IV. Germany: Commission of Experts of the German Society of Sports Medicine and Prevention. Deutsche Gesellschaft für Sportmedi(2002).
23. Heyward VH, Gibson AL. Advanced Fitness Assessment and Exercise Prescription. 7th ed. Dusseldorf: Human Kinetics publishers (2013).
24. Burtis CAE, Ashwood ERE. Tietz Textbook of Clinical Chemistry. 3rd ed. W.B. Saunders Company, Elsevier (1999). p. 31–1202.
25. WU AHB. Section II- general clinical tests. In: Wu AHB, editors, Tietz Clinical Guide to Laboratory Tests. Philadelphia, PA: W.B. Saunders Company, Elsevier. p. 31–1202.
27. Smith JF, Brouk MJ. Factors affecting dry matter intake by lactating dairy cows. Kans Agric Exp Stn res rep. (2000). 54–58. doi: 10.4148/2378-5977.3198
28. Siciliano RA, Reale A, Mazzeo MF, Morandi S, Silvetti T, Brasca M. Paraprobiotics: a new perspective for functional foods and nutraceuticals. Nutrients. (2021) 13:1–19. doi: 10.3390/nu13041225
29. Dias M de LLA, Salgado SM, Guerra NB, Livera AVS, Andrade SAC, Ximenes GN da C. Phisicochemical, sensory, and microbiological evaluation and development of symbiotic fermented drink. Food Sci Technol. (2013) 33:805–11. doi: 10.1590/S0101-20612013000400030
30. Johnson Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. (2011) 4:177–84. doi: 10.1177/1756283X11398736
31. Branca F, Rossi L. The role of fermented milk in complementary feeding of young children: lessons from transition countries. Eur J Clin Nutr. (2002) 56:S16–20. doi: 10.1038/sj.ejcn.1601676
32. Darwish AMG, Soliman TN, Elhendy HA, El-kholy WM. Nano-encapsulated Iron and folic acid-fortified functional yogurt enhance anemia in albino rats. Front Nutr. (2021) 8:1–23. doi: 10.3389/fnut.2021.654624
33. El-Azeem ASA, Hegazy AM, Badawy IH, Ibrahim GA, Shafei KE, Sayed HSE, et al. Effectiveness of functional wheat - fermented milk beverage against tannic acid induced anemia. Res J Pharm Biol Chem Sci. (2016) 7:2622–32. Available online at: https://www.rjpbcs.com/pdf/2016_7(3)/%5B321%5D.pdf
34. García-Burgos M, Moreno-Fernandez J, Díaz-Castro J, Alféreza MJM, López-Aliaga I. Fermented goat's milk modulates immune response during iron deficiency anemia recovery. J Sci Food Agric. (2021) 1–10. doi: 10.1002/jsfa.11448
35. Mann B, Athira S, Sharma R, Kumar R, Sarkar P. Bioactive peptides from whey proteins. In: Deeth HC, Bansal N, editors. Whey Proteins, From Milk to Medicine. London: Academic Press, Elsevier (2018). p 519–47.
36. Allam MGM, Darwish AMG, Ayad EHE, Shokery ES, Mashaly RE, Darwish SM. In vivo evaluation of safety and probiotic traits of isolated enterococcus feacium strain KT712. Res J Microbiol. (2016) 11:169–77. doi: 10.3923/jm.2016.169.177
37. Mazzuca F, Roberto M, Arrivi G, Sarfati E, Schipilliti FM, Crimini E, et al. Clinical impact of highly purified, whey proteins in patients affected with colorectal cancer undergoing chemotherapy: preliminary results of a placebo-controlled study. Integr Cancer Ther. (2019) 18:1–11. doi: 10.1177/1534735419866920
38. Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev. (2011) 32:97–102.
39. Wrone EM, Carnethon MR, Palaniappan L, Fortmann SP. Association of dietary protein intake and microalbuminuria in healthy adults : third national health and nutrition examination survey. Am J Kidney Dis. (2003) 41:580–7. doi: 10.1053/ajkd.2003.50119
Keywords: high-protein pro-yoghurt, athletes' anemia, athletic performance, urinary albumin, randomized placebo controlled
Citation: Gomaa MAE, Allam MG, Haridi AAIM, Eliwa A-EM and Darwish AMG (2022) High-Protein Concentrated Pro-Yogurt (Pro-WPI) Enriched With Whey Protein Isolate Improved Athletic Anemia and Performance in a Placebo-Controlled Study. Front. Nutr. 8:788446. doi: 10.3389/fnut.2021.788446
Received: 02 October 2021; Accepted: 13 December 2021;
Published: 20 January 2022.
Edited by:
Angel Gil-Izquierdo, Spanish National Research Council (CSIC), SpainReviewed by:
José Miguel Martínez Sanz, University of Alicante, SpainCopyright © 2022 Gomaa, Allam, Haridi, Eliwa and Darwish. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amira M. G. Darwish, YW1pcmFnZGFyd2lzaEB5YWhvby5jb20=
†ORCID: Mohamed A. E. Gomaa orcid.org/0000-0001-5777-9041
Marwa G. Allam orcid.org/0000-0002-6653-1213
Abdallah A. I. M. Haridi orcid.org/0000-0002-4607-0771
Alaa-Eldin M. Eliwa orcid.org/0000-0002-8212-0318
Amira M. G. Darwish orcid.org/0000-0003-3586-1575
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.