
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 22 December 2021
Sec. Nutritional Epidemiology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.782270
This article is part of the Research TopicInsights in Nutritional EpidemiologyView all 17 articles
Background: The association between circulating vitamin D levels and risk of vitiligo was inconsistent among observational studies, and whether these observed associations were causal remained unclear. Therefore, we aimed to evaluate the effect of vitamin D on the risk of vitiigo using meta-analysis and Mendelian randomization (MR).
Methods: At the meta-analysis stage, literature search was performed in PubMed and Web of Science to identify eligible observational studies examining the association of circulating 25-hydroxyvitamin D [25(OH)D] or 25-hydroxyvitamin D3 [25(OH)D3] levels with risk of vitiligo up to April 30, 2021. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) of 25(OH)D and 25(OH)D3 in patients with vitiligo relative to controls were pooled. Then at the MR stage, genetic instruments for circulating 25(OH)D (N = 120,618) and 25(OH)D3 (N = 40,562) levels were selected from a meta-analysis of genome-wide association studies (GWAS) of European descent, and summary statistics of vitiligo were obtained from a meta-analysis of three GWASs including 4,680 cases and 39,586 controls. We used inverse-variance weighted (IVW) as main method, followed by weighted-median and likelihood-based methods. Pleiotropic and outlier variants were assessed by MR-Egger regression and MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test.
Results: In the meta-analysis, patients with vitiligo had a lower level of circulating 25(OH)D compared with controls [SMD = −1.40; 95% confidence interval (CI): −1.91, −0.89; P < 0.001], while no statistically significant difference of 25(OH)D3 between vitiligo cases and controls was found (SMD = −0.63; 95% CI: −1.29, 0.04; P = 0.064). However, in the MR analyses, genetically predicted 25(OH)D [odds ratio (OR) = 0.93, 95% CI = 0.66–1.31, P = 0.66] and 25(OH)D3 levels (OR = 0.95, 95% CI = 0.80–1.14, P = 0.60) had null associations with risk of vitiligo using the IVW method. Sensitivity analyses using alternative MR methods and instrumental variables (IV) sets obtained consistent results, and no evidence of pleiotropy or outliers was observed.
Conclusion: Our study provided no convincing evidence for a causal effect of 25(OH)D or 25(OH)D3 levels on the risk of vitiligo. Further longitudinal and experimental studies, as well as functional studies are warranted to elucidate the role of vitamin D in the development of vitiligo.
Vitiligo is an acquired pigmented skin disease caused by the loss of melanocyte function, which is characterized by localized or generalized complete depigmentation of skin mucosa. It can occur in all parts of the body, commonly in finger back, wrist, forearm, face, neck and around genitalia, affecting both men and women (1). Globally, the prevalence of vitiligo was estimated to be ranging from a low of 0.5% to a high of 2.0% in adults, and the peak onset period is between 10 and 30 years of age (2). Although vitiligo does not bring fatal risk, it is often accompanied by a variety of autoimmune system-related diseases, such as Hashimoto's thyroiditis, diabetes, Addison's disease and so on (3–6). Therefore, it is necessary to further explore the influence of modifiable risk factors on the incidence of vitiligo, which may provide new ideas for its prevention and treatment.
Previous studies have identified a series of nutritional factors associated with vitiligo, such as selenium (7), copper, and zinc (8). Recently, vitamin D deficiency has been reported to be a potential risk factor for vitiligo (9). With usual physiological intake, the majority of vitamin D is converted to 25-hydroxyvitamin D (25(OH)D) and released into the blood, thus circulating 25(OH)D concentrations can reflect vitamin D status in the human body. As the main metabolite of 25(OH)D, the levels of circulating 25-hydroxy vitamin D3 [25(OH)D3] is also widely accepted as an indicator of overall vitamin D status (10). Though no randomized controlled trials have been conducted to investigate the preventive role of vitamin D on the risk of vitiligo to date (11), numerous observational epidemiological studies have evaluated circulating 25(OH)D and 25(OH)D3 levels in patients with vitiligo relative to controls. For instance, Atazadeh et al. (12) conducted a case-control study with 90 vitiligo patients and 90 healthy controls in Iran, and found that the median (interquartile range) of circulating 25(OH)D level was lower in patients with vitiligo than in matched healthy controls [28.50 (22.52–33.20) ng/mL vs. 29.10 (27.40–35.70) ng/mL, P = 0.01). Another case-control study (13) conducted in India with 100 vitiligo patients and 100 healthy controls suggested that circulating 25(OH)D3 levels were lower in vitiligo patients than controls (16.17 ± 8.63 vs. 25.49 ± 1.02 ng/ml, P < 0.001). However, Saniee (14) recruited 98 patients with vitiligo and 98 matched healthy controls and measured 25(OH)D levels, but did not detect statistically significant differences between patients with vitiligo and normal subjects (22.37 ± 10.78 vs. 26.16 ± 17.11 ng/ml, P = 0.19). Similarly, Ustun et al. also found no statistically significant association (15). These inconsistent results may be due to differences in the study population, sample size, measurement methods and so on. Besides, findings from traditional observational studies are susceptible to bias such as confounding and reverse causation, therefore, it remains unclear whether the observed association was causal or not.
Mendelian Randomization (MR) study utilizes genetic variants such as single nucleotide polymorphisms (SNPs), which act as instrumental variables (IVs) to estimate the potential causal relationship between exposure and outcome (16). Since genotypes are randomly distributed in the process of gamete formation, the relationship between exposure and outcome of interest will not be biased by common confounding factors, such as postnatal environment, socio-economic status and behavioral factors in conventional observational studies. Moreover, MR study has unique advantages in causal inference because genetic variation is inherited from parents and remains unchanged after birth, so the association between genetic variation and outcome is reasonable in time sequence.
Therefore, in the present study, we first performed a systematic review and meta-analysis of existing observational evidence on the association of circulating 25(OH)D or 25(OH)D3 levels with risk of vitiligo, and then performed MR analysis to further investigate whether there was evidence for a causal relationship of circulating 25(OH)D and 25(OH)D3 levels with risk of vitiligo.
The overall design of the present study is shown in Figure 1. We first systematically searched PubMed and Web of Science up to April 30, 2021 to identify observational studies examining the association of circulating 25(OH)D or 25(OH)D3 levels with risk of vitiligo. The keywords included vitiligo AND (vitamin D OR 25-hydroxyvitamin D OR 25-dihydroxyvitamin D3 OR 25(OH)D OR 25(OH)D3 OR calcidiol).
The inclusion criteria were: (i) The study was cross-sectional, case-control or cohort design; (ii) The original study reported standardized mean difference (SMD) and 95% confidence interval (95% CI) of circulating 25-hydroxyvitamin D or 25-hydroxyvitamin D3 between patients with vitiligo and controls, or provided sufficient data to calculate SMD and 95% CI; (iii) When two or more literatures recruited overlapped study population, the literature with the largest sample size was included. The exclusion criteria were: (i) Review or meta-analysis; (ii) Full text is unavailable; (iii) Data provided in the literature were insufficient.
Relevant information was extracted and crosschecked by two researchers independently (Liu K and Chen W), and discussed with the third researcher (Ye D) in case of disagreement. The extracted information included the first author, year of publication, region, type of study design, study period, sample size, gender and age of the study participants. Two researchers (Liu K and Chen W) independently scored the quality of the included articles using Newcastle Ottawa scale (NOS) (17). The scores ranging from 7 to 9 were regarded as high quality, 5–6 as moderate quality, and ≤4 as low quality.
The mean and standard deviations (SD) of circulating levels of 25 (OH) D and 25 (OH) D3 between patients with vitiligo and controls were used to calculate SMDs and 95% CIs. If the study provided with median and range, we computed mean and SD using previously described formulas (18, 19). If odds ratio (OR) was provided, we computed SMD using the formulae of SMD= (20). The heterogeneity among studies was tested by Cochrane's Q test and I2 statistics. If I2 < 50% and P > 0.10, a fixed-effects model was used; otherwise, a random-effects model was applied. Sensitivity analysis was performed to evaluate the stability of the association by sequential removal of each study from the analysis. Subgroup analysis was performed in terms of region, publication year, quality score and study design, and meta-regression analysis was conducted for the above factors, respectively. Funnel plot was generated, and Begg's test (21) and Egger's test (22) were used to evaluate potential publication bias.
All statistical analyses were performed using STATA (version 14.0). Two-sided P-values < 0.05 were considered statistically significant.
Genetic association data of vitiligo were obtained from a meta-analysis of three genome-wide association studies (GWAS) involving 4,680 vitiligo cases and 39,586 controls of European ancestry (23). A total of 48 genetic variants associated with risk of vitiligo were identified, accounting for 17.4% of heritability. Detailed information of the above studies has been described in the previous article (23). All study participants provided written informed consent and the study was carried out under the jurisdiction of each local institutional review board.
IVs for 25(OH)D and 25(OH)D3 were derived from a large-scale GWAS including 120,618 and 40,562 participants of European descent, respectively (24). A total of ten and seven independent SNPs (r2 threshold < 0.1) associated with circulating 25(OH)D and 25(OH)D3 levels at genome-wide significance level (P < 5 × 10−8) were identified, respectively. None of the SNPs overlapped or were in linkage disequilibrium (r2 < 0.1) with known risk loci of vitiligo. The variance explained for total 25(OH)D by the ten lead SNPs was 3.95%, and the variance explained for 25(OH)D3 by the seven SNPs was 4.58%. The details of GWAS studies and datasets used in the present study are listed in Supplementary Table 1.
We calculated F-statistics to quantify the strength of the IVs, with the equation of F = R2 × (n – 2)/(1 – R2), in which R2 represents the variance explained by the IVs and n indicates the sample size (25). We used inverse-variance weighted (IVW) method to evaluate the potential causal relationship of circulating 25(OH)D and 25(OH)D3 levels with risk of vitiligo as the main analysis. IVW method combines the causal effect estimates of multiple single genetic IVs by Wald ratio method (26). The estimated effect size by IVW method is essentially the regression coefficient of weighted regression without considering the intercept term (27). Moreover, weighted-median and likelihood-based methods were used to evaluate the robustness of the IVW method. For the weighted median method, the consistent estimation of the causal effect can be obtained as long as the weight of the causal effect calculated by the effective IV reaches 50% (25). Moreover, the likelihood-based method was used to evaluate the casual relationship under the assumption of a linear association between the risk factor and the outcome variables (28).
In addition, we conducted MR-Egger regression analysis to analyze the effect of potential pleiotropy on causal estimation. The intercept term of MR-Egger represents the average pleiotropic effect of genetic variation. If the intercept is different from zero, it indicates that there is evidence of directed pleiotropy (29). Also, we performed Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) method (30) to detect and correct for horizontal pleiotropic outliers. It conducts a global test of heterogeneity by regressing the SNP-outcome association on the SNP-exposure association and comparing the observed distance of each SNP from the regression with that expected under the null hypothesis of no pleiotropy.
We further performed “leave-one-out” method as sensitivity analyses to test the reliability of the association of genetically predicted circulating 25(OH)D and 25(OH)D3 with risk of vitiligo. Specifically, we removed each SNP and combined the effect estimates of the remaining SNPs using the IVW method. The fluctuation of the results before and after removing the SNP reflects the stability of the association. Finally, considering the influence of pleiotropy on the results, associated phenotypes of the SNPs used as IVs were manually scanned through the GWAS Catalog (https://www.ebi.ac.uk/gwas, accessed on May 15, 2021). SNPs associated with other traits at genome-wide significance were excluded from the IVs and MR analyses were subsequently performed using the remaining SNPs.
R (version 4.0.5) with packages “MendelianRandomization” and “MR-PRESSO” was used for MR analysis. P-values < 0.05 were considered statistically significant.
The flowchart of literature inclusion and exclusion is shown in Figure 1. Briefly, a total of 125 articles were retrieved in the initial stage, with 15 articles and 5 articles investigating the association of 25(OH)D (12, 14, 31–43) and 25(OH)D3 (13, 15, 44–46) with risk of vitiligo included in the final analysis, respectively. In total, we collected the data of 987 patients and 770 controls for 25(OH)D, and 305 patients and 321 controls for 25(OH)D3 between 2012 and 2020 in this meta-analysis. Overall, there were 14 case-control studies and one cross-sectional study for 25(OH)D, and 4 case-control studies and one cross-sectional study for 25(OH)D3. As assessed by NOS, the quality of the included studies was high in general. The basic information of the literature included is shown in Table 1.
Statistically significant heterogeneity was observed among studies for the association of 25(OH)D (I2 = 95.5%, P < 0.001) and 25(OH)D3 (I2 = 93.3%, P < 0.001) with risk of vitiligo, respectively. Therefore, we used random-effects models to combine the effect sizes. As shown in Figure 2 Patients with vitiligo had a lower level of 25(OH)D compared with controls (SMD = −1.40; 95% CI: −1.91, −0.89; P < 0.001). However, the differences of circulating 25(OH)D3 levels in cases and controls did not reach statistical significance (SMD = −0.63; 95% CI: −1.29, 0.04; P = 0.064).
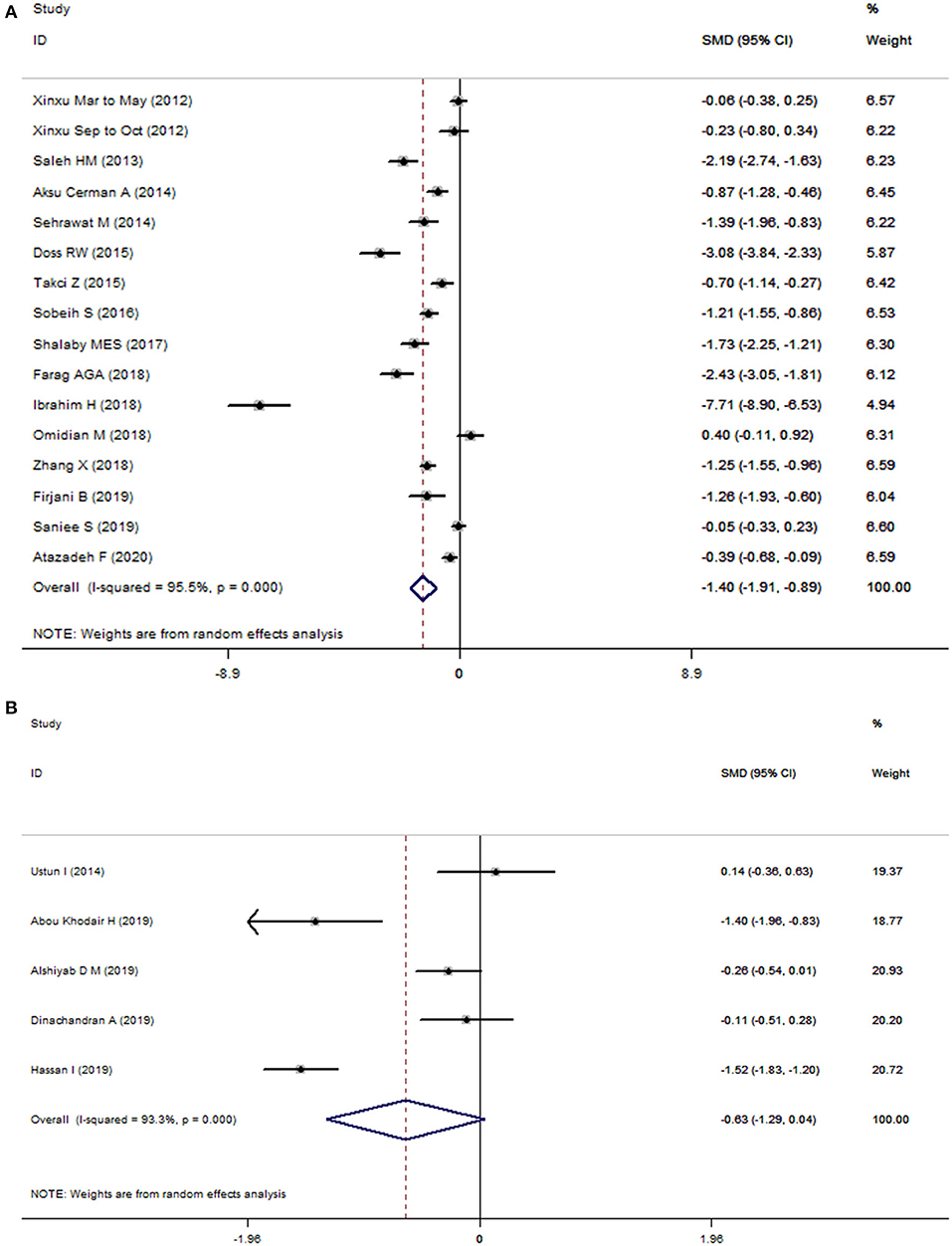
Figure 2. Forest plots of 25(OH)D (A) and 25(OH)D3 (B) in vitiligo patients relative to controls. The horizontal lines correspond to the study-specific standardized mean differences (SMD) and 95% confidence intervals (CI), respectively. The area of the squares reflects the study-specific weight.
Consistently, subgroup analysis revealed associations between circulating levels of 25(OH)D and risk of vitiligo in different strata. The levels of 25(OH)D3 in patients with vitiligo was significantly lower than that of the control group in the subgroups of studies with a high-quality score (SMD = −0.81; 95% CI: −1.56, −0.07; P = 0.032), published in 2017 and before (SMD = −0.81; 95% CI: −1.56, −0.07; P = 0.032), and in Asian populations (SMD = −1.40; 95% CI: −1.96, −0.83; P < 0.001). However, meta-regression analysis did not find potential sources of heterogeneity from region, publication year, quality score, study type and sample size for 25(OH)D and 25(OH)D3 (Supplementary Table 2). The Begg's test (P = 0.010) and Egger's test (P = 0.003) suggest evidence of potential publication bias in 25(OH)D, and the funnel plot was not symmetrical (Supplementary Figure 1A). For 25(OH)D3, the Begg's test (P = 0.806) and Egger's test (P = 0.966) did not suggest evidence of publication bias (Supplementary Figure 1B). Sensitivity analysis omitting one study at a time suggested none of these studies had a strong effect on the combined effect estimates (Supplementary Figure 2).
Supplementary Table 3 presents detailed information of IVs and their effect estimates with 25(OH)D, 25(OH)D3 and vitiligo, respectively. The F-statistic was 495.99 for 25(OH)D and 278.07 for 25(OH)D3, suggesting the IVs were unlikely to suffer from weak instrument bias.
As shown in Table 2, genetically predicted circulating 25(OH)D (OR = 0.93, 95% CI = 0.66–1.31, P = 0.663) and 25(OH)D3 levels (OR = 0.95, 95% CI = 0.80–1.14, P = 0.595) were not associated with altered risk of vitiligo using the IVW method. Similar results were obtained using the weighted-median approach and maximum-likelihood method. The intercept from the MR-Egger regression analysis did not reach statistical significance [25(OH)D: P = 0.614, 25(OH)D3: P = 0.061], suggesting no apparent evidence of directional pleiotropy. Additionally, no outlier SNPs were detected using MR-PRESSO test, and the causal effect estimates of 25(OH)D (P = 0.529) and 25(OH)D3 (P = 0.215) with risk of vitiligo were not statistically significant.
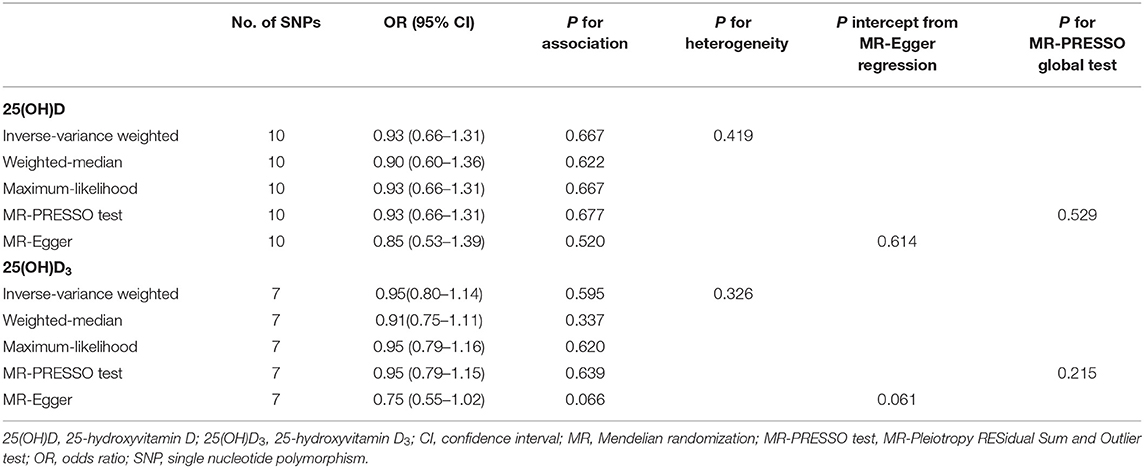
Table 2. Association of genetically predicted circulating levels of 25(OH)D and 25(OH)D3 levels with risk of Vitiligo.
In the sensitivity analyses, we performed “leave-one-out” analyses to identify potential influencing SNPs. We found that the risk estimates of genetically predicted 25(OH)D and 25(OH)D3 with risk of vitiligo did not change substantially after excluding one single SNP at a time (Supplementary Figure 3). Then we excluded potential pleiotropic SNPs and used the remaining seven and five SNPs as IVs for 25(OH)D and 25(OH)D3. Details of the excluded SNPs and their associated phenotypes according to the GWAS Catalog are shown in Supplementary Table 4. Consistently, we did not observe statistically significant association of genetically predicted circulating levels of 25(OH)D (OR = 0.98, 95% CI = 0.63–1.53, P = 0.920) or 25(OH)D3 (OR = 1.16, 95% CI = 0.85–1.58, P = 0.360) with risk of vitiligo (Supplementary Table 5).
To date, vitiligo is a disease that cannot be completely cured. Patients often encounter psychological difficulties due to discrimination, such as shame, depression and anxiety, which usually lead to inferiority and social isolation (47, 48), and have an adverse impact on the quality of life (49). At present, the pathogenic mechanism of vitiligo is not completely clear. There are studies suggesting that vitamin D may increase the melanogenesis and tyrosinase content of human melanocytes through its anti-apoptotic effect, thus preventing the loss of skin pigment (50). In addition, vitamin D can also inhibit the autoimmune pathway in the pathogenesis of vitiligo by inhibiting the expression of interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor α (TNF-α) and tumor necrosis factor γ (TNF-γ), regulating the mature differentiation and activation of dendritic cells and inhibiting antigen presentation (51). For prevention and early diagnosis of vitiligo, the present study investigated the role of circulating 25(OH)D and 25(OH)D3 on the risk of vitiligo by using meta-analysis and Mendelian randomization, however, we did not find convincing evidence to support a protective role of vitamin D supplementation on the risk of vitiligo.
The results from the meta-analysis indicated that patients with vitiligo had a lower level of 25(OH)D compared with controls. Consistently, a meta-analysis conducted by Zhang et al. (52) in 2018 including 679 patients and 537 controls reported similar results (SMD = −0.94, 95% CI: −1.39, −0.48, P = 0.0001). Our updated meta-analysis included five additional recent published studies with a sample size of 987 patients and 770 controls for 25(OH)D, 305 patients and 321 controls for 25(OH)D3, and therefore had increased statistical power. Similarly, we also found heterogeneity in the studies included in the meta-analysis. However, meta-regression did not suggest potential sources of heterogeneity, and subgroup analysis consistently revealed associations between circulating levels of 25(OH)D and risk of vitiligo in different strata. Publication bias was detected for the association between 25(OH)D and risk of vitiligo, suggesting that our results may be more based on positive results, resulting in overestimation of the summary effect. Previously, no meta-analysis has focused on the association between 25(OH)D3 and risk of vitiligo. In the present study, we found no statistical differences in circulating levels of 25(OH)D3 between cases and controls. However, the number of included studies was relatively small, more studies are warranted for obtaining stable results. Additionally, owing to the limitations inherent in observational studies, high heterogeneity among studies may be due to differences in the study design, characteristics of the study population such as age and ethnicity, use of drugs and so on. Finally, all of the included studies were cross-sectional studies and case-control studies with relatively small study sample sizes, which is weak in demonstrating causality, since it is difficult to judge the time sequence of exposure and disease (53). Therefore, low vitamin D levels could also be a consequence of already developed vitiligo.
In order to overcome the limitations inherent in conventional observational studies, we adopted a two-sample MR method which used genetic variants as IVs of exposures to further investigate the relationship of 25(OH)D and 25(OH)D3 levels with risk of vitiligo. MR approach offers great opportunities to the etiological research of diseases, provided that the following three assumptions are satisfied (54). The first assumption is that the IVs must be associated with the exposure of interest. To ensure this, we used independent loci associated with circulating 25(OH)D and 25(OH)D3 levels achieving genome-wide significance level (P < 5 × 10−8) as IVs, which was identified from the largest GWAS to date. The second assumption is that the IVs must not be associated with potential confounders of the exposure-outcome association. Since genotypes are randomly allocated during gamete formation, MR analyses using genetic variants as IVs to a large extent solve the problem of confounding in conventional observational studies. The third assumption is that the IVs influence the outcome only through the risk factor. To ensure this, we excluded potential pleiotropic SNPs and retained those solely associated with 25(OH)D and 25(OH)D3 levels in the MR sensitivity analyses to evaluate the robustness of our results. We also performed different MR methods to test for potential pleiotropy. We did not observe evidence of directional pleiotropy for the causal association between 25(OH)D, 25(OH)D3 and risk of vitiligo in any of the above MR approaches. However, the estimate of MR studies reflect the effects of lifelong interference, whereas that of observational studies reflects more acute effects.
The limitations of the present study should be noted. First, the data for the meta-analysis were from observational studies, in which there could be potential confounding factors in the baseline characteristics of the selected population. The statistical ability of the small sample is limited, thus the well-designed and large sample studies are necessary to validate the findings. Second, heterogeneity in meta-analysis is a potential problem that affects the results of statistical analysis. In the present study, subgroup analysis and meta-regression did not indicate sources account for the heterogeneity observed in the study. In addition, publication bias was found in the results of 25(OH)D, which indicated that the results of meta-analysis were affected by publication bias. Third, the variance explained by IVs is limited, with 3.95% total 25(OH)D and 4.58% 25(OH)D3, respectively. Besides, the number of reported cases in the outcome dataset is relatively small, which could affect the statistical power in MR study. However, it is the largest sample size in the outcome database available at present, which needs more vitiligo GWASs with larger sample size to explore the association. Finally, because our MR analyses were restricted to participants of European ancestry, it is unclear whether our findings can be extrapolated to other study populations. Therefore, further prospective studies and functional studies in vivo and in vitro are needed to elucidate the exact role of 25(OH)D and 25(OH)D3 in the occurrence of vitiligo.
Our study showed that although there seemed to be an inverse association between vitiligo and 25(OH)D level based on meta-analysis of observational studies, MR analysis pointed to a lack of causal association. In addition, meta-analysis and MR study did not provide evidence of an associations between 25(OH)D3 level and risk of vitiligo. The findings suggested there is no convincing evidence that vitamin D may help to prevent vitiligo.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JS performed the literature review, conducted data analysis, interpreted findings, and drafted the manuscript. KL and WC carried on data analysis and interpreted findings of meta-analysis. BL and HY mainly conducted on the data collation of the Mendelian randomization study. JS, LL, and XS can take responsibility for statistical reports, tables, and figures of the data analysis. YM and DY directed analytic strategy, supervised the study from conception to completion and revised drafts of the manuscript. All authors have read and approved the final manuscript.
This work was supported by grants from the National Natural Science Foundation of China (81973663), the Natural Science Foundation of Zhejiang Province (LQ21H260001 and LQ20H260008), the Talent Project of Zhejiang Association for Science and Technology (2018YCGC003), the Foundation of Zhejiang Chinese Medical University (2020ZG01 and 2020ZG16), the Research Project of Zhejiang Chinese Medical University (2021JKZKTS004A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors sincerely thank the researchers and participants of the original GWAS for their collection and management of large-scale data resources, as well as those who actively participated in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.782270/full#supplementary-material
CI, confidence interval; GWAS, genome-wide association study; IV, instrumental variable; IVW, inverse-variance weighted; IL-6, interleukin-6; IL-8, interleukin-8; MR, mendelian randomization; MAF, minor allele frequency; NOS, newcastle ottawa scale; OR, odds ratio; PRESSO, Pleiotropy RESidual Sum and Outlier; SD, standard deviation; SMD, standardized mean difference; SNP, single nucleotide polymorphism; TNF-α, tumor necrosis factor α; TNF-γ, tumor necrosis factor γ; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3.
1. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. (2015) 386:74–84. doi: 10.1016/S0140-6736(14)60763-7
2. Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. (2020) 236:571–92. doi: 10.1159/000506103
3. Taieb A, Picardo M. Clinical practice. Vitiligo N Engl J Med. (2009) 360:160–9. doi: 10.1056/NEJMcp0804388
4. Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. (2017) 35:117–28. doi: 10.1016/j.det.2016.11.002
5. Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. (2007) 356:1216–25. doi: 10.1056/NEJMoa061592
6. Sandru F, Carsote M, Albu SE, Dumitrascu MC, Valea A. Vitiligo and chronic autoimmune thyroiditis. J Med Life. (2021) 14:127–30. doi: 10.25122/jml-2019-0134
7. Huo J, Liu T, Huan Y, Li F, Wang R. Serum level of antioxidant vitamins and minerals in patients with vitiligo, a systematic review and meta-analysis. J Trace Elem Med Biol. (2020) 62:126570. doi: 10.1016/j.jtemb.2020.126570
8. Zeng Q, Yin J, Fan F, Chen J, Zuo C, Xiang Y, et al. Decreased copper and zinc in sera of Chinese vitiligo patients: a meta-analysis. J Dermatol. (2014) 41:245–51. doi: 10.1111/1346-8138.12392
9. Piotrowska A, Wierzbicka J, Zmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. (2016) 63:17–29. doi: 10.18388/abp.2015_1104
10. Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc. (2017) 76:392–9. doi: 10.1017/S0029665117000349
11. Watabe A, Yamasaki K, Asano M, Kanbayashi Y, Nasu-Tamabuchi M, Terui H, et al. Efficacy of oral cholecalciferol on rhododendrol-induced vitiligo: a blinded randomized clinical trial. J Dermatol. (2018) 45:456–62. doi: 10.1111/1346-8138.14244
12. Atazadeh F, Fazeli Z, Vahidnezhad H, Namazi N, Younespour S, Youssefian L, et al. Increased level of cathelicidin (LL-37) in vitiligo: possible pathway independent from vitamin D receptor gene polymorphism. Exp Dermatol. (2020) 29:1176–85. doi: 10.1111/exd.14200
13. Hassan I, Bhat YJ, Majid S, Sajad P, Rasool F, Malik RA, et al. Association of vitamin D receptor gene polymorphisms and serum 25-hydroxy vitamin D levels in vitiligo - a case-control study. Indian Dermatol Online J. (2019) 10:131–8. doi: 10.4103/idoj.IDOJ_97_18
14. Saniee S. Zinc, vitamin D and TSH levels in vitiligo patients. Erciyes Med J. (2019) 41:148–52. doi: 10.14744/etd.2019.40316
15. Ustun I, Seraslan G, Gokce C, Motor S, Can Y, Ugur Inan M, et al. Investigation of vitamin D levels in patients with vitiligo vulgaris. Acta Dermatovenerol Croat. (2014) 22:110–3.
16. Katan MB. Commentary: Mendelian randomization, 18 years on. Int J Epidemiol. (2004) 33:10–11. doi: 10.1093/ije/dyh023
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Yehuda S. Nutrients, brain biochemistry, and behavior: a possible role for the neuronal membrane. Int J Neurosci. (1987) 35:21–36. doi: 10.3109/00207458708987106
19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
20. Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. (2000) 19:3127–31. doi: 10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101 doi: 10.2307/2533446
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. (2016) 48:1418–24. doi: 10.1038/ng.3680
24. Zheng JS, Luan J, Sofianopoulou E, Sharp SJ, Day FR, Imamura F, et al. The association between circulating 25-hydroxyvitamin D metabolites and type 2 diabetes in European populations: a meta-analysis and Mendelian randomisation analysis. PLoS Med. (2020) 17:e1003394. doi: 10.1371/journal.pmed.1003394
25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
26. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
27. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
28. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium E-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. doi: 10.1007/s10654-015-0011-z
29. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
30. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
31. Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. (2014) 170:1299–304. doi: 10.1111/bjd.12980
32. Farag AGA, Haggag MM, Muharram NM, Mahfouz R, Elnaidany NF, Abd El Ghany HM. Is vitamin D a participant in narrow-band ultraviolet B-induced pigmentation in patients with vitiligo? J Egypt Womens Dermatol Soc. (2018) 15:30–4. doi: 10.1097/01.EWX.0000525983.54753.6
33. Ibrahim H, El Taieb M, El Gamel Z, El Saied AR. Effect of narrow-band ultraviolet B on the serum of 25-hydroxyvitamin D in vitiligo patients. J Cosmet Dermatol. (2018) 17:911–6. doi: 10.1111/jocd.12515
34. Omidian M, Asadian T. Evaluation of serum vitamin D levels in patients with vitiligo undergoing NBUVB (narrowband UV-B) therapy. Jundishapur J Nat Pharm Prod. (2017) 13:e65045. doi: 10.5812/jjnpp.65045
35. Saleh HM, Abdel Fattah NS, Hamza HT. Evaluation of serum 25-hydroxyvitamin D levels in vitiligo patients with and without autoimmune diseases. Photodermatol Photoimmunol Photomed. (2013) 29:34–40. doi: 10.1111/phpp.12016
36. Sehrawat M, Arora TC, Chauhan A, Kar HK, Poonia A, Jairath V. Correlation of vitamin D levels with pigmentation in vitiligo patients treated with NBUVB therapy. ISRN Dermatol. (2014) 2014:493213. doi: 10.1155/2014/493213
37. Sobeih S, Mashaly HM, Gawdat H, Amr K, Hamid MF, Shaalan E. Evaluation of the correlation between serum levels of vitamin D and vitamin D receptor gene polymorphisms in an Egyptian population. Int J Dermatol. (2016) 55:1329–35. doi: 10.1111/ijd.13363
38. Takci Z, TekIN Ö, ErtuGRul DT, KaradaG AS, Akin KO. A case-control study: evaluation of vitamin D metabolism in patients with vitiligo. Turkish J Med Sci. (2015) 45:837–41. doi: 10.3906/sag-1405-17
39. Xu X, Fu WW, Wu WY. Serum 25-hydroxyvitamin D deficiency in Chinese patients with vitiligo: a case-control study. PLoS ONE. (2012) 7:e52778. doi: 10.1371/journal.pone.0052778
40. Zhang X, Wang W, Li Y, Wang H, Liu R, Zhu L. Serum 25-hydroxyvitamin D status in chinese children with vitiligo: a case-control study. Clin Pediatr. (2018) 57:802–5. doi: 10.1177/0009922817734362
41. Shalaby MES, Ibrahim S, Shorbagy M, Ahmed R. Evaluation of serum level of 25-hydroxy vitamin D in vitiligo patients. Gulf J Dermatol Venereol. (2017) 24:45–9.
42. Amer AM, Galal Khater EM, Marei AM, A Firjani BA. Evaluation of serum level of 25-hydroxy vitamin D in vitiligo patients. Zagazig Univ Med J. (2019) 25:935–40. doi: 10.21608/zumj.2019.10995.11390
43. Doss RW, El-Rifaie AA, Gohary YM, Rashed LA. Vitamin D receptor expression in vitiligo. Indian J Dermatol. (2015) 60:544–8. doi: 10.4103/0019-5154.169123
44. Alshiyab DM, Al-Qarqaz FA, Heis LH, Muhaidat JM, Eddin WS, Atwan AA. Assessment of serum vitamin D levels in patients with vitiligo in Jordan: a case-control study. Dermatol Res Pract. (2019) 2019:2048409. doi: 10.1155/2019/2048409
45. Dinachandran A, Pillai R. A Study to assess the association of vitamin D (serum 25 hydroxy vitamin D3) and vitiligo. IP Indian J Clin Exp Dermatol. (2019) 5:6–8. doi: 10.18231/2581-4729.2019.0002
46. Abou Khodair H, Amer AW-A, Abd Al Samee HS, Gad NFA. Assessment of serum vitamin D level before and after narrowband therapy in vitiligo. Egypt J Hosp Med. (2019) 74:310–7. doi: 10.21608/ejhm.2019.23052
47. Kostopoulou P, Jouary T, Quintard B, Ezzedine K, Marques S, Boutchnei S, et al. Objective vs. subjective factors in the psychological impact of vitiligo: the experience from a French referral centre. Br J Dermatol. (2009) 161:128–33. doi: 10.1111/j.1365-2133.2009.09077.x
48. Porter J, Beuf AH, Nordlund JJ, Lerner AB. Psychological reaction to chronic skin disorders: a study of patients with vitiligo. Gen Hosp Psychiatry. (1979) 1:73–7. doi: 10.1016/0163-8343(79)90081-1
49. Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes. (2003) 1:58. doi: 10.1186/1477-7525-1-58
50. AlGhamdi K, Kumar A, Moussa N. The role of vitamin D in melanogenesis with an emphasis on vitiligo. Indian J Dermatol Venereol Leprol. (2013) 79:750–8. doi: 10.4103/0378-6323.120720
51. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. (2000) 164:2405–11. doi: 10.4049/jimmunol.164.5.2405
52. Zhang JZ, Wang M, Ding Y, Gao F, Feng YY, Yakeya B, et al. Vitamin D receptor gene polymorphism, serum 25-hydroxyvitamin D levels, and risk of vitiligo: a meta-analysis. Medicine. (2018) 97:e11506. doi: 10.1097/MD.0000000000011506
53. Schulz KF, Grimes DA. Case-control studies: research in reverse. Lancet. (2002) 359:431–4. doi: 10.1016/S0140-6736(02)07605-5
Keywords: 25-hydroxyvitamin D, 25-hydroxyvitamin D3, Mendelian randomization, meta-analysis, vitiligo
Citation: Song J, Liu K, Chen W, Liu B, Yang H, Lv L, Sun X, Mao Y and Ye D (2021) Circulating Vitamin D Levels and Risk of Vitiligo: Evidence From Meta-Analysis and Two-Sample Mendelian Randomization. Front. Nutr. 8:782270. doi: 10.3389/fnut.2021.782270
Received: 24 September 2021; Accepted: 29 November 2021;
Published: 22 December 2021.
Edited by:
Mauro Serafini, University of Teramo, ItalyReviewed by:
Hui Lu, Capital Medical University, ChinaCopyright © 2021 Song, Liu, Chen, Liu, Yang, Lv, Sun, Mao and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding Ye, eWVkaW5nQHpjbXUuZWR1LmNu; Yingying Mao, bXl5QHpjbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.