
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 24 November 2021
Sec. Nutritional Epidemiology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.778031
This article is part of the Research Topic Glucosinolate Metabolites: Bioavailability, Bioactivity and Clinical Variability View all 7 articles
 Yi-Fan Wei1,2†
Yi-Fan Wei1,2† Ying-Ying Hao3†
Ying-Ying Hao3† Song Gao3
Song Gao3 Xiu-Qin Li3
Xiu-Qin Li3 Fang-Hua Liu1,2
Fang-Hua Liu1,2 Zhao-Yan Wen1,2
Zhao-Yan Wen1,2 Han-Yuan Wang1,2
Han-Yuan Wang1,2 Shuang Zhang1,2
Shuang Zhang1,2 Shi Yan1,2
Shi Yan1,2 Meng Luan1,2,3
Meng Luan1,2,3 Yu-Hong Zhao1,2
Yu-Hong Zhao1,2 Ting-Ting Gong3*‡
Ting-Ting Gong3*‡ Qi-Jun Wu1,2*‡
Qi-Jun Wu1,2*‡Background: The associations of the consumption of cruciferous vegetables (CVs) and their bioactive components, isothiocyanates (ITCs), with ovarian cancer (OC) mortality have been unclear, owing to limited studies and inconsistent findings. To date, no studies have evaluated these associations among Chinese patients with OC. This study aims to provide more evidence indicating the relationships of pre-diagnosis CVs and ITC intake with OC survival.
Methods: We examined the associations of pre-diagnosis CV and ITC intake with OC mortality in a hospital-based cohort (n = 853) of Chinese patients with epithelial OC between 2015 and 2020. Pre-diagnosis dietary information was evaluated with a validated food frequency questionnaire. Deaths were ascertained until March 31, 2021 via medical records and active follow-up. The associations were examined with the Cox proportional hazards model, adjusted for potential confounders, and stratified by menopausal status, residual lesions, histological type, and body mass index (BMI).
Results: During a median follow-up of 37.2 months (interquartile: 24.7–50.2 months), we observed 130 deaths. The highest tertile of total CV intake was associated with better survival than the lowest tertile intake [hazard ratio (HR) = 0.57, 95% confidence interval (CI) = 0.33–0.98, p trend < 0.05]. In addition, higher intake of ITCs from CVs was associated with better survival (HRT3VS.T1 = 0.59, 95% CI = 0.36–0.99, p trend = 0.06). Significant inverse associations were also observed for subgroup analyses stratified by menopausal status, residual lesions, histological type, and BMI, although not all associations showed statistical significance.
Conclusion: Increasing pre-diagnosis consumption of CVs and ITCs was strongly associated with better survival in patients with OC.
Ovarian cancer (OC) is a gynecological malignant tumor with insidious onset, rapid development, and poor prognosis (1). Worldwide, 313,959 women were diagnosed with OC, and 207,252 new deaths due to this disease occurred in 2020 (2). In China, there were ~25,000 new cases and 22,000 new deaths in 2015 (3). Fewer than half of affected women survive beyond 5 years after diagnosis because of the predominance of aggressive high-grade serous carcinomas and the absence of specific early symptoms and effective early detection strategies (1). Emerging evidence suggests that maximal cytoreductive surgery (4), statin use (5), obesity (6), and vegetable consumption (7) might affect OC survival.
Previous research has suggested that of vegetables, particularly cruciferous vegetables (CVs), might increase OC survival (8). CVs come from plants in the Cruciferae and Brassicaceae families, which account for a large proportion of vegetables, e.g., broccoli, brussels sprouts, cabbage, cauliflower, collard greens, kale, kohlrabi, mustard, rutabaga, turnips, bok choy, and Chinese cabbage (9). Isothiocyanates (ITCs) are formed from the hydrolysis of glucosinolates in plants through the action of the enzyme myrosinase and the gut microbiota, and they are abundant in CVs (10, 11). Different glucosinolates in CVs produce distinct ITCs (11). Some ITCs are volatile and become hydrolyzed at higher cooking temperatures (12, 13). Previous epidemiological studies have indicated that CVs are inversely associated with several health outcomes including cardiovascular disease (14), type 2 diabetes mellitus (15), gastrointestinal cancer (16, 17), breast cancer (18), and reproductive cancers (19, 20). Several epidemiological studies have also shown that ITCs might be associated with various cancers (21–23), diabetes (24, 25), and cardiovascular disease (26, 27). Notably, only three observational studies have focused on the association between pre-diagnosis CV intake and OC mortality; however, these findings were inconsistent. For example, two studies found that a diet high in CVs might help improve OC survival (8, 28). However, one study on 811 patients with OC found no association between CV intake and OC survival (29). More importantly, no study has investigated the relationship between pre-diagnosis ITC intake and OC prognosis.
To provide more evidence indicating the relationships of pre-diagnosis CVs and ITC intake with OC survival, we conducted a hospital-based OC follow-up study in China. To our knowledge, this study is the first large-scale prospective study exploring this topic among Chinese women with different dietary habits, as compared with Europeans and Americans.
The ovarian cancer follow-up study (OOPS) is a prospective longitudinal cohort study of patients newly diagnosed with OC. This study aimed to collect demographic, clinical, and lifestyle data from patients with OC to assess their associations with cancer-related outcomes, and was approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China. Information collection on long-term outcomes remains ongoing. Between January 2015 and December 2020, 853 patients with OC who were 18–75 years of age were enrolled. Among them, 796 women agreed to participate, and 744 (93%) returned the completed study questionnaire. We further excluded participants who left 11 (10%) or more food items blank (n = 24) and those with implausible caloric intakes (<500 or >3,500 calories per day) (n = 17). Finally, dietary data were available for 703 women with OC (Figure 1).
Through self-administered questionnaires, we collected data on demographics and lifestyle factors, including educational level, monthly household income, physical activity (work, commuting, household chores, and leisure-time exercise) (30), smoking status, alcohol drinking, and tea drinking. Height and body weight [used to calculate body mass index (BMI)] were measured by trained staff following a standard protocol. In addition, clinical variables were abstracted from the electronic medical records of the Shengjing hospital information system. These variables included age at diagnosis, histological type (serous or non-serous), histopathologic grade (well, moderate, and poorly differentiated), International Federation of Gynecology and Obstetrics (FIGO) stage (I–II, III–IV, and unknown), residual lesions (none, <1, and ≥1 cm), and comorbidities (hypertension, coronary heart disease, diabetes, and so on) (31, 32).
Dietary intake was assessed at recruitment with a 111-item food frequency questionnaire (FFQ), which has been verified to have reasonable reliability and validity. The reproducibility coefficients (spearman correlation coefficients and intraclass correlation coefficients) were above 0.5 for most food groups, and correlation coefficients (spearman correlation coefficients) were between 0.3 and 0.7 for most food groups between the FFQ and weighed diet records. Participants reported their usual frequency of intake for standard serving sizes of food items in the 12 months before OC diagnosis. Frequency categories included almost never, two or three times per month, one time per week, two or three times per week, four to six times per week, one or two times per day, and more than two times per day. In our study, CV intake was calculated by summing the intake amounts of Chinese cabbage, pakchoi, kohlrabi, rape, broccoli, cauliflower, and raphanus sativus. Intake of each CV in grams/day was calculated through multiplying consumption frequencies per day by fitted portion sizes (g/time). Spearman correlation coefficients and intraclass correlation coefficients of reproducibility for CV were 0.61 and 0.53, respectively. The crude and energy-adjusted spearman correlation coefficients of validity for CV were 0.43 and 0.33, respectively. The Chinese Food Composition Tables (33) were used as the nutrient database to calculate the daily ITC intake. ITC intake was calculated by first multiplying the amount (in grams) consumed (for each CV) by its nutrient content per gram and then adding the ITC contributions across all CVs (34–36).
Information on the vital status of OOPS participants was ascertained from medical records and active follow-up. The outcome of focus was death due to any cause. Survival time was defined as the interval between histologic diagnosis and the date of death due to any cause or the date of the last follow-up (March 31, 2021) for patients who were still alive.
Differences in general and clinical characteristics according to CV intake categories were assessed with the χ2 test, except for continuous variables, which were assessed with the one-way ANOVA or the Kruskal–Wallis test. The Kaplan–Meier technique was used to plot crude survival curves and estimate the crude overall survival probabilities. Cox proportional hazards regression models were used to assess the associations of pre-diagnosis CVs and ITC intake with overall survival. The proportional hazards assumption was tested through inclusion of an interaction term between each activity variable and log survival time, and no violations were found (all p > 0.05). CV and ITC intake was categorized by tertile distribution, wherein the lowest tertile served as the reference group. Furthermore, we tested the association between the frequency of specific species CVs (including Chinese cabbage, pakchoi, kohlrabi, rape, broccoli, cauliflower, and raphanus sativus) and OC survival. The linear trend tests were performed by assigning the median value of consumption for each tertile of CVs and ITCs and treating it as a continuous variable in the respective regression model (37). In model 1, we did not adjust any covariates to calculate crude hazard ratio (HR) and 95% confidence interval (CI). In order to account for the influence of age at diagnosis and total energy, we adjusted for age at diagnosis (<50 or ≥50 years) and total energy intake (continuous, kcal) in model 2. In addition, we further adjusted for BMI (continuous, kg/m2), comorbidities (yes or no), dietary changes (yes or no), meat (continuous, g/days), fruit (continuous, g/days), green leafy vegetables (continuous, g/days), alliums (continuous, g/days), soy (continuous, g/days), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), FIGO stage (I–II, III–IV, and unknown), histological type (serous or non-serous), histopathologic grade (well, moderate, and poorly differentiated), menopausal status (yes or no), parity (≤ 1, ≥2), physical activity (continuous), residual lesions (none, <1, and ≥1 cm), and smoking status (yes or no) in model 3 to minimize the impact of clinical characteristics, lifestyle factors, and dietary factors on the survival. Of these covariates, comorbidities were defined as the simultaneous presence of two or more diseases in some individuals (32). In our analysis, the comorbidities include hypertension, coronary heart disease, diabetes, and so on. Dietary change was collected via self-administered questionnaires, and participants were asked whether they have changed their dietary habits recently. Parity was also gathered through self-administered questionnaires. Based on previous study, we assessed physical activity in these OC patients (38). Briefly, participants were asked the usual type and duration of activities related to work, commuting, household chores, and leisure-time exercise during the past 12 months in our study (38). We further used metabolic equivalent tasks (METs) from the 2011 update (30) of a major compendium of physical activities to calculate the amount of physical activity.
Stratified analyses were used to assess potential effect modification by menopausal status (no compared with yes), residual lesions (no compared with yes), histological type (serous compared with non-serous), and BMI (<25 compared with ≥25 kg/m2). Potential interactions of CV and ITC intake with these stratifying variables were assessed by the addition of cross-product terms to the multivariable Cox models. We also analyzed the relationships of pre-diagnosis CVs and ITCs with overall survival in stage III or IV patients with OC. All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided P < 0.05 were considered statistically significant.
The distribution of socio-demographic and clinical characteristics of 703 patients with OC according to tertiles of total CVs is presented in Table 1. The median (interquartile) age at diagnosis was 53.0 (48.0–60.0) years, and no significant difference was observed among the three groups. During a median follow-up of 37.2 months (interquartile: 24.7–50.2 months), we observed 130 deaths, and patients with a higher CV intake had longer follow-up times (p < 0.05). In addition, patients with a higher intake of CVs tended to consume more meat, green leafy vegetables, allium vegetables, fruits, beans and bean products, tea, and total energy. Furthermore, the proportion of postmenopausal women was significantly higher among patients with greater CV intake. Table 2 shows that patients with a non-serous histological subtype, later FIGO stage, and larger residual lesions had poorer survival in this study.
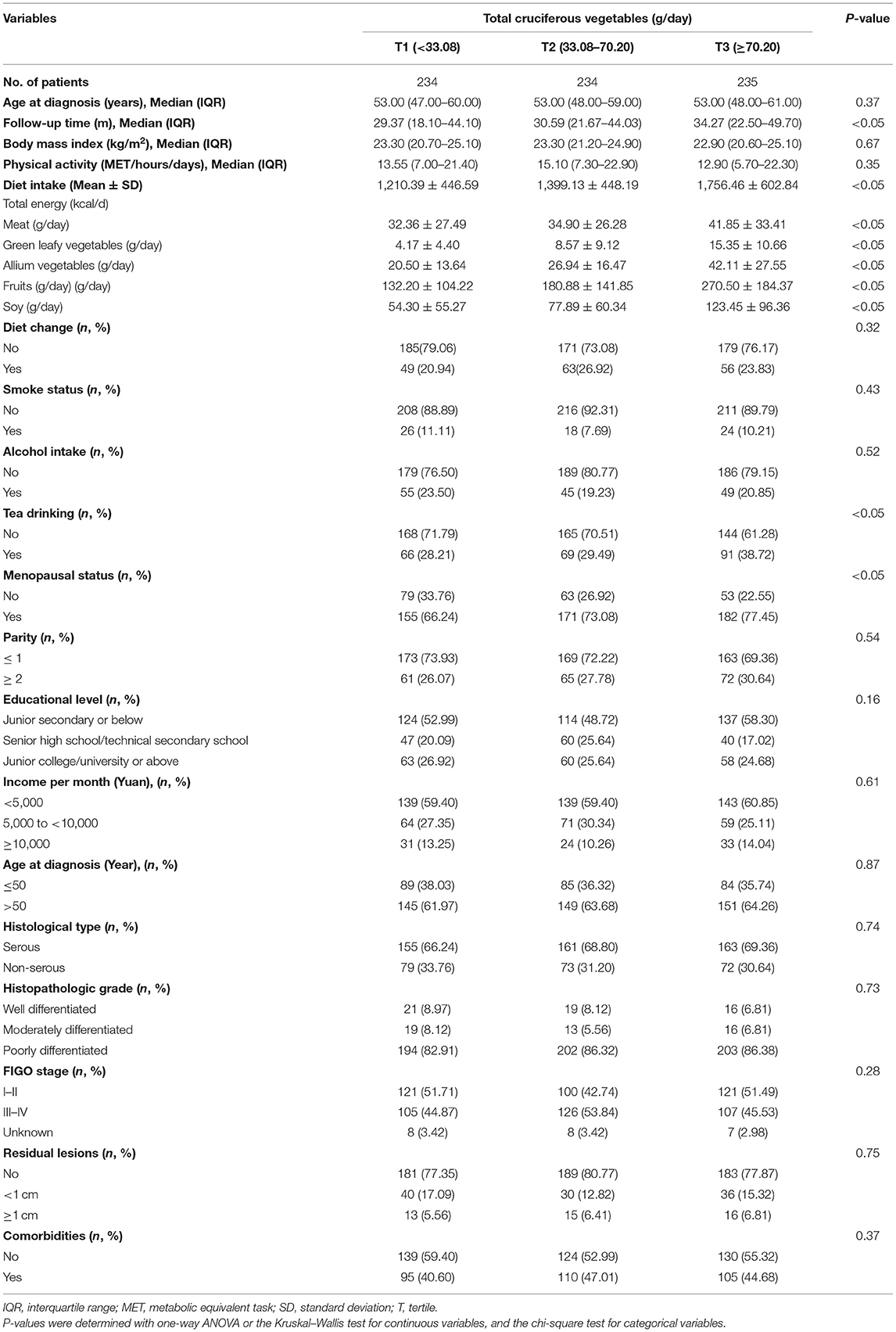
Table 1. General characteristics of ovarian cancer patients according to cruciferous vegetables (N = 703).
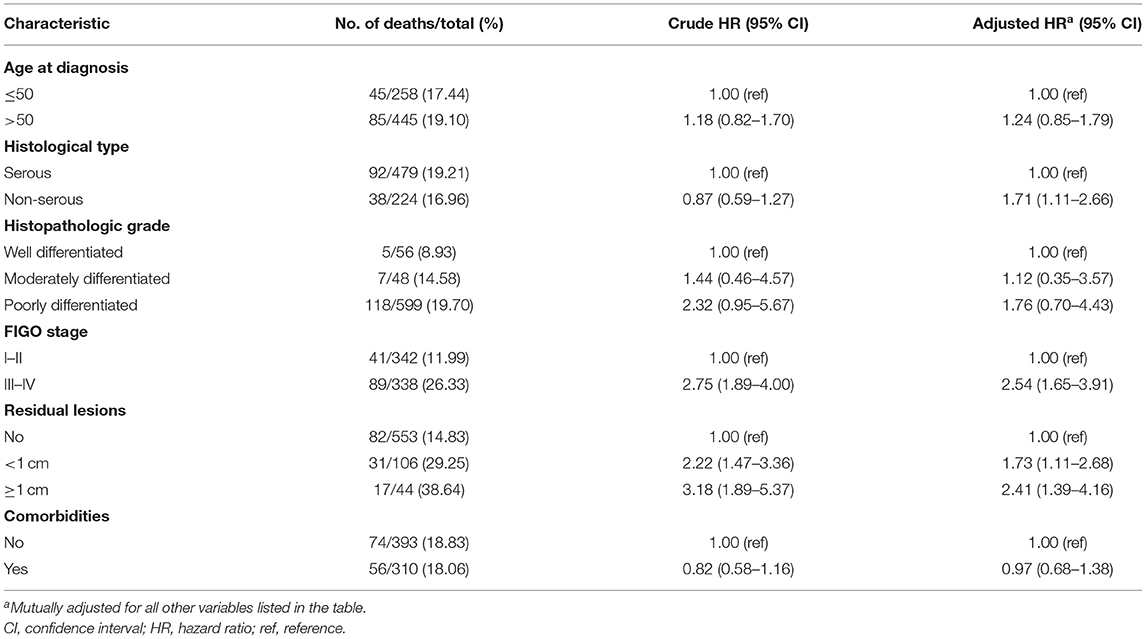
Table 2. Selected clinical characteristics and associations with all-cause mortality among women diagnosed with ovarian cancer (N = 703).
The associations of total CV as well as ITC intake with the overall survival of OC are described in Table 3. The highest tertile of total CV intake was associated with better survival than the lowest tertile intake (HR = 0.57, 95% CI = 0.33–0.98), and a linear trend was also evident (p trend < 0.05). As shown in Figure 2, the OC survival was better in patients with the highest tertile of CV intake than those in the lowest tertile. Moreover, a similar protective effect was observed for ITCs from CV intake (HR T3VS.T1 = 0.59, 95% CI = 0.36–0.99), although we did not observe a significant linear trend (p trend = 0.06). Analyses of specific species of CVs indicated that the significant protective effect of Chinese cabbage, kohlrabi, and pakchoi was consistent with the main findings (Supplementary Table 1).
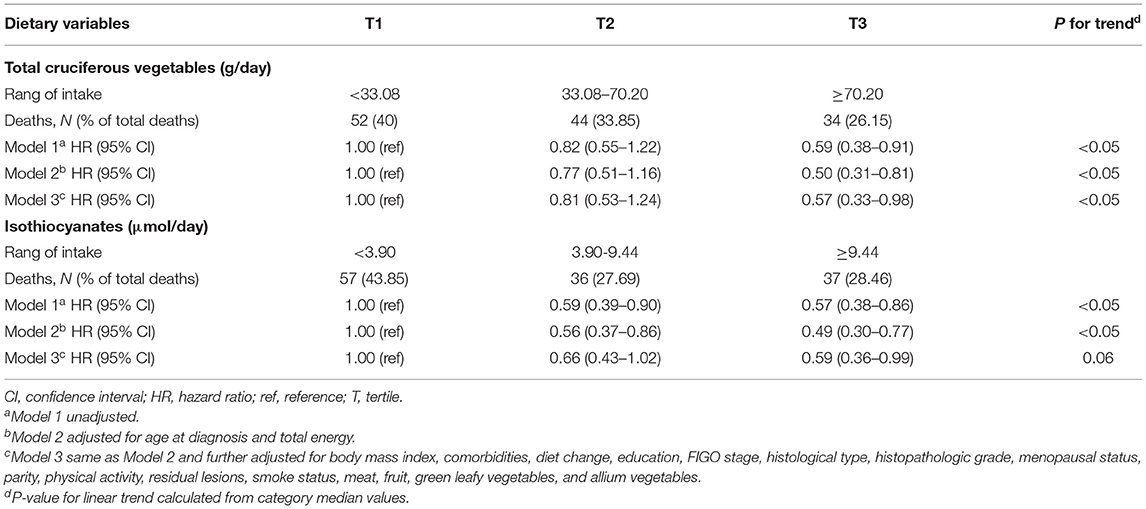
Table 3. Hazard ratio (95% CI) for overall survival among ovarian cancer patients according to cruciferous vegetables and relative nutrient intake.
The relationship between total CV intake and overall survival in patients with OC was evaluated across potential effect-modifying variables, such as menopausal status, residual lesions, histological subtype, and BMI. The better survival advantages associated with the highest total CV intake were present only in postmenopausal patients, patients with serous histological subtype, or patients with BMI <25 (Figure 3). Additionally, we examined the influence of ITC intake on overall OC survival across the aforementioned potential effect-modifying variables that generated similar patterns (Supplementary Figure 1).
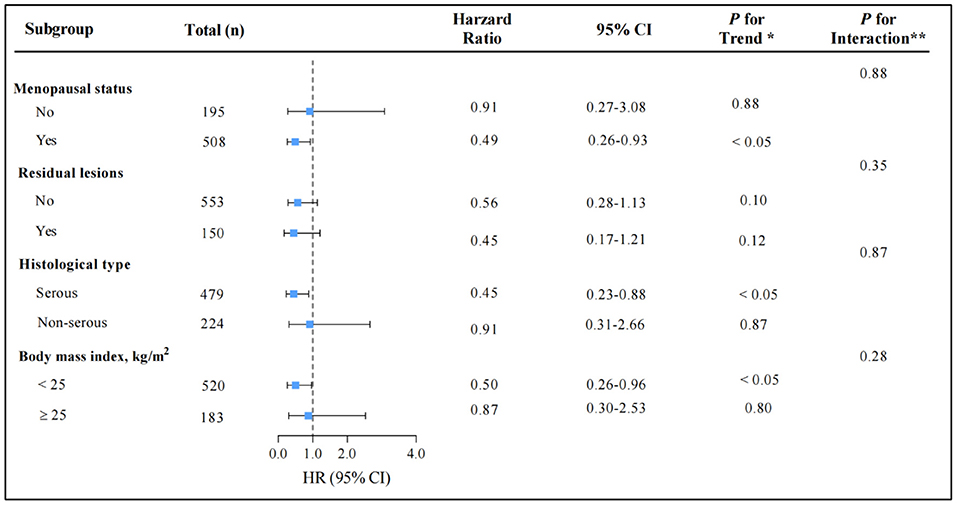
Figure 3. Multivariable hazard ratios (HRs) and 95% CIs for overall survival among ovarian cancer patients across strata of various factors. The analyses used three categories of cruciferous vegetables intake (T1 < 33.08, T2 14.76–90.00, and T3 ≥ 90.00 g/d). The forest plot represents the HRs of the comparison of the highest vs. the lowest of cruciferous vegetables intake. Cox model stratified by menopausal status, residual lesions, histological type, and body mass index, with additional adjustments for age at diagnosis, body mass index, comorbidities, diet change, education, FIGO stage, histological type, histopathologic grade, menopausal status, parity, physical activity, residual lesions, smoke status, meat, fruit, green leafy vegetables, allium vegetables, and total energy. *indicates P for trend across levels of cruciferous vegetables intake. **indicates P for interaction between strata and cruciferous vegetables intake. P-values are two-sided.
The present findings from this prospective cohort study of 853 Chinese women diagnosed with OC supported that pre-diagnosis CV and ITC intake might enhance the survival of patients with OC. Additionally, we observed significant findings in the analysis of specific species of CVs including Chinese cabbage, kohlrabi, and pakchoi.
Evidence from previous studies evaluating pre-diagnosis CV intake in relation to mortality among OC survivors has been limited and inconsistent. Our findings were consistent with some previous results. For example, Dolecek et al. (28) have reported that higher pre-diagnosis CV intake was significantly associated with longer survival in 341 patients with OC (HR = 0.56, 95% CI = 0.37–0.84). In addition, Nagle et al. (8) have presented findings from 609 women with invasive epithelial OC in Australia, in which pre-diagnosis CV consumption was inversely associated with total mortality (HR = 0.75, 95% CI = 0.57–0.98). In contrast, Playdon et al. (29) have reported a null finding (high to low intake, HR = 0.99, 95% CI = 0.77–1.29) in the results of the Australian OC study (n = 811), which recruited 18–79 year old patients with OC between January 2002 and June 2006. However, interestingly, they observed a trend toward lower mortality risk with higher CV intake when the analysis was restricted to high-grade serous OC cases. These inconsistent findings might be attributable to several reasons, including different demographic and clinical characteristics of patients with OC, exposure assessment, dietary habits, and potential confounders adjustment. For example, Playdon et al. included patients with more advanced OC (III–IV: 71.0%) than our study (III–IV: 48.1%). The diagnostic age composition of patients in our study (≤ 50 years: 36.7%) differed from that in the study of Playdon et al. (<50 years: 18.0%). Additionally, although both these studies used an FFQ to collect the diet information, the specific species of CVs differed among them. Of note, the consumption of CVs also differed among these studies. For example, the lower limit of the highest category in the studies of Dolecek et al., Nagle et al., and Playdon et al., was three servings/week (34.28 g/day), 0.83 servings/day (66.40 g/day), and 1.5 servings/day (120.00 g/day), respectively (1 serving equals to 80 g) (39). In contrast, the lower limit of the highest category for CV intake was 70.20 g/day in the present study. Notably, previous evidence has suggested that consumption of CVs differs among Chinese (>100 g/d), Australian (40–80 g/d), and American (25–30 g/d) populations (40). Furthermore, the difference in the potential confounding adjustments among these studies should not be ignored.
The inverse associations of CV and ITC intake with OC mortality are biologically plausible. CVs are good sources of glucosinolates, which can be hydrolyzed by the plant enzyme myrosinase into biologically active compounds such as ITCs. Findings from previous in vivo or in vitro studies have shown that ITCs have cancer-preventive potential (41) through the key mechanisms of inducing carcinogen detoxification and elimination through elevated NRF2 signaling (42, 43), enhancing DNA damage repair (44, 45), and eliminating cancer stem cells (46). Different types of ITCs can also achieve anti-OC ability by inhibiting cell proliferation (47–49) and enhancing cisplatin sensitivity (50, 51). Additionally, CVs contain antioxidants, such as carotenoids, vitamin C, and vitamin E. Antioxidants can counteract the effects of reactive oxygen species, including initiation lipid peroxidation, cell damage, disruption of cell signaling, and increased viral replication and expression, all of which are pivotal events in carcinogenesis (52, 53).
This study has several crucial strengths. First, this is the first investigation to report a survival advantage among patients with OC with higher intake of pre-diagnosis CVs and related ITCs in China. Second, the OOPS study was a well-designed prospective cohort study with high baseline survey participation rates and follow-up retention rates, thus minimizing the potential for recall bias or selection bias. Further, we used a validated FFQ to evaluate dietary information through face-to-face interviews, thus providing stable estimates of CV intake. Third, a wide range of potential confounders associated with OC prognosis were strictly adjusted for, such as residual lesions, histological type, and FIGO stage. Fourth, Asian populations habitually consume large amounts of CVs and other plant-based foods, thus enabling hypotheses associated with the underlying health properties of these foods to be investigated specifically (54).
However, potential study limitations also must be considered. First, FFQ was used to evaluate CV intake of OC patients. The standard size of food share was applied to calculate food intake, but the intake of patients could not be accurately assessed. Thus, information bias is inevitable. However, we used a highly reproducible validated FFQ and selected highly trained and skilled researchers to collect dietary information. Additionally, spearman correlation coefficients and intraclass correlation coefficients of reproducibility for CV were 0.61 and 0.53, respectively. Second, compared with the average intake of CVs (~22.6 g/d) in Western populations (55), the intake in Chinese populations exceeds 100 g/d (40), thus potentially limiting the generalizability of our findings to the consumption of CVs in Western populations. Furthermore, ITC exposure levels are influenced by various cooking methods (9). Boiling CVs leads to a 30–60% loss of complete glutathione S-transferases, owing to leaching and thermal degradation (56). Nevertheless, none of the studies have distinguished CV intake according to food preparation method. Hence, further studies must focus on whether the inverse correlation of CV intake might be influenced by the cooking method. Third, the relationship between CV intake and progression-free survival in patients with OC was not evaluated in our analysis; however, owing to the poor prognosis and short post-progression survival period of this disease, the progression-free survival was similar to overall survival (57, 58). In addition, according to previous similar research (8, 29, 59), we analyze all-cause deaths rather than OC-specific deaths in the present study, because research shows that there is little difference between them (60). Last, although we have comprehensively adjusted for potential confounding covariates in multivariate models, these findings still may be affected by unknown or unmeasured confounders including different clinical treatment and types of mutation (61, 62). Additionally, residual confounding from unmeasured confounders as well as the assessment of dietary intake may also influence the results, since these variables were assessed base on self-administered questionnaires.
CVs are commonly consumed worldwide, and they are rich in nutrients, such as ITCs and antioxidants. In the present study, increasing pre-diagnosis consumption of CVs was strongly associated with better survival in patients with OC. This protective effect was similar in the analysis of ITCs from CVs. Nevertheless, further studies with longer follow-up periods are needed to confirm our findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China. The patients/participants provided their written informed consent to participate in this study.
Q-JW, Y-HZ, and T-TG: contributed to the study design. SG, X-QL, SY, and T-TG: collection of data. Y-FW and F-HL: analysis of data. Y-FW, Y-YH, F-HL, Z-YW, H-YW, SZ, ML, and Q-JW: wrote the first draft of the manuscript and edited the manuscript. All authors have read and approved the final manuscript.
This work was supported by the National Key R&D Program of China (No. 2017YFC0907404 to Y-HZ), the Natural Science Foundation of China (No. 82073647, 81602918 to Q-JW, and 82103914 to T-TG), China Postdoctoral Science Foundation Funded Project (No. 2018M641752 to Q-JW), LiaoNing Revitalization Talents Program (No. XLYC1907102 to Q-JW), Shenyang high level innovative talents support program (No. RC190484 to Q-JW), and 345 Talent Project of Shengjing Hospital of China Medical University (Q-JW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the research team for their daily efforts in material collection and manuscript writing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.778031/full#supplementary-material
CVs, cruciferous vegetables; ITCs, isothiocyanates; OC, ovarian cancer; BMI, body mass index; HR, hazard ratio; CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; OOPS, the ovarian cancer follow-up study; FFQ, food frequency questionnaire.
1. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. (2002) 20:1248–59. doi: 10.1200/JCO.2002.20.5.1248
5. Majidi A, Na R, Dixon-Suen S, Jordan SJ, Webb PM. Common medications and survival in women with ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. (2020) 157:678–85. doi: 10.1016/j.ygyno.2020.03.028
6. Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW, Song JY. Obesity and epithelial ovarian cancer survival: a systematic review and meta-analysis. J Ovarian Res. (2014) 7:41. doi: 10.1186/1757-2215-7-41
7. Hurtado-Barroso S, Trius-Soler M, Lamuela-Raventos RM, Zamora-Ros R. Vegetable and fruit consumption and prognosis among cancer survivors: a systematic review and Meta-Analysis of cohort studies. Adv Nutr. (2020) 11:1569–82. doi: 10.1093/advances/nmaa082
8. Nagle CM, Purdie DM, Webb PM, Green A, Harvey PW, Bain CJ. Dietary influences on survival after ovarian cancer. Int J Cancer. (2003) 106:264–9. doi: 10.1002/ijc.11204
9. Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. (2007) 55:224–36. doi: 10.1016/j.phrs.2007.01.009
10. Liou CS, Sirk SJ, Diaz C, Klein AP, Fischer CR, Higginbottom SK, et al. A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell. (2020) 180:717–28. doi: 10.1016/j.cell.2020.01.023
11. Palliyaguru DL, Yuan JM, Kensler TW, Fahey JW. Isothiocyanates: translating the power of plants to people. Mol Nutr Food Res. (2018) 62:e1700965. doi: 10.1002/mnfr.201700965
12. Wu X, Zhou QH, Xu K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol Sin. (2009) 30:501–12. doi: 10.1038/aps.2009.50
13. Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. (1998) 7:1091–100.
14. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. doi: 10.1093/ije/dyw319
15. Jia X, Zhong L, Song Y, Hu Y, Wang G, Sun S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim Care Diabetes. (2016) 10:272–80. doi: 10.1016/j.pcd.2015.12.004
16. Wu QJ, Yang Y, Wang J, Han LH, Xiang YB. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci. (2013) 104:1067–73. doi: 10.1111/cas.12195
17. Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol. (2013) 24:1079–87. doi: 10.1093/annonc/mds601
18. Farvid MS, Holmes MD, Chen WY, Rosner BA, Tamimi RM, Willett WC, et al. Postdiagnostic fruit and vegetable consumption and breast cancer survival: prospective analyses in the nurses' health studies. Cancer Res. (2020) 80:5134–43. doi: 10.1158/0008-5472.CAN-18-3515
19. Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol. (2012) 19:134–41. doi: 10.1111/j.1442-2042.2011.02906.x
20. Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol. (2012) 23:2198–203. doi: 10.1093/annonc/mdr604
21. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. (2011) 129:2681–93. doi: 10.1002/ijc.25928
22. Ambrosini GL, de Klerk NH, Fritschi L, Mackerras D, Musk B. Fruit, vegetable, vitamin a intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis. (2008) 11:61–6. doi: 10.1038/sj.pcan.4500979
23. Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA. (2001) 285:2975–7. doi: 10.1001/jama.285.23.2975
24. Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr. (2012) 63:767–71. doi: 10.3109/09637486.2012.665043
25. Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract. (2012) 96:348–54. doi: 10.1016/j.diabres.2012.01.009
26. Armah CN, Derdemezis C, Traka MH, Dainty JR, Doleman JF, Saha S, et al. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: evidence from randomised controlled trials. Mol Nutr Food Res. (2015) 59:918–26. doi: 10.1002/mnfr.201400863
27. Mirmiran P, Bahadoran Z, Golzarand M, Zojaji H, Azizi F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H. pylori eradication: a randomized clinical trial in patients with type 2 diabetes. J Diabetes Metab Disord. (2014) 13:64. doi: 10.1186/2251-6581-13-64
28. Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, et al. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J Am Diet Assoc. (2010) 110:369–82. doi: 10.1016/j.jada.2009.11.014
29. Playdon MC, Nagle CM, Ibiebele TI, Ferrucci LM, Protani MM, Carter J, et al. Pre-diagnosis diet and survival after a diagnosis of ovarian cancer. Br J Cancer. (2017) 116:1627–37. doi: 10.1038/bjc.2017.120
30. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DJ, Tudor-Locke C, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12
31. Kurman RJ, Carcangiu ML, Herrington CS, Young RH, eds. WHO: Classification of Tumours of Female Reproductive Organs. (2014). Available online at: https://www.ncbi.nlm.nih.gov/nlmcatalog/101656343.
32. Aragona M. The role of comorbidity in the crisis of the current psychiatric classification system. Philos Psychiatry Psychol. (2009) 16:1–11. doi: 10.1353/ppp.0.0211
33. Yang Y, Wang G, He M, Pan C, Wang Z. China Food Composition (Standard Edition). Beijing: Peking University Medical Press (2018).
34. Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol. (2013) 24:1918–24. doi: 10.1093/annonc/mdt119
35. Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. (1998) 7:775–81.
36. Jiao D, Yu MC, Hankin JH, Low S, Chung F. Total isothiocyanate contents in cooked vegetables frequently consumed in singapore. J Agric Food Chem. (1998) 46:1055–8. doi: 10.1021/jf9706989
37. Yu EY, Wesselius A, Mehrkanoon S, Goosens M, Brinkman M, van den Brandt P, et al. Vegetable intake and the risk of bladder cancer in the BLadder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. BMC Med. (2021) 19:56. doi: 10.1186/s12916-021-01931-8
38. Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: The China Kadoorie Biobank study. Am J Clin Nutr. (2013) 97:487–96. doi: 10.3945/ajcn.112.046854
39. World Health Organization. WHO STEPS Surveillance Manual: The WHO STEPWISE Approach to Chronic Disease Risk Factor Surveillance. (2008). Available online at: http://www.who.int/chp/steps/manual/en/ (accessed September 2015).
40. Lyon. Cruciferous Vegetables, Isothiocyanates and Indoles. Internatioanal Agency for Research on Cancer World Health Organization (2004).
41. Zhang Y, Li J, Tang L. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med. (2005) 38:70–7. doi: 10.1016/j.freeradbiomed.2004.09.033
42. Dinkova-Kostova AT, Fahey JW, Kostov RV, Kensler TW. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci Technol. (2017) 69:257–69. doi: 10.1016/j.tifs.2017.02.002
43. Yang L, Palliyaguru DL, Kensler TW. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol. (2016) 43:146–53. doi: 10.1053/j.seminoncol.2015.09.013
44. Charron CS, Clevidence BA, Albaugh GA, Kramer MH, Vinyard BT, Milner JA, et al. Assessment of DNA damage and repair in adults consuming allyl isothiocyanate or Brassica vegetables. J Nutr Biochem. (2013) 24:894–902. doi: 10.1016/j.jnutbio.2012.06.004
45. Fogarty MC, Hughes CM, Burke G, Brown JC, Davison GW. Acute and chronic watercress supplementation attenuates exercise-induced peripheral mononuclear cell DNA damage and lipid peroxidation. Br J Nutr. (2013) 109:293–301. doi: 10.1017/S0007114512000992
46. Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. (2010) 16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937
47. Gong TT, Guo Q, Li X, Zhang TN, Liu FH, He XH, et al. Isothiocyanate Iberin inhibits cell proliferation and induces cell apoptosis in the progression of ovarian cancer by mediating ROS accumulation and GPX1 expression. Biomed Pharmacother. (2021) 142:111533. doi: 10.1016/j.biopha.2021.111533
48. Koschorke A, Faraci S, Giani D, Chiodoni C, Iorio E, Canese R, et al. Phenethyl isothiocyanate hampers growth and progression of HER2-positive breast and ovarian carcinoma by targeting their stem cell compartment. Cell Oncol (Dordr). (2019) 42:815–28. doi: 10.1007/s13402-019-00464-w
49. Lamy E, Oey D, Eissmann F, Herz C, Munstedt K, Tinneberg HR, et al. Erucin and benzyl isothiocyanate suppress growth of late stage primary human ovarian carcinoma cells and telomerase activity in vitro. Phytother Res. (2013) 27:1036–41. doi: 10.1002/ptr.4798
50. Gong TT, Liu XD, Zhan ZP, Wu QJ. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp Cell Res. (2020) 393:112061. doi: 10.1016/j.yexcr.2020.112061
51. Stehlik P, Paulikova H, Hunakova L. Synthetic isothiocyanate indole-3-ethyl isothiocyanate (homoITC) enhances sensitivity of human ovarian carcinoma cell lines A2780 and A2780/CP to cisplatin. Neoplasma. (2010) 57:473–81. doi: 10.4149/neo_2010_05_473
52. Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Carotenoid, vitamin a, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. (2006) 15:395–7. doi: 10.1158/1055-9965.EPI-05-0835
53. Stanczyk M, Gromadzinska J, Wasowicz W. Roles of reactive oxygen species and selected antioxidants in regulation of cellular metabolism. Int J Occup Med Environ Health. (2005) 18:15–26.
54. Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, et al. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Diet. (2014) 114:700–8. doi: 10.1016/j.jand.2013.12.019
55. Johnston CS, Taylor CA, Hampl JS. More Americans are eating “5 a day” but intakes of dark green and cruciferous vegetables remain low. J Nutr. (2000) 130:3063–7. doi: 10.1093/jn/130.12.3063
56. Zhang Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat Res. (2004) 555:173–90. doi: 10.1016/j.mrfmmm.2004.04.017
57. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
58. Amir E, Seruga B, Kwong R, Tannock IF, Ocana A. Poor correlation between progression-free and overall survival in modern clinical trials: are composite endpoints the answer? Eur J Cancer. (2012) 48:385–8. doi: 10.1016/j.ejca.2011.10.028
59. Dixon SC, Ibiebele TI, Protani MM, Beesley J, DeFazio A, Crandon AJ, et al. Dietary folate and related micronutrients, folate-metabolising genes, and ovarian cancer survival. Gynecol Oncol. (2014) 132:566–72. doi: 10.1016/j.ygyno.2013.12.025
60. Thomson CA, E CT, Wertheim BC, Neuhouser ML, Li W, Snetselaar LG, et al. Diet quality and survival after ovarian cancer: Results from the Women's Health Initiative. J Natl Cancer Inst. (2014) 106:dju314. doi: 10.1093/jnci/dju314
61. Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. (2020) 371:m3773. doi: 10.1136/bmj.m3773
Keywords: cohort, cruciferous vegetables, isothiocyanates, ovarian cancer, prognosis, survival
Citation: Wei Y-F, Hao Y-Y, Gao S, Li X-Q, Liu F-H, Wen Z-Y, Wang H-Y, Zhang S, Yan S, Luan M, Zhao Y-H, Gong T-T and Wu Q-J (2021) Pre-diagnosis Cruciferous Vegetables and Isothiocyanates Intake and Ovarian Cancer Survival: A Prospective Cohort Study. Front. Nutr. 8:778031. doi: 10.3389/fnut.2021.778031
Received: 16 September 2021; Accepted: 26 October 2021;
Published: 24 November 2021.
Edited by:
Donato Angelino, University of Teramo, ItalyCopyright © 2021 Wei, Hao, Gao, Li, Liu, Wen, Wang, Zhang, Yan, Luan, Zhao, Gong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Jun Wu, d3VxakBzai1ob3NwaXRhbC5vcmc=; Ting-Ting Gong, Z29uZ3R0QHNqLWhvc3BpdGFsLm9yZw==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.