
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 11 November 2021
Sec. Nutritional Immunology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.763700
In China, the use of antibiotics growth promoters as feed additives has been banned. The goal of raising dairy heifers is to gain a relatively high body weight on a high-fiber diet at first mating or calving, thus increasing economic benefits. The objective of this experiment was to explore the effects of supplemental Clostridium butyricum (C. butyricum) on growth performance, rumen fermentation and microbiota, and blood parameters in Holstein heifers. Twenty Holstein heifers [mean ± standard deviation (SD); age = 182 ± 4.20 d, body weight = 197.53 ± 5.94 kg, dry matter intake (DMI) = 6.10 ± 0.38 kg] were randomly assigned to one of two diets group for a 42-day feeding period: (1) basal diet (an untreated control group, i.e., the CON group) or (2) basal diet plus daily 2 × 108 (colony-forming unit, CFU) of C. butyricum per kg of DMI per heifer (the CB group). The results demonstrated that C. butyricum supplementation increased the average daily gain from d 21 to 42 and DMI compared to the control group. Supplementation with C. butyricum significantly decreased the molar proportion of acetate and the acetate to propionate ratio but increased the molar proportion of butyrate and propionate. Compared with the control group, the relative abundance of Butyrivibrio fibrisolvens, Ruminococcus albus, Ruminobacter amylophilus, Ruminococcus flavefaciens, and Streptococcus bovis increased during the trial period in the CB group. However, C. butyricum had no significant effect on the blood parameters in Holstein heifers. In conclusion, these results show that feeding C. butyricum can improve growth performance and rumen fermentation without any negative impact on blood parameters in Holstein heifers.
The heifer stage is a vigorous period of growth and development for dairy cows because muscles, bones, and organs grow rapidly during this period. Cultivation at this stage is not only related to the development of the quality of the cow's body and the normal performance of lactation performance (1) but also consumes many costs. Raising dairy heifers aims to achieve a relatively high body weight gain with high-fiber diet at first mating or calving, thus increasing economic benefits (2). The use of antibiotics has been used in the past to improve their growth performance; however, with the ban of antimicrobial feed additives in china due to the problem of antibiotic residues and environmental pollution, there has been an increasing interest by ruminant nutritionists to find substitutes for antibiotics. Many microbial species have been approved as feed additives, such as Clostridium butyricum, which can improve digestibility and growth performance by improving intestinal health (3–6).
Clostridium butyricum (C. butyricum) is a gram-positive endophytic bacterium with anaerobic probiotics properties and can produce short-chain unsaturated fatty acids, especially butyric acid (7). A key feature of this species is that it can produce endospores, unlike Lactobacillus and Bifidobacterium, as well as survive relatively high bile concentrations and low pH (8), hence increasing their survivability in the rumen. Currently, C. butyricum is widely used in the production of aquatic and monogastric animals. Several studies have shown that supplementation with C. butyricum can improve the growth performance of kuruma shrimp (9), Miichthys miiuy (10) and tilapia (4), increasing their antioxidant or immune capacity. It has also been used as a dietary probiotic to benefit immune function and, more importantly, regulate the balance of intestinal flora in broiler chickens (11). In addition, the supplementation of less digestible diets with C. butyricum in weaned piglets has been shown to influence their growth positively (12). Therefore, we envisage that C. butyricum will have a similar positive effect on heifers fed high-fiber diets. Research on C. butyricum in dairy cows has focused mainly on immune regulation, milk composition improvement and milk production (13, 14); however, there are few studies on growth and development indicators for growth stages, such as in calves and heifers. Studies have shown that microbial feed additives such as yeast can improve the productive performance of ruminants by improving the activity and growth rate of rumen microorganisms (15). We hypothesize that C. butyricum can also affect rumen fermentation by adjusting the relative abundance of rumen microbiota, thereby improving the growth performance of heifers. Therefore, the objective of this study was to evaluate the effects of dietary supplementation with C. butyricum on growth performance, rumen fermentation, rumen microbiota and blood parameters in Holstein heifers.
The Animal Care Advisory Committee of the Northeast Agricultural University approved all animal procedures and uses (protocol number: NEAU-[2011]-9, Harbin, China).
Twenty Holstein heifers [mean ± standard deviation (SD); age = 182 ± 4.20 d, body weight (BW) = 197.53 ± 5.94 kg, dry matter intake (DMI) = 6.10 ± 0.38 kg] were blocked into 10 groups based on BW, DMI and age, and heifers within a block were randomly allocated to one of two diets group (10 calves per group): (1) an untreated control group (the CON group) and (2) a group treated daily with 2 × 108 CFU per kg of DMI per heifer (the CB group). The C. butyricum LXKJ-1 was provided by Hubei Greensnow Biological Biotechnology Co., Ltd. (Wuhan, China; patent number: ZL 2016 1 0927003. 9), preservation number is CCTCC NO. M 2016130 in the China Center for Type Culture Collection, and the bacterial concentration reached 1 × 109 CFU/g. Before the morning feeding, C. butyricum LXKJ-1 (2 × 108 CFU/kg DMI) was individually hand - mixed with 200 g of the total mixed ration (TMR) feed, and the other was not (control). The ingredients and nutritional composition of the diet are given in Table 1. Diet [forage: concentrate = 50: 50, dry matter (DM) basis] was compounded according to the NRC recommendations (2001) to meet the nutrient requirements of heifers. Each heifer was individually kept in a tie stall pen in a barn, fed twice daily for at 06:00 and 18:00 with free access to water throughout the 42-day feeding trial. Based on the feed intake of the cow the day before, the amount of feed offered was adjusted daily to allow for at least 5% refusal (on an as-fed basis). The feed was pushed up at least 10 times per day.
The experiment began when heifers were 6 months of age (Day 0). BW and DMI were measured on days 0, 1, 2, then on days 20, 21, 22 and finally on days 40, 41, 42. DMI was obtained by recording the weight of offered and refusal diet of individual heifers. The average daily gain (ADG), [(kg of final BW – kg of initial BW)/experimental days] and feed efficiency (kg of ADG/kg of the DMI) were then calculated (16).
Before the morning feeding, blood samples were collected from the jugular vein using 10-mL evacuated blood-collection tubes containing heparin on days 0, 1, 2, then on days 20, 21, 22 and finally on days 40, 41, 42, and were centrifuged at 3,000 × g at 4°C for 10 min. Plasma was collected and stored at −40°C for further analysis. Plasma concentrations for blood urea nitrogen (BUN), cholesterol (CHOL), glucose (GLU), triglyceride (TG), and total protein (TP) levels were determined on a fully automated biochemical analyzer using standard commercial kits supplied from Biosino Bio-tec (Beijing, China). Concentrations of catalase (CAT), glutathione peroxidase (GSH-PX), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and total superoxide dismutase (T-SOD) in plasma were determined by colorimetry using standard commercial kits supplied from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China). Plasma concentrations of immunoglobulin A (IgA), immunoglobulin G (IgG) and immunoglobulin M (IgM) were analyzed using commercial ELISA kits (Abnova Corporation, Taipei, Taiwan, China). Three hours after the morning feeding on d 0, 1, 2, 20, 21, 22, 40, 41, and 42 of the experiment, a stomach tube equipped with a 200-mL syringe (Shanghai Syringe Factory Sales Company, Shanghai, China) was used to collect rumen fluid samples from each heifer. To prevent saliva contamination during rumen fluid collection, the first 100 ml of liquid collected was discarded. The collected rumen fluid was filtered through 4 layers of cheesecloth, and the pH was immediately measured using a pH meter (Sartorius Basic pH Meter, Germany). An aliquot (5 mL) of rumen filtrate was acidified with 1 mL of 250 g/kg metaphosphoric acid and stored at −20°C for analysis of ammonia-N (NH3-N), volatile fatty acid (VFA), and microbial crude protein (MCP) concentrations. The VFA concentration was measured by gas chromatography (GC-8A; Shimadzu Corp., Kyoto, Japan) (17). The Ammonia-N was determined using the phenol/hypochlorite method (18), while the ruminal MCP concentration was determined using the spectrophotometric method (19).
Total DNA were extracted from the rumen contents by the modified bead-beating protocol (20). The real-time PCR was carried out using a real-time PCR machine (ABI PRISM 7500 SDS thermal cycler, Applied Biosystems, Foster City, CA, USA) using SYBR Green Supermix (TaKaRa Biotechnology Co., Ltd. Dalian, China). All the operations were carried out according to the manufacturer's instructions. The PCR primer sets used are shown in Table 2. The group-specific primers for total bacteria (reference genes) and species-specific primers for Butyrivibrio fibrisolvens (B. fibrisolvens), Fibrobacter succinogenes (F. succinogenes), Prevotella ruminicola (P. ruminicola), Ruminococcus albus (R. albus), Ruminobacter amylophilus (R. amylophilus), Ruminococcus flavefaciens (R. flavefaciens), and Streptococcus bovis (S. bovis) were designed according to the methods described previously (21, 22). Relative gene expression of microbes was calculated using the 2−ΔΔCt method as follows: Relative quantification = 2−[(Cttargetgene−Ctreferencegene)treatmentgroup−(Cttargetgene−Ctreferencegene)controlgroup] (23), where Ct represents the threshold cycle.
Data were analyzed using SAS software (version 9.4, SAS Institute Inc., Cary, NC). Data on ADG and feed efficiency were analyzed using the one-way ANOVA procedure with C. butyricum treatment used as the main factor. Data on growth performance, DMI, plasma parameters, rumen fermentation parameters and microbes were analyzed using the PROC MIXED program of SAS software. A randomized block design with repeated measures was used, with time, treatment, and interaction of treatment × time as fixed effects and cow within treatment as a random effect. The data obtained from 0 day were added to the model as covariates in the statistical analysis. The level of significance was set at P <0.05, and differences were considered statistical trends when 0.05 < P ≤ 0.10. Standard errors of the mean are reported.
As shown in Table 3, BW (231.41 vs. 233.93 kg; P = 0.048) increased with the administration of C. butyricum and was influenced by time (P <0.0001) and treat × time (P = 0.0001). DMI was increased with increasing C. butyricum supplementation dose (6.76 vs. 7.12 kg; P <0.0001) and was influenced by time (P <0.0001), but not by treat × time (P = 0.66). C. butyricum supplemented diet significantly increased ADG from day 21 to 42 (1.14 vs. 1.22 kg; P = 0.0003) and there was no differences in feed efficiency between the different treatments over the entire test period.
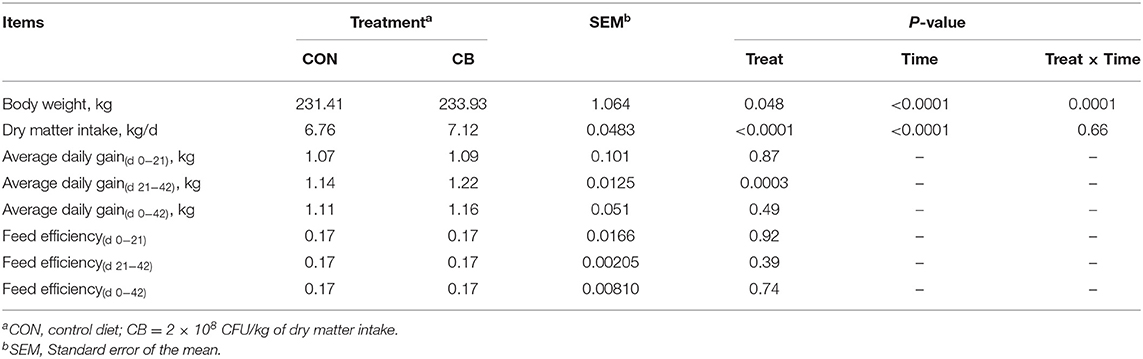
Table 3. Effect of Clostridium butyricum on growth performance, feed efficiency, and average daily gain in heifers.
The results of plasma parameters are presented in Table 4. Within the experiment, no significant differences were observed on biochemical, antioxidant, and immunological levels in heifers between the two groups, and they were not affected by time and treat × time. However, glucose (6.13 vs. 6.28 mmol/L; P = 0.08) trended to increase with C. butyricum supplementation.
Table 5 shows the results for ruminal pH, ammonia-N and VFA concentrations. There were no significant differences in pH, ammonia-N and MCP concentration with C. butyricum supplementation. The TVFA concentration was not affected by C. butyricum supplementation (53.91 vs. 54.10 mM; P = 0.75) but was influenced by time (P <0.0001). With increasing C. butyricum supplementation, molar proportion of ruminal propionic acid was increased (21.74 vs. 23.54 mol/100 mol; P = 0.001) and the acetate to propionate ratio were decreased (2.68 vs. 2.37; P = 0.0002) but time (P = 0.77 or P = 0.75, respectively) and treatment × time (P = 0.99 or P = 0.32, respectively) had no influence. Furthermore, the molar proportion of butyric acid increased (15.28 vs. 17.15 mol/100 mol; P <0.0001) with increasing C. butyricum supplementation and was affected by time (P = 0.0002) and treat × time (P <0.0001). In addition, the molar proportion of acetic acid was decreased (57.79 vs. 55.29 mol/100 mol; P = 0.0003) and was influenced by treat × time (P = 0.006).
The relative abundance of ruminal microbiota is presented in Table 6. The C. butyricum had no effect on the relative abundance of F. succinogenes and P. ruminicola in the CB group. And C. butyricum significantly increased the relative abundance of R. flavefaciens (1.03 vs. 1.91; P = 0.0001), R. albus (1.02 vs. 1.67; P = 0.04) and S. bovis (1.00 vs. 2.06; P = 0.003) and was influenced by time (P = 0.001, P = 0.003 or P <0.0001, respectively) and treat × time (P = 0.001, P = 0.003 or P <0.0001, respectively). The relative abundance of B. fibrisolvens and R. amylophilus increased (1.01 vs. 1.53; P = 0.02 or 1.00 vs. 1.53; P = 2.06, respectively) with C. butyricum supplementation and was not influenced by time (P = 0.68 or P = 0.89, respectively) and treat × time (P = 0.73 or P = 0.89, respectively).
Under the premise of being healthy, obtaining the highest weight at the lowest cost is one of the most important goals when heifers are at the age of first mating or calving. With the gradual withdrawal of feed antibiotics, probiotic feed additives have become increasingly popular. The increase in the number of beneficial bacteria in the feces, improvement of intestinal histology, and the enhancement of intestinal digestive enzyme activity may all be associated to the potential of C. butyricum HJCB998 to enhance the intestinal absorption capacity of animals, thereby improving growth performance (3). Studies for monogastric (11, 24) and ruminant (6, 14) have confirmed the positive effects of C. butyricum on the production performance and total tract apparent digestibility. Therefore, although apparent digestibility was not measured in this study, the positive effect of C. butyricum LXKJ-1 on growth performance can be inferred from the increasing in ADG and DMI of dairy heifers. Although the digestive system of monogastric animals is different from that of ruminants, a large number of positive research results on monogastric animals has given us confidence in its application in ruminants. In a related study, the feed-gain ratio of pig was reduced with Clostridium butyricum UCN - 12 supplementation, which could translate into reduce feed costs during production (12). The above studies provide theoretical references for C. butyricum LXKJ-1 regarding increased body weight in heifers. In this experiment, C. butyricum LXKJ-1 significantly increased the DMI, ADG and BW of heifers. In addition, the results showed that the improvement of rumen fermentation by C. butyricum LXKJ-1 may provide more energy for the growth of heifers. C. butyricum could produce a large amount of short-chain fatty acids during anaerobic fermentation, including propionic acid and butyric acid (25) which serves as energy source for cells. Therefore, C. butyricum UCN-12 not only increases the molar proportion of propionic acid in the rumen but is also a butyric acid-producing probiotic typically implicated in the production of butyric acid (5). As specific nutrients and energy components, propionic acid and butyric acid could also provide more energy for heifers, thereby improving their growth performance.
For heifers, ensuring the health and improving growth performance are equally important. Plasma biochemical indicators can reflect the body health condition and metabolic level of heifers; therefore, for the application of new feed additives, it is essential to verify the effects of additives on blood biochemical indicators (26). BUN is an indicator of protein and amino acid metabolism in the body. TP reflects the protein absorption and reflects the level of immunity (27). The content of TG and CHOL in plasma is also an essential indicator of the blood lipid level of the animal. In this study, the addition of C. butyricum LXKJ-1 in the diet did not affect heifers' protein and fat metabolism. However, the increasing trend of blood GLU levels were observed after feeding C. butyricum LXKJ-1 related to increased molar proportion of propionate, a glucogenic precursor formed in the rumen and increase blood glucose availability via gluconeogenesis in the liver (28). The antioxidant system can prevent animals from being harmed by free radicals and environmental stimuli generated. Enhancing the immune response can promote the improvement of the disease resistance of the animal body and improve the growth performance. Antioxidant enzyme activity and immunoglobulin content are essential indicators that reflect the body's antioxidant capacity and immune function. Kohiruimaki et al. (13) found that adding C. butyricum Miyairi 588 can enhance the number of CD4+ T cells and improve the immunity of transition dairy cows. However, in this experiment, C. butyricum LXKJ-1 did not seem to affect the antioxidant and immune functions of heifer. The difference between the results of this experiment and previous studies may be due to by differences in C. Clostridium species, animal species and experimental period. Considering the importance of C. butyricum LXKJ-1 to improve the antioxidant and immune capacity of animals to replace feed antibiotics, the efficacies of C. butyricum LXKJ-1 to improve immunological functions need further investigation.
After verifying the safety of new feed additives, we want to further unravel the reason why C. butyricum LXKJ-1 improved the performance of heifers. For ruminants, VFAs are the main source of energy. Therefore, it is necessary to deeply study the influence of C. butyricum LXKJ-1 on rumen fermentation and rumen microbiota of heifer. The improvement of rumen fermentation and the regulation of the relative numbers of cellulolytic bacteria and amylolytic bacteria by C. butyricum LXKJ-1 in the rumen are important findings of this experiment. We found that total VFAs concentration in the rumen is affected by time, which may be related to the increase in DMI with the extension of the experimental period. It has been previously reported that feeding C. butyricum to dairy cows affected the production of VFAs in the rumen (14); moreover, previous experiments have suggested that microbial feed additives can affect rumen VFA production by adjusting the number of rumen microbes (26, 29). In this experiment, supplementation with C. butyricum LXKJ-1 significantly increased the number of several major cellulolytic bacteria and amylolytic bacteria and had a greater impact on the relative abundance of two amylolytic bacteria S. bovis and R. amylophilus. This is likely the main reason why C. butyricum LXKJ-1 could influence rumen fermentation and increase the molar proportion of ruminal propionic acid in heifers. In addition, we found that C. butyricum LXKJ-1 decreased the molar proportion of acetic acid and the ratio of acetate to propionate in the rumen, which may be caused by the increase in the propionic acid production. At the same time, we also found that supplementation with Clostridium butyricum also significantly improved relative abundance of R. flavefaciens, R. albus, and B. fibrisolvens. There were time or treatment × time effects for total VFA concentration, the molar proportion of butyric acid and the relative abundance of R. flavefaciens, S. bovis, R. albus as expected in growing heifers. The significant effects of time and interaction demonstrated the continuous effect of C. butyricum LXKJ-1 on rumen fermentation and relative abundance of microbiota. The regulatory mechanism of C. butyricum LXKJ-1 on the number of rumen microbes is still unclear. However, there are reports in the literature that C. butyricum can regulate the number of bacteria in the intestine and feces in broiler chickens (C. butyricum HJCB998) (11), sows (C. butyricum UCN-12) (5) and tilapia (China Center for Type Culture Collection accession NO. M2014537) (4). Some microbial feed additives contain different enzymes, vitamins, and some unidentified cofactors that may enhance the microbial activity and growth rate in the rumen (15). C. butyricum, in addition to the production of short-chain fatty acids during metabolism, also produces some nutritional factors, such as enzymes (exo-pectate lyase, pectin methylesterase, and endo-pectate lyase) and vitamins (vitamin B and E), which may provide favorable conditions for the growth of rumen microorganisms (30–32). Therefore, the regulatory mechanism of C. butyricum LXKJ-1 impact on rumen microbes needs further study. In addition, increase in the relative expression of rumen bacteria may also increase the ruminal degradation of protein and carbohydrates in the diet (33), thereby increasing total tract apparent digestibility (6, 26).
In summary, as shown in Figure 1, the research indicates that C. butyricum LXKJ-1 can improve the rumen fermentation parameters by adjusting the number of rumen microbiota, thereby improving the growth performance of the heifers. Therefore, this study provides a theoretical grounding for enhancing the growth performance of heifers by C. butyricum supplements.
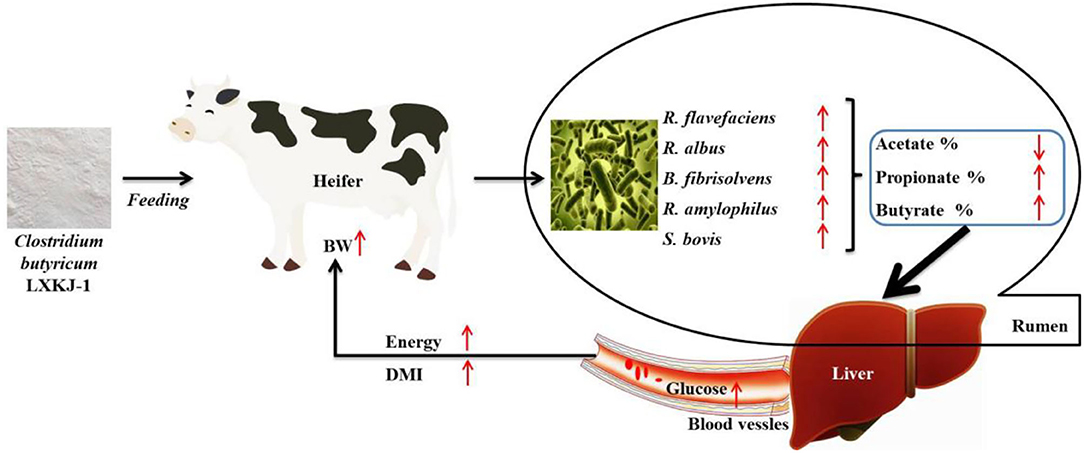
Figure 1. Pathway of Clostridium butyricum regulation of rumen fermentation to improve the growth performance of heifer.
Dietary supplementation with C. butyricum could increase BW, DMI and enhance the rumen fermentation functions by increasing the abundance of rumen microbiota and improving molar proportion of propionate and butyrate without any negative impact on blood parameters in heifers. Under the experimental conditions, C. butyricum is an effective microbial feed additive that could be used in the production of heifers.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
All animal procedures and uses were approved by the Animal Care Advisory Committee, Northeast Agricultural University.
YL and YW conceived, designed the experiments, and wrote the paper. YW, JL, and YL conducted the experiments. XD and YZ supervised the work. All authors reviewed the manuscript.
This study was financially supported by the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2020095).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Hubei Greensnow Biological Biotechnology Co., Ltd. (Wuhan, China) for donating the Clostridium butyricum used in this study.
1. Heinrichs AJ. Raising dairy replacements to meet the needs of the 21st century. J Dairy Sci. (1993) 76:3179–87. doi: 10.3168/jds.S0022-0302(93)77656-0
2. Qiao GH, Shao T, Yang X, Zhu XQ, Li JH, Lu Y. Effects of supplemental Chinese herbs on growth performance, blood antioxidant function and immunity status in Holstein dairy heifers fed high fibre diet. Ital J Anim Sci. (2013) 12:116–20. doi: 10.4081/ijas.2013.e20
3. Wang WW, Wang J, Zhang HJ, Wu SG, Qi GH. Effects of Clostridium butyricum on production performance and intestinal absorption function of laying hens in the late phase of production. Anim Feed Sci Technol. (2020) 264:114476. doi: 10.1016/j.anifeedsci.2020.114476
4. Li HQ, Zhou Y, Ling HY, Luo L, Qi DS, Feng L. The effect of dietary supplementation with Clostridium butyricum on the growth performance, immunity, intestinal microbiota and disease resistance of tilapia (Oreochromis niloticus). PloS ONE. (2019) 14:e0223428. doi: 10.1371/journal.pone.0223428
5. Cao M, Li Y, Wu QJ, Zhang P, Li WT, Mao ZY, et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J Anim Sci. (2019) 8 3426-3439. doi: 10.1093/jas/skz186
6. Cai LY, Yu JK, Hartanto R, Qi DS. Dietary supplementation with Saccharomyces cerevisiae, Clostridium butyricum and their combination ameliorate rumen fermentation and growth performance of heat-stressed goats. Animals. (2021) 11:2116. doi: 10.3390/ani11072116
7. Duan YF, Dong HB, Wang Y, Zhang Y, Zhang JS. Effects of the dietary probiotic Clostridium butyricum on intestine digestive and metabolic capacities, SCFA content and body composition in marsupenaeus japonicus. J Ocean Univ China. (2018) 17:690–696. doi: 10.1007/s11802-018-3464-3
8. Kong Q, He GQ, Jia JL, Zhu QL, Ruan H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbio. (2011) 62:512–7. doi: 10.1007/s00284-010-9737-8
9. Duan YF, Zhang Y, Dong HB, Wang Y, Zhang JS. Effect of the dietary probiotic Clostridium butyricum on growth, intestine antioxidant capacity and resistance to high temperature stress in kuruma shrimp Marsupenaeus japonicus. J Therm Biol. (2017) 66:93–100. doi: 10.1016/j.jtherbio.2017.04.004
10. Song ZF, Wu TX, Cai LS, Zhang LJ, Zheng XD. Effects of dietary supplementation with Clostridium butyricum on the growth performance and humoral immune response in Miichthys miiuy. J Zhejiang Univ Science B. (2006) 7:596–602. doi: 10.1631/jzus.2006.B0596
11. Yang CM, Cao GT, Ferket PR, Liu TT, Chen AG. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. (2012) 91:2121–9. doi: 10.3382/ps.2011-02131
12. Chen L, Li S, Zheng J, Li WT, Jiang XM, Zhao XL, et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Anim.Sci Biotechnol. (2018) 9:957–70. doi: 10.1186/s40104-018-0275-8
13. Kohiruimaki M, Ohtsuka H, Tanami E, Kitagawa M, Masui M, Ando T, et al. Effects of active egg white product / Clostridium butyricum Miyairi 588 additive on peripheral leukocyte populations in periparturient dairy cows. J Vet Med Sci. (2008) 70:321–3. doi: 10.1292/jvms.70.321
14. Jin GL, Choi SK, Choi SH, Song MK. Effect of microbial additives on metabolic characteristics in sheep and milking performance of lactating dairy cows. J Anim Sci Technol. (2007) 49:819–28. doi: 10.5187/JAST.2007.49.6.819
15. Ghazanfar S, Anjum MI, Azim A, Ahmed I. Effects of dietary supplementation of yeast (Saccharomyces cerevisiae) culture on growth performance, blood parameters, nutrient digestibility and fecal flora of dairy heifers. J Anim Plant Sci. (2015) 25:53–9.
16. Jiang X, Xu HJ, Cui ZQ, Zhang YG. Effects of supplementation with Lactobacillus plantarum 299v on the performance, blood metabolites, rumen fermentation and bacterial communities of preweaning calves. Livest Sci. (2020) 239:104120. doi: 10.1016/j.livsci.2020.104120
17. Stewart CS, Duncan SH. The effect of avoparcin on cellulolytic bacteria of the ovine rumen. Microbiology. (1985) 131:427–35. doi: 10.1099/00221287-131-3-427
18. Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. (1980) 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
19. Zinn RA, Owens FN. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Can J Anim Sci. (1986) 66:157–66. doi: 10.4141/cjas86-017
20. Yu ZT, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. (2004) 36:808–12. doi: 10.2144/04365ST04
21. Denman SE, Mcsweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. (2009) 58:572–82. doi: 10.1111/j.1574-6941.2006.00190.x
22. Khafipour E, Li SC, Plaizier JC, Krause DO. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Applied and Environ Microbiol. (2009) 22:7115–24. doi: 10.1128/AEM.00739-09
23. Kim WY, Hanigan MD, Lee SJ, Lee SM, Kim DH, Hyun JH. Effects of Cordyceps militaris on the growth of rumen microorganisms and in vitro rumen fermentation with respect to methane emissions. J Dairy Sci. (2014) 97:7065–75. doi: 10.3168/jds.2014-8064
24. Zhan HQ, Dong XY, Li LL, Zheng YX, Gong YJ, Zou XT. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult Sci. (2018) 98:896–903. doi: 10.3382/ps/pey436
25. Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. (2002) 217:133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x
26. Li Y, Sun YK, Li X, Zhang GN, Xin HS., Xu HJ, et al. Effects of Acremonium terricola culture on performance, milk composition, rumen fermentation and immune functions in dairy cows. Anim Feed Sci Technol. (2018) 240:40–51. doi: 10.1016/j.anifeedsci.2018.03.015
27. Kaneko J. Clinical biochemistry of domestic animals. Am J Archaeol. (2008) 116:1970. doi: 10.1016/B978-0-12-370491-7.X0001-3
28. Jiang X, Liu X, Liu S, Li Y, Zhao HB, Zhang YG. Growth, rumen fermentation and plasma metabolites of Holstein male calves fed fermented corn gluten meal during the postweaning stage. Anim Feed Sci Technol. (2019) 249:1–9. doi: 10.1016/j.anifeedsci.2019.01.012
29. Yeo JM, Lee SJ, Lee SM, Shin SH, Lee SH, Ha JK, et al. Effects of Cordyceps militaris mycelia on in vitro rumen microbial fermentation. Asian-Australas J Anim Sci. (2009) 22:201–5. doi: 10.5713/ajas.2009.80579
30. Nakajima N, Ishihara K, Tanabe M, Matsubara K, Matsuura Y. Degradation of pectic substances by two pectate lyases from a human intestinal bacterium, Clostridium butyricum-beijerinckii group. J Biosci Bioeng. (1999) 88:331–3. doi: 10.1016/S1389-1723(00)80020-1
31. Howie JW, Baker F. Rumen and caecal microorganisms as symbionts. Proc R Soc B. (1952) 139:193–6. doi: 10.1098/rspb.1952.0005
32. Araki Y, Andoh A, Fujiyama Y, Takizawa J, Takizawa W, Bamba T. Oral administration of a product derived from Clostridium butyricum in rats. Int J Mol Med. (2002) 9:53. doi: 10.3892/ijmm.9.1.53
Keywords: heifer, Clostridium butyricum, growth performance, rumen fermentation, rumen microbiota
Citation: Li Y, Wang Y, Lv J, Dou X and Zhang Y (2021) Effects of Dietary Supplementation With Clostridium butyricum on the Amelioration of Growth Performance, Rumen Fermentation, and Rumen Microbiota of Holstein Heifers. Front. Nutr. 8:763700. doi: 10.3389/fnut.2021.763700
Received: 24 August 2021; Accepted: 19 October 2021;
Published: 11 November 2021.
Edited by:
Julio Villena, CONICET Centro de Referencia para Lactobacilos (CERELA), ArgentinaReviewed by:
Sandra Quilodrán-Vega, University of Concepción, ChileCopyright © 2021 Li, Wang, Lv, Dou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujing Dou, ZG91eGl1amluZ0BuZWF1LmVkdS5jbg==; Yonggen Zhang, emhhbmd5b25nZ2VuQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.