
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 30 September 2021
Sec. Nutrigenomics
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.751010
This article is part of the Research TopicThe Epigenetic Mechanism of Intestinal Immune Response and Its Nutritional RegulationView all 8 articles
Animal antimicrobial peptides (AMPs), known as broad-spectrum and high-efficiency antibacterial activity, are important effector molecules in innate immune system. AMPs not only have antimicrobial, antiviral and antitumor effects but also exhibit important effects in vivo, such as anti-inflammatory response, recruiting immune cells, promoting epithelial damage repair, and promoting phagocytosis of bacteria. However, research on the application of AMPs is incomplete and controversial. This review mainly introduces the classification of AMPs, biological functions, as well as the mechanisms of action, expression rules, and nutrition regulation from three perspectives, aiming to provide important information for the application of AMPs.
The indiscriminate use of antibiotics has caused bacteria to develop resistance and super bacteria that endangers human health. Therefore, researchers are desperately searching for alternatives to antibiotics. Recently, antimicrobial peptides (AMPs) have received extensive attention because of their broad antimicrobial spectrum, low resistance to drug resistance, and no residue formation.
AMPs, also known as host defense peptides, are a key barrier in the body to prevent the invasion of foreign pathogenic bacteria. Mature AMPs generally contain 12–100 amino acid residues and have positively charged and amphiphilic molecular structures, which facilitates their interaction with the cellular targets (like negatively charged microbial membranes or others) (1). However, as studies on AMPs continue to be deepen, researchers have found that they are not omnipotent. Some bacteria can still develop resistance to AMPs, and certain AMPs can also partly kill probiotics in vivo. Some researchers found that when S. aureus was experimentally exposed to pexiganan, cross-resistance occurred (2). In this review, we summarized the classification, biological functions, active mechanism and expression of AMPs.
Based on structural differences, AMPs can be roughly divided into three categories: polyamino acids, short-chain AMPs, and lipopeptides.
Polyamino acids are polymers formed by the condensation of amino groups and carboxyl groups between amino acid molecules. Cationic polyamino acids, such as polylysine, polyarginine, and polyhistidine, have bactericidal effects and have attracted a lot of attention due to their unique antibacterial mechanism and low resistance to bacterial resistance. The bacterial cell membrane can be depolarized and ruptured by polylysine through electrostatic interactions, leading to the death of bacteria (3, 4). Polyarginine contains a guanidine group, which can ionize in an alkaline environment, penetrate the outer membrane of bacteria, dissolve cells; on the other hand, bacterial contents and nucleic acids can bind to polyarginine, causing the change of replication and transcription of target cells genes (5). In acidic conditions, polyhistidine can interact efficiently with the outer membrane of anionic cells, thereby exerting an antibacterial effect (6). Short-chain AMPs are peptides that are shorter than 15 amino acids, usually chemically synthetic, and have lower production costs and optimization potential compared to other AMPs. At present, the common short-chain AMPs mainly include those that with positively charged amino acids and hydrophobic amino acids. Most short-chain AMPs commonly exhibit positive charge and hydrophobicity. While bacterial cell membranes are mostly negatively charged, which can generate electrostatic affinity with the positive charges, and easily anchored by the hydrophobicity and overall amphiphlicity of peptides, leading to the destruction of the cell membrane, outflow of bacterial contents, and eventually, death (7). Lipopeptides, amphiphilic substances, composed of peptide base connected to one or more lipid chains, usually self-assemble and form certain aggregation structures (8). Active substances can be transported into cells in the form of endocytosis through lipopeptides, and the cell walls of bacteria will also can be destroyed by lipopeptides (8, 9). Based on the shape, they can be divided into two types: cyclic lipopeptides and linear lipopeptides.
According to different origins, AMPs usually have the following four categories: microbial AMPs, animal AMPs, plant AMPs, and other AMPs. AMPs from different origins have different antibacterial effects. Here we mainly discuss animal AMPs, which can be divided into insect AMPs, mammalian AMPs, amphibian AMPs, avian AMPs, and fish AMPs.
Insect AMPs are basic peptides with low molecular weight, good water solubility, strong thermal stability, no immunogenicity, and resistance to hydrolysis. They do not damage the normal cells of higher animals but have strong and broad-spectrum antibacterial, anticancer, and antiviral abilities (10). Mammalian AMPs, divided into defensins and cathelicidins, mainly exist in neutrophils and epithelial cells of skin and mucosa. Among them, defensins have been studied the most and constitute most of the AMPs in the AMP family. Amphibian AMPs, composed of 5–60 amino acids, usually have good water solubility, good thermal stability, and the ability to tolerate proteases. They are mostly composed of a single peptide chain, and some also have a special disulfide bond structure (11). Avian defensins are significant different from mammalian defensins in coding. Human β-defensin is encoded by only two exons, while avian β-defensin is encoded by four exons, where the first one encodes the 5'UTR region of the defensin gene, the second encodes signal and part of the precursor, the third encodes the rest of the precursor, and the third and fourth encode mature peptides (12). But AvBD12 has only three exons, which may be the last two exons fused into one during evolution (13). Moreover, in the avian cathelicidin family, the domain of the signal peptide cathelicidins are highly conserved, but the mature peptides are highly differentiated (13, 14). Fish AMPs are positively charged short-chain amino acids that participate in the host's defense mechanism. Although fish AMPs are divided into defensins and cathelicidins similar to mammals, fish also secrete specific AMPs with high salt tolerance, which might be associated with the high salt of sea water (15).
AMPs have negatively charged electrostatic interactions and interactions with specific intracellular targets, which make the integrity of microbial cell membranes destroyed and the synthesis of cell proteins, DNA and RNA inhibited (16). AMPs mostly exert antibacterial effects via their secondary structure, which mainly includes α helices and β sheets. In aqueous solution, most α-AMPs are in random helical conformation, which can resume amphipathic conformations, highly structured, in membrane mimetic environment. In contrast, β-AMPs are quite more ordered in both above cases (17). It makes α-AMPs bind to bacterial membranes that the electrostatic interaction between cationic residues on the peptide and anionic lipids on the target membrane (18). β-AMPs have a linear butyl side chain, which can interact with membrane lipids through the hydrophobic subunit Bu to kill bacteria by disrupting the integrity of the bacterial membrane (19). Moreover, many studies have shown that it's essential for AMPs to ploy biological activities and primary mechanism of action that the positive charges and hydrophobic properties (Figure 1A). The electrostatic interaction, that between AMPs and the negatively charged bacterial membrane, can be promoted by the positive charges; while the bilayer of the bacterial membrane can be inserted by AMPs though hydrophobicity, which leads to the cell membrane be disrupted and permeabilitied, and the bacterial contents be leakaged and death, eventually (20–22). On the other hand, some AMPs, which can make membrane transporters blocked and cell division inhibited and can interact with nucleic acids and/or with the protein biosynthesis process, function differently and act in a non-lytic manner, preferring intracellular targets (23–25). Some membrane-active AMPs, such as indolicidin and LL-37, may penetrate the cell, interact with intracellular targets and bind to the DNA (as many cationic AMPs do) (26, 27). Other AMPs, such as the proline-rich drosocin (in the non-glycosylated form), enter the cell through transporters and act on specific intracellular proteins, without significantly perturbing its membranes (28).
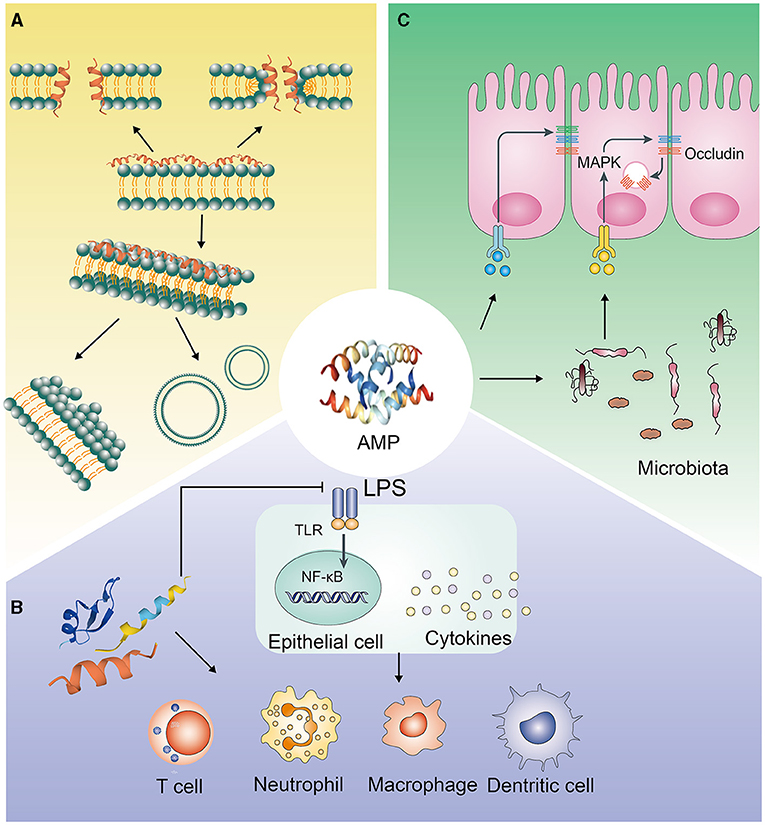
Figure 1. Antimicrobial pathways of AMPs. (A) Interacting with pathogenic bacteria directly; (B) Eliminating pathogenic bacteria through immune regulation; (C) Inhibiting the invasion of pathogenic bacteria by regulating the intestinal barrier.
The antibacterial effect of AMPs is also related to their concentration. AMPs are usually located on the surface of the cell membrane, and when the peptide concentration reaches a certain threshold, the bacterial cell membrane is destroyed (29). In locations when AMPs' physiological concentration are lower than micromolar levels, other functions such as immune regulation might be more important than killing bacterial directly. Animals generate a suite of AMPs, which act additively and often even synergistically in most cases. Hence, although the concentration of each AMP might be below the activity threshold, bacteria might still be killed by the AMP mixture (30, 31). It indicates a cooperative interaction of the AMPs molecules with the lipid monolayer that the surface pressure of the mixture is sigmoidal increasing by increasing AMPs' concentration (32). Interestingly, when the concentration is too high, AMPs may start to aggregate before attaching to the cell membrane, suggesting that their potency to disrupt the cell membrane might be reduced (33). High concentrations of AMPs were shown to increase the intestinal permeability and imbalance of intestinal bacteria in mice (34).
Research on the mechanism of action of AMPs, which resist fungi, has also been performed. Some studies have found that the intracellular targets can be affected by AMPs though ROS production, programmed cell death, mitochondrial dysfunction, disruption of cation homeostasis, ATP efflux, cell cycle impairment, autophagy, and vacuolar dysfunction. AMPs, which finish interacting with the target, can be internalized or can remain outside the fungal cell (35).
The AMPs' concentration in the body is lower than 2 μg/ml, which is lesser than the concentration required for bactericidal effects but is sufficient to regulate immune cell function in the physiological environment (36). Many studies have shown that immunomodulatory activity is the main biological function of AMPs (Figure 1B).
The immunomodulatory functions of AMPs mainly include: (1) Regulating the level of inflammation in vivo. Excessive activation and amplification of the innate immune system can cause damage to the host, and AMPs can inhibit excessive inflammation in vivo. The mechanisms of AMPs regulating inflammation are very complicated. LL-37 can prevent the translocation of the NF-κB subunit p65 translocation, activate MAPK and PI3K signaling pathways, and selectively upregulate the expression of anti-inflammatory factors, or directly bind to LPS, preventing its interaction with LPS binding protein, thereby inhibiting the activation of TLR4 and the downstream signaling pathways (37, 38). In B lymphocytes, mouse and human neutrophils, and dendritic cells, the abnormally high expression of proinflammatory factors, induced by LPS, can be reduced by LL-37 (36, 37, 39). Moreover, AMPs, such as C14-R1 and C12-R2, can kill bacteria via prom0oting ROS generation and causing oxidative damage (40). (2) Indirectly playing a chemotactic role by inducing or increasing the secretion of chemokines. At low physiological concentrations, AMPs can induce the chemotaxis of immune effector cells and the production of chemokines. For example, human defensins can stimulate the transcription and production of IL-8 in bronchial epithelial cells, via inducing degranulation and activation of mast cells to recruit neutrophils (37). When the physiological concentration is little higher, AMPs directly act as chemokines, recruiting granulocytes to the infection site to initiate innate and adaptive immune responses. LL-37 can mediate FPR2 receptors and CXCR2 to increase calcium efflux, thereby facilitating chemotaxis of peripheral blood monocytes and neutrophils. Moreover, LL-37 can also activate FPR2 receptors to induce monocyte chemotaxis (41, 42). Similarly, hBD-2 and hBD-3 can chemoattract monocytes through CCR2 (43). (3) Initiating and regulating specific immunity. If the innate immunity is unable to eliminate the infection, AMPs initiate and expand the host's specific immune response by signal transmission pathways, acting as a signal transduction bridge between innate immunity and specific immunity. Injection of PTd and AMP HH2-CpG can increase the secretion level of IgG by 100 times, and hence, increase the antibody level of immunoglobulin subtypes IgG2a and IgG1 (44). (4) Directly enhancing the ability to resist bacterial infections. CRAMP knockout mice are more likely to show skin necrosis due to Streptococcal A infection and are more likely to show urinary system infections (45, 46). (5) Activating immune cell function through specific receptors. For example, LL-37, formed a complex with DNA of host cell, activates plasma cell-like DC cells through the TLR-9 signaling pathway, making IFN-γ produced and autoimmune T cells activated (47, 48). Moreover, porcine cathelicidin protegrin-1 maintains barrier function by accelerating the migration of porcine epithelial cell, depending on the activation of insulin-like growth factor-1 receptors, to modulate immune activity (49).
Epithelial cells in the animal intestine, urinary tract, and respiratory tract can express AMPs. In recent years, many studies have shown that AMPs play an important role in animal mucosal and skin defense. The role of AMPs is not only to kill pathogenic bacteria but also to enhance the body's resistance to pathogenic microorganisms by enhancing the barrier function of epithelial tissue (Figure 1C). For example, LL-37 can induce the expression of various cell growth factors such as vascular endothelial growth factor and keratinocyte growth factor, stimulate the growth of intestinal epithelial cells, and ensure the integrity of the intestinal epithelial tissue (50). LL-37 can also increase the hardness of alveolar epithelial cells by interacting with fibrous actin, thereby enhancing the body's defense against Pseudomonas aeruginosa. Cathelicidin can increase the expression of epithelial mucins MUC1 and MUC2 via the MAPK signaling pathway and promote the repair of epithelial injury (51). Cathelicidin-WA can also promote the absorption of long-chain fatty acids by intestinal epithelial cells via the PPAR-γ signaling pathway to strengthen the intestinal barrier (52). Moreover, AMPs have also been shown to facilitate the absorption of skin-derived toxins to protect frogs from predators, as AMPs can permeabilize the epithelial tissue to enable fast transmembrane transport for co-secreted toxins (53). Meanwhile, during thermal injury, β-defensin-2 can bind to C1q to make the classical pathway inhibited, with the result that it can be reduced that the overactivation of complement cascade by human β-defensin-2 (54). Tight junctions between intestinal epithelial cells can regulate the permeability of the intestinal mucosal barrier and maintain the tightness of the intestinal epithelial cells. They are a vital part of the intestinal mucosal barrier. In recent years, studies have shown that AMPs can regulate the expression of tight junction proteins and affect the permeability of the intestinal mucosal barrier. Human defensin-1 can increase the expression of the tight junction proteins occludin and claudin-1 in epidermal keratinocytes and reduce cell permeability (55). Similarly, the AMPs, cathelicidin-BF, mastoparan X, and lactoferrin-derived peptide-20, can increase the expression of IPEC-J2 tight junction protein zonula occludens-1, occludin, and claudin-1 in rat intestinal epithelial cells, maintain the normal shape of the tight junction structure, and protect the integrity of the intestinal barrier (56–60). Moreover, the AMP microcin J25 can improve intestinal mucosal morphology and strengthen the intestinal barrier in mice, along with a reduction in intestinal permeability (34, 61). Our research results also showed that injection of PR-39 significantly alleviated the damage to the mouse intestine caused by Salmonella infection and dextran sodium sulfate (DSS) induction and maintained the structural integrity of the intestine and intestinal homeostasis (62). Additionally, it has been reported that AMPs can interact with intestinal microbes to regulate the structure of intestinal flora, maintain intestinal homeostasis, and strengthen the intestinal barrier (63, 64). However, AMPs such as melittin, which can open the tight junctions rapidly between cells and cause a sudden increase in cell permeability, have the opposite effect on epithelial cells (65).
AMPs show different expressions in different growth and developmental stages of organisms. Our research group studied the expression patterns of the β-defensin family in the intestines of Jinhua pigs and Landrace pigs of different ages and found that the expression of pBD-1 and pBD-3 genes in the intestines of piglets showed an increasing trend with the increase in age. At the age days of 20, 40, and 60, the expression levels of pBD-1, pBD-2, and pBD-3 in the intestine of Jinhua pigs were higher than those of Landrace pigs (66). Other studies have shown that weaning can significantly affect the expression of AMPs PR-39 and PG−1 mRNA in piglets (67). In other animals, the expression of Cathelicidin AMPs also shows developmental changes with age. The CRAMP gene of cathelicidin AMPs in the mouse intestine is highly expressed in young mice, and its expression level gradually decreases with the proliferation and differentiation of epithelial cells (68). AMPs, as the effector molecules, are significant for innate immunity. When foreign bacteria infect, the body expresses AMPs to play a corresponding immunomodulatory effect. The body has different immune responses to bacteria of different serotypes and shows selectivity. For example, Salmonella typhimurium can specifically upregulate the gene expression of porcine defensin-1 and defensin-2 in the epithelial cell line of the pig colon, while Salmonella cholerae do not stimulate the expression of AMPs (69). The infection of foreign pathogenic microorganisms and their toxins can increase the expression of β-defensin in piglets or the piglet jejunal epithelial cell line IPEC-J2 (70). For example, S. typhimurium can increase the gene and protein expression of PR-39 and protegrin in pig bone marrow cells, and the infection of Streptococcus ATCC19714 can upregulate the gene expression of oral β-defensin 1 in pigs (71). We found that E. coli K88 infection promoted the expression of the PR-39 gene in the bone marrow, spleen, and ileum of Jinhua pigs and Landrace pigs and increased the expression of the PR-39 gene in the thymus, liver, and lung of piglets (72). The mechanism of action probably depends on the FOXO6-METTL3-m6A-GPR161 signaling axis (73).
It is more economical and effective to regulate the expression of endogenous AMPs through nutritional means, among which, VD3 and butyric acid are the most important regulators. VD3 can induce the expression of AMPs in a variety of human cell lines and primary cells (74). VD3 can inhibit bacteria by killing them through the effect of LL-37 and the maturation of phagosomes; additionally, it can induce the expression of HBD-2 and has a dose-dependent effect (68). Retinoic acid, metabolized by VA, can induce the expression of PR-39 in pigs (70). Additionally, bacterial polysaccharides can increase the expression levels of PR-39 in the bone marrow and liver and hepcidin in the liver (75). Arginine, isoleucine, leucine, and valine can modulate the expression of intestinal endogenous β-defensins in porcine through multiple pathways (76, 77).
To minimize the excessive use of antibiotics, the emergence of AMPs might solve key problems such as bacterial resistance and drug residues. However, the application of AMPs in the aquaculture industry still faces many challenges. With the continuous advancement in science and technology, the technical barriers to the application of AMPs in the aquaculture industry might be gradually removed. By constructing a suitable expression system and improving the expression strategy, many recombinant AMPs with low cost, high yield, and excellent activity can be obtained. Through genetic and artificial modifications, the antimicrobial function of AMPs can be maximized. The use of amidation, cyclic methods such as D-amino acid substitutions, and coating can improve the stability and safety of AMPs in the body. A comprehensive analysis of AMPs from the structural features and pharmacokinetics to the immunomodulatory mechanisms is benefit to the development of safer and more efficient AMPs for health. In addition, with the deepening of molecular directed evolution and systematic molecular evolution research in AMPs, more high-quality AMPs will be gradually developed, and the application of AMPs will be more and more extensive.
XZ and XC: designed the study. TG and JF: wrote the manuscript. XZ, TG, and LS: critically made comments to the manuscript. All authors have read and agreed to the published version of the manuscript.
This study was supported by the National Natural Science Foundation of China (key program, 31630075, 32002185), the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ21C170002), and the Guangdong Basic and Applied Basic Research Fund Project under grant (number 2019A1515110092, 2019A1515110696).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. (2020) 19:311–32. doi: 10.1038/s41573-019-0058-8
2. Habets MGJL, Brockhurst MA. Therapeutic antimicrobial peptides may compromise natural immunity. Biol Lett. (2012) 8:416–8. doi: 10.1098/rsbl.2011.1203
3. Xi Y, Song T, Tang S, Wang N, Du J. Preparation and antibacterial mechanism insight of polypeptide-based micelles with excellent antibacterial activities. Biomacromolecules. (2016) 17:3922–30. doi: 10.1021/acs.biomac.6b01285
4. Shao Z, Yang Y, Fang S, Li Y, Chen J, Meng Y. Mechanism of the antimicrobial activity of whey protein-epsilon-polylysine complexes against Escherichia coli and its application in sauced duck products. Int J Food Microbiol. (2020) 328:108663. doi: 10.1016/j.ijfoodmicro.2020.108663
5. Li J, Liu S, Lakshminarayanan R, Bai Y, Pervushin K, Verma C, et al. Molecular simulations suggest how a branched antimicrobial peptide perturbs a bacterial membrane and enhances permeability. Biochim Biophys Acta Biomem. (2013) 1828:1112–21. doi: 10.1016/j.bbamem.2012.12.015
6. Holdbrook DA, Singh S, Choong YK, Petrlova J, Malmsten M, Bond PJ, et al. Influence of pH on the activity of thrombin-derived antimicrobial peptides. Biochim Biophys Acta Biomemb. (2018) 1860:2374–84. doi: 10.1016/j.bbamem.2018.06.002
7. Yan Y, Li Y, Zhang Z, Wang X, Niu Y, Zhang S, et al. Advances of peptides for antibacterial applications. Colloids Surfaces B Biointerfaces. (2021) 202:111682. doi: 10.1016/j.colsurfb.2021.111682
8. Hamley IW. Lipopeptides: from self-assembly to bioactivity. Chem Commun. (2015) 51:8574–83. doi: 10.1039/C5CC01535A
9. Reinhardt A, Neundorf I. Design and application of antimicrobial peptide conjugates. Int J Mol Sci. (2016) 17:701. doi: 10.3390/ijms17050701
10. Manniello MD, Moretta A, Salvia R, Scieuzo C, Lucchetti D, Vogel H, et al. Insect antimicrobial peptides: potential weapons to counteract the antibiotic resistance. Cell Mol Life Sci. (2021) 78:4259–82. doi: 10.1007/s00018-021-03784-z
11. Varga JFA, Bui-Marinos MP, Katzenback BA. Frog skin innate immune defences: sensing and surviving pathogens. Front Immunol. (2019) 9:3128. doi: 10.3389/fimmu.2018.03128
12. Hellgren O, Ekblom R. Evolution of a cluster of innate immune genes (beta-defensins) along the ancestral lines of chicken and zebra finch. Immunome Res. (2010) 6:3. doi: 10.1186/1745-7580-6-3
13. Cuperus T, Coorens M, van Dijk A, Haagsman HP. Avian host defense peptides. Dev Comp Immunol. (2013) 41:352–69. doi: 10.1016/j.dci.2013.04.019
14. Wang Y, Wang M, Shan A, Feng X. Avian host defense cathelicidins: structure, expression, biological functions, and potential therapeutic applications. Poult Sci. (2020) 99:6434–45. doi: 10.1016/j.psj.2020.09.030
15. Li Y, Xiang Q, Zhang Q, Huang Y, Su Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides. (2012) 37:207–15. doi: 10.1016/j.peptides.2012.07.001
16. Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. (2013) 6:1543–75. doi: 10.3390/ph6121543
17. Ciumac D, Gong H, Hu X, Lu JR. Membrane targeting cationic antimicrobial peptides. J Colloid Interface Sci. (2019) 537:163–85. doi: 10.1016/j.jcis.2018.10.103
18. Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Am J Neuroradiol. (2018) 39:E61–76. doi: 10.3174/ajnr.A5638
19. Zhang Q, Ma P, Xie J, Zhang S, Xiao X, Qiao Z, et al. Host defense peptide mimicking poly–peptides with fast, potent and broad spectrum antibacterial activities. Biomater Sci. (2019) 7:2144–51. doi: 10.1039/C9BM00248K
20. Wang J, Dou X, Song J, Lyu Y, Zhu X, Xu L, et al. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med Res Rev. (2019) 39:831–59. doi: 10.1002/med.21542
21. Yang Y, Wu D, Wang C, Shan A, Bi C, Li Y, Gan W. Hybridization with insect cecropin A (1-8) improve the stability and selectivity of naturally occurring peptides. Int J Mol Sci. (2020) 21:1470. doi: 10.3390/ijms21041470
22. Li J, Han Y, Qu G, Cheng J, Xue C, Gao X, et al. Molecular dynamics simulation of the aggregation behavior of N-Dodecyl-N, N-Dimethyl-3-Ammonio-1-Propanesulfonate/sodium dodecyl benzene sulfonate surfactant mixed system at oil/water interface. Colloids Surfaces Physicochem Eng Aspects. (2017) 531:73–80. doi: 10.1016/j.colsurfa.2017.07.088
23. Ongey EL, Pflugmacher S, Neubauer P. Bioinspired designs, molecular premise and tools for evaluating the ecological importance of antimicrobial peptides. Pharmaceuticals. (2018) 11:68. doi: 10.3390/ph11030068
24. Armengol E, Domenech O, Fuste E, Perez-Guillen I, Borrell JH, Sierra JM, et al. Efficacy of combinations of colistin with other antimicrobials involves membrane fluidity and efflux machinery. Infect Drug Resist. (2019) 12:2031–8. doi: 10.2147/IDR.S207844
25. Zhao Y, Chen Z, Cao Z, Li W, Wu Y. Defensins, a novel type of animal toxin-like potassium channel inhibitor. Toxicon. (2019) 157:101–5. doi: 10.1016/j.toxicon.2018.11.304
26. Savini F, Loffredo MR, Troiano C, Bobone S, Malanovic N, Eichmann TO, et al. Binding of an antimicrobial peptide to bacterial cells: interaction with different species, strains and cellular components. Biochim Biophys Acta Biomemb. (2020) 1862:183291. doi: 10.1016/j.bbamem.2020.183291
27. Casciaro B, Lin Q, Afonin S, Loffredo MR, de Turris V, Middel V, et al. Inhibition of Pseudomonas aeruginosa biofilm formation and expression of virulence genes by selective epimerization in the peptide Esculentin-1a(1-21)NH2. FEBS J. (2019) 286:3874–91. doi: 10.1111/febs.14940
28. Cardoso MH, Meneguetti BT, Costa BO, Buccini DF, Oshiro KGN, Preza SLE, et al. Non-lytic antibacterial peptides that translocate through bacterial membranes to act on intracellular targets. Int J Mol Sci. (2019) 20:4877. doi: 10.3390/ijms20194877
29. Yount NY, Yeaman MR. Immunocontinuum: perspectives in antimicrobial peptide mechanisms of action and resistance. Protein Peptide Lett. (2005) 12:49–67. doi: 10.2174/0929866053405959
30. Hancock REW, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. (2016) 16:321–34. doi: 10.1038/nri.2016.29
31. Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. (2020) 368:487. doi: 10.1126/science.aau5480
32. Zhang L, Rozek A, Hancock RE. Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem. (2001) 276:35714–22. doi: 10.1074/jbc.M104925200
33. Haney EF, Wu B, Lee K, Hilchie AL, Hancock REW. Aggregation and its influence on the immunomodulatory activity of synthetic innate defense regulator peptides. Cell Chem Biol. (2017) 24:969. doi: 10.1016/j.chembiol.2017.07.010
34. Yu H, Shang L, Zeng X, Li N, Liu H, Cai S, et al. Risks related to high-dosage recombinant antimicrobial peptide microcin J25 in mice model: intestinal microbiota, intestinal barrier function, and immune regulation. J Agric Food Chem. (2018) 66:11301–10. doi: 10.1021/acs.jafc.8b03405
35. Struyfs C, Cammue BPA, Thevissen K. Membrane-interacting antifungal peptides. Front Cell Dev Biol. (2021) 9:649875. doi: 10.3389/fcell.2021.649875
36. Nijnik A, Hancock R. Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg Health Threats J. (2009) 2:e1. doi: 10.3402/ehtj.v2i0.7078
37. Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. (2002) 106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x
38. Mookherjee N, Brown KL, Bowdish DME, Doria S, Falsafi R, Hokamp K, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. (2006) 176:2455–64. doi: 10.4049/jimmunol.176.4.2455
39. Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C, et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. (2006) 18:1729–36. doi: 10.1093/intimm/dxl107
40. Zhong C, Zhang F, Zhu N, Zhu Y, Yao J, Gou S, et al. Ultra-short lipopeptides against gram-positive bacteria while alleviating antimicrobial resistance. Eur J Med Chem. (2021) 212:113138. doi: 10.1016/j.ejmech.2020.113138
41. Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. (2000) 192:1069–74. doi: 10.1084/jem.192.7.1069
42. Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol. (2009) 39:3181–94. doi: 10.1002/eji.200939496
43. Roehrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol. (2010) 184:6688–94. doi: 10.4049/jimmunol.0903984
44. Gracia A, Polewicz M, Halperin SA, Hancock REW, Potter AA, Babiuk LA, et al. Antibody responses in adult and neonatal BALB/c mice to immunization with novel Bordetella pertussis vaccine formulations. Vaccine. (2011) 29:1595–604. doi: 10.1016/j.vaccine.2010.12.083
45. Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. (2001) 414:454–7. doi: 10.1038/35106587
46. Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka LU, Ehren I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. (2006) 12:636–41. doi: 10.1038/nm1407
47. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. (2007) 449:564–6. doi: 10.1038/nature06116
48. Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. (2012) 280:22–35. doi: 10.1016/j.cellimm.2012.11.009
49. Penney J, Li J. Protegrin 1 enhances innate cellular defense via the insulin-like growth factor 1 receptor pathway. Front Cell Infect Microbiol. (2018) 8:331. doi: 10.3389/fcimb.2018.00331
50. Otte J-M, Zdebik A-E, Brand S, Chromik AM, Strauss S, Schmitz F, et al. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul Peptides. (2009) 156:104–17. doi: 10.1016/j.regpep.2009.03.009
51. Tai EKK, Wong HPS, Lam EKY, Wu WKK, Yu L, Koo MWL, et al. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. (2008) 104:251–8. doi: 10.1002/jcb.21615
52. Zong X, Cao X, Wang H, Xiao X, Wang Y, Lu Z. Cathelicidin-WA facilitated intestinal fatty acid absorption through enhancing PPAR-gamma dependent barrier function. Front Immunol. (2019) 10:1674. doi: 10.3389/fimmu.2019.01674
53. Raaymakers C, Verbrugghe E, Hernot S, Hellebuyck T, Betti C, Peleman C, et al. Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat Commun. (2017) 8:1495. doi: 10.1038/s41467-017-01710-1
54. Milner SM, Bhat S, Buja M, Gulati S, Poindexter BJ, Bick RJ. Expression of human beta defensin 2 in thermal injury. Burns. (2004) 30:649–54. doi: 10.1016/j.burns.2004.06.001
55. Goto H, Hongo M, Ohshima H, Kurasawa M, Hirakawa S, Kitajima Y. Human beta defensin-1 regulates the development of tight junctions in cultured human epidermal keratinocytes. J Dermatol Sci. (2013) 71:145–8. doi: 10.1016/j.jdermsci.2013.04.017
56. Han F, Lu Z, Liu Y, Xia X, Zhang H, Wang X, et al. Cathelicidin-BF ameliorates lipopolysaccharide-induced intestinal epithelial barrier disruption in rat. Life Sci. (2016) 152:199–209. doi: 10.1016/j.lfs.2016.03.041
57. Feng J, Wang L, Xie Y, Chen Y, Yi H, He D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int Immunopharmacol. (2020) 85:106658. doi: 10.1016/j.intimp.2020.106658
58. Zhao X, Wang L, Zhu C, Xia X, Zhang S, Wang Y, et al. The antimicrobial peptide mastoparan X protects against enterohemorrhagic Escherichia coli O157:H7 infection, inhibits inflammation, and enhances the intestinal epithelial barrier. Front Microbiol. (2021) 12:644887. doi: 10.3389/fmicb.2021.644887
59. Zong X, Hu W, Song D, Li Z, Du H, Lu Z, et al. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem Pharmacol. (2016) 104:74–82. doi: 10.1016/j.bcp.2016.01.009
60. Zong X, Cao X, Wang H, Zhao J, Lu Z, Wang F, et al. Porcine lactoferrin-derived peptide LFP-20 modulates immune homoeostasis to defend lipopolysaccharide-triggered intestinal inflammation in mice. Br J Nutr. (2019) 121:1255–63. doi: 10.1017/S0007114519000485
61. Shang L, Yu H, Liu H, Chen M, Zeng X, Qiao S. Recombinant antimicrobial peptide microcin J25 alleviates DSS-induced colitis via regulating intestinal barrier function and modifying gut microbiota. Biomed Pharmacother. (2021) 139:111127. doi: 10.1016/j.biopha.2020.111127
62. Tang ZR, Deng H, Zhang XL, Zen Y, Xiao DF, Sun WZ, et al. Effects of orally administering the antimicrobial peptide buforin II on small intestinal mucosal membrane integrity, the expression of tight junction proteins and protective factors in weaned piglets challenged by enterotoxigenic Escherichia coli. Anim Feed Sci Technol. (2013) 186:177–85. doi: 10.1016/j.anifeedsci.2013.10.012
63. Zhang Y, Chen S, Zong X, Wang C, Shi CY, Wang FQ, et al. Peptides derived from fermented soybean meal suppresses intestinal inflammation and enhances epithelial barrier function in piglets. Food Agr Immunol. (2020) 31:120–35. doi: 10.1080/09540105.2019.1705766
64. Zong X, Fu J, Xu BC, Wang YZ, Jin ML. Interplay between gut microbiota and antimicrobial peptides. Anim Nutr. (2020) 6:389–96. doi: 10.1016/j.aninu.2020.09.002
65. Maher S, Feighery L, Brayden DJ, McClean S. Melittin as an epithelial permeability enhancer I: investigation of its mechanism of action in Caco-2 monolayers. Pharmaceut Res. (2007) 24:1336–45. doi: 10.1007/s11095-007-9288-2
66. Gao Y, Han F, Huang X, Rong Y, Yi H, Wang Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci. (2013) 91:5614–25. doi: 10.2527/jas.2013-6528
67. Fan F, Wu Y, Liu J. Expression and purification of two different antimicrobial peptides, PR-39 and Protegrin-1 in Escherichia coli. Protein Express Purif. (2010) 73:147–51. doi: 10.1016/j.pep.2010.05.012
68. Menard S, Foerster V, Lotz M, Guetle D, Duerr CU, Gallo RL, et al. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. (2008) 205:183–93. doi: 10.1084/jem.20071022
69. Veldhuizen EJA, Koomen I, Ultee T, van Dijk A, Haagsman HP. Salmonella serovar specific upregulation of porcine defensins 1 and 2 in a jejunal epithelial cell line. Vet Microbiol. (2009) 136:69–75. doi: 10.1016/j.vetmic.2008.09.072
70. Wu H, Zhang GL, Minton JE, Ross CR, Blecha F. Regulation of cathelicidin gene expression: induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar typhimurium infection. Infect Immun. (2000) 68:5552–8. doi: 10.1128/IAI.68.10.5552-5558.2000
71. Su J, Zhang ZW, Han YH, Li S, Xu SW. Expression and identification of porcine beta-defensin 1 in Escherichia coli and up-regulation by streptococcus infection in porcine tongue in vivo. Int J Peptide Res Therapeut. (2012) 18:145–52. doi: 10.1007/s10989-011-9287-3
72. Gombart AF, O'Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)(2)D-3 in various tissues. J Steroid Biochem Mol Biol. (2007) 103:552–7. doi: 10.1016/j.jsbmb.2006.12.095
73. Zong X, Wang H, Xiao X, Zhang Y, Hu Y, Wang F, et al. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m(6)A-GPR161 signalling axis. RNA Biol. (2021) 18:576–86. doi: 10.1080/15476286.2020.1820193
74. Gao Y, Rong Y, Wang Y, Xiong H, Huang X, Han F, et al. Expression pattern of porcine antimicrobial peptide PR-39 and its induction by enterotoxigenic Escherichia coli (ETEC) F4ac. Vet Immunol Immunopathol. (2014) 160:260–5. doi: 10.1016/j.vetimm.2014.05.012
75. Lu Z, Jin M, Huang M, Wang Y, Wang Y. Bioactivity of selenium-enriched exopolysaccharides produced by Enterobacter cloacae Z0206 in broilers. Carbohydrate Polymers. (2013) 96:131–6. doi: 10.1016/j.carbpol.2013.03.063
76. Ren M, Zhang S, Liu X, Li S, Mao X, Zeng X, et al. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous beta-defensin expression through the Sirt1/ERK/90RSK pathway. J Agric Food Chem. (2016) 64:3371–9. doi: 10.1021/acs.jafc.6b00968
77. Lan J, Dou X, Li J, Yang Y, Xue C, Wang C, et al. l-arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-kappaB and MAPK pathways and stimulating beta-defensin expression in vivo and in vitro. J Agric Food Chem. (2020) 68:2648–63. doi: 10.1021/acs.jafc.9b07611
Keywords: antimicrobial peptides, biological function, classification, nutrition regulation, expression mechanism
Citation: Gong T, Fu J, Shi L, Chen X and Zong X (2021) Antimicrobial Peptides in Gut Health: A Review. Front. Nutr. 8:751010. doi: 10.3389/fnut.2021.751010
Received: 31 July 2021; Accepted: 03 September 2021;
Published: 30 September 2021.
Edited by:
Deguang Song, Yale University, United StatesReviewed by:
Xingjun Feng, Northeast Agricultural University, ChinaCopyright © 2021 Gong, Fu, Shi, Chen and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Chen, Y2hlbnhpbjEyMTBAMTYzLmNvbQ==; Xin Zong, em9uZ3hpbkB6anUuZWR1LmNu
†Those authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.