- 1The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, The State and Shandong Province Joint Key Laboratory of Translational Cardiovascular Medicine, Department of Cardiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China
- 3Shandong Provincial Clinical Research Center for Emergency and Critical Care Medicine, Institute of Emergency and Critical Care Medicine of Shandong University, Chest Pain Center, Qilu Hospital of Shandong University, Jinan, China
- 4Department of Emergency Medicine, Qilu Hospital of Shandong University, Jinan, China
- 5Key Laboratory of Emergency and Critical Care Medicine of Shandong Province, Key Laboratory of Cardiopulmonary-Cerebral Resuscitation Research of Shandong Province, Shandong Provincial Engineering Laboratory for Emergency and Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China
- 6Department of Gastroenterology, Qilu Hospital of Shandong University, Jinan, China
- 7Department of Anesthesiology, Qilu Hospital of Shandong University, Jinan, China
- 8Department of Respiratory and Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China
- 9Department of Poisoning and Occupational Diseases, Qilu Hospital of Shandong University, Jinan, China
- 10Department of Endocrinology, Qilu Hospital of Shandong University, Jinan, China
- 11Department of Nephrology, Qilu Hospital of Shandong University, Jinan, China
- 12Department of Pharmacology, School of Basic Medical Science, Shandong University, Jinan, China
Background and Aim: Lymphocytes play an important role in fighting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. Low total lymphocyte count (TLC), which contributes to poor clinical outcomes, is common in persons with coronavirus disease 2019 (COVID-19). The current explanation for the cause of low TLC is that it is directly related to the invasiveness of SARS-CoV-2, which attacks lymphocytes. We hypothesized that malnutrition contributes to the development of low TLC in early-stage COVID-19.
Methods: We prospectively enrolled 101 patients with confirmed COVID-19. On their first day of hospitalization, we collected baseline and laboratory data, including clinical symptoms; the Sequential Organ Failure Assessment, Nutrition Risk Screening 2002 and Subjective Global Assessment were used to assess the malnutrition status of the patients. Multivariable logistic regression was used to identify independent risk factors for low TLC and severe COVID-19.
Results: Malnutrition was associated with lower TLC in COVID-19. Fifty-nine (58.4%) of the patients showed low TLC, 41 (40.6%) were at risk for malnutrition, and 18 of them were malnourished. Low TLC was an independent risk factor for severe COVID-19. Compared to patients with normal TLC, those with low TLC more often presented with anorexia, malnutrition, higher SOFA scores (P < 0.05) and comorbidities (diabetes and malignancies). Malnutrition (OR: 3.05, 95% CI: 1.5–6.19, P = 0.006) and SOFA scores (OR: 1.51, 95% CI: 1.04-2.43, P = 0.042) were identified as independent risk factors for low TLC.
Conclusions: Malnutrition was common among our patients with early-stage COVID-19, and it contributed to the occurrence of low TLC.
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues its rapid spread globally. A lymphocyte is a type of immune cell that plays an important role in fighting SARS-CoV-2 infection, and low total lymphocyte count (TLC) is common in COVID-19, ranging from 35 to 82% of patients (1, 2). Low TLC also contributes to poor clinical outcomes (2). Non-survivors showed severe lymphopenia over time (3). Increasing the TLC level may be a key therapeutic target for these infections, but until now, the cause of low TLC has been thought to be directly related to the invasive nature of SARS-CoV-2, which attacks immune cells (1, 4).
However, the cause of lymphopenia could be multifactorial, and malnutrition is a main contributor (5). Intensive care unit (ICU) stay, polymorbidity, and older age are associated with high risk for malnutrition, representing a relevant risk factor for higher morbidity in COVID-19 (6). The high incidence of gastrointestinal symptoms in COVID-19 may further increase malnutrition risk (7, 8). A recent study reported that 52.7% of elderly patients with COVID-19 are malnourished, and that 27.5% is at risk for malnutrition (9). Lymphopenia may occur when there is lack of protein and other nutrients that are necessary to produce enough lymphocytes. Significant decrease in TLC has been observed in elderly patients who are malnourished (9), indicating a possible correlation between TLC and malnutrition in persons with COVID-19. In this study, we aimed to establish a multivariable model and determine whether malnutrition is an independent risk factor for low TLC in COVID-19. Another goal of this study was to produce evidence for the pathogenesis of lymphopenia in early-stage COVID-19 and demonstrate the feasibility of elevating TLC levels through nutrition management as a treatment for affected patients.
Materials and Methods
We prospectively enrolled adult patients admitted to the COVID-19 infection ward of a designated hospital in Wuhan who have confirmed COVID-19 from February 6 to March 20, 2020. On the first day of hospitalization, baseline data, clinical symptoms from disease onset to hospitalization, and laboratory data of the patients were collected. The Sequential Organ Failure Assessment (SOFA)1, which measures the degree of dysfunction of six organ systems (respiratory, liver, cardiovascular, renal, coagulation, and central nervous system), was conducted. The Nutrition Risk Screening 2002 (NRS-2002) was performed to identify patients at risk for malnutrition. Patients with an NRS-2002 score ≥ 3 were further assessed using the Subjective Global Assessment (SGA) (6). Malnutrition status was divided into three categories: (1) no risk for malnutrition (NRS-2002 score <3), (2) risk for malnutrition (NRS-2002 score ≥ 3 with Subjective Global Assessment [SGA] A classification) and (3) malnourished (NRS-2002 score ≥ 3 and SGA B or C classification). Hospitalized patients were excluded from the study if they (1) were lost to follow-up or had insufficient information; or (2) history of cardiac arrest, death within 24 h or length of stay of less than 72 h, or with do-not-resuscitate orders. To verify the correlation between malnutrition and low TLC in non-hospitalized patients, the non-hospitalized patients with sufficient clinical information (such as NRS-2002 scores and results of routine blood tests) were enrolled at the same time. Their malnutrition status was classified as no risk for malnutrition (NRS-2002 score <3) and risk for malnutrition (NRS-2002 score ≥ 3).
Data on the use of lymphopenia-inducing drugs (corticosteroids, cytotoxic drugs, thymic hormones, and interferons) (10) within 2 weeks and coexisting comorbidities related to lymphopenia (5) were also collected as confounding factors. The study was approved by the ethics committee of Qilu Hospital. Clinical outcomes of the hospitalized patients consisted of acute respiratory distress syndrome (ARDS), severe COVID-19, ICU care, and mortality. The outcomes of ARDS and severe COVID-19 were defined according to criteria from the World Health Organization (WHO) (11, 12). Mortality was defined as death, and ICU care was defined as the day of admittance to the ICU during the in-hospital care of a patient.
SPSS 16.0 (SPSS Inc, Chicago, IL, United States) was used for data analysis. Multivariable logistic regression analysis was performed to identify independent risk factors associated with low TLC and severe COVID-19, and odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. Variables with P ≤ 0.2 on the univariate analyses were included in the multivariable model. P < 0.05 was considered statistically significant. Univariate logistic regression was used to assess the consistency of the relationships between the categories of malnutrition status and low TLC with clinical outcomes.
Results
A total of 101 cases were enrolled. As shown in Table 1, the mean age of the enrolled participants is 65.3 ± 13 years, and 59.4% are male. The mean SOFA score on the first day of hospitalization was 2.4 ± 1.3, and 59 (58.4%) of the patients showed low TLC on admission. The most common comorbidities were hypertension (n = 26, 25.7%), cardiovascular disease (n = 13, 12.9%), and diabetes (n = 13, 12.9%). A total of 41 (40.6%) patients were at risk for malnutrition (NRS-2002 score ≥ 3); 18 of them were malnourished, and 23 were at risk for malnutrition but were not malnourished. As shown in Supplementary Figure 1, low TLC and malnourishment are associated with most worst clinical outcomes. Low TLC, instead of malnourishment, was identified as an independent risk factor for severe COVID-19 (Supplementary Tables 1, 2) using the multivariate logistic model.
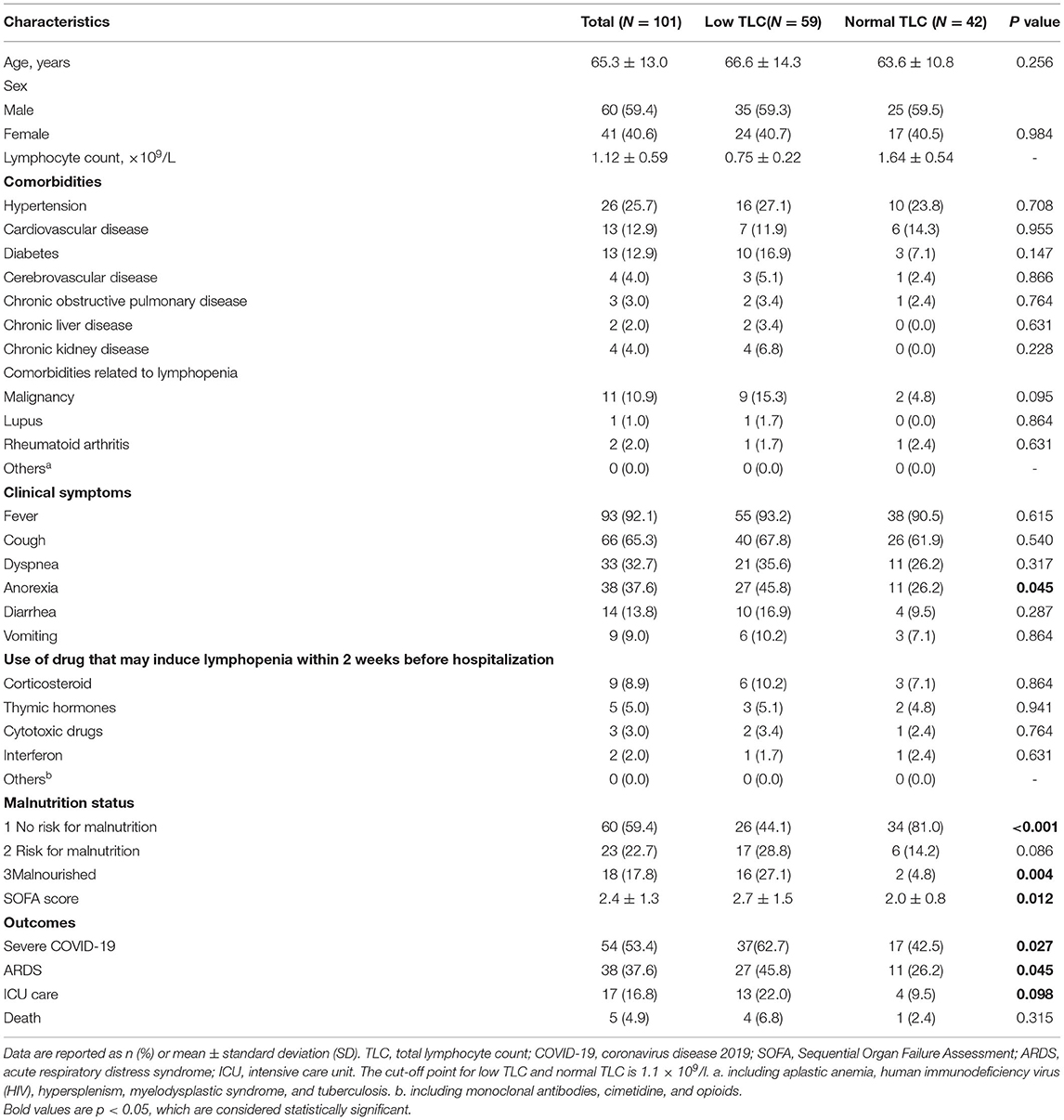
Table 1. Univariate analysis of the risk factors for low TLC in hospitalized patients with early-stage COVID-19.
Compared to patients with normal TLC, those with low TLC more often presented with anorexia from the onset of the disease. They were more often malnourished and had higher SOFA score but less often well-nourished (P < 0.05, Table 1). The univariate analysis of the risk factors for TLC identified five indicators (i.e., comorbid diabetes and malignancy, anorexia, higher SOFA score, and malnutrition), which were included in the multivariate analysis. A similar trend in malnutrition was found among the cases that were not hospitalized (Supplementary Table 3).
The multivariate logistic regression identified the SOFA score (OR: 1.51, 95% CI: 1.04–2.43, P = 0.042) and a trend toward malnutrition (OR: 3.05, 95% CI: 1.5–6.19, P = 0.006) as independent risk factors for low TLC (Table 2). Compared to patients who were not at risk for malnutrition (set as a reference), the risk for low TLC increased 3.67- and 7.63-fold in patients who were at risk for malnutrition and those who were malnourished.
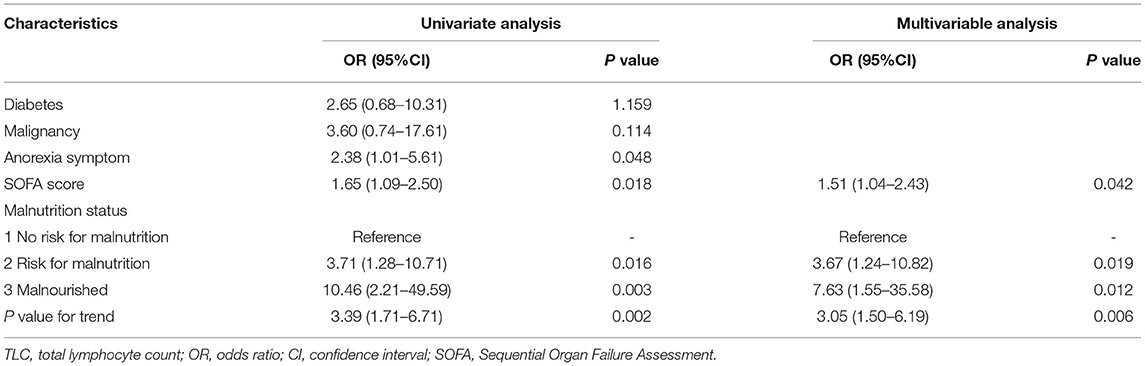
Table 2. Multivariate logistic regression analysis of the risk factors for low TLC in hospitalized patients with early-stage COVID-19.
Discussion
Our study found that malnutrition was common among our patients with early-stage COVID-19, and that it contributed to their low TLC. The data suggest that malnutrition is another important cause of lymphopenia, in addition to the invasive nature of SARS-CoV-2, which attacks immune cells.
Previous studies have identified lymphopenia as a significant prognostic marker for COVID-19 and TLC as a variable associated with disease severity and mortality (4, 13, 14). Appropriate functions of lymphocytes, such as cytotoxicity, antibody production, and immune response regulation, play critical roles in restricting SARS-CoV-2 infection. Lymphopenia is a hallmark of predisposition to severe disease, with incidence of about 85% in patients with severe COVID-19 (15). In this study, low TLC was also verified to be an independent risk factor for severe COVID-19 in hospitalized patients. An understanding of the pathogenesis of low TLC may facilitate the development and testing of therapeutic targets for COVID-19 management.
Malnutrition has been observed frequently in persons with infectious diseases such as COVID-19 (9, 16, 17). Thus, nutritional support is probably a reasonable approach to improve their prognosis (18, 19). The causes of malnutrition in COVID-19 are multifactorial. Polymorbidity, older age (6), gastrointestinal symptoms (7, 8), and catabolism stress induced by infection, which are commonly found in COVID-19, may contribute to patients' deteriorated nutritional status in COVID-19. Sufficient protein and nutrients are necessary to produce lymphocytes, as protein–energy malnutrition leads to reduced production of lymphocytes (20). TLC is also a popular serum marker, which may be useful for determining nutritional status. Levels of TLC have been found to vary with the degree of malnutrition; for example, TLV levels <1,500/mm3 show a strong correlation with malnutrition, and levels <900/mm3 reflected severe malnutrition (21, 22). Consequently, immune function is affected, thereby increasing the risk of infection due to impaired cell-mediated immunity and cytokine, complement, and phagocyte function (23). Ensuring adequate nutrition in early-stage COVID-19 might increase the level of TLC and improve immune function and prognosis, but it needs to be verified by further intervention studies. Previous studies have found that malnutrition is associated with worse clinical outcomes (9, 16, 17), and nutritional support probably improves the prognosis (18, 19) of infectious diseases (including COVID-19). In this study, we also found that malnourishment was associated with the worse clinical outcomes in the univariate model (Supplementary Figure 1). However, malnutrition was not found to be an independent risk factor for severe COVID-19 in the multivariate analysis. Considering the significant correlation between malnutrition and low TLC, the prognostic value of malnutrition, with respect to COVID-19, may be partly attributable to its contribution to lymphopenia.
In this study, SOFA score was identified as an independent risk factor for low TLC, and the reason was probably related to the invasiveness of SARS-CoV-2. Infection with SARS-CoV-2 can damage multiple organ systems, including the respiratory (24), liver (25), cardiovascular (26), renal (27), coagulation (28), and central nervous systems (29). Given that SOFA score is calculated based on the degree of dysfunction of the above organ systems, higher SOFA score may indicate higher level of invasiveness of SARS-CoV-2 and damage to organ systems. Furthermore, SOFA score has been found to be associated with poor prognosis for COVID-19 (30).
Our study had some limitations. First, it was limited by the observational nature of the data. Further intervention research is needed regarding the effectiveness of nutrition support on lymphocyte count in patients with COVID-19. Second, because of the small sample size of this study, a multivariate model was not established to determine the association of lymphopenia and malnutrition with all clinical outcomes. Further study with a large sample size is warranted.
Our study indicated that malnutrition was common among our patients with early-stage COVID-19, and that it is an important but generally unrecognized cause of lymphopenia. Ensuring adequate nutrition in early-stage COVID-19 may prevent low TLC; therefore, this hypothesis needs to be verified through further intervention studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Qilu Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HW, YC, and YL concept and design. All authors: acquisition, analysis, or interpretation of the data. HW and NZ drafting of the manuscript. HW and JF statistical analysis. HW and YC obtained funding. XH and ZH contributed to the collection of additional data and analysis in the revision. All authors approved the final version of the manuscript and are responsible for the content.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81873927, 82072231), Key Research Plan of Shandong Province (2020SFXGFY03), Taishan Scholars Program of Shandong Province (tsqn202103165), Clinical Research Center of Shandong University (2020SDUCRCC013), and China Postdoctoral Science Foundation (2018M632685).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.739216/full#supplementary-material
Footnotes
References
1. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
2. Guan WJ Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. (2020). doi: 10.1056/NEJMoa2002032
3. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
4. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. (2020). doi: 10.1016/j.ebiom.2020.102763
5. Naeim F, Rao PN, Grody WW. Atlas of Hematopathology: Morphology, Immunophenotype, Cytogenetics, and Molecular Approaches. London, UK: Elsevier Health Science. (2013).
6. Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. (2020) 142:75–84. doi: 10.26800/LV-142-3-4-13
7. Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol. (2020) 18:1636. doi: 10.1016/j.cgh.2020.03.043
8. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. (2020) 158:2294–7. doi: 10.1053/j.gastro.2020.03.020
9. Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. (2020) 74:871–5. doi: 10.1038/s41430-020-0642-3
10. Gergely P. Drug-induced lymphopenia: focus on CD4+ and CD8+ cells. Drug Saf. (1999) 21:91–100. doi: 10.2165/00002018-199921020-00003
11. World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected 2020;1–21. Available online at: https://www.who.int/publications/i/item/10665-332299 (accessed December 20, 2021).
12. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) [Internet]. Available online at: https://www.mdcalc.com/sequential-organfailure-assessment-sofa-score#pearls-pitfalls
13. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. (2020) 8:36. doi: 10.1186/s40560-020-00453-4
14. Lee J, Park SS, Kim TY, Lee DG, Kim DW. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers (Basel). (2021) 13:471. doi: 10.3390/cancers13030471
15. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
16. Li Y, Tong S, Hu X, Wang Y, Lv R, Ai S, et al. The relationship between nutritional status and the prognosis of COVID-19: A retrospective analysis of 63 patients. Medicine (Baltimore). (2021) 100:e25287. doi: 10.1097/MD.0000000000025287
17. Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. (2004) 12:417–23. doi: 10.1016/j.tim.2004.07.007
18. Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. (2020) 39:1631–8. doi: 10.1016/j.clnu.2020.03.022
19. Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition. (2020) 74:110835. doi: 10.1016/j.nut.2020.110835
20. Saito H, Nomura K, Hotta M, Takano K. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. (2007) 40:575–9. doi: 10.1002/eat.20417
21. Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition. (2000) 16:131–40. doi: 10.1016/S0899-9007(99)00251-8
22. Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition. (2001) 17:496–8. doi: 10.1016/S0899-9007(01)00558-5
23. Saunders J, Smith T. Malnutrition: causes and consequences. Clin Med (Lond). (2010) 10:624–7. doi: 10.7861/clinmedicine.10-6-624
24. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
25. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. (2020) 40:998–1004. doi: 10.1111/liv.14435
26. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. (2020) 17:259–60. doi: 10.1038/s41569-020-0360-5
27. Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. (2020) 24:155. doi: 10.1186/s13054-020-02872-z
28. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb Haemost. (2020) 120:949–56. doi: 10.1055/s-0040-1710317
29. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central Nervous System Involvement by Severe Acute Respiratory Syndrome Coronavirus−2 (SARS-CoV-2). J Med Virol. (2020) 92:699–702. doi: 10.1002/jmv.25915
Keywords: malnutrition, COVID-19, lymphocyte, SARS-CoV-2, lymphopenia
Citation: Zhang K, Qin W, Zheng Y, Pang J, Zhong N, Fei J, Li Y, Jian X, Hou X, Hu Z, Li C, Wang H and Chen Y (2022) Malnutrition Contributes to Low Lymphocyte Count in Early-Stage Coronavirus Disease-2019. Front. Nutr. 8:739216. doi: 10.3389/fnut.2021.739216
Received: 10 July 2021; Accepted: 03 December 2021;
Published: 06 January 2022.
Edited by:
Clelia Madeddu, University of Cagliari, ItalyReviewed by:
Maria Montserrat Diaz Pedrosa, State University of Maringá, BrazilDavide Firinu, Università di Cagliari, Italy
Copyright © 2022 Zhang, Qin, Zheng, Pang, Zhong, Fei, Li, Jian, Hou, Hu, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang, d2FuZ2hhbzM0QDEyNi5jb20=; Yuguo Chen, Y2hlbjkxOTA4NUBzZHUuZWR1LmNu
†These authors have contributed equally to this study
 Kai Zhang1†
Kai Zhang1† Yue Zheng
Yue Zheng Hao Wang
Hao Wang Yuguo Chen
Yuguo Chen