
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 29 September 2021
Sec. Clinical Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.736884
Background: Low Geriatric Nutritional Risk Index has been identified as an index of impaired nutritional state. The objective of the meta-analysis was to assess the association of the Geriatric Nutritional Risk Index (GNRI) with adverse outcomes in patients with coronary artery disease (CAD).
Methods: Relevant studies were identified by comprehensively searching PubMed and Embase databases in May 2021. Studies assessing the association of GNRI with all-cause mortality or major adverse cardiovascular events (MACEs) in patients with CAD were included. The predictive value of GNRI was summarized by pooling multivariable adjusted risk ratios (RR) with 95% confidence intervals (CI) per GNRI point decrease or the lowest vs. the highest GNRI group.
Results: A total of eight studies involving 9277 patients with CAD were analyzed. Meta-analysis showed that the lowest GNRI was associated with a higher risk of all-cause mortality (RR 2.10; 95% CI 1.68–2.63) and MACEs (RR 2.84; 95% CI 1.56–5.16), respectively. Furthermore, per point decrease in GNRI was associated with 8 and 10% additional risk of all-cause mortality and MACEs. Subgroup analysis indicated that the value of low GNRI in predicting all-cause mortality was not affected by subtype of patients or follow-up duration.
Conclusion: Low GNRI score at baseline was associated with a higher risk of all-cause mortality and cardiovascular events in patients with CAD. The nutritional state estimated by the GNRI score could provide important predictive information in patients with CAD.
Geriatric Nutritional Risk Index, created by Bouillanne et al., was calculated by serum albumin level and the ratio of actual to ideal body weight (1). This new nutritional tool is designed to predict the risk of morbidity and survival in hospitalized elderly patients who find it difficult to obtain the normal weight. Under this simple nutritional tool, low GNRI score reflects poor nutritional status (2). Thereafter, this nutritional tool has been widely applied to evaluate the association of nutritional status and its adverse outcomes in various populations.
Despite the prevalence of nutritional deficiency remaining unclear in patients with coronary artery disease (CAD), malnutrition was reported to be at 38.7% with Controlling Nutritional status (CONUT) scores and 64% with GNRI scores among non-ST-elevated myocardial infarction (NSTEMI) (3). Poor nutritional status in patients with CAD is associated with unfavorable outcomes. Increasing pieces of evidence have suggested that malnutrition, estimated by low GNRI, was associated with an increased risk of mortality in patients with heart failure (4), hemodialysis (5), and various malignancies (6). Several studies (7–11) have investigated the associations of GNRI with adverse outcomes in patients with CAD. However, the predictive value of GNRI was not fully established in this population due to the presence of conflicting results (3, 12).
There is no previous meta-analysis specifically focused on the predictive value of GNRI in patients with CAD. We hypothesized that low GNRI scores may be associated with adverse outcomes. To summarize the available evidence, we performed this meta-analysis to assess the value of baseline GNRI in predicting adverse outcomes in patients with CAD in terms of all-cause mortality and major adverse cardiovascular events (MACEs).
This study was reported according to the guideline of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (13). Relevant studies were identified by comprehensively searching PubMed and Embase databases through May 2021. The following keywords in combination were applied for the literature search: “geriatric nutritional risk index” OR “GNRI” AND “coronary artery disease” OR “coronary heart disease” OR “acute coronary syndrome” OR “myocardial infarction” OR “unstable angina pectoris” (Supplementary Text 1). In addition, we also reviewed the reference lists of pertinent articles to identify any possible missing studies.
Studies satisfying all the following criteria were included: (1) cohort studies recruiting patients with stable or acute CAD, (2) assessing the association of GNRI with all-cause mortality or major adverse cardiovascular events [(MACEs) including death, revascularization, no-fatal myocardial infarction, stroke, heart failure, or cerebrovascular attack], and (3) providing multivariable adjusted risk ratio (RR), hazard ratio (HR), or odds ratio (OR) with 95% confidence intervals (CI) per GNRI point decrease or the lowest vs. the highest GNRI group.
Studies reporting in-hospital outcomes were excluded.
The following information was collected from the included studies: the surname of the first author, published year, region, study design, subtypes of patients, sample sizes, percentage of men, age at baseline, cutoff value of the highest GNRI, definition of MACEs, time of follow-up, endpoints, most comprehensively adjusted risk summary, and adjusted confounders. The methodological quality of included studies was judged using the Newcastle–Ottawa Scale (NOS) (14). Studies with overall NOS ≥7 points were deemed to have high-quality. The above procedures were performed by two independent authors and disagreements were settled through discussion.
The STATA 12.0 (Stata Corporation, College Station, TX, USA) was applied to perform the meta-analysis. The predictive value of GNRI was calculated by pooling multivariable adjusted RR with 95% CI per point decrease in GNRI or the lowest vs. the highest category of GNRI. The I2 statistic and the Cochrane Q test were used to judge the heterogeneity, with statistical significance set at I2 ≥ 50% or p <0.10. We chose a random effects model in case of significant heterogeneity. Otherwise, a fixed-effect model was selected. To investigate the robustness of the pooling risk estimate, we conducted the sensitivity analysis by sequentially removing each study to recalculate the risk summary. Meanwhile, we performed the subgroup analyses according to the subtypes of patients with CAD, sample sizes, median or mean age, and length of follow-up. Both Begg's test (15) and Egger's test (16) were planned to examine the likelihood of publication bias when the outcomes included more than 10 studies.
Figure 1 summarizes the process of study selection. A total of eight studies (7–12, 17, 18) involving 9,277 patients with CAD were included in this meta-analysis. The main characteristics of these eligible studies are summarized in Table 1. The included studies were published from 2016 to 2021, with sample sizes ranging between 206 and 2853. All the included studies were performed in Asian countries (Republic of Korea, Japan, and China). Five studies (7–9, 11, 18) were based on patients with total CAD and three studies (10, 12, 17) were based on the acute stage of CAD. The median or mean age of the patients ranged from 60 to 74 years. The time of follow-up ranged between 1 and 7.4 years. Regarding the methodological quality, the included studies were grouped as high quality (Supplementary Table 1).
The predictive value of GNRI by categorical analysis for all-cause mortality was available in six studies (7, 9, 10, 12, 17, 18). As shown in Figure 2, there was no evidence of significant heterogeneity (I2 = 0%; p =0.771). The adjusted pooled RR of all-cause mortality was 2.10 (95% CI 1.68–2.63) when compared the lowest to the highest GNRI category. The value of the lowest GNRI in predicting all-cause mortality was consistently observed across multiple subgroups (Supplementary Table 1). In addition, three studies (9, 12, 17) assessed the predictive value of GNRI by continuous data for all-cause mortality. As shown in Figure 3, the pooled RR of all-cause mortality was 1.08 (95% CI 1.02–1.14) per point decrease in GNRI, with significant heterogeneity (I2 = 80.3%; p = 0.006). Sensitivity analysis indicated that removal of individual study each time did not significantly alter the originally statistical significance of the pooling risk estimate (data not shown).
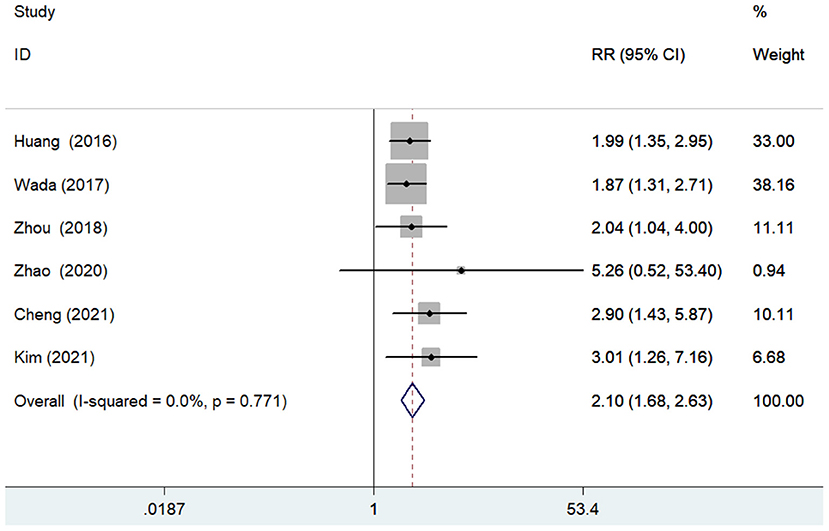
Figure 2. Forest plots showing the pooled RR and 95% CI of all-cause mortality for the lowest vs. the highest GNRI category. RR, risk ratio; CI, confidence interval; GNRI, Geriatric Nutritional Risk Index.
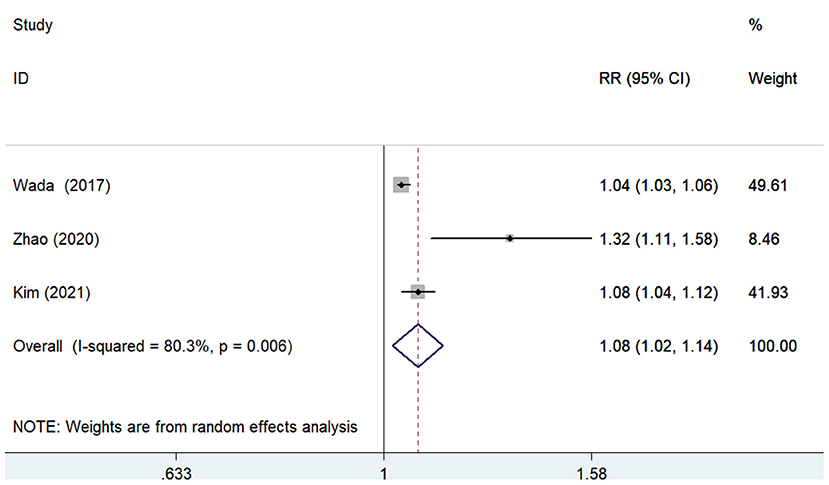
Figure 3. Forest plots showing the pooled RR and 95% CI of all-cause mortality for per point decrease in GNRI. RR, risk ratio; CI, confidence interval; GNRI, Geriatric Nutritional Risk Index.
The predictive value of GNRI for MACEs by categorical analysis was available in three studies (8, 12, 18). As shown in Figure 4, there was evidence of significant heterogeneity (I2 = 75.3%; p = 0.018). The adjusted pooled RR of MACEs was 2.84 (95% CI 1.56–5.16) when compared the lowest to the highest GNRI category. In addition, three studies (11, 12, 17) evaluated the value of GNRI by continuous data in predicting MACEs. As shown in Figure 5, the pooled RR of MACEs was 1.10 (95% CI 1.04–1.16) per point decrease in GNRI, with significant heterogeneity (I2 = 91.8%; p < 0.001). Sensitivity analysis suggested that the originally statistical significance of the pooling risk estimate did not significantly change when excluded from the individual study each time (data not shown).
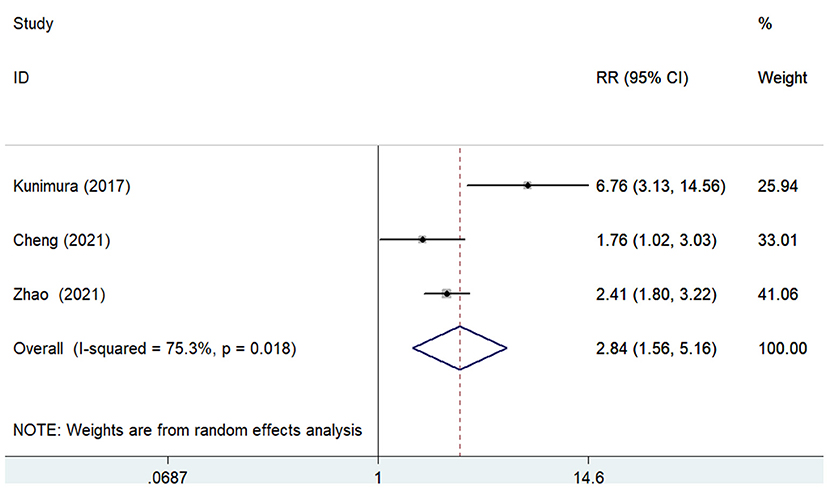
Figure 4. Forest plots showing the pooled RR and 95% CI of major adverse cardiovascular events for the lowest vs. the highest GNRI category. RR, risk ratio; CI, confidence interval; GNRI, Geriatric Nutritional Risk Index.
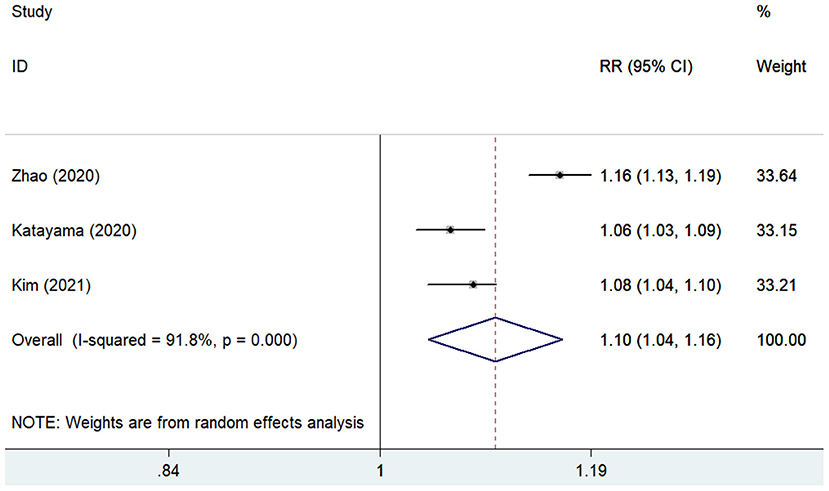
Figure 5. Forest plots showing the pooled RR and 95% CI of major adverse cardiovascular events for per point decrease in GNRI. RR, risk ratio; CI, confidence interval; GNRI, Geriatric Nutritional Risk Index.
We did not conduct the Begg's test and the Egger's test to check the publication bias because of the less than recommended arbitrary number of 10 studies (19).
This is the first meta-analysis to evaluate the predictive value of GNRI in patients with CAD. The main findings of our meta-analysis indicated that a low GNRI score was an independent predictor risk all-cause mortality and MACEs in patients with CAD. The patients with CAD who had the lowest GNRI score had a 2.84-fold and 2.10-fold increased risk of MACEs and all-cause mortality, respectively. Moreover, per point decrease in GNRI score was associated with 10 and 8% higher risk of MACEs and all-cause mortality, respectively. Together with these findings, the nutritional status, estimated by the GNRI, may provide important predictive value in patients with CAD.
Geriatric Nutritional Risk Index score was also associated with a 3.16-fold higher risk of bleeding event in patients undergoing percutaneous coronary intervention (PCI) with an oral anticoagulant during a 3-year follow-up (20). In addition, each 1-point decrease in GNRI score significantly increased 6.5% higher risk of cardiovascular mortality in patients with ST-segment elevation myocardial infarction (STEMI) during the 12.4 month follow-up period (21). Apart from the long-term outcomes, GNRI (<92) at admission was an independent factor influencing post-myocardial infarction complications (OR 2.13; 95%CI 1.61–2.84) and in-hospital death (OR 2.48; 95%CI 1.55–3.95) in patients with acute myocardial infarction (22). The findings above further supported the predictive role of GNRI in patients with CAD.
Several nutritional scoring systems, including the prognostic nutritional index (PNI), CONUT, triglycerides-total cholesterol-body weight index (TCBI), Mini Nutritional Assessment (MSA), Graz Malnutrition Screening (GMS), and GNRI have been introduced for evaluating nutritional state in clinical practice. Malnutrition determined by the PNI (23), TCBI (24, 25), and CONUT (3) was also significantly associated with adverse outcomes in patients with CAD (23). However, there is no consensus on which tools have the best predictive role in patients with CAD. GNRI is a simple tool for assessing the nutritional status of the aging population (1). Considering that the majority of included patients with CAD were from the elderly population, GNRI may be the best tool for evaluating nutritional status in these patients. Therefore, our meta-analysis only focused on the predictive role of GNRI in patients with CAD. Among patients with acute myocardial infarction, GNRI was reported to have the best value in predicting all-cause mortality than the TCBI and PNI scoring systems (17). Using the area under the curve analysis, the GNRI score had a stronger predictive value for cardiovascular death than those of the PNI and CONUT score (21). Our future study will further evaluate the predictive value of malnutrition defined by other nutritional tools in patients with CAD or compare which tool has the best predictive value. It should be noted that combined, the different scoring systems could achieve the greatest incremental value in predicting adverse cardiovascular outcomes (26).
Coronary artery disease includes a heterogeneous population. Results of subgroup analysis suggested that the association between low GNRI and all-cause mortality was stronger in patients with acute coronary syndrome (ACS) compared with those with stable CAD. The value of low GNRI in predicting all-cause mortality weakened with the lengthening of the follow-up time in the subgroup analysis. It is noteworthy that these results were established on the small number of studies analyzed. Future studies are necessary to confirm the present findings.
Despite diet nutrition being recommended in patients with CAD as secondary prevention, nutritional support is often neglected by physicians (27). This meta-analysis underlines the importance to evaluate the nutritional state in patients with CAD. Nutritional deficiency estimated by the GNRI may provide important predictive information in patients with CAD regardless of the stage (acute or stable). Malnutrition is a modifiable risk factor. CAD patients with malnutrition should be given closer monitoring, dietary intervention, and intensive treatment. However, whether nutritional intervention can improve the prognosis of CAD patients with malnutrition has not been demonstrated in clinical trials.
There are several potential limitations in the current meta-analysis. First, the majority of the analyzed studies adopted the retrospective design, and selection bias of this type of study may have been committed. Second, the included studies selected different thresholds of low GNRI scores, which makes it difficult for clinicians to identify those in need of nutritional supplementation. Third, there was significant heterogeneity in pooling MACE subtypes. Different subtypes of patients with CAD, thresholds of low GNRI score, definitions of MACEs, or intervals of follow-up duration may be correlated to the observed heterogeneity. Fourth, GNRI score of 92–98, 82–91, and <82 reflects the mild, moderate, and severe malnutrition, respectively. However, most of the included studies reported the predictive value of GNRI based on the single cutoff and not by nutritional status, which prevents the evaluation of the prediction of malnutrition defined by GNRI <92. Future studies should further assess the association of the different degrees of malnutrition with adverse outcomes in patients with CAD. Fifth, considering all patients were from East Asia, generalization of the current findings to western countries should be done with caution. Finally, we did not examine the publication bias tests due to the less than recommended arbitrary minimum number of studies.
This meta-analysis consolidates the growing evidence that a lower GNRI score at baseline is an independent predictor of all-cause mortality and MACEs in patients with CAD regardless of the acute stage of stable phase. Nutritional status estimated by the GNRI score may play an important role in the risk classification of patients with CAD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CFM: study conception, design, and revising the article critically for important intellectual content. YF and LH: acquisition of data, analysis, and interpretation of data. YF and YJZ: statistical analysis. YF: drafting the article. All authors read and approved the final version of the study to be published.
This study was supported by (1) Jiangsu Innovative Team Leading Talent Fund (CXTDC2016006, QNRC2016446), (2) Jiangsu 333 Talent Fund (BRA2020016), and (3) Suqian Science and Technology Support Project Fund (K201907).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.736884/full#supplementary-material
1. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
2. Cereda E, Pedrolli C. The Geriatric Nutritional Risk Index. Curr Opin Clin Nutr Metab Care. (2009) 12:1–7. doi: 10.1097/MCO.0b013e3283186f59
3. Kalyoncuoglu M, Katkat F, Biter HI, Cakal S, Tosu AR, Can MM. Predicting one-year deaths and major adverse vascular events with the controlling nutritional status score in elderly patients with non-ST-elevated myocardial infarction undergoing percutaneous coronary intervention. J Clin Med. (2021) 10:2247. doi: 10.3390/jcm10112247
4. Li H, Cen K, Sun W, Feng B. Prognostic value of Geriatric Nutritional Risk Index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. (2021) 33:1477–86. doi: 10.1007/s40520-020-01656-3
5. Xiong J, Wang M, Zhang Y, Nie L, He T, Wang Y, et al. Association of Geriatric Nutritional Risk Index with mortality in hemodialysis patients: a meta-analysis of cohort studies. Kidney Blood Press Res. (2018) 43:1878–89. doi: 10.1159/000495999
6. Lv GY, An L, Sun DW. Geriatric Nutritional Risk Index predicts adverse outcomes in human malignancy: a meta-analysis. Dis Markers. (2019) 2019:4796598. doi: 10.1155/2019/4796598
7. Huang BT, Peng Y, Liu W, Zhang C, Chai H, Huang FY, et al. Nutritional state predicts all-cause death independent of comorbidities in geriatric patients with coronary artery disease. J Nutr Health Aging. (2016) 20:199–204. doi: 10.1007/s12603-015-0572-2
8. Kunimura A, Ishii H, Uetani T, Aoki T, Harada K, Hirayama K, et al. Impact of Geriatric Nutritional Risk Index on cardiovascular outcomes in patients with stable coronary artery disease. J Cardiol. (2017) 69:383–8. doi: 10.1016/j.jjcc.2016.09.004
9. Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, et al. Prognostic impact of the Geriatric Nutritional Risk Index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. (2017) 119:1740–5. doi: 10.1016/j.amjcard.2017.02.051
10. Zhou XR, Chen QJ, Zhao L, Li XM, Liu F, Xiang Y, et al. Geriatric Nutritional IUsk Index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Chin J Arterioscler. (2018) 26:906–11.
11. Katayama T, Hioki H, Kyono H, Watanabe Y, Yamamoto H, Kozuma K. Predictive value of the Geriatric Nutritional Risk Index in percutaneous coronary intervention with rotational atherectomy. Heart Vessels. (2020) 35:887–93. doi: 10.1007/s00380-020-01558-4
12. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of Geriatric Nutritional Risk Index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Nutr Metab Cardiovasc Dis. (2020) 30:1685–96. doi: 10.1016/j.numecd.2020.05.016
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
14. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (assessed August 25, 2021).
15. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
17. Kim HR, Kang MG, Kim K, Koh JS, Park JR, Hwang SJ, et al. Comparative analysis of three nutrition scores in predicting mortality after acute myocardial infarction. Nutrition. (2021) 90:111243. doi: 10.1016/j.nut.2021.111243
18. Cheng L, Rong J, Zhuo X, Gao K, Meng Z, Wen X, et al. Prognostic value of malnutrition using Geriatric Nutritional Risk Index in patients with coronary chronic total occlusion after percutaneous coronary intervention. Clin Nutr. (2021). 40:4171–9 doi: 10.1016/j.clnu.2021.01.042
19. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
20. Yoshida R, Ishii H, Morishima I, Tanaka A, Morita Y, Takagi K, et al. Impact of nutritional and inflammation status on long-term bleeding in patients undergoing percutaneous coronary intervention with an oral anticoagulant. J Atheroscler Thromb. (2019) 26:728–37. doi: 10.5551/jat.47654
21. Jia Y, Gao Y, Li D, Cao Y, Cheng Y, Li F, et al. Geriatric Nutritional Risk Index score predicts clinical outcome in patients with acute st-segment elevation myocardial infarction. J Cardiovasc Nurs. (2020) 35:E44–52. doi: 10.1097/JCN.0000000000000674
22. Yoo SH, Kook HY, Hong YJ, Kim JH, Ahn Y, Jeong MH. Influence of undernutrition at admission on clinical outcomes in patients with acute myocardial infarction. J Cardiol. (2017) 69:555–60. doi: 10.1016/j.jjcc.2016.05.009
23. Wada H, Dohi T, Miyauchi K, Jun S, Endo H, Doi S, et al. Relationship between the prognostic nutritional index and long-term clinical outcomes in patients with stable coronary artery disease. J Cardiol. (2018) 72:155–61. doi: 10.1016/j.jjcc.2018.01.012
24. Doi S, Iwata H, Wada H, Funamizu T, Shitara J, Endo H, et al. A novel and simply calculated nutritional index serves as a useful prognostic indicator in patients with coronary artery disease. Int J Cardiol. (2018) 262:92–8. doi: 10.1016/j.ijcard.2018.02.039
25. Maruyama S, Ebisawa S, Miura T, Yui H, Kashiwagi D, Nagae A, et al. Impact of nutritional index on long-term outcomes of elderly patients with coronary artery disease: sub-analysis of the SHINANO 5 year registry. Heart Vessels. (2021) 36:7–13. doi: 10.1007/s00380-020-01659-0
26. Wada H, Dohi T, Miyauchi K, Endo H, Tsuboi S, Ogita M, et al. Combined effect of nutritional status on long-term outcomes in patients with coronary artery disease undergoing percutaneous coronary intervention. Heart Vessels. (2018) 33:1445–52. doi: 10.1007/s00380-018-1201-x
Keywords: geriatric nutritional risk index, coronary artery disease, mortality, cardiovascular events, meta-analysis
Citation: Fan Y, He L, Zhou Y and Man C (2021) Predictive Value of Geriatric Nutritional Risk Index in Patients With Coronary Artery Disease: A Meta-Analysis. Front. Nutr. 8:736884. doi: 10.3389/fnut.2021.736884
Received: 06 July 2021; Accepted: 30 August 2021;
Published: 29 September 2021.
Edited by:
Jasminka Z. Ilich, Florida State University, United StatesReviewed by:
Yoshiyuki Ikeda, Kagoshima University, JapanCopyright © 2021 Fan, He, Zhou and Man. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changfeng Man, Y2hhbmdmZW5nbWFuQG5qbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.