- 1Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
- 2Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Department of Medicine, University of Alberta, Edmonton, AB, Canada
- 4Isfahan Gastroenterology and Hepatology Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 5Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 6Department of Psychiatry, Psychosomatic Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 7Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Background: The association between meat consumption and mental disorders is less investigated in Iranian population. We examined the association between meat consumption and prevalence of symptoms of depression, anxiety, and psychological distress in Iranian adults.
Methods: This cross-sectional study included 3,362 participants aged 18–55 years old. A dish-based 106-item semiquantitative food frequency questionnaire (FFQ) was used to assess usual dietary intake of study population. Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire (GHQ), all validated in Iranian population, were applied to collect data on symptoms of anxiety, depression, and psychological distress, respectively.
Results: The prevalence of symptoms of depression, anxiety, and psychological distress in the study population was 28.6, 13.6, and 22.6%, respectively. After considering potential confounders, individuals in the top quartile of red meat intake had 43% increased risk of depression symptoms [odds ratio (OR) = 1.43; 95% CI: 1.09–1.89] compared to those in the first quartile. No significant relation was observed between red meat intake and anxiety or psychological distress symptoms. White meat consumption was not associated with mental disorders. Stratified analysis by sex showed that male participants in the highest quartile of red meat intake had 92% greater risk of depression symptoms (95% CI: 1.17–3.15) than those individuals in the lowest category. Red and white meat intake was not associated with mental disorders in women. In overweight or obese individuals, despite lack of any association between red meat intake and mental disorders, high intake of white meat was associated with a lower odds of psychological distress symptoms (OR = 0.64; 95% CI: 0.42–0.99) and a lower risk of depression symptoms (OR = 0.68; 95% CI: 0.45–1.00). In normal-weight participants, those in the highest quartile of red meat intake had greater odds for depression symptoms than those in the lowest quartile (OR = 1.66; 95% CI: 1.14–2.42).
Conclusions: We found that red meat consumption was associated with increased risk of depression symptoms, especially in men, and normal-weight participants. In overweight or obese participants, white meat intake was inversely associated with psychological distress symptoms.
Introduction
Mental disorders are a growing public health concern (1). Anxiety and depression are two most common mental disorders (2). In 2015, World Health Organization (WHO) reported that 44.3 million people suffer from depression, and 37.3 million people suffer from anxiety worldwide (3). The prevalence of anxiety and depression symptoms among Iranian general population is estimated to be 42 and 37.2%, respectively (4–6). Previous studies on different populations demonstrated that anxiety and depression were associated with an increased risk of several chronic diseases, including hemorrhagic stroke, myocardial infarction, diabetes, cancer, and irritable bowel syndrome (7–10). The economic burden of depression in the US was estimated to be 83.1 billion dollars in 2000 and 118 billion dollars in 2004 in Europe (11, 12).
Several modifiable and non-modifiable risk factors were associated with anxiety and depression prevalence rates (4, 13). For example, unhealthy dietary intake could significantly increase the risk of mental disorders (14–16). Red or processed meat intake has rarely been investigated in relation to psychological disorders (17–20). Given that red or processed meat consumption is associated with elevated levels of pro-inflammatory cytokines, they might also involve in the development of mental disorders (8).
A recently published meta-analysis, which included three cross-sectional, three cohort, and two case–control studies, examined the relation between meat consumption and depression (17). Most included cross-sectional and case–control studies in that meta-analysis revealed no significant association between meat consumption and depression (21–26), whereas, included cohort studies (26–28) reported that meat consumption was associated with a 13% greater risk of depression (17). It is noteworthy that some cross-sectional studies reported increased risk of depression among individuals with a rare meat consumption as less than once a week compared to moderate levels of intake (21, 23). Higher intake of meat, compared to moderate consumption, was also associated with a high prevalence of depression (23). There is no investigation in this regard in Iranian population. Given the high prevalence of depressive symptoms among Iranians and the different dietary pattern of this population compared to others, the aim of this investigation was to evaluate the association between red and white meat intake and symptoms of mental disorders in a large group of Iranian adults.
Materials and Methods
Study Design
The data of this cross-sectional study was derived from the Study on the Epidemiology of Psychological-Alimentary Health and Nutrition (SEPAHAN), a cross-sectional study started in 2010, with two main goals of assessing the link of functional gastrointestinal disorders (FGIDs) and their symptoms with lifestyle and nutritional aspects, and also psychological features. The study was carried out among the Iranian adults with the age of 18–55 years, who worked in 50 health centers affiliated to Isfahan University of Medical Sciences (IUMS). In the first step, questionnaires on sociodemographic factors, dietary habits, and dietary intake were sent to 10,087 individuals, a group of 8,691 participants returned the questionnaires (response rate: 86.16%). In the second step, which was done 1 month later, a validated questionnaire on psychological distress and mental disorders was distributed among the study population (response rate: 64.64%). When the demographic data between individuals who returned the completed questionnaires in the first step was compared to those who returned the completed questionnaires in both phases, no significant difference was found. After integrating data from the two steps, complete information was available for 3,846 participants. Some data were not usable either due to incomplete questionnaires in step 1 or 2 (n = 998, 9.9%), or lack of identification code in one of these steps (n = 884, 9.0%), or incomplete questionnaires on the exposure, outcome, or covariates (n = 789, 7.6%). Moreover, participants who reported their calorie intake outside the range of 800–4,200 kcal/day were excluded (n = 484, 4.8%) (29) because energy intake outside this range is unlikely to be true for relatively inactive women and active men (29). Finally, data of 3,362 adults were used for the present analysis. Written informed consent was obtained from each participant. The study protocol was approved by the bioethics committee of Food Security Research Center, Isfahan University of Medical Sciences, Iran (no. 299021 and ethical code IR.MUI.RESEARCH.REC.1399.221).
Assessment of Meat Intake
We used a validated dish-based 106-item semiquantitative food frequency questionnaire (FFQ), designed for Iranian adults, to compute red and white meat intake (30). This FFQ included five groups of dishes and foods; two of these categories—mixed dishes (cooked or canned, 29 items) and other food items (fast foods and other miscellaneous foods, 36 items)—were used to calculate meat intake of the participants. In brief, participants were asked to report their frequency of dietary intake of foods and mixed dishes over the last year, with nine various responses from “never or less than once a month” to “12 or more times per day.” Then, the daily grams of each food item were calculated using the household measures. An Iranian-version of Nutritionist IV software (First Databank, San Bruno, CA, USA) was used to achieve the daily average nutrient intake for each participant. The applied FFQ could reasonably classify usual intake of foods and food groups in Iranian adults, based on our previous investigations (31, 32). In this study, red meat consumption was calculated by summing up the intake of red meat (beef, veal, mutton, and lamb), processed meat (sausages, hamburgers, and hot dogs), and visceral meats (lamb's liver, kidney, and heart). White meat consumption included all kinds of fish, chicken, and poultry.
Assessment of Outcomes
To assess the anxiety and depression symptoms, we used Hospital Anxiety and Depression Scale (HADS), which was validated in Iranian population (33). HADS is a short and efficient questionnaire to assess psychological disorders symptoms and to determine the scale of the symptoms of anxiety disorders and depression. It includes 14 items and composes of two sections: anxiety and depression. Each item includes a Likert-type scale (0–1–2–3), in the present study in both sections, anxiety and depression symptoms, the points of 0–7 were considered as “normal” and the points of 8 or more were interpreted as having symptoms of psychological disorders. The Cronbach's alpha coefficient was 0.78 for anxiety subscale and 0.86 for depression subscale, based on the validation study of HADS among Iranian adults (33). To define psychological distress symptoms, we used an Iranian-validated version of General Health Questionnaire (GHQ), a short and simple to use questionnaire that has 12 items with a Likert-type scale (less than usual, no more than usual, rather more than usual, or much more than usual). Responders were asked to report whether they recently have a specific symptom of psychological distress. In the current study, the bimodal scoring method with the score of 0–0–1–1 was applied and provided scores ranging from 0 to 12. Scoring of 4 or more was defined as having symptoms of high levels of psychological distress. Lower scores represented low levels of psychological distress in individuals. The Cronbach's alpha coefficient for psychological distress among Iranians was 0.87 (34).
Assessment of Confounders
To obtain data on age, sex, education, marital status, smoking status, family size, socioeconomic status (SES), diabetes, antidepressant medication, and dietary supplement use, a self-administered questionnaire was applied. Anthropometric information (including height and weight) was collected by using a validated self-reported questionnaire (35). Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. Participants were divided into two groups according to their BMI: normal weight (<25 kg/m2) and overweight or obese (≥25 kg/m2). General Practice Physical Activity Questionnaire (GPPAQ), a valid simple four-level physical activity index, was used to estimate the activity levels of participants (36).
Statistical Methods
First, we obtained energy-adjusted amounts of red and white meat intake through residual method. Then, participants were classified based on energy-adjusted quartiles of red and white meat intake. As it is common in nutritional epidemiology (29), we used quartiles to compare individuals with the highest intake vs. individuals with the lowest intake in relation to the outcomes of the interests. One-way ANOVA was used to compare of continuous variables across different categories of red and white meat intake. Chi-square test was applied to examine the distribution of categorical variables across different categories of red and white meat intake. Analysis of covariance (ANCOVA) with the Bonferroni correction was used to report the mean intake of nutrients and food groups after adjustment of age, sex, and energy intake. The logistic regression was used to assess the relation between red and white meat intake and psychological disorders symptoms. The relations were first examined in crude model. Then, adjustment was done for the confounder variables, including age (years), sex (male/female), energy intake (kcal/day), physical activity (≥1/ <1 h/week), smoking (current smokers/ex-smokers/non-smokers), marital status (single/married), SES [consist of educational level (>diploma/ ≤ diploma), family size (>4/ ≤ 4 members), and house ownership (yes/no)], self-reported diabetes (yes/no), use of antidepressants medications (yes/no), and dietary supplements (yes/no) in Model 1. Dietary intake (including intake of fat, dairy, nuts, soy and legumes, grains, fruit, vegetables, and n-3 fatty acids) and BMI were also considered in Model 2. To determine the trend of odds ratios (ORs) across different levels of red and white meat intake, we considered the quartiles of meat as an ordinal variable. The participants in the first category of meat intake were considered as the reference category in all models. The analyses were also conducted separately by sex and BMI status. Statistical Package for Social Sciences (SPSS Inc., version 18.0, Chicago, IL, USA) was used for all analyses and p-values <0.05 were considered statistically significant.
Results
Mean age and weight of study participants were 36.29 ± 7.87 (SD) years and 68.65 ± 13.18 kg, respectively. The prevalence of symptoms of depression, anxiety, and psychological distress in the study population was 28.6, 13.6, and 22.6%, respectively. General characteristics of study participants and the prevalence of symptoms of depression, anxiety, and high psychological distress across quartiles of energy-adjusted red and white meat intake are provided in Table 1. Participants in highest quartile of red meat intake had higher weight (69.75 ± 14.25 vs. 68.33 ± 12.61, p-value = 0.02), physical activity (16.00 vs. 11.20%, p-value = 0.03), and were less likely to be women (52 vs. 58.5%, p-value <0.001), educated (54.30 vs. 66.70%, p-value <0.001), and more likely to be homeowners (59 vs. 56.70%, p-value = 0.02), compared with those in the lowest quartile. Individuals in the top category of white meat intake had higher weight (69.93 ± 13.13 vs. 68.84 ± 13.04, p-value <0.001) and physical activity (33 vs. 24.40%, p-value <0.001) and were less likely to be women (50.80 vs. 54.60%, p-value <0.001), married (78.80 vs. 80.20%, p-value = 0.01) compared with those in the bottom category. There were no significant differences in other demographic characteristics of participants across quartiles of red and white meat intake. Higher prevalence of depression symptoms (32.6 vs. 25.2%, p-value = 0.01) was observed among individuals in the top quartile of red meat intake compared with those in the bottom quartile. There was no significant difference across quartiles of meat intake with regard to anxiety and psychological distress symptoms. No significant differences were found in the prevalence of symptoms of mental disorders between different levels of white meat intake.
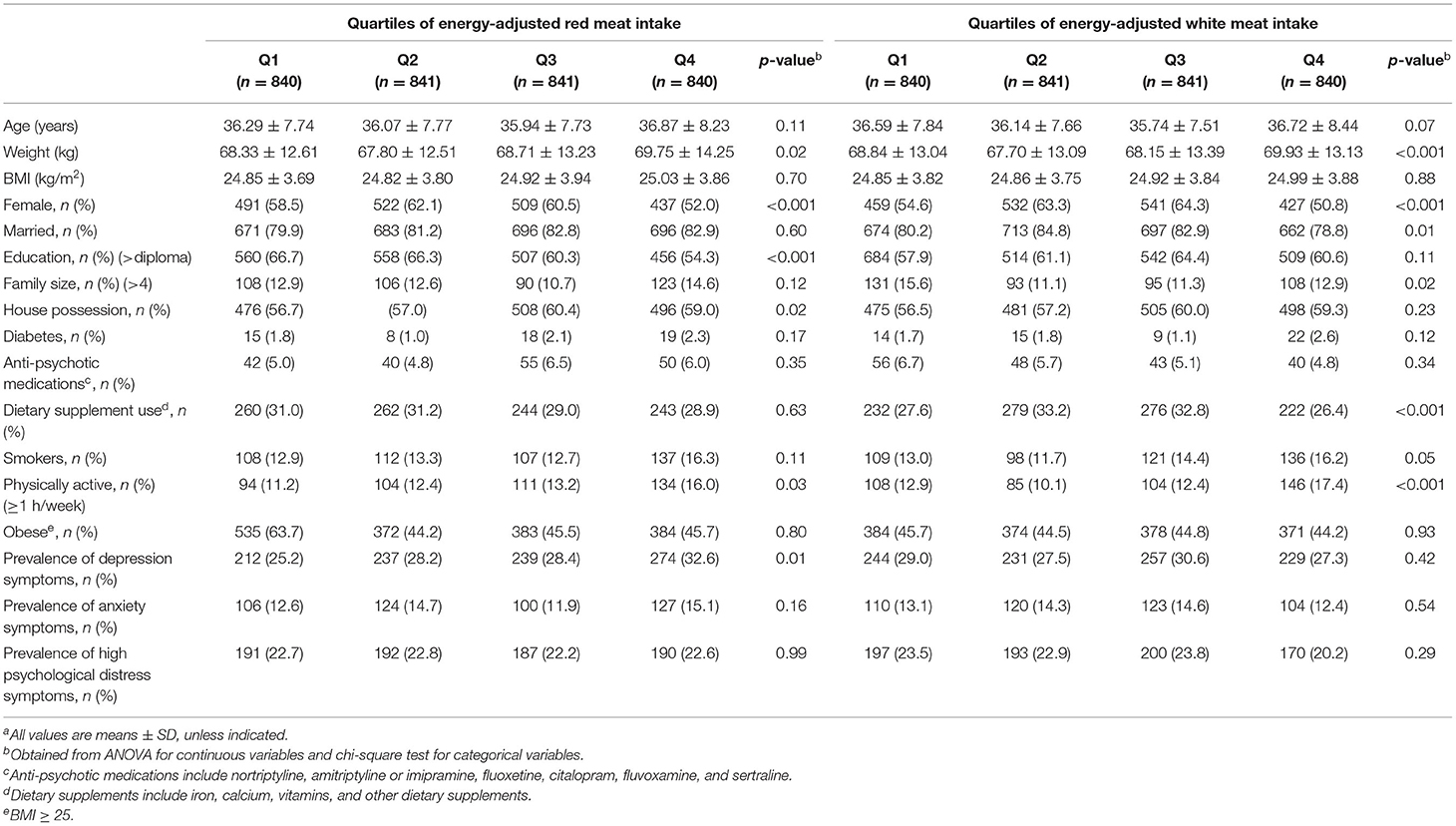
Table 1. General characteristics of study participants and prevalence of symptoms of depression, anxiety, and high psychological distress across quartiles of energy-adjusted red and white meat intakea.
Dietary intake of selected nutrients and food groups of study participants across quartiles of energy-adjusted red and white meat intake is shown in Table 2. In the highest quartile of red meat intake, compared with the lowest quartile, we observed lower intake of energy, carbohydrates, vitamin B1, iron, whole grains, refined grains, and fruits, but higher intake of proteins, fats, omega-3 fatty acids, vitamin B6, vitamin E, vegetables, nuts, soy, and legumes. Individuals with the highest intake of white meat had higher consumption of proteins, fats, omega-3 fatty acids, vitamin B6, vitamin C, vitamin E, vegetables, nuts, soy and legumes and significantly lower intake of energy, carbohydrates, dietary fiber, vitamin B1, iron, whole grains, and refined grains compared with those in the lowest quartile.
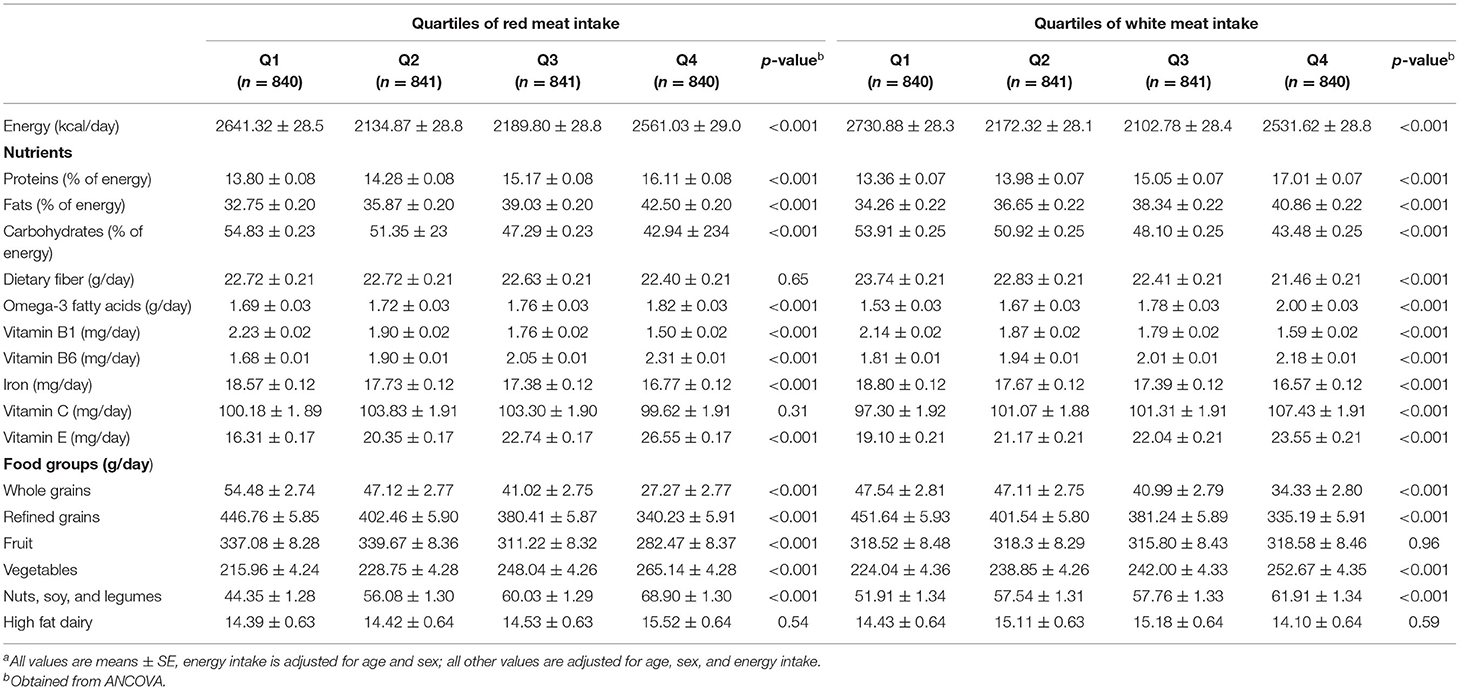
Table 2. Dietary intake of selected nutrients and food groups of study participants across quartiles of energy-adjusted red and white meat intakea.
Multivariable-adjusted ORs and (95% CI) for depression, anxiety, and psychological distress symptoms across quartiles of energy-adjusted red and white meat intake in the whole study population are presented in Table 3. The risk of depression symptoms among individuals in the top quartile of red meat intake was 43% greater than those in the first quartile (OR = 1.43; 95% CI: 1.16–1.78). The association remained significant even after controlling for all potential confounders including BMI (OR = 1.43; 95% CI: 1.09–1.89). No significant relation was observed between red meat intake and anxiety or psychological distress symptoms. Similarly, white meat intake was not associated with depression, anxiety, and psychological distress symptoms either in crude or adjusted models.
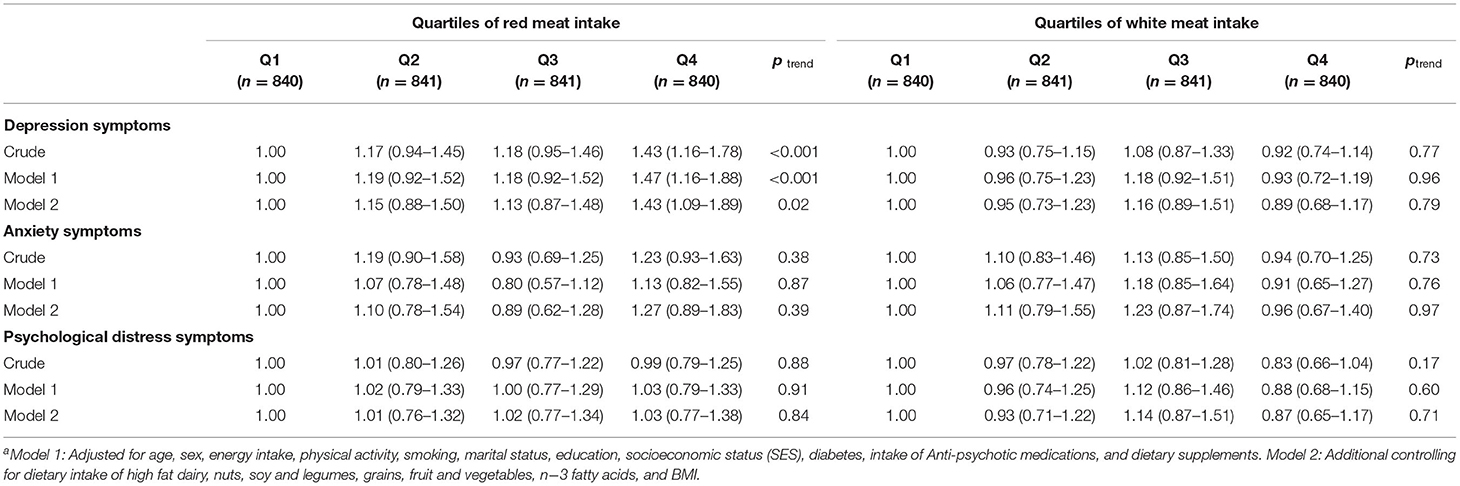
Table 3. Multivariable-adjusted odds ratios and 95% confidence intervals for depression, anxiety, and psychological distress symptoms across quartiles of energy-adjusted red and white meat intake in whole populationa.
Multivariable-adjusted ORs and 95% CI for depression, anxiety, and psychological distress symptoms across quartiles of energy-adjusted red and white meat intake in men and women are provided in Supplemental Tables 1, 2, respectively. Male participants in the highest quartile of red meat intake had higher odds of depression symptoms (OR = 1.75; 95% CI: 1.23–2.51) compared with those in the lowest quartile. After controlling for potential confounders, this association was strengthened (OR = 1.92; 95% CI: 1.17–3.15). Either in crude or in the adjusted models, no significant associations were observed between different levels of red meat intake and odds of anxiety and psychological distress symptoms among male participants. In case of white meat intake, we found that men in the third quartile had higher risk of depression symptoms (OR = 1.64; 95% CI: 1.02–2.63) and psychological distress symptoms (OR = 2.02; 95% CI: 1.20–3.39) compared to those in the first quartile.
Women in the highest quartile of red meat intake had greater odds of depression symptoms (OR = 1.36; 95% CI: 1.04–1.79) than those in the lowest quartile. However, after controlling for dietary intake, omega-3 fatty acids, and BMI, no statistically significant association was seen. No significant association was observed between different levels of red meat intake and odds of anxiety and psychological distress symptoms in women. Moreover, no significant associations were found between different amount of white meat intake and mental disorders symptoms after considering all potential confounders.
Multivariable-adjusted ORs and 95% CI for psychological disorders across quartiles of energy-adjusted red and white meat intake in overweight or obese participants (BMI ≥ 25 kg/m2) and normal-weight participants (BMI <25 kg/m2) are presented in Supplemental Tables 3, 4, respectively. After adjustment for confounding variables, no association was found between red meat intake and symptoms of mental disorders in overweight or obese individuals. However, higher intake of white meat was significantly associated with lower odds of psychological distress symptoms (OR = 0.64; 95% CI: 0.42–0.99) and marginally associated with lower risk of depression symptoms (OR = 0.68; 95% CI: 0.45–1.00) compared to lower intake.
In normal-weight participants, highest quartiles of red meat intake associated with higher odds of depression symptoms in both crude (OR = 1.44; 95% CI: 1.08–1.92) and fully adjusted model (OR = 1.66; 95% CI: 1.14–2.42). No significant relation was observed between white meat intake and symptoms of mental disorders in normal-weight participants.
Discussion
This cross-sectional study showed a significant positive association between red meat intake and the risk of depression symptoms in Iranian adults. This association was seen among male participants, even after adjustment for all potential confounders. But there was no relation in females, after considering confounding factors. In addition, in normal-weight participants, a significant positive association was observed between red meat intake and the odds of depression symptoms. However, analysis in overweight or obese participants showed that white meat intake had an inverse association with odds of psychological distress symptoms and marginally inverse association with depression symptoms. To our knowledge, this study was one of the first investigations in the Middle East, which evaluated the relation between meat intake and mental disorders.
Considering the high prevalence of mental disorders, especially depression all over the world, even a minimal advantage to reduce the prevalence of depression or anxiety would be substantial to the entire population. Our results showed that red and white meat intake might be associated with some mental disorders symptoms.
Previous studies have shown inconsistent results on relation between depression and meat consumption. Two prospective cohort studies indicated a significant association between meat or processed meat consumption (but not fish intake) and depression (26, 27). Whitehall II, another cohort study conducted on 3,486 participants, showed that high adherence to “processed food dietary pattern” including processed meat was associated with an increased odds of depression (37). Similarly, a pilot randomized controlled trials have suggested that restriction of meat, fish, and poultry consumption could improve mental state (38). Zhou et al. (23) in a cross-sectional study indicated that weekly meat intake was positively correlated with depression symptoms. Darooghegi Mofrad et al. (20) have also reported that red meat consumption was associated with higher odds of depression, anxiety, and psychological distress in Tehrani women. In contrast, in Australian women, a “traditional” dietary pattern (characterized by meat and fish, along with vegetables, fruit, and whole grains) was associated with lower odds of depression and anxiety (14). Another cross-sectional study among 11,473 Chinese participants revealed consumption of meat (including fresh and salted meat and fish) less than once a week was significantly associated with increased risk of depression, compared with once a week or more meat intake (21). This effect might be due to family low income, insufficient consumption of fish, or perhaps anemia caused by low meat intake (39). A prospective cohort study with multistage random sampling has also found no significant relation between meat or processed meat intake and depressive symptoms in elders (28). A recently published systematic review has also demonstrated that the prevalence or risk of depression and/or anxiety was significantly greater in meat-abstainers compared to meat-consumers (19). These inconsistencies could be due to different study designs, different populations, and different tools and scales to measure variables.
Previous studies revealed that women were more susceptible to mental disorders; however, we found that red meat intake is related to depression symptoms in men, but not in women. This finding might be because of estrogens that have neuronal protective effects (40). In addition, our analyses showed significantly higher red meat intake in males than females (84.7 vs. 74.4 g/day, p < 0.001) that could be the reason.
Although, previous studies have shown that the prevalence of depression in overweight or obese individuals was higher than in normal-weight participants (41), our findings showed a positive relation between red meat intake and depression symptoms in normal-weight individuals and showed no relation in the overweight or obese participants. Furthermore, in overweight or obese participants, white meat intake showed an inverse association with psychological distress symptoms, whereas, there was no linkage in normal-weight individuals. Future prospective investigations are suggested to shed light on these different associations among overweight or obese individuals vs. normal-weight individuals in relation to depression. In the case of white meat intake, the current study was conducted in one of the central provinces of Iran that is far from the sea and fish was less likely to be consumed by the study population than chicken and poultry; this point might affect our findings. Hence, we made adjustment for omega-3 intake in the analysis. However, the results were still unexplained and further investigations are needed.
The evidence that explains mechanisms of the relation of meat intake and mental health is not completely understood, but some previous studies showed low-grade inflammatory status in depression and anxiety disorders (42–45). Inflammation plays a potential role in the etiology of depression (46); on the other hand, it has indicated that red meat intake could elevate levels of pro-inflammatory cytokines, such as C-reactive protein (CRP), tumor-necrosis factor alpha (TNF-α), and interleukin (IL)-6 (47). Unfortunately, in the current study, it was not possible to evaluate inflammatory and oxidative biomarkers. Although, the involved biochemical pathways are not fully known, some studies blamed total fat and fat types of meat for this condition (17, 48). Diets rich in saturated fatty acid (SFA) or total fats could increase free radical production and elevate oxidative stress and inflammation (49–51). Pro-inflammatory cytokines may disrupt neurotransmitter metabolism pathways, reduce plasma tryptophan level, and prevent brain-derived neurotrophic factor (BDNF) expression (52, 53). BNFD is a peptide critical for optimal neuronal function, which has been indicated to be decreased in depression (54, 55). In addition, endothelium is involved in synthesis and secretion of BDNF; therefore, endothelial dysfunction leading to disturb cell signaling cascades (56). It was suggested that diets high in meat, fish, and poultry increase the risk of inflammation and depression due to n-6 to n-3 fatty acid ratio (57). Plasma concentrations of tryptophan, an essential amino acid to produce serotonin, and large neutral amino acids (LNAAs) could be another mechanism, which may be involved in depression (58). Brain concentrations of tryptophan depend on plasma concentrations of both tryptophan and LNAA, which competes with tryptophan to cross over the blood–brain barrier (59). Some studies have shown a higher reduction in plasma tryptophan than plasma LNAA after consumption of a meal rich in proteins or proteins plus fats, such as meat (60).
The strengths of the present study were collecting data from a large population of Iranian adults and using validated questionnaires to evaluate psychological disorders, physical activity, and dietary intake. Effects of several potential confounders were also taken into account. However, several limitations need to be considered when interpreting our findings. Due to the cross-sectional design of the study, we did not have possibility to have causal relation between meat intake and mental disorders. Large prospective cohort studies are necessary to distinguish a causal relation. In addition, all data derived from FFQs might be invalid and physiologically implausible because memory and recall are not valid tools to collect scientific data (61). Since the number of lean participants was low in our sample (n = 114, 3.4%), we had to include these individuals in the category of normal-weight participants. The prevalence of depression and anxiety symptoms was higher in lean participants than normal-weight individuals (41.2 vs. 30% for depression symptoms and 16.7 vs. 15.0% for anxiety symptoms). Some sort of bias might occur due to this inclusion; however, it would be negligible, and such error would shift the ORs toward the null. Different types of red and white meat were not specifically investigated. Although, we were interested in examining the separate associations between processed meat and red meat intake and outcomes of interest in the current study, low consumption of processed red meat in the study population (6 ± 1 g/day) led us to consider total red meat, rather than processed meat and red meat intake, as the exposure. However, when we excluded processed meat and considered only unprocessed red meat intake, our results did not change. We could not measure biomarkers of inflammation and oxidative status. In addition, it was not possible to assess body resources of micronutrient, such as vitamin D, B6, B12, folate, and zinc, which might involve in mental disorders (18, 62–64). In addition, the study population consisted of a medical university non-academic staff, including crews, employees, and managers. Although the socio-economic status of the study population was representative of general Iranian population, generalization the findings to other populations should be made cautiously.
Conclusions
We found that higher intake of red meat was associated with increased risk of depression symptoms, especially in males. In overweight or obese participants, white meat intake was inversely associated with psychological distress symptoms. Red meat intake was related to increased odds of depression symptoms in normal-weight participants. Further, prospective studies are needed to confirm these findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Isfahan University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SK, AK, PS, HA, AE, and PA contributed in conception, design, data collection, data interpretation, manuscript drafting, approval of the final version of the manuscript, and agreed for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
The financial support for this study was from Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran (Grant No: 299021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank all staff of Isfahan University of Medical Sciences who kindly participated in our study and staff of Public Relations Unit, and other authorities of Isfahan University of Medical Sciences for their excellent cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.710555/full#supplementary-material
Abbreviations
ANCOVA, analysis of covariance; BMI, body mass index; BDNF, drain-derived neurotrophic factor; CES-D, Center for Epidemiologic Studies-Depression; FFQ, food frequency questionnaire; FGIDs, functional gastrointestinal disorders; GHQ, General Health Questionnaire; GPPAQ, General Practice Physical Activity Questionnaire; HADS, Hospital Anxiety and Depression Scale; IUMS, Isfahan University of Medical Sciences; LNAA, large neutral amino acids; OR, odds ratio; SEPAHAN, Study on the Epidemiology of Psychological-Alimentary Health and Nutrition; SES, socioeconomic status; SPSS, Statistical Package for Social Sciences.
References
1. Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet. (2007) 370:859–77. doi: 10.1016/S0140-6736(07)61238-0
2. Compton WM, Conway KP, Stinson FS, Grant BF. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991–1992 and 2001–2002. Am J Psychiatry. (2006) 163:2141–7. doi: 10.1176/ajp.2006.163.12.2141
3. World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization (2017).
4. Valizadeh R, Sarokhani D, Sarokhani M, Sayehmiri K, Ostovar R, Angh P, et al. A study of prevalence of anxiety in Iran: systematic review and meta-analysis. Der Phar Chem. (2016) 8:48–57.
5. Sarokhani D, Parvareh M, Dehkordi AH, Sayehmiri K, Moghimbeigi A. Prevalence of depression among Iranian elderly: systematic review and meta-analysis. Iran J Psychiatry. (2018) 13:55.
6. Mohamadi M, Kamal SHM, Vameghi M, Rafiey H, Sajjadi ASFH. A meta-analysis of studies related prevalence of depression in Iran. J Res Health. (2017) 7:581–93. doi: 10.18869/acadpub.jrh.7.1.581
7. Daskalopoulou M, George J, Walters K, Osborn DP, Batty GD, Stogiannis D, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PLoS ONE. (2016) 11:e0153838. doi: 10.1371/journal.pone.0153838
8. Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology. (2013) 38:1573–85. doi: 10.1016/j.psyneuen.2013.01.002
9. Blanchard EB, Scharff L, Schwarz SP, Suls JM, Barlow DH. The role of anxiety and depression in the irritable bowel syndrome. Behav Res Ther. (1990) 28:401–5. doi: 10.1016/0005-7967(90)90159-G
10. Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. (2002) 53:859–63. doi: 10.1016/S0022-3999(02)00313-6
11. Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. (2003) 64:1465–75. doi: 10.4088/JCP.v64n1211
12. Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. J Ment Health Policy Econ. (2006) 9:87–98.
13. Sarris J, O'Neil A, Coulson CE, Schweitzer I, Berk M. Lifestyle medicine for depression. BMC Psychiatry. (2014) 14:107. doi: 10.1186/1471-244X-14-107
14. Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. (2010) 167:305–11. doi: 10.1176/appi.ajp.2009.09060881
15. Opie R, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. (2017) 20:161–71. doi: 10.1179/1476830515Y.0000000043
16. Ahmadi A, Mohammadi-Sartang M, Nooraliee P, Veisi M, Rasouli J. Prevalence of anxiety and it's relationship with consumption of snacks in high school students in Shiraz. J Shahrekord Univ Med Sci. (2013) 15:83–90.
17. Zhang Y, Yang Y, Xie M-s, Ding X, Li H, Liu Z-c, et al. Is meat consumption associated with depression? A meta-analysis of observational studies. BMC Psychiatry. (2017) 17:409. doi: 10.1186/s12888-017-1540-7
18. Jacka FN, Maes M, Pasco JA, Williams LJ, Berk M. Nutrient intakes and the common mental disorders in women. J Affect Disord. (2012) 141:79–85. doi: 10.1016/j.jad.2012.02.018
19. Dobersek U, Wy G, Adkins J, Altmeyer S, Krout K, Lavie CJ, et al. Meat and mental health: a systematic review of meat abstention and depression, anxiety, and related phenomena. Crit Rev Food Sci Nutr. (2021) 61:622–35. doi: 10.1080/10408398.2020.1741505
20. Darooghegi Mofrad M, Mozaffari H, Sheikhi A, Zamani B, Azadbakht L. The association of red meat consumption and mental health in women: a cross-sectional study. Complement Ther Med. (2021) 56:102588. doi: 10.1016/j.ctim.2020.102588
21. Chen R, Wei L, Hu Z, Qin X, Copeland JR, Hemingway H. Depression in older people in rural China. Arch Intern Med. (2005) 165:2019–25. doi: 10.1001/archinte.165.17.2019
22. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Fish and fat intake and prevalence of depressive symptoms during pregnancy in Japan: baseline data from the Kyushu Okinawa Maternal and Child Health Study. J Psychiatr Res. (2013) 47:572–8. doi: 10.1016/j.jpsychires.2013.01.012
23. Zhou X, Bi B, Zheng L, Li Z, Yang H, Song H, et al. The prevalence and risk factors for depression symptoms in a rural Chinese sample population. PLoS ONE. (2014) 9:e99692. doi: 10.1371/journal.pone.0099692
24. Park Y, Kim M, Baek D, Kim S-H. Erythrocyte n−3 polyunsaturated fatty acid and seafood intake decrease the risk of depression: case-control study in Korea. Ann Nutr Metabol. (2012) 61:25–31. doi: 10.1159/000339264
25. Kim JL, Cho J, Park S, Park E-C. Depression symptom and professional mental health service use. BMC Psychiatry. (2015) 15:261. doi: 10.1186/s12888-015-0646-z
26. Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. Eur J Clin Nutr. (2013) 67:75–82. doi: 10.1038/ejcn.2012.193
27. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, Schlatter J, Lahortiga F, Majem LS, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. (2009) 66:1090–8. doi: 10.1001/archgenpsychiatry.2009.129
28. Tsai AC, Chang T-L, Chi S-H. Frequent consumption of vegetables predicts lower risk of depression in older Taiwanese–results of a prospective population-based study. Public Health Nutr. (2012) 15:1087–92. doi: 10.1017/S1368980011002977
29. Willett WC. Nutritional epidemiology. In: Issues in Analysis and Presentation of Dietary Data, 1-41 & Implications of Total Energy Intake for Epidemiologic Analyses. 3rd ed. New York, NY: Oxford University Press (2012). p. 1–10.
30. Keshteli A, Esmaillzadeh A, Rajaie S, Askari G, Feinle-Bisset C, Adibi P. A dish-based semi-quantitative food frequency questionnaire for assessment of dietary intakes in epidemiologic studies in iran: design and development. Int J Prev Med. (2014) 5:29–36.
31. Barak F, Falahi E, Keshteli AH, Yazdannik A, Saneei P, Esmaillzadeh A. Red meat intake, insulin resistance, and markers of endothelial function among Iranian women. Mol Nutr Food Res. (2015) 59:315–22. doi: 10.1002/mnfr.201400333
32. Saneei P, Esmaillzadeh A, Keshteli AH, Roohafza HR, Afshar H, Feizi A, et al. Combined healthy lifestyle is inversely associated with psychological disorders among adults. PLoS ONE. (2016) 11:e0146888. doi: 10.1371/journal.pone.0146888
33. Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes. (2003) 1:14. doi: 10.1186/1477-7525-1-14
34. Montazeri A, Harirchi AM, Shariati M, Garmaroudi G, Ebadi M, Fateh A. The 12-item General Health Questionnaire (GHQ-12): translation and validation study of the Iranian version. Health Qual Life Outcomes. (2003) 1:66. doi: 10.1186/1477-7525-1-66
35. Aminianfar S, Saneei P, Nouri M, Shafiei R, Hassanzadeh-Keshteli A, Esmaillzadeh A, et al. Validation study of self-reported anthropometric indices among the staff of the Isfahan University of Medical Sciences, Isfahan, Iran. J Isfahan Med Sch. (2015) 33:1318–27.
36. NICE. Promoting and Creating Built or Natural Environments that Encourage and Support Physcial Activity: Scope. London: National Institute for Health and Clinical Excellence (2006).
37. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. (2009) 195:408–13. doi: 10.1192/bjp.bp.108.058925
38. Beezhold BL, Johnston CS. Restriction of meat, fish, and poultry in omnivores improves mood: a pilot randomized controlled trial. Nutr J. (2012) 11:9. doi: 10.1186/1475-2891-11-9
39. Onder G, Penninx BW, Cesari M, Bandinelli S, Lauretani F, Bartali B, et al. Anemia is associated with depression in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. (2005) 60:1168–72. doi: 10.1093/gerona/60.9.1168
40. Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. (1997) 154:1641–7. doi: 10.1176/ajp.154.12.1641
41. Pereira-Miranda E, Costa PR, Queiroz VA, Pereira-Santos M, Santana ML. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. (2017) 36:223–33. doi: 10.1080/07315724.2016.1261053
42. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
43. Bremmer M, Beekman A, Deeg D, Penninx B, Dik M, Hack C, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. (2008) 106:249–55. doi: 10.1016/j.jad.2007.07.002
44. Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. (2009) 39:413–23. doi: 10.1017/S0033291708003723
45. Kim Y-K, Jeon SW. Neuroinflammation and the immune-kynurenine pathway in anxiety disorders. Curr Neuropharmacol. (2018) 16:574–82. doi: 10.2174/1570159X15666170913110426
46. Kohler O, Krogh J, Mors O, Benros ME. Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol. (2016) 14:732–42. doi: 10.2174/1570159X14666151208113700
47. Schwedhelm C, Pischon T, Rohrmann S, Himmerich H, Linseisen J, Nimptsch K. Plasma inflammation markers of the tumor necrosis factor pathway but not c-reactive protein are associated with processed meat and unprocessed red meat consumption in bavarian adults. J Nutr. (2017) 147:78–85. doi: 10.3945/jn.116.237180
48. Sánchez-Villegas A, Verberne L, De Irala J, Ruiz-Canela M, Toledo E, Serra-Majem L, et al. Dietary fat intake and the risk of depression: the SUN Project. PloS ONE. (2011) 6:e16268. doi: 10.1371/journal.pone.0016268
49. Liu T, Zhong S, Liao X, Chen J, He T, Lai S, et al. A meta-analysis of oxidative stress markers in depression. PLoS ONE. (2015) 10:e0138904. doi: 10.1371/journal.pone.0138904
50. Pawels E, Volterrani D. Fatty acid facts, Part I. Essential fatty acids as treatment for depression, or food for mood? Drug News Perspect. (2008) 21:446–51. doi: 10.1358/dnp.2008.21.8.1272136
51. Bilici M, Efe H, Köroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. (2001) 64:43–51. doi: 10.1016/S0165-0327(00)00199-3
52. Hayley S, Poulter M, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor-and cytokine-induced alterations of neuroplasticity. Neuroscience. (2005) 135:659–78. doi: 10.1016/j.neuroscience.2005.03.051
53. Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. (2009) 34:4–20.
54. Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. (2016) 14:721–31. doi: 10.2174/1570159X14666160119094646
55. Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Mol Brain Res. (2005) 136:29–37. doi: 10.1016/j.molbrainres.2004.12.020
56. Uauy R, Aro A, Clarke R, L'abbé M, Mozaffarian D, Skeaff C, et al. WHO scientific update on trans fatty acids: summary and conclusions. Eur J Clin Nutr. (2009) 63(Suppl. 2):S68. doi: 10.1038/ejcn.2009.15
57. Calder PC. n– 3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. (2006) 83:1505–19S. doi: 10.1093/ajcn/83.6.1505S
58. Sánchez-Villegas A, Toledo E, De Irala J, Ruiz-Canela M, Pla-Vidal J, Martínez-González MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. (2012) 15:424–32. doi: 10.1017/S1368980011001856
59. Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. (1972) 178:414–6. doi: 10.1126/science.178.4059.414
60. Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr. (2003) 77:128–32. doi: 10.1093/ajcn/77.1.128
61. Archer E, Marlow ML, Lavie CJ. Controversy and debate: memory based methods paper 3: Nutrition's 'Black Swans': Our reply. J Clin Epidemiol. (2018) 104:130–5. doi: 10.1016/j.jclinepi.2018.07.013
62. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
63. Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. (2010) 92:330–5. doi: 10.3945/ajcn.2010.29413
Keywords: meat intake, mental disorder, depression, anxiety, psychological distress, diet
Citation: Kazemi S, Keshteli AH, Saneei P, Afshar H, Esmaillzadeh A and Adibi P (2021) Red and White Meat Intake in Relation to Mental Disorders in Iranian Adults. Front. Nutr. 8:710555. doi: 10.3389/fnut.2021.710555
Received: 16 May 2021; Accepted: 24 June 2021;
Published: 27 July 2021.
Edited by:
Donato Angelino, University of Teramo, ItalyReviewed by:
Justyna Godos, University of Catania, ItalyUrska Dobersek, University of Southern Indiana, United States
Copyright © 2021 Kazemi, Keshteli, Saneei, Afshar, Esmaillzadeh and Adibi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parvane Saneei, c2FuZWVpcEB5YWhvby5jb20=; c2FuZWVpQG51dHIubXVpLmFjLmly; Ahmad Esmaillzadeh, YS1lc21haWxsemFkZWhAc2luYS50dW1zLmFjLmly
 Shiva Kazemi1,2
Shiva Kazemi1,2 Ammar Hassanzadeh Keshteli
Ammar Hassanzadeh Keshteli Parvane Saneei
Parvane Saneei Ahmad Esmaillzadeh
Ahmad Esmaillzadeh