
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 12 July 2021
Sec. Eating Behavior
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.709683
 Cong Yin1
Cong Yin1 Zihan Li1
Zihan Li1 Yulin Xiang1
Yulin Xiang1 Hongbing Peng1
Hongbing Peng1 Ping Yang1
Ping Yang1 Shijun Yuan1
Shijun Yuan1 Xueyan Zhang1
Xueyan Zhang1 You Wu1
You Wu1 Min Huang2*
Min Huang2* Juan Li1*
Juan Li1*Background: Weight loss by lifestyle modification is the cornerstone therapy of non-alcoholic fatty liver disease (NAFLD). Intermittent fasting has shown favorable effects on body weight (BW) and relevant indicators of NAFLD in several reports.
Objective: To estimate the effects of intermittent fasting on adults with NAFLD.
Materials and methods: Literature searches were conducted on PubMed, EMBASE, Web of Science, Cochrane Library, and ClinicalTrials.gov from inception to May 10, 2021.
Results: A total of six studies involving 417 patients with NAFLD were included. In the meta-analysis, there were significant differences in BW, body mass index (BMI), alanine aminotransferase (ALT), and aspartate transaminase (AST) between the control and fasting group. Up to now, there is no significant difference in triglycerides (TG), total cholesterol (TC), and other metabolic parameters between the two groups.
Conclusions: Intermittent fasting is beneficial for weight management and liver enzyme improvement, but long-term feasibility and safety of intermittent fasting should be conducted in further studies.
Non-alcoholic fatty liver disease (NAFLD) is the predominant cause of chronic liver diseases worldwide, causing considerable liver-related and extrahepatic morbidity and mortality (1). It is a clinical and pathological syndrome characterized by excessive fat deposits in hepatocytes, resulting from causes other than factors including alcohol and liver damage (2). Patients with NAFLD have an increased risk of cardiovascular disease, type 2 diabetes, and even chronic kidney disease, and mortality from liver-related and non-liver-related causes appears more significant than in the general population (3–6). The increasing prevalence of the disease worldwide is thus particularly worrisome. Given that there are currently no drugs approved for the treatment of NAFLD, lifestyle modification (through dietary and exercise interventions) remains the underlying approach for the treatment of NAFLD (7). Paired liver biopsy studies have demonstrated that a ≥5% loss of body weight (BW) is associated with significant reductions in hepatic steatosis (HS), ≥7% loss of weight is associated with a decrease in hepatic inflammation, and a ≥10% loss is associated with a reduction in fibrosis (8).
Since dietary interventions play an important role in weight loss management, it is necessary to study suitable dietary interventions for patients with NAFLD. Intermittent fasting, which includes alternate-day fasting, and other forms of periodic caloric restriction have already received attention from animal research scientists (9, 10). It has been shown that fasting may benefit weight management and improve cardiovascular and metabolic risks (11).
Although there exist some clinical trials assessing the effect of intermittent fasting on NAFLD, there has been no meta-analysis of these clinical trials, and there is a lack of evidence-based medical evidence on the effect of intermittent fasting on NAFLD (12–17). Therefore, in this study, we performed a systematic review and meta-analysis to include clinical trials that adopt fasting intervention for NAFLD and to evaluate the effect of fasting on factors associated with NAFLD.
This research study was performed according to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) (Supplementary Table 1) (18).
We searched PubMed, EMBASE, Cochrane Library, Web of Science, and ClinicalTrials.gov databases to find relevant studies up to May 10, 2021, in all languages using a combination of the main search terms “fasting” and “NAFLD.” The search strategy was designed to be as broad as possible. Then, the reference lists of the relevant studies were checked for additional studies. The detailed search strategy is presented in Supplementary Table 2.
Two authors (YC and LJ) independently reviewed the titles and abstracts. Discrepancies were resolved through discussions among the two authors. The two authors then independently analyzed the full text of the remaining studies to determine the final inclusion.
For this meta-analysis, inclusion criteria were as follows: (1) participants with NAFLD were adult humans aged ≥18 years across different countries; (2) the intervention group underwent fasting intervention alone or in conjunction with other lifestyle interventions; (3) the comparator group underwent no nutritional intervention (“control”) or a different type of nutritional intervention; (4) the full text was available and was written in English; (5) the trial included evaluation of at least one of the following outcomes: BW, body mass index (BMI), waist circumference (WC), fasting blood sugar/glucose (FBS/GLU), homeostasis model assessment of insulin resistance (HOMA-IR), fasting insulin (FINS), alanine aminotransferase (ALT), aspartate transaminase (AST), liver stiffness, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), or low-density lipoprotein cholesterol (LDL-C); and (6) the trial reported the mean value of changes from baseline (or values of post-intervention when unavailable) with SD (or suitable data to determine the parameter: SE or 95% CI). Animal studies and uncontrolled and cross-sectional studies were not included.
Two authors (YC and LJ) extracted data from the selected trials. This process was verified by another investigator (HM). An Excel database was used to extract the following information from the included studies: (1) study characteristics (e.g., names of the authors, year, sample size, and ages of the patients), (2) treatments, (3) methodological aspects, and (4) clinical outcomes. To obtain the data not presented in the original studies, we sent two e-mails 1 week apart to the corresponding authors.
We followed the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias of these included studies, which included detection bias (outcome assessment blinding), selection bias (random sequence generation and allocation concealment), attrition bias (incomplete outcome data), and reporting bias (selective reporting) (19). We assessed every domain of bias in terms of methodological quality and assigned a rating of low or high risk of bias. If there was insufficient information to make an adequate assessment, the domain was evaluated as “unclear”.
A series of calculations were executed to standardize the data in terms of SD as some studies reported CIs. The conversion of 95% CI to SD was calculated by (19). The mean change from baseline to after placebo or treatment was calculated by , and the SD of change was calculated by (19). We calculated SD for the change values by selecting 0.5 as the correlation coefficient (r = 0.5), and to make sure that the meta-analysis was not sensitive to the selected correlation coefficient, all analyses were repeated using correlation coefficients of 0.2 and 0.8.
The mean difference (MD) and its corresponding 95% CIs were calculated using the random effects model, which takes the between-study heterogeneity into account (19). Heterogeneity was assessed statistically using the standard chi-square I2 test. A random effects model using the double-arcsine transformation approach was used. I2 values ≤ 25, 25–50, 50–75, and >75% indicated no, small, moderate, and significant heterogeneities, respectively. To examine the potential sources of between-study heterogeneity, subgroup analyses were performed according to the type of control diet (religious intermittent fasting/modern intermittent fasting regimens). A sensitivity analysis was conducted only when three or more studies were included in the comparison. Sensitivity analysis was used to assess the robustness of the results of meta-analyses by sequentially removing individually included studies. The presence of the publication bias was checked for each outcome through funnel plots. Publication bias was assessed using the Egger regression asymmetry test when the comparison contained at least 10 studies. All statistical analyses were performed using STATA MP, version 16, and two-sided p-values < 0.05 were considered statistically significant.
In the systematic search, a total of 7,531 citations were identified, and the study selection process is illustrated in Figure 1. After the removal of duplicates, 4,634 studies were assessed via their title and abstract for potential eligibility. In total, 4,582 studies were excluded, and 52 studies were retrieved for full-text analysis. Finally, six studies fulfilled the inclusion criteria of being a systematic review, and the characteristics of the included studies are summarized in Table 1.
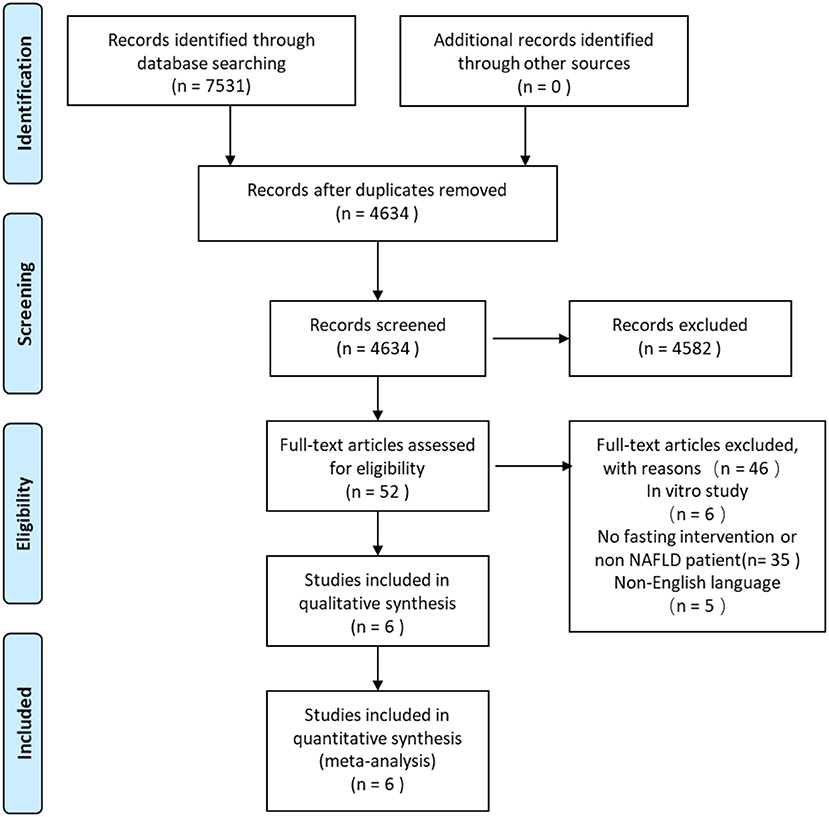
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram for screening and selection process of studies.
Seven datasets [one study (20) had two comparators: control and time-restricted feeding (TRF)] were included in this meta-analysis. The intervention group included 417 participants (range 30–95), and the comparator group included 278 participants (range 9–81). All studies did not distinguish between men and women. All five studies targeted the NAFLD population; only one study had non-alcoholic steatohepatitis (NASH) population in addition to the NAFLD population.
Two of the six studies were randomized controlled trials (RCTs), and on the specific methods of fasting, four used Ramadan fasting, two used alternate-day fasting, and one used TRF. On the days of fasting, the fasting time is almost always 15–16 h/day. Two studies restricted calories, and the other studies did not report caloric restriction. None of the studies cooperated with the exercise intervention. The duration of these researchers ranged from 4 to 12 weeks. BW was measured in all the six studies, BMI was reported in five studies, FBS/GLU, TG, TC, and HDL-C in four studies, WC and ALT in three studies, and AST, liver stiffness, FI, and HOMA-IR in two studies.
One study had two comparators, and two datasets were included (13). Two studies used the same study population, and only one dataset was included (12, 14). One of the remaining three studies did not report BW (16). Therefore, a total of five BW datasets were included in the analysis. Meta-analysis shows that the effect of fasting on BW was statistically significant (MD, −2.45, 95% CI from −3.98 to −0.91, p ≤ 0.00) (Figure 2). Negative values favor fasting because the fasting group experienced more reduction in BW than did the control group.
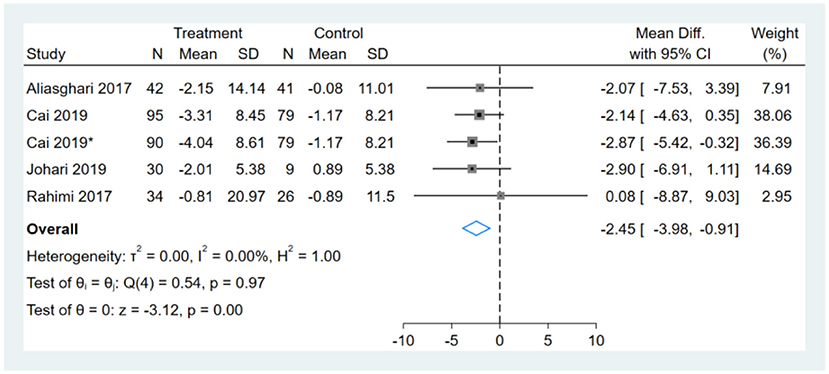
Figure 2. Forest plot analysis showing changes in body weight (BW). *This study has two fasting intervention groups.
One study had two comparators, and two datasets were included (13). Two studies used the same study population, and only one dataset was included (12, 14). Therefore, a total of six BMI datasets were included in the analysis. Meta-analysis shows that the effect of fasting on BMI was statistically significant (MD, −0.50, 95% CI from −0.93 to −0.07, p = 0.02) (Figure 3). Negative values favor fasting because the fasting group experienced more reduction in BMI than did the control group.
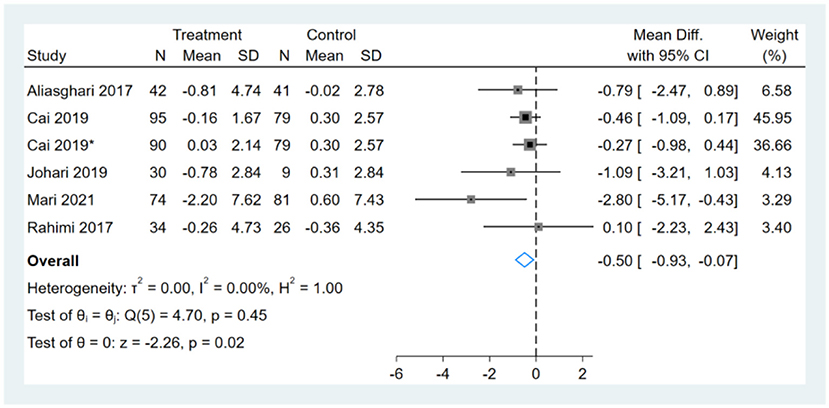
Figure 3. Forest plot analysis showing changes in body mass index (BMI). *This study has two fasting intervention groups.
Two studies reported WC results, one of them had two comparators, and two datasets were included (13). Therefore, a total of three WC datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for WC (Figure 4).
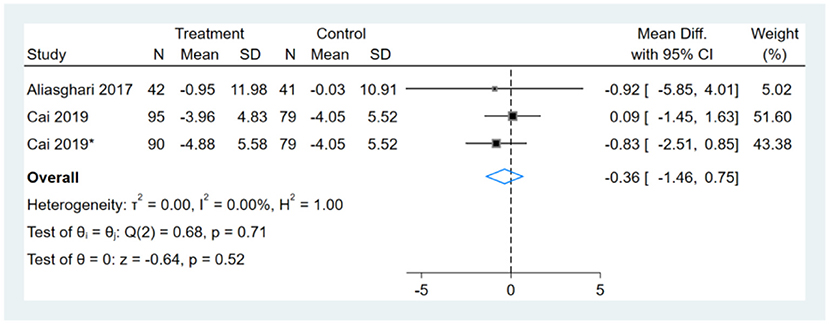
Figure 4. Forest plot analysis showing waist circumference (WC) changes. *This study has two fasting intervention groups.
Three studies reported FBS/GLU results, one of them had two comparators, and two datasets were included (13). Therefore, a total of four FBS/GLU datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for FBS/GLU (Figure 5).
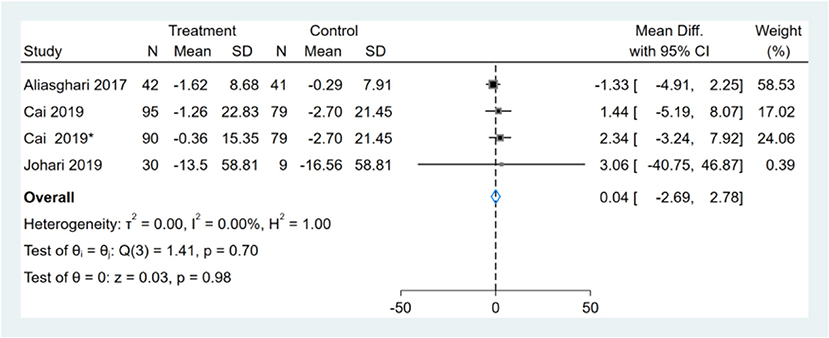
Figure 5. Forest plot analysis showing changes in fasting blood sugar/glucose (FBS/GLU). *This study has two fasting intervention groups.
Only two studies reported HOMA-IR results, and meta-analysis shows that the effect of the fasting intervention was not significant for HOMA-IR (Figure 6).
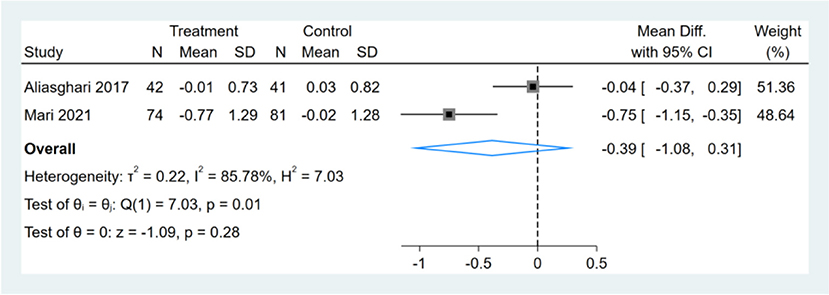
Figure 6. Forest plot analysis showing changes in homeostasis model assessment of insulin resistance (HOMA-IR).
Only two studies reported FINS results, and meta-analysis shows that the effect of the fasting intervention was not significant for FINS (Figure 7).
Three studies were included for analysis, which shows heterogeneity (I2 = 87%). We found the changing trend of ALT before and after the fasting reported in one study was significantly opposite to that in the previous study (17), and the heterogeneity was reduced after the deletion of this study (I2 = 0%). After the removal of the highly heterogeneous study, the remaining two studies were analyzed and showed that the effect of fasting on ALT was statistically significant (MD, −10.54, 95% CI from −14.01 to −7.08, p ≤ 0.00) (Figure 8).
Only two studies reported AST results, and meta-analysis shows that the effect of fasting on AST was statistically significant (MD, −11.31, 95% CI from −14.30 to −8.32, p ≤ 0.00) (Figure 9).
Only one study reported liver stiffness results, which had two comparators, and two datasets were included. Therefore, a total of two liver stiffness datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for liver stiffness (Figure 10).
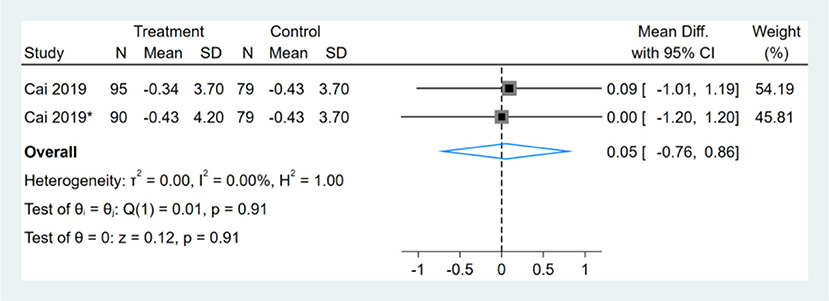
Figure 10. Forest plot analysis showing changes in liver stiffness. *This study has two fasting intervention groups.
Three studies reported TG results, one of them had two comparators, and two datasets were included (13). Therefore, a total of four TG datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for TG (Figure 11).
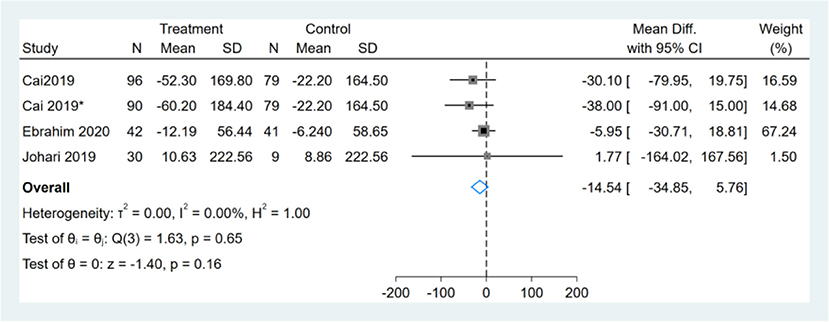
Figure 11. Forest plot analysis showing changes in triglyceride (TG). *This study has two fasting intervention groups.
Three studies reported TC results, one of them had two comparators, and two datasets were included (13). Therefore, a total of four TC datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for TC (Figure 12).
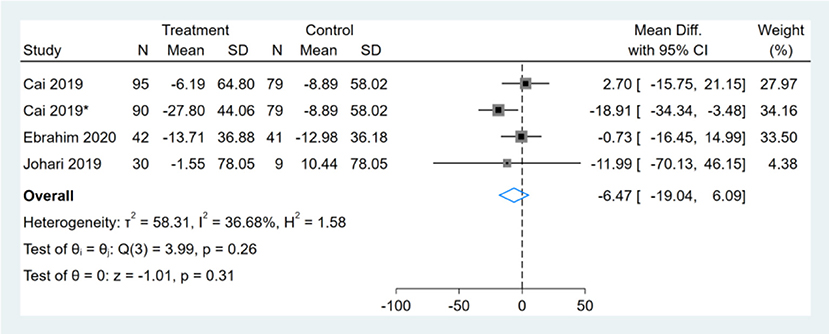
Figure 12. Forest plot analysis showing changes in total cholesterol (TC). *This study has two fasting intervention groups.
Three studies reported LDL-C results, one of them had two comparators, and two datasets were included (13). Therefore, a total of four LDL-C datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for LDL-C (Figure 13).
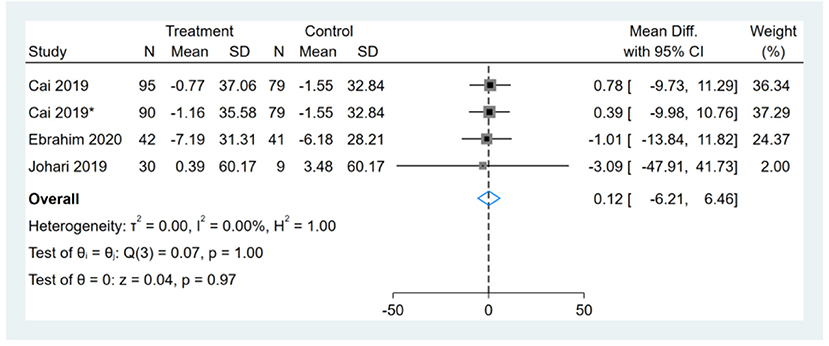
Figure 13. Forest plot analysis showing changes in low-density lipoprotein cholesterol (LDL-C). *This study has two fasting intervention groups.
Three studies reported HDL-C results, one of them had two comparators, and two datasets were included (13). Therefore, a total of four HDL-C datasets were included in the analysis. Meta-analysis shows that the effect of the fasting intervention was not significant for HDL-C (Figure 14).
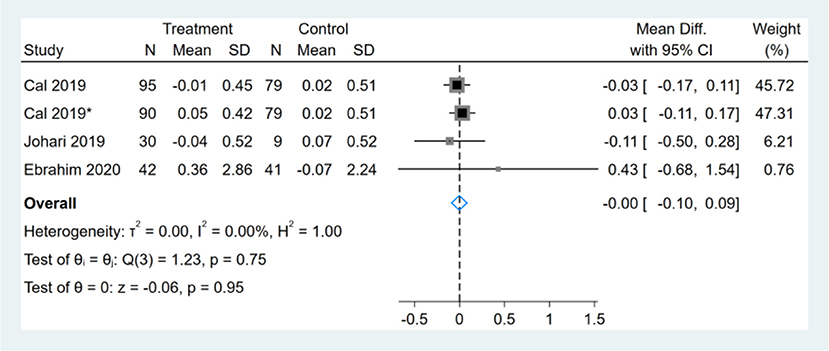
Figure 14. Forest plot analysis showing changes in high-density lipoprotein cholesterol (HDL-C). *This study has two fasting intervention groups.
The pooled effect indicated that fasting significantly reduced the BW in subjects whose type of diet was modern intermittent fasting (−2.56; 95%CI: −4.19 −0.91) but not in the intermittent religious fasting (Table 2). BMI, WC, FBS/GLU, TG, TC, LDL-C, and HDL-C have no difference in the type of diet (Table 2). More investigations are needed as there were only a limited number of trials in each subgroup.
The risk of bias assessment is shown in Figures 15, 16. We assess all the six studies according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions. The blinding of the participants was limited because of the characteristics of the intervention. Consequently, we assess all the six studies as high risk of performance bias. Four studies did neither evaluate the generation of randomization sequence nor adequately conceal allocations.
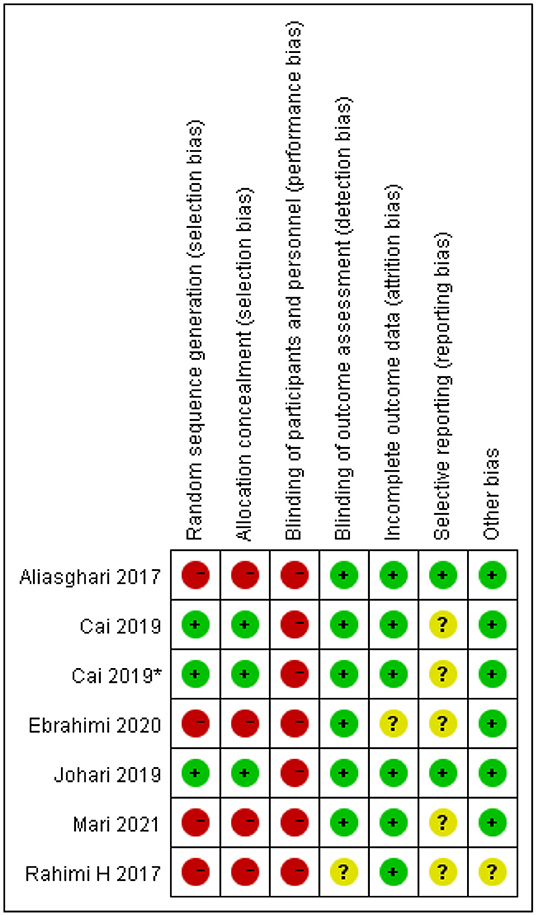
Figure 16. Risk of bias summary identified in the studies included in the meta-analysis. *This study has two fasting intervention groups.
Based on the visual inspection of funnel plots, there may be publication deviations (Figures 17–29). However, the number of included trials was small in each analysis. Thus, further investigations are needed.
Obesity results from excess calories are a significant risk factor for developing insulin resistance (IR), a critical factor in the etiology of metabolic syndrome and type 2 diabetes mellitus (20, 21). In turn, IR promotes the development of NAFLD in a subset of patients that could evolve to a more severe form, i.e., NASH. Unfortunately, science is far from thoroughly clarifying the genesis of NAFLD and suggesting appropriate therapy to reduce or eliminate them drastically (22). At present, there are no approved pharmacological therapies for NAFLD. Many guidelines advocate recommendations focusing on controlling risk factors and lifestyle changes that include dietary and physical activities (15). Dietary restriction increases the endogenous synthesis of fatty acids. Dietary and endogenously synthesized fatty acids contribute to the whole-body fatty acid pool (22, 23). Intermittent fasting is the established diet paradigm that extends life span and health span across species (21, 24).
Diet and exercise are among the most commonly used ones but complicated to maintain due to the ability of each organism to balance the reduced intake of calories with a decrease in resting metabolic rate, thus frustrating the beneficial effect (25). Therefore, based on the medical history and preferences of patients, appropriate individualized diet and exercise prescriptions should be developed. This is best approached with a healthcare team including a physician, registered dietitian, and exercise physiologist. Through this slow and thoughtful process of cycles of weight loss and weight maintenance, it is thought that patients will be able to prevent the more debilitating cycles of rapid weight loss, short-term reductions in metabolic rate, and rapid weight gain (25).
This study is the first time to evaluate the effectiveness of intermittent fasting on weight loss and improvement of liver function-related parameters in patients with NAFLD. On the basis of this meta-analysis, we confirmed an improvement in ALT and AST through fasting compared with a non-fasting control group. This seems to be related to a decrease in BW and a reduction in BMI. Earlier meta-analyses have shown similar results that intermittent fasting was more effective than the control for BMI and BW (9, 10).
Studies in rodents have demonstrated that TRF improves metabolic profiles and reduces the risk of obesity and its related conditions (26, 27). Similarly, all six studies in our meta-analysis that directly compared the fasted group with the non-fasted group showed that fasting was not only beneficial for weight and BMI reduction but also for ALT and AST reduction, with a statistical significance. By pooling the data, we provide clear evidence that fasting can reduce BW, BMI, ALT, and AST. None of the six included studies were confounded by exercise or other interventions. Therefore, intermittent fasting has an independent and significant benefit on weight loss and improvement of liver function in patients with NAFLD. Recent research studies reported similar results that short-term, isocaloric TRF would diminish the severity of NAFLD and improve metabolic parameters associated with liver function in short-term diet-restricted fasted mice (28). The beneficial effects of fasting are often regarded as driven by reductions in BW and/or body fat (29). In addition, the mechanism for the direct hepatic benefit of fasting is considered to be related to its effect on circadian biology, gut microbiome, and modifiable lifestyle behaviors (26, 30–33).
We failed to observe the benefits of fasting on metabolism-related parameters such as TG, TC, LDL-C, HDL-C, FBS/GLU, HOMA-IR, and FINS. Among the included studies, four studies reported improvements in lipid metabolism indicators, such as TG, TC, LDL-C, and HDL-C. In contrast, the above indicators did not show significant differences during statistical analysis by STATA. This may be related to the fact that there are few available literature studies with high heterogeneity among them. Thus, the effect of fasting on lipid metabolism needs to be further confirmed by more clinical RCTs.
This meta-analysis provides evidence for the effect of intermittent fasting on weight reduction and improvement of liver function. However, few included studies and the low sample size would affect and limit the meta-analysis results. Four of the six included studies were religion-related fasting, and only two were RCTs, which is also an important reason for heterogeneity. More studies on large-scale randomized controlled intervention are needed to better inform clinical recommendations for fasting interventions in NAFLD. Long-term control and maintenance of weight are more important, while follow-up support is lacking in the six studies included this time. In addition, safety is essential for fasting interventions but is severely lacking.
Intermittent fasting is beneficial for weight management and liver enzyme improvement, but long-term feasibility and safety of intermittent fasting should be conducted in further studies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
CY and JL contributed to conception and design of the study and wrote the first draft of the manuscript. CY and MH organized the database. CY performed the statistical analysis. ZHL, YLX, HBP, PY, XYZ, SJY, and YW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was supported by the Hubei Provincial Natural Science Foundation of China (Grant No. 2017CFB383) and Key project at the central government level: the ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.709683/full#supplementary-material
1. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne C, Targher G. Complications, morbidity and mortality of non-alcoholic fatty liver disease. Metabol Clin Exp. (2020) 2020:154170. doi: 10.1016/j.metabol.2020.154170
2. Xiong Y, Peng Q, Cao C, Xu Z, Zhang B. Effect of different exercise methods on non-alcoholic fatty liver disease: a meta-analysis and meta-regression. Int J Environ Res Public Health. (2021) 18:63242. doi: 10.3390/ijerph18063242
3. Kasturiratne A, Weerasinghe S, Dassanayake A, Rajindrajith S, de Silva A, Kato N, et al. Influence of non-alcoholic fatty liver disease on the development of diabetes mellitus. J Gastroenterol Hepatol. (2013) 28:142–7. doi: 10.1111/j.1440-1746.2012.07264.x
4. Levene A, Goldin R. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. (2012) 61:141–52. doi: 10.1111/j.1365-2559.2011.04145.x
5. Miele L, Targher G. Understanding the association between developing a fatty liver and subsequent cardio-metabolic complications. Expert Rev Gastroenterol Hepatol. (2015) 9:1243–5. doi: 10.1586/17474124.2015.1074860
6. Musso G, Gambino R, Tabibian J, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. (2014) 11:e1001680. doi: 10.1371/journal.pmed.1001680
7. El-Agroudy N, Kurzbach A, Rodionov R, O'Sullivan J, Roden M, Birkenfeld A, et al. Are lifestyle therapies effective for NAFLD treatment? Trends Endocrinol Metabol. (2019) 30:701–9. doi: 10.1016/j.tem.2019.07.013
8. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46. doi: 10.1016/j.jhep.2017.05.016
9. Cho Y, Hong N, Kim K, Cho S, Lee M, Lee Y, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. (2019) 8:101645. doi: 10.3390/jcm8101645
10. Harris L, Hamilton S, Azevedo L, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database Systemat Rev Implementat Rep. (2018) 16:507–47. doi: 10.11124/JBISRIR-2016-003248
11. Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. (2014) 13:38–45. doi: 10.1016/j.arr.2013.11.002
12. Aliasghari F, Izadi A, Gargari B, Ebrahimi S. The effects of ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am Coll Nutr. (2017) 36:640–5. doi: 10.1080/07315724.2017.1339644
13. Cai H, Qin Y, Shi Z, Chen J, Zeng M, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. (2019) 19:219. doi: 10.1186/s12876-019-1132-8
14. Ebrahimi S, Gargari B, Aliasghari F, Asjodi F, Izadi A. Ramadan fasting improves liver function and total cholesterol in patients with nonalcoholic fatty liver disease. Int J Vitamin and Nutr Res. (2020) 90:95–102. doi: 10.1024/0300-9831/a000442
15. Johari M, Yusoff K, Haron J, Nadarajan C, Ibrahim K, Wong M, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep. (2019) 9:11232. doi: 10.1038/s41598-019-47763-8
16. Mari A, Khoury T, Baker M, Said Ahmad H, Abu Baker F, Mahamid M. The impact of ramadan fasting on fatty liver disease severity: a retrospective case control study from Israel. Israel Med Assoc J. (2021) 23:94–8.
17. Rahimi H, Habibi ME, Gharavinia A, Emami Mh, Baghaei A, Tavakol N. Effect of ramadan fasting on alanine transferase (ALT) in nonalcoholic fatty liver disease (NAFLD). J Nutr Fast Health. (2017) 5:107–12. doi: 10.22038/jfh.2017.24588.1089
18. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systemat Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
19. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019). doi: 10.1002/9781119536604
20. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig. (2004) 114:1752–61. doi: 10.1172/JCI21625
21. Johnson A, Olefsky J. The origins and drivers of insulin resistance. Cell. (2013) 152:673–84. doi: 10.1016/j.cell.2013.01.041
22. Tarantino G, Citro V, Capone D. Nonalcoholic fatty liver disease: a challenge from mechanisms to therapy. J Clin Med. (2019) 9:10015. doi: 10.3390/jcm9010015
23. Miyazaki M, Flowers M, Sampath H, Chu K, Otzelberger C, Liu X, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metabol. (2007) 6:484–96. doi: 10.1016/j.cmet.2007.10.014
24. Dias G, Murphy T, Stangl D, Ahmet S, Morisse B, Nix A, et al. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene Klotho. Mol Psychiatry. (2021) 21:1102. doi: 10.1038/s41380-021-01102-4
25. Connolly J, Romano T, Patruno M. Selections from current literature: effects of dieting and exercise on resting metabolic rate and implications for weight management. Family Practice. (1999) 16:196–201. doi: 10.1093/fampra/16.2.196
26. Longo V, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabol. (2016) 23:1048–59. doi: 10.1016/j.cmet.2016.06.001
27. Rothschild J, Hoddy K, Jambazian P, Varady K. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. (2014) 72:308–18. doi: 10.1111/nure.12104
28. Wilson R, Zhang R, Chen Y, Peters K, Sawyez C, Sutherland B, et al. Two-week isocaloric time-restricted feeding decreases liver inflammation without significant weight loss in obese mice with non-alcoholic fatty liver disease. Int J Mol Sci. (2020) 21:239156. doi: 10.3390/ijms21239156
29. Anton S, Moehl K, Donahoo W, Marosi K, Lee S, Mainous A, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. (2018) 26:254–68. doi: 10.1002/oby.22065
30. Patterson R, Sears D. Metabolic effects of intermittent fasting. Ann Rev Nutr. (2017) 37:371–93. doi: 10.1146/annurev-nutr-071816-064634
31. Longo V, Mattson M. Fasting: molecular mechanisms and clinical applications. Cell Metabol. (2014) 19:181–92. doi: 10.1016/j.cmet.2013.12.008
32. Su J, Wang Y, Zhang X, Ma M, Xie Z, Pan Q, et al. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am J Clin Nutr. (2021) 113:1332–42. doi: 10.1093/ajcn/nqaa388
Keywords: fasting, intermittent fasting, time restricted feeding, non-alcoholic fatty liver disease, systematic review, meta-analysis
Citation: Yin C, Li ZH, Xiang YL, Peng HB, Yang P, Yuan SJ, Zhang XY, Wu Y, Huang M and Li J (2021) Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front. Nutr. 8:709683. doi: 10.3389/fnut.2021.709683
Received: 14 May 2021; Accepted: 11 June 2021;
Published: 12 July 2021.
Edited by:
Nicholas T. Bello, Rutgers, The State University of New Jersey, United StatesReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyCopyright © 2021 Yin, Li, Xiang, Peng, Yang, Yuan, Zhang, Wu, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, bHoxOTgyMDdAMTI2LmNvbQ==; Min Huang, aHVhbmdtMDgwNkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.