
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr. , 07 September 2021
Sec. Nutritional Immunology
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.667188
This article is part of the Research Topic Insights of Gut Microbiota: Probiotics and Bioactive Compounds View all 21 articles
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in preterm infants. The exact mechanism by which NEC develops is poorly understood however there is growing evidence to suggest that perturbations in the early-life gut microbiota composition increase the risk for NEC. Modulation of the gut microbiota with probiotics, prebiotics, or in combination (synbiotics) is an area which has attracted intense interest in recent years. In this narrative review, we present an overview of the role of the gut microbiota in the pathogenesis of NEC. We also examine the evidence currently available from randomized controlled trials, observational studies, systematic reviews, and meta-analysis examining the role of probiotics, prebiotics, and synbiotics in reducing the risk of or preventing NEC. Current clinical practice guidelines with recommendations on the routine administration of probiotics to preterm infants for NEC are also explored.
The early life gut microbiome is a dynamic community of microorganisms that play an important role in infant health. Factors influencing the development of the infant gut microbiota include mode of delivery (caesarean section vs. vaginal birth), gestational age (premature vs. full-term birth), antibiotic use, mode of feeding (formula vs. breastfeeding), and environmental factors (1, 2). Bifidobacterium typically dominate the microbiota in vaginally delivered, breastfed infants. Infants delivered by caesarean section are characterised by reduced Bacteroides and Bifidobacterium and increased colonization by opportunistic pathogens such as Enterococcus, Enterobacter, Clostridium, and Klebsiella species (1–5). Disrupted microbiota acquisition during this critical developmental window may have both short and long-term health implications. Imbalances in the composition of the gut microbiota have been associated with a wide range of diseases including allergic disorders, type 1 diabetes, inflammatory bowel disease, obesity, sepsis, and necrotizing enterocolitis (NEC) (6–10).
With our growing understanding of the role of the microbiome in health and disease, the use of probiotics to promote a healthy microbiome is an active area of research. Probiotics are defined by the FAO/WHO as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (11). Probiotics may positively contribute to host health by modulating immune responses such as inflammation, improving the function of the intestinal mucosal barrier, modulating the expression of host genes, and preventing colonization by pathogenic bacteria. One of the mechanisms through which probiotics influence a range of health parameters is through the production of bioactive compounds. Vitamins, antimicrobial peptides, conjugated linoleic acid (CLA), exopolysaccharides, gamma aminobutyric acid (GABA), and short-chain fatty acids (SCFAs) are all examples of microbially produced bioactive compounds (12). SCFAs including acetate, propionate, and butyrate are crucial for gut health and can modulate metabolic activity including colonocyte function, gut homeostasis, and the immune system (13). While CLA has immunomodulating properties, reducing the proinflammatory cytokines (14).
Prebiotics are defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (15). Prebiotics specifically stimulate the growth of beneficial microbes including bifidobacteria and lactobacilli. Prebiotics are naturally found in human milk (HM), which contains over 200 human milk oligosaccharides (HMOs) (16). HMOs can increase the proportion of HMO-consuming bifidobacteria and Bacteroides in breast-fed infants. Infant formula are now often supplemented with prebiotics and probiotics to mimic the functional effects of HMOs and HM bacteria (17). A synbiotic is defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (18). A synbiotic may be classified as complementary or synergistic. In a complementary synbiotic the probiotic and prebiotic provide a health benefit together but are not co-dependent. In synergistic synbiotics, the prebiotic is chosen based on its ability to be selectively utilized by the probiotic.
One particular area that has produced positive results in probiotic intervention studies is the prevention of NEC in preterm infants (19). NEC is a serious acquired disease of the gastrointestinal tract, characterized by acute intestinal necrosis. The incidence rate of NEC is reported as approximately 5–10% of very preterm or very low birth weight (VLBW) infants (20, 21). A 2020 systematic review reported that seven out of 100 VLBW infants in the neonatal intensive care unit (NICU) are likely to develop NEC (22). The mortality rate is reported at 20–30%, and infants who survive NEC have a greater risk of neurodevelopmental delays (23). Preterm infants represent a particularly vulnerable group especially those weighing <1,500 g, VLBW infants, and <1,000 g, extremely low birth weight (ELBW).
The pathogenesis of NEC is complex and the exact etiology remains unknown; however, immaturity of the intestinal barrier and immune system are thought to contribute (24). The intestinal microbiome is also believed to contribute to the pathogenesis of NEC. Experiments using animal models have shown that NEC does not occur in germ-free mice and toll-like receptor targeted knockout mice strongly suggesting that the gut microbiome is critical for NEC development (25–28). Studies using 16S rRNA gene sequencing have reported a reduction in microbial community diversity, decreases in Firmicutes, and an increase in Proteobacteria in the stool of NEC patients (29–31). Proteobacteria contain numerous gram-negative pathogens with high levels of lipopolysaccharide (LPS). TLR4 recognizes LPS and TLR4 activation leads to inhibition of mucosal repair. Breakdown of the gut barrier and translocation of pathogenic bacteria leads to an increased inflammatory response, resulting in NEC (32). Patients with NEC have been reported to have higher levels of LPS in their plasma (25). In addition, intraperitoneal injection of LPS to rats and mice has been demonstrated to induce intestinal injury and shock (33). Several studies have linked colonization by clostridia with NEC and pointed toward a potential deleterious role in the pathogenesis of NEC (34, 35). The exact mechanism is unclear but it is thought that lactose fermentation leading to an overproduction of butyric acid and the presence of toxin genes may play a role (36, 37).
Olm et al. performed metagenomic analysis of faecal samples from premature infants to identify microbial features predictive of NEC (38). Samples collected prior to NEC onset contained significantly higher Klebsiella, bacteria encoding fimbriae, and secondary metabolite gene clusters related to bacteriocin production and quorum sensing. Bacterial replication rates were measured from metagenomic data by determining the difference in DNA sequencing coverage between the origin and the terminus of replication. Replication rates, particularly that of Enterobacteriaceae, were significantly higher two days prior to NEC diagnosis. Microbiome analysis of faecal samples may not accurately represent the bacterial communities at the site of injury, the intestinal mucosa. A study by Romano-Keeler et al. examined the microbiome in both NEC tissue and faecal samples in surgical patients with and without NEC (39). The authors reported a tissue-specific overrepresentation of Firmicutes, specifically Staphylococcus and Clostridium and a lower abundance of Actinomyces and Corynebacterium in NEC.
EPIPAGE 2, a prospective cohort study in France, assessed nutritional strategies and the gut microbiota as risk factors for NEC (40). Slower rates of progression of enteral feeding and less favorable direct-breastfeeding policies were associated with a higher risk of NEC. An association between Clostridium neonatale and Staphylococcus aureus with NEC was also noted. Interestingly, no relation between antibiotic treatment and the onset of NEC was observed. This is in contrast to several studies which have reported that early antibiotic use in preterm infants increases the risk of NEC (41–43). Acid-suppressive medications such as histamine-2 receptor antagonists and proton pump inhibitors (PPIs) are routinely used for the treatment of upper gastrointestinal bleeding or gastroesophageal reflux in preterm infants. Exposure to these acid-suppressive medicines has been associated with an increased risk of NEC (44, 45). Changes in gut microbiota composition related to PPI therapy have been well-documented (46, 47). Feeding with HM provides beneficial bacteria and essential prebiotic substances including non-digestible HMOs, immunoprotective IgA, and lactoferrin, and has been reported to reduce the risk of development of NEC (48–50). HM may also protect against NEC through the presence of epidermal growth factor, which attenuates TLR4 signaling via activation of the phosphoinositide 3-K signaling pathway (51).
Due to the role of the intestinal microbiome in the pathogenesis of NEC, dietary supplementation with probiotics to modulate the intestinal microbiome has been proposed as a strategy to reduce the risk of NEC and associated morbidity and mortality. An overview of the characteristics of randomized controlled trials (RCTs) evaluating probiotics, prebiotics, and synbiotics for NEC are shown in Table 1. Of the thirty-four RCTs evaluating probiotics for NEC, seventeen reported significant beneficial effects, eleven reported no health benefit, and six reported a trend to prevent NEC. The Probiotics in Preterm Infants (PiPs) trial, the largest trial to date of a probiotic intervention, assessed the effectiveness of Bifidobacterium breve BBG-001 to reduce NEC, late onset sepsis (LOS), and death in 1,315 preterm infants in the UK (60). The trial did not find a significant reduction in NEC and the authors did not recommend the routine use of probiotics in this population. An important limitation to note of this trial was the high rates of cross-colonization in the placebo group which may have confounded the results. The ProPrems RCT compared daily administration of a probiotic mixture containing Bifidobacterium infantis, Streptococcus thermophilus, and Bifidobacterium lactis with placebo in 1,099 very preterm infants (70). Infants receiving the probiotic mixture had a significantly lower incidence of NEC (stage 2 or greater) compared to control infants. The 2015 ProPre-Save RCT evaluated the efficacy of probiotic alone, prebiotic alone, or combined (synbiotic), on the prevention of NEC in 400 VLBW infants (88). Infants were randomized to either a control group or one of three study groups. The study groups were administered probiotic (B. lactis), prebiotic (inulin), or synbiotic (B. lactis plus inulin) for up to eight weeks. The probiotic and synbiotic groups had a lower incidence of NEC compared to the prebiotic and control groups. The study groups had reduced mortality, reduced nosocomial sepsis, faster time to reach full enteral feeding, and shorter NICU duration compared to the control group. Another large RCT randomly assigned 750 preterm infants to receive Lactobacillus reuteri DSM 17938 or placebo (76). Here, a non-significant 40% decrease in NEC was reported in the probiotic group compared with control group.
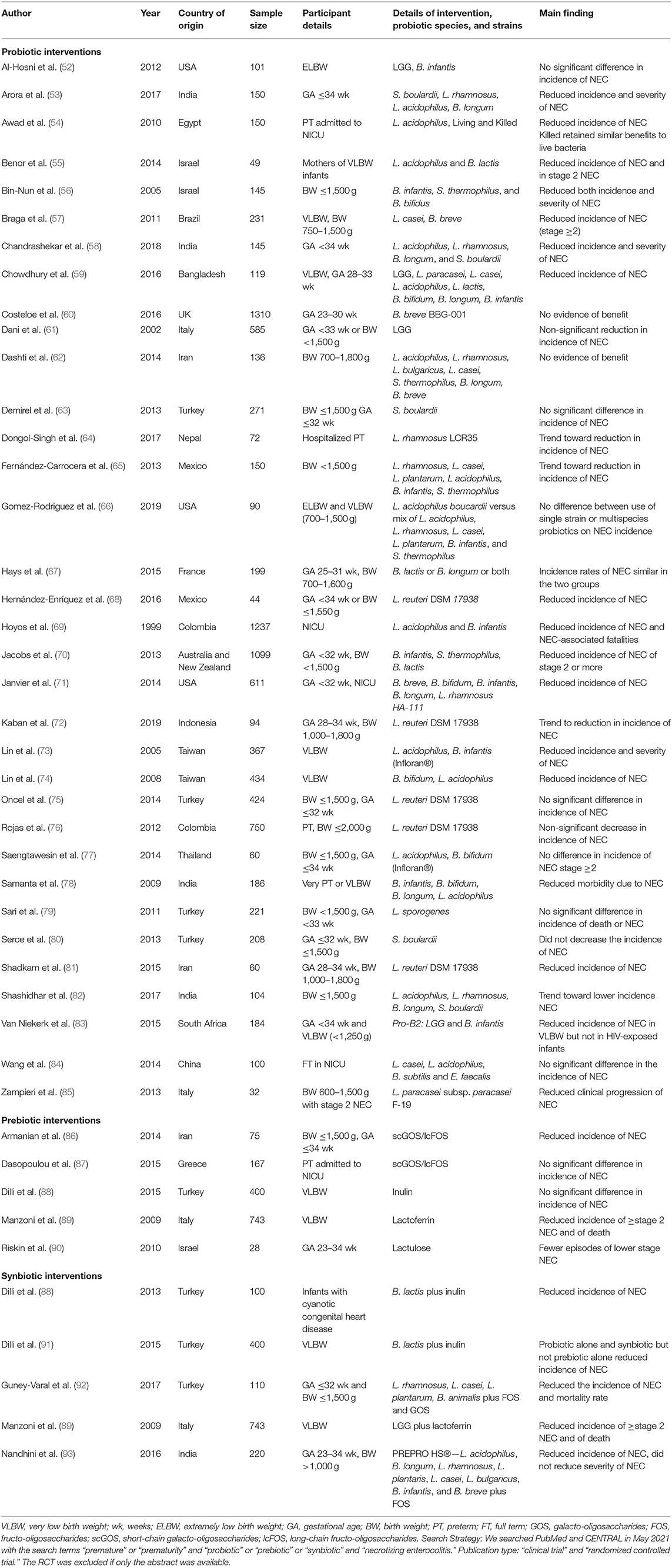
Table 1. Characteristics of randomized controlled trials evaluating probiotics, prebiotics, and synbiotics for NEC.
A 2016 retrospective multi-center study examined data from 10,890 preterm infants from 44 NICUs in Germany with routine use of a dual strain probiotic (Infloran™, Lactobacillus acidophilus and B. infantis) (94). Infloran administration significantly reduced the incidence of NEC, mortality after NEC, overall mortality, and nosocomial bloodstream infection. Subgroup analysis in ELBW infants revealed that these effects were even more pronounced in these infants. Gray et al. performed a multi-center cohort study of 78,076 preterm infants from 289 NICUs in the United States from 1997 to 2016 (95). The most commonly administered probiotic was Lactobacillus (71%), followed by Ultimate Flora (Bifidobacterium and Lactobacillus), ABC Dophilus (Bifidobacterium, Lactobacillus, and Streptococcus), and Align (Bifidobacterium). Probiotic administration increased over time and was associated with a decrease in the incidence of NEC and death. In contrast to other studies reporting that probiotics reduce Candida colonization, an increase in Candida infection was observed here. The authors state that confirmatory reports are required to determine if the findings are clinically significant. Probiotic use was not associated with an increase in bloodstream infection or meningitis. Concerning the safety of probiotics in preterm infants, their use has very rarely been associated with deleterious side effects such as bacterial sepsis due to probiotic translocation (61, 96, 97). The cost benefits ratio is very much in favor of probiotics considering the data from the numerous preterm infants who have receive such supplementation (98). In Canada, a 2019 retrospective cohort study evaluated the effect of probiotic administration on extremely preterm infants (<29 weeks gestational age) admitted to NICU (99). 3093 infants were included in the analysis with 652 infants receiving probiotic preparations, either Florababy (B. breve, Bifidobacterium bifidum, B. infantis, Bifidobacterium longum, and Lactobacillus rhamnosus GG) or Biogaia (L. reuteri). Probiotic use was associated with a significant reduction in the rate of NEC and mortality but not in the rate of LOS.
A 2020 Cochrane review of 56 RCTs (n = 10,812) compared probiotic supplementation with placebo in very preterm or VLBW infants (100). This review reported that probiotics may reduce the risk of NEC and probably reduces mortality for very preterm or VLBW infants. The evidence for this was assessed as low certainty due to weaknesses in trial design particularly with regards measures used to blind clinicians and caregivers to the intervention. Small-study bias was also a concern with most of the included trials small in size (median n = 149). Heterogeneity of the probiotic interventions used in RCTs was reported by the authors as the main challenge in applying the findings of the review. Additionally, the authors noted that few trials provided data for extremely preterm or ELBW infants.
A 2020 systematic review and network meta-analysis (NMA) analyzed data from 63 RCTs (n = 15,712) to assess the effectiveness of various single-strain and multi-strain probiotics for the prevention of NEC mortality and morbidity (101). High-certainty evidence indicated that combinations of Bifidobacterium and Lactobacillus were most effective for the prevention of mortality and stage 2 NEC. Moderate-certainty evidence suggested that B. lactis, L. rhamnosus, and L. reuteri prevent stage 2 NEC. Moderate-certainty evidence also indicated that B. lactis and L. reuteri reduced hospital stay. Low-certainty evidence suggested that combinations of Bacillus and Enterococcus; Lactobacillus, Bifidobacterium, and Enterococcus; and Bifidobacterium and S. thermophilus may prevent stage 2 NEC. Important limitations as noted by the authors were the lack of available data comparing the effects of different probiotic strains with each other and the lack of strain level information in many of the trials. Most recently in 2021, a NMA of 51 RCTs (n = 10,664) examined the role of probiotics in preventing NEC (102). L. acidophilus LB was ranked as the best supplementation option for reducing NEC risk in preterm infants. The NMA also included a subgroup analysis of type of feeding; exclusively HM fed vs. formula fed (alone or combination with HM). The administration of B. lactis Bb-12/B94 was associated with a reduced risk of NEC stage ≥2 in exclusively HM-fed infants and non-exclusively HM-fed infants. The relative size effect favored exclusively HM-fed infants.
Chi et al. also employed a NMA approach based on 45 RCTs (n = 12,320) to compare probiotic, prebiotic, and synbiotics for premature infants (103). The RCTs included strains of Bifidobacterium, Lactobacillus, Enterococcus, Streptococcus, Bacillus, and Saccharomyces, alone and in combination. Supplementation with Bifidobacterium plus Lactobacillus was associated with lower rates of morbidity and mortality in NEC. Lactobacillus plus prebiotic was associated with lower rates of NEC morbidity and had the highest probability of having the lowest rate of NEC. Bifidobacterium plus prebiotic had the highest probability of having the lowest rate of mortality. The authors found that the efficacy of single strain supplements was limited and recommended the use of synbiotics particularly those including both Bifidobacterium and Lactobacillus. A limitation of this NMA was the insufficient data available for extremely preterm or ELBW infants. A 2018 meta-analysis used 18 RCTs (n = 1,322) to evaluate whether prebiotics alone could reduce the incidence of sepsis, NEC, and mortality in preterm infants (104). Participants who received prebiotics showed significant decreases in the incidence of sepsis and mortality; however, there was no significant differences between intervention and control groups in relation to the morbidity rate of NEC.
In 2020, the European Society of Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) published a position paper aiming to provide recommendations relating to the use of probiotics in preterm infants (105). A conditional recommendation for the use of L. rhamnosus GG ATCC 53103 or the combination of B. lactis BB-12, B. infantis BB-02, and S. thermophilus TH-4 was made. It was advised that strains should be selected based on proven effectiveness and an established safety profile. With regard dosage, similar doses as administered in relevant RCTs were recommended. Due to limitations in currently available data, an optimal start of treatment or total duration was not indicated. The paper highlights that probiotics are typically marketed as nutritional supplements and as a result are loosely regulated. Product safety and quality is therefore of concern especially in a vulnerable population such as preterm infants with immature immune systems. ESPGHAN recommended more stringent controls and that probiotic strains be manufactured according to current Good Manufacturing Practice (cGMP) to ensure strain identity, purity, and viability. The probiotic strains should not include any plasmids containing transferable antibiotic resistance genes and local microbiologists must have the ability to routinely detect probiotic sepsis. The panel also recommended against the use of probiotic strains that produce d-lactate, as their potential risks are uncertain. Also in 2020, the American Gastroenterological Association (AGA) published their clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders (106). The guidelines conditionally recommended probiotics for the prevention of NEC in preterm infants <37 weeks gestational age and low birth weight. The AGA reported that specific probiotics can prevent mortality and severe NEC (stage 2 or greater), reduce days required to reach full feeds, and decrease the duration of hospitalization. The committee identified significant heterogeneity between studies, variability in the strains studied, and a lack of consistent harms reporting as significant knowledge gaps.
Most recently, a 2021 clinical report by the American Academy of Pediatrics (AAP) recommended against the routine administration of probiotics to preterm infants, particularly those whose birth weight is <1,000 g, for the treatment or prevention of NEC (107). The AAP highlights that probiotic products in the US are classified as dietary supplements and are not subject to approval by the US Food and Drug Administration (FDA). As a result, manufacturers can bypass FDA safety, efficacy, and manufacturing standards. The AAP notes that despite the inconsistent data on their safety and efficacy, probiotics are increasingly given to preterm infants in the US with approximately 10% of extremely low gestational age infants receiving a probiotic preparation while in the NICU. The academy advises that centres using probiotics obtain informed consent from parents after discussing the risks and benefits. They also recommended that centres should conduct surveillance to assess the impact of probiotics on the centres microbiota, which could potentially affect all infants, and should carefully document adverse events, outcomes, and safety.
There is mounting evidence supporting the use of probiotics to decrease the risk of NEC in preterm infants. Several large RCTs have demonstrated that the relative risk for NEC can be reduced using probiotic formulations. It is important to note that some meta-analyses have reported low to moderate level of certainty about the effects of probiotic supplementation on the risk of NEC and the largest RCT to date found no reduction in NEC incidence following supplementation with a single-strain probiotic. A confounding factor in this RCT was the high rates of cross-colonization found in the placebo group. In addition, not all probiotics used in preventing NEC may be equally effective. Therefore, further carefully designed and conducted large-scale RCTs are necessary to determine optimal strains as well as optimal timing and dosing. Furthermore, detailed information about the study population needs to be included such as type of feeding, antibiotic usage, gender, and ethnicity. Data on the particularly vulnerable extremely preterm infants and ELBW infants is limited and more RCTs focused specifically on these groups are needed. Prebiotic and synbiotic interventions are scarcely investigated in RCTs to date and further trials evaluating their efficacy are required. There are conflicting recommendations from experts as to the administration of probiotics to preterm infants for NEC. Concerns about the safety and purity of commercially available probiotics appears to be the greatest hurdle to overcome in terms of the widespread implementation of probiotics in NICU. Many probiotic products are sold as dietary supplements and are not produced under strict quality control conditions. Probiotics which are licensed as a drug by national regulatory authorities should be recommended.
KM: conceptualization, original draft preparation, review, and editing. RPR and CS: conceptualization, supervision, review, and editing. CAR and EMD: review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Science Foundation Ireland grant number SFI/12/RC/2273-P2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–5. doi: 10.1073/pnas.1002601107
2. Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. (2015) 3:17. doi: 10.3389/fped.2015.00017
3. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562:583–8. doi: 10.1038/s41586-018-0617-x
4. Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O'Shea CA, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET cohort. Microbiome. (2017) 5:4. doi: 10.1186/s40168-016-0213-y
5. Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574:117–21. doi: 10.1038/s41586-019-1560-1
6. Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. (2015) 26:26050. doi: 10.3402/mehd.v26.26050
7. Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. (2017) 81:e00036–17. doi: 10.1128/MMBR.00036-17
8. Neu J, Pammi M. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin Perinatol. (2017) 41:29–35. doi: 10.1053/j.semperi.2016.09.015
9. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. (2020) 51:102590. doi: 10.1016/j.ebiom.2019.11.051
10. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of gut microbiota in Inflammatory Bowel Disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. (2019) 8:126. doi: 10.3390/pathogens8030126
11. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
12. Indira M, Venkateswarulu TC, Abraham Peele K, Nazneen Bobby M, Krupanidhi S. Bioactive molecules of probiotic bacteria and their mechanism of action: a review. 3 Biotech. (2019) 9:306. doi: 10.1007/s13205-019-1841-2
13. Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. (2019) 33:13546–59. doi: 10.1096/fj.201901433R
14. Kim JH, Kim Y, Kim YJ, Park Y. Conjugated linoleic acid: potential health benefits as a functional food ingredient. Annu Rev Food Sci Technol. (2016) 7:221–44. doi: 10.1146/annurev-food-041715-033028
15. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
16. Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. (2011) 108(Suppl 1):4653–8. doi: 10.1073/pnas.1000083107
17. Salminen S, Stahl B, Vinderola G, Szajewska H. Infant formula supplemented with biotics: current knowledge and future perspectives. Nutrients. (2020) 12:1952. doi: 10.3390/nu12071952
18. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. (2020) 17:687–701. doi: 10.1038/s41575-020-0344-2
19. Deshmukh M, Patole S. Current status of probiotics for preterm infants. Indian J Pediatr. (2021) 88:703–8. doi: 10.1007/s12098-021-03736-2
20. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364:255–64. doi: 10.1056/NEJMra1005408
21. Rich BS, Dolgin SE. Necrotizing Enterocolitis. Pediatr Rev. (2017) 38:552–9. doi: 10.1542/pir.2017-0002
22. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing Enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20:344. doi: 10.1186/s12887-020-02231-5
23. Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23:426–32. doi: 10.1016/j.siny.2018.08.005
24. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13:590–600. doi: 10.1038/nrgastro.2016.119
25. Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res. (2011) 69:183–8. doi: 10.1203/PDR.0b013e3182093280
26. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. (2012) 143:708.e5–18.e5. doi: 10.1053/j.gastro.2012.05.053
27. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. (2007) 179:4808–20. doi: 10.4049/jimmunol.179.7.4808
28. Jilling T, Simon D, Lu J, Meng FJ Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. (2006) 177:3273–82. doi: 10.4049/jimmunol.177.5.3273
29. Neu J. Necrotizing enterocolitis: a multi-omic approach and the role of the microbiome. Dig Dis Sci. (2020) 65:789–96. doi: 10.1007/s10620-020-06104-w
30. Denning NL, Prince JM. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol Med. (2018) 24:4. doi: 10.1186/s10020-018-0002-0
31. Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. (2017) 5:31. doi: 10.1186/s40168-017-0248-8
32. Hackam DJ, Sodhi CP. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol. (2018) 6:229.e1–38.e1. doi: 10.1016/j.jcmgh.2018.04.001
33. Yan X, Managlia E, Tan XD, De Plaen IG. Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. (2019) 317:G57–66. doi: 10.1152/ajpgi.00332.2018
34. Butel MJ, Aires J. Editorial Commentary: Neonatal necrotizing enterocolitis: a clostridial disease? Clin Infect Dis. (2015) 61:1116–8. doi: 10.1093/cid/civ469
35. Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect. (2016) 22:37–45. doi: 10.1016/j.cmi.2015.10.014
36. Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, et al. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin Infect Dis. (2015) 61:1107–15. doi: 10.1093/cid/civ468
37. Waligora-Dupriet AJ, Dugay A, Auzeil N, Huerre M, Butel MJ. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res. (2005) 58:629–35. doi: 10.1203/01.PDR.0000180538.13142.84
38. Olm MR, Bhattacharya N, Crits-Christoph A, Firek BA, Baker R, Song YS, et al. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci Adv. (2019) 5:eaax5727. doi: 10.1126/sciadv.aax5727
39. Romano-Keeler J, Shilts MH, Tovchigrechko A, Wang C, Brucker RM, Moore DJ, et al. Distinct mucosal microbial communities in infants with surgical necrotizing enterocolitis correlate with age and antibiotic exposure. PLoS ONE. (2018) 13:e0206366. doi: 10.1371/journal.pone.0206366
40. Roze JC, Ancel PY, Lepage P, Martin-Marchand L, Al Nabhani Z, Delannoy J, et al. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr. (2017) 106:821–30. doi: 10.3945/ajcn.117.152967
41. Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. (2014) 165:23–9. doi: 10.1016/j.jpeds.2014.01.010
42. Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. (2009) 123:58–66. doi: 10.1542/peds.2007-3423
43. Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. (2011) 159:392–7. doi: 10.1016/j.jpeds.2011.02.035
44. Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. (2006) 117:e137–42. doi: 10.1542/peds.2005-1543
45. Patole S. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis: a case of excessive collateral damage? Pediatrics. (2006) 117:531–2. doi: 10.1542/peds.2005-2230
46. Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. (2019) 25:2706–19. doi: 10.3748/wjg.v25.i22.2706
47. Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med. (2014) 34:771–85. doi: 10.1016/j.cll.2014.08.008
48. Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. (2009) 29:57–62. doi: 10.1038/jp.2008.117
49. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. (2019) 7:CD002971. doi: 10.1002/14651858.CD002971.pub3
50. Patel AL, Panagos PG, Silvestri JM. Reducing incidence of necrotizing enterocolitis. Clin Perinatol. (2017) 44:683–700. doi: 10.1016/j.clp.2017.05.004
51. Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. (2015) 8:1166–79. doi: 10.1038/mi.2015.30
52. Al-Hosni M, Duenas M, Hawk M, Stewart LA, Borghese RA, Cahoon M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol. (2012) 32:253–9. doi: 10.1038/jp.2011.51
53. Arora S, Khurana MS, Saini R. To study the role of probiotics in the prevention of necrotizing enterocolitis in preterm neonates. Int J Contemp Pediatr. (2017) 4. doi: 10.18203/2349-3291.ijcp20173787
54. Awad H, Mokhtar H, Imam SS, Gad GI, Hafez H, Aboushady N. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pak J Biol Sci. (2010) 13:253–62. doi: 10.3923/pjbs.2010.253.262
55. Benor S, Marom R, Ben Tov A, Armoni Domany K, Zaidenberg-Israeli G, Dollberg S. Probiotic supplementation in mothers of very low birth weight infants. Am J Perinatol. (2014) 31:497–504. doi: 10.1055/s-0033-1353490
56. Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. (2005) 147:192–6. doi: 10.1016/j.jpeds.2005.03.054
57. Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. (2011) 93:81–6. doi: 10.3945/ajcn.2010.29799
58. Chandrashekar GS, Shettigar S, Varghese TC. Role of probiotics in prevention of necrotizing enterocolitis in preterm neonates. Indian J Child Health. (2018) 5:112–5.
59. Chowdhury T, Ali MM, Hossain MM, Singh J, Yousuf AN, Yasmin F, et al. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak. (2016) 26:770–4.
60. Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Probiotics in Preterm Infants Study Collaborative Group. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. (2016) 387:649–60. doi: 10.1016/S0140-6736(15)01027-2
61. Dani C, Coviello CC, Corsini II, Arena F, Antonelli A, Rossolini GM. Lactobacillus sepsis and probiotic therapy in newborns: two new cases and literature review. AJP Rep. (2016) 6:e25–9. doi: 10.1055/s-0035-1566312
62. Dashti AS, Afjeh SA, Basiry A, Shirvani F, Seifi K, Taheri ZM. Prophylactic probiotics for prevention of necrotizing enterocolitis (NEC) in low birth weight neonates. Arch Pediatr Infect Dis. (2013) 2:174–9.
63. Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr. (2013) 102:e560–5. doi: 10.1111/apa.12416
64. Dongol Singh SS, Klobassa DS, Resch B, Urlesberger B, Shrestha RP. Placebo controlled introduction of prophylactic supplementation of probiotics to decrease the incidence of necrotizing enterocolitis at Dhulikhel Hospital in Nepal. Kathmandu Univ Med J (KUMJ). (2017) 15:319–23.
65. Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, Gallardo-Sarmiento RB, Garcia-Perez CS, Montano-Rodriguez R, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. (2013) 98:F5–9. doi: 10.1136/archdischild-2011-300435
66. Gomez-Rodriguez G, Amador-Licona N, Daza-Benitez L, Barbosa-Sabanero G, Carballo-Magdaleno D, Aguilar-Padilla R, et al. Single strain versus multispecies probiotic on necrotizing enterocolitis and faecal IgA levels in very low birth weight preterm neonates: A randomized clinical trial. Pediatr Neonatol. (2019) 60:564–9. doi: 10.1016/j.pedneo.2019.02.005
67. Hays S, Jacquot A, Gauthier H, Kempf C, Beissel A, Pidoux O, et al. Probiotics and growth in preterm infants: a randomized controlled trial, PREMAPRO study. Clin Nutr. (2016) 35:802–11. doi: 10.1016/j.clnu.2015.06.006
68. Hernández-Enríquez NP, Rosas-Sumano AB, Monzoy-Ventre MA, Galicia-Flores L. Lactobacillus reuteri DSM 17938 in preventing necrotizing enterocolitis in preterm newborns. Pilot study of efficacy and safety. Rev Mex Pediatr. (2016) 83:37–43.
69. Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. (1999) 3:197–202. doi: 10.1016/s1201-9712(99)90024-3
70. Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. (2013) 132:1055–62. doi: 10.1542/peds.2013-1339
71. Janvier A, Malo J, Barrington KJ. Cohort study of probiotics in a North American neonatal intensive care unit. J Pediatr. (2014) 164:980–5. doi: 10.1016/j.jpeds.2013.11.025
72. Kaban RK, Wardhana, Hegar B, Rohsiswatmo R, Handryastuti S, Amelia N, et al. Lactobacillus reuteri DSM 17938 improves feeding intolerance in preterm infants. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:545–53. doi: 10.5223/pghn.2019.22.6.545
73. Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. (2005) 115:1–4. doi: 10.1542/peds.2004-1463
74. Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. (2008) 122:693–700. doi: 10.1542/peds.2007-3007
75. Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F110–5. doi: 10.1136/archdischild-2013-304745
76. Rojas MA, Lozano JM, Rojas MX, Rodriguez VA, Rondon MA, Bastidas JA, et al. Prophylactic probiotics to prevent death and nosocomial infection in preterm infants. Pediatrics. (2012) 130:e1113–20. doi: 10.1542/peds.2011-3584
77. Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai. (2014) 97(Suppl 6):S20–5.
78. Samanta M, Sarkar M, Ghosh P, Ghosh J, Sinha M, Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr. (2009) 55:128–31. doi: 10.1093/tropej/fmn091
79. Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr. (2011) 65:434–9. doi: 10.1038/ejcn.2010.278
80. Serce O, Benzer D, Gursoy T, Karatekin G, Ovali F. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev. (2013) 89:1033–6. doi: 10.1016/j.earlhumdev.2013.08.013
81. Shadkam NM, Jalalizadeh F, Nasiriani K. Effects of probiotic lactobacillus reuteri (DSM 17938) on the incidence of necrotizing enterocolitis in very low birth weight premature infants. Iran J Neonatol (IJN). (2015) 6:15–20.
82. Shashidhar A, Suman Rao PN, Nesargi S, Bhat S, Chandrakala BS. Probiotics for promoting feed tolerance in very low birth weight neonates - a randomized controlled trial. Indian Pediatr. (2017) 54:363–7. doi: 10.1007/s13312-017-1106-2
83. Van Niekerk E, Nel DG, Blaauw R, Kirsten GF. Probiotics Reduce necrotizing enterocolitis severity in HIV-exposed premature infants. J Trop Pediatr. (2015) 61:155–64. doi: 10.1093/tropej/fmv004
84. Wang Y, Gao L, Zhang YH, Shi CS, Ren CM. Efficacy of probiotic therapy in full-term infants with critical illness. Asia Pac J Clin Nutr. (2014) 23:575–80. doi: 10.6133/apjcn.2014.23.4.14
85. Zampieri N, Pietrobelli A, Biban P, Soffiati M, Dall'agnola A, Camoglio FS. Lactobacillus paracasei subsp. paracasei F19 in Bell's stage 2 of necrotizing enterocolitis. Minerva Pediatr. (2013) 65:353–60.
86. Armanian AM, Sadeghnia A, Hoseinzadeh M, Mirlohi M, Feizi A, Salehimehr N, et al. The Effect of Neutral Oligosaccharides on Reducing the Incidence of Necrotizing Enterocolitis in Preterm infants: A Randomized Clinical Trial. Int J Prev Med. (2014) 5:1387–95.
87. Dasopoulou M, Briana DD, Boutsikou T, Karakasidou E, Roma E, Costalos C, et al. Motilin and gastrin secretion and lipid profile in preterm neonates following prebiotics supplementation: a double-blind randomized controlled study. JPEN J Parenter Enteral Nutr. (2015) 39:359–68. doi: 10.1177/0148607113510182
88. Dilli D, Aydin B, Fettah ND, Ozyazici E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. (2015) 166:545.e1–51.e1. doi: 10.1016/j.jpeds.2014.12.004
89. Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev. (2014) 90(Suppl 1):S60–5. doi: 10.1016/S0378-3782(14)70020-9
90. Riskin A, Hochwald O, Bader D, Srugo I, Naftali G, Kugelman A, et al. The effects of lactulose supplementation to enteral feedings in premature infants: a pilot study. J Pediatr. (2010) 156:209–14. doi: 10.1016/j.jpeds.2009.09.006
91. Dilli D, Aydin B, Zenciroglu A, Ozyazici E, Beken S, Okumus N. Treatment outcomes of infants with cyanotic congenital heart disease treated with synbiotics. Pediatrics. (2013) 132:e932–8. doi: 10.1542/peds.2013-1262
92. Guney-Varal I, Koksal N, Ozkan H, Bagci O, Dogan P. The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: A randomized controlled trial in a tertiary care unit. Turk J Pediatr. (2017) 59:13–9. doi: 10.24953/turkjped.2017.01.003
93. Nandhini LP, Biswal N, Adhisivam B, Mandal J, Bhat BV, Mathai B. Synbiotics for decreasing incidence of necrotizing enterocolitis among preterm neonates - a randomized controlled trial. J Matern Fetal Neonatal Med. (2016) 29:821–5. doi: 10.3109/14767058.2015.1019854
94. Denkel LA, Schwab F, Garten L, Geffers C, Gastmeier P, Piening B. Protective effect of dual-strain probiotics in preterm infants: a multi-center time series analysis. PLoS ONE. (2016) 11:e0158136. doi: 10.1371/journal.pone.0158136
95. Gray KD, Messina JA, Cortina C, Owens T, Fowler M, Foster M, et al. Probiotic use and safety in the neonatal intensive care unit: a matched cohort study. J Pediatr. (2020) 222:59.e1–64.e1. doi: 10.1016/j.jpeds.2020.03.051
96. Singh B, Shah PS, Afifi J, Simpson CD, Mitra S, Dow K, et al. Probiotics for preterm infants: a national retrospective cohort study. J Perinatol. (2019) 39:533–9. doi: 10.1038/s41372-019-0315-z
97. Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Stoen R, Klingenberg C. Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerg Infect Dis. (2016) 22:1664–6. doi: 10.3201/eid2209.160033
98. Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F217–8. doi: 10.1136/archdischild-2011-300838
99. Athalye-Jape G, Patole S. Probiotics for preterm infants - time to end all controversies. Microb Biotechnol. (2019) 12:249–53. doi: 10.1111/1751-7915.13357
100. Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. (2020) 10:CD005496. doi: 10.1002/14651858.CD005496.pub5
101. Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, McMaster Probiotic P, et al. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. (2020) 159:467–80. doi: 10.1053/j.gastro.2020.05.096
102. Beghetti I, Panizza D, Lenzi J, Gori D, Martini S, Corvaglia L, et al. Probiotics for preventing necrotizing enterocolitis in preterm infants: a network meta-analysis. Nutrients. (2021) 13:192. doi: 10.3390/nu13010192
103. Chi C, Li C, Buys N, Wang W, Yin C, Sun J. Effects of probiotics in preterm infants: a network meta-analysis. Pediatrics. (2021) 147:e20200706. doi: 10.1542/peds.2020-0706
104. Chi C, Buys N, Li C, Sun J, Yin C. Effects of prebiotics on sepsis, necrotizing enterocolitis, mortality, feeding intolerance, time to full enteral feeding, length of hospital stay, and stool frequency in preterm infants: a meta-analysis. Eur J Clin Nutr. (2019) 73:657–70. doi: 10.1038/s41430-018-0377-6
105. van den Akker CHP, van Goudoever JB, Shamir R, Domellof M, Embleton ND, Hojsak I, et al. Probiotics and preterm infants: a position paper by the european society for paediatric gastroenterology hepatology and nutrition committee on nutrition and the european society for paediatric gastroenterology hepatology and nutrition working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr. (2020) 70:664–80. doi: 10.1097/MPG.0000000000002655
106. Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. (2020) 159:697–705. doi: 10.1053/j.gastro.2020.05.059
Keywords: microbiome, prebiotic, probiotic, synbiotic, necrotizing enterocolitis
Citation: Murphy K, Ross RP, Ryan CA, Dempsey EM and Stanton C (2021) Probiotics, Prebiotics, and Synbiotics for the Prevention of Necrotizing Enterocolitis. Front. Nutr. 8:667188. doi: 10.3389/fnut.2021.667188
Received: 18 February 2021; Accepted: 09 August 2021;
Published: 07 September 2021.
Edited by:
Ana Griselda Binetti, CONICET Instituto de Lactología Industrial (INLAIN), ArgentinaReviewed by:
Maria Guadalupe Vizoso Pinto, CONICET Higher Institute of Biological Research (INSIBIO), ArgentinaCopyright © 2021 Murphy, Ross, Ryan, Dempsey and Stanton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Stanton, Y2F0aGVyaW5lLnN0YW50b25AdGVhZ2FzYy5pZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.