- 1Department of Nutrition and Food Technology, Faculty of Agriculture, Jordan University of Science and Technology, Irbid, Jordan
- 2Department of Animal Production, Faculty of Agriculture, Jordan University of Science and Technology, Irbid, Jordan
- 3Department of Clinical Nutrition and Dietetics, Faculty of Allied Health Science, The Hashemite University, Zarqa, Jordan
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder triggered by several factors, including those of genetic and environmental nature. ASD can alter communication, behavior, and children's nutritional status, placing them at high risk for nutritional imbalances. Therefore, this study aims to assess preschool autistic children's nutritional status as compared to that of Typically Developing (TD) children of the same age. The study also revealed some of the ASD risk factors among the Jordanian population. It included 52 ASD and 51 TD children (3–6 years), and considered sociodemographic, obstetric, and nutritional factors of the two groups, stratified by gender. Nutritional status was evaluated through a comprehensive questionnaire, 3-day food record, and anthropometric and biochemical measurements. Differences between groups were identified using the chi-square and independent-sample t-test. The logistic regression model was used after the adjustment of confounders to detect an autistic child's determinants. The study showed little difference between ASD and TD children with respect to nutrients' intake inadequacy and biochemical-nutritional deficiencies, but did reveal gender-based differences. Autistic girls were at higher risk of inadequate carbohydrate intake, while autistic boys were at higher risk of inadequate vitamin E, vitamin K, and fluoride compared to TD children. More autistic children had been treated in neonatal care units after birth than had TD children. The regression analysis revealed that lower maternal education level (OR, 12.25; 95% CI, 1.18–126.91), vaginal delivery (OR, 0.273; 95% CI, 0.105–0.712), family history of autism (OR, 0.189; 95% CI, 0.059–0.612), and taking dietary supplements during pregnancy (OR, 4.665; 95% CI, 1.158–18.79) were all determinants of ASD in children. In conclusion, maternal nutrition, postnatal conditions, and nutritional status might be contributors to ASD in children. Pre-school children are at high risk for developing nutritional deficiencies. It is therefore important to maintain optimal nutritional status in pregnant patients, and in children after delivery and during early childhood. Future studies that investigate the role of nutrient deficiencies and nutritional interventions in ASD are necessary. Also required are studies that focus on gender differences in the prevalence of ASD, types and severity of symptoms, and ASD nutrition-related problems.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by lack of social and emotional interactions (1), repetitive behaviors, and restricted verbal and non-verbal linguistic skills (2). During the past several years, the number of ASD cases has increased, with a prevalence of 62 per 10,000 and a higher occurrence in boys compared to girls (ratio of 5:1) (3, 4). Autism is not well-understood and its occurrence is attributed to several environmental (5), genetic (6), neurological, gastrointestinal, and immunological factors (7). Many ASD children suffer from multiple nutrition-related issues such as maldigestion, malabsorption (8), abnormal fatty acid and amino acid metabolism, or multiple food intolerances (9). Also, ASD children display eating problems, food selectivity and increased refusal of new food items, which could place them at greater risk for nutritional excess or/and deficiencies (10, 11). Inadequacies of several nutrients were reported in ASD children were reported (12). As such, a comprehensive array of symptoms in the digestive tract in ASD children suggest the need for introducing nutritional interventions that could decreasing some ASD symptoms that may be associated with imbalanced nutrient intake and/or utilization (13–15).
Nutrition assessment is the first step in identifying nutrition-related problems and, thus, implementing a nutritional intervention to restore healthy nutritional status (16, 17). Data from the nutritional assessment domains showed differences in the levels of some nutrients and antioxidants when comparing ASD children to TD (Typically Developing) children from several countries (11, 18–21). Differences in the measured parameters suggest association with several factors, including health conditions present in the ASD children, nutrition care provided by the caregivers, and/or population-based factor (22–24). In addition, it is critical to evaluate nutritional status and identify children's nutrition-related problems early in life as it can have long-term impact on their health if left uncorrected (25). Therefore, this study evaluated the nutritional status of pre-school-aged ASD children using nutritional assessment domains (anthropometric measurements, dietary intake of macro-micronutrients, and the levels of common biochemical parameters) and compared them to that of TD children of the same age. These domains were compared according to gender. This study also identifies demonstrated some of ASD risk factors common in the Jordanian population.
Materials and Methods
Study Design and Participants
This case-control study of ASD children compared to their TD counterparts was conducted in Amman (Jordan), between July 2018 and February 2019. The study population included pre-school children aged 3–6 years and their mothers. The caregivers/parents signed the consent of participation with detailed information about the study's purpose, all measurements, and laboratory tests. Children were included in the study if they were previously diagnosed with ASD. Children with severe health issues and illnesses or those with a confirmed intake of supplements (vitamins and minerals) in the last 2 months were excluded from the study. The investigator visited 19 special needs centers in Amman, where most of these centers were at the time of the study. Fourteen centers agreed to take part in the study, however, after conducting thorough interviews with the centers' directors, only 8 centers had ASD children meeting the study inclusion criteria. The investigator met with the caregivers of 69 ASD children from these centers; 14 did not meet the inclusion criteria and 3 refused to participate in the study with a response rate of 94.6%. The number of ASD girls treated in the special needs centers was low. The maximum realizable number of ASD girls participated in this study. The final sample included 52 ASD cases (37 boys and 15 girls). For TD children, 51 children were recruited from two kindergartens in Amman (26 boys and 25 girls). Parents met with the principal investigator to complete questionnaires that gathered information about sociodemographics, maternal history, pregnancy and delivery, and child health and well-being, particularly surrounding their eating habits. Additional questions addressed the child's main ASD characteristics and symptoms. The study protocol was approved by the Institutional Review Board at Jordan University of Science and Technology (JUST) (Approval # 57/118/2018).
Anthropometric Measurements
Weight and height were measured using an electronic scale attached to a height-measuring rod (AutoSike EBS-300RT digital scale with stadiometer, China). Weight was measured to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm according to a standard procedure (26). The BMI was calculated using Quetelet's formula [(weight (kg)/height2 (m2)] (27). Children's height, weight, and BMI were subsequently plotted on their CDC clinical growth charts (children ages 2–20 years) (28). Children were classified as being obese (≥95th percentile), overweight (>85th and <95th percentile), normal (>5th to <85th percentiles), and underweight (<5th Percentile) according to CDC criteria (29). The z-scores of weight-for-age (WAZ), height-for-age (HAZ), and BMI-for-Age (BAZ) were calculated and classified according to the WHO criteria: Stunted with HAZ <-2, Underweight with WAZ <-2, Wasted with BAZ <-2, At risk of overweight, overweight and obese with BAZ >1, >2, and >3, respectively (30).
Dietary Intake
The dietary intake of children was assessed using a 3-days food record (2 weekdays and 1 weekend) which is considered the method of choice for assessing nutrient intake among autistic children (31, 32). Through interviews, the dietitian used standard household measuring tools and food models to educate the parents about the portion size and help them estimate the children's consumption. The dietician analyzed dietary intake using a Computerized Nutrient Analysis Program “ESHA software” (version 10.63). Several Jordanian dishes and local foods were not registered in the ESHA database. Nutrient data for these items were derived from the food composition tables related to local foods in Jordan (3) and the Middle East (4), and were inserted into the database. Nutritional adequacy was determined by comparing the estimated intake with the Acceptable Macronutrient Distribution Range (AMDR) and Dietary Reference Intakes (DRIs), including recommended dietary allowances (RDAs) and adequate intakes (AIs) (33). The total energy requirement (TEE) for each child was calculated based on the WHO recommendations according to age, weight, height, gender and activity level (34). Reference values for macronutrients and micronutrients for children between 1 and 3 years and children between 4 and 6 years were drawn from the dietary guidelines for Americans (35). In this study, children's intake of a nutrient was considered inadequate if the mean intake was <67% of the RDA/AI (36–38).
Biochemical Parameters Determination
Blood samples (5 ml) were collected from each participant in EDTA blood tubes and transferred to the JUST laboratory for subsequent analysis. Red Blood Cell (RBCs) and White Blood Cell (WBCs) counts were determined by the Coulter method (39). Hemoglobin (Hgb) levels were measured by the photometric method (40). Ferritin and 25-hydroxyl vitamin D were measured by an immunoassay analyzer (Access 2) using the Access 2 ferritin kit and the Access 2 25(OH) Vitamin D kit (Beckman Coulter, Brea, CA, USA, RRID:SCR_008940). For calcium determination, a photometric color test was done using clinical chemistry analyzer (Beckman Coulter AU analyzer, USA, RRID: SCR_008940). Alkaline Phosphatase (ALP) was evaluated using the kinetic change in color test as described by the International Federation for Clinical Chemistry (41). For analyzing inorganic phosphorous, a photometric UV test was used (42). The measured values for ASD and TD children were compared with the reference ranges obtained from the analytical laboratory at Jordan University of Science and Technology health center.
Data Analysis
Data was analyzed using the Statistical Package for Social Sciences “SPSS software” (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp, RRID: SCR_019096). Continuous variables were described using mean and standard deviation, and categorical variables were described using percentage. Chi-square (χ2) was performed to test the differences between ASD and TD children. An independent-samples t-test was performed to compare the mean and standard deviation of continuous variables between the two groups. Logistic regression models were used to detect determinants of having an autistic child by taking the TD children as the reference category. For each variable, the number of non-missing values are used. We specify the missing = listwise sub-command to exclude data if there is a missing value for any variable in the list. So, by default, missing values are excluded, and percentages are based on the number of non-missing values. Correlations are computed based on the number of pairs with non-missing data. For regression analysis, if values of any of the variables included in the analysis equation are missing, the entire case is excluded from the analysis. Multivariate-adjusted ORs with 95% CIs were estimated as increased risk for having an autistic child (OR > 1). Findings with a p < 0.05 were considered statistically significant.
Results
Participants' Characteristics
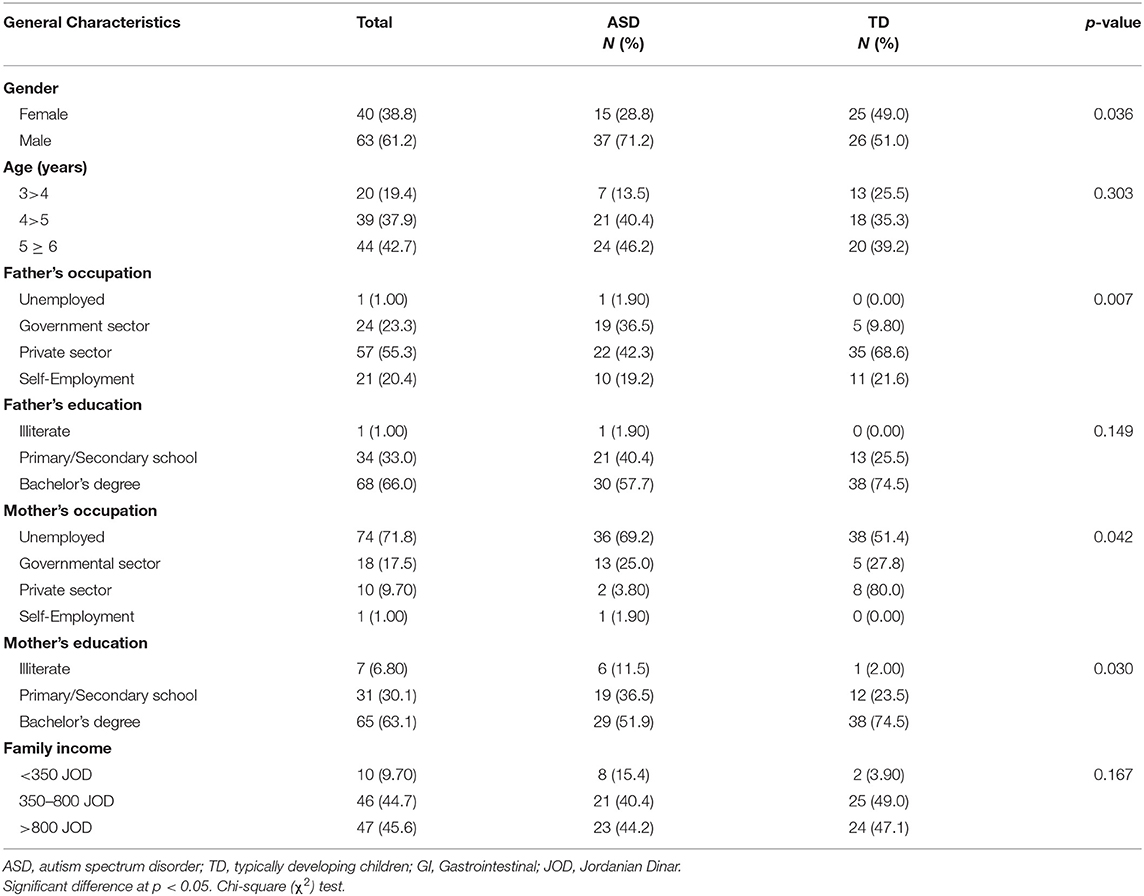
The final sample included 103 ASD and TD children with a mean age of (4.40 ± 0.80) and (4.18 ± 0.81), respectively (Data is not shown in tables). The general characteristics of the sample are shown in Table 1. A higher percentage of children were from male gender (61.2%), aged ≥5 years (42.7%), their family's average monthly income is >800 JD (45.6%). A significant difference shown between ASD and TD children; male gender is the dominant in ASD group (71.2%, P =0.036). The two groups differ significantly in their fathers' (P = 0.007) and mothers' (P = 0.042) occupation, with more ASD parents either unemployed or working in the governmental sector. ASD parents had a lower education level compared to TD parents. However, the difference between the groups was significant only for the mother's education level (P = 0.030).
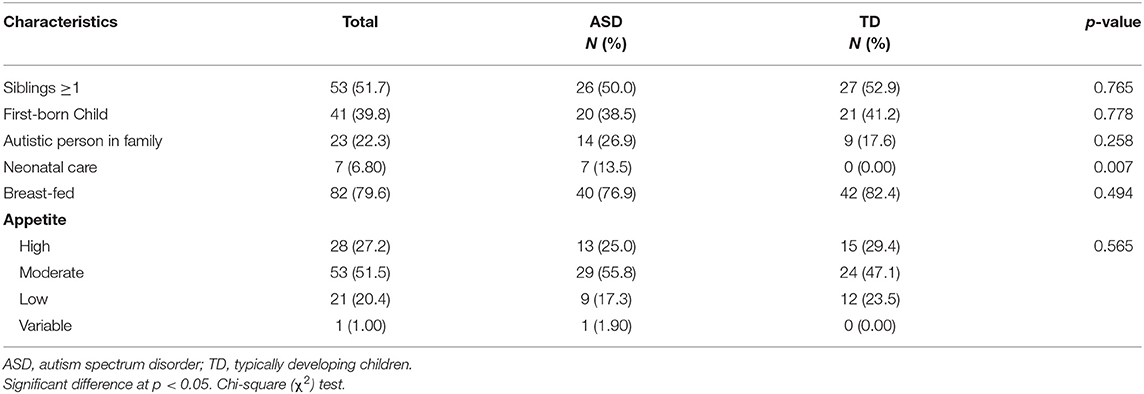
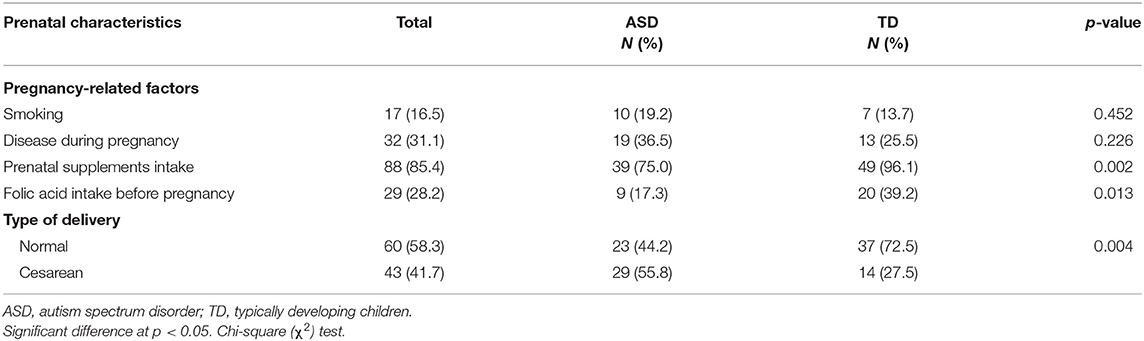
A comparison of some postnatal characteristics between ASD and TD children are summarized in Table 2. These characteristics included the family history of autism, neonatal care, breast feeding, if the child has ≥1 sibling or if he/she was the first child in his/her family, as well as his/her general appetite. These variables were analyzed and the difference between ASD and TD children was studied. Children's characteristics did not vary significantly between the two groups except for neonatal care; 13.5% of ASD children spent time in the neonatal unit, while none of the TD children did (P = 0.007). Some pregnancy-related factors are presented in Table 3; the results indicated that more ASD children were born through cesarean section compared to TD children (55.8 vs. 27.5%, P = 0.004). TD mothers were more committed to the intake of prenatal supplements (96.1 vs. 75.0%, P = 0.002) and folic acid (39.2 vs. 17.3%, P = 0.013) compared to ASD mothers.
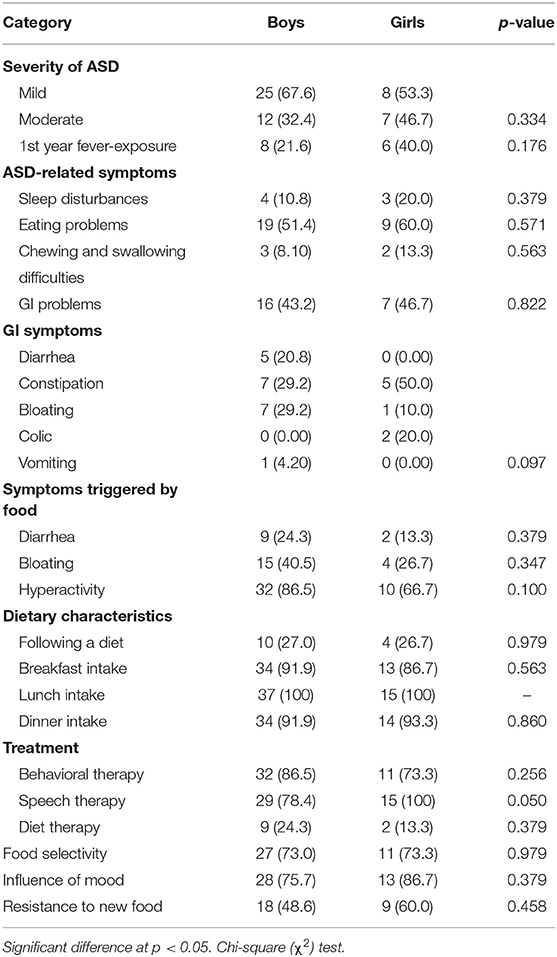
There were gender differences among ASD groups, however, these differences did not vary significantly for all the characteristics and symptoms except for speech therapy as a treatment type (Table 4). All ASD girls were treated by speech therapy compared to 78.4% of ASD boys (P = 0.050), and more boys received diet therapy compared to girls (24.3 vs. 13.3%, P = 0.379). The severity of ASD was more mild in boys (67.6%), and more moderate in girls (46.7%). Also, the ASD girls experienced fever more frequently during their first year of life. For ASD symptoms, girls (86.7%) were more affected by their mood than boys (75.7%), and the presence of ASD-related symptoms such as sleep disturbances, eating problems, and chewing difficulties which were also higher in girls compared to boys. Some Gastrointestinal (GI) symptoms were more prevalent in ASD boys, such as diarrhea, bloating, and vomiting. Also, ASD-related symptoms, including diarrhea, bloating, and hyperactivity, were more likely to be triggered by food in ASD boys compared to ASD girls. The results revealed that ASD girls (60.0%) resist new foods more than do ASD boys (48.6%).
Anthropometric Measurements
The mean values for weight-for-age, stature-for-age, BMI-for-age, and z-scores are shown in Table 5. The results showed no significant differences in these parameters between ASD and TD groups. The mean values for these parameters were within the normal ranges. However, the average means for WAZ and HAZ were lower in TD boys compared to ASD boys, which may put more TD boys at risk of underweight and stunting than ASD boys. For BAZ, ASD boys had a higher mean, putting more of them at risk of overweight, compared to TD boys. On the other, ASD girls had lower means for WAZ, HAZ, and BAZ than TD girls. The findings from this study may point toward higher risk of undernutrition for ASD girls compared to ASD boys such as underweight (WAZ: −0.350 ± 1.68 and −0.023 ±1.57, respectively), and stunting (HAZ: −0.620 ± 1.36 and −0.135 ± 1.73, respectively).
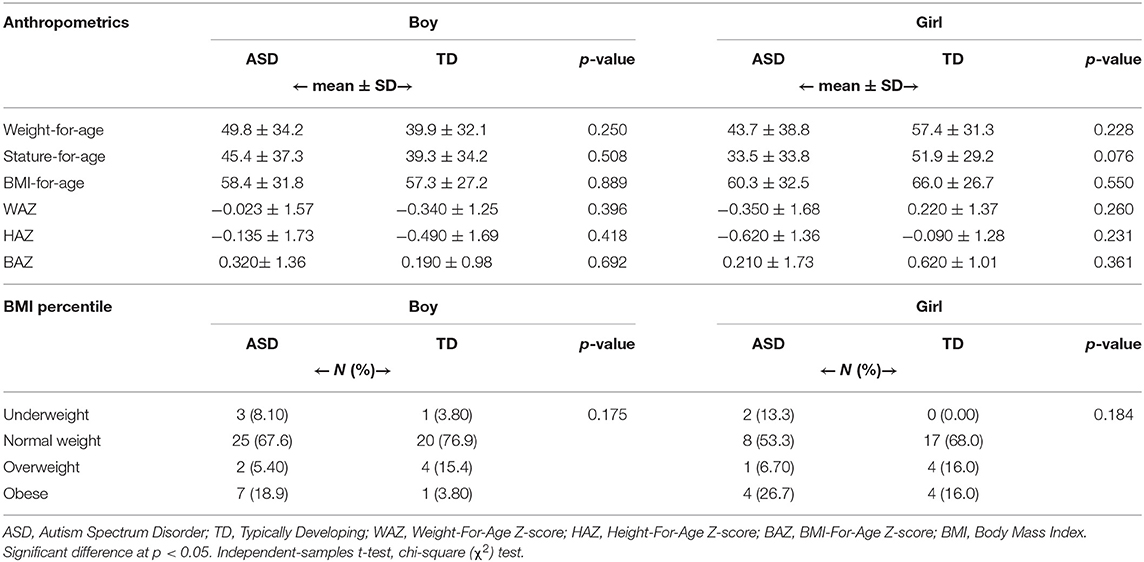
Table 5. Anthropometric data: percentiles of weight-for-age, height-for-age, BMI-for-age, and Z-scores of ASD and TD children stratified by gender.
For BMI classifications, insignificant differences indicated between both ASD and TD groups in each gender category (P = 0.175 for boys and P = 0.184 for girls) as presented in Table 5. The prevalence of underweight was higher among ASD boys and girls (8.10 and 13.3%) compared to TD boys and girls (3.80 and 0.00%, respectively). Further, more ASD children were obese (18.9% in boys and 26.7% in girls) compared to TD children (3.80% in boys and 16.0% in girls). Both underweight and obesity were higher in ASD girls compared to ASD boys.
Dietary Intake
Food records were collected to assess the nutritional status and identify dietary factors associated with autism incidence among our study population. A comparison of the inadequate daily energy and macronutrient intake between ASD and TD children is presented in Table 6. The 3-day food record showed that ASD girls had a significantly higher inadequacy from carbohydrate intake than TD girls (26.7 vs. 4.0%, P = 0.036). Other macronutrients intake did not vary significantly between the two groups. Nonetheless, more ASD boys did not meet the requirements for the daily energy and protein intake (27.8 and 19.4%, respectively) compared to TD boys (19.2 and 11.5%, respectively). The inadequate intake of carbohydrates and fats was seen in ASD girls more than ASD boys, who showed a higher percentage of inadequate protein intake.
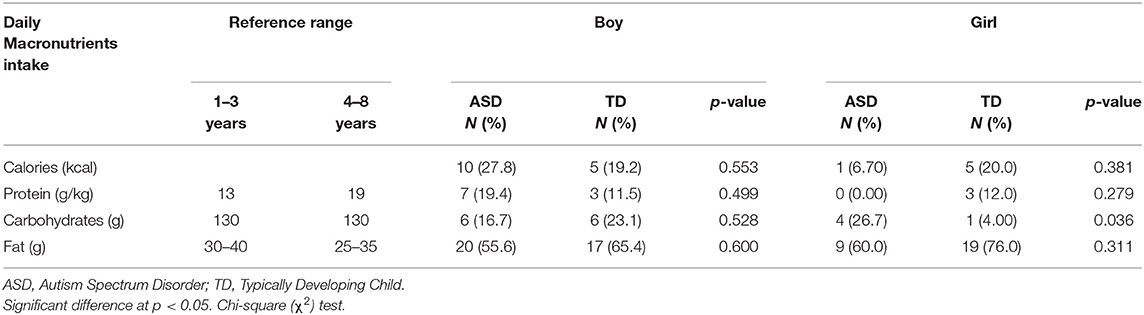
Table 6. Comparison of the inadequate daily energy and macronutrient intake between ASD and TD children stratified by gender.
Micronutrient intake was analyzed to complete the assessment of the children's nutritional status. Gender differences among our study population between both ASD and TD groups were studied for inadequate intake of vitamins (Table 7) and minerals (Table 8). ASD boys exhibit significantly higher percentages of inadequacy of vitamin E (94.4 vs. 73.1%, P = 0.015) and vitamin K (86.1 vs. 61.5%, P = 0.026) than do TD boys. The results showed higher rates of inadequacy of vitamin B2 and vitamin B6 intake among ASD boys compared to TD boys, while more ASD girls failed to meet the daily needs of pantothenic acid, and biotin compared to TD girls. All children, ASD and TD, fell short of the requirements for vitamin A, vitamin D, vitamin B12, and folate.
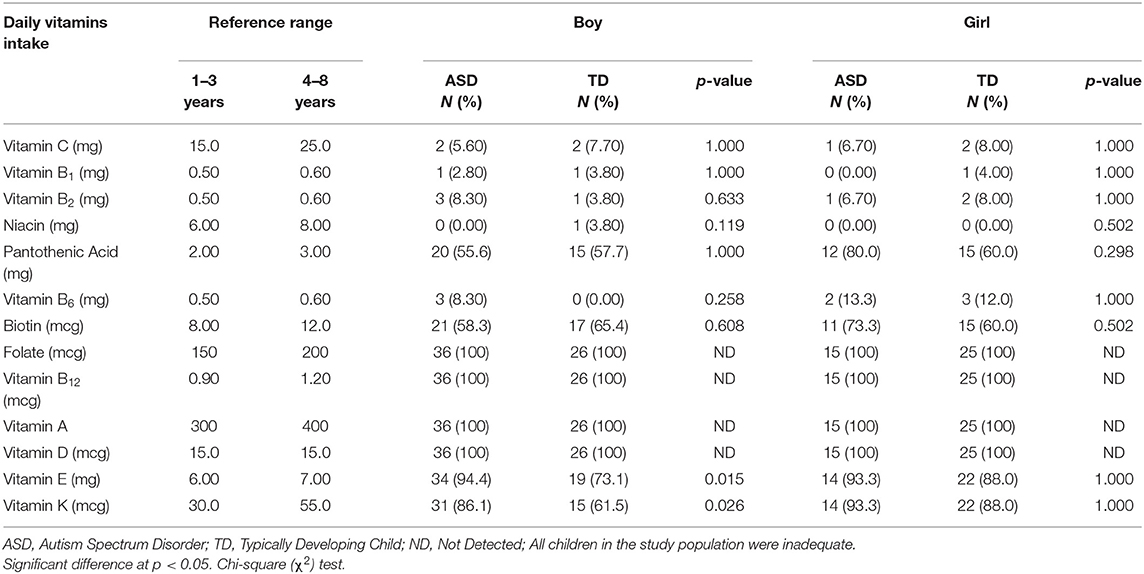
Table 7. Comparison of the inadequate vitamin intake between ASD and TD children stratified by gender.
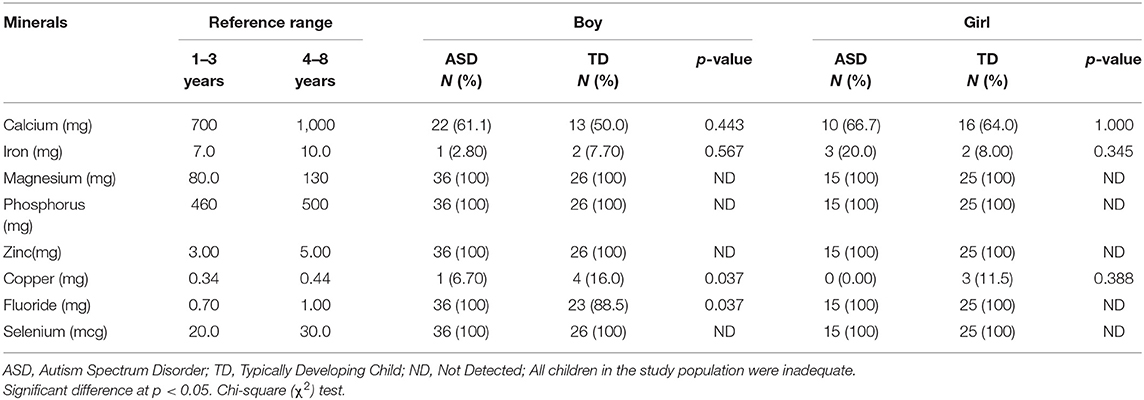
Table 8. Comparison of the inadequate mineral intake between ASD and TD children stratified by gender.
As shown in Table 8, all children failed to meet their requirements from magnesium, phosphorus, zinc, and selenium. More ASD boys (61.1%) and girls (66.7%) failed to meet the daily requirements for calcium compared to TD boys and girls (50.0 and 64.0%). Also, more ASD girls (20.0%) had an inadequate iron intake than did TD girls (8.0%). However, these differences were not statistically significant. Boys showed a significant difference in fluoride; all ASD boys fell short of the fluoride requirements which was significantly higher when compared to TD boys (100 vs. 88.5%, P = 0.037). On the other hand, copper consumption among our study population was nearly better; frequency of inadequate copper intake was low among all groups; it was significantly lower among ASD boys than TD boys (6.70 vs. 16.0%, P = 0.037).
Biochemical Parameters Determination
Biochemical analysis was performed to identify the differences between ASD and TD children. Values for each child were compared to the established reference values for each individual parameter to identify the abnormal results and the frequency of deficiency among the study population. Biochemical and nutrition-related, parameter levels are shown in Table 9 and Supplementary Table 1. CBC analysis showed that ASD boys had a significantly higher deficiency in MCHC (14.3 vs. 0.0%, P = 0.044) and HCT (60.0 vs. 11.5%, P < 0.001) than did TD boys, with a lower mean of serum HCT (36.6 ± 2.89 vs. 38.7 ± 2.03, P = 0.002). ASD girls had a significantly lower deficiency in MCV compared to TD girls (40.0 vs. 72.0%, P = 0.046). More ASD boys were deficient in HCT, MCV, MCH, and MCHC compared to ASD girls, and only ASD boys (5.7%) had a deficiency in HGB when compared to all other groups (0.0%). Children from the two groups had normal RBC levels. Although not significant, the mean RBC level was lower in ASD children than TD children, and lower in ASD girls compared to ASD boys. Whereas, there was no deficiency in RDW-CV in any of the groups, the mean RDW-CV was significantly higher in ASD boys when compared to TD boys (14.0 ± 1.31 vs. 13.1 ± 0.61, P = 0.004). In contrast, the majority of ASD and TD children had a deficiency in MCH; insignificantly higher in ASD girls than TD girls (60 vs. 84%). The deficiency in serum ferritin was more common among ASD boys (71.4%) and girls (46.7%) than in TD boys and girls (50.0 and 20.0%, respectively), but the difference was insignificant between ASD and TD children when not separated by gender. Ferritin deficiency was higher in boys from the two groups than in girls. Children were not deficient in serum levels for phosphorus, ALP, or calcium except for ASD boys, 2.9% of whom were deficient in calcium. Most of the children from the two groups were deficient in vitamin D3; though the difference was not significant vitamin D3 deficiency was more common among the TD boys and girls compared to ASD boys and girls.
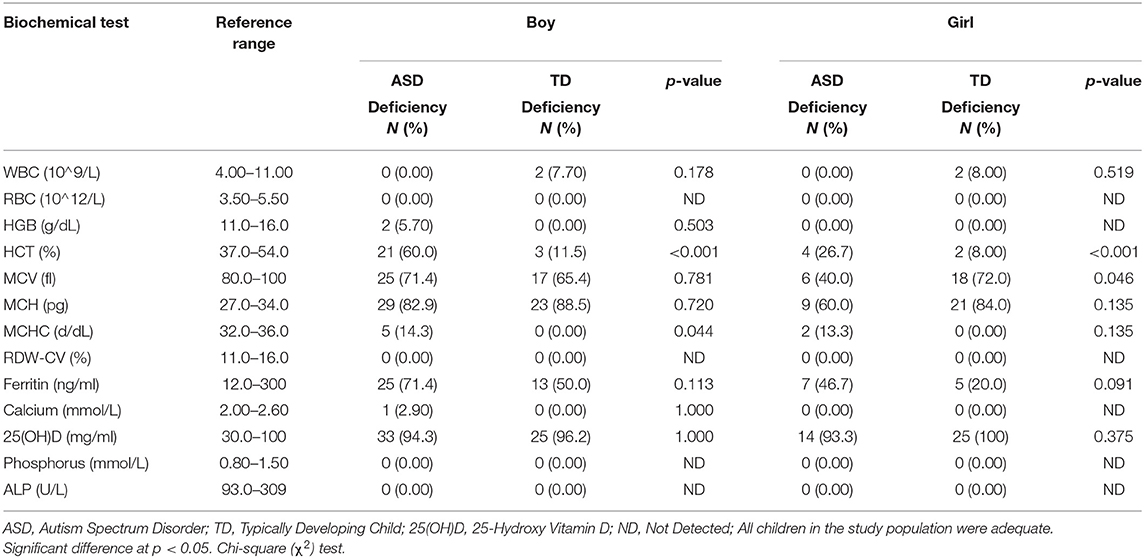
Table 9. Comparison of the biochemical and nutritional deficiencies between ASD and TD children stratified by gender.
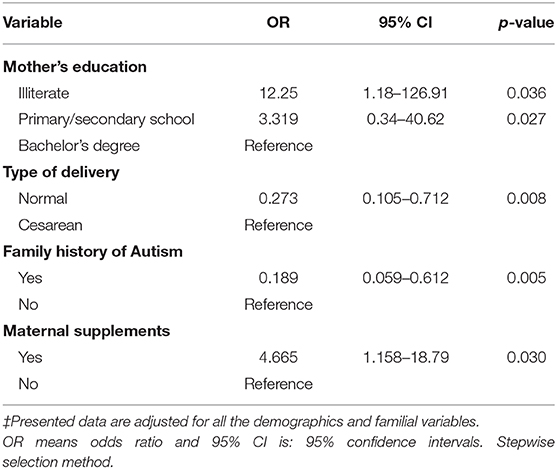
Determinants of Autism Among Pre-school Children
Table 10 shows the adjusted association between some variables and autism presence using the stepwise selection method. Entry testing was based on the significance of the score statistic, and removal testing was based on the probability of a likelihood-ratio statistic based on conditional parameter estimates. Maternal education level, child's delivery method, family history of autism, and taking nutritional supplements during pregnancy were all predictors for having an autistic child. Results showed that illiterate mothers were 12 times more likely to have an autistic child than mothers who attained university education levels (OR = 12.25; CI 95% 1.18, 126.91), while mothers with lower education levels (i.e., primary and secondary school) were 3 times more likely to have an autistic child when compared to mothers with higher education levels (OR = 3.319; CI 95% 0.34, 40.62). Children who were delivered vaginally were less likely to be autistic than children delivered by cesareans births with an odds ratio = 0.273 (CI 95% 0.105, 0.712). Interestingly, families with autism history were less likely to have autistic children than were the families with no autism history, with an odds ratio = 0.189 (CI 95% 0.059, 0.612). Finally, mothers who took nutritional supplements during pregnancy were about 5 times more likely to have an autistic child than those who did not take supplements (OR = 4.665; CI 95% 1.16, 18.79).
Discussion
Several genetic and environmental factors are extensively bound with ASD (43). Our study discusses some environmental and obstetric factors related to ASD among Jordanian children. Similar to previous studies conducted globally, we found a higher prevalence of ASD among boys compared to girls hence the high recruitment of male participants than girls. Male gender might be a determinant for autism. The risk of disease is 4 times higher in genetically susceptible males than females (44). Beggiato et al. (45) reported that girls were more likely to be underidentified by some diagnostic instruments for ASD, and thus, the lower rates of ASD diagnosis compared to boys.
In this study, lower education level among parents correlates with a higher risk of having an autistic child. The results were consistent with a comparative study between ASD and TD children in China (15). In India, lower education level among ASD mothers, but not fathers, was associated with higher ASD incidence in offspring. However, the difference was not significant (46). Conversely, another Chinese study reported higher parental education level for parents in the ASD group than in the TD group (47).
The current study supports findings of previous studies that show being the first child in the family, cesarean delivery (48), and treatment in the neonatal care unit were associated with increased risk of ASD (49). Other factors such as family history, did not statistically differ between the two groups in our study. Contrary to expectation, the final regression analysis of our results showed that familial history of autism, while significant was inversely related to the risk of ASD in the offspring. Our results contradicted previous studies that confirmed higher ASD risk with increased family incidence of ASD (50, 51). Further investigation is merited. This study did not reveal any significant differences between the ASD and TD children who were breastfed, though our study demonstrated a similar trend as that in an Indian study wherein the lack of breastfeeding was significantly higher among ASD children (46). Unexpectedly, Dodds (44) reported that breastfeeding at the time of discharge might be associated with increased ASD risk.
In our study, the mothers of ASD children reported a higher occurrence of disease during pregnancy than mothers of TD children. Although our results were not statistically significant, they align with those of a study in which increased presence during pregnancy of medical conditions such as anemia, heart, pulmonary and renal diseases were associated with an increased risk of ASD in children (44). In line with previous studies (48, 52), smoking during pregnancy was not significantly associated with ASD. However, a higher percent of ASD children were born to smoking mothers in Jordan, which might correlated instead with socioeconomic status (53).
Another obstetric factor relating to decreased risk of autism is the intake of folic acid and other maternal supplements. Levine et al. (50) reported that folic acid and/or multivitamin intake during pregnancy correlates with a lower risk of ASD. The rationale behind this decrease in ASD risk might be the correction of nutritional deficiencies, and folate deficiency in particular. Mothers of ASD children might have serum autoantibodies directed at the folate receptor alpha (FRα) on the placental and the blood-brain barriers, causing an impairment in transferring folate to the fetus. Thus, folic acid supplementation plays a role in reducing ASD severity (54). Calcium supplementation in preparation for pregnancy coincided with a lower risk of ASD among Chinese children (47). Interestingly, the final analysis of our results showed that the mothers who took nutritional supplements during pregnancy were about 5 times more likely to have an autistic child than those who did not take supplements. Raghavan et al. (55) reported that adequate intake of supplements during pregnancy is linked with a decreased risk of ASD. However, both the inadequate intake and excessive intake are related to increased ASD risk in the offspring.
Some ASD-related symptoms observed in this study such as sleep disturbances, mood swings, and GI abnormality agrees with the literature (46). The most common GI abnormalities in our study were constipation, followed by bloating and diarrhea. Similarly, 22.1% of ASD children in Italy suffered from constipation, followed by painful bowel movements and cramps. These GI abnormalities might be associated with an increased risk of sleeping disturbances (56). ASD girls in our study were affected by mood and sleep disturbances more than boys, though the difference was not statistically significant. These results are consistent with previous research that indicates more anxiety, depression, and sleep abnormalities among ASD girls than TD boys (57). Another ASD-related characteristic that was significantly higher among ASD children than TD children (data not shown) is increased food selectivity and resistance to trying new foods. These behaviors were similar to ASD children's behaviors in the United States (58) and China (59). Most ASD children in our study were regularly taking their main meals, as opposed to 80% of ASD children who were skipping their regular meals in a study of Indian children (60).
In our study, there were higher WAZ, HAZ, and BAZ among ASD boys compared to TD boys. In Egypt, similar results were found among ASD children compared to TD children (61). Our study's results showed ASD girls to have lower WAZ, HAZ and BAZ compared to TD girls, which is consistent with the results from a study of ASD children in China (15, 59). Barnhill (21) and Malhi (12) reported no significant differences between ASD and TD children in weight, height, and BMI in the United States and India. Our results showed a higher prevalence of malnutrition, including underweight and overweight, in ASD children compared to TD children. Correspondingly, in Spain, ASD children had a significantly higher prevalence of underweight than TD children (11). Likewise, Egan et al. reported that the prevalence of overweight and obesity among ASD children was higher than population norms (62). Contrary to our study, one study found a lower prevalence of obesity among ASD children than TD children in China (15). Our results might be explained by increased consumption of unhealthy snacks and artificial sweeteners, and the decreased consumption of vegetables among ASD children in Jordan (data from the 3-day food record). Sleep disturbance, irregular eating habits, low physical activity, and psychopharmacologic medicine might also contribute to the observed increase in overweight or obesity in ASD children (63).
Although most children in the current study did meet their daily energy requirements, more ASD boys and TD girls failed to meet the daily energy needs than did other groups. Previous studies from China and the United States reported that ASD children had lower intakes of energy and all macronutrients than TD children (15, 64). We also found that ASD girls failed to meet the requirements for carbohydrate intake more frequently than did TD girls. The same intake pattern was found among children in Spain (11). In Egypt, more ASD children failed to meet the recommendations for protein intake than did TD children (61); we observed this among ASD boys in this study. More TD children in our study did not meet the total fat intake requirements compared with ASD children. Similarly, another study found that more ASD children had excessive fat intake than TD children (11).
Most children's intake from vitamin E and K was below the requirements, it being even lower among ASD children. Previous studies agreed that most children failed to meet vitamin E requirements (21), and that vitamin K intake was inadequate among ASD children (58). But, in Spain, most children had an adequate intake of vitamin E and vitamin K, with ASD children having a higher intake (11). ASD boys in our study had lower intake of vitamin B2 and B6. Our results align with previous studies among ASD children in the United States and Spain (11, 21, 64). ASD girls in our study had a lower intake of biotin, which is consistent with observations of a previous study in Spain (11). Girls also had a lower intake of vitamin B6 In contrast with a previous study that reported a higher intake of vitamin B6 among ASD children (11). All children had an inadequate intake of vitamin A in this study. This inadequacy of vitamin A intake is higher relative to another study, as nearly half of ASD and TD children had inadequate vitamin A intake (15). Other studies showed that ASD children exhibited lower intake of vitamin A than TD children (11, 21). All subjects in our study had inadequate vitamin D intake. The results were consistent with a study in which 2% of ASD children had adequate vitamin D intake compared to 0% among TD children (21). A lower percentage of inadequacy was found among children in Spain, but it was insignificantly higher among TD children (11). Several previous studies pointed to the lower intake of folic acid and vitamin B12 among ASD children (12, 21, 61, 64); however, we found that all children had inadequate intake of these two vitamins.
In this study, we found inadequate mineral intake (including magnesium, phosphorus, zinc, and selenium) in all children, with no difference between ASD and TD children. While there was no difference in the intake of zinc and phosphorus between the two groups in Egypt, magnesium, and selenium intake was lower among the ASD group (61). Conversely, ASD children in Spain had a higher zinc and magnesium intake compared to TD children (11). ASD children in the United States had a lower intake of zinc and a higher intake of selenium, which was inadequate in most children (21). Another study in the United States reported that ASD boys consumed less phosphorus and selenium compared to their healthy counterparts (64). While magnesium inadequacy was shared among all groups (10), it was higher among TD children (58). Our study further showed that children had inadequate fluoride intake similar to Spanish children (11). Our results showed a higher copper inadequacy among TD children, different from the copper intake among Indian children (12). As expected from a previous study (10), our study showed that calcium inadequacy was common among children, with a higher prevalence among ASD than TD children. Previous studies reported a lower intake of calcium and iron among ASD children (21, 61, 64). Neumeyer and colleagues reported that the inadequate intake of calcium, phosphorus, and protein among ASD boys was significantly related to lower bone mineral density (64) that may increase the risk of fractures among ASD children (65). Our study showed a higher inadequacy of iron intake among ASD compared to TD girls, though the result was insignificant. We also found higher inadequacy of iron intake among TD boys compared to ASD boys. In Spain, ASD children also exhibited higher intake of iron compared to TD children (11).
The differences in nutrient intake and nutritional adequacies among ASD and TD children from different countries might be affected by the dietary assessment tool used in the study (58). These differences can also be related to the amount of food intake and limited food choices among ASD children. For example, in India, 79% of ASD children had improper feeding behavior, such as selecting certain food items or having inadequate intake (12). This inadequate intake among children might be correlated with nutritional inadequacies (10). In our study, ASD children consumed few sources of vitamins and minerals, particularly seafood, eggs, and vegetables. A previous study found that the limited consumption of fruits, vegetables, meat, and poultry among ASD children was related to lower intake of B vitamins and micronutrients (60).
As HGB, HCT and MCV are lower among pre-school children, and specifically in ASD children (66), it was essential to assess these levels among ASD children in Jordan. Our results agreed with a previous study that indicated lower HCT among ASD children compared to TD children (66, 67). In our study, there was a minimal HGB deficiency among ASD boys. Similarly, in China only 2.08% of ASD children below age 6 exhibited HGB deficiency, while it was absent among older children (15). In contrast, other studies reported a significantly lower HGB level among ASD as opposed to TD children. They reported lower MCV among ASD children as a group, while this study found this in ASD boys, but not ASD girls (66, 67). Our results indicated that the mean level of WBC was lower among ASD than TD children, and the deficiency was higher among TD children; however, the difference was not significant in our study and previous studies (68, 69). ASD boys had a significantly higher mean for RDW than did TD boys. The results were consistent with a previous study among ASD children in Turkey (68). Other studies have indicated higher levels of RDW among TD children (67, 69). Our study findings showed that ferritin deficiency was higher among ASD children compared to TD children, and higher in ASD boys than ASD girls. These results among our study population were similar to those among children in the United States (67); indicating that the risk of iron deficiency and iron-deficiency anemia among ASD children is higher (20). Low ferritin levels among ASD children might be related to lower intake of dietary iron that results from restricted food choices (56).
In our study, calcium deficiency was present among 2.9% of ASD boys only. This result correspond with observations by Adams (20) whose study reported calcium in the serum of ASD and TD children in the normal range. Likewise, Neumeyer (64) found serum calcium level to be the same among the two groups. However, ASD children in Egypt had a lower serum level of calcium than the TD group (61). Nearly all children in our study presented with vitamin D3 deficiency, serum levels being lower than 30 mg/ml. This is a higher percentage compared to previous studies in the United States and Egypt in which 62 and 57% of children (respectively), had vitamin D deficiency (54, 70). There was no significant difference in the severity of vitamin D3 deficiency between ASD and TD children, but the incidence was higher among the TD girls compared to ASD girls. Conversely, studies reported a lower serum level of vitamin D3 among ASD children compared with TD children (59). Notably, Saad and colleagues reported that the level of vitamin D deficiency is associated with the severity of ASD symptoms (70). Vitamin deficiency among ASD children might be caused by inadequate dietary vitamin D intake due to the picky eating behavior among ASD children (59).
Conclusion
Limitations of our study include the season that blood was drawn (winter, between January and February), affecting serum 25(OH)D levels because of the lack of sunlight exposure. However, vitamin D3 deficiency has become widespread for the rise in urbanized lifestyle and decreased sunshine exposure. And although the 3-day record was favorable as an assessment tool for children's dietary intake, the risk of underestimation or overestimation of certain food items is present. Nonetheless, our study effectively indicated the strong correlation between some environmental factors and the risk of ASD. Mothers' nutritional status during pregnancy and postnatal conditions could be strong contributors to autism spectrum disorder. Hence, maternal nutrition should be considered essential in trying to reduce the risk of ASD. Our assessment of children's nutritional status indicated that malnutrition, including underweight, overweight, obesity, and inadequate nutrient intake, was present in ASD and TD children. Planned intervention is needed to prevent malnutrition and ensure adequate nutrient intake in this age group, aiming to prevent any future developmental issues. The similarity in nutritional deficiencies between ASD and TD children may indicate that nutrition is not the main contributor to ASD. However, it might be part of a complex interplay between genetics, maternal, and environmental factors that leads to ASD among children. Moreover, the correction of these deficiencies might be necessary to reduce the severity of ASD and its related symptoms. We recommend influencing food selectivity and refusal among ASD children by starting with the family's favorite food items and considering the child's sensitivity to texture, smell, and flavor preferences. An individualized diet plan should also be prescribed for each autistic child according to the present ASD-related symptoms. The current study also revealed that ASD males and females might differ in the prevalence of the disease, type and severity of symptoms, and ASD nutrition-related problems. More studies are essential to explore the role of gender as a determinant factor for the severity of ASD and its related syndrome. Also, further studies are still needed to understand better the role of nutrients and other determinants of ASD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board at Jordan University of Science and Technology, Irbid, Jordan. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
HA: study, concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding, administrative, technical support, and study supervision. AA: conducted the study, data collection and interpretation, and drafting of the manuscript. KA: data interpretation and critical revision of the manuscript for important intellectual content. MO: study design, administrative and material support and study supervision. IA-S: statistical analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. All authors agree to be accountable for the content of the work.
Funding
This study was supported by the Deanship of Scientific Research at Jordan University of Science and Technology grant (20180473).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank the children's parents and the managers of the special need centers and kindergarten for their time and efforts given in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.627011/full#supplementary-material
References
1. El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. (2017) 32:1935–41. doi: 10.1007/s11011-017-0088-z
2. Manzi B, Loizzo AL, Giana G, Curatolo P. Autism and metabolic diseases. J Child Neurol. (2008) 23:307–14. doi: 10.1177/0883073807308698
3. Elsabbagh M, Divan G, Koh Y, Kim Y, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. (2012) 5:160–79. doi: 10.1002/aur.239
4. Ranjan S, Nasser J. Nutritional status of individuals with autism spectrum disorders: do we know enough? Adv Nutr. (2015) 6:397–407. doi: 10.3945/an.114.007914
5. Karimi P, Kamali E, Mousavi S, Karahmadi M. Environmental factors influencing the risk of autism. J Res Med Sci. (2017) 22:27. doi: 10.4103/1735-1995.200272
6. Connolly J, Hakonarson H. Etiology of autism spectrum disorder: a genomics perspective. Curr Psychiatry Rep. (2014) 16:501. doi: 10.1007/s11920-014-0501-9
7. Dawson G, Rice C. The complex etiology of autism presents challenges in risk communication. Pediatrics. (2016) 137:e20152703. doi: 10.1542/peds.2015-2703
8. Sassano A, Katsoulidis E, Antico G, Altman J, Redig A, Minucci S, et al. Suppressive effects of statins on acute promyelocytic leukemia cells. Cancer Res. (2007) 67:4524–32. doi: 10.1158/0008-5472.CAN-06-3686
9. Xu G, Snetselaar L, Jing J, Liu B, Strathearn L, Bao W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw Open. (2018) 1:e180279. doi: 10.1001/jamanetworkopen.2018.0279
10. Tanoue K, Minami T, Syou N, Fujita J, Toyohara K, Kato H, et al. Food repertoire and nutritional deficiency in japanese children with autism spectrum disorders. Jpn J Child Adolesc Psychiatry. (2017) 58:389–97. doi: 10.20615/jscap.58.3_389
11. Marí-Bauset S, Llopis-González A, Zazpe I, Marí-Sanchis A, Suarez-Varela M. Comparison of nutritional status between children with autism spectrum disorder and typically developing children in the Mediterranean Region (Valencia, Spain). Autism. (2017) 21:310–22. doi: 10.1177/1362361316636976
12. Malhi P, Venkatesh L, Bharti B, Singhi P. Feeding problems and nutrient intake in children with and without autism: a comparative study. Indian J Pediatr. (2017) 84:283–8. doi: 10.1007/s12098-016-2285-x
13. Adams J, Audhya T, Geis E, Gehn E, Fimbres V, Pollard E, et al. Comprehensive nutritional and dietary intervention for autism spectrum disorder—a randomized, controlled 12-month trial. Nutrients. (2018) 10:369. doi: 10.3390/nu10030369
14. Adams J, Audhya T, McDonough-Means S, Rubin R, Quig D, Geis E, et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatrics. (2011) 11:111. doi: 10.1186/1471-2431-11-111
15. Liu X, Liu J, Xiong X, Yang T, Hou N, Liang X, et al. Correlation between nutrition and symptoms: nutritional survey of children with autism spectrum disorder in Chongqing, China. Nutrients. (2016) 8:294. doi: 10.3390/nu8050294
16. Daradkeh G, Musthafa ME, Guizani N. Handbook for Nutritional Assessment Through Life Cycle. New York, NY: Nova Science Publishers, Inc. (2016).
17. Lee R, Nieman D. Nutritional Assessment. 5th ed. New York, NY: McGraw-Hill Higher Education (2010).
18. Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in saudi autistic children. Clin Biochem. (2009) 42:1032–40. doi: 10.1016/j.clinbiochem.2009.03.011
19. Priya M, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res. (2011) 142:148–58. doi: 10.1007/s12011-010-8766-2
20. Adams J, Audhya T, McDonough-Means S, Rubin R, Quig D, Geis E, et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab. (2011) 8:34. doi: 10.1186/1743-7075-8-34
21. Barnhill K, Gutierrez A, Ghossainy M, Marediya Z, Devlin M, Sachdev P, et al. Dietary status and nutrient intake of children with autism spectrum disorder: a case-control study. Res Autism Spect Disord. (2018) 50:51–9. doi: 10.1016/j.rasd.2018.03.002
22. Chaidez V, Hansen R, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. (2014) 44:1117–27. doi: 10.1007/s10803-013-1973-x
23. Crowell J, Keluskar J, Gorecki A. Parenting behavior and the development of children with autism spectrum disorder. Compr Psychiatry. (2019) 90:21–9. doi: 10.1016/j.comppsych.2018.11.007
24. Garcia J, Hahs-Vaughn D. Health factors, sociability, and academic outcomes of typically developing youth and youth with autism spectrum disorder: a latent class analysis approach. J Autism Dev Disord. (2020). doi: 10.1007/s10803-020-04572-7. [Epub ahead of print].
25. Kawicka A, Regulska-Ilow B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz Państw Zakł Hig. (2013) 64:1–12.
26. Keys A, Fidanza F, Karvonen M, Kimura N, Taylor H. Indices of relative weight and obesity. J Chron Dis. (1972) 25:329–43. doi: 10.1016/0021-9681(72)90027-6
27. Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. (1985) 9:147–53.
28. Centers for Disease Control and Prevention, National Center for Health Statistics. Clinical Growth Charts Website of Center for Disease Control and Prevention. CDC (2017). Available online at: https://www.cdc.gov/growthcharts/clinical_charts.html (accessed September 22, 2020).
29. Centers for Disease Control and Prevention. Nutritional Status Indicators. CDC (2016). Avaialble online at: https://www.cdc.gov/nccdphp/dnpao/growthcharts/training/overview/page5_1.html (accepted September 22, 2020).
30. World Health Organization. Training Course on Child Growth Assessment WHO Official Website. WHO (2008). Available online at: https://www.who.int/childgrowth/training/module_c_interpreting_indicators.pdf?ua=1 (accessed September 22, 2020).
31. Crawford P, Obarzanek E, Morrison J, Sabry Z. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9-and 10-year-old girls. J Am Diet Associ. (1994) 94:626–30. doi: 10.1016/0002-8223(94)90158-9
32. Holdeman N. (2010). Validation of a Food Frequency Questionnaire to a 3-Day Diet Record in Children With Autism Spectrum Disorder. [Master's thesis], The Ohio State University (Ohio), Columbus, OH.
33. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press (1997).
34. United Nations University, World Health Organization. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome: Food & Agriculture Org (2004).
35. Dietary Guidelines Advisory C. Dietary Guidelines for Americans 2015-2020. Washington, DC: Government Printing Office (2015).
36. Dixon L, Breck A, Khan L. Comparison of children's food and beverage intakes with national recommendations in New York city child-care centres. Public Health Nutr. (2016) 19:2451–7. doi: 10.1017/S1368980016001129
37. Rossouw C. (2005). Eating Habits and Nutrient Intakes of 10-15 Year Old Children in the North West Province. [dissertation], North-West University, Potchefstroom.
38. Kleinman R, Hall S, Green H, Korzec-Ramirez D, Patton K, Pagano M, et al. Diet, breakfast, and academic performance in children. Ann Nutr Metab. (2002) 46 (Suppl. 1):24–30. doi: 10.1159/000066399
39. Igout J, Fretigny M, Vasse M, Callat M, Silva M, Willemont L, et al. Evaluation of the coulter LH 750 haematology analyzer compared with flow cytometry as the reference method for WBC, platelet and nucleated RBC count. Clin Lab Haematol. (2004) 26:1–7. doi: 10.1111/j.0141-9854.2003.00577.x
40. Sari M, Pee S, Martini E, Herman S, Bloem M, Yip R. Estimating the prevalence of anaemia: a comparison of three methods. Bull World Health Organ. (2001) 79:506–11.
41. McComb RB, Bowers GN, Posen S. (editors). Measurement of alkaline phosphatase activity. In: Alkaline phosphatase. Boston, MA: Springer (1979). p. 289–372. doi: 10.1007/978-1-4613-2970-1_7
42. Dryer RL, Tammes AR, Routh JI. The determination of phosphorus and phosphatase with N-phenyl-p-phenylendediamine. J Biol Chem. (1957) 225:177–83. doi: 10.1016/S0021-9258(18)64920-8
43. Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. (2014) 43:443–64. doi: 10.1093/ije/dyt282
44. Dodds L, Fell D, Shea S, Armson B, Allen A, Bryson S, et al. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. (2011) 41:891–902. doi: 10.1007/s10803-010-1114-8
45. Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, et al. Gender differences in autism spectrum disorders: divergence among specific core symptoms. Autism Res. (2017) 10:680–9. doi: 10.1002/aur.1715
46. Geetha B, Sukumar C, Dhivyadeepa E, Reddy J, Balachandar V. Autism in India: a case–control study to understand the association between socio-economic and environmental risk factors. Acta Neurol Belgica. (2019) 119:393–401. doi: 10.1007/s13760-018-01057-4
47. Li YM, Shen YD, Li YJ, Xun GL, Liu H, Wu RR, et al. Maternal dietary patterns, supplements intake and autism spectrum disorders: a preliminary case-control study. Medicine. (2018) 97:e13902. doi: 10.1097/MD.0000000000013902
48. Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. (2009) 123:1293–300. doi: 10.1542/peds.2008-0927
49. Polo-Kantola P, Lampi K, Hinkka-Yli-Salomäki S, Gissler M, Brown A, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatrics. (2014) 164:358–65. doi: 10.1016/j.jpeds.2013.09.044
50. Levine S, Kodesh A, Viktorin A, Smith L, Uher R, Reichenberg A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. (2018) 75:176–84. doi: 10.1001/jamapsychiatry.2017.4050
51. Xie S, Karlsson H, Dalman C, Widman L, Rai D, Gardner R, et al. Family history of mental and neurological disorders and risk of autism. JAMA Netw Open. (2019) 2:e190154. doi: 10.1001/jamanetworkopen.2019.0154
52. Tran P, Lehti V, Lampi K, Helenius H, Suominen A, Gissler M, et al. Smoking during pregnancy and risk of autism spectrum disorder in a finnish national birth cohort. Paediatr Perinat Epidemiol. (2013) 27:266–74. doi: 10.1111/ppe.12043
53. Caramaschi D, Taylor A, Richmond R, Havdahl K, Golding J, Relton C, et al. Maternal smoking during pregnancy and autism: using causal inference methods in a birth cohort study. Transl Psychiatry. (2018) 8:262. doi: 10.1038/s41398-018-0313-5
54. Ramaekers V, Sequeira J, DiDuca M, Vrancken G, Thomas A, Philippe C, et al. Improving outcome in infantile autism with folate receptor autoimmunity and nutritional derangements: a self-controlled trial. Autism Res Treat. (2019) 2019:7486431. doi: 10.1155/2019/7486431
55. Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, et al. Maternal multivitamin intake, plasma folate and vitamin B12 levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol. (2018) 32:100–11. doi: 10.1111/ppe.12414
56. Prosperi M, Santocchi E, Balboni G, Narzisi A, Bozza M, Fulceri F, et al. Behavioral phenotype of ASD preschoolers with gastrointestinal symptoms or food selectivity. J Autism Dev Disord. (2017) 47:3574–88. doi: 10.1007/s10803-017-3271-5
57. Hartley S, Sikora D. Sex differences in autism spectrum disorder: an examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. J Autism Dev Disord. (2009) 39:1715–22. doi: 10.1007/s10803-009-0810-8
58. Johnson C, Handen B, Mayer-Costa M, Sacco K, Disabilities P. Eating habits and dietary status in young children with autism. J Dev Phys Disabil. (2008) 20:437–48. doi: 10.1007/s10882-008-9111-y
59. Guo M, Zhu J, Yang T, Lai X, Lei Y, Chen J, et al. Vitamin A and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutr Neurosci. (2019) 22:637–47. doi: 10.1080/1028415X.2017.1423268
60. Siddiqi S, Urooj A, D'Souza M. Dietary patterns and anthropometric measures of Indian children with autism Spectrum disorder. J autism Dev Disord. (2019) 49:1586–98. doi: 10.1007/s10803-018-3850-0
61. Meguid NA, Anwar M, Bjørklund G, Hashish A, Chirumbolo S, Hemimi M, et al. Dietary adequacy of Egyptian children with autism spectrum disorder compared to healthy developing children. Metab Brain Dis. (2017) 32:607–15. doi: 10.1007/s11011-016-9948-1
62. Egan A, Dreyer M, Odar C, Beckwith M, Garrison C. Obesity in young children with autism spectrum disorders: prevalence and associated factors. Harv Rev Psychiatry. (2013) 9:125–31. doi: 10.1089/chi.2012.0028
63. Curtin C, Jojic M, Bandini L. Obesity in children with autism spectrum disorders. Harv Rev Psychiatry. (2014) 22:93–103. doi: 10.1097/HRP.0000000000000031
64. Neumeyer A, Sokoloff N, McDonnell E, Macklin E, McDougle C, Holmes T, et al. Nutrition and bone density in boys with autism spectrum disorder. J Acad Nutr Diet. (2018) 118:865–77. doi: 10.1016/j.jand.2017.11.006
65. Neumeyer A, O'Rourke J, Massa A, Lee H, Lawson E, McDougle C, et al. Brief report: bone fractures in children and adults with autism spectrum disorders. J Autism Dev Disord. (2015) 45:881–7. doi: 10.1007/s10803-014-2228-1
66. Gunes S, Ekinci O, Celik T. Iron deficiency parameters in autism spectrum disorder: clinical correlates and associated factors. Ital J Pediatrics. (2017) 43:86. doi: 10.1186/s13052-017-0407-3
67. Alkaissi A, Russo S, Ghawadra S. Association between autism spectrum disorder and iron deficiency in children diagnosed autism spectrum disorder in the Northern West bank. Med Nurs. (2015) 16:1–10.
68. Hesapcioglu S, Kasak M, Kurt A, Ceylan M. High monocyte level and low lymphocyte to monocyte ratio in autism spectrum disorders. Int J Dev Disabil. (2019) 65:73–81. doi: 10.1080/20473869.2017.1371369
69. Kutlu A, Cevher Binici NJAJoPAPD. Does increased neutrophil-lymphocyte ratio predict autism spectrum disorder? Int J Dev Disabil. (2018) 19:607–14. doi: 10.5455/apd.296339
Keywords: autism spectrum disorder, nutritional status, children, maternal, determinant factors, Jordan
Citation: Alkhalidy H, Abushaikha A, Alnaser K, Obeidat MD and Al-Shami I (2021) Nutritional Status of Pre-school Children and Determinant Factors of Autism: A Case-Control Study. Front. Nutr. 8:627011. doi: 10.3389/fnut.2021.627011
Received: 16 November 2020; Accepted: 29 January 2021;
Published: 19 February 2021.
Edited by:
Nafisa M. Jadavji, Midwestern University, United StatesReviewed by:
Michele Roccella, University of Palermo, ItalySabika Allehdan, The University of Jordan, Jordan
Copyright © 2021 Alkhalidy, Abushaikha, Alnaser, Obeidat and Al-Shami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hana Alkhalidy, aGFhbGtoYWxpZHlAanVzdC5lZHUuam8=
 Hana Alkhalidy
Hana Alkhalidy Amal Abushaikha
Amal Abushaikha Khadeejah Alnaser
Khadeejah Alnaser Mohammad D. Obeidat2
Mohammad D. Obeidat2