- 1Clinical Nutrition and Metabolism, Department of Public Health and Caring Sciences, Uppsala University, Uppsala, Sweden
- 2Norslund-Svärdsjö Academic Health Care Center, Center for Clinical Research Dalarna, Falun, Sweden
- 3Division of Family Medicine and Primary Care, Department of Neurobiology, Care Sciences and Society (NVS), Karolinska Institute, Huddinge, Sweden
Background: Non-alcoholic fatty liver disease (NAFLD) is associated with dyslipidemia and increased cardiovascular disease risk. Dietary choices may produce profound effects on blood lipids. Thus, the purpose of this study was to investigate which foods modify blood lipids in NAFLD.
Methods: Systematic review of published systematic reviews and randomized controlled trials (RCTs). Searches were performed in PubMed, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials, from inception through March 2020. Studies in populations with NAFLD, which provided data on foods or dietary patterns and blood lipids were included, but not weight loss diets, supplements, nor individual nutrients. The strength of evidence was evaluated using The Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
Results: No relevant systematic reviews were identified. Eleven RCTs were included in the qualitative synthesis. Two RCTs were included in meta-analyses, regarding the comparison between Mediterranean and Low-fat diets, in which there were no clear effects on either high-density lipoprotein cholesterol or triglycerides, with Low evidence. From single RCTs, there was Moderate evidence for reduced triglycerides by a healthy dietary pattern, compared with usual care; and for reduced total cholesterol by a probiotic yogurt, enriched with Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12, compared with conventional yogurt. For all other comparisons, the evidence was considered as Low or Very low.
Conclusion: Few studies were identified which reported effects of foods on blood lipids in subjects with NAFLD. The possible beneficial effect of probiotics warrants further study. PROSPERO identifier: CRD42020178927.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a prevalent and emerging public health concern, closely associated with obesity, metabolic syndrome, insulin resistance, and type 2 diabetes (1). Individuals with NAFLD frequently present with dyslipidemia, typically elevated fasting plasma triglycerides and/or low high-density lipoprotein (HDL) cholesterol, but not necessarily elevated low-density lipoprotein (LDL) cholesterol (2); and may have high, or even very high, cardiovascular disease (CVD) risk (3). Dietary choices may produce profound and rapid effects on plasma lipoproteins, even under isocaloric conditions, and is thus possible to determine with high evidence in well-conducted short-term randomized controlled trials (RCTs). When several foods with cholesterol-lowering effects are combined (e.g., in the Portfolio diet) in strictly controlled settings, effects approaching 30% may be achieved on the group level (4). However, the effects of dietary fat quality (e.g., saturated and polyunsaturated fat) on LDL cholesterol and levels of apolipoprotein B are less pronounced in individuals with obesity than without (5). Considering the strong association between NAFLD and obesity, it can thus be speculated that the effect of dietary fat type may be less effective in individuals with compared to without NAFLD (due to concomitant overweight), however this requires further investigation. Factors proposed to blunt the effectiveness of dietary fat modification on LDL cholesterol in obesity (and thus speculatively in NAFLD) are increased inflammation, insulin resistance and endogenous cholesterol synthesis (6). Triglycerides may also be causally associated with CVD risk and can be clearly affected by diet and/or weight loss, although by distinctly different food choices than regarding hypercholesterolemia (7). The mechanisms through which foods influence plasma lipoproteins are partly unknown and may also be independent of the overall dietary fatty acid composition (8). The main routes involve intestinal absorption and excretion of lipids (including bile acids), or hepatic synthesis or clearance from the circulation. However, it is unknown whether hepatic steatosis modifies these effects. For instance, foods affecting gut microbiota (e.g., probiotics and prebiotics) may be of special importance. Also, foods affecting intestinal cholesterol uptake (e.g., foods enriched with phytosterols and/or stanols) could theoretically have less importance in NAFLD compared with foods believed to mainly exert direct effects on the liver, e.g., foods high in polyunsaturated fatty acids (PUFA). Thus, we aimed to systematically evaluate the literature for the effects of foods on blood lipids in persons with NAFLD.
Methods
A systematic search for systematic reviews and RCTs was performed, in PubMed, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials, from inception through March 2020. The PICO and full search strategy are provided in Table 1. Included search terms were selected based on dyslipidemia guidelines and studies in other populations. The target population was adults with NAFLD, not taking lipid-lowering medication. In order to be included, studies must include information on NAFLD diagnosis or provide other data of liver fat content of participants, and a clear majority of participants must conform with inclusion criteria. Interventions of interest were foods and dietary patterns, but not single nutrients (not clearly associated with specific foods), supplements, nor weight loss diets. As comparison, other foods or background diets were acceptable, but not different doses of the same food. The outcomes of interest were LDL cholesterol, total cholesterol, HDL cholesterol, apolipoprotein B, apolipoprotein AI, triglycerides, non-HDL cholesterol, oxidized LDL cholesterol, and other lipoproteins and subfractions. If data was not reported, authors were not contacted for additional information, however, for included studies, if data was ambiguous, authors were contacted for clarification. Bibliographies of included RCTs were screened for additional studies. Language was restricted to English. The study protocol is available at www.crd.york.ac.uk/PROSPERO, identifier CRD42018089661. No ethics approval was required.
Study Selection, Data Extraction, Risk of Bias, and Evidence Assessments
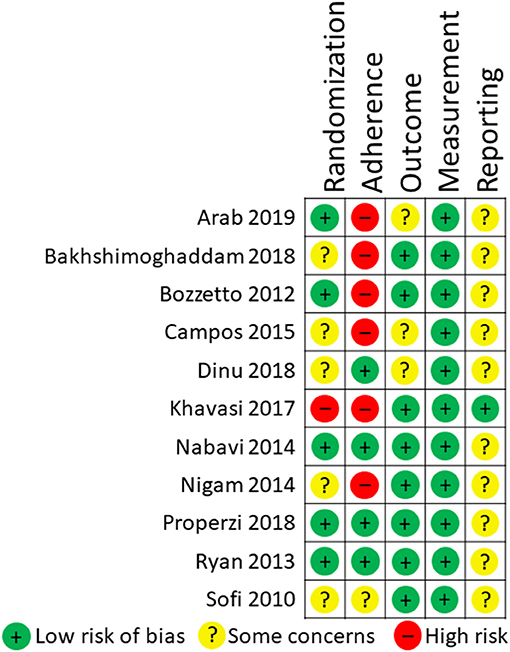
Two reviewers screened abstracts and in cases of disagreement, the paper was included for full-text review. From RCTs that fulfilled inclusion criteria, data was extracted by at least two researchers using standardized forms including: author, year, study type (parallel/crossover), population (NAFLD/other), n analyzed overall and per group, % male, age in years (mean ± SD and/or range), intervention/daily dose (mean ± SD and/or range), control/daily dose (mean ± SD and/or range), effects on lipids (mean difference, 95%CI and/or end mean for intervention and control groups), duration, country, and funding. Risk of bias in RCTs was assessed by two authors using the SBU Risk of Bias tool for intervention studies (version 6 May 2020). The quality of evidence was evaluated using The Grading of Recommendations Assessment, Development, and Evaluation (GRADE), by pre-determined criteria for risk of bias, inconsistency, indirectness, imprecision, publication bias, large effects, dose response relationships, and opposing bias. For instance, evidence was downgraded (-1) for inconsistency if results were not replicated, as consistent results of low heterogeneity are impossible to attain from a single RCT. In indeterminate cases, discussions were extended among all authors and overall judgments were employed.
Data Synthesis and Analysis
Results from included RCTs were included in meta-analyses, when considered appropriate, using ReviewManager 5.3 software. The Cochrane Handbook (9) was adhered to. In cases of at least moderate (I2>50%) unexplained heterogeneity, random effects models were preferred. If mean changes from baseline and their standard deviations were unavailable and impossible to calculate, end-of-study means and standard deviations were used. Cross-over studies were included together with parallel studies, with modified weights, calculated from available data. In cases where the variances of mean differences were unavailable, a conservative correlation R = 0.5 was imputed and standard deviations adjusted accordingly. Mg/dL was converted to mmol/L by multiplication with 0.02586 for cholesterol and 0.01129 for triglycerides. Data is presented as mean±SD. P-values > 0.05 were considered as non-significant (NS).
Results
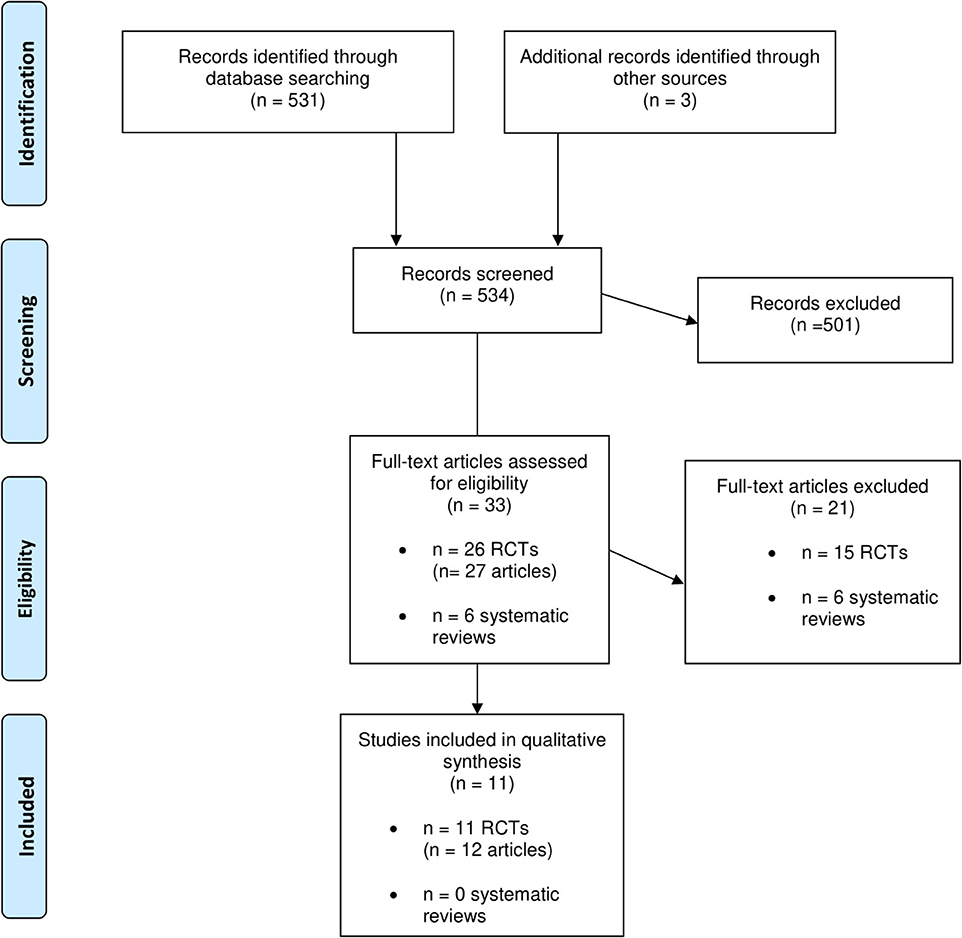
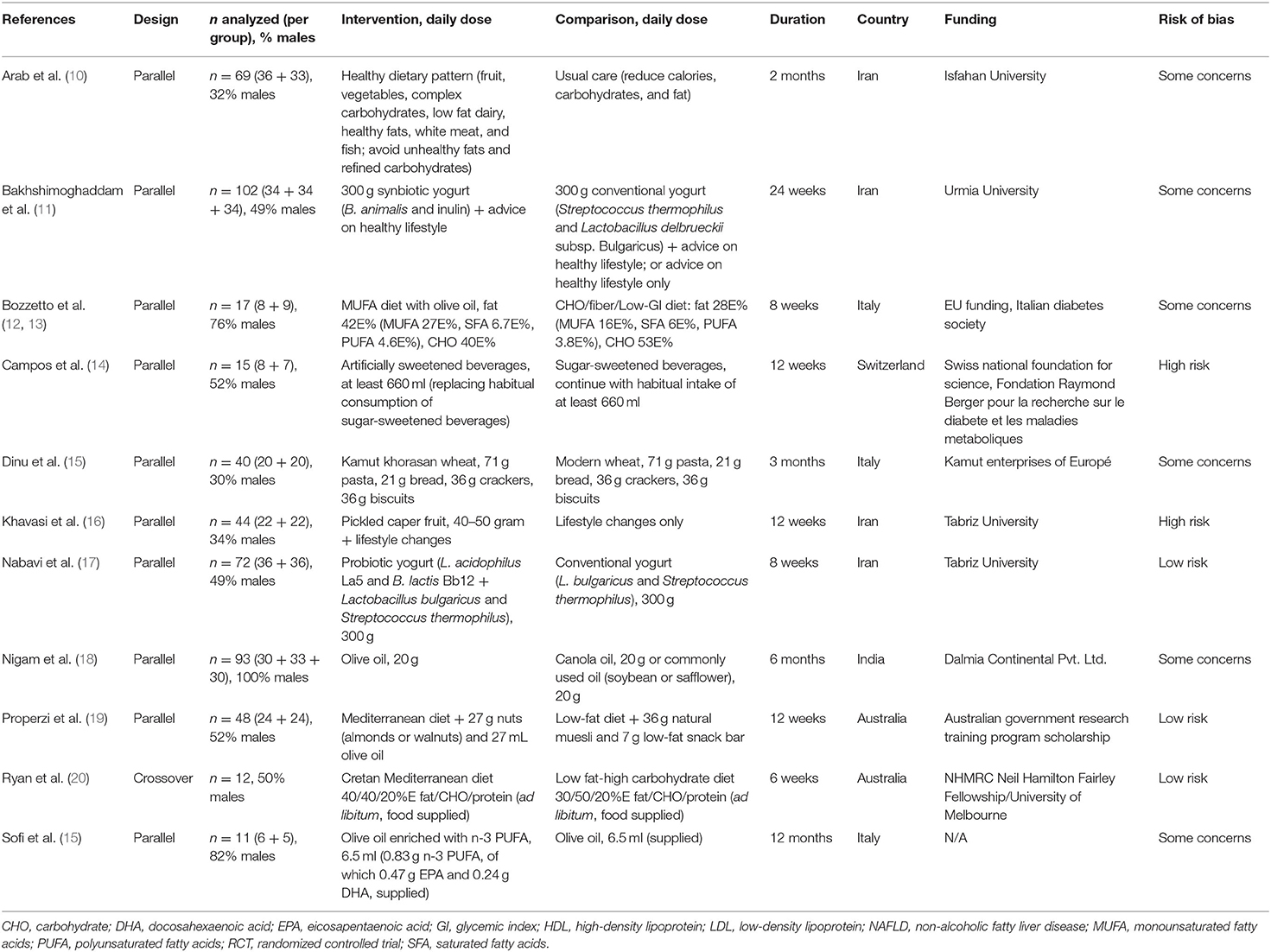
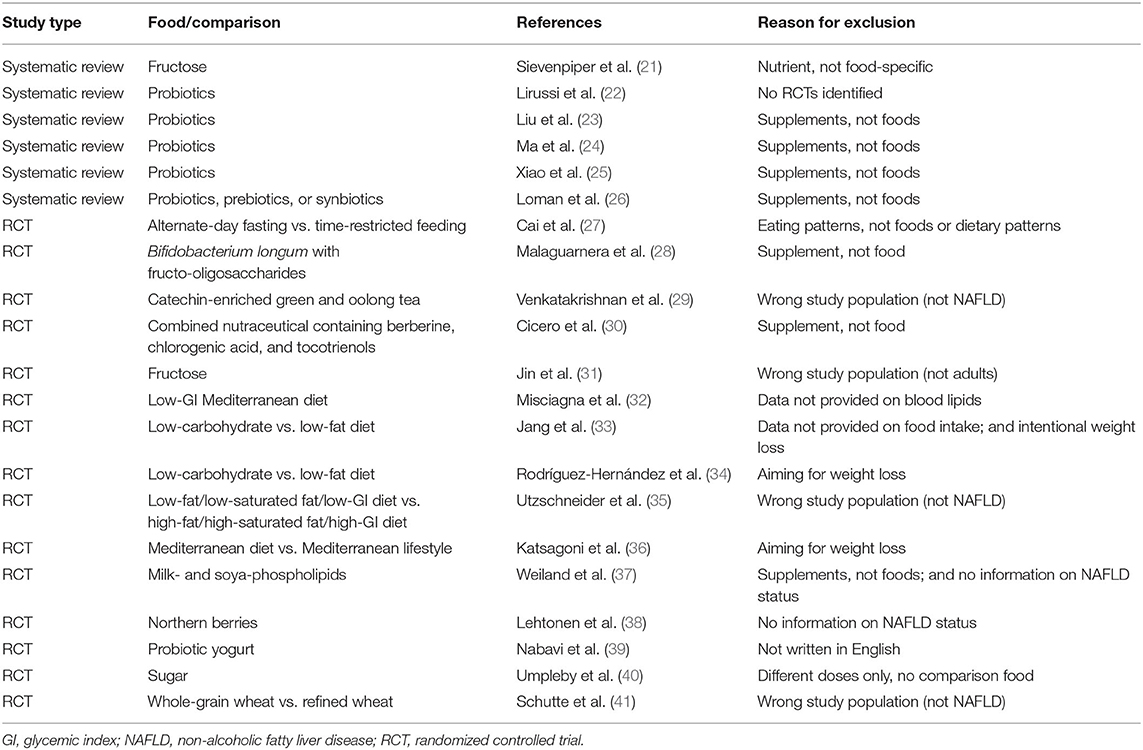
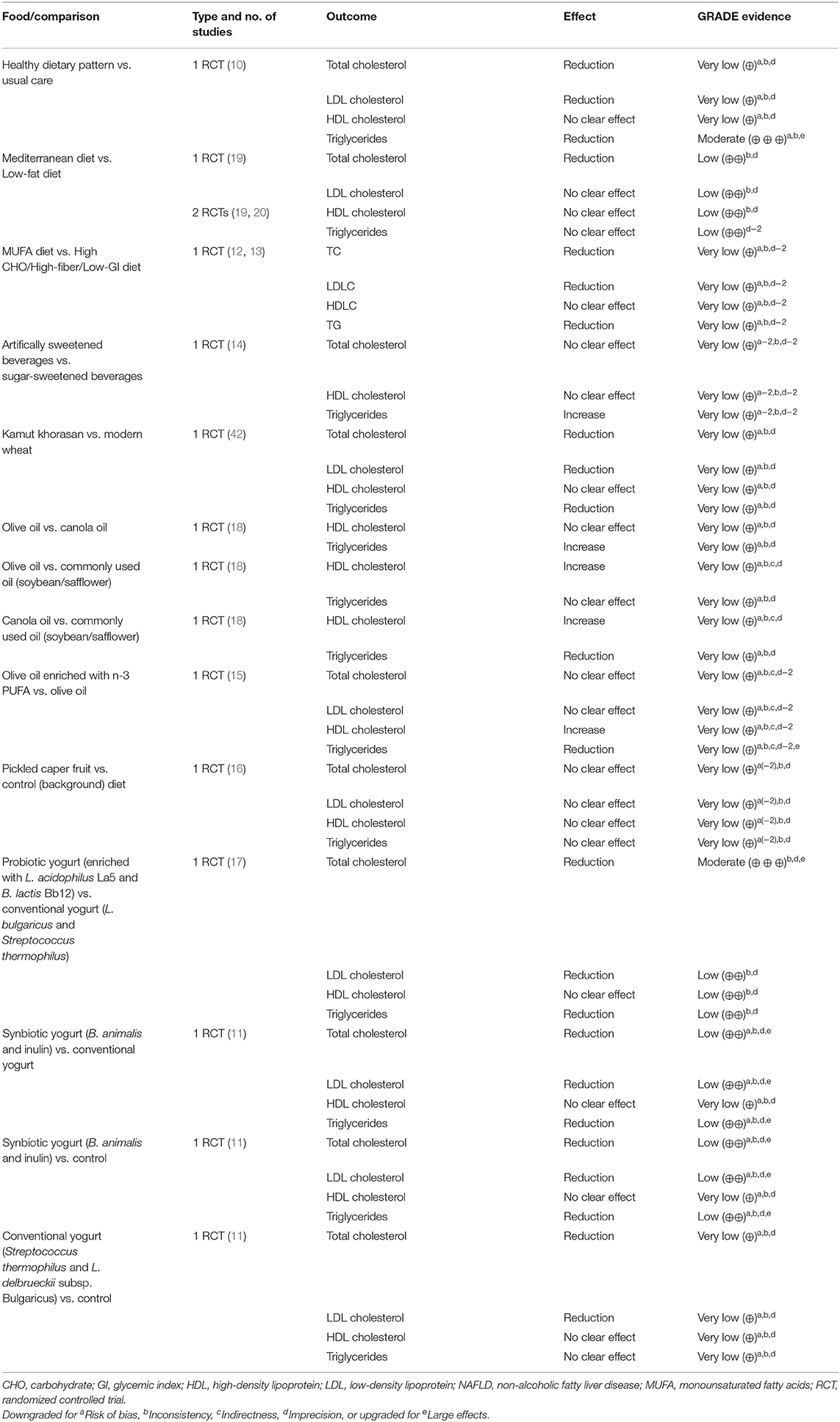
Six systematic reviews and 26 RCTs were evaluated in full text. No systematic reviews and 11 RCTs (12 publications) fulfilled inclusion criteria (Figure 1). The characteristics of the 11 included RCTs are presented in Table 2. The excluded papers are presented, with reasons, in Table 3. The overall risk of bias was considered as Low in three RCTs, Some concerns in six RCTs, and High in two RCTs (Table 2 and Figure 2). Four RCTs compared dietary patterns and the remaining seven compared food items. The overall results on blood lipids and the evidence gradings for each available outcome are presented in Table 4. Two studies were considered appropriate to include in meta-analyses, as they both compared a Mediterranean with a Low-fat dietary pattern (Figure 3).
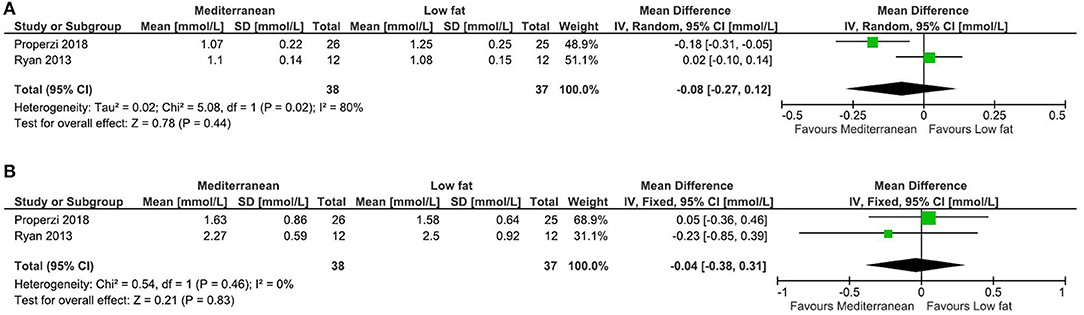
Figure 3. Meta-analysis of Mediterranean vs. Low fat dietary pattern for blood lipids in NAFLD. (A). Forest plot for the effects on HDL Cholesterol. (B). Forest plot for the effects on Triglycerides. Ryan et al. (20) is a cross-over study and its variance has been adjusted accordingly, in order to increase its weighting.
Dietary Patterns
Healthy Dietary Pattern vs. Usual Care
In the RCT by Arab et al. (10), there were significant reductions in both total cholesterol (from 5.56 ± 1.6 to 5.01 ± 1.8 mmol/l in intervention vs. from 4.81 ± 0.92 to 4.91 ± 0.89 mmol/l in control group, P = 0.04) and triglycerides (from 2.46 ± 1.9 to 1.86 ± 0.98 vs. from 2.11 ± 1.7 to 2.21 ± 1.4 mmol/L, P = 0.002). There was a tendency toward reduced LDL cholesterol (from 3.11 ± 1.0 to 2.83 ± 1.1 vs. from 2.87 ± 0.84 to 2.96 ± 0.79 mmol/L, P = 0.46), but no clear effect for HDL cholesterol (from 1.20 ± 0.29 to 1.16 ± 0.20 vs. from 1.12 ± 0.26 to 1.07 ± 0.20 mmol/L, P = 0.12). The quality of evidence for the effect on triglycerides was considered as Moderate, upgraded for large effects, whereas all other comparisons were further downgraded to Very low evidence, due to high imprecision.
Mediterranean Diet vs. Low-Fat Diet
From the RCTs by Properzi et al. (19) and Ryan et al. (20), effects on HDL cholesterol and triglycerides were combined in meta-analyses, but there were no clear effects (Figure 3). Effects on total and LDL cholesterol were only reported in the study by Properzi et al. (19), with an indicated slight reduction for total cholesterol (from 4.78 ± 1.3 to 4.53 ± 1.3 vs. from 5.23 ± 0.90 to 5.15 ± 1.1 mmol/L, NS), but no meaningful effect on LDL cholesterol (from 2.83 ± 1.2 to 2.73 ± 1.1 vs. from 3.20 ± 0.78 to 3.17 ± 0.86 mmol/L, NS). The evidence was considered as Low for all outcomes.
High-Monounsaturated Fatty Acid Diet vs. High-Carbohydrate, High-Fiber, Low-Glycemic Index Diet
In the RCT by Bozzetto et al. (12, 13), there were tendencies (all NS) toward reductions after the high-monounsaturated fatty acid (MUFA) diet vs. high-carbohydrate (high-CHO) diet, for total (from 4.32 ± 0.65 to 4.29 ± 0.59 vs. from 4.06 ± 0.98 to 4.24 ± 1.2 mmol/L) and LDL cholesterol (from 2.85 ± 0.52 to 2.82 ± 0.54 vs. from 2.53 ± 0.75 to 2.77 ± 1.0 mmol/L), and for triglycerides (from 1.38 ± 0.42 to 1.29 ± 0.37 vs. from 1.24 ± 0.77 to 1.48 ± 1.2 mmol/L). There was no clear effect on HDL cholesterol (from 0.91 ± 0.2 to 0.93 ± 0.1 vs. from 0.96 ± 0.2 to 0.96 ± 0.2 mmol/L). The evidence was considered as Very low. In the report from 2014 (10), results were reported on postprandial lipids combined with 19 (8 + 11 per group) additional subjects that were also prescribed exercise training. Post-prandial (after 2, 4, and 6 h, presented as figure) triglycerides and cholesterol in plasma and chylomicrons/very-low-density lipoproteins were increased after the MUFA diet (P<0.05) and reduced after the high-CHO diet (P < 0.05). We did not perform evidence gradings for these outcomes, as they were not clearly pre-specified.
Foods
Artificially vs. Sugar-Sweetened Beverages
In the RCT by Campos et al. (14), triglycerides tended to increase by artificially (from 1.35 ± 0.2 to 1.5 ± 0.3 mmol/L) vs. sugar-sweetened beverages (from 2.0 ± 0.6 to 1.3 ± 0.2 mmol/L), however based on only 8+7 individuals with steatosis, and the evidence was graded as Very low. There were no clear effects on total (from 4.3 ± 0.3 to 4.2 ± 0.4 vs. from 4.3 ± 0.3 to 4.2 ± 0.2 mmol/L) or HDL cholesterol (from 1.15 ± 0.08 to 1.10 ± 0.07 vs. from 1.11 ± 0.07 to 1.14 ± 0.08 mmol/L), also with Very low evidence.
Kamut Khorasan vs. Modern Wheat
In the RCT by Dinu et al. (42), there were tendencies toward reductions in total (from 5.89 ± 1.0 to 5.39 ± 0.94 vs. from 5.51 ± 0.65 to 5.56 ± 0.73 mmol/L, P = 0.096) and LDL cholesterol (from 3.76 ± 0.94 to 3.46 ± 0.85 vs. from 3.36 ± 1.1 to 3.37 ± 0.92 mmol/L, P = 0.12), and in triglycerides (from 1.57 ± 0.97 to 1.36 ± 0.51 vs. from 1.41 ± 0.56 to 1.50 ± 0.57 mmol/L P = 0.16), in the kamut vs. modern wheat group. There was no clear effect on HDL cholesterol (from 1.43 ± 0.33 to 1.39 ± 0.35 vs. from 1.45 ± 0.44 to 1.37 ± 0.35 mmol/L, P = 0.99). The evidence was considered as Very low for all outcomes.
Olive Oil vs. Canola Oil vs. Commonly Used Oil (Soybean or Safflower)
The RCT by Nigam et al. (18) had three parallel arms, which besides dietary oils received similar lifestyle counseling including exercise. For HDL cholesterol, there were tendencies toward increases for olive (from 0.98 ± 0.11 to 1.07 ± 0.12 mmol/L) and canola (from 1.02 ± 0.14 to 1.05 ± 0.15 mmol/L), vs. commonly used oil (from 1.02 ± 0.13 to 0.91 ± 0.14 mmol/L), but no meaningful difference for olive vs. canola oil. For triglycerides, there were tendencies toward an increase for olive (from 2.04 ± 0.93 to 1.92 ± 0.14 mmol/L) vs. canola oil (from 2.11 ± 0.93 to 1.75 ± 0.55 mmol/L), toward no clear effect for olive vs. commonly used oil (from 2.08 ± 1.1 to 2.06 ± 1.2 mmol/L), and toward a reduction for canola vs. commonly used oil. The evidence was considered as Very low for all comparisons and outcomes.
Olive Oil Enriched With n-3 PUFA vs. Olive Oil
In the RCT by Sofi et al. (15), there was an increase in HDL cholesterol (from 1.15 ± 0.14 to 1.56 ± 0.24 vs. from 1.13 ± 0.12 to 1.07 ± 0.05 mmol/L, P = 0.03) and a reduction in triglycerides (from 1.86 ± 0.97 to 1.50 ± 0.72 vs. from 1.61 ± 0.28 to 1.84 ± 0.34 mmol/L, P = 0.04) in the enriched vs. regular olive oil group. No clear differences were seen for total (from 5.53 ± 0.72 to 5.51 ± 0.60 vs. from 5.11 ± 0.57 to 4.99 ± 0.58 mmol/L, P = 0.9) or LDL cholesterol (from 3.40 ± 0.64 to 3.45 ± 0.84 vs. from 3.24 ± 0.50 to 3.08 ± 0.60 mmol/L, P = 0.2). The evidence was considered as Very low for all outcomes.
Pickled Caper Fruit vs. No Food
In the RCT by Khavasi et al. (16), there were no clear effects on total (from 4.95 ± 1.2 to 4.75 ± 1.2 vs. from 4.31 ± 0.96 to 3.94 ± 0.93 mmol/L, P = 0.09), LDL (from 2.90 ± 0.93 to 2.67 ± 0.73 vs. from 2.00 ± 1.2 to 1.68 ± 1.1 mmol/L, P = 0.03), or HDL cholesterol (from 0.99 ± 0.21 to 1.01 ± 0.22 vs. from 1.14 ± 0.13 to 1.14 ± 0.16 mmol/L, P = 0.3), or on triglycerides (from 2.14 ± 0.63 to 2.15 ± 0.90 vs. from 2.54 ± 1.0 to 2.44 ± 1.1 mmol/L, P = 0.053). Some data on variances were estimated because of implausible data in table. The evidence was considered as Very low for all outcomes.
Probiotic (L. acidophilus La5 and B. lactis Bb12) vs. Conventional Yogurt
In the RCT by Nabavi et al. (17), there was a reduction in total (from 5.08 ± 1.0 to 4.46 ± 1.1 vs. from 5.14 ± 0.82 to 5.25 ± 0.87 mmol/L) and LDL cholesterol (from 3.11 ± 0.91 to 2.58 ± 0.56 vs. from 2.88 ± 0.77 to 2.85 ± 0.70 mmol/L), and at least a tendency for reduced triglycerides (from 2.19 ± 0.72 to 1.95 ± 0.77 vs. from 2.23 ± 0.87 to 2.33 ± 0.90 mmol/L) by the probiotic vs. conventional yogurt (P-values N/A for mean differences in change). No clear effect was observed for HDL cholesterol (from 1.23 ± 0.28 to 1.27 ± 0.32 vs. from 1.24 ± 0.25 to 1.31 ± 0.25 mmol/L). The evidence was Moderate for total cholesterol (upgraded for large effects) and Low for all other outcomes.
Synbiotic (B. animalis and Inulin) vs. Conventional Yogurt vs. No Food
In the RCT by Bakhshimoghaddam et al. (11), there were reductions in total and LDL cholesterol, and triglycerides by the synbiotic compared with conventional yogurt or control group (data presented as graphs), with Low evidence. For HDL cholesterol, there was no clear effect for any comparison, with Very low evidence. For the conventional yogurt compared with control group, there were reductions in total and LDL cholesterol, but not for HDL cholesterol or triglycerides, with Very low evidence for all outcomes.
Discussion
In this systematic review of the effects of foods on blood lipids in individuals with NAFLD, no systematic reviews and only 11 RCTs fulfilled the inclusion criteria. No foods or dietary patterns modifies blood lipids with High quality evidence in NAFLD. With Moderate evidence, a Healthy dietary pattern reduces fasting triglycerides, compared with standard care. Also with Moderate evidence, a probiotic yogurt, enriched with Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12, decreases total cholesterol, compared with conventional yogurt, and with Low evidence, reduces LDL cholesterol and triglycerides. Also with Low evidence, a synbiotic yogurt containing Bifidobacterium animalis and inulin reduces total and LDL cholesterol, and triglycerides. Finally, with Low evidence, a Mediterranean dietary pattern (high in unsaturated fatty acids) reduces total cholesterol (but not other lipoproteins) compared with a Low-fat dietary pattern. For all other comparisons, the evidence quality was considered as Very low for all effects, or lack thereof.
The limited number of studies discovered was somewhat surprising, given the prevalence and clinical significance of NAFLD. One plausible reason may be that, in most studies on diet and CVD risk factors, liver fat content is not easily attainable and thus overlooked. Also, many patients with NAFLD go undiagnosed, as it usually presents with little symptoms. The effect of dietary fat type (e.g., saturated/polyunsaturated fat) on plasma lipoproteins is diminished in individuals with obesity compared with normal body weight (5). Considering the strong association between NAFLD and obesity, it can thus be speculated that the effect of dietary fat type may be less effective in individuals with compared to without NAFLD (due to concomitant overweight), however this requires further investigation. Factors proposed to blunt the effectiveness of dietary fat modification on LDL cholesterol in obesity (and thus speculatively in NAFLD) are increased inflammation, insulin resistance, and endogenous cholesterol synthesis (6).
An RCT testing the effects of a synbiotic supplement (fructo-oligosaccharides plus B. animalis) on liver fat content and liver fibrosis in NAFLD was published during the writing of this manuscript (43). This study showed that this synbiotic combination altered the fecal microbiome but had no effect on liver fat, fibrosis, or plasma lipoproteins compared to prebiotic fructo-oligosaccharides only. This is in contrast to the results reported by Bakhshimoghaddam (11) where a similar synbiotics was administered in the form of yogurt. Whether the mode of administration of synbiotics (supplement vs. yogurt/whole food) modifies the effects on plasma lipoproteins requires further investigation but is not unlikely considering potential matrix effects when part of a whole food.
The present systematic review has limitations. Studies on dietary supplements and individual nutrients were not included in our searches, which could have been relevant e.g.. for determining which bacterial strains (in probiotics) may provide beneficial effects on plasma lipoproteins. Also, we did not search gray literature or contact authors for additional information, and only studies published in English were included. Thus, we cannot rule out that additional relevant studies may exist. In addition, the present study could not confidently determine effect sizes or required daily doses for the included foods, because of the scarce data available.
Conclusions
The results from the included RCTs are mostly in line with current guidelines for the treatment of dyslipidemia or prevention of CVD (7, 44) in other populations. However, it is not possible from the present data to determine which foods significantly modify blood lipids in NAFLD. The possible beneficial effect of probiotics enriched with certain bacterial strains on plasma lipoproteins warrants further study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
The study was conceptualized by DI. AR and FR performed screening. AR, FR, and DI performed data extraction. Risk of bias was assessed by FR and DI. Data analysis and grading of the evidence was performed by DI, with support from AR and FR. The paper was written by AR, FR, and DI. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nature reviews gastroenterology & hepatology. (2017) 14:32–42. doi: 10.1038/nrgastro.2016.147
2. Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. (2016) 65:1109–23. doi: 10.1016/j.metabol.2016.05.003
3. Stols-Goncalves D, Hovingh GK, Nieuwdorp M, Holleboom AG. NAFLD and atherosclerosis: two sides of the same dysmetabolic coin? Trends Endocrinol Metab. (2019) 30:891–902. doi: 10.1016/j.tem.2019.08.008
4. Kendall CW, Jenkins DJ. A dietary portfolio: maximal reduction of low-density lipoprotein cholesterol with diet. Curr Atheroscler Rep. (2004) 6:492–8. doi: 10.1007/s11883-004-0091-9
5. Sundfor TM, Svendsen M, Heggen E, Dushanov S, Klemsdal TO, Tonstad S. BMI modifies the effect of dietary fat on atherogenic lipids: a randomized clinical trial. Am J Clin Nutr. (2019) 110:832–41. doi: 10.1093/ajcn/nqz113
6. Flock MR, Green MH, Kris-Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. (2011) 2:261–74. doi: 10.3945/an.111.000422
7. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.15829/1560-4071-2020-3826
8. Vors C, Le Barz M, Bourlieu C, Michalski MC. Dietary lipids and cardiometabolic health: a new vision of structure-activity relationship. Curr Opin Clin Nutr Metab Care. (2020) 23:451–9. doi: 10.1097/MCO.0000000000000693
9. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020). (2020). Available online at: http://www.training.cochrane.org/handbook (accessed October 1, 2020).
10. Arab A, Hadi A, Moosavian SP, Rafie N, Hajianfar H. The effect of nutrition education program on overweight/obese patients with non-alcoholic fatty liver disease: a single-blind parallel randomized controlled trial. Clin Nutr Res. (2019) 8:238–46. doi: 10.7762/cnr.2019.8.3.238
11. Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr. (2018) 148:1276–84. doi: 10.1093/jn/nxy088
12. Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. (2012) 35:1429–35. doi: 10.2337/dc12-0033
13. Bozzetto L, Annuzzi G, Costabile G, Costagliola L, Giorgini M, Alderisio A, et al. A CHO/fibre diet reduces and a MUFA diet increases postprandial lipaemia in type 2 diabetes: no supplementary effects of low-volume physical training. Acta Diabetol. (2014) 51:385–93. doi: 10.1007/s00592-013-0522-6
14. Campos V, Despland C, Brandejsky V, Kreis R, Schneiter P, Chiolero A, et al. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obesity. (2015) 23:2335–9. doi: 10.1002/oby.21310
15. Sofi F, Giangrandi I, Cesari F, Corsani I, Abbate R, Gensini GF, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr. (2010) 61:792–802. doi: 10.3109/09637486.2010.487480
16. Khavasi N, Somi MH, Khadem E, Faramarzi E, Ayati MH, Fazljou SMB, et al. Effect of daily caper fruit pickle consumption on disease regression in patients with non-alcoholic fatty liver disease: a double-blinded randomized clinical trial. Adv Pharm Bull. (2017) 7:645–50. doi: 10.15171/apb.2017.077
17. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. (2014) 97:7386–93. doi: 10.3168/jds.2014-8500
18. Nigam P, Bhatt S, Misra A, Chadha DS, Vaidya M, Dasgupta J, et al. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol Ther. (2014) 16:255–61. doi: 10.1089/dia.2013.0178
19. Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. (2018) 68:1741–54. doi: 10.1002/hep.30076
20. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. (2013) 59:138–43. doi: 10.1016/j.jhep.2013.02.012
21. Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A. Fructose vs. glucose and metabolism: do the metabolic differences matter? Curr Opin Lipidol. (2014) 25:8–19. doi: 10.1097/MOL.0000000000000042
22. Lirussi F, Mastropasqua E, Orando S, Orlando R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. (2007) CD005165. doi: 10.1002/14651858.CD005165.pub2
23. Liu L, Li P, Liu Y, Zhang Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta-analysis. Dig Dis Sci. (2019) 64:3402–12. doi: 10.1007/s10620-019-05699-z
24. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. (2013) 19:6911–8. doi: 10.3748/wjg.v19.i40.6911
25. Xiao MW, Lin SX, Shen ZH, Luo WW, Wang XY. Systematic review with meta-analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol Res Pract. (2019) 2019:1484598. doi: 10.1155/2019/1484598
26. Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. (2018) 76:822–839. doi: 10.1093/nutrit/nuy031
27. Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. (2019) 19:219. doi: 10.1186/s12876-019-1132-8
28. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. (2012) 57:545–53. doi: 10.1007/s10620-011-1887-4
29. Venkatakrishnan K, Chiu HF, Cheng JC, Chang YH, Lu YY, Han YC, et al. Comparative studies on the hypolipidemic, antioxidant and hepatoprotective activities of catechin-enriched green and oolong tea in a double-blind clinical trial. Food Funct. (2018) 9:1205–13. doi: 10.1039/C7FO01449J
30. Cicero AF, Rosticci M, Parini A, Morbini M, Urso R, Grandi E, et al. Short-term effects of a combined nutraceutical of insulin-sensitivity, lipid level and indexes of liver steatosis: a double-blind, randomized, cross-over clinical trial. Nutr J. (2015) 14:30. doi: 10.1186/s12937-015-0019-y
31. Jin R, Welsh JA, Le NA, Holzberg J, Sharma P, Martin DR, et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. (2014) 6:3187–201. doi: 10.3390/nu6083187
32. Misciagna G, Del Pilar Díaz M, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinici trial. J Nutr Health Aging. (2017) 21:404–12. doi: 10.1007/s12603-016-0809-8
33. Jang EC, Jun DW, Lee SM, Cho YK, Ahn SB. Comparison of efficacy of low-carbohydrate and low-fat diet education programs in non-alcoholic fatty liver disease: a randomized controlled study. Hepatol Res. (2018) 48:E22–29. doi: 10.1111/hepr.12918
34. Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. (2011) 10:486–92. doi: 10.1016/S1665-2681(19)31517-0
35. Utzschneider KM, Bayer-Carter JL, Arbuckle MD, Tidwell JM, Richards TL, Craft S. Beneficial effect of a weight-stable, low-fat/low-saturated fat/low-glycaemic index diet to reduce liver fat in older subjects. Br J Nutr. (2013) 109:1096–104. doi: 10.1017/S0007114512002966
36. Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, et al. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr. (2018) 120:164–75. doi: 10.1017/S000711451800137X
37. Weiland A, Bub A, Barth SW, Schrezenmeir J, Pfeuffer M. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men-two double-blind, randomised, controlled, clinical trials. J Nutr Sci. (2016) 5:e21. doi: 10.1017/jns.2016.9
38. Lehtonen HM, Suomela JP, Tahvonen R, Vaarno J, Venojärvi M, Viikari J, et al. Berry meals and risk factors associated with metabolic syndrome. Eur J Clin Nutr. (2010) 64:614–21. doi: 10.1038/ejcn.2010.27
39. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-jafarabadi M. The effects of probiotic yogurt on metabolic factors in nonalcoholic fatty liver disease. SJKU. (2016) 20:12–25. doi: 10.22102/20.6.12
40. Umpleby AM, Shojaee-Moradie F, Fielding B, Li X, Marino A, Alsini N, et al. Impact of liver fat on the differential partitioning of hepatic triacylglycerol into VLDL subclasses on high and low sugar diets. Clin Sci. (2017) 131:2561–73. doi: 10.1042/CS20171208
41. Schutte S, Esser D, Hoevenaars FPM, Hooiveld GJEJ, Priebe MG, Vonk RJ, et al. A 12-wk whole-grain wheat intervention protects against hepatic fat: the Graandioos study, a randomized trial in overweight subjects. Am J Clin Nutr. (2018) 108:1264–74. doi: 10.1093/ajcn/nqy204
42. Dinu M, Whittaker A, Pagliai G, Giangrandi I, Colombini B, Gori AM, et al. A khorasan wheat-based replacement diet improves risk profile of patients with nonalcoholic fatty liver disease (NAFLD): a randomized clinical trial. J Am Coll Nutr. (2018) 37:508–14. doi: 10.1080/07315724.2018.1445047
43. Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. (2020) 158:1597–610.e7. doi: 10.1053/j.gastro.2020.01.031
44. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000725
Keywords: NAFLD, steatosis, cholesterol, triglycerides, diet, food
Citation: Rosqvist F, Rydell A and Iggman D (2020) The Effects of Foods on Blood Lipids in Non-alcoholic Fatty Liver Disease (NAFLD)—A Systematic Review and Meta-Analysis. Front. Nutr. 7:613221. doi: 10.3389/fnut.2020.613221
Received: 01 October 2020; Accepted: 24 November 2020;
Published: 16 December 2020.
Edited by:
Anne Marie Minihane, University of East Anglia, United KingdomReviewed by:
Wendy Louise Hall, King's College London, United KingdomAmalia Gastaldelli, National Research Council (CNR), Italy
Copyright © 2020 Rosqvist, Rydell and Iggman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Iggman, david.iggman@regiondalarna.se
 Fredrik Rosqvist
Fredrik Rosqvist