
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 15 December 2020
Sec. Nutritional Epidemiology
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.600449
This article is part of the Research TopicAnalyzing the Relationship Between Dietary Patterns, Health Outcomes and Individual Food ChoicesView all 23 articles
Ultra-processed foods (UPFs) are popular in the United States. In recent years, there has been an increasing interest in the health impact of UPF. This study is conducted to assess the association between UPF consumption and depressive symptoms among United States adults. Data were collected from the National Health and Nutrition Examination Survey 2011–2016. Dietary data were obtained through 24-h dietary recall interviews. Depressive symptoms were detected by a nine-item Patient Health Questionnaire; participants with more than 10 points were diagnosed with depressive symptoms. Results of logistic regression revealed a positive association between UPF consumption and depressive symptoms. The study suggests that UPF may increase the risk of depressive symptoms, particularly in people with less exercise.
Food processing aims to improve food availability, safety, digestibility, transportability, and storage life (1). Since the mid-nineteenth century, the mechanization of the food industry has made it possible to produce, transport, and sell processed foods on a large scale. To better understand the impact of the nature, purpose, and extent of food processing on human health and disease, a novel food classification method—NOVA (a name, not an acronym) was proposed. An updated version of NOVA classified all foods into four groups (2): (1) unprocessed or minimally processed foods; (2) processed culinary ingredients; (3) processed foods; (4) ultra-processed foods (UPFs) and drink products. Among them, UPFs are attracting increasing attention.
UPFs are essentially industrial formulations mostly or entirely made from industrial ingredients, with little or no whole foods. They often contain substances not used in home cooking, especially the additives for sensory properties of food (3). Typical UPFs include carbonated beverages, bagged snacks, mass-produced packaged bread and buns, and ice cream. Because of their super-palatability, convenience, and long storage life, UPFs dominate the food supply in high-income countries, particularly in the United States (US), where UPFs account for 57.5% of total energy intake (4). At the same time, UPF consumption is increasing rapidly in middle-income countries (5).
UPF producers prioritize taste, cost, storage, and stability during transport, whereas neglecting nutritional quality (6). UPFs are common in the western dietary pattern and generally rich in total fat, saturated fat, added sugar, and salt, whereas poor in fiber and vitamin density (7, 8), which is detrimental to mental health (9, 10). Beyond poor nutritional quality, UPFs also contain all kinds of additives, along with neo-formed contaminants produced during food processing and packaging (11–13), some of which may have an adverse effect on intestinal flora (14, 15), inducing the development of inflammation-associated diseases (16, 17), such as depression.
In recent years, the impact of high UPF consumption has aroused widespread public concerns, stimulating extensive researches to investigate adverse health outcomes related to UPF. Researches have demonstrated an association between UPF consumption and increased risk of all-cause mortality (18–20), cancer (21), type 2 diabetes (22), and cardiovascular diseases (23). Additionally, positive associations with frailty (24), overweight/obesity (25) were reported in other studies. Among these studies, two European studies explored the association between UPF and mental disorders (26, 27). However, both two studies were conducted in a population with relatively low UPF consumption. There is a lack of a large-scale study to assess the association between UPF consumption and depressive symptoms in the US population. Thus, we conducted this study to evaluate the relationship between UPF consumption and depressive symptoms in US adults aged more than 20 years.
This study used data collected from the National Health and Nutrition Examination Survey (NHANES), which is administered by the National Centers for Health Statics at the Centers for Disease Control and Prevention. NHANES is conducted to assess the health and nutritional status of the US population of all ages. Data are collected using a complex, multistage probability sampling design to make the sample nationally representative. Participants received a detailed interview in their home and physical examination, dietary survey, and clinical laboratory tests at a mobile examination center on another day. All participants provided written informed consent, and the Research Ethics Review Board approved the study protocol.
Data from three survey cycles (2011–2012, 2013–2014, and 2015–2016) were analyzed in this study. A total of 29,902 respondents participated in three survey cycles. The response rates of data collected through interviews in the three survey cycles were 72.6, 71.0, and 61.3%, respectively. In this study, we excluded 12,854 participants younger than 20 years old, 298 pregnant or lactating females, 2,438 participants with an unfinished depression questionnaire, and 675 participants without 24-h recall data. Finally, 13,637 individuals were included in our study (Figure 1).
Interviewer-administered 24-h dietary recall interview was used to estimate the consumption of foods and beverages. The validity of 24-h dietary recall has been proved in biomarker-based studies (28, 29). All participants are eligible for an in-person dietary interview by trained interviewers in the mobile exam center. The effective Automated Multiple-Pass Method was used to collect dietary data (30). The Automated Multiple-Pass Method is computerized with a five-step multiple-pass approach: collect a quick recall list of foods consumed the previous day, probe for forgotten foods, collect time and occasion of eating, collect detailed information of consumed foods, and final probe. Daily intakes of nutrients and energy were calculated based on self-reported consumed foods, according to the guidance of the Food and Nutrient Database for Dietary Studies.
Twenty-four-hour dietary recall is not representative of usual dietary habits. For reflecting participants' diet more precisely, a sensitivity analysis was conducted (Supplementary Table 2). During the dietary survey, all participants were asked, “was the amount of food that you ate yesterday much more than usual, usual, or much less than usual;” only participants who answered “usual” were included in the sensitivity analysis.
NOVA classifies all foods into four categories according to the degree of processing (2). In this study, we mainly follow UPF with interest. UPFs are manufactured industrial foods, which usually contain abundant fat, saturated fat, sugar, and salt. Generally, UPFs do not contain or only contain a small percentage of unprocessed or minimally processed foods. According to the NOVA food classification system, we classified all food items as UPF or non-UPF. To ensure the accuracy of food classification and the consistency with other studies, we referred to the published literature (31, 32). The details of food classification are shown in the Supplementary Material.
The proportion of UPF in total energy intake (%UPF) was calculated to reflect participants' UPF consumption. UPF consumption was divided into quartiles as the exposure variable.
The nine-item Patient Health Questionnaire (PHQ-9) was used to detect depressive symptoms in NHANES. PHQ-9 consists of nine items, all based on the description of depression in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition. Each item has four options: “No” (0 points), “several days” (1 point), “more than half of the time” (2 points), and “almost every day” (3 points). The total scores are the sum of the scores of all the items, ranging from 0 to 27. In the present study, participants whose PHQ-9 score ≥ 10 were classified as depressive symptoms. This criterion has been confirmed to have good specificity and sensitivity (33). Additionally, in sensitive analysis, individuals who self-reported using antidepressants have also seemed depressive symptoms for testing the stability of the results (Supplementary Table 3).
For controlling the potential confounding factors, we adjusted some covariates in multivariate models. Sociodemographic characteristics included sex, age (20–44 years, 45–59 years, 60 years, or older), race (Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, or other races), educational level (below high school, high school, or over high school), annual family income (<$20,000, $20,000–<$45,000, $45,000–<$75,000, ≥$75,000), and marital status (married/living with a partner, divorced/separated/widowed/single). Body mass index (BMI) was calculated as weight (kilogram) divided by height squared (square meter) and categorized as underweight or normal weight (<25 kg/m2), pre-obesity (25–<30 kg/m2), or obesity (≥30 kg/m2).
Lifestyle characteristics were also considered. Physical activities were evaluated by the Global Physical Activity Questionnaire. Activity levels were divided into active (more than 300 min of moderate-intensity physical activity a week), moderately active (150–300 min of moderate-intensity, or 75–150 min of vigorous-intensity aerobic physical activity per week), and active (<150 min of moderate-intensity physical activity a week). With regard to smoking status, participants were categorized as current smoker, former smoker, and never smoker. Drinking alcohol was defined if they had at least 12 alcohol drinks a year.
In addition, we adjusted some chronic diseases. Blood pressure was measured in the mobile exam center and calculated by the mean of three blood pressure measurements; hypertension was defined as systolic pressure ≥ 130 mmHg and diastolic pressure ≥ 80 mmHg. About two-thirds of NHANES participants did not finish fasting blood glucose measures, so the definition of diabetes was based on self-reported clinical diagnosis. Heart disease and chronic bronchitis were also self-reported.
According to the official guidance of NHANES, we constructed new simple weights by taking one-third of the 2-year weights. New weights were used in an analysis to make an estimate representative of the US civilian non-institutionalized resident population.
We used weighted percentages or means for describing categorical and continuous variables, respectively. For comparing the distribution of sociodemographic characteristics, lifestyle, and dietary intake between the depressive symptoms group and non-depressive symptoms group, we used Cochran–Mantel–Haenszel chi-square test for categorical variables and Student's t-test for continuous variables. The multiple adjusted logistic regression model was used to calculate the odds ratios (ORs) and 95% confidence intervals (95% CIs) for depressive symptoms according to UPF consumption, with the lowest quartile as reference. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, race, educational level, annual family income, marital status, BMI, physical activity, smoking, drinking, hypertension, diabetes, heart disease, and chronic bronchitis. The significance of the linear trend was calculated using the median value of each quartile as a continuous variable in each model. In addition, we conducted stratified analyses to test differed associations among people with different physical activity levels. We also assessed the dose–response relationship by restricted cubic spline with knots at the 5th, 25th, 50th, 75th, and 95th percentiles of the exposure distribution, adjusted for all covariates. Stata 15.0 was used for organizing the data and statistical analyses. All reported probabilities (p-values) were two-sides with a statistical significance level of 0.05.
Table 1 described the demographic and behavioral characteristics of the 13,637 participants included in this analysis. In the analytic sample, participants consumed an average of 1,201 kcal/day of UPF consumption, equivalent to 55% of total energy intake. Depressed individuals tended to consume more UPF. Among all included participants, 1,208 (8.9%) of them met the definition of depressive symptoms. Women had a significantly higher prevalence (11.3%) of depressive symptoms than men (6.4%). Compared with individuals without depressive symptoms, those with depressive symptoms (PHQ-9 score ≥ 10) were middle-aged, less education, lower-income, more obese, and living alone. Depressed individuals were also physically inactive, and they were more likely to smoke. In addition, elevated UPF consumption was associated with low dietary quality (Table 2). People with high UPF consumption tended to intake fewer vitamins and trace elements (n-3 fatty acid, dietary fiber, vitamin C, vitamin E, folate, calcium, and zinc) but more saturated fats, sugars, and energy.
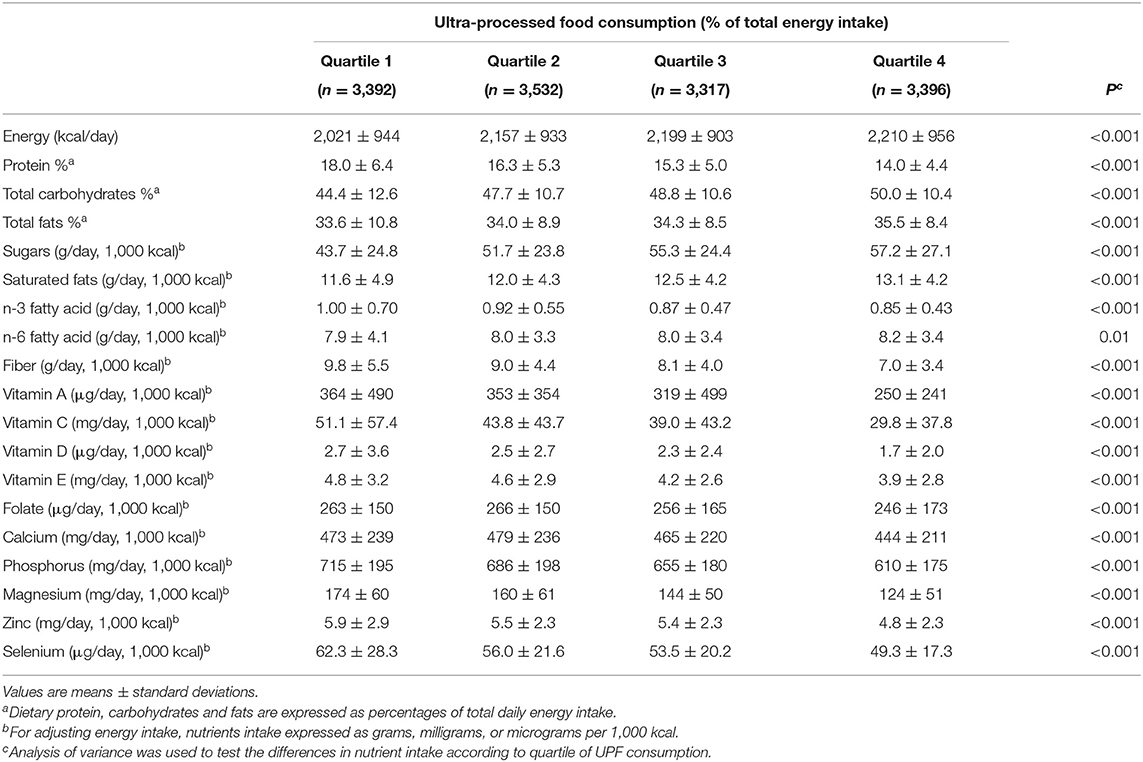
Table 2. Nutrient intake according to quartiles of UPF consumption among US adults aged 20 years, NHANES 2011–2016 (N = 13,637).
Table 3 presents the association between UPF consumption and depressive symptoms. Without adjusting any covariates, a significantly positive association (p = 0.002) was observed between UPF consumption and depressive symptoms; the crude OR with 95% CI was 1.43 (1.10–1.85) for the highest vs. lowest quartile. Model 1 adjusted age and sex, showing the same results as the unadjusted model. Further adjusting for BMI, race, marital status, educational level, family income, smoking, drinking, hypertension, diabetes, heart disease, and chronic bronchitis, the results were still stable; the OR (95% CI) of UPF consumption and depressive symptoms was 1.34 (1.00–1.78) for the highest vs. lowest quartile in the fully adjusted model. Dose–response relationship between UPF consumption and depressive symptoms is shown in Figure 2. In the restricted cubic spline model, a positively linear association was found between the two (p for non-linearity = 0.34). Stratified analyses were performed to assess whether this association was modified by physical activities in Table 4. In models 2 and 3, this positive association between UPF consumption and depressive symptoms was only significant in people with poor physical activity. Among physically active people, the effect on depressive symptoms of UPF was small and not significant.
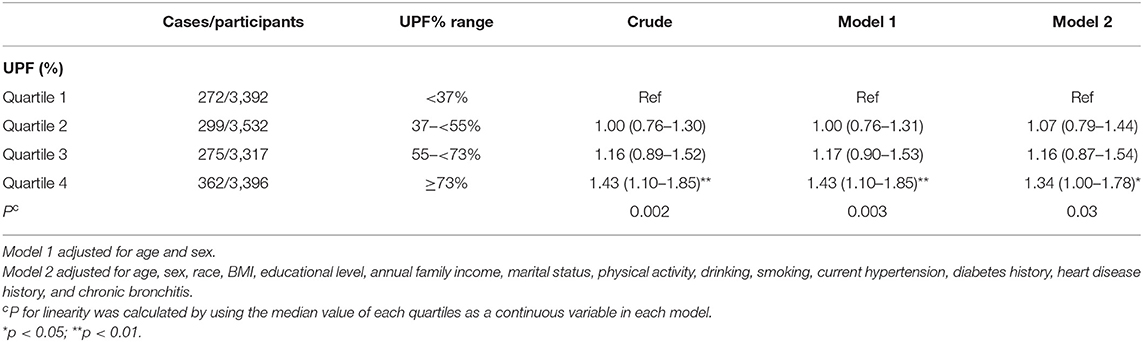
Table 3. Weighted odds ratios (95% confidence intervals) for depressive symptoms across quartiles of UPF% (N = 13,637).
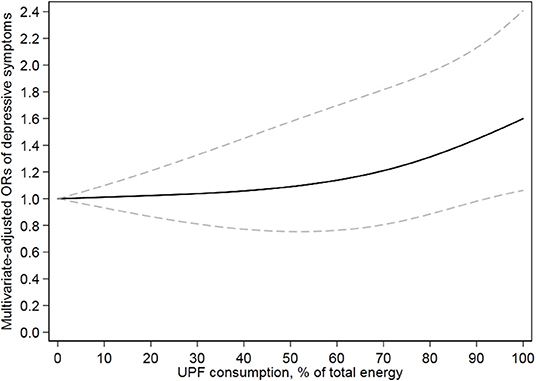
Figure 2. Dose–response relationship of ultra-processed food and risk of depressive symptoms. Model adjusted for age, sex, race, BMI, educational level, annual family income, marital status, physical activity, drinking, smoking, current hypertension, diabetes history, heart disease history, and chronic bronchitis. Solid line and dash line represent the estimated relative risks and their 95% CIs, respectively.
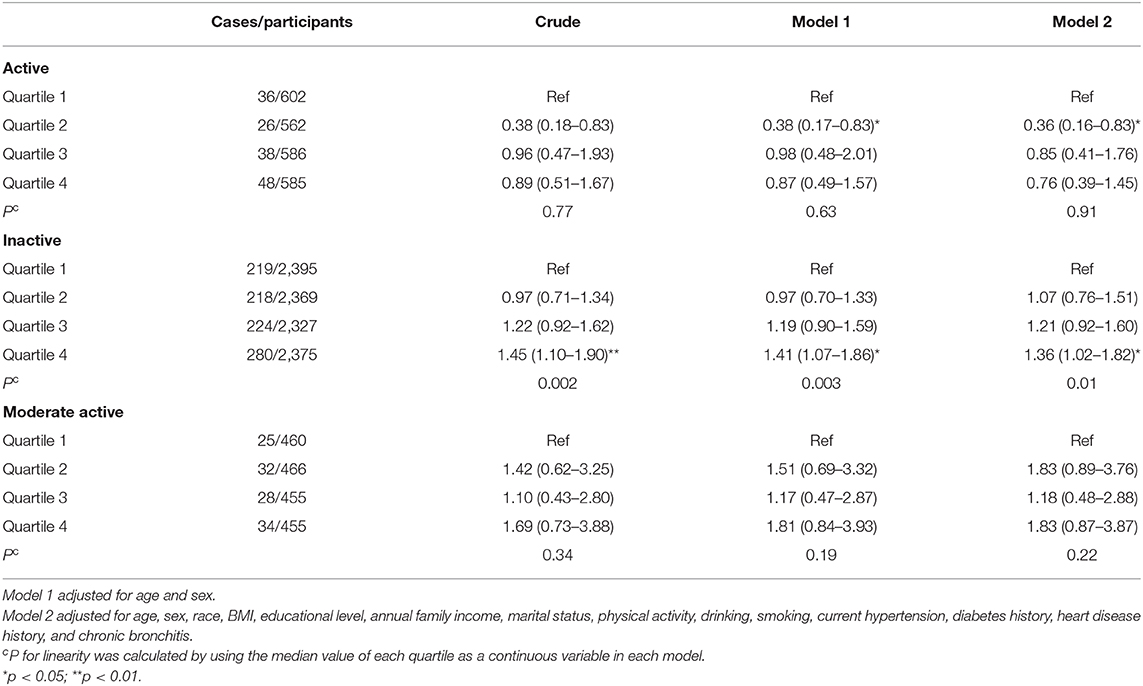
Table 4. Weighted odds ratios (95% confidence intervals) for depressive symptoms according to quartiles of UPF%, stratified by physical activity levels.
Sensitivity analysis only including participants with self-reported “usual intake” showed similar results (Supplementary Table 2). In addition, when both participants with PHQ-9 score ≥ 10 and antidepressant users considered as depressive symptoms (Supplementary Table 3), the positive association between UPF consumption and depressive symptoms was more significant.
In this study, we found UPF consumption was positively associated with depressive symptoms in US adults. After adjusting for sociodemographic characteristics, health behaviors, and chronic disease, participants whose UPF contributed more than 73% of total energy intake had a 35% higher risk of depressive symptoms compared with whose UPF contributed <34% of total energy intake.
Several studies evaluated the effect of UPF or partial components on depressive symptoms. One study from the French NutriNet-Santé cohort reported that high UPF consumption was positively associated with depressive symptoms (27). Another prospective study from the Spain SUN cohort, although conducted in specific university graduates, also found a consistent positive association (26). Nevertheless, both studies were conducted in a population with relatively low UPF consumption; 32% contribute to the total energy in the NutriNet-Santé study, and 276 g/day in the SUN study (vs. 55% and 943 g/day in this study). Our results showed that this positive association still existed in the US population with relatively high UPF consumption. In addition, in Whitehall's study (34), a dietary pattern, mainly containing some typical UPF, for instance, sweetened desserts, fried food, processed meat, refined grains, and high-fat dairy products, was associated with the increased risk for depressive symptoms. In contrast, an association between “processed” pattern and depressive symptoms was non-significant in another UK longitudinal study (35).
This positive association between UPF consumption and depressive symptoms could be explained by the following reasons. First, as a typical part of western dietary pattern, although the nutritional value of different types varies greatly, UPF is often accompanied by low diet quality. A NHANES study reported an inverse dose–response association between UPF and overall diet quality (4). Another study also suggested that reducing the intake of UPF was a potentially effective measure to improve the nutritional quality (8). Low diet quality is widely recognized as a risk factor for depression (36). In this study, as the increase of UPF consumption, the content of nearly all “healthy nutrients,” such as zinc, iron, copper, selenium, dietary fiber and vitamins, also presented the obvious declined trend, many of which are considered to be protective factors of depression (37, 38).
Beyond limited nutritional intakes, high consumption of UPF interfered with the intake of “healthy foods” or minimally processed foods (39), declining the diet quality indirectly. Besides, food additives and neo-formed contaminants derived from processing may also contribute to depressive symptoms. Phthalates and bisphenols are widely used as plasticizers in food packaging; a recent study reported that UPF consumption was associated with higher urinary phthalate metabolites concentrations; some researchers believe that exposure to phthalates would increase the risk of depressive symptoms. Moreover, in a study of Korean teenagers and children, artificial sweetener consumption was related to the increased θ-β ratio (ratios of the θ and β waves in the frontocentral brain areas), which is considered to be linked to some negative emotions, including depressive symptoms (40).
The adverse effect of UPF on the gut microbiome might also contribute to depressive symptoms. As the “virtual endocrine organ” (41), the gut microbiome ferments dietary fiber into short-chain fatty acids that are beneficial to normal intestinal function (42). Poor nutritional quality of UPF may lead to a reduction of probiotics (43). Additionally, some additives could also impact the composition and function of the gut microbiome. An animal experiment found that food-grade titanium dioxide, as a whitening agent, could affect bacterial metabolism and promote biofilm formation to impact bacterial function, although it had little effect on gut microbial composition (44). Impaired gut microbiome may cause intestinal metabolism disorder and inflammatory bowel disease and then affect the central nervous system through the microbiome–gut–brain axis (45, 46), leading to the increased risk of depressive symptoms.
In this study, the association between UPF and depressive symptoms is more significant among inactive people, which may be mediated by obesity. Previous literature has described a positive association between UPF and obesity (25, 47, 48).
This study has several advantages. First, the sample from NHANES is large-size and nationally representative, in favor of reliable results. Additionally, we adjusted for many potential related factors of depressive symptoms in logistic regression models for reducing the interference of covariates as far as possible. However, we have to admit that there are some limitations to this study. Reverse causality is a major limitation of this study; a cross-sectional study was restricted to make causal inferences. Second, food processing methods are many and varied; the degree of processing is difficult to quantify. For some foods, such as canned fruits, it is hard to classify them precisely. Third, the dietary survey in this study was not specially designed to distinguish the degree of food processing; a certain degree of misclassification bias existed inevitably. Fourth, one single 24-h dietary recall may not reflect participants' daily diet precisely (49), and the accuracy of a 24-h dietary recall interview is largely dependent on participants' memory. Poor memory of depressive participants may lead to low dietary intake reporting, making the positive association null. Additionally, the PHQ-9 depression scale is a self-assessment scale reflecting the recent mental state of subjects, not a clinical diagnostic standard for depression. Compared with healthy subjects, people with depressive symptoms may be more reluctant to reply to the scale and cooperate with the research survey, resulting in non-response bias. Indeed, in NHANES, some adults did not respond to the PHQ-9.
In conclusion, a positive association was found between UPF consumption and the risk of depressive symptoms in this study. It is warranted to confirm this cross-sectional association prospectively in the US population. Besides, not only the nutritional quality but also non-nutritional factors may play a role in this positive association. Further studies will be needed to explore specific food additives or neo-formed contaminants' impact on depressive symptoms.
Publicly available datasets were generated in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving human participants were reviewed and approved by NCHS Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
LZ and DZ: conceptualization. LZ and XY: data curation. JS: methodology. LZ: writing—original draft. DZ: writing—review and editing. All authors: contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thanks to all individuals at the National Center for Health Statistics of the Centers for Disease Control and Prevention who collected data and making the datasets of NHANES available on the website.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.600449/full#supplementary-material
UPF, ultra-processed foods; NHANES, National Health and Nutrition Examination Surveys; FNDDS, Food and Nutrient Database for Dietary Studies; PHQ-9, Nine-item Patient Health Questionnaire; BMI, Body mass index; OR, odds ratio; CI, confidence intervals; PUFAs, polyunsaturated fatty acids.
1. Ludwig DS. Technology, diet, and the burden of chronic disease. JAMA. (2011) 305:1352–3. doi: 10.1001/jama.2011.380
2. Monteiro C, Cannon G, Levy R, Moubarac J-C, Jaime P, Martins A. NOVA. The star shines bright. Position paper 2. World Nutr. (2016) 7:28–38. Available online at: https://www.worldnutritionjournal.org/index.php/wn/article/view/5/4
3. Monteiro CA, Levy RB, Claro RM, Castro IRRd, Cannon G. A new classification of foods based on the extent and purpose of their processing. Cad Saude Publica. (2010) 26:2039–49. doi: 10.1590/s0102-311x2010001100005
4. Martinez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metrics. (2017) 15:6. doi: 10.1186/s12963-017-0119-3
5. Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obesity Rev. (2013) 14(Suppl. 2):21–8. doi: 10.1111/obr.12107
6. Moodie R, Stuckler D, Monteiro C, Sheron N, Neal B, Thamarangsi T, et al. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. (2013) 381:670–9. doi: 10.1016/S0140-6736(12)62089-3
7. Luiten CM, Steenhuis IH, Eyles H, Ni Mhurchu C, Waterlander WE. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets. Public Health Nutr. (2016) 19:530–8. doi: 10.1017/s1368980015002177
8. Rauber F, da Costa Louzada ML, Steele EM, Millett C, Monteiro CA, Levy RB. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients. (2018) 10:587. doi: 10.3390/nu10050587
9. Owen L, Corfe B. The role of diet and nutrition on mental health and wellbeing. Proc Nutr Soc. (2017) 76:425–6. doi: 10.1017/s0029665117001057
10. Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, et al. Dietary patterns and depression risk: a meta-analysis. Psychiatry Res. (2017) 253:373–82. doi: 10.1016/j.psychres.2017.04.020
11. Scrinis G, Monteiro CA. Ultra-processed foods and the limits of product reformulation. Public Health Nutr. (2018) 21:247–52. doi: 10.1017/s1368980017001392
12. Martinez Steele E, Monteiro CA. Association between dietary share of ultra-processed foods and urinary concentrations of phytoestrogens in the US. Nutrients. (2017) 9:209. doi: 10.3390/nu9030209
13. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US national health and nutrition examination survey, 2013–2014. Environ Int. (2019) 131:105057. doi: 10.1016/j.envint.2019.105057
14. Laudisi F, Stolfi C, Monteleone G. Impact of food additives on gut homeostasis. Nutrients. (2019) 11:2334. doi: 10.3390/nu11102334
15. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. (2015) 519:92–6. doi: 10.1038/nature14232
16. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. (2012) 36:764–85. doi: 10.1016/j.neubiorev.2011.12.005
17. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
18. Rico-Campà A, Martínez-González MA, Alvarez-Alvarez I, Mendonça RD, de la Fuente-Arrillaga C, Gómez-Donoso C, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ (Clin Res ed). (2019) 365:l1949. doi: 10.1136/bmj.l1949
19. Blanco-Rojo R, Sandoval-Insausti H, López-Garcia E, Graciani A, Ordovás JM, Banegas JR, et al. Consumption of ultra-processed foods and mortality: a national prospective cohort in Spain. Mayo Clin Proc. (2019) 94:2178–88. doi: 10.1016/j.mayocp.2019.03.035
20. Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Public Health Nutr. (2019) 22:1777–85. doi: 10.1017/s1368980018003890
21. Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Allès B, Méjean C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ (Clin Res ed). (2018) 360:k322. doi: 10.1136/bmj.k322
22. Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the Nutrinet-Santé prospective cohort. JAMA Intern Med. (2019) 180:283–91. doi: 10.1001/jamainternmed.2019.5942
23. Srour B, Fezeu LK, Kesse-Guyot E, Alles B, Mejean C, Andrianasolo RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante). BMJ (Clin Res ed). (2019) 365:l1451. doi: 10.1136/bmj.l1451
24. Sandoval-Insausti H, Blanco-Rojo R, Graciani A, López-García E, Moreno-Franco B, Laclaustra M, et al. Ultra-processed food consumption and incident frailty: a prospective cohort study of older adults. J Gerontol A Biol Sci Med Sci. (2020) 75:1126–33. doi: 10.1093/gerona/glz140
25. Juul F, Martinez-Steele E, Parekh N, Monteiro CA, Chang VW. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. (2018) 120:90–100. doi: 10.1017/s0007114518001046
26. Gomez-Donoso C, Sanchez-Villegas A, Martinez-Gonzalez MA, Gea A, Mendonca RD, Lahortiga-Ramos F, et al. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN Project. Eur J Nutr. (2020) 59:1093–103. doi: 10.1007/s00394-019-01970-1
27. Adjibade M, Julia C, Alles B, Touvier M, Lemogne C, Srour B, et al. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Sante cohort. BMC Med. (2019) 17:78. doi: 10.1186/s12916-019-1312-y
28. Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. (2011) 174:591–603. doi: 10.1093/aje/kwr140
29. Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. (2003) 158:1–13. doi: 10.1093/aje/kwg092
30. Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr. (2006) 136:2594–9. doi: 10.1093/jn/136.10.2594
31. Martínez Steele E, Baraldi L, Louzada M, Moubarac J, Mozaffarian D, Monteiro C. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. (2016) 6:e009892. doi: 10.1136/bmjopen-2015-009892
32. Martínez Steele E, Juul F, Neri D, Rauber F, Monteiro C. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med. (2019) 125:40–8. doi: 10.1016/j.ypmed.2019.05.004
33. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
34. Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. (2009) 195:408–13. doi: 10.1192/bjp.bp.108.058925
35. Northstone K, Joinson C, Emmett P. Dietary patterns and depressive symptoms in a UK cohort of men and women: a longitudinal study. Public Health Nutr. (2018) 21:831–7. doi: 10.1017/s1368980017002324
36. Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. (2018) 226:346–54. doi: 10.1016/j.jad.2017.09.022
37. Xu H, Li S, Song X, Li Z, Zhang D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition (Burbank, Los Angeles County, Calif). (2018) 54:48–53. doi: 10.1016/j.nut.2018.03.009
38. Li Z, Wang W, Xin X, Song X, Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. (2018) 228:68–74. doi: 10.1016/j.jad.2017.12.004
39. Canella DS, Louzada MLdC, Claro RM, Costa JC, Bandoni DH, Levy RB, et al. Consumption of vegetables and their relation with ultra-processed foods in Brazil. Rev Saude Publica. (2018) 52:50. doi: 10.11606/s1518-8787.2018052000111
40. Saad JF, Kohn MR, Clarke S, Lagopoulos J, Hermens DF. Is the theta/beta EEG marker for ADHD inherently flawed? J Atten Disord. (2018) 22:815–26. doi: 10.1177/1087054715578270
41. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. (2014) 28:1221–38. doi: 10.1210/me.2014-1108
42. Shepherd SJ, Lomer MCE, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. (2013) 108:707–17. doi: 10.1038/ajg.2013.96
43. Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. The microbiome of uncontacted Amerindians. Sci Adv. (2015) 1:e1500183. doi: 10.1126/sciadv.1500183
44. Pinget G, Tan J, Janac B, Kaakoush NO, Angelatos AS, O'Sullivan J, et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front Nutr. (2019) 6:57. doi: 10.3389/fnut.2019.00057
45. Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. (2016) 158:52–62. doi: 10.1016/j.pharmthera.2015.11.012
46. Koopman M, El Aidy S, consortium MI. Depressed gut? The microbiota-diet-inflammation trialogue in depression. Curr Opin Psychiatry. (2017) 30:369–77. doi: 10.1097/YCO.0000000000000350
47. Beslay M, Srour B, Méjean C, Allès B, Fiolet T, Debras C, et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med. (2020) 17:e1003256. doi: 10.1371/journal.pmed.1003256
48. Sandoval-Insausti H, Jiménez-Onsurbe M, Donat-Vargas C, Rey-García J, Banegas JR, Rodríguez-Artalejo F, et al. Ultra-processed food consumption is associated with abdominal obesity: a prospective cohort study in older adults. Nutrients. (2020) 12:2368. doi: 10.3390/nu12082368
Keywords: depressive symptoms, ultra-processed food, dose-response, cross-sectional study, NHANES
Citation: Zheng L, Sun J, Yu X and Zhang D (2020) Ultra-Processed Food Is Positively Associated With Depressive Symptoms Among United States Adults. Front. Nutr. 7:600449. doi: 10.3389/fnut.2020.600449
Received: 30 August 2020; Accepted: 19 November 2020;
Published: 15 December 2020.
Edited by:
Francesco Visioli, University of Padua, ItalyReviewed by:
Cinzia Ferraris, University of Pavia, ItalyCopyright © 2020 Zheng, Sun, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongfeng Zhang, emhhbmdkZjE5NjFAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.