
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 17 December 2020
Sec. Food Chemistry
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.596823
 Yue-Yue Sheng1
Yue-Yue Sheng1 Jing Xiang1
Jing Xiang1 Ze-Shi Wang1
Ze-Shi Wang1 Jing Jin2
Jing Jin2 Ying-Qi Wang1
Ying-Qi Wang1 Qing-Sheng Li1
Qing-Sheng Li1 Da Li1
Da Li1 Zhou-Tao Fang1
Zhou-Tao Fang1 Jian-Liang Lu1
Jian-Liang Lu1 Jian-Hui Ye1
Jian-Hui Ye1 Yue-Rong Liang1*
Yue-Rong Liang1* Xin-Qiang Zheng1*
Xin-Qiang Zheng1*Theacrine, i.e., 1,3,7,9-tetramethyluric acid, is one of the major purine alkaloids found in leaf of a wild tea plant species Camellia kucha Hung T. Chang. Theacrine has been attracted great attentions academically owing to its diverse health benefits. Present review examines the advances in the research on the health beneficial effects of theacrine, including antioxidant effect, anti-inflammatory effect, locomotor activation and reducing fatigue effects, improving cognitive effect, hypnotic effect, ameliorating lipid metabolism and inhibiting breast cancer cell metastasis effect. The inconsistent results in this research field and further expectations were also discussed.
Theacrine is a purine alkaloid found in Camellia kucha Hung T. Chang (a wild tea plant species, formerly named as Camellia assamica var. kucha) (1, 2), and its full name is 1,3,7,9-tetramethyl-1H-purine-2,6,8(3H,7H,9H)-trione, with chemical formula C9H12N4O3 and molecular weight 224.22 (Figure 1). It was recently reported that theacrine was detected in Camellia sinensis var. puanensis (3), Ilex vomitoria (4), and Camellia gymnogyna (5, 6). The content of theacrine in tender shoots with two leaves and a bud of C. kucha (Kucha) was 1.3–3.4% based on dry weight (DW). Pure theacrine could be obtained by separating from Kucha leaf through high-speed counter-current chromatography using eluent solvent system composed of hexane/dichloromethane/methanol/water (1/5/4/2, v/v/v/v) (7). Theacrine is a specific purine alkaloid in Kucha and no or little theacrine was detected in the leaf of Camellia sinensis which is usually used for processing green tea or black tea. Both theacrine and caffeine are detected in leaf of C. kucha. Though it is considered that theacrine is biosynthesized from caffeine catalyzed by N-methyltransferase using S-adenosyl-L-methionine (SAM) as methyl donor (Figure 2), the exact molecular mechanism of theacrine metabolism in Kucha is still unclear (8–10). A theacrine synthase in C. kucha (CkTcS) has been identified recently (11). The CkTcS possesses novel N9-methyltransferase activity using 1,3,7-trimethyluric acid but not caffeine as a substrate to biosynthesize theacrine, during which the C8 oxidation of caffeine molecule takes place prior to N9-methylation (8).
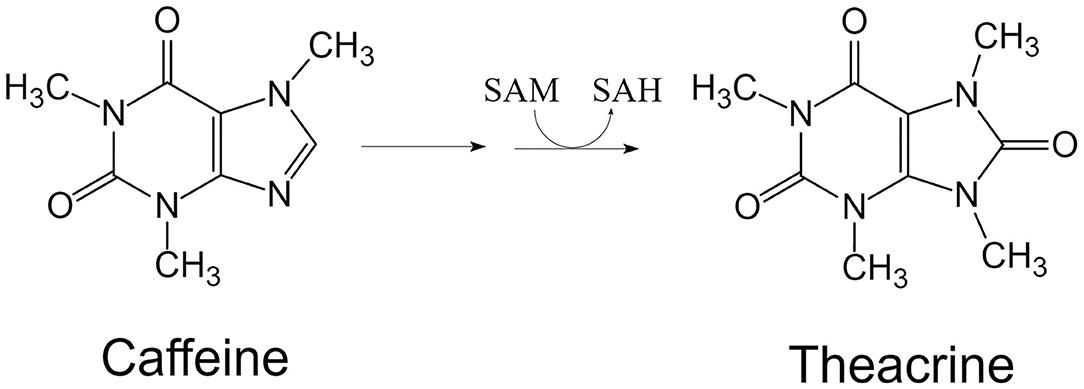
Figure 2. Theacrine is biosynthesized from caffeine catalyzed by N-methyltransferase using SAM as methyl donor. SAM, S-adenosyl-L-methionine; SAH, S-adenosyl-L-homocysteine.
Theacrine has been attracted great attentions academically owing to its diverse healthy benefits (1–3), including reducing inflammation (12), providing stimulatory effects without adaptation or habituation (13), antioxidant effect (14), enhancing sustained energy (15), promoting positive mood elevation, and mental clarity (15–17) and etc. However, these researches lack systematic summarization and some important mechanisms remain to be further investigated. In this review, we summarized the achievements in the study on health benefits of theacrine and the possible mechanisms behind the observed associations (Table 1), so as to encourage the scientific community to pay more attention to the nutraceutical properties of theacrine.
Acute toxicity test (14 d) on mice using extracts of Kucha showed that the semi-lethal dose (LD50) of Kucha extracts and theacrine was 5,600 mg/kg bw/day (12) and 810.6 mg/kg bw/day (22), respectively. Four-week oral administration of pure theacrine by rats at dose up to 150 mg/kg bw/day, showed that the appearance and behavior, body weights, organ coefficient, hematological and biochemical parameters of the tested rats showed as normal as control group. Pathological examination showed no changes induced by the drug toxicity, and no delayed toxic reaction was observed after stopped giving drug (40). A 90-day oral toxicological evaluation showed that the no observed adverse effect level (NOAEL) of theacrine was 180 mg/kg bw/day, as at this dose there were no toxicologically relevant treatment-related findings in male or female animals (41).
Oral administration test on sixty healthy men and women using TeaCrine®, a nature-identical, chemically equivalent bioactive version of theacrine, confirmed that daily supplementation theacrine up to 300 mg/kg bw/day for more than 8 weeks was clinically safe, without habituating neuro-energetic effects (13). Tests on one-hundred twenty-five men and women (mean age 23.0 years, height 169.7 cm, body mass 72.1 kg; n = 25 persons/group) showed that oral consumption of a mixture containing methylliberine (100–150 mg) and theacrine (25–50 mg) showed no negative effect on the health over 4 weeks of continuous oral administration (42).
The leaf of C. kucha containing 2.86% (W/W) theacrine and 2.13% (W/W) caffeine had stronger antioxidant activity than Camellia sinensis (Longjing tea) containing 4.64% (W/W) caffeine but no theacrine (14). The IC50 value (inhibitory concentration in mg/mL of tea necessary to reduce the absorbance by 50%) of C. kucha in scavenging DPPH radicals was 0.79 ± 0.022 mg/mL, being lower than that of C. sinensis (0.91 ± 0.015 mg/mL). The TEAC value (Trolox equivalent antioxidant capacity, TEAC (mg/g) = IC50(trolox)/IC50(sample) × 1,000) of C. kucha in scavenging DPPH radicals (436.78 ± 12.44 mg/g) was higher than that of C. sinensis (376.12 ± 5.81 mg/g) (14). The TEAC value of C. kucha in FRAP assay (493.67 ± 8.02 mg/g) was also higher than that of C. sinensis (438.05 ± 9.54 mg/g) (14). These suggest that C. kucha containing theacrine had stronger DPPH and FRAP radical scavenging activities than C. sinensis without theacrine. Purine alkaloids including caffeine, theophylline, theobromine and theacrine, which are detected in C. kucha, showed comparatively lower antioxidant activities in oxygen radical absorbance capacity (ORAC) and cellular antioxidant activity assay (CAA) than Trolox. The ORAC values of theabromine, theophylline, caffeine and theacrine were ~65, ~37, ~35, and ~25% of that of Trolox (18). Though theacrine showed lower activity in ORAC and CAA than theophylline and theobromine, it could significantly improve vessel density on chorioallantoic membrane and myocardial apoptosis in chick embryo model (18). These properties are related to its upregulating expression of sirtuin which is a group of highly conserved NAD-dependent histone deacetylases in mammals and has regulatory effects on cell survival, proliferation, metabolism, death and aging, as well as longevity of organs. Theacrine upregualted the expression of sirtuin family member SIRT3, which in turn activated the SIRT3/FOXO3/SOD2 signaling pathway, resulting in improvement of the intervertebral disc degeneration (19) and the cardiac function of ovariectomized mice with myocardial infarction (20) by alleviating oxidant stress. Furthermore, the antioxidant capacity of theacrine was also due to its strengthening the antioxidant system in vivo, such as elevating activities and gene expressions of superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), as well as reducing activity of xanthine oxidase, resulting in significant decrease in the content of malondialdehyde (MDA) and increase in ORAC level in plasma and liver of oxidant-stressed mice (21).
Oral administration of theacrine by mice (8–32 mg/kg bw/day) significantly inhibited the ear edema induced by xylene and hind paw edema induced by λ-carrageenan in a dose dependent manner (22). Oral administration of theacrine showed potent anti-inflammatory activities. Theacrine inhibited the xylene-induced ear edema, in which the inhibition percentages were 10.1, 12.9, and 45.2% at doses 8, 16, and 32 mg/kg, respectively, compared with the control. Maximal inhibitory rates of anti-edema effect of theacrine at doses 8, 16 and 32 mg/kg were observed at the 2 h, with inhibitory rates being 14.1, 16.6, and 20.1% (P < 0.05), respectively, compared with 18.8% of indomethacin (22). Theacrine showed inflammatory cell infiltration and focal necrosis of hepatocytes. Oral administration of theacrine (10, 20, 30 mg/kg DW/day) for 7 consecutive days was found to reduce hepatic mRNA levels of inflammatory mediators (IL-6, IL-1β, TNF α, and IFN γ), decreased the levels of plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and reversed the histologic damages in liver of restraint stressed mice (43). Oral administration of theacrine (12.5–25.0 mg/kg bw/day) showed therapeutic effect on arthritis in SD rat (43). An aqueous formula consisting of bioactive form of theacrine, liberine and methylliberine was used as dietary supplement for reducing inflammation, pain, depression, and alleviating deleterious effects of hyperglycemia and sleep deprivation (43).
Theacrine shows anti-inflammation effect by interfering with the actions of inflammatory mediators such as histamine, serotonin, and bradykinin (22). The inflammatory mediators histamine, serotonin, and bradykinin induce edema by promoting vasodilatation and increasing vascular permeability (44). λ-Carrageenan-induced edema is a biphasic event, in which the early phase (90–180 min) of the inflammation is due to the release of histamine, serotonin and similar substances, and the later phase (270–360 min) is associated with the activation of kinin-like substances (45). Theacrine functions on the first phase of inflammation by interacting with histamine and serotonins and other similar mediators. Furthermore, theacrine has a membrane stabilizing effect, results in reduction of capillary permeability (22). The anti-inflammation mechanism of theacrine is also involved in its enhancement of transforming growth factor-β (TGF-β) mediated shifts via TGF-β/SMAD pathway (23). Biological signals for TGF-β are transduced through transmembrane serine/threonine kinase receptors to a family of intracellular mediators known as SMADs. Theacrine upregulated the expression of RNA and protein of SMAD3, p-ERK and p-p38, but downregulated the expression of nuclear factor-kB (NF-kB) and interleukin-6 (IL-6) in Freund's incomplete adjuvant (FIA)-induced SD rats (23).
There were many studies showing that theacrine activated locomotor and reduced fatigue. In comparison to placebo under double-blind conditions, oral administration of TeaCrine® at a dose equivalent 200 mg theacrine resulted in more energy, less fatigue, better concentration and mood (15). Dietary supplement with 5–850 mg theacrine would improve mood, energy and focus, accompanying with reduction of anxiety or fatigue (25). A formula containing theacrine and caffeine with weight ratio 2:1 to 4:1 induced an increase in energy of at least 8%, accompanying with a decrease in fatigue at least 6% (26). A pre-workout supplement of 10 mg theacrine improved selective attention (27). 27–38% improvements in time-to-exhaustion were observed in participants with supplement of 275 mg theacrine, leading athletes to sustain greater focus under fatigue for long periods (16). Sense of energy was significantly increased from baseline to 2 h post-ingestion in treatments of caffeine and combination of theacrine + caffeine; meanwhile, oral administration of 125 mg theacrine produced a significant rise in energy in 3 h post-ingestion, with little impact on heart rate (HR) and blood pressure (BP) (17). The mechanism of theacrine activating locomotor and reducing fatigue is likely involved in its action as an adenosine receptor antagonist. There was study showing that pre-treatment with theacrine (24 or 48 mg/kg, i.p.) significantly attenuated the motor depression induced by the adenosine receptor A1 agonist (N6-cyclopentyladenosine, CPA; 0.1 mg/kg, i.p.) and receptor A2A agonist (2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine; 0.2 mg/kg, i.p.), but this effect was reduced by both antagonists D1R SCH23390 (0.1 or 0.05 mg/kg, i.p.) and D2R eticlopride (0.1 mg/kg, i.p.), indicating that theacrine is likely acting as an adenosine receptor antagonist (24).
Test on mice showed that oral administration of theacrine (5, 10, 15 mg/kg) significantly reversed learning and memory impairment caused by central fatigue (28). Combination of theacrine (125 mg) and caffeine (150 mg) moderately improved cognitive performance as assessed by trail making test (TMT) (17) and showed modest cognitive benefits during complex decision making, potentially due to overlapping peak concentrations or enhanced bioavailability (26, 27). The improved cognitive accuracy at end-of-game in all conditions may indicate a training effect in highly skilled players for allocation of resources (16).
The cognitive improving effect of theacrine was associated with its regulating brain glucose metabolism, inhibiting phosphodiesterases and restoring levels of fatigue-related neurotransmitters in the brains, such as 5-hydroxytryptamine (5-HTP) and dopamine as well as their metabolites (28).
Parkinson's disease (PD) is a long-term degenerative disorder of the central nervous system that mainly affects the motor system, affecting about 1% of adults older than 60 years (28). Oxidative damage of dopaminergic neurons is the fundamental causes of PD. Tests using multiple animal models of PD (6-OHDA-treated rats and in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice and zebrafish) and 1-methyl-4-phenylpyridinium (MPP+)-treated SH-SY5Y cells showed that theacrine had the potency to relieve PD by retrieving the apoptosis of dopaminergic neurons and activating SIRT3 which deacetylating SOD2 and restoring mitochondrial functions (46).
Theacrine is structurally similar to caffeine (Figure 2), but in fact its physiological effects are quite different from the latter. Theacrine is shown to be stimulatory when used in higher doses, however, it can actually have a sedative effect when used in low doses such as the amount consumed through tea leaves of C. kucha. Oral administration of theacrine (10 and 30 mg/kg) could significantly prolong the sleeping time induced by pentobarbital (p < 0.01), while caffeine and theobromine exhibited an inverted effect on mice. This suggests that theacrine possesses potent sedative and hypnotic properties and its central nervous system effects is different from those of caffeine and theobromine (29). Furthermore, caffeine has been demonstrated to show signs of tolerance build-up in as little as 4 days, while theacrine shows no such adaptation (13).
Intragastric administration of theacrine (3, 10, 30 mg/kg BW/day) showed antidepressant effects on mice, including shortening the immobility time in the tail suspension test (P < 0.05), enhancing the rotatory locomotor activities during forced swimming test (P < 0.05), increasing the mortality induced by yohimbine and the head-twitching number induced by 5-HTP (p < 0.01). Theacrine could also markedly improve such symptoms as hypothermia (P < 0.01), akinesia (P < 0.05), and eye ptosis of mice (P < 0.01) induced by reserpine, which may be contributed to its influence on monoamine neurotransmitter (30). Sleep parameter analysis by electroencephalogram (EEG) and electro-magnetic gun (EMG) showed that theacrine (3.0 mg/kg, i.g.) significantly enhanced pentobarbital-induced sleep in mice by shortening wake time and increasing non-rapid-eye-movement sleep (NREMS) time, but it had no effect on rapid-eye-movement sleep (REMS). Meanwhile, theacrine markedly attenuated caffeine (a non-selective antagonist of adenosine receptor)-induced insomnia (31). Theacrine worked as a non-selective adenosine receptor agonist to induce a hypnotic effect through the adenosine system. There was study showing that theacrine significantly reversed the decrease in sleeping time in mice pretreated with the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and the A2A receptor antagonist 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-(4,3-e)-1,2,4-triazolo[1,5-c]pyrimidine (SCH 58261), during which theacrine markedly increased the adenosine content but inhibited adenosine deaminase activities in the hippocampus of rats (31). These suggested that theacrine mediates the adenosine system to augment pentobarbital-induced sleep.
An in vitro test showed that theacrine up-regulated SIRT3, LCAD, FABP4 and LPL gene expression (32). In vivo test showed that theacrine (10 mg/kg) up-regulated SIRT3 gene expression in liver, but 40 mg/kg of theacrine showed inhibition on SIRT3. Low-dose theacrine (10 and 20 mg/kg) up-regulated hepatic LCAD, FABP4, HTGL, and ATP5c mRNA expression, while high-dose theacrine (40 mg/kg) down-regulated their levels. It shows that theacrine has an effect on fatty acid metabolism through regulating fatty acid metabolism related genes, and the mechanism may be related with its up-regulation of SIRT3 expression (32). Extract (50–200 μg/mL) of C. kucha with 1.44% (W/W/) theacrine showed significant suppressive effect on lipid droplet accumulation and triglyceride (TG) level in 3T3-L1 cells in a dose dependent manner by suppressing the expression of genes involving in lipid metabolism, including FAS, FAT, SCD-1, LPL, SREBP-1c, and ACC (33). Test on C57B/6J mice showed that theacrine significantly decreased hepatic TG content (p < 0.01) and ameliorated hepatic steatosis in mice (34). Acylcarnitine metabolism disorder contributes significantly to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Theacrine ameliorated acylcarnitine metabolism disorder in high-fat diet (HFD)-fed mice, resulting in suppression of hepatic steatosis and liver inflammation and improvement of energy expenditure through the SIRT3/LCAD signaling pathway (35).
The underlying mechanism that theacrine ameliorates high fat diet induced hepatic steatosis in mice involves in SIRT3/AMP-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) pathway, during which the activation of ACC by theacrine depends on the phosphorylation of AMPK, but the activation of SIRT3 by theacrine is independent of the phosphorylation of AMPK (34). Theacrine promotes acylcarnitine metabolism in non-alcoholic fatty liver disease (NAFLD) through the SIRT3/LCAD signaling pathway, in which the target of theacrine's activities on NAFLD is identified as SIRT3 (35). Theacrine up-regulated the expression of SIRT3 as well as the phosphorylation of AMPK and ACC (28). Theacrine activated the mitochondrial deacetylase SIRT3 and consequently, the increased activity of long-chain acyl coenzyme A (CoA) dehydrogenase (LCAD) through deacetylation (32, 35). Peroxisome proliferator-activated receptor γ (PPAR γ) and CCAAT/enhancer-binding protein α (C/EBP α) are considered to be the key regulators of adipogenesis (47, 48). Theacrine suppressed the lipid droplet accumulation in 3T3-L1 adipocytes by downregulation of the expression of major transcription factors of adipogenesis pathway including PPAR γ and C/EBP α (33). Furthermore, theacrine also decreased the mRNA and protein levels of fatty acid synthase, fatty acid translocase, steroylcoenzyme A desaturase-1, lipoprotein lipase and acetyl-CoA carboxylase-1 (33).
Theacrine also inhibited transforming growth factor-β (TGF-β) induced cell adhesion, migration, and invasion, showing antimetastatic potential implications for disease management in breast cancer (36). Epithelial-to-mesenchymal transition (EMT), whereby epithelial cells transform into mesenchymal cells, is shown to be related to cancer and cancer cell metastasis. The EMT process is coordinated by the loss of expression of epithelial marker such as Occludin and E-cadherin, accompanying with gain of mesenchymal markers such as Vimentin, Fibronectin and N-cadherin, which promote tumor progression, cell invasion, and metastasis (49). The EMT markers expression is associated with poor prognosis of breast cancer patients (50, 51). TGF-β-induced EMT has been recognized as an emerging mechanism underlying its metastasis-promoting function (52). The regulators of EMT have become attractive targets for developing anti-metastasis therapies. Theacrine reversed EMT, resulting in downregulation of mesenchymal markers including Fibronectin, Vimentin, N-cadherin, Twist, and Snail and upregulation of epithelial markers such as Occludin and E-cadherin in human breast cancer MDA-MB-231 cells. Also, theacrine attenuated TGF-β-induced EMT, cell adhesion, migration, and invasion in the MDA-MB-231 cells. These suggest that theacrine shows suppressive effects on TGF-β-induced progression and breast cancer cell metastasis by reversing the EMT process (36).
Furthermore, there was study revealing that theacrine showed anti-virus activity. Theacrine at 50 μM dosage significantly inhibited the expression of NS1 protein in human influenza virus A/Puerto Rico/8/34 at 12 hpi, and 100 μM theacrine significantly suppressed the virus progeny production (37).
Theacrine is a purine alkaloid found in Camellia kucha and it shows diverse health benefits. Theacrine upregulates the expression of SIRT3 and activates the SIRT3/FOXO3/SOD2 signaling pathway, resulting in antioxidant efficiency. Theacrine upregulates the expression of RNA and protein of SMAD3, p-ERK and p-p38 and downregulates the expression of NF-kB and IL-6, showing anti-inflammatory effects. Theacrine acts as an adenosine receptor antagonist to play a role in the locomotor activation and fatigue reduction. Theacrine regulates brain glucose metabolism, inhibits phosphodiesterases and restores the levels of 5-HTP and dopamine to improve cognitive capacity. Theacrine possesses potent sedative and hypnotic properties and its central nervous system effects through mediating the adenosine system. Theacrine ameliorates lipid metabolism by activating SIRT3/AMPK/ACC pathway and downregulating the mRNA and protein levels of fatty acid synthase, fatty acid translocase, steroylcoenzyme A desaturase-1, lipoprotein lipase and acetyl-CoA carboxylase-1. Theacrine shows suppressive effects on TGF-β-induced progression and breast cancer cell metastasis by reversing EMT, which in turn leads to the downregulation of mesenchymal markers and upregulation of epithelial markers, and also attenuating TGF-β-induced EMT, cell adhesion, migration, and invasion. The bioactivities of theacrine are shown in Figure 3.
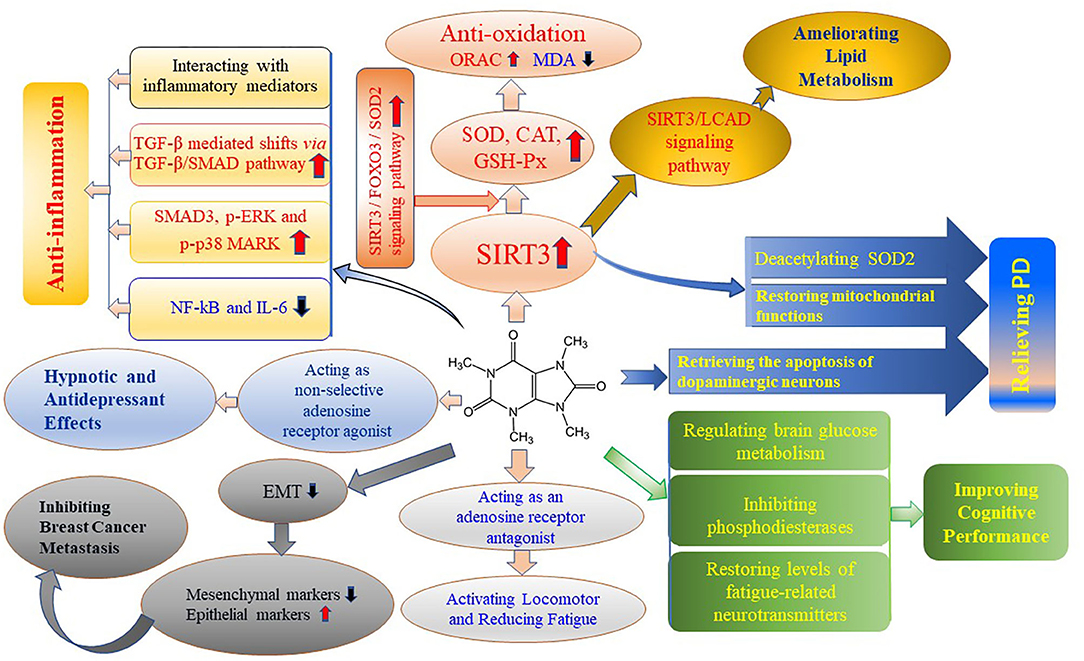
Figure 3. Bioactivities of theacrine. The end of each direction shows one bioactivity of theacrine, in which the intermediates show the actions of theacrine or signaling pathways. CAT, Catalase; EMT, Epithelial-to-mesenchymal transition; FOXO3, Forkhead Box O3, a protein coding gene, including DNA-binding transcription factor activity and protein kinase binding; GSH-Px, Glutathione peroxidase; IL-6, interleukin-6; LCAD, Lightweight chemical agent detector; NF-kB, Nuclear factor-kb; ORAC, oxygen radical absorbance capacity; PD, Parkinson's disease; p-ERK, phosphorylated extracellular regulated protein kinases; p-p38 MARK, phosphorylated mitogen-activated protein kinases; MDA, malondialdehyde; SIRT3, Sirtuin-3, a major mitochondria NAD+-dependent deacetylase; SMAD, the proteins are required for the transmission of the TGF-β signal to the nucleus; SOD, Superoxide dismutase; TGF-β, Transforming growth factor-β. The red up arrow means increase in the indicators and the blue down arrow means decrease in the indicators.
However, there is still a long way to go to the clinical application of theacrine. First, the mechanism underlying the physiological activities of theacrine remains to be further explored. Theacrine has similar chemical structure as caffeine, but how their physiological activities are differentiated greatly? The mechanism why theacrine was stimulatory at higher doses, but it could actually have a sedative effect at low doses remains to be clarified (30). Further studies need to elucidate the mechanisms by which theacrine downregulated the EMT markers (36). Second, drug–drug interaction between theacrine and other compounds needs to be further investigated. It was found that co-administration of theacrine and caffeine resulted in a clinically significant pharmacokinetic interaction, viz., caffeine co-administration increased maximum plasma concentration and the area under the curve of theacrine without altering theacrine half-life in comparison with the corresponding pharmacokinetic parameters when theacrine is administered alone (53). There was pharmacokinetic interaction between methylxanthines and theacrine in humans when theacrine (50 mg), methylliberine (100 mg) and caffeine (150 mg) were orally administered (54). More work needs to probe how the enhanced oral bioavailability was happened. Third, some inconsistent results remain to be reconfirmed. It was reported that acute intake of the theacrine-containing dietary supplement did not improve cognitive performance statistically though it favorably impacted multiple subjective feelings related to energy and mood (38). There was study showing that neither theacrine (300 mg), caffeine (300 mg) and nor combination of both theacrine (150 mg) and caffeine (150 mg) improved muscular strength, power, or endurance performance in resistance-trained men when consumed 90 min pre-exercise (39). Caffeine treatment led to a marked increase in the ambulatory activity accompanied with decreasing of the immobility time in forced swimming test at both 10 and 30 mg/kg. Under the same conditions, neither theacrine nor theobromine showed obvious excited efficacy (29, 55). The clarification of these issues would be greatly helpful for developing clinical applications of theacrine.
Y-RL, Y-YS, and X-QZ conceived the project and references collection. Y-YS introduction. JX toxicological evaluation of theacrine. Z-SW and JJ anti-oxidant effects. Y-QW anti-inflammatory effects. Q-SL and Z-SW locomotor activation and reducing fatigue. Z-TF and DL improving cognitive performance. J-LL and J-HY ameliorating lipid metabolism. Y-RL inhibiting breast cancer cell metastasis and abstract. X-QZ conclusions and future expectations. All authors contributed to the article and approved the submitted version.
This research was financially supported by grants from the Science Technology Department of Zhejiang Province (Project No. 2016C02053-5) and the Ningbo Municipal Bureau of Science and Technology (Project No. 2017C10001), China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors appreciated the Science Technology Bureau of Ningbo City and the Science Technology Department of Zhejiang Province for offering financial support to the present project.
5-HT, 5-Hydroxytryptamine; 5-HTTP, 5-Hydroxytryptophan; 6-OHDA, 6-Hydroxydopamine; AAPH, 2,20-Azobis(2-amidinopropane) dihydrochloride; ACC, Acetyl-coa carboxylase; ALT, Alanine aminotransferase; AMP, Adenosine 5'-monophosphate; AMPK, AMP-activated protein kinase; AST, Aspartate aminotransferase; ATP, Adenosine triphosphate; C/EBP a, CCAAT/enhancer-binding protein a; CAA, Cellular antioxidant activity assay; CAT, Catalase; C/EBP α, CCAAT/enhancer-binding protein α; CkTcS, Theacrine synthase in C. Kucha; CoA, Coenzyme a; CPA, Cyclopentyladenosine; DA, Dopamine; DDPH, Diphenyl-2-picrylhydrazyl; DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; DW, Dry weight; EEG, Electroencephalogram; EMG, Electro-magnetic gun; EMT, Epithelial-to-mesenchymal transition; FABP, Fatty acid-binding protein; FAS, Fatty acid synthase; FAT, Fatty acid translocase; FIA, Freund's incomplete adjuvant; FOXO3, Forkhead Box O3, a protein coding gene, including DNA-binding transcription factor activity and protein kinase binding; FRAP, Ferric reducing ability of plasma; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; GSH-Px, Glutathione peroxidase; HFD, High fat diet; Hpi, Hour post-infection; IFN, Interferon; IL, Interleukin; LCAD, Lightweight chemical agent detector; LPL, Lipoprotein lipase; LTL, Liver triglyceride lipase; MDA, Malondialdehyde; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; NAFLD, Nonalcoholic fatty liver disease; NF-kB, Nuclear factor-kb; NOAEL, No observed adverse effect level; NREMS, Non-rapid-eye-movement sleep; ORAC, Oxygen radical absorbance capacity; PD, Parkinson's disease; PPAR, Peroxisome proliferator-activated receptor; p-ERK, phosphorylated extracellular regulated protein kinases; p-p38 MARK, phosphorylated mitogen-activated protein kinases; PWS, Preworkout supplements; REMS, Rapid-eye-movement sleep; SAM, S-adenosyl-L-methionine; SCD, Stearoyl-coa desaturase; SIRT3, Sirtuin-3 (SIRT3), a major mitochondria NAD+-dependent deacetylase; SOD, Superoxide dismutase; SMAD, SMAD are the proteins required for the transmission of the TGF-β signal to the nucleus.; SREBP-1c, Sterol regulatory element binding transcription factor 1c; TEAC, Trolox equivalent antioxidant capacity; TG, Triglyceride; TMT, Trail making test; TNF, Tumor necrosis factor; TNSALP, Tissue-non-specific alkaline phosphatase; TGF-β, Transforming growth factor-β; TNBC, Triple negative breast cancer.
1. Shi XG, Zheng XQ, Song XH, Wang YY, Ye CX. A new combination of Kucha. Acta Sci Nat Univ Sunyatseni. (2008) 47:129–30.
2. Ye CX, Ashihara H, Zheng XQ. New discovery of pattern of purine alkaloids in wild tea trees. Acta Sci Nat Univ Sunyatseni. (2003) 42:62–5.
3. Li YF, Ouyang SH, Chan, YQ, Wang TM, Li WX, et al. A comparative analysis of chemical compositions in Camellia sinensis var. puanensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chem. (2017) 216:282–8. doi: 10.1016/j.foodchem.2016.08.017
4. Negrin A, Long CL, Motley TJ, Kennelly EJ. LC-MS metabolomics and chemotaxonomy of caffeine-containing holly (Ilex) species and related taxa in the Aquifoliaceae. J Agric Food Chem. (2019) 67:5687–99. doi: 10.1021/acs.jafc.8b07168
5. Jin JQ, Jiang CK, Yao MZ, Chen L. Baiyacha, a wild tea plant naturally occurring high contents of theacrine and 3″-methylepigallocatechin gallate from Fujian, China. Sci Rep. (2020) 10:9715. doi: 10.1038/s41598-020-66808-x
6. Teng J, Yan CY, Zeng W, Zhang YQ, Zeng Z, Huang YH. Purification and characterization of theobromine synthase in a Theobromine-Enriched wild tea plant (Camellia gymnogyna Chang) from Dayao Mountain, China. Food Chem. (2020) 311:125875. doi: 10.1016/j.foodchem.2019.125875
7. Cheng Y, Yan Z, Lu J, Ye C, Wang D. Isolation and preparation of theacrine by high-speed counter-current chromatography from Camellia assamica var. Kucha. Acta Sci Nat Univ Sunyatseni. (2010) 49:65–9.
8. Zheng XQ, Ye CX, Kato M, Crozier A, Ashihara H. Theacrine (1, 3, 7, 9-tetramethyluric acid) synthesis in leaves of a Chinese tea, Kucha (Camellia assamica var. Kucha). Phytochemistry. (2002) 60:129–34. doi: 10.1016/S0031-9422(02)00086-9
9. Kato M, Ashihara H. Biosynthesis and catabolism of purine alkaloids in camellia plants. Nat Prod Commun. (2008) 3:1429–35. doi: 10.1177/1934578X0800300907
10. Wang S, Chen J, Ma J, Jin J, Chen L, Yao M. Novel insight into theacrine metabolism revealed by transcriptome analysis in bitter tea (Kucha, Camellia sinensis). Sci Rep. (2020) 10:6286. doi: 10.1038/s41598-020-62859-2
11. Zhang YH, Li YF, Wang YJ, Tan L, Cao ZQ, Xie C, et al. Identification and characterization of N9-methyltransferase involved in converting caffeine into non-stimulatory theacrine in tea. Nat Commun. (2020) 11:1473. doi: 10.1038/s41467-020-15324-7
12. Wang D, Lu J, Cheng Y, Shi X, Song X, Ye C. Primary studies on acute toxicity and sedative/hypnotic activity of Camellia kucha. Acta Sci Nat Univ Sunyatseni. (2010) 49:76–79.
13. Taylor L, Mumford P, Roberts M, Hayward S, Mullins J, Urbina S, et al. Safety of TeaCrineⓇ, a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use. J Int Soc Sport Nutr. (2016) 13:2. doi: 10.1186/s12970-016-0113-3
14. Li, K, Shi, X, Yang X, Wang Y, Ye C, Yang Z. Antioxidative activities and the chemical constituents of two Chinese teas, Camellia kucha and C. ptilophylla. Int J Food Sci Technol. (2012) 47:1063–71. doi: 10.1111/j.1365-2621.2012.02942.x
15. Ziegenfuss TN, Habowski SM, Sandrock JE, Kedia AW, Kerksick CM, Lopez HL. A two-part approach to examine the effects of theacrine (TeaCrine®) supplementation on oxygen consumption, hemodynamic responses, and subjective measures of cognitive and psychometric parameters. J Diet Suppl. (2017) 14:9–24. doi: 10.1080/19390211.2016.1178678
16. Bello ML, Walker AJ, McFadden BA, Sanders DJ, Arent SM. The effects of Theacrine and caffeine on endurance and cognitive performance during a simulated match in high-level soccer players. J Int Soc Sport Nutr. (2017) 17:35.
17. Butawan M, Stockton MB, Bloomer RJ. Effects of a single dose of TeaCrine®, caffeine, or their combination on subjective feelings, cognitive performance, and hemodynamics in men and women. J Int Soc Sport Nutr. (2017) 14 (Suppl. 2):31. doi: 10.1186/s12970-017-0188-5
18. Tsoi B, Yi RN, Cao LF, Li SB, Tan RR, Chen M, et al. Comparing antioxidant capacity of purine alkaloids: A new, efficient trio for screening and discovering potential antioxidants in vitro and in vivo. Food Chem. (2015) 176:411–9. doi: 10.1016/j.foodchem.2014.12.087
19. Zhou TY, Wu, YG, Zhang YZ, Bao YW, Zhao Y. SIRT3 retards intervertebral disc degeneration by anti- oxidative stress by activating the SIRT3/FOXO3/SOD2 signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23:9180–8. doi: 10.26355/eurrev_201911_19408
20. Song LL, Zhang Y, Zhang XR, Song YN, Dai HZ. Theacrine attenuates myocardial fibrosis after myocardial infarction via the SIRT3/beta-catenin/PPAR gamma pathway in estrogen-deficient mice. Eur Rev Med Pharmaco Sci. (2019) 23:5477–86. doi: 10.26355/eurrev_201906_18217
21. Li WX, Li YF, Zhai YJ, Chen WM, Kurihara H, He RR. Theacrine, a purine alkaloid obtained from Camellia assamica var. kucha, attenuates restraint stress-provoked liver damage in mice. J Agric Food Chem. (2013) 61:6328–35. doi: 10.1021/jf400982c
22. Wang YY, Yang XR, Zheng XQ, Li J, Ye, CX, et al. Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities. Fitoterapia. (2010) 81:627–31. doi: 10.1016/j.fitote.2010.03.008
23. Gao M, Zheng J, Zheng C, Huang ZY, Huang QW. Theacrine alleviates chronic inflammation by enhancing TGF-beta-mediated shifts via TGF-beta/SMAD pathway in Freund's incomplete adjuvant-induced rats. Biochem Biophys Res Commun. (2020) 552:743–8. doi: 10.1016/j.bbrc.2019.11.126
24. Feduccia AA, Wang YY, Simms JA, Yi HY, Li R, Bjeldanes L, et al. Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: involvement of adenosine and dopamine receptors. Pharmacol Biochem Behav. (2012) 102:241–8. doi: 10.1016/j.pbb.2012.04.014
25. Lopez HL, Wells S, Ziegenfuss TN. Dietary Supplement for the Theacrine-Containing Supplement Include Improvement of one of Mood, Energy, Focus, concentration or Sexual Desire or a Reduction of One of Anxiety or Fatigue Comprises, a Nutraceutically Acceptable Carrier. US Patent, US2019374546-A1, Morganville, NJ.
26. Lopez HL, Wells S, Ziegenfuss TN. Improving Physical Performance of Human and Treating and Improving Physical or Mental Performance in Human, Involves Administering Human With Synergistic Composition Orally, Where Composition Comprises Theacrine and Caffeine. US Patent, US2018104248-A1. Morganville, NJ (2018).
27. Koozehchian MS, Earnest CP, Jung YP, Collins PB, O'Connor A, Dalton R, et al. Dose response to one week of supplementation of a multi-ingredient preworkout supplement containing caffeine before exercise. J Caffeine Res. (2017) 7:81–94. doi: 10.1089/jcr.2017.0001
28. Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. (2004) 363:1783–93. doi: 10.1016/S0140-6736(04)16305-8
29. Xu JK, Kurihara H, Zhao L, Yao XS. Theacrine, a special purine alkaloid with sedative and hypnotic properties from Cammelia assamica var. kucha in mice. J Asian Nat Prod Res. (2007) 9:665–72. doi: 10.1080/10286020601103155
30. Xie G, Wu MZ, Wang YR, Gao YT, Li LD, Zhou HL, et al. Experimental study of theacrine on antidepressant effects. Chin Pharmacol Bull. (2009) 25:1160–3.
31. Qiao HY, Ye XS, Bai XY, He J, Li TL, Zhang J, Zhang WK, Xu JK. Theacrine: a purine alkaloid from Camellia assamica var. kucha with a hypnotic property via the adenosine system. Neurosci Lett. (2017) 659:48–53. doi: 10.1016/j.neulet.2017.08.063
32. Xu J, Zhai YJ, Wang GE, Yang Y, Li YF, Kurihara H, Qin SJ, He RR. Effects of theacrine on fatty acid metabolism related gene regulated by SIRT3. Tradit Chin Drug Res Clin Pharmacol. (2014) 25:410–4. doi: 10.3969/j.issn.1003-9783.2014.04.006
33. Li KK, Wong HL, Hu T, Zhang C, Han XQ, Ye CX, et al. Impacts of Camellia kucha and its main chemical components on the lipid accumulation in 3T3-L1 adipocytes. Int J Food Sci Technol. (2016) 51:2546–55. doi: 10.1111/ijfs.13236
34. Xu J, Wang GE, Zhang SJ, He RR, Hiroshi K, Qin SJ. Theacrine ameliorates high fat diet induced hepatic steatosis in mice via SirT3/AMPK/ACC pathway. Chin Pharmacol Bull. (2014) 30:791–5.doi: 10.3969/j.issn.1001-1978.2014.06.012
35. Wang GE, Li YF, Zhai YJ, Gong L, Tian JY, Hong M, Yao N, et al. Theacrine protects against nonalcoholic fatty liver disease by regulating acylcarnitine metabolism. Metab Clin Exp. (2018) 85:227–39. doi: 10.1016/j.metabol.2018.04.011
36. Ko JH, Yang MH, Baek SH, Nam D, Jung SH, Ahn KS. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA-MB-231 cells. Phytother Res. (2019) 33:1934–42. doi: 10.1002/ptr.6389
37. Lin PR, Kuo PC, Li YC, Jhuo CF, Hsu WL, Tzen JTC. Theacrine and strictinin, two major ingredients for the anti-influenza activity of Yunnan Kucha tea. J Ethnopharmacol. (2020) 262:113190. doi: 10.1016/j.jep.2020.113190
38. Kuhman DJ, Joyner KJ, Bloomer RJ. Cognitive performance and mood following ingestion of a theacrine-containing dietary supplement, caffeine, or placebo by young men and women. Nutrients. (2015) 7:9618–32. doi: 10.3390/nu7115484
39. Cesareo KR, Mason JR, Saracino PG, Morrissey MC, Ormsbee MJ. The effects of a caffeine-like supplement, TeaCrine®, on muscular strength, endurance and power performance in resistance-trained men. J Int Soc Sport Nutr. (2019) 16:47. doi: 10.1186/s12970-019-0316-5
40. Wang YY, Yang XR, Mo F, Shi XQ, Ye CC, Song XH, et al. Long-term toxicity study of theacrine in rats. Lishizhen Med Mater Med Res. (2010) 21:2439–41. doi: 10.3969/j.issn.1008-0805.2010.10.009
41. Clewell A, Hirka G, Glavits R, Palmer PA, Endres JR, Murbach TS, et al. A 90-day oral toxicological evaluation of the methylurate purine alkaloid theacrine. J Toxicol. (2016) 2016:6206859. doi: 10.1155/2016/6206859
42. VanDusseldorp TA, Stratton MT, Bailly AR, Holmes AJ, Alesi MG, Feito Y, et al. Safety of short-term supplementation with methylliberine (Dynamine®) alone and in combination with TeaCrine® in young adults. Nutrients. (2020) 12:654. doi: 10.3390/nu12030654
43. Owoc J. Stable Aqueous Compositions Used for Administering Bioactive Form of Theacrine to Mammalian Subject for e.g. Reducing Inflammation, Pain and Depression, Comprise Theacrine, Liberine and Methylliberine. US Patent, US2015238494-A1; US9468645-B2. Weston, FL (2015).
44. Carlson RP. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacological agents. Agents Actions. (1985) 17:197–204. doi: 10.1007/BF01966592
45. Olajide OA, Makinde MJ, Awe SO. Effects of the aqueous extract of Bridelia ferruginea stem bark on carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol. (1999) 66:113–7. doi: 10.1016/S0378-8741(99)00006-9
46. Duan, WJ, Liang, L, Pan, MH, Lu, DH, Wang, T.M, Li, et al. Theacrine, a purine alkaloid from Kucha, protects against Parkinson's disease through SIRT3 activation. Phytomedicine. (2020) 77:153281. doi: 10.1016/j.phymed.2020.153281
47. Spiegelman BM. PPAR-g: adipogenic regulator and thiazolidinedione receptor. Diabetes. (1998) 47:507–14. doi: 10.2337/diabetes.47.4.507
48. Cowherd RM, Lyle RE, McGehee REJ. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. (1999) 10:3–10. doi: 10.1006/scdb.1998.0276
49. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. (2002) 2:442–54. doi: 10.1038/nrc822
50. Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, et al. Delta EF1 is a transcriptional repressor of Ecadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. (2005) 24:2375–85. doi: 10.1038/sj.onc.1208429
51. Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, et al. Snail Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. (2005) 103:1631–43. doi: 10.1002/cncr.20946
52. Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. (2010) 15:169–90. doi: 10.1007/s10911-010-9181-1
53. He H, Ma D, Crone LB, Butawan M, Meibohm B, Bloomer RJ, Yates CR. Assessment of the drug-drug interaction potential between theacrine and caffeine in humans. J Caffeine Res. (2017) 7:95–102. doi: 10.1089/jcr.2017.0006
54. Wang YH, Mondal G, Butawan M, Bloomer RJ, Yates CR. Development of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for characterizing caffeine, methylliberine, and theacrine pharmacokinetics in humans. J Chromatogr B. (2020) 1155:122278. doi: 10.1016/j.jchromb.2020.122278
55. Li YF, Chen M, Wang C, Li XX, Ouyang SH, He CC, Mao ZF, Tsoi B, Kurihara H, He RR. Theacrine, a purine alkaloid derived from Camellia assamica var. kucha, ameliorates impairments in learning and memory caused by restraint-induced central fatigue. J Funct Food. (2015) 16:472–83. doi: 10.1016/j.jff.2015.05.003
Keywords: Camellia sinensis, antioxidant, anti-inflammation, locomotor, cognition, lipid metabolism, metastasis, hypnosis
Citation: Sheng Y-Y, Xiang J, Wang Z-S, Jin J, Wang Y-Q, Li Q-S, Li D, Fang Z-T, Lu J-L, Ye J-H, Liang Y-R and Zheng X-Q (2020) Theacrine From Camellia kucha and Its Health Beneficial Effects. Front. Nutr. 7:596823. doi: 10.3389/fnut.2020.596823
Received: 20 August 2020; Accepted: 30 November 2020;
Published: 17 December 2020.
Edited by:
Dejan S. Stojkovic, University of Belgrade, SerbiaReviewed by:
Catarina Lourenço-Lopes, University of Vigo, SpainCopyright © 2020 Sheng, Xiang, Wang, Jin, Wang, Li, Li, Fang, Lu, Ye, Liang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue-Rong Liang, eXJsaWFuZ0B6anUuZWR1LmNu; Xin-Qiang Zheng, eHF6aGVuZ0B6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.