
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 15 January 2021
Sec. Neuroenergetics and Brain Health
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.587367
This article is part of the Research TopicPolyphenols’ Action on the BrainView all 9 articles
 Imran Imran1*
Imran Imran1* Sana Javaid1,2
Sana Javaid1,2 Aroosa Waheed1
Aroosa Waheed1 Muhammad Fawad Rasool3
Muhammad Fawad Rasool3 Abdul Majeed3
Abdul Majeed3 Noreen Samad4
Noreen Samad4 Hamid Saeed5
Hamid Saeed5 Faleh Alqahtani6*
Faleh Alqahtani6* Mohammed M. Ahmed6
Mohammed M. Ahmed6 Faten Abdullah Alaqil6
Faten Abdullah Alaqil6Grewia asiatica L. fruit natively called phalsa is a popular berry of Pakistan and widely consumed in the form of fresh juices and carbonated drinks in the summer season. The berry is enriched with antioxidants such as phenols, flavonoids, anthocyanins, and vitamin C. Scientifically, it is the least explored berry in terms of neuromodulatory activities, and therefore, in the designed study, chronically fed rats with the different dilutions (5%−30%) of fruit juice were subjected to behavioral assessment for anxiety, depression, and cognition (spatial memory) followed by biochemical analysis of isolated brains. Results revealed a prominent impact of 20 and 30% dilutions of fruit exudate as treated animals showed anxiolytic behavior to central zone (P < 0.05) of open field test (OFT) and open arms of elevated plus maze (EPM) (P < 0.05) in anxiety models. Overall, immobility of rats treated with a higher concentration of exudate in forced swim test (FST) was reduced (P < 0.05) presenting antidepressant-like activity. Moreover, in learning and memory experimental models, the treated animals reversed scopolamine-induced amnesic effects as evident from improved step-through latencies (P < 0.05 vs. scopolamine; passive avoidance test), spontaneous alternation behavior (P < 0.05 vs. scopolamine; Y-maze test), discrimination index (P < 0.05 vs. scopolamine; novel object recognition test), and escape latencies (P < 0.05 vs. scopolamine; Morris water maze). Biochemical studies of isolated brains from treated rats demonstrated significantly elevated levels of superoxide dismutase and glutathione peroxidase (P < 0.05), whereas levels of acetylcholinesterase and malondialdehyde level (P < 0.05) were reduced, indicating its potential to reduce oxidative damage in the brain and modulation with the cholinergic system. The outcomes of studies support the benefits of phytoconstituents possessed by G. asiatica fruit in the amelioration of neurological disorders that could be due to their antioxidative capacity or due to interaction with GABAergic, serotonergic, and cholinergic systems in the brain.
It is well-known that healthy nutrition is an important key to healthy living. The therapeutic importance of nutritional substances has been accepted for years. A recent survey reports that 33% of Americans tend to include those components in their daily diet that prove beneficial for their health as well (1). Hippocrates' philosophy also claims that interventions made in a patient's nutrition can help him recover from ailment (2). Fruits include various nutrients that help promote health and protect against diseases (3). When taken as a whole, they work best due to the synergistic effect of all of their unique constituents. The presence of several bioactive constituents in fruits tends to boost the overall health of consumers. That is why the fruit is designated as one of the most valuable nutritional sources rich in fiber, vitamins, carbohydrates, minerals, and antioxidants, which are crucial for a healthy lifestyle (4).
Grewia is the only genus of “Tiliaceae” that yields edible fruit and consists of almost 150 species scattered throughout the world (5). Out of these, about 10 species are found in Pakistan (6). Grewia asiatica L. is one variety that grows wild and is cultivated as well, providing a sour, acidic fruit used in South Asian countries (7). The fruit of this plant is locally known as phalsa. This small, purplish, and sour fruit is a seasonal gift of nature and becomes excessively available in District Multan, Pakistan, from late April or early May and is available until June. The fruit is commonly used to make juice, squash, and carbonated drinks, which are used as healthiest beverages in the summer due to their soothing effect (6).
Grewia asiatica L. not only is the source of a delicious edible fruit but also possesses a variety of medicinal benefits as its fruit holds anti-inflammatory, antimicrobial, anticancer, and antidiabetic characteristics (8). Its fruit owns nutritionally significant amino acids, i.e., threonine and methionine (6) and various vitamins (6). Chemical analysis of seeds reports the presence of oil comprising palmitic, stearic, oleic, and linoleic acids (9). Analysis of the presence of micronutrients reveals that iron is found in the highest concentration (10). Besides these, various compounds and secondary metabolites are reported in fruits of G. asiatica, i.e., naringenin-7-O-β-D-glucoside, pelargonidin 3,5-diglucoside, cyanidin-3-glucoside quercetin, quercetin 3-O-β-D-glucoside, catechins, and tannins (11). When evaluated for anthocyanin content, the occurrence of cyanidin 3-O-(6”-acetyl-glucoside), peonidin-3-O-glucoside, and pelargonidin 3-O-(6”-acetyl-glucoside) was exposed, which also impart pharmacological benefits to this fruit (12).
Fruits of the various plants have demonstrated potential in resolving brain-related disorders (13–15), so the current need is to evaluate the fruit of local plant G. asiatica to cure neurological diseases that have become much more prevalent in Pakistan (16). There are more than 600 types of neurological disorders (17), but the most prevalent ones are dementia, Alzheimer's disease, epilepsy, schizophrenia, and Parkinson's disease (18). Symptoms of almost all these neurological disorders are structural, functional, or biochemical abnormalities, leading to frequently prominent consequences such as loss of coordination, seizures, confusion, and reduced cognition (19). Challenges in treating neurological disorders include high costs (20) and low tolerability of therapeutic drugs due to related side effects (21). These limitations provide researchers the impetus to discover alternative options to provide improved medication adherence and remedial outcomes in patients. According to WHO, more than 70% of the population of developed countries still rely on natural remedies due to their safety, cost-effectivity, and easy availability (22). It has also been reported that the youth believe that choosing food wisely will have a positive therapeutic effect on their lives (1).
The current investigational study has been planned to evaluate the impact of the freshly made dilutions of G. asiatica fruit (phalsa) juice on learning, anxiety, and depression-like behavior by employing different animal behavioral models. Additionally, biochemical evaluation of brain activity was performed through a series of experiments. We expected to scientifically prove for the first time the ameliorative potential of this indigenous plant berry on brain functions.
The fresh mature fruit of G. asiatica L. was purchased locally (15 kg) in May from Multan, Pakistan. After authentication through an expert botanist Dr. Zafar Ullah Zafar (associate professor, Institute of Pure and Applied Biology, BZU Multan), voucher no. R. R. Stewart 473 was submitted into the herbarium. The fruit was thoroughly washed with water to remove impurities and blended in water (1 kg fruit berries:0.5 L water) using a commonly used food blender to make a concentrated fruit juice (exudate). Freshly prepared juice was first filtered through a sieve to remove seed debris and then stored into non-reacting glass bottles at 4°C in the refrigerator. Then, 5, 10, 20, and 30% dilutions were made by mixing prepared exudate with water.
Sprague-Dawley (SD) male rats (150–250 g) were included in the current study. Animals were purchased from the National Institute of Health, Islamabad, and housed in the animal house (Faculty of Pharmacy, Bahauddin Zakariya University, Multan) under strictly regulated maintenance conditions (25°C, 12-h light/dark cycle; lights on 8:00 am) and provided with standardized rodent chow to eat. Animals were adapted to experimentation sites 60 min before the test. All behavioral experiments were carried out from 8:00 a.m. to 6:00 p.m. The intensity of aversive stimuli was kept at a minimum in behavioral tests to avoid any carryover effects of one test on to the next. All animal studies were performed after availing authorization from the Department of Pharmacology Ethical Committee BZU, Multan (EC/12-PHL-2017 dated 22-05-2017) and were accomplished by following instructions of the Institute of Laboratory Animal Resources (ILAR), Commission on Life Sciences, National Research Council (NRC, 1996).
Piracetam, chloral hydrate, scopolamine, 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), 2-thiobarbituric acid (TBA), and acetylthiocholine iodide were purchased from Sigma-Aldrich, USA. Diazepam and fluoxetine were procured from Roche Pharma (Switzerland) and Hilton Pharma (Pakistan), respectively. Isoflurane (Forane® Abbots Laboratories, USA) was a kind gift by Ahsan Medicine Agency, Multan, intended for the purpose of induction of anesthesia. All drugs and chemicals used in studies were highly purified and of research-grade.
Randomly selected animals were divided into nine different groups (6–8 animals/group). Group 1 was considered as a control group and simply fed on tap water throughout the period of the study. Rats of groups 2 to 5 were administered ad libitum 5, 10, 20, and 30% dilutions (v/v in tap water) of G. asiatica exudate (Gr.Ex) for 28 days via standardized water bottles placed in the cages. After 28 days of consumption of juice, the behavioral studies were initiated, but animals of groups 2–5 were given free access to mentioned dilutions of Gr.Ex until day 41 of the experiment (Figure 1). In all behavioral experiments, the dilutions of Gr.Ex were withdrawn an hour before the commencement of the experiment.
During the behavioral test for anxiolytic activity, diazepam [2 mg/kg, intraperitoneally (i.p.)] was administered acutely 30–60 min before to one positive control group 6A (n = 6). Fluoxetine at the dose of 20 mg/kg i.p. was given once a day for 1 week (days 25–31) to one separate group of rats designated as group 6B (n = 6) to work as a positive control in the forced swimming test. For studies affiliated with the learning and memory tasks, we introduced two new separate groups of rats such as group 6C and group 7. Group 6C was a positive control to which piracetam 200 mg/kg was administered i.p. once daily for 1 week from days 26 to 32. Scopolamine (1 mg/kg, i.p.) was acutely administered 30 min before to animals of Groups 2–5, 6C, and 7. The day-wise scheme of designed experiments and animal grouping is summarized in Figure 1.
Here, 500 mL of freshly prepared 5, 10, 20, and 30% G. asiatica fruit juice was individually mixed with 500 mL of methanol (100%) for 3–4 days at 25°C. The mixture was shaken intermittently to allow maximum extraction of active constituents. After this, the procedures of filtration and successive evaporation were carried out. The extracts were obtained with a yield of 0.62, 1.37, 2.69, and 4.05% for 5, 10, 20, and 30% dilutions of fruit pulp, respectively. The prepared extracts were kept at a minimal temperature in the refrigerator until further experimentation.
Gr.Ex was evaluated for phenolic compounds by adopting the Folin–Ciocalteu method (23). For assessment, 200 μl of each methanolic extract (1 mg/ml) was increased to 3 mL by using distilled water to which 0.5 mL of Folin–Ciocalteu reagent (50% v/v) was inoculated. After 3 min, 2 mL of 20% sodium bicarbonate (w/v) was introduced, and the resulting solution was reserved in the dark for an hour. The absorbance of this mixture was checked at 650 nm, and phenolic content was computed through the calibration curve. Outcomes had been articulated as mg of gallic acid equivalent (mg GAE) per gram of dry weight.
The aluminum chloride method was employed during this testing (24). In brief, 1 mL of test extract (1 mg/ml) was mingled with 4 mL of water followed by the addition of 0.3 mL of sodium nitrate (5%), and the components were allowed to mix for 5 min. Next, 0.3 mL of aluminum chloride (10%) was added, and the mixture was allowed to incubate at room temperature for 6 min. Afterward, 1 mL of sodium hydroxide (1 M) was inoculated and the volume of the mixture was made 10 mL by using distilled water. Absorbance was measured at 510 nm. The entire testing was conducted thrice using quercetin as a standard, and conclusions were represented as quercetin equivalent per (mg QE) gram of dry weight (24).
The anthocyanin content was evaluated by taking 1 mL of extract (1 mg/ml) to the flask to prepare two dilutions (25). One dilution was made with potassium chloride buffer (pH 1.0) and the other with sodium acetate buffer (pH 4.5), and both solutions were allowed to equilibrate for 15 min. The absorbance of each dilution was noted at 510 and 700 nm using blank distilled water as a control.
The absorbance of the diluted sample was calculated using the formula:
While anthocyanin content was figured out through the given formula:
where MW denotes molecular weight, DF is the dilution factor, and ε is the molar absorptivity.
The plant's antioxidant capacity was assessed through 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays.
Grewia asiatica was evaluated for antioxidant potential by adding 1 mL of methanolic DPPH (0.1 mM) to 3 mL of different concentrations (5–30 μg/ml) of each methanolic extract under study (26). The solution was mixed by vigorous shaking and kept at 25°C for 0.5 h. After that, absorbance was noted at 517 nm. Whole experiments were conducted in n = 6 while using ascorbic acid as the standard. The outcomes were indicated as IC50, while % DPPH inhibition was computed by the given equation:
where A0 is the absorbance of only methanol (control), and A1 is the absorbance in the presence of test or standard sample.
The radical hunting potential of G. asiatica fruit pulp was estimated through the method described (27). For this assay, ABTS+ was freshly prepared by combining 7 mM of ABTS solution with 2.45 mM potassium persulfate. This blend was maintained in the dark for 16 h. Later, this solution was diluted with methanol until the absorbance reaches 0.700 at 734 nm. Afterward, 3.995 mL of this ABTS+ solution was added to 5 μl of methanolic extract and mixed thoroughly. After 0.5 h, absorbance was recorded at 734 nm. All experimentation was repeated thrice while using the Trolox as standard and IC50 was expressed. In addition, % scavenging effect was determined through the given calculation:
where AB is the absorbance of only methanol (control), and AA is the absorbance in the presence of test compound or standard.
The anti-cholinesterase capability of Gr.Ex was evaluated by the method described (28). For the assay, 100 μl of DTNB (3 mM), 40 mL of tris buffer (50 mM, pH 8.0), and 20 μl of acetylcholinesterase (AChE) (0.26 U/ml) were added to 96-well plates. Later, 20 μl of test extract in different concentrations (25–500 μg/ml) solubilized in a buffer comprising not more than 10% methanol were added to these wells. After allowing the incubation for 0.25 h, the absorbance was analyzed at 412 nm to consider as blank. To initiate the enzymatic reaction, 20 μl of acetylthiocholine was added and absorbance was checked after 5 min for 20 min. All experiments were repeated thrice using physostigmine as a standard, and outcomes were expressed as % inhibition (29, 30) calculated by the formula:
where ΔA is the absorbance of the blank, and ΔB is the absorbance of the sample.
The open field test (OFT) utilizes the innate exploratory behavior of rats to evaluate anxiety, as they are naturally frightened of lighted areas but tend to explore them as well (31, 32). The test apparatus consists of a poly-acrylate-made square-shaped field (80 × 80 cm) having walls high enough (40 cm) to avoid animal escape during the test. Each animal was individually placed in the arena of the open field. Parameters such as the number of entries in the central zone and time spent there were monitored for 5 min and subsequently analyzed by ANY-maze software (V 6.1, Stoelting Co., USA). The arena was cleaned thoroughly and wiped with 70% isopropyl alcohol to remove any residual smell of the previous session.
The elevated plus maze test (EPM) is a dependable and trustworthy test employed by scientists (33, 34) to evaluate anxiolytic behavior in rodents. The test is conducted in a 50-cm-high plus (+)-shaped apparatus (110 × 10 cm) having bare and walled arms. Rats naturally prefer to stay in closed areas but attempt to explore open zones at the same time. On test day, each animal was positioned to face exposed and unprotected arms to allow the exploration of the whole maze for 5 min. The open and closed arm visits, as well as time spent there, were observed by ANY-maze software (V 6.1, Stoelting Co., USA).
The forced swimming test (FST) is based on the rodent's reaction to the threat of drowning, and the resulting response is used to estimate their vulnerability to depression-like behavior (35). The animals are subjected to forced swimming in a deep acrylic glass tank (34.5 cm × 23.5 cm) filled with water to the level that they can easily float without touching their paws to the bottom of the tank. Rats were individually observed for a duration of 5 min for their movements and immobility, and these parameters were evaluated with the help of behavioral assessment software ANY-maze V 6.1, Stoelting Co., USA) to assess the antidepressant-like potential of Gr.Ex.
The step-through passive avoidance test (ST-PAT) uses the conceptualization of placing rodents into the aversive bright zone from which they try to escape into an innately preferred dark environment where an unpleasant stimulus is delivered (36, 37). The test was conducted in an apparatus having two compartments of the same dimensions (20 cm × 20 cm × 20 cm) interconnected by an opening (10 cm × 10 cm). One compartment was illuminated with a bulb (100 W; halogen lamp) as a light source, and the other was a dark chamber having stainless steel floor to provide electric foot shock. The sequence of ST-PAT was divided into three segments, i.e., (1) training phase, (2) short-term memory assessed after 1 h of shock, and (3) memory retrieval assessed after 24 h of shock. In the training phase of 5 min, all animals received an electric shock of 0.5 mA for 1 s (Coulbourn Instruments Animal Shocker, USA) through the stainless-steel floor to all animals. Subsequent to the training phase, the animals of different groups (2–5, 6C, and 7) were given scopolamine (1 mg/kg, i.p.) 30 min before the judgment of memory associated with aversive stimuli (1 h post-shock session). The same procedure for the memory retention was repeated with all animals after 24 h of shock but without the amnesic drug. Total step-through latencies and duration in the dark compartment in 1 and 24 h post-shock sessions were noted for the duration of 5 min.
This memory test was conducted in a trio-arm (A, B, and C) maze (55 cm × 10 cm × 15 cm) arranged adjacently at 120° (38, 39). After 30 min of scopolamine administration, rats were placed in any arm of the maze and allowed to explore spontaneously. Entries in each arm with a sequence of arm entries (ABC, BCA, CBA, etc.) were observed for 5 min to estimate their spontaneous alternation behavior. Animals showing increased alternation behavior indicated enhanced spatial memory.
% Spontaneous alternation was determined through the formula:
The novel object recognition (NOR) test operates on rodent's instincts to discover new surroundings for memory assessment (40, 41). The test apparatus comprises an open box (80 cm × 80 cm × 40 cm) in which animals were placed for two consecutive sessions, i.e., one comprising two identical objects and another session in which one old object was replaced by a new one. Animals were initially acclimatized in the behavioral room for at least 60 min. Subsequently, rats in the open field were exposed with familiar objects for the duration of 5 min. The animals were permitted to investigate these similar objects through sniffing or touching at a short distance (<2 cm). After 1 h, a new object was presented in the cage replacing one of the previously explored objects, and animal behavior was recorded for 5 min. The time taken by animals to explore the familiar and new object was noted to calculate the discrimination index.
The Morris water maze (MWM) test is employed to estimate memory and spatial learning in rodents (42–44). The test apparatus comprises a spherical tub-like container (150 cm diameter and 50 cm height) having a smooth internal surface. Water with some opacifying agent (milk or non-toxic dye) was filled in this tank up to 32 cm high, and a square platform (10 × 10 cm) made of plastic was placed 2 cm below the water surface. Various distinct geometrical signs (▴, □, ◦, —) were displayed on the inner side of the water tank (proximal cues) and simultaneously mounted on the stands in the surroundings of the tank (distal cues) to provide spatial reference points to the animals (44, 45). Initial training trials (training days) of animals were carried out without amnesic drug for the first 2 days (four trails/animal/day of 2-min duration) by placing a platform in the southwest (SW) quadrant of the tank. During the training session, if the animal could not locate the platform within 120 s, then the rats were slightly pushed toward the platform and made them stay on it for 10 s for the formation of spatial memory. For the next 3 days (experimental trials), the rats were individually tested to locate the hidden platform for 2 min in scopolamine-induced amnesic condition (escape latencies). After 24 h of completion of the experimental trial, animals were tested in the water maze without platform (probe day), and entries in the targeted platform quadrant, swimming time, and distance traveled were observed to assess reference memory associated with the previous positioning of the platform. Scopolamine in the experimental and probe sessions was administered 30 min before to the animals of groups 2–5, 6C, and 7. Ad libitum Gr.Ex dilutions (5, 10, 20, and 30%) were withdrawn 1 h before the start of any session of the MWM test. The recorded videos were subsequently accessed with the help of behavioral tracking software, i.e., ANY-maze version 6.1, Stoelting Co., USA.
For biochemical assays, animals were deeply anesthetized in an anesthesia induction chamber (World Precision Instruments, USA) by the inflow of 5% isoflurane v/v mixed with synthetic air (Lab-Gas, Multan, Pakistan) by Rodent Anesthesia Vaporizing System (Kent Scientific Corporation USA). The flow of isoflurane vapors was kept constant for 1–2 min until animals were deeply anesthetized with loss of all reflexes and subsequently decapitated by rodent guillotine to isolate brains.
Animals were randomly selected from all treatment groups immediately at the end of the MWM task. They were sedated with isoflurane and decapitated for brain extraction. Isolated brains were appropriately stored in normal saline at 8°C. For brain homogenate preparation, 0.3 g of the brain sample was centrifuged at 12,000 g with 3 mL of phosphate buffer (pH 7.4) for 20 min at low temperature (46). The obtained homogenate was further assayed to investigate the level of AChE, malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in the animal brain.
Brain homogenate was assayed for AChE activity according to the method described (28). Brain homogenate (0.4 ml) was combined with DTNB (100 μl) and 0.1 M phosphate buffer having pH 8 (2.6 ml). The absorbance of this mixture was noted at 412 and taken as control. Later, ATC (5.2 μl) was added to the cuvette, and the change in absorbance was noted for 10 min.
After minor modifications in the procedure prescribed (47), 2 mL mixture of 15% trichloroacetic acid (TCA) and 0.38% thiobarbituric acid (TBA) were mixed with 100–500 μl of the homogenized brain. The resulting combination was thoroughly mixed and boiled on a water bath for 15–20 min. This mixture was then chilled at 4°C, followed by subsequent centrifugation (3,500 rpm) for 10–15 min. The absorbance of the supernatant obtained was checked at 532 nm.
This assay was performed by following the procedure described (48). Based upon the conversion of nitroblue tetrazolium (NBT) to insoluble formazan, 0.5 mL of the homogenized brain was mixed with a solution containing 0.4 mL of NBT (24 μM), 1 mL of sodium bicarbonate (50 mM), and 0.2 mL of ethylenediaminetetraacetic acid (EDTA) (0.1 mM). Later, 0.4 mL of hydroxylamine hydrochloride (1 mM) was inoculated to initiate the reaction, and absorbance was monitored for 5 min at 560 nm.
The test was performed by following the illustrated method (46). Here, 0.3 mL of brain homogenate was combined with 0.1 mL of sodium azide (10 mM), 0.1 mL of H2O2 (1 mM), 0.3 mL phosphate buffer (pH 7.4, 0.1 M), and 0.2 mL of reduced glutathione (2 mM). The resultant blend was incubated for 0.25 h at 37°C, and 0.5 mL of 5% TCA was added to stop the reaction. Centrifugation was done for 5 min at 1,500 g, and separated supernatant was removed. Phosphate buffer (0.2 ml) and DTNB (0.7 ml) were reacted with supernatant obtained (0.1 ml) to note the absorbance at 420 nm.
The data were expressed as mean ± SEM (n = 6 for behavioral studies and n = 3 for biochemical studies). The data normality was checked through the Kolmogorov–Smirnov test. Analysis of most of the parameters of the behavioral and biochemical investigation was done through one-way ANOVA with Tukey's post hoc test. However, latencies checked in PAT and MWM and duration in the dark in PAT were evaluated by two-way ANOVA and Tukey's post hoc test.
When methanolic extracts prepared from the different concentrations of fruit pulp were evaluated for the presence of phytoconstituents, the results demonstrated the amount of content was dependent on the concentration (5, 10, 20, and 30%) of pulp used. Phenolic content recorded was 15.96 ± 1.56, 65.23 ± 1.58, 94.23 ± 2.69, and 123.90 ± 1.33 mg GAE/g with 5, 10, 20, and 30% Gr.Ex, respectively The Tukey's test revealed that the phenolic content variation was concentration-dependent. The content in 10, 20, and 30% Gr.Ex was significantly higher (P < 0.0001) as compared to 5, 10, and 20% Gr.Ex, respectively (Figure 2A).
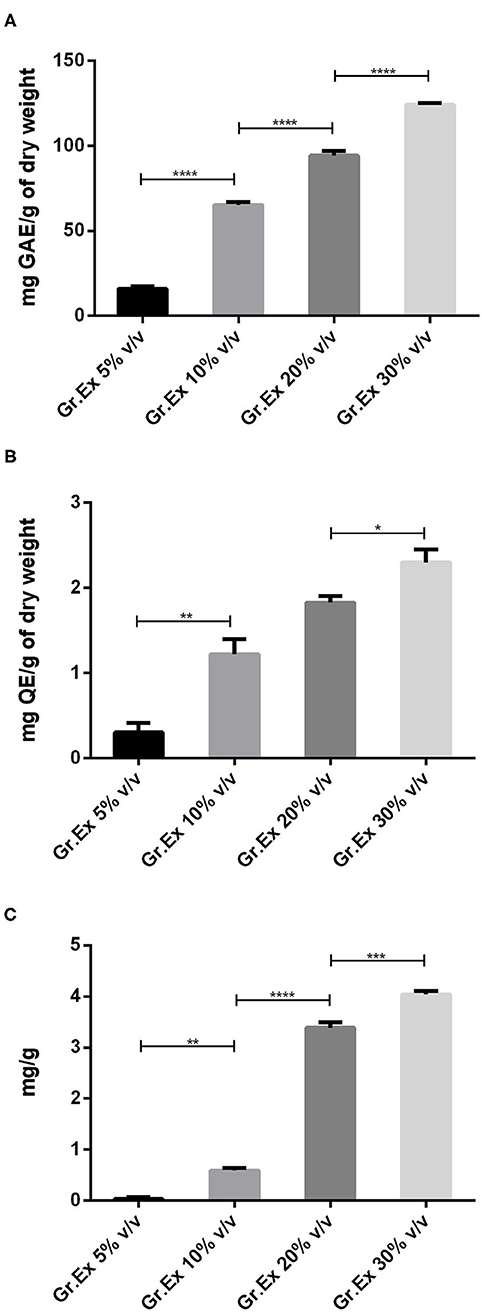
Figure 2. Assessment of total phenol (A), flavonoid (B), and anthocyanin (C) content in methanolic extracts of different concentrations of fruit pulp of Grewia asiatica. Data expressed as mean ± SEM (n = 3) and evaluated by one-way ANOVA followed by Tukey's test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, comparison between 5 and 10%, 10 and 20%, and 20 and 30% dilutions of G. asiatica exudate (Gr.Ex).
Similarly, total flavonoid contents calculated for all prepared extracts were ranging from 0.1 to 2.5 mg QE/g, and maximal content was discovered with 30% Gr.Ex, 2.3 ± 0.15 mg QE/g. The flavonoid content in 10% Gr.Ex was significantly higher (P = 0.006) as compared to 5% (Figure 2B). The same concentration-dependent rise in anthocyanin content was recorded with significant anthocyanin variation in 10% concentration (P = 0.001) as compared to 5% Gr.Ex. Similarly, the content fluctuated between 3.20 and 4.12 mg/g for 20 and 30% Gr.Ex (Figure 2C).
In vitro studies revealed the presence of phenolic, flavonoid, and anthocyanin constituents in the methanolic extract of G. asiatica fruit pulp. So, further testing for antioxidant potential was carried out as the plants rich with the abovementioned constituents yield efficient radical scavenging potential. Antioxidant activities were performed through DPPH and ABTS methods, which are among the most reliable in vitro methods to estimate the radical hunting capacity of a test substance. In both DPPH and ABTS, the maximum percentage inhibition (>60%) was observed for 30% Gr.Ex, with P = 0.048 and P = 0.001, as compared to lower concentrations, respectively (Figures 3A,B). The outcomes of antioxidant assays found promising to the previously reported studies (6, 49) explain that the fruit of G. asiatica was enriched with phytoconstituents that impart this oxidative stress-combating characteristics, which are very essential to deal with a range of diseases.
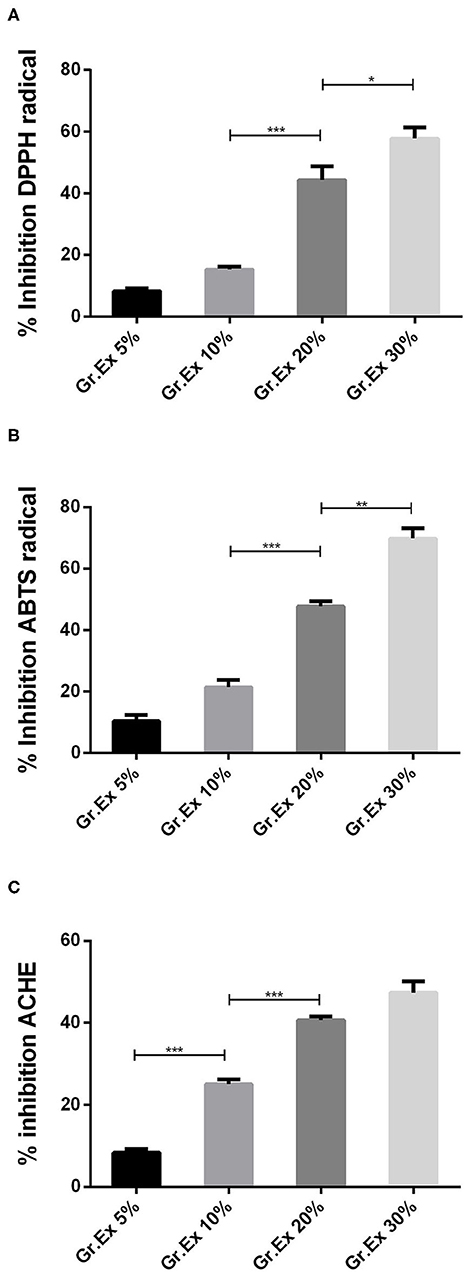
Figure 3. In vitro evaluation of 1,1-diphenyl-2-picrylhydrazyl (DPPH) (A), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (B), and acetylcholinesterase (AChE) (C) % inhibition capacity of methanolic extracts prepared for different concentrations of fruit juice of Grewia asiatica. Data expressed as mean ± SEM (n = 3) and evaluated by one-way ANOVA followed by Tukey's test. *P < 0.05, **P < 0.01, ***P < 0.001, comparison between 5 and 10%, 10 and 20%, and 20 and 30% dilutions of G. asiatica exudate (Gr.Ex).
The AChE inhibitory action by methanol extract of different concentrations of G. asiatica fruit pulp demonstrated % inhibition in the range of 8.33–50.66% in a concentration-dependent manner (Figure 3C). The % inhibition of AChE was maximum (50.66%) with 30% Gr.Ex, though the activity of the enzyme was inhibited with lower concentrations (5, 10, and 20%) too. This anti-cholinesterase potential is reported to be correlated with the presence of phenolic and flavonoid derivatives (29) in the fruit of G. asiatica.
Animals were tested in an open field to evaluate the effect of Gr.Ex on anxiety in animals. The exposure to open areas compels animals to stay in peripheries due to anxiety developed in exposed areas as evident from the behavior of animals in the control group. ANOVA revealed a significant difference among all groups for central zone entries [F(5, 30) = 30.14, P < 0.0001] and time spent in the central area [F(5, 30) = 23.74, P < 0.0001]. Compared to the control group, animals treated with diazepam (2 mg/kg) demonstrated a significant increase in the number as well as the duration of central zone visits (P < 0.0001). Similarly, animals treated with 20 and 30% dilution of Gr.Ex exhibited an increased preference for the central area of the field. The number of entries into the central zone was increased in animals treated with 20% (P = 0.0002) and 30% (P < 0.0001) of Gr.Ex (Figure 4A). Likewise, the animals treated with 20 and 30% of Gr.Ex spent more time in the central area of field as compared to control animals, with P = 0.0071 and P < 0.0001, respectively (Figure 4B). However, these results were not significant in animals treated with 5 and 10% dilutions of Gr.Ex.
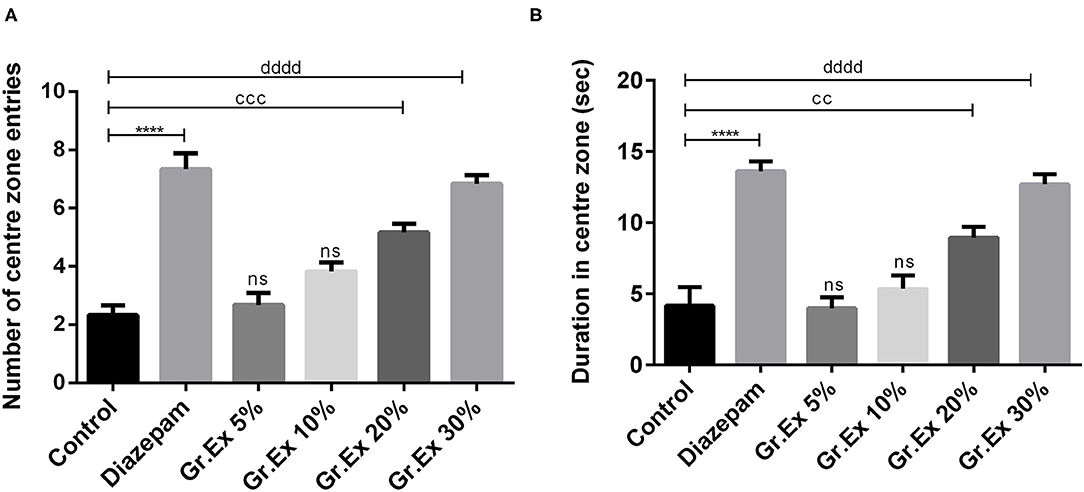
Figure 4. Anxiolytic effect of various dilutions of Grewia asiatica exudate (Gr.Ex) and diazepam on the (A) number of center zone entries and (B) duration in the center zone in the open field test. Data expressed as mean ± SEM (n = 6) and evaluated by one-way ANOVA followed by Tukey's test. ****P < 0.0001, comparison between control animals and diazepam group. ccP < 0.01, cccP < 0.01, comparison between control animals and 20% Gr.Ex-treated group. ddddP < 0.0001, comparison between control animals and 30% Gr.Ex-treated group.
In the EPM test, ANOVA exposed a significant difference between all groups for open arm entries [F(5, 30) = 10.27, P < 0.0001] and time spent in open arms [F(5, 30) = 33.26, P < 0.0001]. Rats treated with 20 and 30% dilutions of Gr.Ex exhibited significantly increased preference for the open arms, as their entries in open arms of the maze were increased, withP < 0.05, as compared to control animals (Figure 5A). Similarly, their duration of stay in the open arms of the maze was also increased in a concentration-dependent manner by 20% (P = 0.03) and 30% (P = 0.0002) of Gr.Ex (Figure 5B). The animals treated with Gr.Ex responded the same way as animals treated with diazepam did (P < 0.05), revealing the dose-dependent anxiolytic-like potential of Gr.Ex.
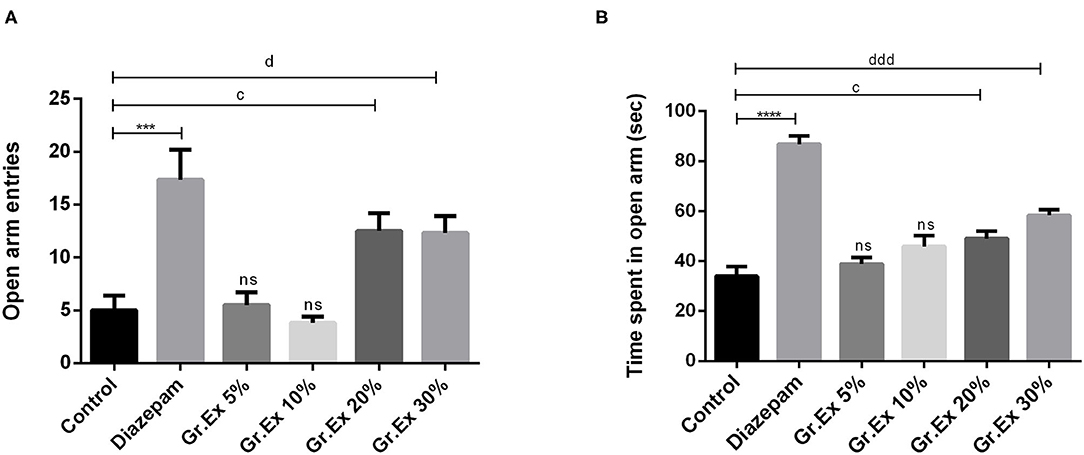
Figure 5. Anxiolytic effect of various dilutions on (A) open arm entries and (B) time spent in the open arm in the elevated plus maze test. Data expressed as mean ± SEM (n = 6) and evaluated by one-way ANOVA followed by Tukey's test. ***P < 0.001, ****P < 0.0001, comparison between control animals and diazepam group. cP < 0.05, comparison between control animals and 20% Grewia asiatica exudate (Gr.Ex)-treated group. dP < 0.05, dddP < 0.0001, comparison between control animals and 30% Gr.Ex-treated group.
Outcomes demonstrated noticeable dissimilarity for immobility time [F(5, 30) = 8.01, P < 0.0001] among all groups, as shown in Figure 6. The immobility time of rats was reduced by standard antidepressant drug, fluoxetine, with P = 0.0005. Similarly, the immobility was reduced in rats treated with 20 and 30% dilutions of Gr.Ex, with P = 0.01 and P = 0.006, respectively, as compared to the control animals. However, the results for observed parameter remained unchanged at lower dilutions, i.e., 5 and 10% of Gr.Ex.
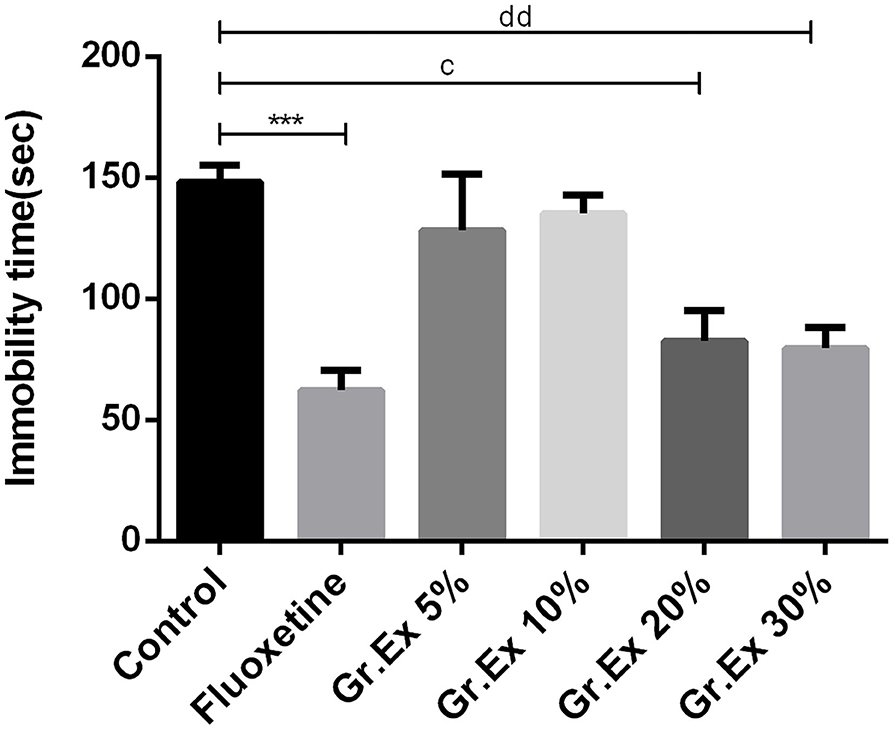
Figure 6. Antidepressant-like effect by various dilutions of Grewia asiatica exudate (Gr.Ex) on immobility of rats in the forced swimming test. Data expressed as mean ± SEM (n = 6) and evaluated by one-way ANOVA followed by Tukey's test. ***P < 0.001, comparison between control animals and fluoxetine group. cP < 0.05, comparison between control animals and 20% Gr.Ex-treated group. ddP < 0.01, comparison between control animals and 30% Gr.Ex-treated group.
The PAT revealed the memory-enhancing potential of Gr.Ex, as there was improved learning (1 h post-shock trial) and memory retention (24 h post-shock trial). Results demonstrated a significant difference among all groups for step-through latency [F(6, 35)= 197.0, P < 0.0001] and for the animal's stay in the dark compartment [F (6, 70) = 47.71, P < 0.0001]. The animals treated with scopolamine showed reduced latencies to enter the dark compartment (P < 0.0001) as compared to control animals. Piracetam treatment led to a significant increase in latency to enter the darkroom as compared to the scopolamine-treated group (P < 0.0001) in both 1 and 24 h post-shock trials. Similarly, scopolamine-induced short latencies were reversed in 1 h post-shock trial by 20 and 30% of Gr.Ex, with P = 0.0001 and P < 0.0001, respectively. The same outcomes were observed in 24 h post-shock trial in which a concentration-dependent effect was observed by 10% (P = 0.0001), 20% (P < 0.0001), and 30% (P < 0.0001) of Gr.Ex (Figure 7A).
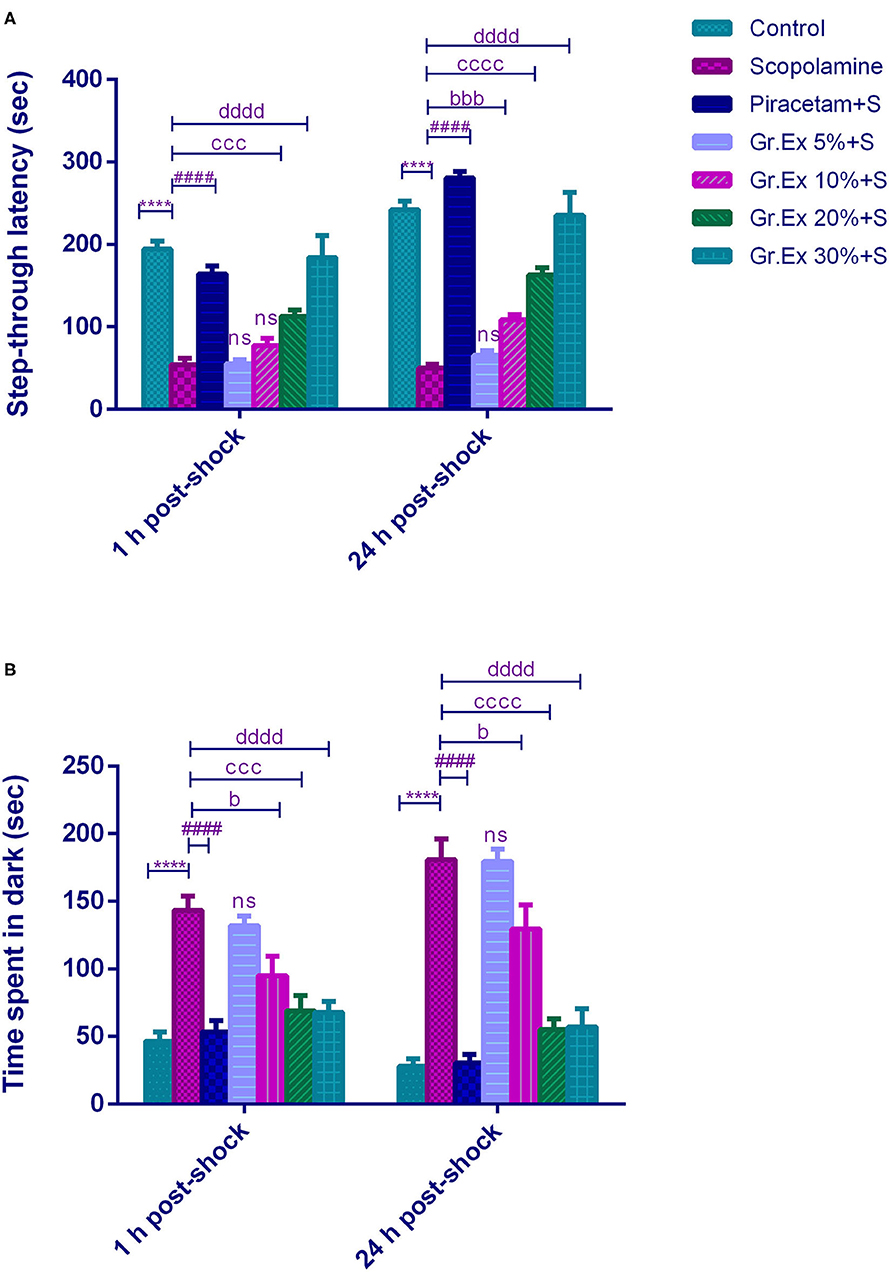
Figure 7. Effect of Grewia asiatica exudate (Gr.Ex) on (A) step-through latencies and (B) time spent in the dark in the passive avoidance test. Data expressed as mean ± SEM (n = 6) and evaluated by two-way ANOVA followed by Tukey's test. ****P < 0.0001, comparison of control animals with scopolamine-treated group. ####P < 0.0001, comparison between scopolamine- and piracetam-treated groups. bP < 0.05, bbbP < 0.001, comparison between scopolamine- and 10% Gr.Ex-treated groups. cccP < 0.001, ccccP < 0.0001, comparison between scopolamine- and 20% Gr.Ex-treated groups. ddddP < 0.0001, comparison between scopolamine- and 30% Gr.Ex-treated groups.
Likewise, scopolamine-treated amnesic rats spent more time in the dark compartment (P < 0.0001) as compared to the control group in both trials. The piracetam-treated animals notably decreased their stay in this shock zone (P < 0.0001) as compared to scopolamine-treated rats. The Gr.Ex also exhibited improved memory retrieval as animals' stay in the dark compartment was reduced in animals treated with at 10, 20, and 30% Gr.Ex dilutions as compared to scopolamine-treated animals, with P = 0.036, P = 0.0001, and P < 0.0001, respectively, in 1 h post-shock trial. The same concentration-dependent outcomes were observed at 24 h where Gr.Ex-treated rats spent lesser time in the dark compartment at dilution 10% (P = 0.02), 20% (P < 0.0001), and 30% (P < 0.0001) (Figure 7B).
The Y-maze test was employed to evaluate the effect of the Gr.Ex treatment on memory, as increased spontaneous alternation is an indication of enhanced remembrance of already visited arms of the maze (Figure 8). ANOVA disclosed noticeable variations in the gain of a spontaneous alternation score by animals of all treated groups [F(6, 35) = 7.359, P < 0.0001]. Scopolamine administration induced amnesia in experimental rats, as their score of %SAP was less than that of the control group (P = 0.006). Piracetam treatment considerably reversed this memory loss effect of scopolamine (P = 0.002). Likewise, the administration of 30% dilution of Gr.Ex prominently increased spontaneous alternation behavior in rats (P = 0.019), but results were not significant at lower concentrations of Gr.Ex.
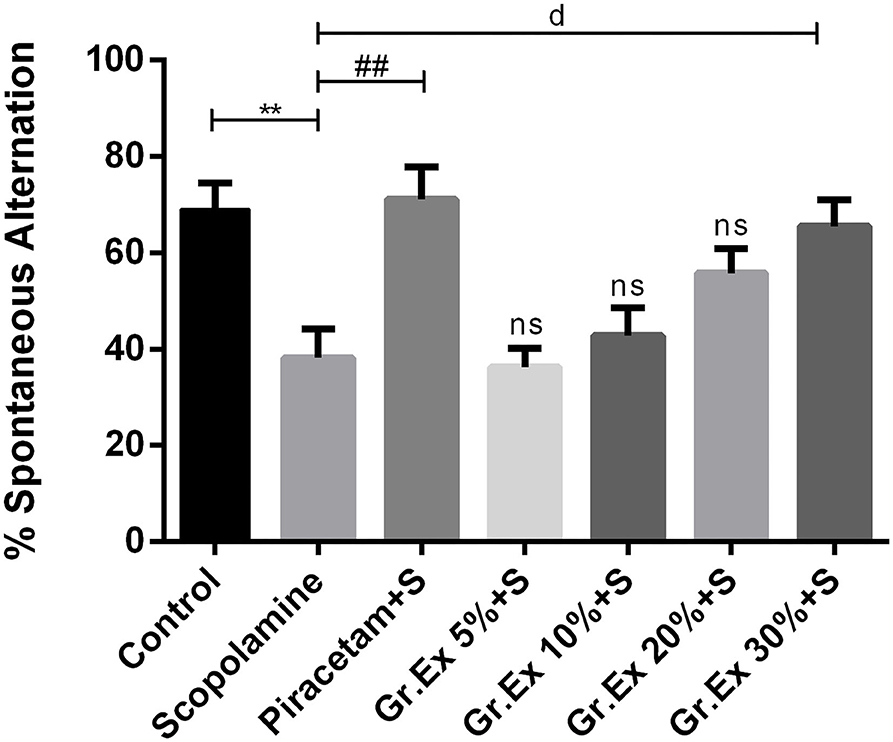
Figure 8. Effect of Grewia asiatica exudate (Gr.Ex) on spontaneous alternation behavior in the Y-maze task. Data expressed as mean ± SEM (n = 6) and evaluated by one-way ANOVA followed by Tukey's test. **P < 0.01, comparison of control animals with scopolamine-treated group. ##P < 0.01, comparison between scopolamine- and piracetam-treated groups. dP < 0.05, comparison between scopolamine and 30% Gr.Ex-treated groups.
Animals treated with Gr.Ex 20 and 30% spent more time in the exploration of a novel object as compared to the scopolamine group, with P = 0.02 and P = 0.002, respectively (Figure 9A). However, scopolamine-induced memory deficit was evident from the behavior of rats who spent less time with the new object compared to the control group (P = 0.03). Similarly, data of the discrimination index demonstrated significant differences between all groups during the trial [F(6, 35)= 26.63, P < 0.0001]. Reduced discrimination index exhibited by amnesic rats was reverted by piracetam (P < 0.0001) and Gr.Ex (P = 0.03) (Figure 9B). However, 5 and 10% dilution did not produce significant effects.
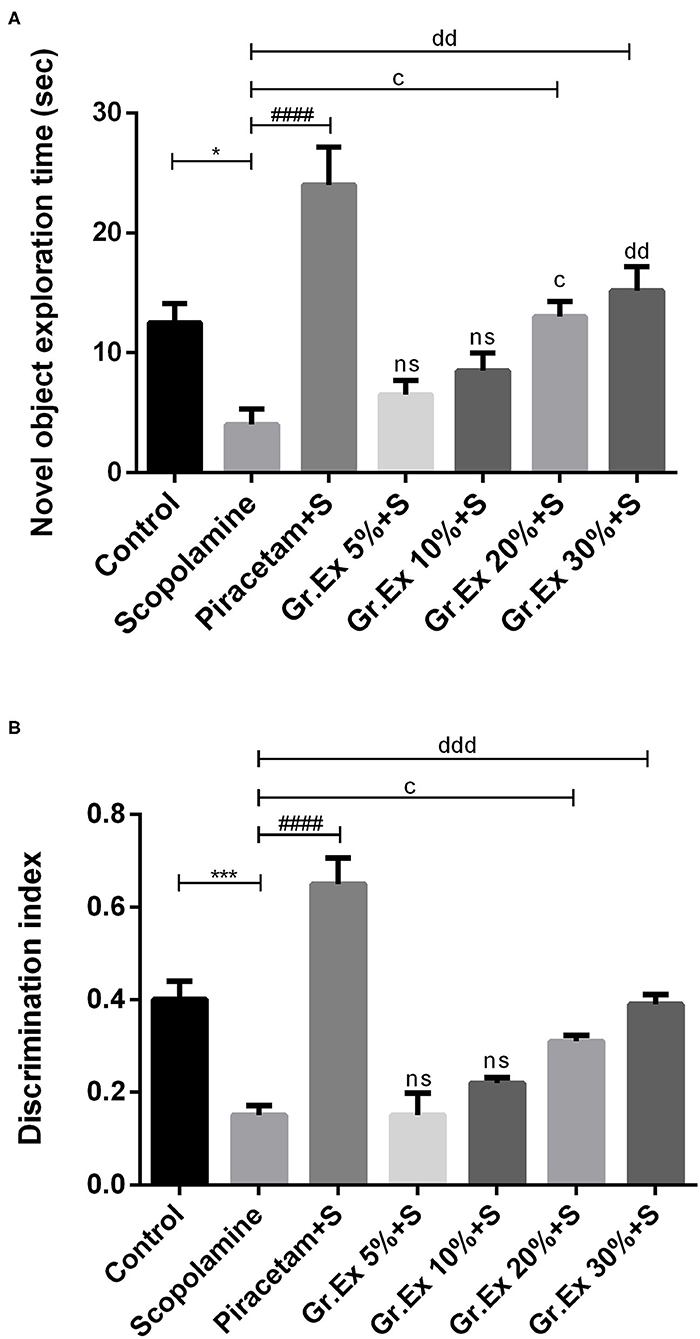
Figure 9. Impact of Grewia asiatica exudate (Gr.Ex) on (A) novel object exploration time and (B) discrimination index in the novel object recognition test. Data expressed as mean ± SEM (n = 6) and evaluated by one-way ANOVA followed by Tukey's test. *P < 0.05, ***P < 0.001, comparison between control animals and scopolamine-treated group. ####P < 0.0001, comparison between scopolamine- and piracetam-treated groups. cP < 0.05, comparison between scopolamine- and 20% Gr.Ex-treated groups. ddP < 0.01, dddP < 0.001, comparison between scopolamine- and 30% Gr.Ex-treated groups.
The MWM test was employed to evaluate the impact of Gr.Ex on scopolamine-induced amnesia, and outcomes were compared with those of piracetam, a nootropic drug. Animals of the control group could locate the hidden platform quickly during training in 3 consecutive days. However, treatment with scopolamine induced prolonged escape latencies in rats (P < 0.0001). This amnesic effect of scopolamine was reversed by 20 and 30% Gr.Ex in a concentration-dependent manner, with P = 0.003 and P = 0.0001, respectively, the way piracetam did (P < 0.0001) (Figure 10A), and ANOVA revealed significant intergroup differences for escape latencies [F(6, 105) = 367.8, P < 0.0001]. Results collected on probe day revealed that Gr.Ex-treated rats had developed spatial memory, as they showed a preference for the quadrant where the platform was previously situated. As compared to the scopolamine-amnesic rats, the animals treated with 20 and 30% Gr.Ex demonstrated an increased number of entries, with P = 0.005 and P = 0.0003, respectively (Figure 10B). The swimming time in the platform quadrant was also prolonged in a concentration-dependent pattern, showing the better remembrance of platform quadrant in animals exposed to 20% (P = 0.003) and 30% (P < 0.0001) Gr.Ex (Figure 10C). Scopolamine-treated rats showed impaired memory, as they were unable to locate the platform quadrant as compared to the control group (P < 0.0001), while piracetam reversed these effects (P < 0.0001). Similarly, amnesic rats traveled significantly long distances to reach the target quadrant than the control group (P = 0.0007), which was shortened by piracetam (P < 0.0001) and Gr.Ex (20 and 30%), with P = 0.003 (Figure 10D). However, the effects were not prominent in rats exposed to low concentrations (5 and 10%) of Gr.Ex.
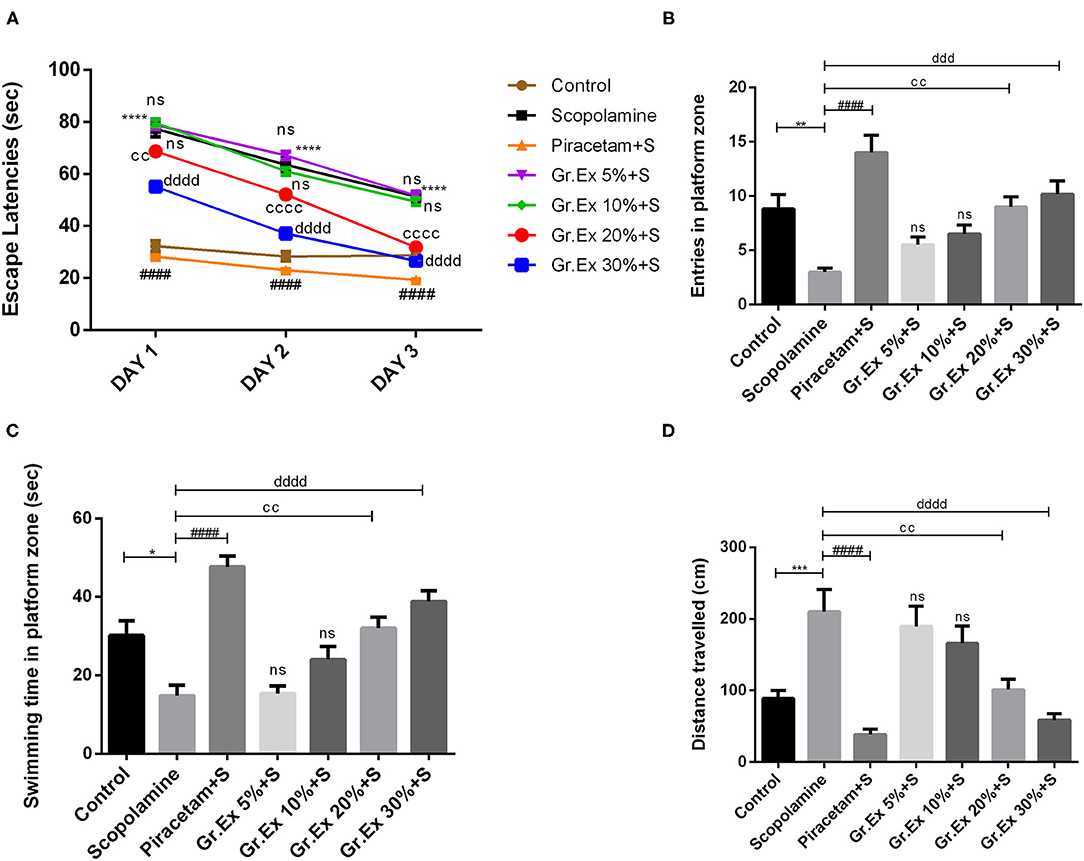
Figure 10. Effect of Grewia asiatica exudate (Gr.Ex) on (A) escape latencies, (B) entries in the platform zone, (C) swimming time in the platform zone, and (D) distance traveled in the Morris water maze test. Data expressed as mean ± SEM (n = 6). Escape latencies were evaluated by two-way ANOVA, while one-way ANOVA followed by Tukey's test was used to evaluate other parameters. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, comparison between control animals and scopolamine-treated group. ####P < 0.0001, comparison between scopolamine- and piracetam-treated groups. ccP < 0.01, ccccP < 0.0001, comparison between scopolamine- and 20% Gr.Ex-treated groups. dddP < 0.001, ddddP < 0.0001, comparison between scopolamine- and 30% Gr.Ex-treated groups.
A significant difference was revealed by ANOVA among the impact of different treatments on brain AChE level [F(6, 14) = 10.26, P < 0.001]. Tukey's post hoc test unveiled that the administration of scopolamine resulted in significantly increased AChE level than the control group (P = 0.005), but piracetam normalized these effects with P = 0.0003. Similarly, the elevated AChE levels were reversed by 20 and 30% of Gr.Ex, with P = 0.023 and P = 0.003, respectively (Figure 11A).
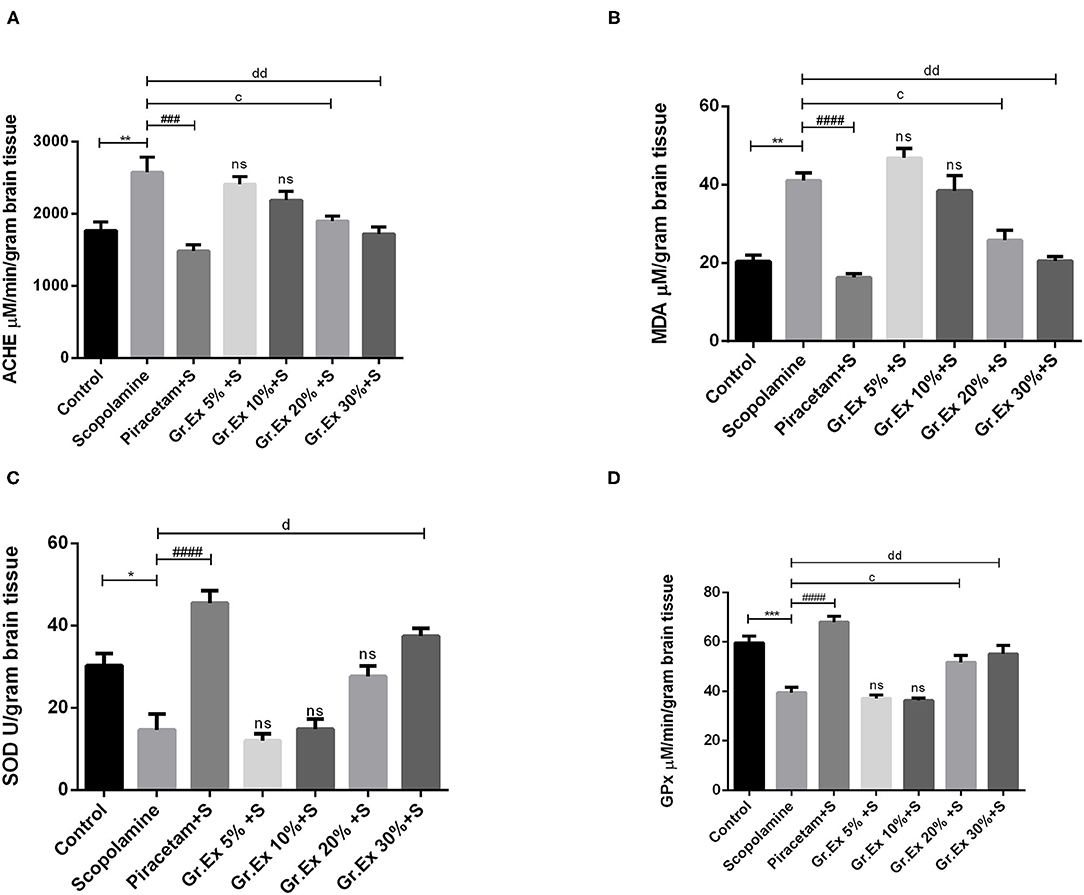
Figure 11. Evaluation of biochemical activity in the brain. Levels of acetylcholinesterase (A), malondialdehyde (B), superoxide dismutase (C), and glutathione peroxidase (D). Data expressed as mean ± SEM (n = 3) and evaluated by one-way ANOVA followed by Tukey's test. *P < 0.05, **P < 0.01, ***P < 0.001, comparison between control animals and scopolamine-treated group. ###P < 0.001, ####P < 0.0001, comparison between scopolamine- and piracetam-treated groups. cP < 0.05, comparison between scopolamine and 20% Grewia asiatica exudate (Gr.Ex)-treated groups. dP < 0.05, ddP < 0.01 comparison between scopolamine- and 30% Gr.Ex-treated groups.
Biochemical studies of brain homogenate demonstrated significant variation among all groups [F(6, 7) = 27.12, P < 0.001]. There was a prominently increased MDA level in scopolamine-treated rats as compared to the control group (P = 0.004), revealing scopolamine-induced lipid peroxidation in the brain. Administration of Gr.Ex reverted these consequences at 20% (P = 0.02) and 30% (P = 0.004) dilutions the way piracetam did (P = 0.001) (Figure 11B).
SOD is a mitochondrial enzyme that causes the conversion of toxic superoxides to hydrogen peroxide and protects neurons from oxidative damage. Biochemical studies established a noteworthy difference for SOD levels among all treated groups [F(6, 14) = 22.35, P < 0.0001]. SOD was significantly decreased after scopolamine administration (P = 0.01), which may cause oxidative stress and neuronal damage. These unpleasant effects were resolved by piracetam (P < 0.0001) and 30% dilution of Gr.Ex (P = 0.0005) (Figure 11C).
GPx is another defense enzyme against oxidative stress in the brain, which is reduced after scopolamine exposure (P = 0.0006). The intergroup variation for GPx levels in the brain was significant [F(6, 14) = 26.6, P < 0.0001]. Piracetam induced improving impact on GPx enzyme level (P < 0.0001), and comparable outcomes were also observed with 20% (P = 0.03) and 30% (P = 0.005) dilutions of Gr.Ex (Figure 11D). However, results obtained from animals treated with 5 and 10% dilutions of Gr.Ex did not produce any significant effects on the mentioned brain enzymes.
Phenols are secondary metabolites present commonly in a variety of fruits (50). Among their different classes, flavonoids and phenolic acids are categorized as major dietary phenolic compounds (51). Besides this, they are famously known to possess the capacity to combat the oxidative stress built during pathological changes or the natural aging process by reducing the lipid peroxidation in tissues (52). Due to this marvelous potential, they are known to play a role in the protection against a range of diseases including cardiovascular, neurological, and metabolic disorders (51).
In the current investigation, the 70% methanolic extract of different concentrations of G. asiatica (5, 10, 20, and 30%) was inquired through in vitro testing for the presence of phenols, flavonoids, and anthocyanins, and results revealed the presence of these constituents in the fruit of G. asiatica. The correlation of radical scavenging potential with these phytoconstituents was also confirmed through antioxidant assays, and results were following the previous claims (6, 49), providing the hint that the fruit may prove beneficial in managing neurological sufferings (53).
A majority of the world's populace still chooses natural remedies for a range of ailments, as cost and the unpleasant side effects associated with synthetic compounds make it difficult for them to gain adequate treatment. Current researchers are making continuous efforts to examine the curative benefits possessed by nutritional resources (54). Being enriched with a range of phytoconstituents and vitamins, plants own marvelous medicinal properties (55) that may help alleviate the health sufferings of humankind.
Grewia asiatica is a nutritional plant that has remarkable medicinal benefits against diabetes, cancer, fever, and aging (6). The fruit exudate of G. asiatica has been evaluated in the current study for possible remedial potential in neurological disorders, as the occurrence of anthocyanins, flavonoids, and phenols is confirmed by our in vitro analysis. We conducted a series of behavioral tests combined with the biochemical evaluation to see the effectiveness of chronic exposure of Gr.Ex on various parameters of anxiety, depression-like behavior, and memory deficit in rodents.
At higher concentrations, Gr.Ex produced a prominent anxiolytic effect in rats, as evident from their activity in the behavioral tests for anxiety. Animals administered with 20 and 30% dilutions of Gr.Ex were daring enough to visit the central zone and open arms successively in both tests. Diazepam was used as a standard treatment that produces an anxiolytic effect by promoting GABAergic neurotransmission in the brain. As Gr.Ex is enriched with valuable phytoconstituents, the anxiolytic-like effect might be linked to the synergistic interaction of its phytochemical constituents. Flavonoids, a family of polyphenolic compounds, are claimed to interact with GABA receptors, thus modulating GABA levels, resulting in neuronal inhibition (56). Our findings are consistent with the previous studies where the ameliorative effect of polyphenol-rich Aronia melanocarpa berries on anxiety was explored by allowing young rats to consume the berry juice for 1 month, and their behavioral analysis demonstrated the positive anxiolytic outcomes (57). Similarly, the rats administered with primary polyphenol in tea (epigallocatechin-3-gallate) for several days revealed a significant anxiolytic effect in open field and light/dark tests (58).
The antidepressant activity was evaluated through an FST in rats, where immobility time was the noted parameter, taking fluoxetine as a positive control that acts as an inhibitor of serotonin reuptake (59). The treatment with different dilutions of Gr.Ex exerted a notable antidepressant-like effect in a concentration-dependent manner, as the immobility of rats was decreased after their exposure to a higher concentration of Gr.Ex. These characteristics might be existing in Gr.Ex due to the presence of flavonoids that may cause inhibition of monoamine oxidases and cause increased levels of monoamines in the brain (59). Besides this, flavonoids are also believed to boost the levels of brain-derived neurotrophic factor (BDNF), an important biomarker of depression, found in the hippocampus; BDNF is a neurotrophin that is known to help in neuronal survival and protection (60). In 2018, a study investigated the association of nutritional polyphenol with symptoms of depression in a cohort of adults, and the outcomes revealed the inverse relationship between both, i.e., the increased dietary flavonoids intake may result in reduced depression-like signs (61). Another study recently reported that naturally-derived polyphenols exert antidepressant-like effects in rats through modulation of the microbiota–gut–brain axis (62). Several lines of criticism are in debate over the recent years with FST or tail suspension test as a tool to judge the antidepressant-like activity (63). According to the researchers, there are possibilities of the uncertainty of the acquired results, animal peculiar behavior in the tank (becomes motionless to get the attention of the experimenter to be pulled out) and terrifying situation for small animals.
Acetylcholine is the major neurotransmitter that acts on widely distributed cholinergic receptors in the brain, and appropriate cholinergic neurotransmission is fundamental for the formation of memory (64). AChE hydrolyzes the acetylcholine, thus diminishing cholinergic neurotransmission. Hence, the assessment of the in vitro and ex vivo activity of this AChE proves valuable to assess cholinergic activity in the brain and can be further correlated with cognitive function.
The nonselective antimuscarinic drug, scopolamine, passes through the blood–brain barrier (BBB) and mimics the memory impairment symptoms of Alzheimer's disease and aging in rats (65) by impairing cholinergic neuronal transmission (66). In behavioral studies, scopolamine-induced memory deficit was efficiently reverted to normal in rats after Gr.Ex (20 and 30%) treatment. Scopolamine-induced shorter latencies to enter the dark compartment were normalized by Gr.Ex in the PAT. Animals, treated with fruit exudate, remembered the previously visited arm in Y-maze and formerly explored objects in the novel object recognition test. Similarly, the inability to locate the platform in the MWM test after scopolamine administration was reversed by Gr.Ex, as evidenced from shorter escape latencies and preference for quadrant on probe day. Biochemical evaluation of the brain homogenate also demonstrated that scopolamine-induced elevated AChE activity was normalized by Gr.Ex, suggesting that inhibition of AChE might be one of the possible mechanisms behind this memory improvement.
Flavonoids present in Gr.Ex might inhibit AChE (67), leading to elevated acetylcholine levels in the hippocampus, which in turn may reduce brain pathological changes (68) by downstreaming the neuronal and glial cells responsible for immune responses happening in the brain (69). Flavonoids also bind and activate cAMP response element-binding proteins (CREBs), leading to overexpression of genes influencing memory by strengthening neurotransmission and flow of information (70).
Our findings are in agreement with the previous findings where scientists evaluated the role of a polyphenol-rich extract from grape and blueberry (PEGB) in ameliorating age-related cognitive decline. The mice fed with PEGB for several weeks showed improved spatial learning, memory, and cognition (71, 72), suggesting that polyphenol supplementation can halt the age-related cognitive deficit. In another study, the cognitive function in mice, given coffee polyphenols daily for 5 months, was evaluated using different behavioral tests, and outcomes supported the role of polyphenols in preventing cognitive dysfunction by reducing the amyloid β (Aβ) plaques in the hippocampus (73). Phenol and flavonoids present in Gr.Ex could prevent the activation of caspase-3, resulting in antiapoptotic actions and neuronal protection (74). Additionally, they are well-known to promote neuronal proliferation by stimulating the release of neurotrophic factors, thus they may revert the stress-, anxiety-, and aging-impaired adult hippocampus neurogenesis (75).
Scopolamine administration builds oxidative stress in the brain, as evident from reduced SOD and GPx, and increases lipid peroxidation, as marked by elevated MDA (76). Anthocyanins, a major group of polyphenols, are well-known to possess antioxidant potential, which plays a neuroprotective role by reducing intracellular ROS levels. The brain is rich in oxidizable fatty acids and comparatively low antioxidant resistance, which makes the organ more susceptible to oxidative stress. Overproduction of ROS deteriorates cellular components of neurons, leading to apoptosis and damaged learning process (77), as pathogenesis of a range of neurological diseases has a strong association with oxidative stress (78). Furthermore, they also increase neuronal signaling in the hippocampus (79), the part of the brain that processes short-term, long-term, and spatial memory (80). So, these constituents could be credited with the outcomes of the existing study, as many studies have reported their beneficial neuroprotective role (14, 81).
The fruit of G. asiatica is also rich in vitamin C (6), which is known to play a crucial role in neuronal maturation and neurotransmission (82). It possesses excellent radical scavenging potential (83) that combats the deteriorating ROS partaking in neurological disorders. Acting as a cofactor in the biosynthesis of carnitine (82), it participates in the production of acetylcholine (84) that improves remembrance by supporting cortical and hippocampal synaptic plasticity. Ascorbic acid is known to enhance the BDNF expression that augments neurogenesis and improves neuronal plasticity essential for learning and memory (85).
Current findings established the antioxidant, anti-cholinesterase, and neuromodulatory role of phenols, flavonoids, and anthocyanins present in the nutritional fruit of G. asiatica. Chronic administration of Gr.Ex led to a reduction in anxiety and depression-like behavior as well as reversal of scopolamine-induced cognitive impairment in rats. Ex vivo biochemical studies also provided supported the observed outcomes. The effects were more pronounced at higher concentrations of fruit exudate, and reported phytoconstituents might be credited to this phenomenal potential of this fruit. However, molecular mechanisms behind these neuroprotective characteristics need to be further explored and investigated in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All animal studies were performed after availing authorization from the Department of Pharmacology Ethical Committee BZU, Multan (EC/12-PHL-2017 dated 22-05-2017) and were accomplished by following instructions of the Institute of Laboratory Animal Resources (ILAR), Commission on Life Sciences, National Research Council (NRC, 1996).
AW, SJ, MR, AM, FA, and II intellectualized the study design, performed the experiment, and analyzed the data and figures. II, MR, NS, HS, MA, FAA, and FA contributed to the assessment of data and statistical analysis. NS, II, and AW performed the biochemical assay. All authors contributed equally in the preparation of the manuscript and approved the final manuscript with consent.
This work was funded by the Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia, through research supporting project number RSP-2020/131.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are thankful to Mr. Muhammad Imran (animal house attendant) who devotedly worked for the welfare of laboratory animals. Furthermore, the authors extend their appreciation to the Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia, for funding this work through research supporting project number RSP-2020/131.
1. Von Essen E, Mårtensson F. Young adults' use of food as a self-therapeutic intervention. Int J Qual Stud Health Well-being. (2014) 9:23000. doi: 10.3402/qhw.v9.23000
3. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. (2012) 3:506–16. doi: 10.3945/an.112.002154
4. Alasalvar C, Salvadó JS, Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chem. (2020) 314:126192. doi: 10.1016/j.foodchem.2020.126192
5. Ullah W, Uddin G, Siddiqui BS. Ethnic uses, pharmacological and phytochemical profile of genus Grewia. J Asian Nat Prod Res. (2012) 14:186–95. doi: 10.1080/10286020.2011.639764
6. Zia-Ul-Haq M, Stanković MS, Rizwan K, De Feo V. Grewia asiatica L., a food plant with multiple uses. Molecules. (2013) 18:2663–82. doi: 10.3390/molecules18032663
7. Moosa A, Farzand A, Ahmad T, Khan SA, Gleason ML, Abbas H, et al. First report of fusarium rot of phalsa (Grewia asiatica) Caused by Fusarium solani in Pakistan. Plant Dis. (2019) 103:2476. doi: 10.1094/pdis-04-19-0688-pdn
8. Khattab HAH, El-Shitany NA, Abdallah IZA, Yousef FM, Alkreathy HM. Antihyperglycemic potential of Grewia asiatica fruit extract against streptozotocin-induced hyperglycemia in rats: anti-inflammatory and antioxidant mechanisms. Oxid Med Cell Longev. (2015) 2015:549743. doi: 10.1155/2015/549743
10. Jyoti S, Shalini P, Satya KC, Gyanendra R. Nutritional and medicinal potential of Grewia subinaequalis DC. (syn. G. asiatica.) (Phalsa). J Med Plants Res. (2015) 9:594–612. doi: 10.5897/jmpr2015.5724
11. Khan RS, Asghar W, Khalid N, Nazir W, Farooq M, Ahmed I, et al. Phalsa (Grewia asiatica L) fruit berry a promising functional food ingredient: a comprehensive review. J Berry Res. (2019) 9:179–93. doi: 10.3233/JBR-180332
12. Talpur FN. Analysis and characterization of anthocyanin from phalsa (Grewia asiatica). MOJ Food Process Technol. (2017) 5:299–305. doi: 10.15406/mojfpt.2017.05.00127
13. Agarwal P, Holland TM, Wang Y, Bennett DA, Morris MC. Association of strawberries and anthocyanidin intake with alzheimer's dementia risk. Nutrients. (2019) 11:3060. doi: 10.3390/nu11123060
14. Shukitt-Hale B, Lau FC, Josep JA. Berry fruit supplementation and the aging brain. J Agric Food Chem. (2008) 56:636–41. doi: 10.1021/jf072505f
15. Miller MG, Shukitt-Hale B. Berry fruit enhances beneficial signaling in the brain. J Agric Food Chem. (2012) 60:5709–15. doi: 10.1021/jf2036033
16. Wasay M, Ali S. Growing burden of neurological diseases in Pakistan–need for a national health survey. J Pak Med Assoc. (2010) 60:249–50. Available online at: https://jpma.org.pk/PdfDownload/1988
17. Siuly S, Zhang Y. Medical big data: neurological diseases diagnosis through medical data analysis. Data Sci Eng. (2016) 1:54–64. doi: 10.1007/s41019-016-0011-3
18. Chin JH, Vora N. The global burden of neurologic diseases. Neurology. (2014) 83:349–51. doi: 10.1212/WNL.0000000000000610
19. Bergen DC, Silberberg D. Nervous system disorders: a global epidemic. Arch Neurol. (2002) 59:1194–6. doi: 10.1001/archneur.59.7.1194
20. Fitzgerald S. Neurologists tackle high costs of prescription drugs. Neurol Today. (2017) 17:1. doi: 10.1097/01.nt.0000516003.92876.07
21. Khawam EA, Laurencic G, Malone DA. Side effects of antidepressants: an overview. Cleve Clin J Med. (2006) 73:351–61. doi: 10.3949/ccjm.73.4.351
22. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Neurol. (2014) 4:177. doi: 10.3389/fphar.2013.00177
23. Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. (2015) 9:449–54. doi: 10.1016/j.jtusci.2014.11.001
24. Mathur R, Vijayvergia R. Determination of total flavonoid and phenol content in Mimusops elengi Linn. Int J Pharm Sci Res. (2017) 8:5282–5. doi: 10.13040/IJPSR.0975-8232.8(12).5282-85
25. Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J. (2012) 19:215–21. Available online at: http://ifrj.upm.edu.my/19%20(01)%202011/(28)IFRJ-2011-060%20Sudarat.pdf
26. Chandra Shekhar T, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. Leaves. Am J Ethnomed. (2014) 1:244–9.
27. Adebiyi OE, Olayemi FO, Ning-Hua T, Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef Univ J Basic Appl Sci. (2017) 6:10–4. doi: 10.1016/j.bjbas.2016.12.003
28. Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. (1961) 7:88–95. doi: 10.1016/0006-2952(61)90145-9
29. Nguyen TK, Im KH, Choi J, Shin PG, Lee TS. Evaluation of antioxidant, anti-cholinesterase, and anti-inflammatory effects of culinary mushroom Pleurotus pulmonarius. Mycobiology. (2016) 44:291–301. doi: 10.5941/MYCO.2016.44.4.291
30. Elufioye TO, Chinaka CG, Oyedeji AO. Antioxidant and anticholinesterase activities of Macrosphyra Longistyla (DC) hiern relevant in the management of Alzheimer's disease. Antioxidants. (2019) 8:400. doi: 10.3390/antiox8090400
31. Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. (2015) 6:e52434. doi: 10.3791/52434
32. Radhakrishnan A, Gulia KK. Categories of wistar rats based on anxiety traits: a study using factor and cluster method. Ann Neurosci. (2018) 25:234–40. doi: 10.1159/000492268
33. Rogóz Z, Skuza G. Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol Rep. (2011) 63:1547–52. doi: 10.1016/S1734-1140(11)70719-8
34. García-Ríos RI, Mora-Pérez A, González-Torres D, Carpio-Reyes R de J, Soria-Fregozo C. Anxiolytic-like effect of the aqueous extract of Justicia spicigera leaves on female rats: a comparison to diazepam. Phytomedicine. (2019) 55:9–13. doi: 10.1016/j.phymed.2018.07.007
35. Campus P, Colelli V, Orsini C, Sarra D, Cabib S. Evidence for the involvement of extinction-associated inhibitory learning in the forced swimming test. Behav Brain Res. (2015) 278:348–55. doi: 10.1016/j.bbr.2014.10.009
36. Nassiri-Asl M, Zamansoltani F, Javadi A, Ganjvar M. The effects of rutin on a passive avoidance test in rats. Prog Neuro-Psychopharmacology Biol Psychiatry. (2010) 34:204–7. doi: 10.1016/j.pnpbp.2009.11.006
37. Ghasemi S, Moradzadeh M, Hosseini M, Beheshti F, Sadeghnia HR. Beneficial effects of Urtica dioica on scopolamine-induced memory impairment in rats: protection against acetylcholinesterase activity and neuronal oxidative damage. Drug Chem Toxicol. (2019) 42:167–75. doi: 10.1080/01480545.2018.1463238
38. Hollander J, McNivens M, Pautassi RM, Nizhnikov ME. Offspring of male rats exposed to binge alcohol exhibit heightened ethanol intake at infancy and alterations in T-maze performance. Alcohol. (2019) 76:65–71. doi: 10.1016/j.alcohol.2018.07.013
39. Jung WR, Kim HG, Kim KL. Ganglioside GQ1b improves spatial learning and memory of rats as measured by the Y-maze and the Morris water maze tests. Neurosci Lett. (2008) 439:220–5. doi: 10.1016/j.neulet.2008.05.020
40. Vogel-Ciernia A, Wood MA. Examining object location and object recognition memory in mice. Curr Protoc Neurosci. (2014) 2014:8.31.1–17. doi: 10.1002/0471142301.ns0831s69
41. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat Protoc. (2013) 8:2531–7. doi: 10.1038/nprot.2013.155
42. Wang W, Liu L, Jiang P, Chen C, Zhang T. Levodopa improves learning and memory ability on global cerebral ischemia-reperfusion injured rats in the Morris water maze test. Neurosci Lett. (2017) 636:233–40. doi: 10.1016/j.neulet.2016.11.026
43. Gacar N, Mutlu O, Utkan T, Komsuoglu Celikyurt I, Gocmez SS, Ulak G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol Biochem Behav. (2011) 99:316–23. doi: 10.1016/j.pbb.2011.05.017
44. Malik H, Javaid S, Fawad Rasool M, Samad N, Rizwan Ahamad S, Alqahtani F, et al. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of ficus benghalensis in behavioral experimental models. Medicina. (2020) 56:144. doi: 10.3390/medicina56030144
45. Angelucci MEM, Cesário C, Hiroi RH, Rosalen PL, Da Cunha C. Effects of caffeine on learning and memory in rats tested in the Morris water maze. Brazilian J Med Biol Res. (2002) 35:1201–8. doi: 10.1590/S0100-879X2002001000013
46. Haider S, Naqvi F, Batool Z, Tabassum S, Sadir S, Liaquat L, et al. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res Bull. (2015) 115:1–8. doi: 10.1016/j.brainresbull.2015.04.001
47. Chow CK, Tappel AL. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. (1972) 7:518–24. doi: 10.1007/BF02533017
48. Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. (2002) 50:81–6. doi: 10.1021/jf010865b
49. Qamar M, Akhtar S, Ismail T, Sestili P, Tawab A, Ahmed N. Anticancer and anti-inflammatory perspectives of Pakistan's indigenous berry Grewia asiatica Linn (Phalsa). J Berry Res. (2020) 10:115–31. doi: 10.3233/JBR-190459
50. Minatel IO, Borges CV, Ferreira MI, Gomez HAG, Chen C-YO, Lima GPP. Phenolic compounds: functional properties, impact of processing and bioavailability. In: Soto Hernández M, editor. Phenolic Compounds - Biological Activity. London: InTechOpen (2017). doi: 10.5772/66368
51. King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. (1999) 99:213–8. doi: 10.1016/S0002-8223(99)00051-6
52. Afanas'ev IB, Dcrozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. (1989) 38:1763–69. doi: 10.1016/0006-2952(89)90410-3
53. Delanty N, Dichter MA. Antioxidant therapy in neurologic disease. Arch Neurol. (2000) 57:1265–70. doi: 10.1001/archneur.57.9.1265
54. Bayarsukh N. Strengthening the conservation and sustainable use of plant genetic resources for food and agriculture in Mongolia. Mong J Agric Sci. (2015) 15:173–9. doi: 10.5564/mjas.v15i2.567
55. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. (2017) 6:42. doi: 10.3390/plants6040042
56. Hanrahan JR, Chebib M, Johnston GAR. Flavonoid modulation of GABA A receptors. Br J Pharmacol. (2011) 163:234–45. doi: 10.1111/j.1476-5381.2011.01228.x
57. Tomić M, Ignjatović DD, Tovilović-Kovačević G, Krstić-Milošević D, Ranković S, Popović T, et al. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking: Aronia melanocarpa berry juice. Food Funct. (2016) 7:3111–20. doi: 10.1039/c6fo00321d
58. Wang J, Li P, Qin T, Sun D, Zhao X, Zhang B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav. (2020) 10:e01633. doi: 10.1002/brb3.1633
59. Lochmann D, Richardson T. Selective serotonin reuptake inhibitors. In: Barrett JE, Editor. Handbook of Experimental Pharmacology. New York, NY: Springer LLC (2019). p. 135–44. doi: 10.1007/164_2018_172
60. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. (2010) 70:289–97. doi: 10.1002/dneu.20758
61. Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary polyphenol intake and depression: results from the mediterranean healthy eating, lifestyle and aging (MEAL) study. Molecules. (2018) 23:999. doi: 10.3390/molecules23050999
62. Donoso F, Egerton S, Bastiaanssen TFS, Fitzgerald P, Gite S, Fouhy F, et al. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology. (2020) 116:104673. doi: 10.1016/j.psyneuen.2020.104673
63. Reardon S. Depression researchers rethink popular mouse swim tests. Nature. (2019) 571:456–7. doi: 10.1038/d41586-019-02133-2
64. Donovan NJ, Amariglio RE, Zoller AS, Rudel RK, Gomez-Isla T, Blacker D, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. (2014) 22:1642–51. doi: 10.1016/j.jagp.2014.02.007
65. Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behav Brain Res. (2011) 221:389–411. doi: 10.1016/j.bbr.2010.11.036
66. Lee B, Shim I, Lee H, Hahm DH. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. (2011) 21:874–83. doi: 10.4014/jmb.1104.04012
67. Khan H, Marya, Amin S, Kamal MA, Patel S. Flavonoids as acetylcholinesterase inhibitors: current therapeutic standing and future prospects. Biomed Pharmacother. (2018) 101:860–70. doi: 10.1016/j.biopha.2018.03.007
68. Thameem Dheen S, Kaur C, Ling E-A. Microglial activation and its implications in the brain diseases. Curr Med Chem. (2007) 14:1189–97. doi: 10.2174/092986707780597961
69. Liu H, Leak RK, Hu X. Neurotransmitter receptors on microglia. Stroke Vasc Neurol. (2016) 1:52–8. doi: 10.1136/svn-2016-000012
70. Spencer JPE. The impact of fruit flavonoids on memory and cognition. Br J Nutr. (2010) 104:S40–7. doi: 10.1017/S0007114510003934
71. Bensalem J, Dudonné S, Gaudout D, Servant L, Calon F, Desjardins Y, et al. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J Nutr Sci. (2018) 7:10. doi: 10.1017/jns.2018.10
72. Bensalem J, Servant L, Alfos S, Gaudout D, Layé S, Pallet V, et al. Dietary polyphenol supplementation prevents alterations of spatial navigation in middle-aged mice. Front Behav Neurosci. (2016) 10:9. doi: 10.3389/fnbeh.2016.00009
73. Ishida K, Yamamoto M, Misawa K, Nishimura H, Misawa K, Ota N, et al. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci Res. (2020) 154:35–44. doi: 10.1016/j.neures.2019.05.001
74. Hussain G, Zhang L, Rasul A, Anwar H, Sohail MU, Razzaq A, et al. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer's and Parkinson's diseases: an update of recent data. Molecules. (2018) 23:814. doi: 10.3390/molecules23040814
75. Dias GP, Cavegn N, Nix A, Do Nascimento Bevilaqua MC, Stangl D, Zainuddin MSA, et al. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev. (2012) 2012:541971. doi: 10.1155/2012/541971
76. Shekhar C, Kumar S. Kinetics of butyrylcholinesterase inhibition by an ethanolic extract of Shorea robusta. Int J Pharm Sci and Res. (2014) 5:975–8. Available online at: http://www.ijpsr.info/docs/IJPSR14-05-08-019.pdf
77. Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. (1997) 23:134–47. doi: 10.1016/S0891-5849(96)00629-6
78. Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. (2014) 12:140–7. doi: 10.2174/1570159x11666131120230309
79. Barros D, Amaral OB, Izquierdo I, Geracitano L, do Carmo Bassols Raseira M, Henriques AT, et al. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav. (2006) 84:229–34. doi: 10.1016/j.pbb.2006.05.001
80. Opitz B. Memory function the hippocampus. In: Szabo K, Hennerici MG, editors. The Hippocampus in Clinical Neuroscience. Karger Berlin (2014). 51–9. doi: 10.1159/000356422
81. Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. (2005) 8:111–20. doi: 10.1080/10284150500078117
82. Travica N, Ried K, Sali A, Hudson I, Scholey A, Pipingas A. Plasma Vitamin C concentrations and cognitive function: a cross-sectional study. Front Aging Neurosci. (2019) 11:72. doi: 10.3389/fnagi.2019.00072
83. Zaidi SMKR, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin Chim Acta. (2004) 340:229–33. doi: 10.1016/j.cccn.2003.11.003
84. Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. (2009) 46:719–30. doi: 10.1016/j.freeradbiomed.2008.12.018
Keywords: Grewia asiatica, amnesia, anxiety, depression, Morris water maze, escape latency
Citation: Imran I, Javaid S, Waheed A, Rasool MF, Majeed A, Samad N, Saeed H, Alqahtani F, Ahmed MM and Alaqil FA (2021) Grewia asiatica Berry Juice Diminishes Anxiety, Depression, and Scopolamine-Induced Learning and Memory Impairment in Behavioral Experimental Animal Models. Front. Nutr. 7:587367. doi: 10.3389/fnut.2020.587367
Received: 25 July 2020; Accepted: 01 December 2020;
Published: 15 January 2021.
Edited by:
Pauline Lafenetre, Université de Bordeaux, FranceReviewed by:
Clara Rossetti-Marcon, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2021 Imran, Javaid, Waheed, Rasool, Majeed, Samad, Saeed, Alqahtani, Ahmed and Alaqil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imran Imran, aW1yYW4uY2hAYnp1LmVkdS5waw==; Faleh Alqahtani, YWZhbGVoQGtzdS5lZHUuc2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.