
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr. , 28 October 2020
Sec. Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.582387
 Beate Brandl1,2
Beate Brandl1,2 Thomas Skurk1,2
Thomas Skurk1,2 Rachel Rennekamp2
Rachel Rennekamp2 Anne Hannink3
Anne Hannink3 Eva Kiesswetter3
Eva Kiesswetter3 Jessica Freiherr4,5
Jessica Freiherr4,5 Susanne Ihsen6†
Susanne Ihsen6† Jutta Roosen7
Jutta Roosen7 Martin Klingenspor1,8
Martin Klingenspor1,8 Dirk Haller1,9
Dirk Haller1,9 Dietmar Krautwurst10
Dietmar Krautwurst10 Thomas Hofmann10,11
Thomas Hofmann10,11 Jakob Linseisen12,13
Jakob Linseisen12,13 Dorothee Volkert3
Dorothee Volkert3 Hans Hauner1,2,14*
Hans Hauner1,2,14*Introduction: Nutritional habits and requirements are changing over the lifespan, but the dynamics of nutritional issues and the diet-health relationship in the major stages of the human life cycle are not sufficiently understood. A human phenotyping research platform for nutrition studies was established to recruit and phenotype selected population groups across different stages of life. The project is the backbone of the highly interdisciplinary enable competence cluster of nutrition research aiming to identify dietary determinants of a healthy life throughout the lifespan and to develop healthier and tasty convenience foods with high consumer acceptance.
Methods: The phenotyping program included anthropometry, body composition analysis, assessment of energy metabolism, health and functional status, multisensory perception, metabolic phenotyping, lifestyle, sociodemography, chronobiology, and assessment of dietary intake including food preferences and aversions.
Results: In total, 503 healthy volunteers at four defined phases of life including 3–5-year old children (n = 44), young adults aged 18–25 years (n = 94), adults aged 40–65 years (“middle agers,” n = 205), and older adults aged 75–85 years (n = 160) were recruited and comprehensively phenotyped. Plasma, serum, buffy coat, urine, feces and saliva samples were collected and stored at −80°C. Significant differences in anthropometric and metabolic parameters between the four groups were found. A major finding was the decrease in fat-free mass and the concomitant increase in % body fat in both sexes across the adult lifespan.
Conclusions: The dataset will provide novel information on differences in diet-related parameters over the lifespan and is available for targeted analyses. We expect that this novel platform approach will have implications for the development of innovative food products tailored to promote healthy eating throughout life.
Trial registration: DRKS, DRKS00009797. Registered on 20 January 2016, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009797.
Dietary habits have substantial impact on the onset and course of many chronic diseases (1). Nutrition behavior, however, is subject to multiple influences such as age, sociodemographic factors, and sensory food preferences among many others (2, 3). Although only limited knowledge about specific nutrient requirements and food selection in dependence of life stage is available, substantial differences in food selection and consumption may exist due to many complex interactions and temporal changes. Whereas food selection in young children may depend on parents' nutrition attitudes (4), young adults leaving their familial environment may develop their own lifestyle under strong peer-group influence (5, 6). Middle-aged adults following a Western dietary pattern and a sedentary lifestyle are at high risk of developing lifestyle-associated chronic diseases. After retirement, dietary requirements and habits may differ from younger age groups with a focus on the relief of physical impairments (7).
During these developmental stages dietary habits play a substantial role for subjective well-being and the objective health status. Of central importance, the progression of caloric overnutrition owing to energy dense foods and other external adverse influences begins already early in life (8). Furthermore, due to the increasing number of old and very old people, prevention of age-related chronic diseases and sustained independence in daily life are of utmost individual and public health interest (9). Thus, the challenge to improve dietary habits in a given age group covers a broad range of specific aspects. It is obvious that each life stage has its own needs, but the dynamics of food selection in the major stages of the human life cycle are not sufficiently understood (10, 11). It was, therefore, the rationale of this project to establish a unique phenotyping platform combining expertise from different disciplines, such as nutrition science, sensory science and consumer science among many others to get a better understanding of the diet-health relationship by a deep and comprehensive characterization of individuals in defined stages of life.
Thus, the central goal of the project is to characterize the dietary requirements and preferences and their relationship to health parameters in defined age groups. This knowledge may facilitate the development of age-group specific and, eventually, personalized dietary strategies for innovative health promotion and disease prevention. In this context, an additional goal was to establish a common databank and biobank that can be used for answering many associated research questions and which is accessible for external collaborators.
The study protocol was approved by the ethical committee of the Faculty of Medicine of the Technical University of Munich in Germany (approval no. 452/15) and Friedrich-Alexander-Universität Erlangen-Nürnberg (approval no. 291/15B). The guidelines of the International Conference on Harmonization of Good Clinical Practice and the World Medical Association Declaration of Helsinki (in the revised version of Seoul, South Korea 2008) were considered. All study participants have given written informed consent.
This cross-sectional study was performed between February 2016 and February 2018 in two centers in Freising and Nuremberg, Germany. A random sample of volunteers from the general population was recruited by advertising in kindergarten, newspapers and other media in the region of Freising and Nuremberg, Germany. Aim and procedures were explained during a presentation of the scientific staff at information meetings.
The eligibility of the volunteers was assessed using a detailed screening procedure either face-to-face or via telephone. Furthermore, an equal gender partition was planned for each age group. Moreover, in the age group 40–65 years half of the participants were selected with an elevated waist circumference (≥ 102 cm in males and ≥ 88 cm for females) indicating a moderately elevated cardiovascular and metabolic risk (Figure 1).
General inclusion criteria were healthy non-smoking community-dwelling Caucasians, body mass index (BMI) 18.5–30.0 (−35.0) kg/m2, Mini Mental State Examination (MMSE) ≥ 24 points (12) (the latter in older adults only). Explicit exclusion criteria in the children's group were chronic diseases such as asthma, endocrine diseases, and genetic syndromes such as trisomy 21. In the group of young adults and middle agers the following exclusion criteria were considered: pregnancy, chronic infections such as human immunodeficiency virus infection, endocrine diseases such as diabetes mellitus, history of myocardial infarction or stroke, untreated hypertension (>160/95 mmHg), heart failure, any reported cancer within the last 3 years, severe lung, liver, kidney disease, autoimmune disease, stomach ulcer, diagnosed psychological or neurological disease, blood transfusion in the last 3 months, immobility, unintended or intentional weight loss of more than 5% in the previous 3 months, and participation in intervention studies. In the age group 75–85 years, the same exclusion criteria were applicable as for the young adults and the middle-aged group with the addition: need of care (classification into a care level according to SGB §XI).
Finally, the four age groups included 44 3- to 5-year old children, 94 young adults aged 18–25 years, 205 adults aged 40–65 years (“middle agers”) and 160 older persons aged 75–85 years. In the group of middle agers 97 subjects were at risk for cardiometabolic disease defined by a waist circumference of ≥ 102 cm in males and ≥ 88 cm in females.
All participants underwent a comprehensive phenotyping program (Figure 2). An overview of measurements and questionnaires in the respective age group is depicted in Tables 1, 2. All measurements and sampling processes were performed using established standard operation procedures (SOPs) by trained personnel.
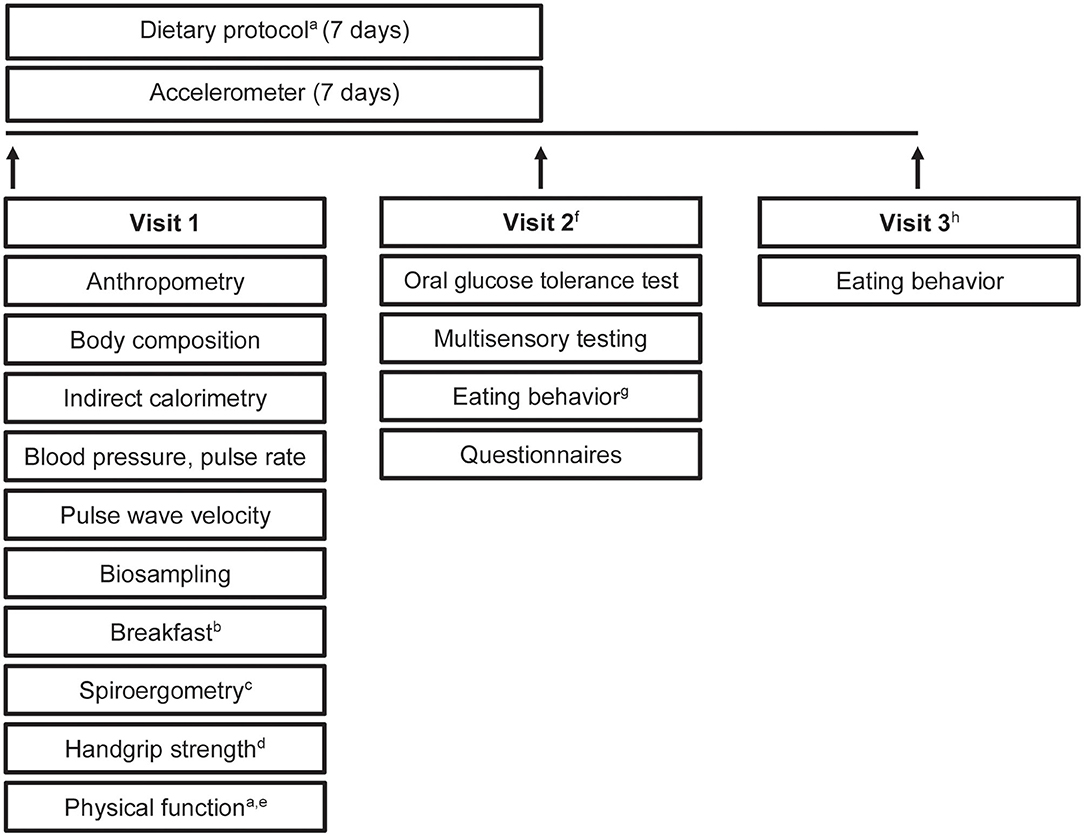
Figure 2. Study design. Timeline and examinations. a, only in older participants; b, standardized breakfast; c, only in young adults and middle agers; d, not in children; e, physical function included tests of the Short Physical Performance Battery and Fullerton Senior Fitness Test Battery; f, young adults, middle agers, older adults were invited for visit 2; g, eating habits were recorded by using a food frequency questionnaire and a 24-h food list; h, visit 3 included a web-based 24-h food list which could be filled at home.
All anthropometric and clinical parameters were measured in the morning following an overnight fast. In the group of 3–5-year-old participants, head circumference was determined. Furthermore, the skinfold thickness was measured at the triceps, subscapular, and suprailiacal location with a skinfold caliper (Holtain Ltd., Crosswell, UK). In the older persons, the upper arm circumference and calf circumference were additionally measured. Body composition and weight were measured using the Seca mBCA 515 device (Seca GmbH & Co KG, Hamburg, Germany). In addition, Bod Pod (COSMED, Fridolfing, Germany) was used to assess body composition (Freising only).
Resting metabolic rate (RMR) was calculated based on gas exchange measurements performed by indirect calorimetry under a canopy hood (COSMED Indirect calorimetry, Fridolfing, Germany). Data acquisition was performed during 30 min under thermoneutral conditions. Furthermore, participants wore standard accelerometry devices to monitor their everyday physical activity for seven days all day long (ActiGraph wGT3X-BT, Pensacola, FL, USA). Recorded data was analyzed using ActiLife software (version 6.13.3; Acti-Graph Corp., Pensacola, FL).
Systolic and diastolic blood pressure and pulse rate were assessed using Omron M8 comfort (Mannheim, Germany), or Maxi Stabil 3 (WelchAllyn GmbH &Co. KG; Hechingen, Germany). Pulse wave velocity (PWV) was measured using Arteriograph (TensioMed Ltd, Budapest, Hungary) with the corresponding TensioMed analysis software (version 3.0.0.4) (Freising only). Spiroergometry was performed with the MetaLyser 3B-R3 (CORTEX Biophysik GmbH, Leipzig, Germany) to measure ventilatory thresholds (VT1, VT2) and O2peak. The respiratory gases were measured during a physical ramp stress until exhaustion or up to 300 watts in the young adults and middle-aged groups. Physical function of the older adults was assessed by the Short Physical Performance Battery (SPPB) (balance, gait speed, chair-rise) (13, 14), and the Fullerton Senior Fitness Test Battery (30 s chair stand test, 30 s arm curl test, chair sit and reach test, back stretch test, 8-Foot Up-and-Go test, 6 min walk) (15, 16). Handgrip strength was measured in young adults, middle agers and older adults by using a hydraulic hand grip dynamometer (JAMAR Model 5030J1, Ludwig Bertram GmbH, Isenhagen, Germany).
Visual functions were assessed using the Landolt ring test. Additionally, red-green color blindness was checked with the Ishihara test. Hearing ability was checked using a standard audiometer (MAICO ST 20, MAICO Diagnostics GmbH, Berlin Germany). To test olfactory performance, the 40-item Monell Extended Sniffin' Sticks Identification Test (MONEX-40) was used (17). Finally, participants had to rate six recombinants of chemically defined fragrances regarding their intensity and pleasantness.
Following a 12-h overnight fast an oral glucose tolerance test (oGTT) was performed. After taking a baseline blood sample in the fasting state, volunteers received 75 g glucose in a volume of 300 ml water (Carl Roth GmbH & Co. KG, Karlsruhe, Germany). After 30, 60, 90, 120, 180, and 240 min blood was drawn and plasma glucose levels were determined (HemoCue Glucose 201+, plasma-calibrated, Ängelholm, Sweden).
The collection of biomaterials was an important part of the enable phenotyping activities. As summarized in Table 1, plasma, serum, dried blood spots, urine, and feces were collected in the volunteers from the different age groups. In the children, collection of blood was not possible, but saliva was stored for genetic analyses.
All blood samples were collected in the fasting state. Routine laboratory parameters were analyzed by a certified laboratory (SynLab; Munich, Germany). Additional blood samples were collected, immediately centrifuged (2.500 g for 10 min at 20°C) and subsequently stored in aliquots at −80°C for further analyses. Buffy coats were collected for DNA extraction. Dried blood spots were prepared by pipetting 75 μl whole EDTA-blood to the center of each circle without touching the filter paper (Whatman® 903 Protein Saver Card, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). The filter cards were left 4 h to dry in a horizontal position protected from light. After drying, samples were bagged together with desiccant (Silica gel, Carl Roth, Karlsruhe, Germany) and stored at −20°C for later measurements. Urine was collected from all participants in the morning. Osmolarity was measured by using an automatic digital osmometer (OM 815, Vogel GmbH & Co. KG, Fernwald, Germany) and samples were aliquoted and stored at −80°C until analysis. Participants collected stool in two tubes, whereby only one tube contained 8 ml DNA stabilization buffer (Stratec Molecular GmbH, Berlin, Germany). Participants were instructed to bring the samples to the study center within 6 h for storage at −80°C. Children collected 1 ml saliva using a saliva collection kit (Oragene DNA, OG-500, DNA Genotek, Ottawa, Canada). Samples were also stored at −80°C.
In the four age groups, eating/dietary habits were recorded in a web-based manner by using a food frequency questionnaire (FFQ2) (18). Moreover, a 24-h food list to identify foods consumed during the previous 24 h was filled in twice with a lag time of 3 months (19). In addition, the older participants were instructed to record their food consumption for 7 days in an open diary. Energy content and nutrient composition of the diets were calculated using EBISpro software (EBISpro 2016, Willstätt-Legelshurst, Germany). Furthermore, meal habits, appetite (20, 21), life events (22), eating motives (23), and food preferences/aversions were assessed by specific and validated questionnaires.
Validated questionnaires to assess the health status (20, 24–29), lifestyle (26, 30), sociodemography (20, 29), and chronobiology (31, 32) were applied in all age groups. Table 2 summarizes the parameters assessed by the various questionnaires.
A good clinical practice compliant (GCP), validated study data bank with a remote data entry system (electronic Case Report Form, eCRF) was set up by a trained data manager from the Munich Study Centre (MSZ, Technical University of Munich) according to established SOPs. Consistency and plausibility were checked automatically by the system. During the study period, regular internal monitoring was performed to ascertain high quality of data entry.
Data were analyzed in the R programming environment. Results are presented as mean ± SD. p-values < 0.05 are regarded as statistically significant. The effect of different age groups was analyzed with a one-way ANOVA test for normally distributed data and a Kruskal-Wallis test in case of non-normal data. In each case a post-hoc test was performed to analyze all pairwise comparisons. The post-hoc tests were done with an appropriate adjustment for multiple testing. Moreover, in each cohort, differences between females and males were assessed by using Mann-Whitney U test.
Selected anthropometric and metabolic characteristics of the study participants in the four age groups are presented in Table 3. Additional detailed information about anthropometry, body composition and laboratory analysis between females and males in each group are shown in the Supplementary Tables 1, 4.
BMI percentile and percentage fat mass were increased significantly from young adults to middle agers up to older adults (each p < 0.001). As expected, percentage fat free mass significantly decreased from young adults to the middle agers and to the age group between 75 and 85 years (p < 0.05, Figures 3A–C).
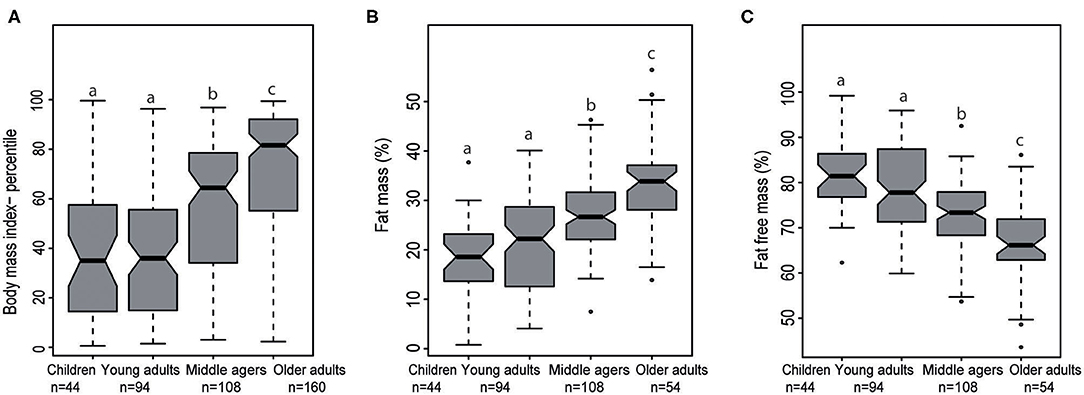
Figure 3. Notched Box plots of (A) body mass index percentile in the four stages of life, (B) fat mass (%), and (C) fat free mass (%) analyzed by air displacement plethysmography. Middle agers fulfilled our criteria for “cardiometabolic risk” were not taken into account. The effect of different age groups was analyzed with a one-way ANOVA test for normally distributed data and a Kruskal-Wallis test in case of non-normal data. In each case a post-hoc test was performed to analyze all pairwise comparisons. The post-hoc tests were done with an appropriate adjustment for multiple testing. Labeled means in a row without a common superscript letter differ, P < 0.05.
Regarding liver enzymes, aspartate aminotransferase was not significantly different across the three adult age groups. Alanine aminotransferase was significantly lower in older adults compared to middle agers (p < 0.05). Gamma-glutamyltransferase levels were significantly lower in young adults compared to middle agers (p < 0.01) and to older adults (p < 0.05). Furthermore, young adults had a mean total cholesterol level of 179.3 ± 31.6 mg/dl that was significantly lower than in the middle-aged (224.4 ± 39.5 mg/dl) and in the older group (220.9 ± 43.2 mg/dl, each p < 0.05). Regarding triglycerides and HDL-cholesterol, no significant differences could be detected between the three age groups. In young adults, a mean LDL-cholesterol level of 102.2 ± 26.8 mg/dl was measured. In contrast, middle agers and older adults had significantly higher LDL-cholesterol levels (each p < 0.05). Fasting plasma glucose and insulin levels significantly increased across the three age groups, from 76.6 ± 5.8 mg/dl up to 89.1 ± 9.4 mg/dl, and from 3.2 ± 1.8 μU/ml to 5.1 ± 4.0 μU/ml, respectively (p < 0.05). In contrast, thyroid- stimulating hormone (TSH) levels were highest in the young adults with 1.8 ± 0.9 μU/ml and were significantly lower in middle agers and older adults, respectively (each p < 0.05).
In the group of 3–5 years old children (n = 44, 24 boys, and 20 girls) there were no differences between both sexes in any of the anthropometric characteristics presented in Supplementary Table 1.
Ninety-four young adults (48 males, 46 females) at a mean age of 22.2 ± 2.0 years participated in the study (Supplementary Table 2). All metabolic parameters were in the normal range, whereas female participants exhibited higher total cholesterol and HDL-cholesterol levels than male participants (p < 0.001). All fasting blood glucose levels were within the normal range (<100 mg/dl) and fasting insulin was higher in female than in male participants (p < 0.05).
In the group of middle agers, 108 volunteers (56 males, 52 females) with normal BMI (<25.0 kg/m2) participated (Supplementary Table 3). Two male participants had a fasting plasma glucose value beyond 100 mg/dl. Moreover, 81 of 108 participants (41 males, 40 females) had a total cholesterol level above 200 mg/dl.
Additionally, 97 participants (46 males, 51 females) of this age group fulfilled our criteria for “cardiometabolic risk” and had an elevated body weight (BMI > 25 kg/m2). Minor deviations from the normal ranges were more frequent than in the normal weight group as shown in Supplementary Table 3.
One hundred-sixty community-dwelling participants (mean age 78.2 ± 2.8 years, mean BMI 26.5 ± 4.0 kg/m2) without physical and mental impairments were included (Table 3). Lipid abnormalities were frequently seen in this group. One hundred five participants had a total cholesterol level above 200 mg/dl. Sixteen (12 males, four females) of the 160 participants had a fasting plasma glucose beyond 100 mg/dl.
Dietary intake of participants was calculated based on FFQ2 and up to two 24 h food lists. Details are given elsewhere (33). Within young adults, middle agers, and older adults energy intake, fat intake (except unsaturated fatty acids), carbohydrate and protein intake did not differ significantly (Table 4). In contrast, fiber intakes in young adults was higher than in middle agers (p = 0.03).
The enable cluster represents a consortium of experts and institutions committed to create interdisciplinary collaborations focussing on food, nutrition, metabolism, energy balance and healthy aging. The cluster comprises academic research groups with expertise in the aforementioned fields together with consumer and social sciences, information and communication technologies, and food industry (http://www.enable-cluster.de).
As a unique feature and guiding principle, the enable cluster is addressing associations between nutrition behavior and quality and diet-related health issues in defined age groups, ranging from early childhood over young adulthood, middle age to older age, thereby covering life stages, in which individuals are exposed to different living conditions and environments. For this purpose, four defined age groups of healthy individuals were recruited and extensively phenotyped using highly standardized operating procedures. The major goal of the project is to compare determinants of food selection, eating behavior, and cardiometabolic disease risks across the four age groups in future analyses.
The current analysis contains a limited number of anthropometric, metabolic and dietary data across the four healthy age groups indicating how these parameters differ across age groups and sex. There is a clear trend toward an increase in percentage body fat mass and, in parallel, a decrease in percentage fat free mass in both sexes depending on the age group. The analysis of metabolic parameters showed age-dependent modest deteriorations particularly in glucose and lipid metabolism suggesting a slow decline in cardiometabolic health. It is unclear how dietary habits and patterns are accountable for these differences and if these alterations will require and benefit from modifications in dietary intake. Furthermore, hypotheses regarding the relationships between sensory qualities and eating behavior as well as metabolic health can be addressed. In the enable database potential links between gut microbiota, resting metabolic rate, and diet can be investigated in well-characterized participants and in different stages of life. In addition, within these age groups we are planning to link omics data (metabolomics, genetics and epigenetics) with clinical parameters, dietary patterns, or microbiota parameters to identify novel associations and to explore mechanistic links. More information on single research projects is available on the cluster web page (http://www.enable-cluster.de).
A strength of the project is that all assessments are based on defined SOPs and validated tools, e.g., questionnaires. A limitation may be that the groups were strictly defined to reduce heterogeneity. Due to the high selection bias, findings cannot be directly transferred to the general population. It is also important to note that the study is cross-sectional and does not allow conclusions on the development of certain parameters during lifetime, as the phenotypes in the specific age groups may have been influenced by former and no longer existing conditions. Irrespective of such limitations, the comparison of lifestyle and health characteristics across four age groups may provide valuable information for the development of age-specific recommendations for a health-promoting diet and lifestyle.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Faculty of Medicine of the Technical University of Munich in Germany. The patients/participants provided their written informed consent to participate in this study.
HH, DV, TS, JF, MK, and DH: conceived the experiments. HH, DV, TS, and BB: designed the phenotyping program. BB: performed study management and data management. BB, AH, and RR: were responsible for data collection and entry. JF: provided materials for sensory testing. TH, DK, and JF: were in charge of the chemosensory pheno-/genotyping to unravel food preference and aversion in the four age groups. BB, EK, DV, JR, SI, and JL: arranged the questionnaires. BB, TS, DV, and HH: wrote the manuscript. All authors critically read the manuscript and approved the final version.
This work was funded by a grant of the German Ministry for Education and Research (BMBF, grant no. 01EA1409A). No influence on the design, conduct, analyses, interpretation or publication of the trial exist. The preparation of this paper was supported by the enable Cluster and is cataloged by the enable Steering Committee as enable 41 (http://enable-cluster.de).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Margot Maier, Sandra Eckardt, Rebecca Cruiz-Aguilar, and Karin Lietzau for outstanding support with phenotyping the four age groups, data collection and monitoring, Irmgard Sperrer and Manuela Hubersberger for excellent technical assistance, Johanna Thalhuber and Dr. Kerstin Dressel for support in the recruitment of participants, and Dr. Alfred Zollner and Adrian Balteanu for excellent support in data management. We also thank Dr. Stephanie Brandt, Childrens' Hospital of the University of Ulm, for her valuable contribution calculating BMI-SDS values.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.582387/full#supplementary-material
eCRF: electronic Case Report Form; FFQ: Food Frequency Questionnaire; GCP: Good Clinical Practice; MetS: Metabolic Syndrome; MMSE: Mini-Mental State Examination; MONEX: Monell Extended Sniffin’ Sticks Identification Test; MSZ: Munich Study Centre; RMR: resting metabolic rate; SGB: social security statute book; SOP: standard operation procedure; SPPB: Short Physical Performance Battery; TSH, thyroid- stimulating hormone; VT: ventilatory threshold.
1. Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Glob Heart. (2016) 11:393–7. doi: 10.1016/j.gheart.2016.10.024
2. Köster EP. Diversity in the determinants of food choice: A psychological perspective. Food Qual Pref. (2009) 20:70–82. doi: 10.1016/j.foodqual.2007.11.002
3. Stok FM, Hoffmann S, Volkert D, Boeing H, Ensenauer R, Stelmach-Mardas M, et al. The DONE framework: Creation, evaluation, and updating of an interdisciplinary, dynamic framework 2.0 of determinants of nutrition and eating. PLoS ONE. (2017) 12:e0171077. doi: 10.1371/journal.pone.0171077
4. Scaglioni S, Cosmi V, de Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children's eating behaviours. Nutrients. (2018) 10:706. doi: 10.3390/nu10060706
5. Campbell KJ, Crawford DA, Salmon J, Carver A, Garnett SP, Baur LA. Associations between the home food environment and obesity-promoting eating behaviors in adolescence. Obesity. (2007) 15:719–30. doi: 10.1038/oby.2007.553
6. Das JK, Salam RA, Thornburg KL, Prentice AM, Campisi S, Lassi ZS, et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann N Y Acad Sci. (2017) 1393:21–33. doi: 10.1111/nyas.13330
7. Inzitari M, Doets E, Bartali B, Benetou V, Di Bari M, Visser M, et al. Nutrition in the age-related disablement process. J Nutr Health Aging. (2011) 15:599–604. doi: 10.1007/s12603-011-0053-1
8. Güngör NK. Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol. (2014) 6:129–43. doi: 10.4274/jcrpe.1471
9. World Health Organization. World Report on Ageing and Health. (2015). Available online at: https://www.who.int/ageing/events/world-report-2015-launch/en/ (accessed October 11, 2020).
10. Nicklaus S. The role of food experiences during early childhood in food pleasure learning. Appetite. (2016) 104:3–9. doi: 10.1016/j.appet.2015.08.022
11. Anzman-Frasca S, Ventura AK, Ehrenberg S, Myers KP. Promoting healthy food preferences from the start: a narrative review of food preference learning from the prenatal period through early childhood. Obes Rev. (2018) 19:576–604. doi: 10.1111/obr.12658
12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
13. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.M85
14. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. (2000) 55:M221–31. doi: 10.1093/gerona/55.4.M221
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Activ. (1999) 7:129–61. doi: 10.1123/japa.7.2.129
16. Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Activ. (1999) 7:162–81. doi: 10.1123/japa.7.2.162
17. Freiherr J, Gordon AR, Alden EC, Ponting AL, Hernandez MF, Boesveldt S, et al. The 40-item Monell extended Sniffin' sticks identification test (MONEX-40). J Neurosci Methods. (2012) 205:10–6. doi: 10.1016/j.jneumeth.2011.12.004
18. Nöthlings U, Hoffmann K, Bergmann MM, Boeing H. Fitting portion sizes in a self-administered food frequency questionnaire. J Nutr. (2007) 137:2781–6. doi: 10.1093/jn/137.12.2781
19. Freese J, Feller S, Harttig U, Kleiser C, Linseisen J, Fischer B, et al. Development and evaluation of a short 24-h food list as part of a blended dietary assessment strategy in large-scale cohort studies. Eur J Clin Nutr. (2014) 68:324–9. doi: 10.1038/ejcn.2013.274
20. Ravens-Sieberer U, Gosch A, Abel T, Auquier P, Bellach BM, Bruil J, et al. Quality of life in children and adolescents: a European public health perspective. Soz Praventivmed. (2001) 46:294–302. doi: 10.1007/BF01321080
21. Wilson M-MG, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. (2005) 82:1074–81. doi: 10.1093/ajcn/82.5.1074
22. Dohrenwend BS, Askenasy AR, Krasnoff L, Dohrenwend BP. Exemplification of a method for scaling life events: the PERI life events scale. J Health Soc Behav. (1978) 19:205. doi: 10.2307/2136536
23. Renner B, Sproesser G, Strohbach S, Schupp HT. Why we eat what we eat. The Eating Motivation Survey (TEMS). Appetite. (2012) 59:117–28. doi: 10.1016/j.appet.2012.04.004
24. McWhinnie JR. Disability assessment in population surveys: results of the O.E.C.D. Common Development Effort. Rev Epidemiol Sante Publique. (1981) 29:413–9.
25. Robine J-M, Jagger C. Creating a coherent set of indicators to monitor health across Europe: the Euro-REVES 2 project. Eur J Public Health. (2003) 13:6–14. doi: 10.1093/eurpub/13.suppl_1.6
26. Cox B, van Oyen H, Cambois E, Jagger C, Le Roy S, Robine J-M, et al. The reliability of the Minimum European Health Module. Int J Public Health. (2009) 54:55–60. doi: 10.1007/s00038-009-7104-y
27. Denkinger MD, Weyerhäuser K, Nikolaus T, Coll-Planas L. Reliabilität der deutschen Kurz-Version des “Late Life Function and Disability Instrument”. Ein sinnvoller und praktikabler Fragebogen zur Bestimmung der körperlichen Funktion und Beeinträchtigung älterer. Personen Z Gerontol Geriatr. (2009) 42:28–38. doi: 10.1007/s00391-008-0550-y
28. Huisman M, Poppelaars J, van der Horst M, Beekman ATF, Brug J, van Tilburg TG, et al. Cohort profile: the longitudinal aging study Amsterdam. Int J Epidemiol. (2011) 40:868–76. doi: 10.1093/ije/dyq219
29. Robert Koch Institut, Department of Epidemiology and Health Monitoring. German Health Interview and Examination Survey for Adults (DEGS1). Public0020Use File 1 (2015). doi: 10.7797/16-200812-1-1-1
30. TNS Inf ratest Sozialforschung. SOEP 2015 – Erhebungsinstrumente 2015 (Welle 32) des Sozioökonomischen Panels: Personenfragebogen, Altstichproben. SOEP Survey Papers 274: Series A. Berlin: DIW/SOEP (2015).
31. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. (1976) 4:97–110.
32. Allebrandt KV, Roenneberg T. The search for circadian clock components in humans: new perspectives for association studies. Braz J Med Biol Res. (2008) 41:716–21. doi: 10.1590/S0100-879X2008000800013
Keywords: metabolic phenotyping, nutrition, enable-cluster, cohort, biosamples
Citation: Brandl B, Skurk T, Rennekamp R, Hannink A, Kiesswetter E, Freiherr J, Ihsen S, Roosen J, Klingenspor M, Haller D, Krautwurst D, Hofmann T, Linseisen J, Volkert D and Hauner H (2020) A Phenotyping Platform to Characterize Healthy Individuals Across Four Stages of Life - The Enable Study. Front. Nutr. 7:582387. doi: 10.3389/fnut.2020.582387
Received: 23 July 2020; Accepted: 17 September 2020;
Published: 28 October 2020.
Edited by:
Faidon Magkos, University of Copenhagen, DenmarkReviewed by:
Sergio Polakof, Institut National de la Recherche Agronomique (INRA), FranceCopyright © 2020 Brandl, Skurk, Rennekamp, Hannink, Kiesswetter, Freiherr, Ihsen, Roosen, Klingenspor, Haller, Krautwurst, Hofmann, Linseisen, Volkert and Hauner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans Hauner, aGFucy5oYXVuZXJAdHVtLmRl
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.