
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 16 November 2020
Sec. Nutrition and Sustainable Diets
Volume 7 - 2020 | https://doi.org/10.3389/fnut.2020.576532
This article is part of the Research Topic Food and Nutrition Security: Underutilized Plant and Animal-Based Foods View all 20 articles
Underutilized or traditional leafy vegetables are grown in the wild and cultivated. They are consumed as nutritional accompaniments to staples, either raw (fresh), cooked, or in a dried form, through custom, habit, and tradition. These traditional leafy vegetables are natural rich sources of phytochemicals and nutritional compounds. Over time, the keenness for consumption of traditional vegetables has become less popular. Poor nutrient diets are the main cause of mortality and morbidity, especially in developing countries, where the problem is predominant due to poverty. Consumption of traditional vegetables can assist in the prevention of chronic disease development, as they contain various bioactive compounds that exhibit multiple health benefits. Traditional leafy vegetables play a vital role in combatting hunger, food insecurity, and malnutrition, and most are suitable for food intervention programs. African nightshade (Solanum family) is one such commonly consumed traditional leafy vegetable. During dry seasons, communities often face shortages of vegetables; thus, the preservation of edible leaves is one strategy to help overcome this problem. The adoption of solar drying and fermentation are traditional methods to extend the availability of African nightshade vegetables. Additionally, the agronomy practices and postharvest processing methods affect the phytochemicals and nutritional compounds of African nightshade accessions. This mini-review provides information on changes in phytochemicals, nutrition, and antinutritive compounds with different postharvest processing methods and irrigation. The review provides the justification to promote the cultivation for consumption, by identifying the potential African nightshade accessions that are rich in phytonutritional compounds. This mini-review summarizes and discusses the major information on (i) the micro- and macronutrients present in Solanum retroflexum, the most commonly consumed nightshade species compared with other traditional vegetables in Southern Africa, (ii) the composition of phytochemical compounds present in different nightshade accessions, (iii) the impact of irrigation on phytochemical composition in different nightshade species, and (iv) the impact of postharvest processing on phytochemicals and antinutritive compounds in S. retroflexum. Inclusion of African nightshade, especially S. retroflexum, with the main staple foods can improve protein, iron, and calcium levels in daily diets, which will help to improve people's health and well-being.
Consumer preference for the intake of fruit and vegetables in the daily diet is increasing, and the World Health Organization (1, 2) recommends a minimum of 400 g of fruit and vegetables, or five portions, per day, excluding starchy tubers. The United States Department of Agriculture (USDA) guidelines (2011) (3) state that an individual must consume at least one cup (~237 g) of raw or cooked vegetables or two cups of raw leafy greens daily. In developing countries, particularly Africa and Asia, consumers need to meet the minimal requirement of caloric values, because micronutrient deficiency, referred to as hidden hunger, is prevalent (4). Micronutrient deficiency is due to a lack of dietary intake of calcium (Ca), iron (Fe), zinc (Zn), potassium (K), magnesium (Mg), iodine (I), copper (Cu), and selenium (Se). Additionally, vitamin A deficiency remains a common health-associated problem in South Asia and sub-Saharan Africa (5). Women of reproductive age (≥15–49 years) in Ethiopia, Kenya, Nigeria, and South Africa suffer from anemia (18–51%), iron deficiency (9–18%), and iron deficiency anemia (10%), as well as vitamin A (4–22%), iodine (22–55%), zinc (34%), and folate (46%) deficiency (6). Consequently, it is worth including underutilized African leafy vegetables in a diet diversification strategy for the sub-Saharan African population to fight against hidden hunger (7). The most commonly grown traditional African leafy vegetables in the sub-Saharan African region are of the Amaranthus species: wild mustard (Brassica spp.), African nightshade (Solanum spp.), sweet potatoes (Ipomoea batatas), spider flower (Cleome gynandra), Jew's mallow (Corchorus olitorius and Corchorus tridens), cowpeas (Vigna unguiculata), pumpkins (Cucurbita pepo, Cucurbita maxima, and Cucurbita moschata), melons (Citrullus lanatus and Cucumis melo), and balsam pear (Momordica balsamina). Of these, wild mustard and African nightshade are widely consumed (7). Once cooked, the leaves are an accompaniment to the staple starch-based maize meal and tomato relish; sometimes the leaves are fermented with milk. These food preparation methods help reduce the bitter-tasting compounds in the leaves derived from anti-nutrients, such as solanaceous glycoalkaloids (8).
Nightshade plants, propagated via the seeds (9), are an annual plant growing to almost 75 cm in height (9), with a simple leaf morphology—alternate margins with blunt teeth and slightly hairy. Solanum retroflexum (Figure 1), which is endemic to South Africa, belongs to the Solanaceae family and known as Black nightshade, or nastergal, umsobo, and muxe in the region; it is a popular leafy vegetable consumed in the Southern and Eastern parts of Africa. Other nightshade vegetables consumed in the sub-Saharan region are S. retroflexum, Solanum americanum, Solanum nigrum, Solanum scabrum, and Solanum villosum (8, 10); S. scabrum Mill (mnavu), a broad-leafed type of nightshade, is popular in West, Central, and East Africa (11).
In Southern Africa, subsistence farmers cultivate nightshade vegetables on a small scale and market them to generate income and improve their livelihoods. African nightshade does not need extensive fertilizer application, thrives in drought, and is less prone to pest attack; therefore, it is cost effective to produce and environmental-friendly when compared with commercial leafy vegetables (12). Currently, there are efforts to increase production, and linking these vegetables to agro-processing and the supply chain will alleviate hunger and improve nutrition and the rural economy. Unfortunately, postharvest losses of vegetables are high during marketing, primarily due to lack of cost-effective cold chain infrastructure; therefore, the traditional ways to preserve and reduce food loss are by adopting drying or fermentation technologies. Traditionally, drying was by the sun or shade drying; however, in Asian and African countries, community cooperatives have recently had cost-effective solar dryers erected. The two agro-processing technologies, solar drying, and fermentation, play a major role in facilitating the food available and sustaining food security in rural regions. These technologies are considered as recommended strategies to improve the bioavailability of micronutrients, and sometimes they can reduce the antinutritive compounds (13, 14). Considering the aforementioned, this review summarizes the research-based information on phytochemical nutritional properties of African nightshade species and the changes in phytonutritional components during irrigation or postharvest processing. In addition, this review discusses the antinutritive components in S. retroflexum species and changes of these compounds during postharvest processing and safety for consumption.
Figure 2 shows the iron (Fe) content in African nightshade species S. retroflexum (per 100 g fresh weight, FW) compared with other traditional vegetables in the Southern African region. The iron content in African nightshade was higher than that in other traditional vegetables in the Southern African region. A 100 g (FW) portion of African nightshade S. retroflexum contained 7.2 mg of iron, whereas other traditionally southern African vegetables, such as C. tridens (wild jute), Amaranthus cruentus (pigweed), C. olitorius (Jew's mallow), V. unguiculata (cowpea), C. lanatus (tsamma melon leaves), C. gynandra (spider flower), Amaranthus hybridus (cockscomb), and Bidens pilosa (black jack), contained 6.3, 5.1, 3.6, 4.7, 6.4, 2.1, 4.1, and 2.0 mg, respectively, in 100 g (FW) portion (7, 15).
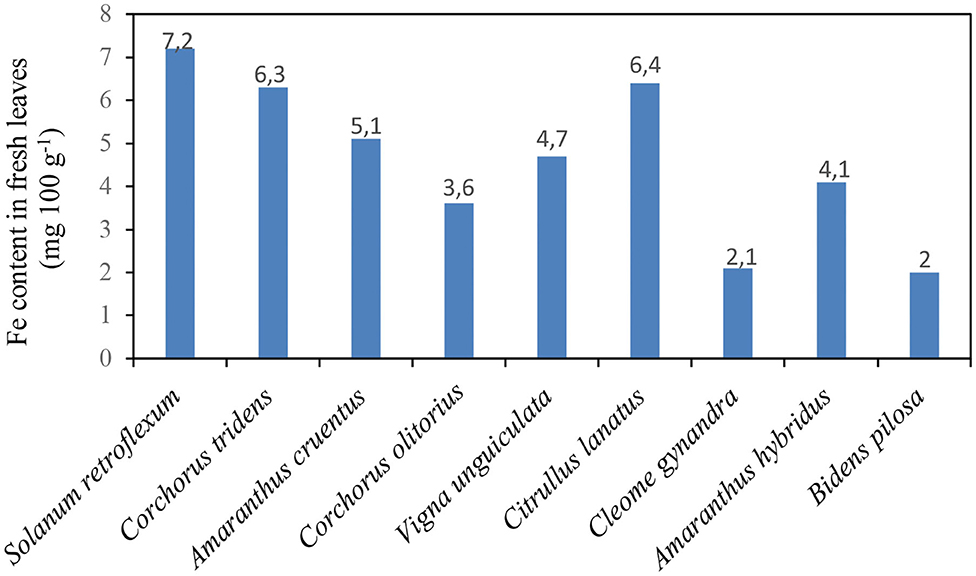
Figure 2. Iron content in Solanum retroflexum leaves compared with the other traditional leafy vegetables Source (15).
Iron is regarded as an essential trace element for many bodily functions, such as biosynthesis of hemoglobin and activity of the central nervous system, and it also participates in the oxidation of macronutrients (e.g., carbohydrates, proteins, and fats) (16). The recommended daily intake (RDI) of iron is 8 mg day−1 for adults (17). Leaves of S. villosum were reported to contain 12 mg 100 g−1 iron on dry weight (DW) basis (15, 18). S. villosum contained higher iron levels than C. gynandra (spider plant) (48.6 mg 100 g−1) and A. cruentus (Madiira AM 38) (52.66 mg 100 g−1) at level-three maturity stages (7, 15, 18). This information suggests the best harvesting time for optimal Fe levels to the consumer.
The calcium content in a 100 DW portion of S. villosum is 442 mg, which was higher than the amount reported in S. retroflexum (199 mg), C. maxima (pumpkin leaves) (177 mg), and B. pilosa (black jack) (162 mg). Calcium is necessary for the maintenance of strong bones and teeth; 99% of calcium in the human body is used for this function (19). The RDI of calcium is 1,200 mg for adults (20).
The magnesium content in a 100 DW portion of S. retroflexum (92 mg) was reportedly higher than that of wild jute (80.9 mg), Jew's mallow (87 mg), cowpea (62 mg), pumpkin leaves (67 mg), tsamma melon leaves (59 mg), spider flower (76 mg), and black jack (79 mg) (7, 15, 21). Manganese content in S. retroflexum (2,080 μg 100 g−1) was higher than that found in spider flower (580 μg 100 g−1), tsamma melon leaves (760 μg 100 g−1), pumpkin leaves (540 μg 100 g−1), Jew's mallow (790 μg 100 g−1), cockscomb (4.1 μg 100 g−1), and black jack (2.5 μg 100 g−1) (21). Magnesium functions as a cofactor for more than 300 enzymatic reactions, and the RDI of magnesium is 420 mg for adult males and 320 mg for adult females (22).
The copper content in S. retroflexum (0.16 mg 100 g−1) was higher than that found in cowpea (0.14 mg 100 g−1). Copper is an essential mineral needed for growth and multiple functions, such as cardiovascular integrity, lung elasticity, neovascularization, neuroendocrine activity, and iron metabolism (23). The WHO guidelines advise 30 μg kg−1 of body weight per day−1, which is about 2 mg per day−1 for the average adult (23).
When compared with the commonly consumed exotic leafy vegetables, spinach, lettuce, kale, mustard green, rapini (broccoli raab), and Swiss chard greens (3), S. retroflexum (15) revealed higher levels of calcium, magnesium, iron, and copper (Table 1).
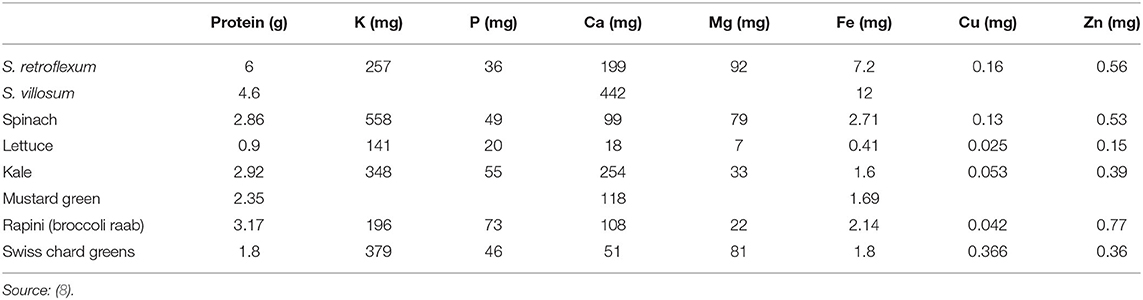
Table 1. Minerals and protein in African nightshade (per 100 g fresh weight) compared with other commercial vegetables.
African nightshade S. retroflexum contained higher amounts of proteins (6%) than other African traditional vegetables (Table 1), such as C. maxima (pumpkin leaves) (4.24%), A. cruentus (pigweed) (3.49%), C. olitorius (Jew's mallow) (5.19%), V. unguiculata (cowpea) (4.7%), C. maxima (pumpkin leaves) (2.9%), C. lanatus (tsamma melon leaves) (3.5%), C. gynandra (spider flower) (5%), B. pilosa (black jack) (6%), and A. hybridus (cockscomb) (5%) (7, 15). Conversely, S. villosum showed lower levels of proteins (4.6%) than S. retroflexum, but similar levels to pumpkin and cowpea leaves (7, 15, 18).
In addition, a 100 g portion of S. retroflexum and S. villosum leaves had a sugar content of 1.02 and 1.15 g, respectively. For fat content, S. retroflexum contained monounsaturated fatty acids and omega 3 and 6 fatty acids at concentrations of 2.61, 0.33, and 0.63 g in a 100 g portion, respectively (24). Most importantly, due to its higher levels of proteins and iron, African nightshade is an important nutritional source for African people (11).
Phytochemicals, plant-based non-nutritive compounds that contribute toward biological activity, aid in protecting the body against non-communicable diseases (25). An ~120 g portion of fruits or vegetables provides 100 different phytochemicals (26), among which phenolic compounds are the most abundant functional compounds. Aerial parts, especially the leaves, of different nightshade plants, S. nigrum, S. scabrum, S. americanum, S. villosum, and S. retroflexum, predominantly contain chlorogenic acid (caffeoylquinic acid), which belongs to the group of hydroxycinnamic acids (8) and is an ester of caffeic and quinic acids. Chlorogenic acid is a pronounced phenolic compound in Amaranthus leaves, including the red (Amaranthus tricolor) and green (Amaranthus lividus) genotypes (27), and a major phenolic compound in lettuce. Caffeoylmalate was detected in S. scabrum and S. retroflexum (27, 28). Kaempferol glycoside derivatives in S. scabrum leaves were mainly kaempferol-3-diglucoside, kaempferol-3-glucosylrhamnogalactoside, and kaempferol-3-rhamnosyl-rhamnogalactoside (isomers) (28) (Table 2). It was found that S. retroflexum leaves contained the following kaempferol derivatives: kaempferol-3-O-sinapoyldihexoside-hexoside, kaempferol-3-O-rutinoside, and kaempferol-dihexoside (28) (Table 2). The concentration of kaempferol derivatives in fresh S. retroflexum was at lower concentrations than that in S. scabrum (Table 2). Isorhamnetin-O-hexoside and rutin were found in S. retroflexum leaves (28). S. scabrum leaves contained mainly non-acylated quercetin glycosides, such as quercetin-3-neohesperidoside-7-glucosylrhamnoside (isomers), quercetin-3-rutinoside-7-rhamnosylglucoside, quercetin-3-galactorhamnoside, quercetin-3-rhamnosylgalactoside, quercetin-3-pentosylglucoside, and quercetin-3-pentosylrutinoside (28); conversely, quercetin-3-O-xylosyl-rutinoside was only detected in S. retroflexum leaves (28). Among the 11 phenolic compounds found in S. retroflexum leaves, rutin was the predominant compound.
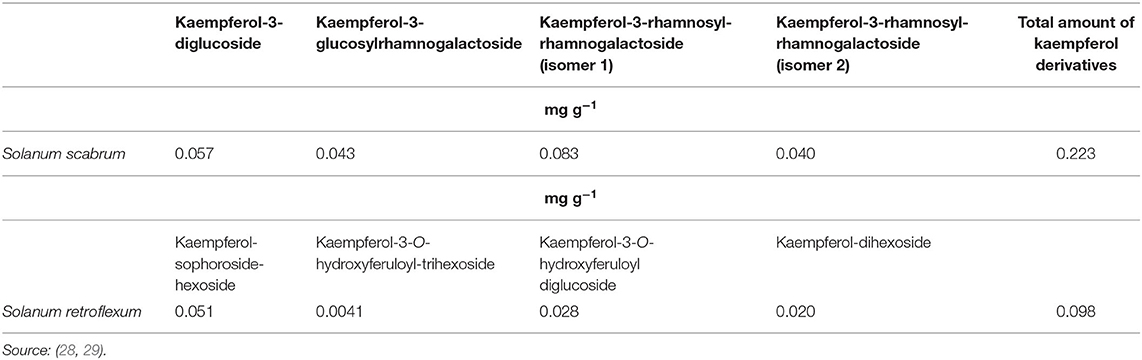
Table 2. Concentration of different kaempferol derivatives present in Solanum scabrum and Solanum retroflexum species.
Vitamin E content in S. nigrum and S. scabrum varied from 92.0 to 229.7 μg g−1 and from 90.4 to 192.5 μg g−1 on DW basis, respectively (8). S. nigrum (PI 312110, USDA) contained the highest amount of vitamin E of all African nightshade species (8), and S. scabrum (SS 04.2, World Vegetable Center [WAC] East and Southern Africa, Arusha, Tanzania) contained the lowest (8). In S. americanum (PI 268152, USDA) and S. villosum (Grif 16939, USDA), the vitamin E content was 145.5 and 114.3 μg g−1, respectively (8).
Total carotenoid content in S. scabrum leaves ranged from 586 to 691 μg g−1 on DW basis and 0.733 μg g−1 in S. retroflexum leaves on FW basis (8, 30, 31). The β-carotene content in the leaves of S. nigrum and S. scabrum species differed from 28.1 to 141.7 μg g−1 DW and from 55.1 to 96.0 μg g−1 DW, respectively (8). S. villosum (Grif 16939, USDA) reportedly contained the highest total carotenoids of 138.1 μg g−1 DW, whereas the lowest amount of 65.2 μg g−1 DW was found in S. scabrum (SS 04.2, WAC) (8).
African traditional leafy vegetables are rich in vitamin A and meet more than 75% of the recommended dietary allowance (RDA) (15). The vitamin A content in African nightshade (422 μg retinol activity equivalent, RAE) is greater than that in Jew's mallow (329 μg RAE), pumpkin leaves (325 μg RAE), and tsamma melon leaves (375 μg RAE) (15, 32).
Based on Yuan's et al. (8) findings, vitamin E, and total phenols contributed toward the antioxidant property [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), ABTS] of the accessions of African nightshade S. nigrum PI 312110 and PI 381290, obtained from the USDA collection. S. scabrum (BG 16, Nduruma, WAC) showed the highest ABTS activity of 25 Trolox equivalent antioxidant capacity (TEAC) mg g−1 DW (8). Different African nightshade species from the USDA collection showed ABTS activity, on DW basis, in the following order: S. scabrum (25.00 TEAC mg g−1; BG 16, Nduruma), >S. americanum (24.81 TEAC mg g−1; PI 268152, USDA), >S. nigrum (23.93 TEAC mg g−1; PI 381290, USDA), ≥S. nigrum (23.45 TEAC mg g−1; PI 312110, USDA), >S. scabrum (22.46 TEAC mg g−1; SS 49 Olevolosi, WVC), S. scabrum (21.36 TEAC mg g−1; SS 52, WVC), ≥S. scabrum (21.26 TEAC mg g−1; Grif 14198, USDA), >S. scabrum (19.14 TEAC mg g−1; BG-29, WVC), >S. nigrum (18.34 TEAC mg g−1; PI 381289, USDA), >S. scabrum (17.92 TEAC mg g−1; Ex Hai, WVC), >S. scabrum (16.22 TEAC mg g−1; PI 643126, USDA), and >S. nigrum (15.49 TEAC mg g−1; PI 306400, USDA) (8).
Phenolic compounds positively correlated with antioxidant activity (33). Phenolic compounds participate in the antioxidant activity due to their redox properties, predominantly adsorbing, and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (33). Current consumer trend is to replace synthetic antioxidants with natural dietary antioxidants for health benefits. Vitamin E (tocopherols) is important for disease prevention by preventing the breakdown of polyunsaturated fatty acids in membrane lipids and alleviating the oxidative stress (34). Lipophilic antioxidant ABTS activity demonstrated a strong correlation with tocopherols (35). However, correlations need to be established between the antioxidant activity and total phenolic content in different accessions of African nightshade.
Minerals, such as Ca, Mg, K, and Mn, increased during severe stress in irrigation treatment at 30% field capacity, whereas P, Fe, and Zn content was the highest at 90% field capacity (28). Many developing countries adopt intercropping systems for effective use of the land, improving the productivity. The intercropping approach is popular among smallholder farmers (36). It can modify the nutritional composition in the plant parts, including the leaves; however, it did not significantly favor the accumulation of minerals in leaves in S. scabrum (28). The hydroxycinnamic acid derivatives, 3-caffeoylquinic acid, 5-caffeoylquinic acid, 4-caffeoylquinic acid, and caffeoylmalate, were significantly affected by the irrigation levels but not by the intercropping with Brassica carinata leaves (28); likewise, sinapoylmalate and sinapic acid were affected by irrigation. The irrigation treatments significantly affected the concentration of kaempferol-3-diglucoside in leaves (28). Ngwene et al. (28) confirmed that agronomy practices, such as intercropping, late-season drought, or irrigation management, can be adopted as a strategy to boost the levels of some health-related phytochemicals to benefit rural people and food manufacturers. The concentration of chlorogenic acid in S. retroflexum leaves (1.04 μg g−1) was less than that in pak choi (2.03 μg/g), salad spinach (1.59 μg g−1), red amaranth (9.06 μg g−1), and green amaranth (15.34 μg g−1) (37). Chlorogenic acid was mostly detected in S. nigrum, S. scabrum, and S. villosum; however, in some cases, chlorogenic acid was either detected in trace amounts or not detected, depending on the location (8). Quercetin-glucosyl-rhamnosyl-galactoside was detected at higher intensities in S. nigrum, S. scabrum, and S. americanum, but again not in all locations (8).
The other important phytochemicals in leafy vegetables are carotenoids. Low irrigation favored an increase in carotenoid accumulation in intercropped S. scabrum. The β-carotene and lutein contents in S. scabrum leaves increased when the irrigation treatment simulated drought treatment (30% water holding capacity, WHC) (28). The temperature variation during cold winter months and hot summer months did not show significant variation in total carotenoid content in S. retroflexum leaves (27).
Postharvest processing (e.g., drying) and food preparation methods (e.g., cooking or fermentation) have a significant influence on the maintenance of phytochemical content of vegetables (38). Methods of postharvest processing can modify the composition of functional compounds in green leafy vegetables (29). Postharvest drying increased the income generation from traditional vegetable functional food. Global functional foods' market size is rising and expected to increase in 2025 to US$ 275.77 due to the increasing consumer appeal for nutritional and fortifying food additives (39). Traditionally adopted drying methods do not meet the requirement of homogenous quality standards; therefore, the installation of cost-effective solar dryers enables subsistence farmers to perform the postharvest drying at 50°C (30); this is a controlled drying method to prevent the depletion of vitamins and functional compounds.
Solar drying of S. retroflexum leaves at 50°C increased the total carotenoid content by 40%, compared with the raw leaves. Similarly, phenolic metabolites, caffeoylmalic acid, rutin, and kaempferol-3-O-rutinoside revealed a remarkable increase in solar dried leaves. Chlorogenic and neochlorogenic acids substantially increased in solar dried S. retroflexum leaves (Figure 3), and antioxidant activity (FRAP) was enhanced compared with raw leaves (30). However, the temperature during the drying process plays a vital role in the chemical transformation of biochemical metabolites. Higher temperatures accelerate oxidation reactions, which can negatively affect the concentration of phenolic compounds (40). Loss of total polyphenols due to hot air drying have been reported previously (20).
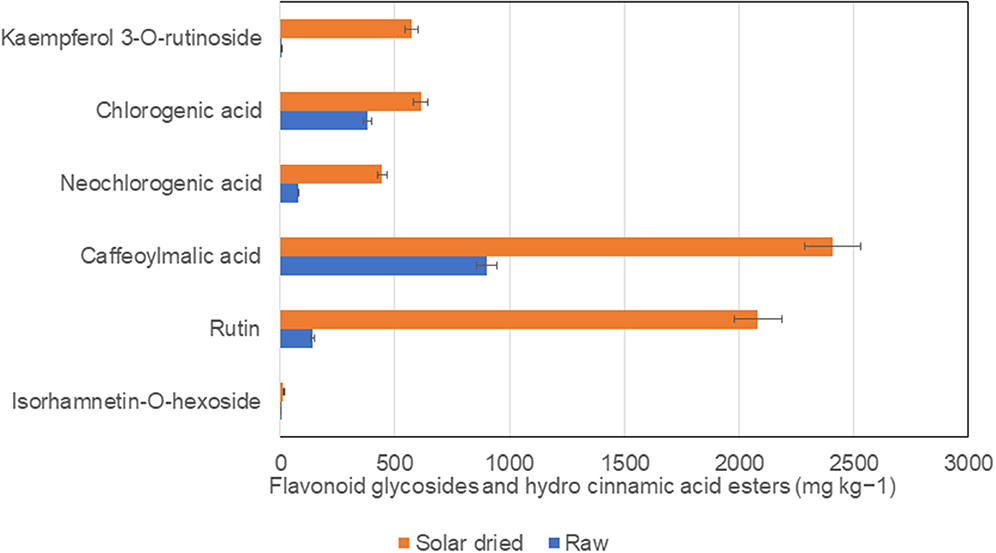
Figure 3. Comparison of phenolic components in solar dried and raw S. retroflexum leaves from Venda, Limpopo, South Africa. Source (24).
The solar dried functional powder of S. retroflexum leaves contains 17.50 g carbohydrate in a 100 g portion; a lower carbohydrate content correlated with a lower calorific content 1,118.67 kJ 100 g−1 (267.36 cal) (Table 3) (30), the protein content is 32.91 g 100 g−1, slightly higher than the solar dried cowpea leafy vegetable (V. unguiculata L.) (29.40 g 100 g−1) and moringa leaves (Moringa oleifera) (28.09–28.99 g 100 g−1) (30, 41), and total dietary fiber is 28.81 g 100 g−1 (30). The solar dried S. retroflexum leaf powder is low in sodium, but the 100 g portion meets the daily requirement of potassium, calcium, and zinc intake; the iron and magnesium contents were ~8- and 2-fold higher than the required amount per day, respectively (Table 3) (30).
In developing a low calorie, meal replacement product, solar dried functional powder of S. retroflexum leaves were utilized as the main ingredient. The final product contained 32.8 g of protein, 12.9 g of dietary fiber, 40 g of total sugar, 40.8 g of carbohydrate, 5.1 g of fat, and 1.4 g monounsaturated fatty acids (Table 4) with 369 cal in a 100 g portion (24). The protein content of African nightshade protein shake meets approximately half the daily requirement, but the available carbohydrate levels are much higher than those prescribed for daily intake (24). Low sodium content intake is preferred by those who suffer from high blood pressure and kidney problems; however, sodium is an important intracellular and extracellular cation that facilitates the regulation of plasma volume and acid–base balance during nerve and muscle contraction (42).
Phenylalanine is an essential amino acid, which acts as a precursor of the amino acid tyrosine. Phenylalanine content in African nightshade powder was 308.7 mg g−1 (Table 4). It is generally recognized as “safe” by the Food and Drug Administration FDA (43). There are unlikely side effects reported at supplement doses of 50–100 mg per kg of body weight (44); therefore, it is possible for patients with amino acid metabolism disorder (phenylketonuria) to avoid the intake of high phenylalanine-containing meals (45). The African nightshade protein shake powder also contains aspartic acid (53.3 mg g−1), a non-essential amino acid used in building proteins; other plant sources of aspartic acid are avocado and asparagus. A serving size of 0.83 g of avocado provides 220 mg of aspartic acid, whereas a comparative portion of the protein shake powder contains 44 mg. Similarly, asparagus contains 5.08 mg g−1 of aspartic acid, which is much lower than the amount present in African nightshade powder (53.3 mg g−1; Table 5) (3). Another important non-essential amino acid in African nightshade powder is glycine, a powerful antioxidant with many health benefits.
Processing African nightshade leaves into powder is a preliminary step in the formulation of instant soups or meals. Evaluations indicated that this product will provide an instant, easy to handle and prepare meal, high in nutritional and sensory quality (46, 47).
Fermentation is a traditional food processing technique, adopted as a preservation technique in Africa, performed with the objective of increasing food safety and make food more edible and appealing in terms of sensory properties, by improving flavor and aromas (32, 48). A frequently reported main effect of lactic fermentation is the improvement of the bioavailability of nutritional components. A popular fermented food on the African continent is Kawal, especially in Sudan, produced by spontaneous fermentation of leguminous leaves (49–51). Bacillus spp. and Lactobacillus plantarum are involved in fermentation, but most antinutritional factors, especially phytic acid content, decreased during the process. Fermentation of African nightshade (S. scabrum) and cowpea leaves, using L. plantarum and Leuconostoc mesenteroides ssp. mesenteroides, for 48 h reduced the pH and inhibited the growth of foodborne pathogens, such as Listeria monocytogenes and Salmonella enterica Enteritidis. Likewise, fermentation of African kale leaves (B. carinata) with L. plantarum BFE 5092 and Lactobacillus fermentum BFE 6620 starter strains inhibited the growth of L. monocytogenes, S. Enteritidis, and other enterobacteria, while maintaining appreciably the concentration of vitamin C (35 mg 100 g−1) in the fermented product (32). Oguntoyinbo et al. (32) demonstrated that controlled fermentation is a promising method to reduce food spoilage and extend shelf life and food safety.
Fermentation of African nightshade S. scabrum, using 3% salt–sugar solution with L. plantarum BFE 5092 and L. fermentum BFE 6620 as starter cultures, had a greater impact on the microbial profile of the fermented product due to the rapid and stable decline of pH and production of lactic acid (52). The fermented product retained substantial levels of vitamins B1, B2, and C, which are sufficient to supplement the RDI and improve the sensory attributes, color, taste, and aroma (52).
L. plantarum 75 enhanced the functional potential of nightshade leaves and improved the bioavailability of phenolic compounds including phenolic acids (gallic, vanillic, 2,5-dihydroxybenzoic, coumaric, ferulic, and ellagic acids) and flavonoids (catechin, quercetin, and luteolin) in the fermented product of S. retroflexum (Figure 4) (53). However, caffeic and ferulic acids were not detected in African nightshade (S. scabrum) after fermenting with L. plantarum 75, possibly due to the different types of microbial metabolism and transformation of the phenolic compounds, which are predominantly determined by the different enzyme systems involved, irrespective of the species (53). Simultaneously, ferulic acid could have been reduced to dihydroferulic acid, whereas caffeic acid possibly metabolized to vinylcatechol, ethylcatechol, or dihydrocaffeic acid (54). Degrain et al. (53) concluded that L. plantarum 75 fermentation improved the extraction of phytochemical components in nightshade leaves and reduced carbohydrate content and calculated energy of the final product (53).
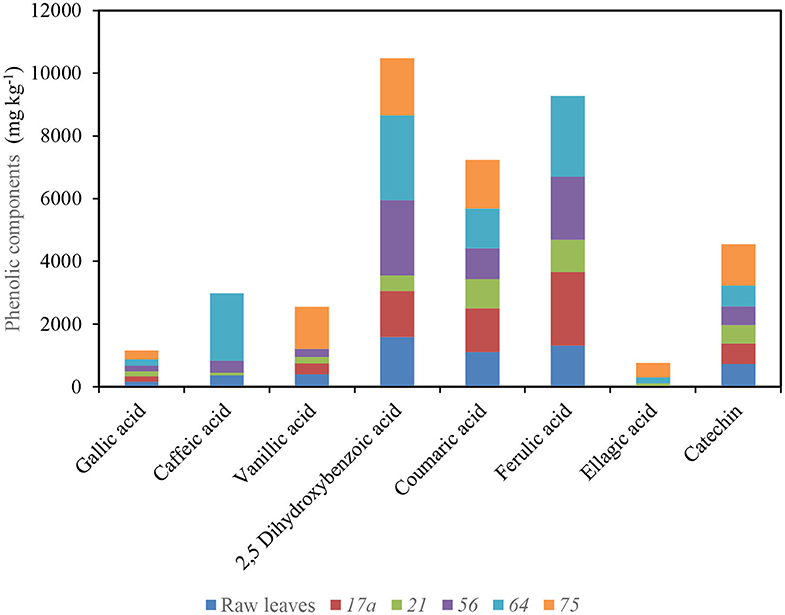
Figure 4. Impact of fermentation with different lactic acid bacterium strains on different phenolic components in African nightshade (S. retroflexum) leaves (75 L. plantarum, 21 and 64 Weissella cibaria, and 56 Leuconostoc pseudomesenteroides). Source (53).
As with solar drying, fermentation of S. retroflexum with L. plantarum 75 improved the antioxidant activity compared with raw leaves (53); flavonoids containing multiple hydroxyl groups mostly have higher antioxidant activities. Many studies have underlined the beneficial health effect of antioxidant-rich foods, such as reducing the risk of non-communicable diseases and premature aging (55). Similarly, S. scabrum leaves fermented with L. plantarum BFE 5092 and BFE 6620 in 2.1 L of a 2.5% brine solution (containing 3% salt and 3% sugar) at 25°C for 144 h revealed an increase in the concentration of caffeoylquinic acid isomers, sinapoylmalate, kaempferol-3-diglucoside, quercetin-3-pentosylrutinoside, sinapic acid, quercetin-3-rutinoside, and caffeoylmalate. The total polyphenols increased in concentration compared with the raw leaves, whereas quercetin-3-glucosyl-rhamnogalactoside and quercetin-3-rhamnosyl-rhamnogalactoside slightly reduced in the fermented leaves compared with the raw leaves (38). This is possibly due to the action of glycosyl hydrolases generated from the fermentation activity of lactobacillus strains on the conversion of flavonoid glycosides to the corresponding aglycones, which shows the higher enhancement of antioxidant activity and the biological bioactivity and benefit to the consumers (56). Coumaric acid levels after fermentation also reduced in S. scabrum leaves (38), possibly due to the coumaric acid acting as an external acceptor of electrons to gain one extra mole of ATP (57).
Proximate analysis of fermented African nightshade (S. retroflexum) leaves with L. plantarum 75 showed 2.51 g carbohydrate content in a 100 g serving portion. Dietary fiber, protein, fat, and sugar contents in a 100 g serving portion of fermented African nightshade vegetable product were 2.52, 3.82, 0.23, and <0.50 g, respectively. However, sodium content (231 g) was higher in the fermented nightshade vegetable product than in the solar dried powder of the same product (53). Sodium content is of great concern, and the daily limit of 2.4 g per day is due to an increase in the prevalence of chronic diseases, such as high blood pressure, which positively correlate to high salt intake (54).
Traditionally, the consumption of African nightshade leaves is after cooking, and the adoption of different cooking methods, such as a boiling and steaming, is to improve palatability and sensory properties. Cooking can enhance the bioavailability of phenolic components and the antioxidant activity (58); however, it can have deleterious effects on the nutrient composition and functional compounds and their bioavailability in different vegetables (59).
Traditional food preparation methods, including blanching, are widely adopted to improve the palatability and reduce the bitterness of African nightshade leaves. Blanching treatments (steam or cook in hot water using plain water or lemon juice) at 95°C for 5 min increased the concentrations of hydroxycinnamic acid derivatives (chlorogenic, neochlorogenic, and cryptochlorogenic acids) and caffeoylmalic acid (60). However, steam blanching in either water or lemon juice at 95°C for 5 min significantly improved the concentration of caffeoylmalic acid. In addition, cooking improved the antioxidant capacity of vegetables (58). The increase of chlorogenic acid concentration in S. retroflexum leaves during blanching treatments could be due to the formation of different caffeoylquinic acid isomers or the hydrolysis of dicaffeoylquinic acid (59). A similar significant increase in caffeoylquinic acid was reported in fried artichokes compared with raw and other cooking methods (60). Transesterification of caffeoylquinic acid is dependent on the pH of the food matrix, as well as temperature and time.
S. scabrum leaves boiled in water demonstrated an increasing trend in the levels of 3-caffeoylquinic acid, 5-caffeoylquinic acid, and 4-caffeoylquinic acid compared with the raw leaves. Boiling also reduced the levels of caffeoylmalate compared with the raw leaves and remarkably reduced the levels of quercetin-3-glucosylrhamnogalactoside, coumaric acid, quercetin-3-rhamnogalactoside, quercetin-3-rhamnosyl-rhamnogalactoside isomers, kaempferol-3-rhamnosyl-rhamnogalactoside, quercetin-3-pentosylrutinoside, and quercetin-3-rutinoside; sinapoylmalate, sinapic acid, kaempferol-3-diglucoside, and kaempferol-3-rhamnosyl-rhamnogalactoside were not detected in the cooked S. scabrum leaves (38). Non-acylated kaempferol diglucosides in broccoli demonstrated higher loss after boiling and minor loss after steaming, but during higher temperature heat treatments, it was expected that kaempferol-3-diglucoside would degrade to its monoglucoside or kaempferol; additionally, 4-O-position had a higher stability against deglucosilation than 3-O-position (58).
Some accessions of African nightshade leaves contain glycoalkaloids, which can cause health concerns. Among the five accessions of African nightshade, S. nigrum reportedly contains solasodine glycosides, including solamargine and solasonine, as in other plants belonging to the family Solanaceae, such as potatoes, tomatoes, and eggplants. Yuan et al. (8) reported the absence of glycoalkaloids in methanol leaf extracts of S. scabrum and S. villosum.
Based on previous reports (8), safe consumption of eggplants was allowed at glycoalkaloid levels ranging from 6.25 to 20.5 mg 100 g−1 FW. Higher levels of glycoalkaloids were detected in commonly consumed African nightshade leaves, which were confirmed as safe for consumption (8).
Steroidal saponins, mass of tigogeninas, were detected in Solanum spp. Tigogenin-5G is detected in most of the African nightshades spp. Dehydrodiosgenin-G-G-Rha-Rha and diosgenin-G-G-Rha-Rha are detected only in S. nigrum from the USDA collection PI 312110. Tigogenin-G-G-Rha-Xyl-Xyl is detectable in all S. nigrum (Kenya) from the USDA collection PI 306400, PI 381289, and PI 381290; S. scabrum SS 5, Ex Ha, SS 49, Olevolosi SS 04.2, BG 16, Nduruma BG-29, Grif 14198, and PI 643126; S. americanum; and S. villosum. Tigogenin-G-G-G is detected mainly in S. nigrum obtained from Kenya, USDA collection PI 30640, and in S. villosum (8). Tigogenin-3G-Xyl-G, tigogenin-5G, and tigogenin-GG-Rha-Xyl-Xyl are detected in S. retroflexum (30). The raw leaves contained 0.45 mg kg−1 of tigogenin and 0.56 mg kg−1 of tigogenin-GG-Rham-Xyl-Xyl, and solar drying increased the levels of tigogenin and tigogenin-GG-Rham-Xyl-Xyl to 70.54 mg kg−1 and 73.92 mg kg−1, respectively (30). Similarly, steam blanching, in water or lemon juice at 95°C for 5 min, increased the peak responses of the tigogenin-5-G, tigogenin-3G-Xyl-G, and tigogenin-GG-Rha-Xyl-Xyl, but the effect was greater in tigogenin-5-G (30). Tigogenin is an important raw material for pharmaceutical use and the synthesis of steroid drugs, demonstrating anti-inflammatory, and anti-diabetic activities (type 2 diabetics) (61).
The raw leaves of S. retroflexum contain other antinutritive compounds, such as tannins (55.4 mg 100 g−1), phytates (88 mg 100 g−1), and oxalates (87.5 mg 100 g−1). Hot water blanching and steam blanching treatments during food preparation help to significantly reduce the levels of these compounds (61).
Tannins, which are polyphenols, can prevent the availability of protein for absorption by forming complexes with proteins (61). Oxalates also prevent the absorption of dietary calcium by binding with Ca2+ (61, 62); furthermore, the insoluble calcium oxalates are stored in the kidney, causing “kidney stones.” The increased oxalate:calcium ratio >9:4 can affect Ca absorption negatively (63). Phytic acid chelates with Zn or phytates, binding with proteins making them unavailable for absorption (62).
Inclusion of African nightshade, especially S. retroflexum, with the main staple food can improve protein, iron, and calcium levels, which will help improve people's health and well-being. It is also possible to use African nightshades as food and medicinal ingredients. The increased polyphenol compounds can contribute to antioxidant activity, and an increased intake of chlorogenic acid correlates with the reduced risk of type 2 diabetes mellitus (64). Available literature suggests that the chlorogenic acid suppresses postprandial hyperglycemia by inhibiting α-glucosidase similar to the α-glucosidase inhibitors, such as acarbose, miglitol, and voglibose (64). At the same time, chlorogenic acid modulates the glucose and lipid metabolism via the activation of adenosine monophosphate-activated protein kinase and stimulates glucose uptake in the skeletal muscle, which shows similar activity as anti-diabetic agents (65).
Solar dried or blanched African nightshade S. retroflexum leaves can be included as a functional ingredient or a functional food to manage type 2 diabetes in rural regions and as a promising potential for the food industry and food manufacturers. For health claims, and to popularize use as an ethnic food, future research on the biological activities of African nightshade leaves on anti-diabetic, anti-proliferative, or anti-inflammatory effects is necessary. Furthermore, in vitro results revealed the chemo-preventive properties in terms of anti-genotoxicity against the liver carcinogen aflatoxin B1 (AFB1) and antioxidant potential, at non-toxic concentrations, of the leaf extract of S. scabrum (38). The authors concluded that although the food preparation and processing methods affected the concentration of phytochemicals, the compositional changes could have acted positively in the observed antioxidant activity and chemo-preventive properties (38).
African nightshade vegetables are rich in minerals and phytochemicals, and the adoption of different food processing or preparation methods can prevent postharvest losses during the supply chain to contribute to food security and reduce hidden hunger. Food processing or preparation methods improved the phytochemicals (functional compounds) in the African nightshade vegetables, and agronomy practices affected the nutritional properties. For all the USDA and WAC African nightshade collections, it is important to profile the mineral composition to select the best accession to benefit consumers. Based on the available literature regarding the antioxidant activity, the recommendation for growers is to use S. scabrum (BG 16, Nduruma, WAC); however, all the accessions need further testing to correlate with the biological actives in order to identify the suitable accession for commercial production and marketing.
DS obtained the funding for the program and conceptualized the research. AP formatted and validated the data. YS under the Australia–Africa partnership program, collaborated with data generation. RS research collaborator, proofread and edited the review. FR research collaborator under the SA-France bilateral program, assisted with the generation of some data and editing of the review. All authors contributed to the article and approved the submitted version.
The Department of Science and Innovation (DSI), the National Research Foundation, South Africa (Grant no. 98352) for Phytochemical Food Network to Improve Nutritional Quality for Consumers, the Australia- Africa Universities Network (AAUN)-Partnership and Research Development Fund 2019, and the Bilateral SA-France Program PROTEA No. 42165TF funded this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. WHO. Fruit and Vegetables for Health. Available online at: https://apps.who.int/iris/handle/10665/43143 (accessed January 18, 2020).
2. WHO. Fruit and Vegetables for Health. Available online at: https://apps.who.int/iris/handle/10665/43143 (accessed January 18, 2020).
3. USDA. Item Clusters, Percent of Consumption, and Representative Foods for Typical Choices Food Patterns. Available online at: https://www.fns.usda.gov/usda-food-patterns (accessed January 18, 2020).
4. Deshpande SS. Fermented Grain Legumes, Seeds and Nuts: a Global Perspective. Rome: FAO. (2000) 142p.
5. Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health. (2015) 3:528–36. doi: 10.1016/S2214-109X(15)00039-X
6. Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, Eilander A. Micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: a systematic review of data from 2005 to 2015. Nutrients. (2017) 9:1096–118. doi: 10.3390/nu9101096
7. Odhav B, Beekrum S, Akula US, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Compost Anal. (2007) 20:430–35. doi: 10.1016/j.jfca.2006.04.015
8. Yuan B, Byrnes D, Giurleo D, Villani T, Simon JE, Wu Q. Rapid screening of toxic glycoalkaloids and micronutrients in edible nightshades (solanum spp.). J Food Drug Anal. (2018) 26:751–60. doi: 10.1016/j.jfda.2017.10.005
9. DAFF. Black Nightshade. Available online at: https://www.dalrrd.gov.za/Portals/0/Brochures%20and%20Production%20guidelines/Brochure%20Black%20Nightshade.pdf (accessed January 18, 2020).
10. Weinberger K. Indigenous Vegetables in Tanzania: Significance and Prospects. Tainan: AVRDC-World Vegetable Center (2004) 600p.
11. Manoko MLK, van den Berg RG, Feron RMC, van der Weerden GM, Mariani C. Genetic diversity of the African hexaploid species Solanum scabrum mill and Solanum nigrum L. (Solanaceae). Genet Resour Crop Evol. (2008) 55:409–18. doi: 10.1007/s10722-007-9248-z
12. Azeez JO, Van Averbeke W, Okorogbona AOM. Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresour Technol. (2010) 101:2499–505. doi: 10.1016/j.biortech.2009.10.095
13. Hotz C, Gibson RS. Traditional food-processing and preparation practices to enhance the bioavailability of micronutients in plant-based diets. J Nutr. (2007) 137:1097–100. doi: 10.1093/jn/137.4.1097
14. Elisha GO, Arnold OM, Christian U, Huyskens-Keil S. Postharvest treatments of African leafy vegetables for food security in Kenya: a review. Afr J Hort Sci. (2016) 9:32–40.
15. Van Jaarsveld P, Faber M, Van Heerden I, Wenhold F, van Rensburg WJ, Van Averbeke W. Nutrient content of eight African leafy vegetables and their potential contribution to dietary reference intakes. J Food Compost Anal. (2014) 33:77–84. doi: 10.1016/j.jfca.2013.11.003
16. Akubugwo IE, Obasi NA, Chinyere GC, Ugbogu AE. Nutritional and chemical value of amaranthus hybridus L. leaves from Afikpo, Nigeria. Afr J Biotechnol. (2007) 6:2833–9. doi: 10.5897/AJB2007.000-2452
17. Meyers LD, Hellwig JP, Otten JJ. Dietary Reference Intakes: the Essential Guide to Nutrient Requirements. National Academies Press (2006).
18. Mamboleo TF, Msuya JM, Mwanri AW. Vitamin C, iron and zinc levels of selected African green leafy vegetables at different stages of maturity. Afr J Biotechnol. Washington, DC (2018) 17:567–73. doi: 10.5897/AJB2017.16346
19. Beto JA. The role of calcium in human aging. Clin Nutr Res. (2015) 4:1–8. doi: 10.7762/cnr.2015.4.1.1
20. Wojdyło A, Lech K, Nowicka P, Hernandez F, Figiel A, Carbonell-Barrachina AA. Influence of different drying techniques on phenolic compounds, antioxidant capacity and colour of ziziphus jujube mill fruits. Molecules. (2019) 24:2361. doi: 10.3390/molecules24132361
21. Mibei EK, Ojijo NKO, Karanja SM, Kinyua JK. Compositional attributes of the leaves of some indigenous African leafy vegetables commonly consumed in Kenya. Ann Food Sci Technol. (2011) 12:146–54. Available online at: www.afst.valahia.ro
22. De Baaij JHF, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev. (2015) 95:1–46. doi: 10.1152/physrev.00012.2014
23. National Research Council. Copper in Drinking Water. Washington, DC: National Academies Press (2000).
24. Managa MG. Influence of Postharvest Processing and Food Preparation Methods on Physicochemical, Phytochemical Properties and Antidiabetic Activity of Traditional Leafy Vegetables. (Ph.D. Thesis), Tshwane University of Technology: South Africa (2020).
25. Abuajah CI, Ogbonna A, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. (2015) 52:2522–9. doi: 10.1007/s13197-014-1396-5
26. Srividya AR, Nagasamy V, Vishnuvarthan VJ. Nutraceutical as medicine: a review. Pharmanest. (2010) 1:132–45.
27. Sarker U, Oba S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of amaranthus leafy vegetable. BMC Plant Biol. (2018) 18:258–72. doi: 10.1186/s12870-018-1484-1
28. Ngwene B, Neugart S, Baldermann S, Ravi B, Schreiner M. Intercropping induces changes in specific secondary metabolite concentration in ethiopian kale (Brassica carinata) and African nightshade (Solanum scabrum) under controlled conditions. Front Plant Sci. (2017) 8:1700–11. doi: 10.3389/fpls.2017.01700
29. Oboh G, Akindahunsi AA. Change in the ascorbic acid, total phenol and antioxidant activity of sun-dried commonly consumed green leafy vegetables in Nigeria. Nutr Health. (2004) 18:29–36. doi: 10.1177/026010600401800103
30. Managa MG, Sultanbawa Y, Sivakumar D. Effects of different drying methods on untargeted phenolic metabolites, and antioxidant activity in Chinese cabbage (Brassica rapa L. subsp. chinensis) and nightshade (Solanum retroflexum Dun.). Molecules. (2020) 25:1326–48. doi: 10.3390/molecules25061326
31. Jiménez-Aguilar DM, Grusak MA. Evaluation of minerals, phytochemical compounds and antioxidant activity of Mexican, central American and African green leafy vegetables. Plant Foods Hum Nutr. (2015) 70:357–64. doi: 10.1007/s11130-015-0512-7
32. Oguntoyinbo FA, Fusco V, Cho GS, Kabisch J, Neve H, Bockelmann W, et al. Produce from Africa's gardens: potential for leafy vegetable and fruit fermentations. Front Microbiol. (2016) 7:981–94. doi: 10.3389/fmicb.2016.00981
33. Katerere DR, Graziani G, Thembo KM, Nyazema NZ, Ritieni A. Antioxidant activity of some African medicinal and dietary leafy African vegetables. Afr J Biotechnol. (2012) 11:4103–8. doi: 10.5897/AJB11.3674
34. Lee YY, Park HM, Hwang TY, Kim SL, Kim MJ, Lee SK, et al. A correlation between tocopherol content and antioxidant activity in seeds and germinating seeds of soybean cultivars. J Sci Food Agric. (2015) 95:819–27. doi: 10.1002/jsfa.6963
35. Boschin G, Arnoldi A. Legumes are valuable sources of tocopherols. Food Chem. (2011) 127:1199–203. doi: 10.1016/j.foodchem.2011.01.124
36. Lithourgidis AS, Dordas CA, Damalas CA, Vlachostergios DN. Annual intercrops: an alternative pathway for sustainable agriculture. Aust J Crop Sci. (2011) 5:396–410. Available online at: https://www.cropj.com/anastasios_5_4_2011_396_410.pdf
37. Khanam UKS, Oba S, Yanase E, Murakami Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J Funct Foods. (2012) 4:979–87. doi: 10.1016/j.jff.2012.07.006
38. Odongo GA, Schlotz N, Baldermann S, Neugart S, Huyskens-Keil S, Ngwene B, et al. African nightshade (Solanum scabrum Mill.): impact of cultivation and plant processing on its health promoting potential as determined in a human liver cell model. Nutrients. (2018) 10:1532–51. doi: 10.3390/nu10101532
39. Report Linker. Functional Foods Market Size, Share & Trends Analysis Report By Ingredient, By Product, By Application And Segment Forecasts, 2019–2025. Available online at: https://www.reportlinker.com/p05767979/Functional-Foods-Market-Size-Share-Trends-Analysis-Report-By-Ingredient-By-Product-By-Application-And-Segment-Forecasts.html?utm_source (assessed January 05, 2020).
40. An K, Zhao D, Wang Z, Wu J, Xu Y, Xiao G. Comparison of different drying methods on Chinese ginger (Zingiber officinale roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. (2016) 197:1292–300. doi: 10.1016/j.foodchem.2015.11.033
41. Seidu JM, Bobobee EYH, Kwenin WKJ, Frimpong R, Kubge SD, Tevor WJ, et al. Preservation of indigenous vegetables by solar drying. ARPN J Agric Biol Sci. (2012) 7:407–15. vailable online at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1066.1605&rep=rep1&type=pdf
42. Akpanyung EO. Proximate and mineral element composition of bouillon cubes produced in Nigeria. Pakistan J Nutr. (2005) 4:327–29. doi: 10.3923/pjn.2005.327.329
43. FDA. US Food and Drug Administration. (2019). Available online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.320 (assessed May 12, 2020).
44. Cohen BE, Elbuluk N, Mu EW, Orlow SJ. Alternative systemic treatments for vitiligo: a review. Am J Clin Dermatol. (2015) 16:463–74. doi: 10.1007/s40257-015-0153-5
45. Tinsley G. Phenylalanine: Benefits, Side Effects and Food. Available online at: https://www.healthline.com/nutrition/phenylalanine#what-is-it (assessed June 01, 2020).
46. Joshi N, Bains K, Kaur H. Evaluation of antioxidant activity of developed instant soup mixes using vegetable leaf powders from unconventional greens. Int J Curr Microbiol App Sci. (2020) 9:711–21. doi: 10.20546/ijcmas.2020.901.077
47. Sudarsan SM, Santhanam SG, Visalachi V. Development and formulation of instant soup mix from sprouted horsegram and radish leaves. Int J Home Sci. (2017) 3:346–9. Available online at: https://www.homesciencejournal.com/archives/2017/vol3issue1/PartF/3-1-62.pdf
48. Oyewole OB. Lactic fermented foods in Africa and their benefits. Food Control. (1997) 8:289–97. doi: 10.1016/S0956-7135(97)00075-3
49. Dirar HA, Harper DB, Collins MA. Biochemical and microbiological studies on Kawal, a meat substitute derived by fermentation of cassia obtusifolia leaves. J Sci Food Agric. (1985) 36:881–92. doi: 10.1002/jsfa.2740360919
50. Taale E, Hissein A, Tankoano A, Aly S. Traditional technologies and probiotic properties of Bacillus strains isolated from Kawal - a chad traditional fermented food condiment. J Food Technol. (2019) 6:57–71. doi: 10.18488/journal.58.2019.62.57.71
51. Algadi MZ, Yousif NE. Anti-nutritional factors of green leaves of cassia obtusifolia and Kawal. J Food Process Technol. (2015) 6:9. doi: 10.4172/2157-7110.1000483
52. Wafula EN, Franz CMAP, Rohn S, Huch M, Mathara JM, Trierweiler B, et al. Fermentation of African leafy vegetables to lower post-harvest losses, maintain quality and increase product safety. Afr J Hortic Sci. (2016)9:1–13.
53. Degrain A, Manhivi V, Remize F, Garcia C, Sivakumar D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African nightshade. Microorganisms. (2020) 8:1324. doi: 10.3390/microorganisms8091324
54. NHS. Salt: The Facts. Available online at: https://www.nhs.uk/live-well/eat-well/salt-nutrition/ (assessed June 07, 2020).
55. Grosso G. Dietary antioxidants and prevention of non-communicable diseases. Antioxidants. (2018) 7:94–96. doi: 10.3390/antiox7070094
56. Maria Landete J, Hernández T, Robredo S, Duenas M, de las Rivas B, Estrella I, et al. Effect of soaking and fermentation on content of phenolic compounds of soybean (Glycine max cv. Merit) and mung beans (Vigna radiata [L] Wilczek). Int J Food Sci Nutr. (2015) 66:203–9. doi: 10.3109/09637486.2014.986068
57. Filannino P, Bai Y, Di Cagno R, Gobbetti M, Gänzle MG. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. (2015) 46:272–9. doi: 10.1016/j.fm.2014.08.018
58. Martínez-Hernández GB, Artés-Hernández F, Colares-Souza F, Gómez PA, García-Gómez P, Artés F. Innovative cooking techniques for improving the overall quality of a kailan-hybrid broccoli. Food Bioproc Tech. (2013) 6:2135–49. doi: 10.1007/s11947-012-0871-0
59. Šilarová P, Boulekbache-Makhlouf L, Pellati F, Ceslová L. Monitoring of chlorogenic acid and antioxidant capacity of Solanum melongena L. (Eggplant) under different heat and storage treatments. Antioxidants. (2019) 8:234–44. doi: 10.3390/antiox8070234
60. Ferracane R, Pellegrini N, Visconti A, Graziani G, Chiavaro E, Miglio C, et al. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J Agric Food Chem. (2008) 56:8601–8. doi: 10.1021/jf800408w
61. Natesh HN, Abbey L, Asiedu S. An overview of nutritional and antinutritional factors in green leafy vegetables. Horticult Int J. (2017) 1:58–65. doi: 10.15406/hij.2017.01.00011
62. Noonan SC, Savage GP. Oxalate content of foods and its effect on humans. Asia Pac J Clin Nutr. (1999) 8:64–74. doi: 10.1046/j.1440-6047.1999.00038.x
63. Welch RM, House WA, Van Campen D. Effects of oxalic acid on availability of zinc from spinach leaves and zinc sulfate to rats. J Nutr. (1977) 107:929–33. doi: 10.1093/jn/107.6.929
64. Bassoli BK, Cassolla P, Borba-Mura GR, Constantin J, Salgueiro-Pagadigorria CL, Bazotte RB, et al. Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: effects on hepatic glucose release and glycaemia. Cell Biochem Funct. (2008) 26:320–8. doi: 10.1002/cbf.1444
Keywords: traditional leafy vegetables, polyphenols, antioxidants, minerals, postharvest processing
Citation: Sivakumar D, Phan ADT, Slabbert RM, Sultanbawa Y and Remize F (2020) Phytochemical and Nutritional Quality Changes During Irrigation and Postharvest Processing of the Underutilized Vegetable African Nightshade. Front. Nutr. 7:576532. doi: 10.3389/fnut.2020.576532
Received: 26 June 2020; Accepted: 28 September 2020;
Published: 16 November 2020.
Edited by:
Rakesh Bhardwaj, National Bureau of Plant Genetic Resources (ICAR), IndiaReviewed by:
Haritha Bollinedi, Indian Agricultural Research Institute (ICAR), IndiaCopyright © 2020 Sivakumar, Phan, Slabbert, Sultanbawa and Remize. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dharini Sivakumar, U2l2YWt1bWFyREB0dXQuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.