
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Nutr. , 12 December 2018
Sec. Clinical Nutrition
Volume 5 - 2018 | https://doi.org/10.3389/fnut.2018.00126
This article is part of the Research Topic Frailty and Herbal Medicines- From Molecular Mechanisms to Clinical Efficacy View all 31 articles
 Nanami Sameshima Uto1
Nanami Sameshima Uto1 Haruka Amitani1,2
Haruka Amitani1,2 Yuta Atobe2
Yuta Atobe2 Yoshihiro Sameshima3
Yoshihiro Sameshima3 Mika Sakaki2
Mika Sakaki2 Natasya Rokot2
Natasya Rokot2 Koji Ataka1
Koji Ataka1 Marie Amitani4
Marie Amitani4 Akio Inui1*
Akio Inui1*Frailty and sarcopenia have recently gained considerable attention in terms of preventive care in Japan, which has an ever-increasing aging population. Sarcopenia is defined as atrophy of skeletal muscles caused by the age-related decrease in growth hormone/insulin-like growth factor and sex hormones. The Japanese Ministry of Health, Labor and Welfare reports that frailty can lead to impairment of both mental and physical functioning. Chronic diseases such as diabetes and dementia may underlie frailty. It is important to prevent progression of frailty and extend the healthy lifespan. In herbal medicine practice, including Japanese Kampo medicine, “Mibyo,” a presymptomatic state, has long been recognized and may be applicable to frailty. Kampo medicines may include several medicinal plants and are thought to have the potential to improve symptoms of frailty, such as loss of appetite and body weight, fatigue, and sarcopenia, as well as anxiety, depression, and cognitive decline. Ninjin'yoeito (Ren Shen Yang Ying Tang) is the most powerful Kampo medicine and has been widely applied to palliative care of cancer patients. This review includes recent anti-aging studies and describes the effects and mechanisms of Ninjin'yoeito (Ren Shen Yang Ying Tang) when used for frailty or to extend a healthy life expectancy.
In Japan, society is aging at an unprecedented rate, substantially changing the social system and disease distribution. Nationwide and community-wide efforts have been made toward ensuring healthy longevity, and paradigm shifts have occurred at various levels. Accordingly, frailty has received attention in preventive medicine practice. The average life span in Japan was reported as 80.98 years in men and 87.14 years in women (Japanese Ministry of Health, Labour and Welfare, 2018). These values continue to increase. The difference between an average life span and healthy life expectancy, namely, the point at which routine daily life becomes limited, is reportedly 8.84 years in men and 12.35 years in women in Japan. These values have remained largely unchanged for a decade. Prevention and treatment of frailty to extend a healthy life expectancy prior to the need for nursing care is a huge challenge in developed societies. In herbal medicine practice, including Kampo medicine in Japan, “Mibyo,” a presymptomatic state, has long been recognized as similarly to frailty. Use of Kampo medicine, especially Ninjin'yoeito (Ren Shen Yang Ying Tang), has been considered for frailty conditions.
At around 60 years old, we may experience rapid loss of muscle mass and a relative increase in fat mass associated with aging, leading to atrophy of the skeletal muscles (sarcopenia) (1–3). These conditions increase the risk of falls and fractures, requiring long-term care.
The Japan Geriatrics Society defined frailty as a state of increased vulnerability in elderly people before the need for long-term care (2014, Figure 1Aa). On the other hand, the Society on Cachexia and Wasting Disorders lists disease progression as one of the diagnostic criteria for frailty (Figure 1Ab), indicating that frailty is more consistent with a syndrome encompassing a variety of physical and mental pathologies, with an emphasis on motor function. The prevalence of frailty is estimated to be about 30% in persons over the age of 80 (2). Frailty can be observed in both malnutrition and overnutrition states and can develop into a vicious cycle known as frailty cycle/cascade, leading to a need for long-term care [(2, 3); Figure 1B]. Physical impairment leads to psychological vulnerability, with depression and cognitive impairment, and vice versa. Depression worsens sarcopenia through excessive secretion of adrenal cortical hormones and/or reduction in physical activity (6–8). Locomotive syndrome is defined as age-related muscle weakness (sarcopenia) and deterioration of motor function due to articular/spinal disease or osteoporosis (4, 9). Although frailty is a psychosomatic pathology and can be divided into physical, social, and cognitive/psychological frailty, locomotive syndrome can be viewed as a clinical condition similarly to physical frailty, with an emphasis on locomotive organs.
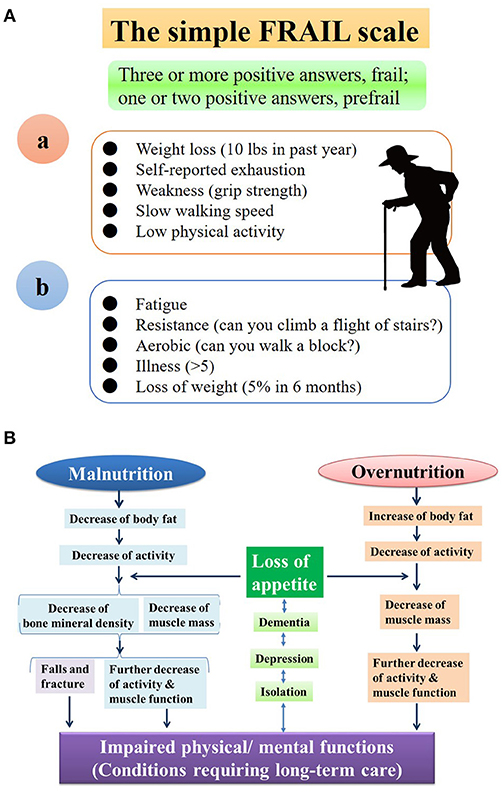
Figure 1. (A) Diagnostic Criteria for Frailty by Fried et al. (4) (a) and Society on Cachexia and Wasting Disorders (b). In both guidelines, frailty is defined as the presence of at least 3 of 5 criteria, with sarcopenia (atrophy of skeletal muscles) as the basis. Frailty may be close to a presymptomatic state Mibyo in Kampo medicine. The Society on Cachexia and Wasting Disorders (a) lists disease aggregation as one of the diagnostic criteria, with frailty cases ranging from mild to severe. Frailty represents a wide range of clinical conditions that encompass emaciation as well as obesity. The appropriate permissions have been obtained from the copyright holders, Sameshima et al. (5). (B) Frailty Cascade/Cycle. Either overnutrition or malnutrition can precipitate frailty, in which both physical and psychological vulnerabilities are likely to be seen. Depression and cognitive impairment are either the causes or results of frailty. The presence of depression, for example, not only has a negative effect on treatment, but also worsens sarcopenia by inducing excessive secretion of adrenal cortical hormones or extreme reduction in physical activity. The appropriate permissions have been obtained from the copyright holders, Kuzuya (2).
Sarcopenia is associated with age-related hormonal changes (decreased growth hormone/insulin-like growth factor [GH/IGF-1] and testosterone) and reduced activity (due to a sedentary lifestyle or osteoarthritis). Cachexia is based on sarcopenia and associated with a variety of diseases that may underlie frailty. Proinflammatory cytokines, including tumor necrosis factor-α, are important in cachexia (10, 11), and may activate the ubiquitin-proteasome system to promote protein catabolism. In contrast, anti-inflammatory cytokines or IGF-1 promote synthesis of muscle proteins or regeneration of muscle fibers. The corticotropin-releasing factor/glucocorticoid system activated by stress or proinflammatory cytokines are other catabolic pathways involving the gut-brain axis [(11); Figure 2A].
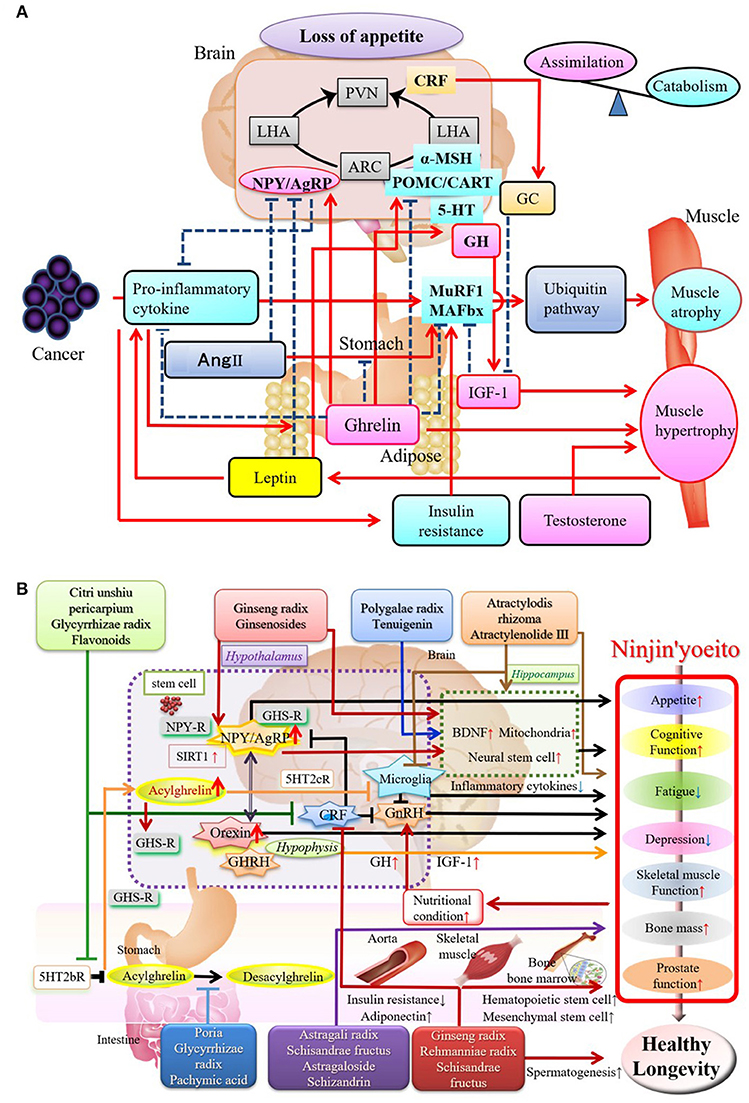
Figure 2. (A) Mechanism of Sarcopenia: Positive and Negative Regulators of Skeletal Muscle. Underlying mechanisms of sarcopenia have become increasingly understood through research on brain-gut interactions. Proinflammatory cytokines activate ubiquitin ligases that cause destruction of muscle. The corticotropin-releasing factor (CRF)/glucocorticoid system, insulin resistance, and decreased androgen levels promote sarcopenia, while the hunger hormone, ghrelin, released from the stomach, and insulin-like growth factor (IGF-1) exerts a trophic action on muscle. MuRF1, muscle ring-finger protein 1; MAFbx, muscle atrophy F-box protein (Atrogin-1); IGF-1, insulin-like growth factor 1; Ang II, angiotensin II; NPY, neuropeptide Y; AgRP, agouti-related peptide; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated peptide; CRF, corticotrophin-releasing factor; 5-HT, serotonin; PVN, paraventricular hypothalamic nucleus; ARC, arcuate nucleus; LHA, lateral hypothalamic area; HC, glucocorticoids; GH, growth hormone. The appropriate permissions have been obtained from the copyright holders, Amitani et al. (11). (B) Components and Active Ingredients of Ninjin'yoeito and their Effects. Many reports have described the role of ginsenosides/saponins from ginseng root on the efficacy of Ninjin'yoeito. Other reported effects include those of ingredients derived from Atractylodes lancea rhizome and Polygala root on energy metabolism and cognition/emotion. C. unshiu peel, Poria, Glycyrrhiza root, and panaxadiol derived from ginseng root improve ghrelin signaling underlying the mechanism of action of Ninjin'yoeito, leading to appetite stimulation and improvement in sarcopenia. Ninjin'yoeito stimulates bone marrow hematopoietic and mesenchymal stem cells that may be involved in repair and regeneration of organs and tissues. The appropriate permissions have been obtained from the copyright holders, Inui (3) and Sameshima et al. (5). GHSR, growth hormone secretagogue receptor; NPY-R, NPY receptor; 5HT2cR, 5-HT2c receptor; BDNF, Brain-derived neurotrophic factor; GnRH, Gonadotropin releasing hormone; GHRH, Growth hormone releasing hormone.
In the search of PubMed electronic database using the key words: “Ninjin youeito” and “human” or “Ninjin'youeito.” Eighteen and eleven literatures were identified, respectively. We excluded literatures written in Japanese, reviews, animal experiments, and in vitro experiments using human cells from the identified literatures. Seven literatures were extracted (Table 1) (12–25).
In elderly individuals, polypharmacy is often problematic and may lead to adverse drug reactions (ADRs). Frailty is likely to involve multiple organ systems and may be a good target for multicomponent herbal medicine. Hozai comprises a group of Kampo formulations that restore vitality to patients who have lost psychological and physical energy due to various diseases including cancer. Hozai formulations include Juzentaihoto, Hochuekkito, and Ninjin'yoeito. Kampo theory may regard frailty as Jinkyo, which means dysfunction of Jin, and is associated with production of Ki. Ki is universal energy and a basic element of life in Kampo theory. Rehmannia root, a component of Ninjin'yoeito, is often used to treat Jinkyo, which is related to frailty, and is contained in Juzentaihoto and Ninjin'yoeito, but not in Hochuekkito. Citrus unshiu peel is contained in Hochuekkito and Ninjin'yoeito, but not in Juzentaihoto. Polygala root and Schisandra fruit are only contained in Ninjin'yoeito. In cancer palliative medicine, Juzentaihoto or Hochuekkito tend to be prescribed initially, and in serious cases are replaced with Ninjin'yoeito.
Among other crude drugs, ginseng has been used since ancient times. Panax ginseng was historically thought to promote immortality, which was sought by the first Qin Emperor. It was imported to Japan in the eighth century, in the era of Emperor Shomu, and has become one of the main components in Ninjin'yoeito. Ninjin'yoeito was frequently used for serious diseases in the Edo Period. The Heji Jufang, compiled during the Song Dynasty, states that Ninjin'yoeito is indicated for weakness due to overwork or illness, dullness of the extremities, sharp musculoskeletal pain, shortness of breath, intense low back pain, emptiness and anxiety, thirst and dry mouth, depressive mood, and lethargy, leading to a condition that is difficult to treat. It is also indicated for lung and large intestine symptoms, including cough, sputum production, diarrhea, and vomiting.
In the Journal of Kampo to Kanyaku, Domei Yakazu, who was committed to the restoration of Kampo Medicine in the twentieth century, during the Showa period, stated that Ninjin'yoeito can be used for cachexia of cancer, suggesting that it is the most powerful Hozai (3). Ninjin'yoeito is now widely used in the field of palliative medicine, including cancer treatment (3, 26, 27). Ninjin'yoeito increases the rate of remission in advanced gynecological cancer, as assessed by positron emission tomography-computed tomography (26). Ninjin'yoeito is used to prevent toxicity (such as impaired hematopoiesis) associated with anticancer drugs or radiotherapy, and can improve appetite, fatigue, general health status, and even survival. Ninjin'yoeito enhances the therapeutic efficacy of melphalan in multiple myeloma and reduces general malaise (27). Ninjin'yoeito also treats decreased appetite and fatigue in Sjögren's syndrome (28). Many reports on the clinical benefits of Ninjin'yoeito describe improvement of general health status in elderly (29) or postoperative patients (30), amelioration of disordered protein synthesis in hepatic cirrhosis (31) or of diabetic complications such as neuropathy (32), and recovery from anemia (33, 34) or thrombocytopenia (31, 35). In chronic obstructive pulmonary disease (COPD), a major underlying cauthese of cachexia, Ninjin'yoeito treats appetite loss, weight loss, and respiratory symptoms, and improves nutritional status and immune function (36). Ninjin'yoeito, but no other Hozai such as Juzentaihoto and Hochuekkito, treated cough, sputum production, and insomnia. Ninjin'yoeito is effective in control of infection after knee joint replacement (37), and increases bone mineral density in postmenopausal women, treats anosmia resistant to glucocorticoid treatment, and is effective in male infertility. Ninjin'yoeito improves cognitive function and depression in patients with Alzheimer's disease when added to treatment with donepezil (18). There are also many reports suggesting its potential usefulness in home health care and frailty (3).
Ninjin'yoeito is composed of 12 crude drugs: peony root, Japanese angelica root, C. unshiu peel, Astragalus root, cinnamon bark, ginseng, Atractylodes rhizome, Glycyrrhiza, Rehmannia root, Schisandra fruit, Poria sclerotium, and Polygala root. The main components of this formulation include glycyrrhizic acid, derived from Glycyrrhiza; paeoniflorin from peony root; ginsenosides from ginseng; hesperidin from C. unshiu peel; atractylenolide III from Atractylodes rhizome; isoastragaloside (HQ1/2) from Astragalus root; tenuigenin from Polygala; and schizandrin from Schisandra fruit (Table 2) (38–58). Glycyrrhizic acid has anti-inflammatory effect and has been clinically applied in treatment of chronic hepatic diseases. Paeoniflorin is known to suppress intracellular Ca2+ influx and relieves muscle pain. In tumor-bearing animal models treated with anticancer drugs, Ninjin'yoeito not only improves food intake and sarcopenia, but also prolongs survival (59, 60). Ninjin'yoeito may improve the signs of aging and significantly extend survival time in approximately 30% of Klotho-deficient senescence-accelerated mice (59, 60).
Ginseng, a component of Ninjin'yoeito, shows antifatigue and antidepressant effects in a forced swim test (61). Ginseng may decrease the signs of aging in a senescence-accelerated mouse (SAMP8) (3). Ginsenosides, active compounds from ginseng, are reported to have a wide variety of effects. Ginsenosides ameliorate memory disturbance induced by amyloid beta (62). In a vascular dementia model (middle cerebral artery ischemia/reperfusion), ginsenoside Rg2 improves hemiplegia and memory impairment (63). These results suggest that ginseng has neuroprotective effects. Ginsenoside Rb2 inhibits the decrease in bone mineral density in the femur and 4th lumbar vertebra in ovariectomized mice through the suppression of oxidative stress and osteoclastic cytokines (64). Ginsenoside Rd ameliorates arteriosclerosis and reduces atherosclerotic plaques through inhibition of voltage-independent Ca channels in Apo-E-deficient mice fed a high-fat diet (65). Protopanaxatriol, a metabolite of ginsenoside Rg2, improves insulin resistance (66). Ginsenoside Rg3 suppresses testosterone-induced prostatic hypertrophy and growth of prostate cancer cells through inhibition of mitogen-activated protein kinase signaling (67).
C. unshiu peel inhibits amyloid beta-induced neurite atrophy and apoptosis of neural cells. Its components, including hesperidin and narirutin, have been reported to improve cognitive function by promoting reformation of the myelin sheath that is lost during aging (18). Hesperidin treats appetite loss and sarcopenia via suppression of the serotonin pathway and recovery of ghrelin secretion in the stomach [Figure 2A; (68)]. The improvement of sarcopenia by ghrelin can be attributed to the activation of the GH/ IGF-1 system (69).
Atractylodes rhizome inhibits cell death by improving mitochondrial activity and intracellular ATP production (70, 71). This protective effect could be important since oxidative stress is considered basic to the pathophysiology of aging (72). Atractylenolide III, a component of Atractylodes rhizome, has been reported to ameliorate depression-like symptoms and memory impairment by increasing the expression level of Ca2+/calmodulin-dependent protein kinase II and Creb and BDNF in the hippocampus (71).
Adiponectin has been reported to have protective effects on atherosclerosis, and mice with over-expressed adiponectin show prolonged survival, even with a high-fat and high-sucrose diet through inhibition of oxidative DNA damage (73). Astragalus root enhances insulin sensitivity via increase of adiponectin, especially its highly-potent high-molecular-weight form (74) and may prevent atherosclerosis.
Polygala root and its main component, tenuigenin, promote the growth and differentiation of hippocampal neural stem cells (75). It has been reported to improve cognitive function in adults and elderly subjects in clinical studies (76, 77), and is approved as an over-the-counter drug.
Schisandra fruit promotes elimination of fatigue substances, such as lactate and ammonia, from the blood, and increases endurance during exercise on a treadmill via upregulation of peroxisome proliferator-activated receptor γ coactivator 1α, an important factor in skeletal muscle metabolism (78). Schisandra fruit increases blood estradiol, uterus estrogen receptor-α and -β, and uterine weight in an ovariectomized post-menopausal model, although it inhibits the proliferation of breast cancer cells (79). Schizandrin is a main component of Schisandra fruit.
Ninjin'yoeito is thus expected to reduce physical and psychological vulnerability related to feeding, immunity, emotion, and cognition, which are oftendisturbed in frailty patients (Figure 2B). Ninjin'yoeito could be widely applicable in mild to severe cases of frailty (3).
Kampo medicines are composed of a wide variety of crude drugs with pleiotropic effects on the psychosomatic syndrome of frailty, and Ninjin'yoeito is expected to form the basis of these medicines. Recently, Kracie Pharma Ltd. reported special drug use survey results on ADRs associated with Ninjin'yoeito Extract Granules in patients aged ≥65 years (80). The population under analysis consisted of 808 patients (210 males and 598 females, mean age of 77.8 ± 7.35 years; 538 and 262 patients with and without comorbidities; and 664 and 130 taking or not taking concomitant drugs). The incidence of ADRs was 3.09% (25 patients), and gastrointestinal disorders were most common, reported by 17 patients (2.10%). Overall, there were no significant sex-related differences, and approximately 70% of the reported ADRs occurred within 2 months of starting Kampo formulation (80). Given the low and similar incidence of ADRs associated with placebo, the medication should even be safe in the elderly.
In addition, the combined use of Ninjin'yoeito with other Kampo medicines may enhance the effects of therapy. The addition of Yokukansan and Yokukansankachimpihange treats the behavioral and psychological symptoms of dementia (BPSD); Rikkunshito is added for gastrointestinal symptoms, Hangekobokuto for aspiration symptoms, Hachimijiogan/Goshajinkigan for prostate symptoms, and Goshajinkigan for osteoarthritis or spondylosis in severe cases with pain or numbness (81–90). Although Kampo medicines are likely to cause fewer ADRs than modern medicine, multi-combination use requires caution and should be limited to 2 medicines.
This review describes the clinical application of Kampo medicine in frailty, with a focus on Ninjin'yoeito. As in metabolic syndrome, prevention and treatment of frailty requires diet/exercise, behavioral modification, and utilization of public healthcare resources. Given the progression to a super-aged society, paradigm shifts at both individual and societal levels are needed. The concept of “Mibyo,” a presymptomatic disease state in Kampo medicine, may be a good place to start and frailty could be an important candidate for intervention. It is important to evaluate this presymptomatic state from a scientific perspective to determine how preventive Kampo medicine should be provided. In western medicine Galen is the first to indicate the importance of diet in slowing the aging process (91), and very recently geroprotectors that delay many diseases related to aging are being considered for healthy longevity (92, 93).
Antiaging studies have rapidly evolved, and the mechanisms behind frailty and aging have become increasingly understood. Ninjin'yoeito acts on hematopoietic stem cells to promote the growth and differentiation of erythrocytes, leukocytes, and platelets in animals and humans (3, 94, 95). We recently found that Polygala root, Schisandra fruit, ginseng, Rehmannia root, and C. unshiu peel, which are characteristic herbal components of Ninjin'yoeito, promote the growth and differentiation of bone marrow-derived mesenchymal stem cells (66). The components of Ninjin'yoeito may thus be important for their effects on stem cells that may migrate and regulate brain functions associated with feeding and emotion (96–98). Ninjin'yoeito also increases hippocampal neural stem cells (75). These effects on tissue stem cells may underlie the pleiotropic actions on Ninjin'yoeito and suggest its use for frailty.
NU wrote the manuscript. NU and AI conceived and organized the structure of the review. NR and KA contributed to the first draft. HA, YA, YS, MS, MA, and AI contributed to critical revision and approved the final manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Morley JE, von Haehling S, Anker SD, Vellas B. From sarcopenia to frailty: a road less traveled. J Cachexia Sarcopenia Muscle (2014) 5:5–8. doi: 10.1007/s13539-014-0132-3
2. Kuzuya M. Frailty: overview and association with nutrition. Jpn J Geriatr. (2014) 51:120–2. (in Japanese). doi: 10.3143/geriatrics.51.120
4. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. Cardiovascular health study collaborative research group. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
5. Sameshima N, Sakaki M, Ishigami M, Morinaga M, Amitani M, Inui A. Frailty and Ninjin-yoei-to. Anti. Aging. Med. (2017) 13:52–60. (in Japanese).
6. Hubbard RE, Lang I. Avoiding depression, dementia, and frailty: do you feel lucky? J Am Med Dir Assoc. (2015) 16:270–1. doi: 10.1016/j.jamda.2015.01.085
7. Kilavuz A, Meseri R, Savas S, Simsek H, Sahin S, Bicakli DH, et al. Association of sarcopenia with depressive symptoms and functional status among ambulatory community-dwelling elderly. Arch Gerontol Geriatr. (2018) 76:196–201. doi: 10.1016/j.archger.2018.03.003
8. Nipp RD, Fuchs G, El-Jawahri A, Mario J, Troschel FM, Greer JA, et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist (2018) 23:97–104. doi: 10.1634/theoncologist.2017-0255
9. Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. (2009) 27:620–8. doi: 10.1007/s00774-009-0080-8
10. Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. (2002) 52:72–91. doi: 10.3322/canjclin.52.2.72
11. Amitani M, Asakawa A, Amitani H, Inui A. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol. (2013) 45:2179–85. doi: 10.1016/j.biocel.2013.07.016
12. Hsiao PJ, Lin KS, Chiu CC, Chen HW, Huang JS, Kao SY, et al. Use of traditional Chinese medicine (Ren Shen Yang Rong Tang) against microinflammation in hemodialysis patients: an open-label trial. Complement Ther Med. (2015) 23:363–71. doi: 10.1016/j.ctim.2015.03.002
13. Xu Y, Chen Y, Li P, Wang XS. Ren Shen Yangrong Tang for fatigue in cancer survivors: a phase I/II open-label study. J Altern Complement Med. (2015) 21:281–7. doi: 10.1089/acm.2014.0211
14. Sato Y, Katagiri F, Inoue S, Itoh H, Takeyama M. Effects of Ninjin-to on levels of calcitonin gene-related peptide and substance P in humanplasma. Biol Pharm Bull. (2004) 27:2032–4. doi: 10.1248/bpb.27.2032
15. Naito T, Itoh H, Nagano T, Takeyama M. Effects of Ninjin-to on levels of brain-gut peptides (motilin, vasoactive intestinal peptide, gastrin, and somatostatin) in human plasma. Biol Pharm Bull. (2001) 24:194–6. doi: 10.1248/bpb.24.194
16. Cyong JC, Ki SM, Iijima K, Kobayashi T, Furuya M. Clinical and pharmacological studies on liver diseases treated with Kampo herbal medicine. Am J Chin Med. (2000) 28:351–60. doi: 10.1142/S0192415X00000416
17. Ito T, Konishi A, Tsubokura Y, Azuma Y, Hotta M, Yoshimura H, et al. Combined use of ninjin'yoeito improves subjective fatigue caused by lenalidomide in patients with multiple myeloma: a retrospective study. Front Nutr. (2018) 5:72. doi: 10.3389/fnut.2018.00072
18. Kudoh C, Arita R, Honda M, Kishi T, Komatsu Y, Asou H, et al. Effect of ninjin'yoeito, a Kampo (traditional Japanese) medicine, on cognitive impairment and depression in patients with Alzheimer's disease: 2 years of observation. Psychogeriatrics (2016) 16:85–92. doi: 10.1111/psyg.12125
19. Kamei T, Kumano H, Iwata K, Nariai Y, Matsumoto T. The effect of a traditional Chinese prescription for a case of lung carcinoma. J Altern Complement Med. (2000) 6:557–9. doi: 10.1089/acm.2000.6.557
20. Kato C, Morishita Y, Fukatsu T. False-positive increase in 1,5-anhydro-D-glucitol due to Kampo (Japanese herbal) medicine. Rinsho Byori. (1996) 44:396–9.
21. Uchiyama Y, Nakajima S, Yoshida K, Mizukawa H, Haruki E. The effect of ninjinyoeito on human aorta endothelial cells. Am J Chin Med. (1994) 22:293–9. doi: 10.1142/S0192415X94000358
22. Uchiyama Y, Nakajima S, Yoshida K, Mizukawa H, Haruki E. The effects of ninjinyoeito on human vascular endothelial cells. Am J Chin Med. (1993) 21:279–89. doi: 10.1142/S0192415X93000339
23. Ogawa R, Toyama S, Matsumoto H. Chronic fatigue syndrome–cases in the Kanebo Memorial Hospital. Nihon Rinsho. (1992) 50:2648–52.
24. Uchiyama Y, Nakajima S, Ohno T, Goto M, Kan M, Haruki E. The effect of Ninjinyoeito on Werner's syndrome skin fibroblasts. Am J Chin Med. (1992) 20:295–305. doi: 10.1142/S0192415X9200031X
25. Hirai K, Tanaka A, Homma T, Mikuni H, Kawahara T, Ohta S, et al. Improvement in frailty in a patient with severe chronic obstructive pulmonary disease after ninjin'yoeito therapy: a case report. Front Nutr. (2018) 5:71. doi: 10.3389/fnut.2018.00071
26. Tanaka T. Antitumor effect by paclitaxel/carboplatin therapy and Ninjin'yoeito for advanced-stage gynecological cancer. Sci Kampo Med. (2011) 35:370–3. (in Japanese).
27. Nomura S, Ishii K, Fujita Y, Azuma Y, Hotta M, Yoshimura Y, et al. Immunotherapeutic effects of Ninjin-youei-to on patients with multiple myeloma. Curr Trends Immunol. (2014) 15:19–27.
28. Katayama I, Murota H, Shirabe H. Effect of Ninjinyoueito in improving quality-of-life measurements for patients with sjogren's syndrome with skin symptoms. Nishi Nihon Hifuka (2008) 70:516–21. (in Japanese). doi: 10.2336/nishinihonhifu.70.516
29. Suzuki S, Aihara F, Shibahara M, Sakai K. Special drug use surveillance for kracie Ninjin'yoeito extract fine granules -a study of safety and efficacy in elderly patients-. Jpn J Med Pharmaceut Sci. (2017) 74:1285–97. (in Japanese).
30. Yoshikawa H, Ikeuchi T, Kai Y. Clinical efficacy of Ninjin-Youei-To for recovery of reduced physical strength of the patients after prostate hypertrophy operation. Jpn J Oriental Med. (1999) 49:617-22. (in Japanese).
31. Iwata K, Kamimura S, Shijo H. Okumura M Administration of Ninjin-yoei-to to liver cirrhosis. Focusing on effects on thrombocytopia. Jpn J Clin Exp Med. (1995) 72:746–50. (in Japanese).
32. Aiso Y, Nagasaka S. Tonyobyoshinkeishougai ni taisuru Ninjin-yoei-to no kouka. Shinki hihu sekigaisen taionkei ' Thermo Focus® ' ni yoru kentou. J New Rem Clin. (2007) 56:2028–32. (in Japanese).
33. Yanagibori A, Miyagi M, Hori M, Otaka K, Matsushima H, Ito M. Effect of Ninjin-yoei-tou on iron-deficiency anemia. Jpn J Clin Exp Med. (1995) 72:2605–8. (in Japanese).
34. Takemura K. Erisuropoechin touyochuu no ketsuekitousekikanja no jinseihinketsu nitausuru Ninjin'yoeito no hojoteki yuukousei ni tsuite. Kampo Newest Ther. (2000) 9:271–4. (in Japanese).
35. Kaibori M, lshizaki M, Matsui K, A-Hon Kwon. Therapeutic effects of combination therapy of traditional Japanese medicine, ninjinyoueito, and sorafenib in recipients with advanced hepoatocellular carcinoma. Jpn J Med Pharmaceut Sci. (2012) 67:445–7. (in Japanese).
36. Kato S, Tamano M, Okamura A, Ozone S, Hoshino T, Takahashi S, et al. Clinical benefits of three major Jingizai formulas in chronic obstructive pulmonary disease. Sci Kampo Med. (2016) 40:172–6. (in Japanese).
37. Kumaki S. Effects of Ninjin'yoeito after knee joint replacement. Prog Med. (2003) 23:2993–6. (in Japanese).
38. Han SY, Kim J, Kim E, Kim SH, Seo DB, Kim JH, et al. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J Ginseng Res. (2018) 42:496–503. doi: 10.1016/j.jgr.2017.06.003
39. Yin L, Guan E, Zhang Y, Shu Z, Wang B, Wu X, et al. Chemical profile and anti-inflammatory activity of total flavonoids from glycyrrhiza uralensis fisch. Iran J Pham Res. (2018) 17:726–34.
40. Chen LG, Jan YS, Tsai PW, Norimoto H, Michihara S, Murayama C, et al. Anti-inflammatory and antinociceptive constituents of atractylodes japonica koidzumi. J Agric Food Chem. (2016) 64:2254–62. doi: 10.1021/acs.jafc.5b05841
41. Uto T, Tung NH, Taniyama R, Miyanowaki T, Morinaga O, Shoyama Y: Anti-inflammatory activity of constituents isolated from aerial part of angelica acutiloba kitagawa Phytother Res. (2015) 29:1956–63. doi: 10.1002/ptr.5490
42. Wan FY, Lv WS, Han L: Determination and pharmacokinetic study of pachymic acid by LC-MS/MS Biol Pharm Bull. (2015) 38:1337–44. doi: 10.1248/bpb.b15-00121
43. Crawford P, Crawford AJ. Edema from taking cinnamon for treatment of diabetes: Similar biochemistry and pathophysiology to thiazolidinedione medications. J Am Bord Fam Med. (2018) 31:809–11. doi: 10.3122/jabfm.2018.05.180024
44. Kim SH, Choung SY. Antihyperglycemic and antihyperlipidemic action of Cinnamomi cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res. (2010) 33:325–33. doi: 10.1007/s12272-010-0219-0
45. Yuan HL, Li B, Xu J, Wang Y, He Y, Zheng Y, et al. Tenuigenin protects dopaminergic neurons from inflammation-mediated damage induced by the lipopolysaccharide. CNS Neurosci Ther. (2012) 18:584–90. doi: 10.1111/j.1755-5949.2012.00347.x
46. Zhang H, Han T, Zhang L, Yu CH, Wan DG, Rahman K, et al. Effects of tenuifolin extracted from radix polygalae on learning and memory: a behavioral and biochemical study on aged and amnesic mice. Phytomedicine (2008) 15:587–94. doi: 10.1016/j.phymed.2007.12.004
47. Ahn KI, Choi EO, Kwon DH, HwangBo H, Kim MY, Kim HJ, et al. Induction of apoptosis by ethanol extract of Citrus unshiu Markovich peel in human bladder cancer T24 cells through ROS-mediated inactivation of the PI3K/Akt pathway. Biosci Trends (2017) 11:565–73. doi: 10.5582/bst.2017.01218
48. Kang S, Song S, Lee J, Chang H, Lee S. Clinical investigations of the effect of Citrus unshiu peel pellet on obesity and lipid profile. Evid Based Complement Alter Med. (2018) 2018:4341961. doi: 10.1155/2018/4341961
49. Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis. (2017) 8:868–86. doi: 10.14336/AD.2017.0816
50. Sun J, Jing S, Jiang R, Wang C, Zhang C, Chen J, et al. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by d-galactose. Clin Interv Aging (2018) 13:829–41. doi: 10.2147/CIA.S163275
51. Hu D, Cao Y, He R, Han N, Liu Z, Miao L, et al. Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Aβ1–42-induced memory impairment in mice. Oxid Med Cell Longev. (2012) 2012:721721. doi: 10.1155/2012/721721
52. Kim C, Shin J, Hwang S, Choi Y, Kim D, Kim C. Schisandrae fructus enhances myogenic differentiation and inhibits atrophy through protein synthesis in human myotubes. Int J Nanomed. (2016) 11:2407–15. doi: 10.2147/IJN.S101299
53. He D, Dai S. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall. A traditional Chinese herbal medicine. Front Pharmacol. (2011) 2:10. doi: 10.3389/fphar.2011.00010
54. Bae J, Kim C, Kim H, Park J, Ahn M. Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovata. J Med Food (2015) 18:224–32. doi: 10.1089/jmf.2014.3144
55. Kitagawa I, Nishimura T, Furubayashi A, Yoshioka I. On the constituents of rhizome of rehmannia glutineosa LIBOSCH. forma hueichingensis HSIAO. YAKUGAKU ZASSHI (1971) 91:593–6. doi: 10.1248/yakushi1947.91.5_593
56. Matsuda H, Fukuda S, Nakanishi J, Fukuda S, Kubo M. Inhibitory effect of oriental medicine “rehmanniae radix” on disseminated intravascular coagulation (DIC). SHOYAKUGAKU ZASSHI (1986) 40:182–7.
57. Kubo M, Asano T, Shimono H, Matsuda H. Studies on rehmanniae radix. I. Effect of 50% ethanolic extract from steamed and dried rehmanniae radix on hemorheology in arthritic and thrombosic rats. Biol Pharmaceut Bull. (1994) 17:1282–6. doi: 10.1248/bpb.17.1282
58. Sasaki H, Nishimura H, Morota T, Chin M, Mitsuhashi H, Komatsu Y, et al. Immunosuppressive principles of rehmannia glutinosa var. hueichingensis. Planta Med. (1989) 55:458–62. doi: 10.1055/s-2006-962064
59. Chiba S, Fujita H, Yomoda S. Effect of ninjin'yoeito on cisplatin-induced amyotropy. Kampo Med. (2017) 68:398.
60. Takahashi R, Chiba S, Takemoto R, Michihara S, Han LK, Hujita H. Ninjin'yoeito improves survival and aging phenotype on accelerated aging model. Jpn J Psychosom Int Med. (2018) 22:16–19. (in Japanese).
61. Hujita H, Murata K. Effect of ginseng on antidepressant action and fatigue Phil Kampo. (2017) 65:24–5. (in Japanese).
62. Rokot NT, Kairupan TS, Cheng KC, Runtuwene J, Kapantow NH, Amitani M, et al. A role of ginseng and its constituents in the treatment of central nervous system disorders. Evid Based Complement Alternat Med. (2016) 2016:2614742. doi: 10.1155/2016/2614742
63. Zhang G, Liu A, Zhou Y, San X, Jin T, Jin Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol. (2008) 115:441–8. doi: 10.1016/j.jep.2007.10.026
64. Huang Q, Gao B, Jie Q, We BY, Fan J, Zhang HY, et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone (2014) 66:306–14. doi: 10.1016/j.bone.2014.06.010
65. Li J, Xie ZZ, Tang YB, Zhou JG, Guan YY. Ginsenoside-Rd, a purified component from panax notoginseng saponins, prevents atherosclerosis in apoE knockout mice. Eur J Pharmacol. (2011) 652:104–10. doi: 10.1016/j.ejphar.2010.11.017
66. Inui A. Ninjin'yoeito for frailty. In: The 22nd Annual Meeting of Japanese Society of Psychosomatic Internal Medicine. Kagoshima (2017).
67. Bae JS, Park HS, Park JW, Li SH, Chun YS. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med. (2012) 66:476–85. doi: 10.1007/s11418-011-0609-8
68. Fujitsuka N, Asakawa A, Morinaga A, Amitani MS, Amitani H, Katsuura G, et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol Psychiatry (2016) 21:1613–23. doi: 10.1038/mp.2015.220
69. Haruta I, Fuku Y, Kinoshita K, Yoneda K, Morinaga A, Amitani M, et al. One-year intranasal application of growth hormone releasing peptide-2 improves body weight and hypoglycemia in a severely emaciated anorexia nervosa patient. J Cachexia Sarcopenia Muscle (2015) 6:237–41. doi: 10.1002/jcsm.12028
70. Fujita H, Yomoda S, Izumi H, Fukunaga K. Effects of Atractylodis lanceae Rhizoma on the content of ATP in the hippocampus. Phil Kampo (2016) 59:25–7. (in Japanese).
71. Fukunaga K, Izumi H, Hujita H, Yomoda S. Mechanisms of amelioration of symptoms of Alzheimer's disease by Yokukansankachimpihange. Phil Kampo (2016) 60:32–3. (in Japanese).
72. McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. (2017) 217:65–77. doi: 10.1083/jcb.201708092
73. Otabe S, Yuan X, Fukutani T, Wada N, Hashinaga T, Nakayama H, et al. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab. (2007) 293:E210–8. doi: 10.1152/ajpendo.00645.2006
74. Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, Wang Y, et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology (2009) 150:625–33. doi: 10.1210/en.2008-0999
75. Chen Y, Huang X, Chen W, Wang N, Li L. Tenuigenin promotes proliferation and differentiation of hippocampal neural stem cells. Neurochem Res. (2012) 37:771–7. doi: 10.1007/s11064-011-0671-3
76. Lee JY, Kim KY, Shin KY, Won BY, Jung HY, Suh YH. Effects of BT-11 on memory in healthy humans. Neurosci Lett. (2009) 454:111–4. doi: 10.1016/j.neulet.2009.03.024
77. Shin KY, Lee JY, Won BY, Jung HY, Chang KA, Koppula S, et al. BT-11 is effective for enhancing cognitive functions in the elderly humans. Neurosci Lett. (2009) 465:157–9. doi: 10.1016/j.neulet.2009.08.033
78. Kim YJ, Yoo SR, Chae C, Jung UJ, Choi MS. Omija fruit extract improves endurance and energy metabolism by upregulating PGC-1α expression in the skeletal muscle of exercised rats. J Med Food (2014) 17:28–35. doi: 10.1089/jmf.2013.3071
79. Kim MH, Choi YY, Han JM, Lee HS, Hong SB, Lee SG, et al. Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)-α/-β. Food Funct. (2014) 5:1594–601. doi: 10.1039/C4FO00133H
80. Suzuki S, Aihara F, Shibahara M, Sakai K. Special drug use surveillance for Kracie Ninjin'yoeito extract fine granules - a study of safety and efficacy in elderly patients. Jpn J Med Pharm Sci. (2017) 74:1285–97. (in Japanese).
81. Okamoto H, Iyo M, Ueda K, Han C, Hirasaki Y, Namiki T. Yokukan-san: a review of the evidence for use of this Kampo herbal formula in dementia and psychiatric conditions. Neuropsychiatr Dis Treat (2014) 10:1727–42. doi: 10.2147/NDT.S65257
82. Saegusa Y, Hattori T, Nahata M, Yamada C, Takeda H. A new strategy using Rikkunshito to treat anorexia and gastrointestinal dysfunction. Evid Based Complement Alternat Med. (2015) 2015:364260. doi: 10.1155/2015/364260
83. Iwasaki K, Wang Q, Nakagawa T, Suzuki T, Sasaki H. The traditional Chinese medicine banxia houpo tang improves swallowing reflex. Phytomedicine (1999) 6:103–6. doi: 10.1016/S0944-7113(99)80043-9
84. Yagi H, Sato R, Nishio K, Arai G, Soh S. Clinical efficacy and tolerability of two Japanese traditional herbal medicines, Hachimi-jio-gan and Gosha-jinki-gan, for lower urinary tract symptoms with cold sensitivity. J Tradit Complement Med. (2015) 5:258–61. doi: 10.1016/j.jtcme.2015.03.010
85. Ishizuka O, Nishizawa O, Hirao Y, Ohshima S. Evidence-based meta-analysis of pharmacotherapy for benign prostatic hypertrophy. Int J Urol. (2002) 9:607–12. doi: 10.1046/j.1442-2042.2002.00539.x
86. Hoshino N, Hida K, Ganeko R, Sakai Y. Goshajinkigan for reducing chemotherapy-induced peripheral neuropathy: protocol for a systematic review and meta-analysis. Int J Colorectal Dis. (2017) 32:737–40. doi: 10.1007/s00384-016-2727-y
87. Cascella M, Muzio MR. Potential application of the Kampo medicine goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy. J Integr Med. (2017) 15:77–87. doi: 10.1016/S2095-4964(17)60313-3
88. Satoh H. Pharmacological characteristics of Kampo medicine as a mixture of constituents and ingredients. J Integr Med. (2013) 11:11–6. doi: 10.3736/jintegrmed2013003
89. Hijikata Y, Miyamae Y, Takatsu H, Sentoh S. Two kampo medicines, jidabokuippo and hachimijiogan alleviate sprains, bruises and arthritis. Evid Based Complement Alternat Med. (2007) 4:463–7. doi: 10.1093/ecam/nel105
90. Kimata Y, Ogawa K, Okamoto H, Chino A, Namiki T. Efficacy of Japanese traditional (Kampo) medicine for treating chemotherapy-induced peripheral neuropathy: a retrospective case series study. World J Clin Cases (2016) 4:310–7. doi: 10.12998/wjcc.v4.i10.310
91. Burstein SM, Finch CE. Longevity examined: an ancient Greek's very modern views on ageing. Nature (2018) 560:430. doi: 10.1038/d41586-018-05986-1
92. Bellantuono I. Find drugs that delay many diseases of old age. Nature (2018) 554:293–5. doi: 10.1038/d41586-018-01668-0
93. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature (2014) 511:405–7. doi: 10.1038/511405a
94. Miura S, Kawamura I, Yamada A, Kawakita T, Kumazawa Y, Himeno K, et al. Effect of a traditional Chinese herbal medicine ren-shen-yang-rong-tang (Japanese name: ninjin-youei-to) on hematopoietic stem cells in mice. Int J Immunopharmacol. (1989) 11:771–80. doi: 10.1016/0192-0561(89)90131-8
95. Zhou Y, Liu J, Cai S, Liu D, Jiang R, Wang Y. Protective effects of ginsenoside Rg1 on aging Sca-1+ hematopoietic cells. Mol Med Rep. (2015) 12:3621–8. doi: 10.3892/mmr.2015.3884
96. Urabe H, Kojima H, Chan L, Terashima T, Ogawa N, Katagi M, et al. Haematopoietic cells produce BDNF and regulate appetite upon migration to the hypothalamus. Nat Commun. (2013) 4:1526. doi: 10.1038/ncomms2536
97. Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, et al. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. (2010) 88:1890–7. doi: 10.1002/jnr.22362
Keywords: herbal medicine, kampo medicine, ninjin'yoeito, frailty, sarcopenia, appetite loss, aging, ghrelin-neuropeptide Y signals
Citation: Uto NS, Amitani H, Atobe Y, Sameshima Y, Sakaki M, Rokot N, Ataka K, Amitani M and Inui A (2018) Herbal Medicine Ninjin'yoeito in the Treatment of Sarcopenia and Frailty. Front. Nutr. 5:126. doi: 10.3389/fnut.2018.00126
Received: 20 July 2018; Accepted: 26 November 2018;
Published: 12 December 2018.
Edited by:
Lidia Santarpia, University of Naples Federico II, ItalyReviewed by:
Miguel Luiz Batista Júnior, University of Mogi das Cruzes, BrazilCopyright © 2018 Uto, Amitani, Atobe, Sameshima, Sakaki, Rokot, Ataka, Amitani and Inui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akio Inui, aW51aUBtLmt1Zm0ua2Fnb3NoaW1hLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.