- 1Department of Physical Education, Faculty of Education, Brandon University, Brandon, MB, Canada
- 2College of Kinesiology, University of Saskatchewan, Saskatoon, SK, Canada
- 3Faculty of Kinesiology & Health Studies, University of Regina, Regina, SK, Canada
Creatine supplementation during resistance training has potential beneficial effects on properties of bone in aging adults. We systematically reviewed randomized controlled trials (RCTs) investigating the effect of creatine supplementation combined with resistance training on bone mineral density (BMD) in aging adults. We searched PubMed and SPORTDiscus databases and included RCTs of ≥3 months duration that examined the combined effect of creatine and resistance training on bone mineral in adults >50 years of age or postmenopausal. Meta-analyses were performed when applicable trials were available on whole body and clinically important bone sites. Five trials met inclusion criteria with a total of 193 participants. Two of the studies reported significant benefits of creatine supplementation and resistance training compared to resistance training alone on bone. Meta-analyses revealed no greater effect of creatine and resistance training compared to resistance training alone on whole body BMD (MD: 0.00, 95% CI −0.01 to 0.01, p = 0.50), hip BMD (MD −0.01, 95% CI −0.02 to 0.01, p = 0.26), femoral neck BMD (MD 0.00, 95% CI −0.01 to 0.01, p = 0.71), and lumbar spine BMD (MD 0.01, 95% CI −0.01 to 0.03, p = 0.32). In conclusion, there is a limited number of RCTs examining the effects of creatine supplementation and resistance training on BMD in older adults. Our meta-analyses revealed no significant effect on whole body, hip, femoral neck, or lumbar spine BMD when comparing creatine and resistance training to resistance training alone. Future longer term (>12 month) trials with higher resistance training frequencies (≥3 times per week) is warranted.
Introduction
Osteoporosis, characterized by a reduction in bone mineral and bone strength, is a leading cause of age-related disability [1]. Healthcare costs linked to treating osteoporosis are in the billions of dollars [2]; therefore, from a healthy aging and economic perspective, lifestyle interventions which decrease osteoporosis are important. It is well-established that physical activity can have a positive effect on bone health [3]. In a Cochrane systematic review which included forty-three randomized controlled trials (RCTs), aerobic, weight bearing, and resistance exercises were all able to enhance bone mineral density (BMD; [4]); however, the most effective exercise intervention for improving femoral neck (hip) BMD was progressive resistance training [4]. This is important as hip fracture results in disability, loss of functionality, and premature death [5]. While resistance training has a positive effect on bone, results are typically small (1–2% per year; [3, 6]) and may not be clinically significant for aging adults.
Creatine is a non-essential nitrogen-containing compound produced endogenously or can be exogenously consumed from foods such as red meat and seafood [7]. Creatine supplementation may have a favorable effect on aging bone (for reviews see [8, 9]). Mechanistically, creatine increases phosphorylcreatine (PCr) stores in aging muscle [10], enhancing the ability to re-synthesize adenosine triphosphate (ATP) which may lead to increased resistance-training capacity [11–13] and greater muscle mass over time in older adults (for reviews see [14–16]). An increase in muscle mass may result in greater muscle pull and stress on bone during resistance exercise leading to bone accretion [17]. We previously showed that aging males who consumed creatine (~8 g·d−1) during 12 weeks of supervised resistance exercise significantly increased upper limb lean tissue mass that was correlated with changes in upper limb bone mineral content. However, creatine had no effect on upper limb BMD [18].
Creatine may also have direct impact on bone turnover. Bone cells rely on the creatine kinase reaction for resynthesis of adenosine triphosphate from phosphorylcreatine and adenosine diphosphate [19]. The addition of creatine to a low serum cell culture medium increases the metabolic activity and differentiation of osteoblast cells involved in bone formation [20]. Stimulating osteoblast cell activity may enhance osteoprotegerin production, a protein which inhibits osteoclast cell activity and decreases bone resorption [21]. In a randomized double-blind study, creatine supplementation of 9 g·d−1 decreased urinary excretion of cross-linked n-telopeptides of Type I collagen, a marker of bone resorption, by ~3.6% compared to a 26% increase in a group of young healthy men and women during an intense 5-week resistance training program [22]. Furthermore, aging males who consumed creatine (~8 g·d−1) during 10–12 weeks of supervised resistance training experienced a significant decrease in bone resorption (cross-linked n-telopeptides of Type I collagen; [23]). Subsequent evidence of an anti-catabolic bone effect from creatine comes from studies involving special populations. Louis et al. [24] investigated the effects of creatine supplementation (3 g·d−1) without structured exercise training in young boys suffering from Duchenne (n = 12) and Becker (n = 3) muscular dystrophy, a condition which leads to accelerated bone loss. Creatine supplementation decreased the urinary excretion of cross-linked n-telopeptides of Type I collagen by 33% compared to placebo. Using a randomized, double-blind, placebo-controlled, cross-over design, Tarnopolsky et al. [29] investigated the effects of creatine supplementation (0.1 g·kg−1·d−1) in young boys (n = 30) with Duchenne muscular dystrophy for 4 months. Creatine supplementation attenuated the increase in urinary excretion of cross-linked n-telopeptides of Type I collagen by 19%. Results across studies indicate that creatine may have anti-catabolic effects on bone which could lead to net bone accretion over time.
In regards to bone mineral and bone strength, creatine supplementation (2% wet weight) given to growing rats (5 weeks of age; n = 16) for 8 weeks increased lumbar BMD and femur bone strength compared to placebo [30]. However, creatine had no effect on BMD in hypertensive male rats, a representative model of osteoporosis [31]. In postmenopausal women, long-term (1 year) creatine supplementation (~10 g·day−1) during resistance training increased femoral shaft subperiosteal width (indicator of bone strength) and preserved femoral neck (hip) BMD compared to placebo [26]. These results may be clinically significant because the femoral neck is often recognized as the most relevant bone site as there is significant trauma when compromised [32]. In contrast, Tarnopolsky et al. [28] found no effect from creatine supplementation (5 g·d−1) on BMD (whole body, hip, lumbar spine) in healthy older adults after 6 months of structured resistance-exercise training. Furthermore, 1-year of low-dose creatine supplementation (1 g·d−1), without resistance training, had no effect on bone health parameters in osteopenic postmenopausal women [33].
Results across studies are mixed as to whether creatine supplementation is effective for increasing bone health in older adults. Variability in bone mineral is typically high in older adults making it difficult to obtain adequate statistical power to detect differences with creatine supplementation in many individual studies. Performing a meta-analysis helps overcome these limitations by assessing large numbers of individuals simultaneously. We therefore performed a meta-analysis to assess the effect of creatine combined with resistance training compared to resistance training alone on BMD in aging adults.
Methods Used to Search, Select, Extract, and Analyze the Data
Our inclusion criteria included (i) Male participants of >50 years of age or postmenopausal females, because this is the age at which BMD starts to precipitously decrease, (ii) an intervention of creatine monohydrate with resistance training, (iii) resistance training with a placebo as a comparator, (iv) measures of whole body BMD or clinically relevant bone sites (i.e., hip, spine, femoral neck, forearm BMD), and (v) RCT design with a minimum of 12 weeks in duration. We searched PubMed and SPORTDiscus databases using the key words “creatine supplementation,” “bone,” and a variety of synonyms for “resistance training” (e.g., “strength training,” “exercise”) on August 27, 2017. Searches were limited to meta-analyses, systematic reviews or RCTs with no date restriction. There were no language restrictions. Bibliographies were also reviewed. Full-text articles selected for inclusion had the following information extracted: authors, country where the study was done, date of publication, information on specific inclusion and exclusion criteria, sample size, proportion of total sample that was female and male, proportion of the total sample completing the study, mean age, details provided about the exercise intervention (frequency, intensity, time, type), duration of study, creatine dose, and administration protocol, intervention and control adherence, adverse outcomes (i.e., nausea, bloating, diarrhea, which may be associated with the experimental protocol) and primary (i.e., whole body BMD and BMD of clinically relevant sites) and secondary outcomes (included other bone health markers as well as muscle mass and strength changes). These secondary outcomes may be associated with improved bone strength.
Means and standard deviations for baseline and post-training measurements were extracted from each study for estimation of mean changes and the standard deviation of mean changes across the interventions. Change scores were calculated as pre-training mean subtracted from post-training mean. Standard deviations (SD) for the change scores were estimated from pre- and post-training standard deviations (SDpre and SDpost) using the following equation derived from the Cochrane Handbook for Systematic Reviews of Interventions [34]:
SD change score = [(SDpre)2 + (SDpost)2 – 2 × (correlation between pre- and post-scores) × SDpre × SDpost]1/2
In this equation we used 0.8 as the assumed correlation between pre- and post-scores.
Heterogeneity was evaluated using χ2 and I2 tests where heterogeneity was indicated by either χ2 p-value equal or <0.1 or I2 test value >75%. When heterogeneity was present we used a random effects model and when heterogeneity was not present we used a fixed-effects model for our meta-analysis. Mean changes and standard deviations for mean changes for individual studies and the pooled effects and their 95% confidence intervals were calculated and Forest plots were generated using Review Manager 5.3 Software. A meta-analysis was done if 3 or more studies examined the same outcome measure. Significance was set at p ≤ 0.05. Risk of bias was also assessed on criteria derived from the Cochrane Handbook for Systematic Reviews of Interventions [34], including random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment or personnel, incomplete outcome data, and selective reporting.
Results
Study Characteristics
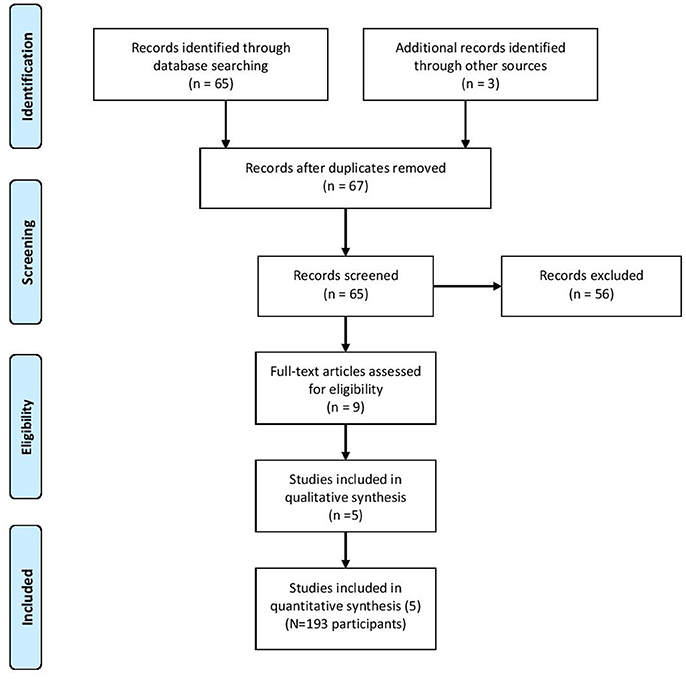
Sixty-seven citations, excluding duplicate entries, were identified as potentially relevant. After the initial screening of title and abstracts, nine full-text articles were retrieved for detailed review (Figure 1). Following full text review, 5 trials met the inclusion criteria with a total of 193 participants. Among these studies there was a large amount of heterogeneity with regards to the duration of the intervention (12 weeks to 1 year), frequency of exercise per muscle per week (1.5–3 times per week), and participant characteristics (Table 1). Two studies reported beneficial effects on at least one marker of bone biology in the creatine plus resistance training group compared to resistance training and placebo [18, 26], while the other 3 reported no effects [25, 27, 28].
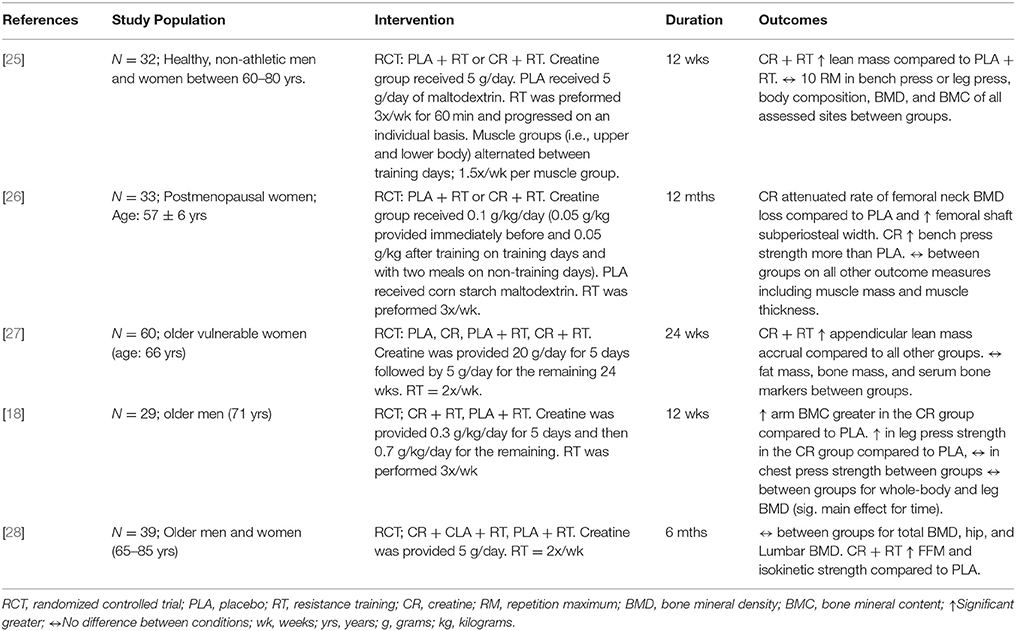
Table 1. Study characteristics and outcomes of research examining the influence of creatine with a resistance training program on bone.
Compliance
All studies reporting on compliance were comparable between the creatine and placebo conditions [18, 25–27]. Average compliance to the exercise interventions ranged from 75 to 95.3% [18, 25–27]. Only one study did not report compliance, however, compliance was monitored [28]. In addition, only one study reported compliance to the supplement and again was comparable between groups (CR = 79% and PLA = 78%; [26]).
Adverse Events
Four studies [18, 25, 27, 28] reported no adverse effects of the experimental intervention. Gualanao et al. [27] further assessed clinical renal and hepatic blood markers and found no effect on urea, creatinine, or creatine kinase. Chilibeck et al. [26] was the only study to report adverse effects that were considered “possibly” or “probably” related to creatine supplementation. Seven participants reported adverse events compared to only 4 in the placebo group. Five participants reported mild gastrointestinal adverse events (i.e., constipation, diarrhea, heartburn, and irritable bowel, and nausea) and two reported muscle cramps (mild to moderate). When both gastrointestinal and musculoskeletal adverse events were grouped, the creatine group had a significantly (p < 0.05) higher number of events compared to placebo. Of note, there were no serious adverse events reported from creatine supplementation.
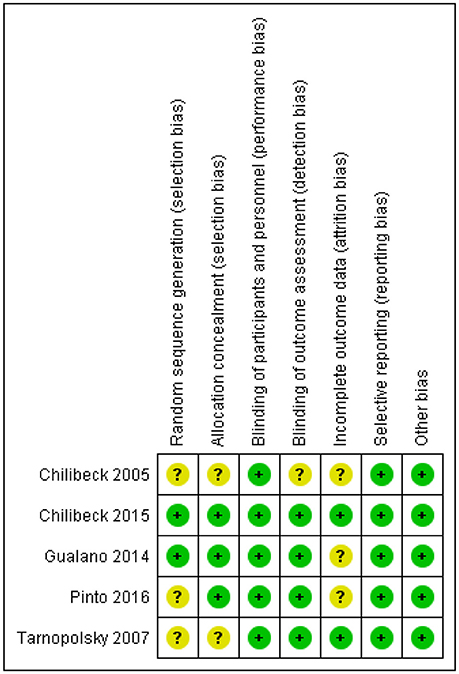
Risk of Bias
One study had a rigorous experimental design and was considered low risk [26]. Four trials [18, 25, 27, 28] were either unclear or did not report on various other potential biases and were considered to be moderate risk (Figure 2).
Meta-Analyses
Mean changes and standard deviations for mean changes for individual studies and pooled effects and their 95% confidence intervals are presented along with Forest plots in Figures 3A–D. There was no greater effect from creatine supplementation during resistance training compared to resistance training and placebo for whole body (p = 0.50), lumbar spine (p = 0.32), hip (p = 0.26), and femoral neck (p = 0.71) BMD. No studies reported changes in forearm BMD.
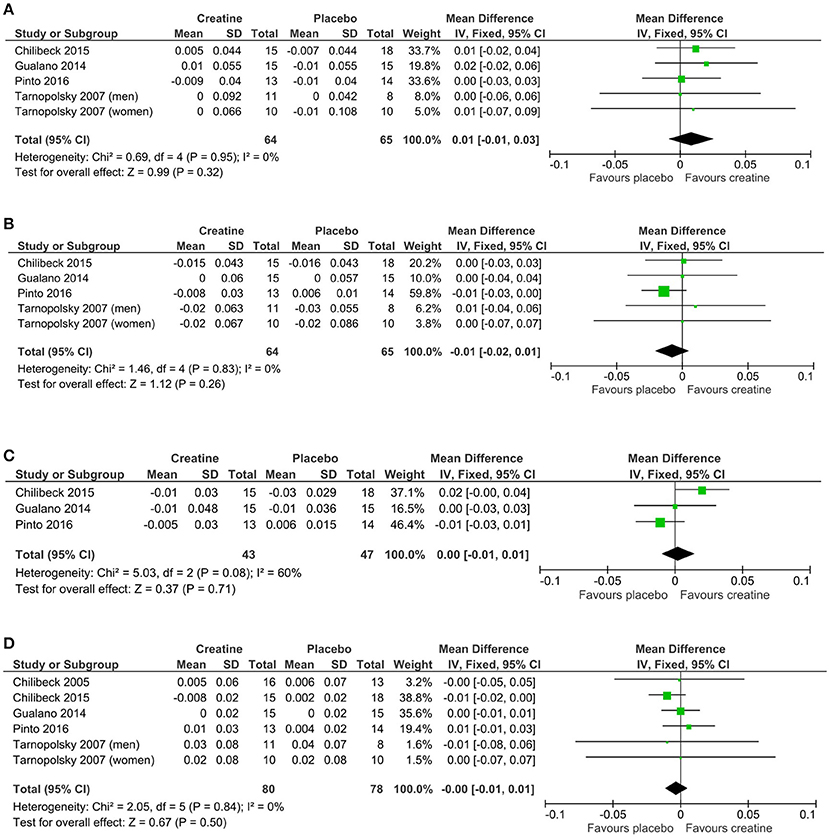
Figure 3. Meta-analyses of creatine and resistance training studies on (A) lumbar spine, (B) hip, (C) femoral neck, and (D) whole body bone mineral density.
Discussion
There is evidence from individual studies that creatine supplementation and resistance training increase properties of aging bone (Table 1), however, our meta-analysis failed to show a greater effect from creatine on BMD compared to placebo. The five studies included in our review had relatively low sample sizes and therefore lacked statistical power individually. Performing a meta-analysis enabled us to determine with greater statistical power whether creatine has a beneficial effect on BMD in aging adults. Despite the greater statistical power, our meta-analysis revealed that creatine in combination with resistance training provided no evidence of benefit on whole body, lumbar spine, hip, or femoral neck BMD compared to resistance training alone.
Mechanistically, creatine supplementation can potentially influence bone turnover both indirectly and directly. Indirectly, creatine can enhance muscle mass and strength adaptations following resistance training [16] and thus increase the pull on bone [18]. In aging males (65–71 years, n = 13–23), creatine supplementation (8 g·d−1) during 12 weeks of resistance training (3 days/week) increased upper limb bone mineral content [18]. These changes in arm bone mineral content were significantly correlated with changes in arm lean tissue mass [18]. With respect to the current meta-analysis, all of the included studies found either an enhanced muscle mass [25, 27, 28] or strength [18, 26, 28] in the creatine group compared to placebo. However, based on the meta-analysis, these adaptations did not translate to greater BMD. Creatine may also have direct impact on bone turnover. Bone cells rely on adenosine triphosphate rephosphorylation via the creatine kinase reaction [19]. Creatine added to a low serum culture medium increased metabolic activity and differentiation of osteoblasts cells [20], which inhibit osteoclast activity and decrease bone resorption [21]. In human studies, urinary excretion of cross-linked n-telopeptides of Type I collagen decreased following creatine consumption [22, 23], suggesting possible anti-catabolic bone effects. However, these biological plausible mechanisms did not result in improved BMD.
In regards to bone mineral and strength there is evidence from individual studies suggesting a possible beneficial effect. Creatine supplementation (10 g·d−1) during 12 months of resistance training (3 days/week) decreased femoral neck bone loss and increased femoral shaft subperiosteal width (indicator of bone strength) in postmenopausal women compared to placebo [26]. In aging males (65–71 years, n = 13–23), creatine supplementation (8 g·d−1) during 10–12 weeks of whole-body resistance-exercise training (3 days/week) increased upper limb bone mineral content compared to older men on placebo during training [18]. Three studies utilized absolute dosing (5g/d; [25, 27, 28]) while two studies utilized relative dosing (0.1g/kg/d; [18, 26]). Both studies using relative dosing found significant effects of creatine on bone mineral. Another methodological difference between studies finding a positive effect compared to no effect of creatine was frequency of training (3 days vs. 1.5–2 days/week/muscle group). Studies utilizing higher frequency of training found positive effects. Future research is required to directly compare higher and lower frequencies with and without creatinine. Other methodological differences included participants' characteristics (osteopenic, postmenopausal, older healthy adults) which may have impacted the findings of the meta-analysis. Lastly, study duration ranged from 12 to 52 weeks. A potential limitation of our inclusion criteria was including studies of 3 months duration. Chilibeck et al. [18], found an effect of creatine and resistance training on bone mineral content after 3 months; however, bone turnover is a relatively slow process and may require 9 months to detect robust changes [26, 35]. The only study investigating BMD in older adults with creatine and resistance over 9 months found positive effects on bone compared to placebo [26]. These methodological differences may have masked the potential effect of creatine on bone mineral in older adults. As such, future research is warranted to further elucidate the optimal dose, training frequency, and study duration.
A potential limitation of the present meta-analysis is the high risk of bias (Figure 2). Only one study demonstrated a low risk of bias [18]. These biases may have led to the equivocal findings. Future research utilizing rigorous methodology is recommended.
Conclusion
There is a limited number of RCTs examining the effects of creatine supplementation and resistance training on bone in older adults. Our meta-analyses revealed no effect on whole body, hip, femoral neck, or lumbar spine BMD when comparing creatine and resistance training compared to resistance training alone. Interestingly, only the studies which used a resistance training frequency of 3 times per muscle group per week in combination with a relative dosing of creatine supplementation found a beneficial effect compared to resistance training alone. Future longer term studies (>12 months) with rigorous methodology utilizing a higher training frequency with and without creatine may be warranted.
Author Contributions
SF, DC, PC: contributed to the conception, design, analysis, interpretation of the work, as well as drafted, revised, and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tarride JE, Guo N, Hopkins R, Leslie WD, Morin S, Adachi JD, et al. The burden of illness of osteoporosis in Canadian men. J Bone Miner Res. (2012) 27:1830–8. doi: 10.1002/jbmr.1615
2. Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int. (2016) 27:3023–32. doi: 10.1007/s00198-016-3631-6
3. Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. (2002) 3:CD000333. doi: 10.1002/14651858.CD000333
4. Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. (2011) 7:CD000333. doi: 10.1002/14651858.CD000333.pub2
5. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. (2005) 16(Suppl 2):S3–7. doi: 10.1007/s00198-004-1702-6
6. Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. (2009) 43:898–908. doi: 10.1136/bjsm.2008.052704
7. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. (2000) 80:1107–213. doi: 10.1152/physrev.2000.80.3.1107
8. Candow DG, Chilibeck PD. Timing of creatine or protein supplementation and resistance training in the elderly. Appl Physiol Nutr Metab. (2008) 33:184–90. doi: 10.1139/H07-139
9. Gualano B, Rawson ES, Candow DG, Chilibeck PD. Creatine supplementation in the aging population: effects on skeletal muscle, bone and brain. Amino Acids (2016) 48:1793–805. doi: 10.1007/s00726-016-2239-7
10. Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci. (2003) 58:11–9. doi: 10.1093/gerona/58.1.B11
11. Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. (2001) 33:2111–7. doi: 10.1097/00005768-200112000-00021
12. Gotshalk LA, Kraemer WJ, Mendonca MA, Vingren JL, Kenny AM, Spiering BA, et al. Creatine supplementation improves muscular performance in older women. Eur J Appl Physiol. (2008) 102:223–31. doi: 10.1007/s00421-007-0580-y
13. Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ. Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc. (2002) 34:537–43. doi: 10.1097/00005768-200203000-00023
14. Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Endocrine (2014) 45:354–61. doi: 10.1007/s12020-013-0070-4
15. Devries MC, Phillips SM. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc. (2014) 46:1194–203. doi: 10.1249/MSS.0000000000000220
16. Chilibeck PD, Mojtaba K, Candow DG, Zello ZA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J Sports Med. (2017) 8:213–24. doi: 10.2147/OAJSM.S123529
17. Chilibeck PD, Sale DG, Webber CE. Exercise and bone mineral density. Sports Med. (1995) 19:103–22. doi: 10.2165/00007256-199519020-00003
18. Chilibeck PD, Chrusch MJ, Chad KE, Shawn Davison K, Burke DG. Creatine monohydrate and resistance training increase bone mineral content and density in older men. J Nutr Health Aging (2005) 9:352–3.
19. Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem. (1994) 133–4:193–220. doi: 10.1007/BF01267955
20. Gerber I, Gwynn I, Alini M, Wallimann T. Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater. (2005) 10:8–22. doi: 10.22203/eCM.v010a02
22. Cornish SM, Candow DG, Jantz NT, Chilibeck PD, Little JP, Forbes S, et al. Conjugated linoleic acid combined with creatine monohydrate and whey protein supplementation during strength training. Int J Sport Nutr Exerc Metab. (2009) 19:79–96. doi: 10.1123/ijsnem.19.1.79
23. Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, et al. Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc. (2008) 40:1645–52. doi: 10.1249/MSS.0b013e318176b310
24. Louis M, Lebacq J, Poortmans JR, Belpaire-Dethiou MC, Devogelaer JP, Van Hecke P, et al. Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve (2003) 27:604–10. doi: 10.1002/mus.10355
25. Pinto CL, Botelho PB, Carneiro JA, Mota JF. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J Cachexia Sarcopenia Muscle (2016) 7:413–21. doi: 10.1002/jcsm.12094
26. Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus-Jenssen L. Effects of creatine and resistance training on bone health in postmenopausal women. Med Sci Sports Exerc. (2015) 47:1587–95. doi: 10.1249/MSS.0000000000000571
27. Gualano B, Macedo AR, Alves CR, Roschel H, Benatti FB, Takayama L, et al. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Exp Gerontol. (2014) 53:7–15. doi: 10.1016/j.exger.2014.02.003
28. Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS ONE (2007) 2:e991. doi: 10.1371/journal.pone.0000991
29. Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology (2004) 62:1771–7. doi: 10.1212/01.WNL.0000125178.18862.9D
30. Antolic A, Roy BD, Tarnopolsky MA, Zernicke RF, Wohl GR, Shaughnessy SG, et al. Creatine monohydrate increases bone mineral density in young Sprague-Dawley rats. Med Sci Sports Exerc. (2007) 39:816–20. doi: 10.1249/mss.0b013e318031fac4
31. Alves CR, Murai IH, Ramona P, Nicastro H, Takayama L, Guimarães F, et al. Influence of creatine supplementation on bone mass of spontaneously hypertensive rats. Rev Bras Reumatol. (2012) 52:453–61. doi: 10.1590/S0482-50042012000300015
32. Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ (2010) 182:1864–73. doi: 10.1503/cmaj.100771
33. Lobo DM, Tritto AC, da Silva LR, de Oliveira PB, Benatti FB, Roschel H, et al. Effects of long-term low-dose dietary creatine supplementation in older women. Exp Gerontol. (2015) 70:97–104. doi: 10.1016/j.exger.2015.07.012
34. Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration (2011). Available online at: http://handbook-5-1.cochrane.org/
Keywords: supplements, creatine, strength, bone, health
Citation: Forbes SC, Chilibeck PD and Candow DG (2018) Creatine Supplementation During Resistance Training Does Not Lead to Greater Bone Mineral Density in Older Humans: A Brief Meta-Analysis. Front. Nutr. 5:27. doi: 10.3389/fnut.2018.00027
Received: 16 November 2017; Accepted: 06 April 2018;
Published: 24 April 2018.
Edited by:
Daniel Moore, University of Toronto, CanadaReviewed by:
Leonidas G. Karagounis, Nestlé Research Center, SwitzerlandEimear Dolan, Universidade de São Paulo, Brazil
Andrea R. Josse, Brock University, Canada
Copyright © 2018 Forbes, Chilibeck and Candow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott C. Forbes, Zm9yYmVzc0BicmFuZG9udS5jYQ==
 Scott C. Forbes
Scott C. Forbes Philip D. Chilibeck2
Philip D. Chilibeck2 Darren G. Candow
Darren G. Candow