
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 03 January 2017
Sec. Gastrointestinal Sciences
Volume 3 - 2016 | https://doi.org/10.3389/fnut.2016.00057
Proanthocyanidins (PACs) are polymers of flavan-3-ols abundant in many vegetable foods and beverages widely consumed in the human diet. There is increasing evidence supporting the beneficial impact of dietary PACs in the prevention and nutritional management of non-communicable chronic diseases. It is considered that PACs with a degree of polymerization >3 remain unabsorbed in the gastrointestinal (GI) tract and accumulate in the colonic lumen. Accordingly, the GI tract may be considered as a key organ for the healthy-promoting effects of dietary PACs. PACs form non-specific complexes with salivary proteins in mouth, originating the sensation of astringency, and with dietary proteins, pancreatic enzymes, and nutrient transporters in the intestinal lumen, decreasing the digestion and absorption of carbohydrates, proteins, and lipids. They also exert antimicrobial activities, interfering with cariogenic or ulcerogenic pathogens in the mouth (Streptococcus mutans) and stomach (Helicobacter pylori), respectively. Through their antioxidant and antiinflammatory properties, PACs decrease inflammatory processes in animal model of gastric and colonic inflammation. Interestingly, they exert prebiotic activities, stimulating the growth of Lactobacillus spp. and Bifidobacterium spp. as well as some butyrate-producing bacteria in the colon. Finally, PACs are also metabolized by the gut microbiota, producing metabolites, mainly aromatic acids and valerolactones, which accumulate in the colon and/or are absorbed into the bloodstream. Accordingly, these compounds could display biological activities on the colonic epithelium or in extra-intestinal tissues and, therefore, contribute to part of the beneficial effects of dietary PACs.
Polyphenols are secondary metabolites synthesized by plants, which are implicated in their protection against microbial pathogens, predators, ultraviolet radiation, and adverse conditions of nutrition and growth (1, 2). They are classified as non-flavonoids and flavonoids, being these latter the major group of phytochemicals present in the human diet. The flavonoid chemical structure is characterized by two aromatic rings connected by a three-carbon bridge (C6–C3–C6) (3, 4). The main subclasses of flavonoids are flavones, flavonols, flavan-3-ols, isoflavones, flavanones, and anthocyanidins (3). The flavan-3-ols (also called flavanols) are the most complex as they include not only simple monomers but also oligomeric and polymeric proanthocyanidins (PACs), known as condensed tannins (3). Accordingly, PAC size is variable and depends on their degree of polymerization (DP), i.e., the amount of monomers of flavan-3-ols incorporated in the molecule. The DP commonly varies between 3 and 11 but can even reach up 50 units or more. For example, the average DP of black gooseberry (Ribes nigrum) PACs is about 48, whereas in wine and beer PACs, it is only 7 and 2, respectively (3, 5). PACs are abundant in many foods and beverages like seeds, barks, fruits, red wine, cider, tea, cocoa, and beer, where they contribute to their bitter taste and astringency (4, 5). Depending on the units of flavanols involved in their structure, PACs are subdivided in different classes. The most abundant consist exclusively of (epi)catechin units and are called procyanidins (PCs), while the less common containing (epi)afzelechin or (epi)gallocatechin subunits are named propelargonidins and prodelphinidins, respectively (3, 4, 6, 7) (Figure 1). PACs may also be classified as type-A or -B according to the interflavanol linkage; type-B PACs are found in a greater abundance and have only one C–C interflavan bond, while type-A are less common and are characterized by an additional ether linkage (7) (Figure 2). Type-B PACs are present in fruits (apples, grapes, pears), legumes, cereals (barley, sorghum), cocoa, and their derived foodstuffs (wine, cider, beer, etc.), while A-type are found in cranberry, cinnamon, apricots, and avocado, among others; some foodstuffs have mixed type-A and -B PACs (7–10).
The beneficial impact of dietary PACs on the risk of cancer (5, 11, 12), cardiovascular diseases (5, 13, 14), and diabetes (12, 15, 16) is supported by a large number of in vitro, animal, clinical, and epidemiological studies. These health-promoting effects suggest that PACs may be absorbed by the intestinal mucosa. However, evidence indicates that only monomers, dimers and, eventually, trimers of flavan-3-ols are absorbable, while oligomers and polymers of higher DP remain unabsorbed and accumulate in the gut lumen. Non-absorbed PACs reach the colon where they are metabolized by the microbiota, producing low molecular weight (LMW) compounds (4, 7, 17–19) that may be absorbed into the circulation. Therefore, the beneficial effect of PACs on human health may be attributed not only to the circulating monomers or dimers of flavanols but also to the microbiota-derived PAC metabolites present in the bloodstream (7). Additionally, PACs and/or their bacterial metabolites may exert health benefits directly in the gastrointestinal (GI) tract through their antioxidant, antiinflammatory, antibacterial, and antiproliferative properties (12).
The present review describes how dietary PACs impact the main physiological processes occurring in the GI tract and exerts protective properties in pathological conditions (Figure 3). More particularly, it addresses the fate of PACs in the different compartments of the GI tract, describing their absorption and metabolism, and their ability to interfere with the processes of digestion and absorption of macronutrients in the intestine and to modulate digestive hormone secretion, hydroelectrolytic epithelial transport, and GI motility. In parallel, this review also describes the protective effects displayed by dietary PACs including the attenuation of enteropathogen deleterious activities and gastric and colonic inflammatory processes. Finally, it addresses their prebiotic effect in the colon, their transformation by the microbiota, and their role in decreasing the risk of colorectal cancer.
Dietary PACs have been shown to bind and precipitate salivary proteins containing high-proline contents, a phenomenon that constitutes the physiologic base of astringency perception after consuming PAC-containing foodstuffs (20). Catechins were detected in saliva for up to 60 min after using a mouth rinsing product containing green tea extract (5 mg/ml) (21); the persistence of these compounds in saliva could favor their antimicrobial activity against some oral bacteria. More particularly, cranberry PACs have been shown to inhibit the adhesion of the cariogenic bacteria Streptococcus mutans to oral epithelial cells, thus preventing the formation of pathogen biofilm in the tooth surface (22, 23). On the other hand, PACs containing galloyl moieties have been recently proposed as a useful tool in restorative and reparative dentistry, due to their ability to act as dentin biomodifiers (24).
Helicobacter pylori is a helix-shaped, microaerophilic, Gram-negative, flagellated bacterium that specifically colonizes the human gastric mucosa. It is considered as the most widespread chronic bacterial pathogen in the world, infecting more than 50% of the human population. Although over 80% of the infected individuals remain asymptomatic, H. pylori is implicated as an etiologic factor in the development of a variety of GI diseases including gastroduodenal ulcers and gastric adenocarcinoma and lymphoma. Accordingly, this pathogen is classified as a class I carcinogen (25–27). H. pylori expresses several virulence factors involved in the initial colonization of the gastric mucosa by the bacteria and in its persistence in the stomach. These factors include (1) adhesins, bacterial surface proteins allowing the adherence of this agent to the gastric epithelial cells, (2) an urease activity that releases ammonia from urea hydrolysis, allowing proton neutralization and the survival of the bacteria in the acidic gastric environment, (3) the vacuolating cytotoxin A (VacA) that alters the mitochondrial function of the epithelial cells and promotes their vacuolization and subsequent apoptosis, in addition to influencing host tolerance by suppressing T cell activation, and (4) the cag pathogenicity island encoding a type IV secretion system, which is involved in the development of inflammatory processes in the mucosa (27–29).
The administration of PACs-rich beverages decreases H. pylori colonization in humans. A prospective, randomized, double-blind, placebo-controlled trial conducted in China in 189 adult patients colonized by H. pylori provided some clinical support for the anti-H. pylori effect of cranberry juice that contains large amounts of A-type PACs (30, 31). The participants were assigned to receive 250 ml of cranberry juice or placebo twice daily for 90 days. H. pylori colonization was determined by 13C-urea breath test after 35 and 90 days of treatment. The eradication of the pathogen was reported in 14.4% of the treated subjects compared with 5.3% in the placebo group. Similar results were reported in a clinical trial carried out in 295 Chilean children colonized by H. pylori. The intake of 200 ml cranberry juice per day for 3 weeks induced the eradication of the pathogen in 16.9% of the treated children vs 1.5% in the placebo group. Interestingly, the coadministration of cranberry juice with the probiotic, L. johnsonii NCC533, eradicated the bacteria in 22.9% of the children (32). The mechanisms associated with the anti-H. pylori activity of cranberry PACs include their ability to inhibit urease activity and prevent bacterial adhesion as well as VacA-induced mucosal damage (Table 1). High molecular mass, non-dialyzable constituents of cranberry juice, subsequently characterized as PACs, have been shown to inhibit the adhesion of clinically isolated H. pylori strains to AGS gastric cell line. As no cross-resistance was detected between the non-dialyzable material and metronidazole, it was suggested that cranberry preparations could improve H. pylori eradication in untreated patients as well as in those under pharmacological treatment (33). In vitro studies consistently indicate that high molecular weight (HMW) components from cranberry inhibit the sialyllactose-specific (S fimbriae) adhesion of H. pylori to immobilized human mucus, erythrocytes, and cultured gastric epithelial cells (34, 35). This anti-adhesion effect was not restricted to cranberry PACs and was also reported with dietary PACs from different sources. Pycnogenol®, a standardized concentrate elaborated from French maritime pine bark and which contains PAC from dimers (mainly type B) to polymers (up to 12 monomeric units of flavanol) (36, 37), inhibited concentration-dependently the adhesion of clinical strains of H. pylori to AGS cells (38). A root extract from Pelargonium sidoides, rich in polymeric PACs (39), has also been shown to prevent H. pylori adhesion to intact human stomach tissue (40–42).
Proanthocyanidins can also interfere with H. pylori by inhibiting its urease activity and/or by inactivating VacA. For example, a methanol extract of Eucalyptus grandis (Myrtaceae) bark was shown to inhibit the urease of clinical strains of H. pylori in a concentration-dependent manner. This effect was attributed to the tannins and triterpene saponins present in the extract (43). Some studies suggest that PAC DP is a determining factor for urease inhibition or their protective effects on VacA-induced mucosal damage. LMW PAC fraction (mean DP of 3) derived from apple peel exhibited an urease inhibition fourfold lower than the high HMW fraction (mean DP of 9.5) (44). More recently, an aqueous extract of Peumus boldus Mol. (Monimiaceae) rich in catechin-derived PCs B3 and C2 was also shown to inhibit H. pylori urease (45). The separation of the extract components according to their molecular weight revealed that the higher the DP, the greater the urease inhibition. Interestingly, the catechin-derived PCs (B3 and C2) were more effective than the epicatechin-derived PCs B2 and C1 in inhibiting the urease, suggesting that not only DP is important but also the chemical nature of the monomer bound to C-4 in the PC structure (45). A structure–activity relationship between PAC DP and their ability to inactivate VacA cytotoxin has also been established with hops extracts (46). Accordingly, the administration of red wine or green tea mixture to H. pylori-infected mice significantly prevented the development of gastritis, limiting the localization of the pathogen and VacA toxin on the surface of the gastric epithelium (47).
On the other hand, exciting advances in the knowledge of the interactions between PCs and target molecules are emerging from molecular docking studies. Such studies, for example, revealed that B-type PCs (catechin dimers) inhibit urease because, according to their docking scores (−6.9 kcal/mol), they fit the binding pocket of the bacterial enzyme, being these interactions energetically favorable (48). Then, molecular docking could contribute to elucidate the interactions between PCs and other cellular or bacterial targets of interest for the protection of the gastric mucosa against H. pylori.
The gastroprotective properties of PAC extracts have been widely studied using different animal models of gastric inflammation induced by ethanol, non-steroidal antiinflammatory drugs (NSAIDs), pylorus ligature, or restrain stress (Table 2). Synthetic PAC oligomers, more particularly those bigger than tetramers, protected the gastric mucosa against ethanol-induced damage, due to their ability to scavenge free radicals and, therefore, prevented the appearance of oxidative damage (49). Such effect could also be due to the protein-binding ability of the oligomers, which would allow them to form a protective layer coating the gastric mucosa. In another study, the administration of a single oral dose of Hawthorn berries extract (a mixture of Crataegus monogyna and C. oxyacantha containing 0.44% PACs) attenuated the intensity of ethanol-induced gastric lesions in rats, an effect similar to that observed with the administration of ranitidine (50). Considering the low proportion of PACs in this extract, it is improbable that these molecules were responsible for this protective effect; however, it is important to mention that Hawthorn berries contain oligomeric PACs and more B-type than A-type (51).
Proanthocyanidins were also protective in gastric damage induced by NSAIDs. Although NSAIDs are widely used for their antipyretic, analgesic, and antiinflammatory properties, their administration is frequently associated with adverse effects mainly affecting the GI mucosa (52, 53). NSAIDs and proton pump inhibitors (like omeprazole and lansoprazole) are frequently coprescribed to minimize NSAID-related adverse effects and the enteropathy induced by such combination, though common, is often clinically silent. Thus, the lesions induced by these drugs in the GI tract could be of considerable clinical importance. Accordingly, bioactive compounds, like PACs, arise as an alternative approach for the management of the adverse effects associated with NSAID therapies (53). The oral intake of PAC extracts from Guazuma ulmifolia (Sterculiaceae) or grape seed for 2–6 days before diclofenac or indomethacin administration in rats, prevented in a dose-dependent manner the development of gastric mucosal damage and attenuated intestinal injury (54–56). These extracts decreased the area of ulceration induced by indomethacin in the stomach by decreasing lipid peroxidation in the mucosa and by increasing the superoxide dismutase and glutathione peroxidase activities as well as the glutathione levels (54). The G. ulmifolia extract was also shown to prevent neutrophil infiltration (reflected by a lower myeloperoxidase activity) in the mucosa (54).
Proanthocyanidin extracts from medicinal plants including Mangaba (Hancornia speciosa) (containing LMW PACs), Curatella americana L. (Dilleneaceae) (containing oligomeric and polymeric PACs), Cecropia glazioui Sneth (Cecropiaceae) (that contains 22% of PACs B2, B3, B5, and C1), and Byrsonima intermedia (containing oligomeric PACs, phenolic acids, and catechin derivatives and flavonoids) have also been evaluated (57–61). The oral administration of a single dose of each extract decreased the gastric lesions induced by indomethacin or piroxicam, hypothermic restraint stress, ethanol, or pylorus ligature, in the same extent that cimetidine, ranitidine, or lansoprazole (57, 58, 60, 61). Interestingly, the extraction step seems to be critical to determine the antiulcer effects of the bioactive compounds; no significant protective effects were observed when plant infusions were used (57, 58).
Another mechanism by which PACs may protect the gastric mucosa is through the modulation of HCl secretion. Plant extracts decreased the secretion of HCl by gastric parietal cells when administered intraduodenally to pylorus-ligated mice (57, 58, 60, 61). The C. glazioui extract reversed the histamine or bethanechol-induced acid secretion to basal values, indicating it inhibits the proton pump. This antisecretory effect was comparable to that observed with the histamine H2 receptor-antagonist, ranitidine (60). It is possible that this effect relies on the type-B2 PACs since these molecules displayed the highest inhibitory activity against the gastric H+, K+-ATPase in vitro, compared with the other type of PACs isolated from C. glazioui (like B3, B5, and C1) (60).
The PAC antiulcer properties are also related to their ability to stimulate mucus synthesis and secretion (62), and to their mucosal repair activity. Indeed, they were shown to accelerate the healing of gastric ulcer induced by acetic acid administration (57, 58, 61). It is probable that nitric oxide synthase (NOS), sulfhydryl compounds (SH), and TRPV-vanilloid receptors were involved in this phenomenon, as pretreatment with NOS inhibitor (L-NAME), SH-blocker (NEM), or TRPV receptor inhibitor (ruthenium red) blocked the PACs protection against ethanol-induced gastric damage (57, 58, 61). The increased expression of key molecules implicated in the restitution of the gastric epithelium during chronic gastroduodenal ulcers arises as another mechanism of protection exerted by PACs. For example, a sea buckthorn extract containing 96.5% PACs was shown to decrease the ulcer index in acetic acid-induced gastric lesions, in association with increased plasma concentrations of epidermal growth factor (EGF) and higher expression of EGF receptor and proliferating cell nuclear antigen in the gastric mucosa (63). These molecules are considered as crucial for the ulcer healing process and are implicated in epithelial restitution and gland reconstruction. The effect of PACs on trefoil peptides, also involved in the epithelial restitution process, has not been studied.
On the other hand, PACs could also exert their protective effects through endocrine and neural mechanisms. A C. americana extract, for example, exhibited ulcer healing properties by increasing the mucosal levels of prostaglandin E2 and somatostatin and decreasing those of gastrin (58). Gravinol S containing 89.3% PAC (25.2% dimers–pentamers, 74.8% oligomers) from grape seeds, when administered ad libitum for 2 weeks, prevented gastric mucosal damage induced by water immersion restraint stress in rats. The authors proposed that PACs inhibit gastrin secretion by G cells and subsequently that of histamine and somatostatin, in addition to increase prostaglandin levels and superoxide dismutase activity in the gastric mucosa (64). Using the same animal model, a single dose of Viburnum opulus (Caprifoliaceae) PAC extract attenuated the gastroduodenal lesions. This effect was abrogated by capsaicin pretreatment, suggesting the implication of the nervous system. Moreover, the administration of this extract was also associated with the stimulation of the nitric oxide system, the increased resistance of the mucus layer, and the stimulation of mucosal superoxide dismutase and catalase activities (65).
A few studies have evaluated the impact of PAC intake on the release of digestive hormones, some of them in relation to GI motility. González-Abuín et al. showed that rats fed a cafeteria diet exhibited a lower density of enteroendocrine cells in the intestinal epithelium and plasma concentrations of active glucagon-like peptide-1 (GLP-1), and that these alterations were prevented with a grape seed PC extract (66). Since GLP-1 is an incretin hormone participating in the regulation of food intake and insulin secretion, it is possible that dietary PACs are beneficial for the control of energetic metabolism in humans. There is no doubt that it is a promising field of research. Serrano et al. showed that the administration of a grape seed proanthocyanidin extract (423 mg phenolics/kg body weight) to rats increased the portal concentrations of active GLP-1 and ghrelin and decreased those of cholecystokinin. These findings were accompanied by a delayed gastric emptying and lower food intake in the treated animals (67). In another study, Ko et al. evaluated the effect of a whole grape juice (with skin and seeds) in rats treated with cisplatin, a chemotherapeutic drug known to provoke acute GI disorders. Cisplatin treatment decreased significantly the rate of gastric emptying compared with the control group, and pretreatment with grape juice (10 ml/kg body weight) prevented this disturbance (68).
Miller et al. evaluated the effect of an extract of Croton palanostigma (100 mg/g of PACs) in the treatment of emesis induced by the administration of morphine-6-glucuronide in adult ferrets. The extract reduced by 77% the morphine-induced vomiting and retching, suggesting that it could suppress the activation of sensory afferent nerve implicated in the emetic reflex (69). According to these observations, Li et al. reported that a grape seed proanthocyanidin extract inhibits in a non-competitive manner the 5-hydroxytryptamine-3 receptors involved in the initiation and coordination of the vomiting reflex, in NCB-20 neuroblastoma cells (70). Taking together, these results suggest that PAC-containing extracts or foodstuffs could be used as a complementary medicine for the management of nausea/emesis, without the side effect usually associated with cannabinoid-based antiemetic agents.
Gastric degradation of PC oligomers has been observed after their incubation in simulated gastric conditions (pH2) in vitro (71). However, these observations were not confirmed by Rios et al., who investigated the gastric stability of PCs in 6 human volunteers after ingestion of cocoa drink containing 733 mg PC polymers and 351 mg flavanol monomers. Gastric samples were collected through a nasogastric tube every 10 min until total gastric emptying (52–60 min), and PCs were quantified. No degradation of these molecules was detected, suggesting their great stability in the stomach environment, and that most of them reach the small intestine intact (72).
Studies in in vitro models or carried out both in humans and animals generally report that PAC monomers, dimers, and eventually trimers may be absorbed in the intestine, while larger polymers remain unabsorbed and accumulate in the gut lumen (73–75). Such absorption is directly proportional to the PACs luminal concentrations, as shown in rats by using intestinal perfusion of different (+)-catechin concentrations (1–100mM) (76). In humans, catechins have been detected in plasma as early as 30 min after drinking green or black tea (77). The intestinal absorption of flavan-3-ols has also been studied in patients with ileostomy after the ingestion of 200 mg of a green tea extract. About 40% of the flavan-3-ols administered were recovered in the ileostomy bag, confirming that substantial amounts of these molecules are absorbed in the small intestine (78). In this study, sulfate, glucuronide, and methylated conjugated metabolites were identified in plasma, all derived from (epi)catechin or (epi)gallocatechin, representing 47 and 26%, respectively, of the parent compounds present in the extract. Data concerning PAC dimers are more controversial, and some authors could not detect any absorption of these compounds in the intestine. Donovan et al., for example, showed that the PAC dimers B1, B2, and B3 were not absorbed in rats neither hydrolyzed to their corresponding monomers in the intestine (79). The absorption of trimers and oligomers is also controversial. Tsang et al. provide evidence that PAC oligomers were not depolymerized to monomers to any extent after ingestion of a grape seed extract (80). In this study, only catechin glucuronides and methylated glucuronide metabolites were detected in plasma as well as in the kidneys and liver. These metabolites were also found in urine with sulfate metabolites and low amounts of the dimers B1, B2, B3, and B4, and the trimer C2. In opposition with these results, Shoji et al. detected oligomers with a mean DP of 2–5 in rat plasma 2 h after the administration of apple PACs with the same DP (81). In another study, 11% of ingested PACs from grape seed extract were recovered in feces, and 71% of them were tetramers to hexamers, suggesting that PACs with more than 3 subunits are more resistant to degradation and accumulate in the colonic lumen when they may be detected in high levels (82). These observations were confirmed by Jimenez-Ramsey et al. and Terrill et al. using 14C-labeled polymeric PACs in chickens and sheep; most of these molecules were not absorbed in the intestine of the animals and were widely recovered in their feces (83, 84). Similar findings were reported in pigs fed grape seed PACs (dimers–pentamers); those were not completely absorbed and remained transiting in the gut lumen for at least 72 h before their fecal excretion (85). In another study in ileostomized subjects, 90% of the PACs consumed as apple juice were found in the ileostomy effluent; however, the DP of the recovered PACs was reduced to 3.4, in comparison to the initial DP of 5.7 in the apple juice. These results, therefore, suggest that part of the oligomeric procyanidins were cleaved into smaller units that, eventually, were absorbed (86).
From these studies it may be concluded that dietary PACs are not affected by their passage across the stomach and that in the small intestine, only monomers, dimers, and eventually trimers may be absorbed to some extent, while larger oligomers and polymers remain in the lumen and accumulate in the colon.
Factors other than DP also affect PAC bioavailability. The fact that flavanols are frequently acylated, especially by gallic acid, reduces their absorption (87) even whether galloylation does not dramatically influence PACs bioavailability as glycosylation with other polyphenols (88). The initial step in the intestinal absorption of dietary flavonoid glucosides is their deglycosylation, which would occur in the enterocyte brush-border membrane through the lactase–phlorizin hydrolase and beta-glycosidase enzymes (89). This event releases a free aglycone that can then enter into epithelial cells either passively or by facilitated diffusion (90). However, flavan-3-ols are the only subclass of flavonoids present in unglycosylated forms in plants, being found naturally as aglycones (91). Therefore, the flavan3-ols are absorbed by the enterocytes without any deconjugation or hydrolysis (88). On the other hand, flavonoids including flavonol-3-ols are generally recognized as xenobiotics by the intestinal detoxification system (92). Accordingly, they may be subjected to Phase II biotransformation (conjugation) in the enterocytes and posteriorly in the hepatocytes, resulting in a series of water-soluble conjugated metabolites including methyl, glucuronide, and sulfate derivatives (93). The role of the small intestine in the glucuronidation and methylation of catechins and that of the liver in their sulfation, methylation, and biliary excretion has been described in rats by Donovan et al. (76). Conjugated compounds are released into the systemic circulation for their further distribution to the body organs and excretion in urine, or are exported into the bile to come back into the intestinal lumen, reaching the colon where they may be metabolized by the microbiota or reabsorbed, leading to an enterohepatic cycling. After the administration of 500 ml of green tea containing 648 µmol of flavan-3-ols in ileostomized subjects, Stalmach et al. quantified the conjugated forms of flavanols present in plasma, urine, and ileal effluents. Sixteen metabolites were detected in plasma and 18 in urine. In the ileal effluents, 70% of the ingested flavan-3-ols were present in their native form and 23 metabolites corresponding to conjugated forms resecreted into the intestinal lumen were detected, mainly sulfate and methyl-sulfate derivatives from epicatechin and epigallocatechin (94). Flavanols seem to be present in plasma as free flavanols as well as sulfate and glucuronide forms, according the type of flavanol (95). In fact, the methylated metabolites of catechin, epicatechin, and epicatechin gallate predominate over the original unmethylated forms in plasma (96). Lee et al. determined flavanol conjugates in plasma after ingestion of green tea in humans. (−)-Epigallocatechin-3-gallate was mainly detected as sulfate conjugate (65%), followed by the free form (20%) and glucuronide form (15%), while (−)-epigallocatechin was mostly found in the glucuronide form (60%) followed by the sulfate form (30%) and the unconjugated (10%). (−)-Epicatechin was exclusively found in the conjugated form, with approximately two-thirds sulfate and one-third glucuronide (97).
As previously stated, most of the ingested PACs remain unabsorbed in the small intestine and accumulate in the colon (98) where they are degraded by the colonic microbiota in low molecular weight aromatic acids, which differ according to their hydroxylation profile and the length of their aliphatic side chain (18). These microbial metabolites are absorbed in the colon and may be also conjugated by the colonocytes or in liver, resulting in glucuronide, methyl, glycine, and sulfate derivatives (99).
The intestinal lumen is the main site of interactions between dietary PACs and nutrients and enzymes. These interactions occur thanks to the chemical structure of PACs and their numerous hydroxyl groups suitable for forming non-specific complexes with proteins, resulting in their precipitation (100). This event occurs preferentially at pH values near the protein isoelectric point. Hagerman and Butler have observed that PAC affinity for proteins was inversely proportional to protein size and depended on their proline content (101). The interaction between PACs and proteins constitutes the base of the tanning process that transforms animal hides into leather through the complexation of skin collagen and the oral sensation of astringency through the complexation of salivary proline-rich glycoproteins. Accordingly, PACs may also interact with the pancreatic enzymes released in the intestinal lumen and with brush-border enzymes and nutrient transporters, thus affecting nutrient bioavailability (102, 103).
Starch constitutes the main source of carbohydrates and energy in the occidental diet, although the disaccharides sucrose and lactose and the monosaccharides glucose and fructose are also present (104). Starch is digested in the intestinal lumen by pancreatic α-amylase and the resulting disaccharides, trisaccharides, and limit dextrin are subsequently digested by the brush-border disaccharidases, maltase-glucoamylase and saccharase-isomaltase, while lactose is hydrolyzed by the lactase-phloridzin hydrolase. The resulting monosaccharides (glucose, galactose, and fructose) are absorbed into the enterocytes through active [sodium–glucose cotransporter 1 (SGLT1)] and facilitated [glucose transporter (GLUT5 and GLUT2)] apical transporters. Dietary polyphenols, including PACs, may delay carbohydrate digestion and reduce postprandial glucose absorption, which represents an alternative approach for diabetes prevention and management (15, 102).
A number of in vitro studies have evaluated the inhibitory activity of different PACs against α-amylase and disaccharidases (Table 3). Fractionated polymeric and oligomeric PACs from peel persimmon inhibited α-amylase and α-glucosidase in vitro. PAC polymers had higher inhibitory activity against α-amylase than oligomers, while the opposite was observed against α-glucosidase. These results suggest that PAC DP is related to the inhibition of these enzymes, and that the oligomers have probably a greater potential than polymers for diabetes prevention or management (105). In another in vitro study, aqueous and alcoholic grape seed extracts were shown to inhibit α-amylase dose dependently, being more elevated the inhibition with the ethanolic extract (around 75%) than with the aqueous (around 52%) at the same concentration (106). The inhibitory effect of four aqueous extracts from different species of cinnamon barks (condensed tannins ranging between 0.12 and 0.15 g catechin equivalent/g extract) against α-amylase, maltase, and sucrase activities was determined in vitro. Thai cinnamon was the most potent maltase inhibitor and Ceylon cinnamon the most efficient in suppressing sucrase and α-amylase. When combined with acarbose (a recognized α-glucosidase inhibitor), all the extracts displayed an additive inhibition against α-amylase, while only the Chinese, Ceylon, and Thai cinnamon extracts (CEs) showed an additive inhibition against sucrase and maltase (107). Interestingly, these results suggest that PACs from the same botanical species may differ in their PAC composition depending on the geographical origin of the plant, probably due to differences in geo-climatic conditions of culture. Pycnogenol® was also shown to inhibit baker’s yeast α-glucosidase more efficiently than green tea extract and acarbose. This could explain the glucose-lowering effects reported with this product in clinical trials with diabetic patients (108, 109). PAC fractions purified from peanut skin also showed an inhibitory activity against maltase and sucrase at a concentration of 1 mg/ml. The strongest maltase inhibition was exerted by the trimeric PAC epicatechin-(2β → O → 7,4β → 8)-[catechin-(6 → 4β)]-epicatechin, while the strongest sucrase inhibition was exhibited by another trimeric PAC, epicatechin-(4β → 8)-epicatechin-(2β → O → 7,4β → 8)-catechin. The inhibitory activity of both compounds was lower than acarbose but higher than that of dimeric PACs (110). Barrett et al. studied the effect of condensed tannins from cranberry, grapes, and cocoa extracts against α-amylase and glucoamylase in vitro, using different tannin:enzyme ratios (0.01:1 to1:1) (103). Cocoa, grape, and cranberry tannins exerted the highest inhibition of α-amylase and glucoamylase at 1:1 ratio (14, 28, and 55%, respectively, for α-amylase and 23, 55, and 41% respectively for glucoamylase). Cocoa, cranberry, and grape tannins also reduced glucoamylase activity in approximately 20% at the 0.01:1 ratio. Grape and cranberry increased their inhibitory activity against the enzyme at 1:1 ratio (55 and 41%, respectively). Accordingly, in this study, the inhibitory effect was strongly dependent on the tannin concentration and HMW tannins (like those present in cranberries) have greater inhibitory capacity than LMW tannins (like those present in cocoa). Similar findings were also described by Tsujita et al. with different fractions of peanut seed skin (111) and almond seed skin (112).
Only one study has evaluated the effect of dietary PACs on lactase activity showing that tea epigallocatechin-3-gallate inhibits lactose hydrolysis by intestinal lactase in vitro (IC50 74µM) at physiological luminal concentrations (113).
Although a great number of studies evaluated the effect of PACs on pancreatic amylase and disaccharidases, most of them were carried out in vitro and have inherent limitations due to the use of porcine or yeast enzymes, whose specificity may differ from these of human origin, and to the method employed for quantifying the enzymatic activity. For example, synthetic substrates are frequently used, that may be affected by the presence of PACs. In addition, they ignore the presence of other proteins normally present in the GI tract, like salivary proline-rich proteins, which may interfere with the inhibition of enzymes by PACs (102).
In vivo studies evaluating the interaction between PACs and the enzymes involved in carbohydrate digestion are scarce. Tomaru et al., for example, observed that dietary supplementation of diabetic obese mice with 0.5 or 1.0% cacao PACs [containing 2.49% catechin, 5.89% epicatechin, 3.93% PC B2, 2.38% PC C1, 3.17% cinnamtannin A2, and 0.48% galactopyranosyl-ent-(−)-epicatechin-(−)-epicatechin] dose dependently prevented the development of hyperglycemia (114). The administration of PAC oligomers from persimmon leaf tea to Wistar rats significantly decreased their blood glucose levels when compared with the placebo group (115).
The intestinal absorption of the monosaccharides resulting from starch and disaccharide digestion is mediated by transporters located in the brush-border membrane, on the apical side of the enterocytes. SGLT1 is an electrogenic transporter that depends on the Na+ gradient and mediates the absorption of glucose and galactose into the enterocyte (15, 102, 116). SGLT1 has a high affinity but a low transport capacity for glucose. GLUT5 is a facilitated transporter involved in fructose absorption (102, 116, 117), while GLUT2 is a facilitated transporter for glucose, galactose, and fructose; contrarily to SGLT1, it has low affinity but high transport capacity for glucose. GLUT2 was first located in the enterocyte basolateral membrane where it mediated the exit of the monosaccharides present in the cells to the systemic circulation during the postprandial period (118). More recently, the presence of GLUT2 has been described in intracellular vesicles. When the intraluminal concentration of glucose increases, i.e., after the intake of a meal rich in carbohydrates, GLUT2-containing vesicles translocate to the apical membrane where it contributes to glucose and fructose absorption. It has been proposed that two-third of the total amounts of glucose absorbed by the intestine in the postprandial period is through GLUT2. GLUT2 would be reinternalized in the cytoplasmic vesicles when the luminal concentration of glucose decreases or through insulin regulation (15, 102, 118–120).
The role of polyphenols in the regulation of the apical transporters has been widely studied (121–127). Several compounds have been shown to interfere with these transporters such as berry anthocyanins, apple polyphenols (phlorizin, quercetin, kaempferol, phloretin, and chlorogenic acid), helichrysum, and grapefruit (kaempferol-3-O-glucoside, chlorogenic acid-3-O-glucoside, naringenin-7-O-glucoside, naringenin diglycoside, kaempferol rutinoside, naringenin-7-O-rutinoside, and quercetin monoglucosides, among others) (121–126). Although the effect of PACs on these transporters was not evaluated so far, it is possible that they may exert certain activity, since flavanol monomers such as catechin, epicatechin, epigallocatechin, epicatechingallate, and epigallocatechingallate have been shown to inhibit SGLT1 or GLUT2 (128, 129). Kobayashi et al. observed that epicatechingallate and epigallocatechingallate (1mM) from tea reduced glucose uptake by rabbit brush-border membrane vesicles by 53 and 35%, respectively, whereas the inhibitory effects of catechin and epigallochatechin were not significant (128). In another study, all these flavanol monomers were shown to inhibit SGLT1-mediated glucose transport into Caco-2 cells (129).
Triglycerides (TG) constitute the majority of the dietary lipids, while the contribution of cholesterol (CS) and phospholipids is much lower. Due to their hydrophobicity, lipids must be solubilized to be digested and posteriorly absorbed (130). Dietary fats are first emulsified in the stomach, a phenomenon that increases the rate of TG hydrolysis by the lipase and the release of diacylglycerol and free fatty acids (FFAs). Once in the small intestine, the fat emulsion is stabilized by bile salts, enabling the action of the colipase/pancreatic lipase (PL), CS ester hydrolase, and phospholipase A2 (PLA2) (130). PL hydrolyzes the TGs, releasing the fatty acids esterified in the carbon 1 and 3 of the molecule, and monoacylglycerol. Regarding CS ester hydrolase and PLA2, these enzymes hydrolyze CS esters and phospholipids, releasing free CS, FFAs, and lysolecithin (130). As these final products of digestion (monoglycerides, FFAs, CS, and lysolecithin) are hydrophobic, they must be incorporated into biliary mixed micelles as they are released in the lumen. This process of solubilization allows them to diffuse across the unstirred water layer until the enterocyte surface, where they are released from the micelles. Posteriorly, they enter into the absorptive cells by passive diffusion according their concentration gradient or by using specific transporters such as FAT/CD36, FATP4, and FABPpm for FFAs and Niemann–Pick C1-like protein 1 (NPC1L1) for CS (131, 132). In the enterocytes, FFAs are reesterified with glycerol or CS, and the resulting TG and CS ester are subsequently incorporated to chylomicrons and exported into lymphatic circulation, to finally end up in the bloodstream. Bile salts are reabsorbed in the terminal ileum through the apical sodium bile acid transporter, they reach the circulation and are taken up by the liver and resecreted by the biliary system. This enterohepatic circulation takes place 10 times per day so that less than 5% of bile acids enter the large intestine during this period for fecal elimination (133).
Similar to carbohydrates, the enzymes and transporters involved in lipid digestion and absorption are subjected to the action of dietary polyphenols. Most of the studies have focused on the inhibitory activity on of these compounds on PL, PLA2, or bile salts, due to their implication in fat absorption and their potential to prevent obesity and its complications. Fewer studies have focused on their interaction with CS esterase or lipid transporters (Table 4).
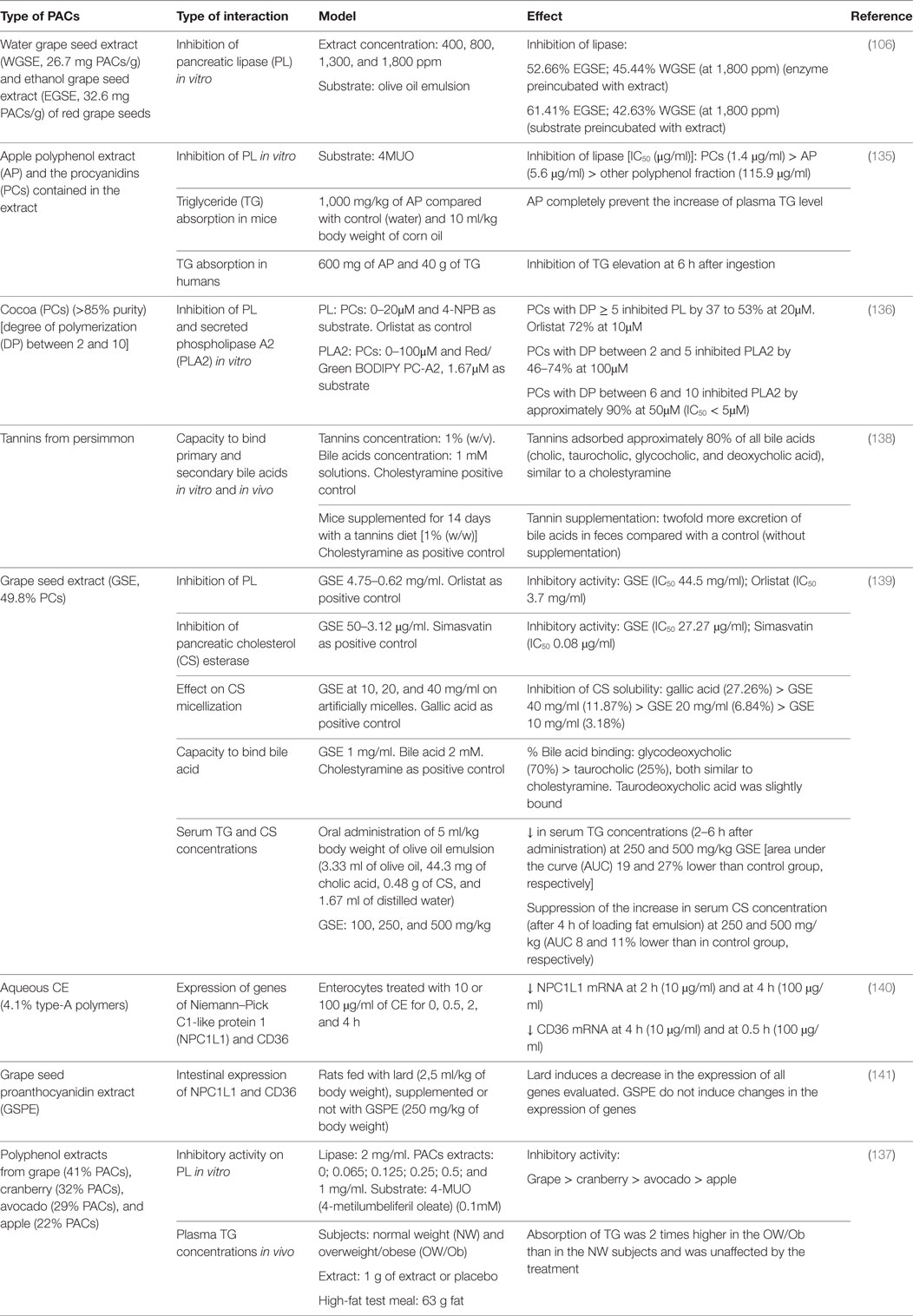
Table 4. Impact of proanthocyanidins on enzymes and transporters involved in the digestion and absorption of lipids.
Hassan reported that an ethanol extract from grape seed was more efficient than a water extract in inhibiting TG hydrolysis by PL in vitro when they were preincubated with the enzyme (52.7 vs 45.4%, respectively) or with its substrate (61.4 vs 42.6%, respectively). Such effect was related to the PACs content of the extracts (106). An apple polyphenol extract containing 65.7% PCs and 12.5% flavan-3-ols almost completely inhibited PL activity in a dose-dependent manner (134). The purified PC fraction showed the higher inhibitory activity, compared to the other polyphenols fractions, and the effect was mainly associated with the DP [DP ≥ 5 had the highest inhibitory activity, especially the heptamer fraction (IC50 = 0.7 μg/ml)]. Results in mice indicate that 1,000 mg/kg of the apple extract fully prevented the increase of plasma TG after the administration of 10 ml corn oil/kg body weight, compared to the control group. In humans, the elevation of postprandial plasma TG was significantly inhibited after the intake of 600 mg apple extract together with 40 g dietary fat (135). In another study, the effect of purified cocoa PCs (>85%; DP between 2 and 10, B-type) against PL and PLA2 was determined in vitro. PCs with DP ≥ 5 inhibited PL, while those with a DP between 2 and 5 inhibited PLA2, and those with DP between 6 and 10 inhibited PLA2 by approximately 90% at 50µM (136). The authors conclude that cocoa PCs has higher inhibitory activity against PLA2 than against PL and suggest that DP is an important factor in determining the potency of these compounds. We recently compared the lipase inhibitory effect of PAC-containing polyphenol extracts from grape, cranberry, avocado, and apple (137). The most to least efficient extracts were grape > cranberry > avocado > apple. The strongest lipase inhibitory activity was exerted by the extract with the higher PAC content and higher DP (9.8) and the only ones containing galloyl moieties. Accordingly, we observed that the PAC content of the extracts correlated (r = 0.85; p < 0.001) with their lipase inhibitory activity, and a similar correlation was also observed when considering the PAC DP. However, when 1 g of the grape extract was administered to normal-weight or overweight/obese subjects simultaneously with a high-fat breakfast, the postprandial increase of plasma TG was not affected compared to the placebo (137). It is important to consider that in humans, PL is released in excess into the intestinal lumen and that, independently of the inhibitory activity exhibited by the extract in vitro against this enzyme, the amounts of PACs ingested may be insufficient to inhibit the enzymatic activity completely.
Another interesting target for PACs to inhibit lipid absorption is bile salts. Matsumoto et al. (138) investigated the ability of persimmon tannins to bind primary and secondary bile acids in vitro and in vivo. These PACs had a high DP and were composed by epicatechin, epigallocatechin, epicatechin-3-O-gallate, and epigallocatechin-3-O-gallate. At a concentration of 1% (w/v), PACs adsorbed approximately 80% of the primary and secondary bile acids in vitro, similar to cholestyramine. In mice fed a diet supplemented with 1% persimmon tannins for 14 days, a twofold increase of fecal bile salt excretion was observed, compared to the control, not supplemented, group. However, this increase remained lower than that observed in the animals treated with cholestyramine. Such interference with bile salts might affect the stabilization of fat emulsion in the intestinal lumen, and/or the formation of biliary micelles.
A grape seed extract (49.8% PCs) was shown to inhibit PL and CS esterase activities, but less than their respective positive controls, orlistat and simvastatin. To study the effect of the extract on CS micellization, the authors evaluated the solubility of CS in artificially prepared micelles in presence of different concentration of the extract. They observed a decrease of CS solubility, but less than with the positive control gallic acid (139). With respect to bile acid binding capacity, the extract binds strongly glycoldeoxycholic and taurocholic acids (70 and 25%, respectively) at a concentration of 1 mg/ml, similar to the effect of cholestyramine at the same concentration. Rats fed a high-fat emulsion with 250 or 500 mg/kg extract showed a significant diminution in the postprandial plasma TG concentrations between 2 and 6 h, as reflected by changes in the area under the curve (AUCTG) (19 and 27% lower than the control group). At these same doses, the extract significantly suppressed the postprandial increase in serum CS concentrations (AUC 11% lower than the control group). It is therefore possible that this extract can be used as therapeutic strategy to prevent hyperlipidemia and obesity due to its capacity to improve plasma lipid profile.
A number of studies have focused on the interactions between polyphenols and intestinal lipids transporters. An aqueous CE containing PACs (4.1% type-A polymers) has been shown to decrease the mRNA levels of CD36 (a FFA transporter) and NPC1L1 (implicated in the intestinal uptake of CS) in small intestine enterocytes (140). In opposition to these results, Quesada et al. did not report any effect of a grape seed extract on the expression of these transporters in rats fed a diet with lard (141).
In our previously described study (137), we also address the capacity of PACs to bind bacterial LPS, as previously by described by Delehanty et al. (142). In fact, it has been proposed that LPS from intestinal Gram-negative bacteria could enter the enterocytes and be incorporated into the chylomicrons to be excreted to the lymphatic system and bloodstream. Accordingly, the presence of dietary lipids in the intestinal lumen would stimulate LPS absorption and, eventually the development of metabolic endotoxemia (143, 144). We confirm that the grape extract bound LPS in vitro, inhibiting its union to polymyxin B and that, when administered to the volunteers after the high-fat meal, it significantly decreased the elevation of postprandial plasma LPS associated with that of TG in the volunteers (137). This phenomenon constitutes a new mechanism by which PACs may decrease systemic inflammation.
In conclusion, some evidence exist that dietary PACs interfere with the different events involved in intraluminal lipid processing, including enzymatic hydrolysis, micellization, and uptake of lipid digestion products by the intestinal epithelial cells. However, most of the studies have focused on the interaction between PACs and PL in vitro and, in some cases, in animal models, which do not accurately represent the situation occurring in the organism. Considering these points, further studies are necessary to elucidate the role of PACs in intestinal fat digestion and absorption in humans.
The digestion of dietary protein occurs first in the gastric and intestinal lumen through the action of HCl/pepsin and pancreatic proteases, including pepsin, trypsin, chymotrypsin, elastase, carboxypeptidase A, B, and aminopeptidase, and concludes with peptidases located in the brush-border membrane and cytoplasm of the enterocytes (130, 145). Pepsin and pancreatic proteases hydrolyze proteins in amino acids, di-, tri-, and oligopeptides. Posteriorly, amino acids are absorbed into the enterocytes through specific transporters according to their chemical structure (146), while di- and tripeptides are transported by the proton-dependent cotransporter, Pept-1 (130, 147). Oligopeptides need further hydrolysis by brush-border proteases to yield absorbable molecules (130). In the absorptive cells, the di- and tripeptides are hydrolyzed to amino acids by cytosolic peptidases, and these are exported to the bloodstream by facilitated diffusion (130).
As previously stated, PACs display a high affinity for proteins, particularly for these with high-proline content. Accordingly, they may affect the bioavailability of dietary proteins by decreasing their digestibility, either directly by binding them or indirectly by inhibiting enzymatic activities (Table 5). These processes contribute to the fact that condensed tannins are sometime considered as antinutritional factors.
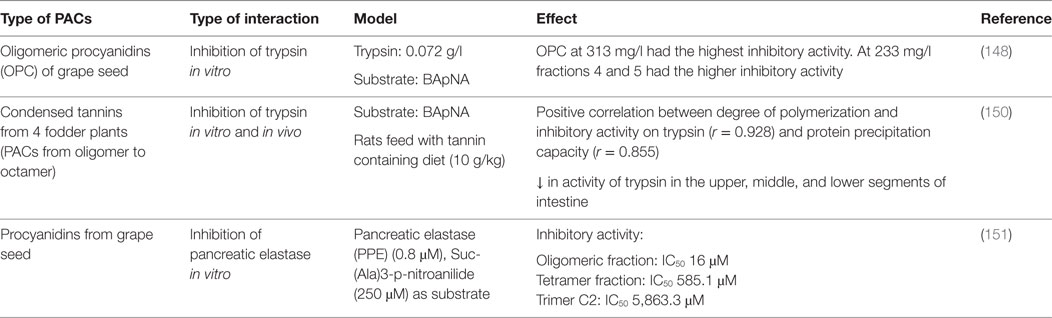
Table 5. Impact of proanthocyanidins on enzymes and transporters involved in the digestion of proteins.
A maximal inhibitory activity of trypsin in vitro was reported for grape seed PACs at a concentration of 313 mg/l; the enzymatic inhibition correlated with PC DP (148). In another study, the same authors investigated the mechanism by which trypsin is inhibited by PC dimer B3 (149). They reported that, at low concentration, specific interactions mediated by hydrogen bonds occur between the hydroxyl groups of the dimer and the amide and carbonyl group of the protein backbone, while at high concentration, those interactions were not specific. Horigome et al. also reported a positive correlation between the PAC DP of four fodder plants and their capacity to precipitate proteins and inhibit trypsin activity (150). In addition, they observed that the preincubation of bovine serum albumin with PACs inhibited its digestion. Rats fed a tannin supplemented diet (10 g/kg) displayed a significantly reduced trypsin activity in their intestine. When antibiotic treated rats (to eliminate the effect of bacterial enzymes) were fed with a tannin supplemented diet (20 g/kg), they observed a significant decrease in nutrient digestibility. Based on these results the authors conclude that the formation of insoluble enzyme–tannin complexes is responsible of the inhibition of trypsin activity. Brás et al. studied the effect of dimer B3, trimer C2, tetramer, and oligomer fractions of PCs on pancreatic elastase activity. The inhibitory activity increased with the PCs molecular weight, being the oligomer fraction the most potent inhibitor (approximately 90%; IC50 = 16μM) (151). In another study, these authors reported that elastase, in the presence of PCs, undergoes slight changes in its secondary (R-helix to β-sheet) and tertiary structures, and forms insoluble aggregates (152).
Fewer studies have addressed the influence of PACs on the others enzymes involved in protein digestion. Uchida et al. showed that the inhibitory activity of condensed tannins from Rhei Rhizoma against the angiotensin converting enzyme increased with the PACs DP (153). The PC B-5 3,3′-di-O-gallate had the highest angiotensin converting enzyme inhibitory activity, and the authors indicate that this activity was protein-specific as this PACs did not inhibit other enzyme activities such as trypsin, chymotrypsin, leucine aminopeptidase, carboxypeptidase A, and urinary kallikrein. No studies were found regarding the interaction between PACs and intestinal amino acids and peptides transporters.
The fate of the PAC-bound proteins in the intestinal and colonic lumen remains unclear. An increased flux of indigested proteins in the colon may result in a major production of toxic metabolites derived from their fermentation by the intestinal microbiota (IM), with negative consequences to the host (154). However, PACs are used in ruminant nutrition to reduce protein degradation in the rumen (155), and it is possible that this phenomenon also occurs in the human colon. PAC-bound proteins, therefore, should be eliminated in stools.
Cássia Santos et al. evaluated the effect of a methanolic extract from leaves of B. intermedia containing catechin derivatives and oligomeric PACs in a rodent model of castor oil-induced diarrhea. The extract was shown to prevent or revert the diarrhea, decreasing fluid accumulation and the emission of watery stools, probably through the stimulation of intestinal opioid receptors, without affecting intestinal motility (156). In a similar study, a fraction from Chiranthodendron pentadactylon flowers rich in flavan-3-ols was evaluated using rat jejunal loops exposed to cholera toxin (3 µg). (−)-Epicatechin showed the best antisecretory activity (ID50 = 8.3 μm/kg), like that of the antisecretory drug loperamide (ID50 = 6.1 µm/kg), and better than (+)-catechin (ID50 = 51.7 μm/kg) and other compounds presents in the extract (flavonol glycosides). Such observations support the traditional use of C. pentadactylon flowers in the treatment of dysentery in the Mexican traditional pharmacopeia (157). In another study, the effect of Aronia melanocarpa fruit juice (rich in condensed tannins) was shown to significantly reduce the intestinal transit in charcoal-administered rats (158). Interestingly, a patent (No US 7,341,744 B1) “Method of treating secretory diarrhea with enteric formulations of proanthocyanidins polymer” was recently registered by Rozhon et al. that describes the pharmaceutical formulation of a proanthocyanidin polymer isolated from Croton spp. or Calophyllum spp., useful for the treatment and prevention of secretory diarrhea (159).
The human colon harbors a highly complex microbial ecosystem that includes bacteria, yeasts, fungi, virus, and phages. More than 1,000 species of bacteria have been described, most of them anaerobic, and with counts reaching 1011–1012/g of intracolonic content (160–166). These microorganisms express a great number of enzymes capable of metabolizing the majority of the substrates reaching the colon, including xenobiotics and PACs (167). The gut microbiota exhibits metabolic, nutritional, and protective functions important for the host health (161, 165). It is involved in energy salvage from the dietary compounds non-digested and absorbed in the small intestine, vitamin synthesis, and in the metabolism of bile salts and xenobiotics. The IM also exerts a protective function, decreasing the risk of pathogen overgrowth in the colonic lumen, stimulating the local immune system and contributing to the development of oral immune tolerance (154, 161, 163). The dominant bacterial phyla constituting the IM are Firmicutes and Bacteroidetes and, in lower proportions, Proteobacteria, Actinobacteria, and Verrucomicrobia (164, 165), while the main bacterial genera are Clostridium, Bacteroides, Prevotella, Eubacterium, Ruminococcus, Fusobacterium, Peptococcus, and Bifidobacterium (160). Some genera are more particularly considered as beneficial for the host like Bifidobacterium, Lactobacillus, Faecalibacterium prausnitzii (a butyrate-producing bacteria), and Akkermansia muciniphila, while others are considered as potentially harmful like Staphylococcus, some species of the Clostridium genus (C. perfringens, C. difficile) and Pseudomonas, which have been associated with diarrhea, systemic infections, liver damage, cancer, and encephalopathy (162). Among the numerous metabolites produced by the IM, the most widely studied are those produced by the fermentation of the dietary fiber in the colon, i.e., the short chain fatty acids (SCFAs) acetate, propionate, and butyrate (161). The decrease of colonic luminal pH induced by SCFAs reduces the risk of pathogen overgrowth and improves mineral solubility and absorption (163). They also act, promoting the integrity of the gut barrier function and host satiety (168). Butyrate is also considered as a preferential substrate for the colonocytes; in addition, it exerts antiinflammatory and antitumoral activities, this latter by favoring the differentiation and apoptosis of epithelial cells. Regarding acetate and propionate, they have been implicated in the regulation of lipid metabolism in the liver (161, 163). However, some of the metabolites produced by the colonic fermentation of amino acids including ammonia, hydrogen sulfide, indol, and phenol compounds, among others, can exert deleterious effects on the colonic mucosa and host health. Indeed, these metabolites affect colonocyte oxidative metabolism and cellular respiration, produce genomic DNA damage, affect the integrity of the barrier function, and also act like pro-carcinogens and promoters of colorectal cancer as well as inflammatory bowel diseases (IBD) (154, 165, 169).
The composition of the IM is influenced by many factors such as the age, gender, and genetic background of the host, the consumption of xenobiotics, antibiotic, and other drugs, the existence of physical or psychological stress, environmental and dietary factors, this latter being probably the most important (160, 161, 164). Accordingly, probiotics and prebiotics have been typically used as a strategy for the nutritional management of the IM composition and its metabolic/immunological activities (165, 170).
As described above, a great proportion of the dietary polyphenols, including PACs, remain undigested in the intestine and reach the colon, where they are used as substrates by specific bacterial populations, stimulating their growth much like prebiotics do. In addition, PACs with high DP exert bacteriostatic and eventually bactericide effects that might also contribute to the modulation of IM composition and bacterial adhesion to colonocytes (164, 171).
Several studies have investigated the effect of PAC intake on the composition of the IM. In an in vitro model of colonic fermentation, the addition of (+) catechin (150 mg/l) for 48 h increased significantly the growth of C. coccoides–E. rectale group, Bifidobacterium spp., and E. coli and inhibited that of C. histolyticum. In the same conditions, the incubation with epicatechin only increased the growth of C. coccoides–E. rectale group (172). In a similar model, a grape seed extract with 28% PACs (600 mg/l) promoted the growth of Lactobacillus/Enterococcus group at 5 and 10 h, while the extract with 78% PAC at the same concentration only decreased C. histolyticum (173). In a recent interventional study, healthy volunteers had to ingest a dairy-based cocoa beverage with high (494 mg) or low (29 mg) flavanol content once a day for 4 weeks (174). The high flavanol beverage was composed of 110 mg monomers (catechin and epicatechin), 99 mg dimers, and 285 mg polymers (trimers to decamers), while the low flavanol beverage only contained 6, 11, and 12 mg of these compounds, respectively. The high flavanol drink significantly increased the counts of Bididobacterium, Lactobacillus, and Enterococcus and decreased those of C. histolyticum, while the low flavanol drink increased C. histolyticum. Both beverages stimulated the growth of the E. rectale/C. coccoides group. Accordingly, cocoa flavanols display a prebiotic potential by promoting a healthy IM in humans. In another human study, the effect of 2-week administration of 0.19 g/day of a PAC-rich grape seed extract (DP 2–15) was evaluated on the IM composition and fecal odor in healthy adults (175). An increase of Bifidobacterium and a non-significant decrease of Enterobacteriaceae were reported. Interestingly, the concentrations of potentially harmful bacterial metabolites such as ammonia, phenol, p-cresol, 4-ethylphenol, indol, and skatol (eventually implicated in the development of colorectal cancer) tended to decrease. Methyl mercaptan gas concentrations and fecal odor decreased significantly. Therefore, in this study, the modification of the IM by PACs was associated with a healthier environment in the colonic ecosystem.
The study of PAC effects on the IM is not restricted to humans. In ruminant nutrition, PACs are recognized to modulate fermentation processes in the rumen, inhibiting methane-producing archaea, decreasing bloating and protein degradation, and favoring the formation of conjugated linoleic acid. Some studies suggest that body weight gain, milk yields, and reproductive performance could be also improved by incorporating tannins in the animal diet, even if there is not yet a clear explanation for these beneficial effects (155).
The enzymatic degradation of flavonoids by the colonic microbiota results in a huge array of new metabolites. Bacterial enzymes may catalyze many reactions including hydrolysis, dehydroxylation, demethylation, decarboxylation, and deconjugation (176). Many flavonoids undergo ring-fission in which their C-ring is degraded, A-ring forms hydroxylated aromatic compounds, and B-ring phenolic acids derivatives (177). Clostridium and Eubacterium have been proposed as the main bacterial genera involved in the metabolism of phenolic compounds including flavan-3-ols (178). It is considered that about 40% of the flavan-3-ols ingested with green tea are converted to phenolic acid metabolites in the colon, which are excreted in urine. About 8% of them are methyl, glucuronide, and sulfate derivatives of flavanols, which reflect the fact that these metabolites were previously absorbed by the colonic epithelium (179).
The first study carried out in humans by Das in 1971 identified 11 metabolites in urine after (+)-catechin intake; among the most important were m-hydroxyphenylpropionic acid, δ-(3,4-dihydroxyphenyl)-γ-valerolactone, and δ-(3-hydroxyphenyl)-γ-valerolactone (180). These results were confirmed more recently by Li et al. who described phenyl-valerolactones as the main tea catechin metabolites produced by gut microorganisms and detected in human urine and blood (181). Consistently, Tzounis et al. reported that the incubation of (−)-epicatechin or (+)-catechin with fecal bacteria led to the generation of 5-(3′,4′-dihydroxyphenyl)-gamma-valerolactone, 5-phenyl-gamma-valerolactone, and phenylpropionic acid (172). Valerolactones were also detected in human urine by Ottaviani et al. after the consumption of flavanols and PCs (182). Aura et al. observed that 3-hydroxyphenyl propionic acid and 3-phenylpropionic acid were the main metabolites originated from (+)-catechin and (−)-epicatechin by human microbiota (183). These results are consistent with those described by Gonthier et al. who also identified urinary and plasma 3-hydroxybenzoic acid and 3-hydroxyhippuric acid in rats fed a diet supplemented with red wine polyphenols (184). Flavanol degradation by the microbiota seems to be a rapid process completed in 4–8 h, as reported in a “pig cecum in vitro model” (185). In another study with pig microbiota, it was shown that about 80% of PC A2 and 40% of cinnamtannin B1 were degraded after 8 h of incubation (186). 3-O-Gallate derivatives of epicatechin and epigallocatechin were extensively metabolized by a human fecal microbiota after 24-h-incubation but remained unaffected in presence of rat fecal microbiota, even after 48-h-incubation. These results suggest differences in the microbiota and their associated metabolic ability between both species (187). Phenylvaleric, phenylpropionic, cinnamic, phenylacetic, and benzoic acids as well as conjugated derivatives of benzoic acid have been identified in urine samples of rats fed with PC dimer B3, trimer C2, and catechin polymer (74). In another study, the main metabolites identified after the in vitro fermentation of purified PAC dimers with a human fecal microbiota were 2-(3,4-dihydroxyphenyl) acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone (167). m-hydroxyphenylpropionic acid, ferulic acid, 3,4-dihydroxyphenylacetic acid, m-hydroxyphenylacetic acid, vanillic acid, and m-hydroxybenzoic acid were identified in urine samples of human volunteers after cocoa intake (188). These results were confirmed by Urpi-Sarda et al. who reported increased urinary concentrations of caffeic acid, ferulic acid, 3-hydroxyphenylacetic acid, vanillic acid, 3-hydroxybenzoic acid, 4-hydroxyhippuric acid, and hippuric acid in humans, while in rats were identified 3,4-dihydroxyphenylpropionic acid, m-coumaric acid, 3-hydroxyphenylacetic acid, protocatechuic acid and vanillic acid (189). These slight differences in the metabolite profile probably reflect interspecies differences in their microbiota composition. Ward et al. detected urinary 3-hydroxyphenylpropionic acid and 4-O-methylgallic acid after regular consumption of PAC-containing grape seed extract in humans (190). Interestingly, Jenner et al. measured the concentrations of dietary polyphenols and their bacterial metabolites in fecal waters from healthy omnivorous subjects under normal diet (i.e., without additional supplementation with fruits and vegetables). The major components detected were phenylacetic acid (479µM), 3-phenylpropionic acid (166µM), 3-(4-hydroxy)-phenylpropionic acid (68µM), 3,4-dihydroxycinnamic acid (52µM), benzoic acid (51µM), 3-hydroxyphenylacetic acid (46µM), and 4–hydroxyphenylacetic acid (19µM). Other phenolic acids ranged between 0.04 and 7µM in fecal waters (19). The bacterial degradation of black tea polyphenol-rich and red wine/grape juice extracts by the colonic microbiota were compared in an in vitro five-stage GI model (SHIME®). The levels of gallic acid and 4-hydroxyphenylpropionic acid remained elevated throughout the colon with red wine/grape juice feeding, while these compounds were consumed in the distal colon and 3-phenylpropionic acid was strongly produced during a polyphenol-rich black tea extract feeding. The gut microbial production of phenolics was dependent on their location in the colon and the extract source (191).
The PAC DP is an important factor affecting the microbial metabolism of these compounds. The presence of PACs with high DP prevents the in vitro microbial metabolism of PACs (192), and A-type PACs are also more resistant to microbial degradation than B-type PACs (193). The main flavanol bacterial metabolites described in these studies are summarized in Table 6.
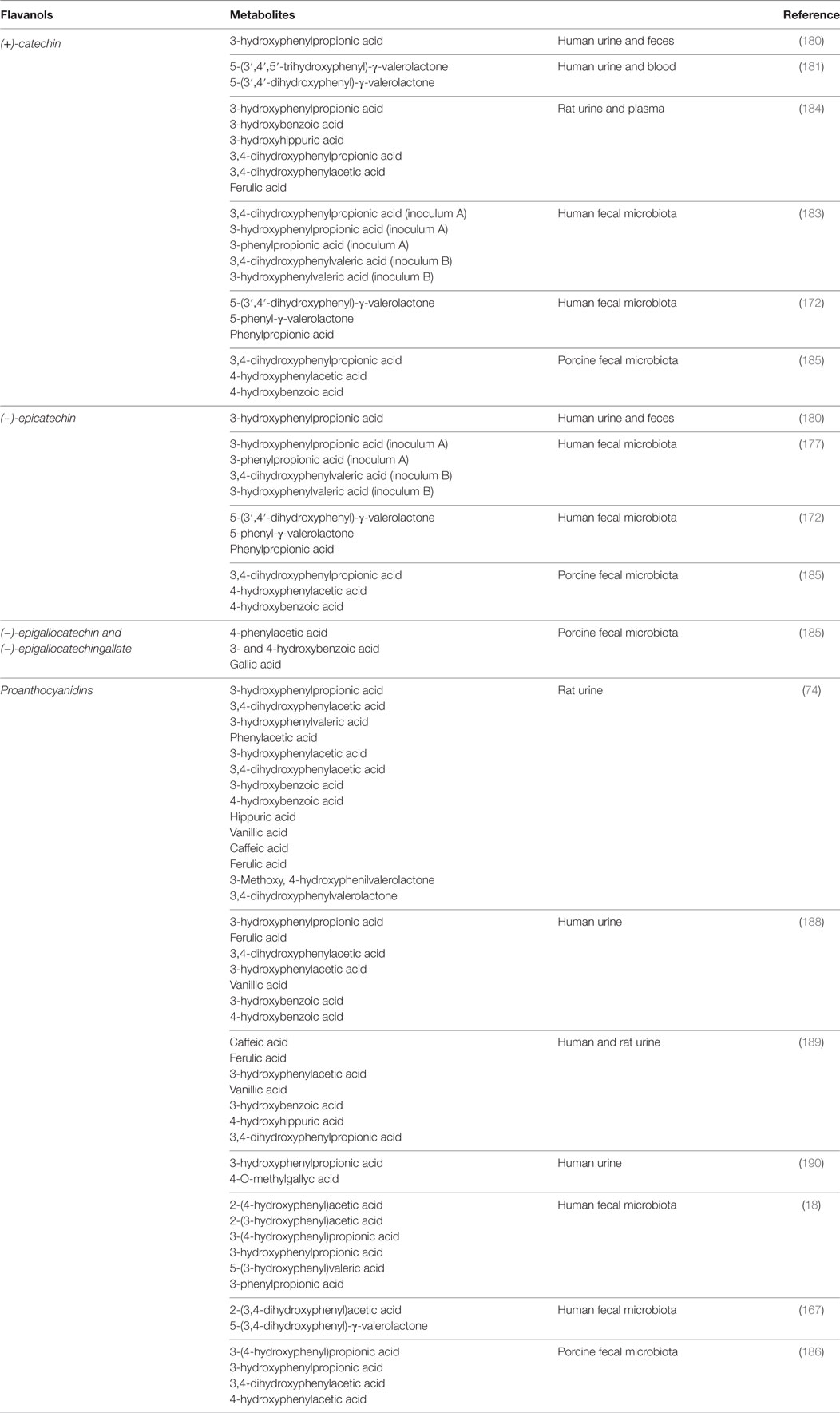
Table 6. Flavanol metabolites identified from microbial conversion (in vitro) or in body fluids (in vivo).
Whether these PAC metabolites can be used by the cells as a source of energy is unclear. A recent study showed that 3,4-dihydroxyphenylacetic acid, a microbial metabolite of quercetin and PACs, can protect against the mitochondrial dysfunction induced by CS in Min6 pancreatic β-cells (194). CS (320µM) decreased the mitochondrial membrane potential, the intracellular concentrations of ATP and the rate of oxygen consumption, while 3,4-dihydroxyphenylacetic acid (100–250µM) prevented, in a concentration-dependent manner, these mitochondrial function alterations. At 250µM, this metabolite was capable of preventing the drop in oxygen consumption and complex I activity, suggesting that 3,4-dihydroxyphenylacetic acid improves the energetic metabolism of these cells.
Inflammatory bowel diseases, mainly Crohn’s disease and ulcerative colitis, are considered as a growing problem of public health in the world. The etiology of these diseases remains poorly understood. Although the mechanisms underlying the occurrence of Crohn’s disease and ulcerative colitis differ, both diseases are characterized by a chronic inflammation and increased oxidative stress in the mucosa, with an inappropriate activation of the immune system (195). Considering that dietary PACs exhibit antiinflammatory, immunomodulatory, and antioxidant properties, a number of studies have tested their impact in animal models of IBD (Table 7).
The oral administration of apple (mainly containing B1, B2, and C1 PACs) or grape seed extract (with 75% PACs: 40% polymers, 14% dimers, 12% trimers, 8% tetramers) has been shown to prevent colonic damage in an experimental colitis model induced by dextran sodium sulfate or oxazolone in rodents (196–199). Accordingly, the mortality rate of the treated animals as well as their weight loss was lower, compared with those receiving the placebo. The grape extract also contributes to the normalization of the ileal villus morphology and mucosal thickness, reducing the histological severity score in the distal ileum and proximal colon (197). It has been postulated that one of the mechanisms implicated in these effects should be the modulation of the proportions of the TCRγδ/TCRαβ intraepithelial lymphocytes. Indeed, these cells play a key role in the maintenance of mucosal homeostasis, contributing to the modulation of the activated release of pro-inflammatory cytokines by epithelial cells and, therefore to the prevention of inflammatory states in the mucosa (196, 200).
Grape seed extract (containing PACs >95%, dimeric >1.8%, oligomers >60%) also exerted a protective effect in recurrent colitis induced by the intracolonic injections of 2,4,6-trinitrobenzene sulfonic acid, reducing the colonic weight/length ratio and the microscopic and macroscopic damage scores (201–204). Such effect relied on the antiinflammatory and antioxidant properties of the extract, as it was shown to inhibit the NF-κB signaling pathway, reducing the expression levels of TNF-α, p-IKKα/β, p-IκBα, and the translocation of NF-κB to the nucleus of colonic epithelial cells (203, 204). As a consequence of these events, the animal treated with the extract exhibited a lower neutrophil infiltration in their colonic mucosa, a decrease of IL-1β concentration, lipid peroxidation, and colonic inducible nitric oxide synthase activity, while the concentrations of IL-2 and IL-4, the antioxidants enzymes activities, and the levels of glutathione increased. These effects were comparable to those obtained with sulfasalazine, the standard drug used for IBD treatment (201–204).
The antiinflammatory properties of polymeric PACs from Pistacia vera L. nuts and apple has been studied in cell models (Caco-2 and T84 cell lines) simulating some conditions of IBD through their activation by pro-inflammatory cytokines such as IL-1β or IFN-γ/IL-1β/TNF-α (205, 206). Apple and pistachio PACs was shown to prevent the cytokine-induced translocation of NF-κB to the nucleus and the subsequent secretion of pro-inflammatory cytokines by these epithelial cells. More specifically, B1 PAC inhibited in a dose-dependent manner the expression of pro-inflammatory genes and repressed NF-κB-, IP-10-, and IL-8-promoters and STAT1-dependent signal transduction (206). The PAC extract from pistachio also contributes to conserve the integrity of IL-1β-stimulated Caco-2 cell monolayers by attenuating the disruption of tight-junctions, preventing the drop in transepithelial electrical resistance and the alterations of the gut barrier function (205).
In a recent in vitro study, we investigated the protective effect of PAC-containing polyphenol extracts from apple, avocado, cranberry, or grape and PACs microbial metabolites on the deleterious effect induced by p-cresol in human colonic epithelial cells (HT-29 and Caco-2). In HT-29 cells, the cranberry and avocado extracts prevented the loss in cell viability (measured as lactate dehydrogenase leakage) and the diminution in ATP contents, while bacterial metabolites only prevented the loss in cell viability. In Caco-2 cells, all extracts and bacterial metabolites prevented the p-cresol-induced alterations of barrier function (measured as transepithelial electrical resistance and fluorescein-dextran transport). These results suggest that PAC-containing polyphenol extracts and PAC metabolites likely contribute to the protection of the colonic mucosa against the deleterious effects of p-cresol (207).
Colorectal cancer is strongly related to dietary habits and is one of the most common cancers worldwide (208). Many studies have assessed the impact of flavonoids including PACs on the risk of this cancer (11, 209–212). In vitro studies using human cells lines derived from colonic adenocarcinoma have reported an inhibitory effect of PACs on cell proliferation and growth (213–215), an increase of apoptosis associated to caspase 3 (213, 215, 216) or caspase 8 activation (217), an arrest of the cell cycle in G1 phase (215), or the suppression of the angiogenic factors vascular endothelial growth factor and angiopoietin 1 (in a model of tumor growth in a xenografted chick chorio-allantoic membrane) (218). In animals, grape seed PACs (0.1–1.0% of diet) inhibited by 72–88% the formation of aberrant colonic crypt foci induced by azoxymethane and by 20–56% the activity of ornithine decarboxylase, an enzyme involved in cell growth and differentiation and related to tumor promotion, in the distal colon (219). In another study using the same model, the administration PACs (0.002%) and PC B2 (0.05%) decreased aberrant crypt foci formation and cell proliferation in the colonic epithelium and increased apoptosis compared with control rats (220).
In humans, a case–control study carried out in Italy reveals a decreased risk of colorectal cancer in the subjects with higher intakes of PACs [odds ratio (OR) = 0.74], being this effect more effective for rectal than for colon cancer (221). Another case–control study in Scotland showed a reduction in the risk of colorectal cancer with the consumption of catechin (OR = 0.68 for the highest vs lowest quartile), epicatechin (OR = 0.74), and PCs (OR = 0.78) (222), while a study in Spain showed a decreased risk in the quartile of highest intake of PAC compared with that of lowest intake [OR = 0.58 CI95% (0.35–0.96), p = 0.02]. In this study, PACs were mainly brought by fruits, wine, and legumes and similar to the Italian study, the protective effect of PACs was more pronounced for rectal than for colon cancer (223). In opposition with these studies, another Scottish case–control study and the prospective Iowa Women’s Health Study did not detect any association between PAC intake and colorectal cancer (224, 225) and Bobe et al., in 1,859 participants of the Polyp Prevention Trial, reported an association between PAC intake and the risk of colorectal adenoma recurrence in men (226).
In conclusion, although in vitro, in vivo, and epidemiologic studies suggest that PACs could act as a chemopreventive agent, further studies are necessary to confirm these results and to clearly establish the subjacent mechanisms implicated and the type and concentration of PACs involved in the protection, prior to use them for the prevention or management of colorectal cancer.
During the postprandial period, high amounts of undigested dietary PACs are found in the gut lumen where they exert great number of activities beneficial for the health host. They contribute to the host defense against pathogens, the modulation of gastric emptying, the inhibition of emetic reflex, and the modulation of the composition of the IM in the colon, in a prebiotic-like manner. They also decrease the inflammatory and pro-oxidant processes occurring in the gastric and colonic mucosa, favoring ulcer healing and contributing to the GI mucosa integrity. In the small intestine, they interfere with the digestion and absorption of carbohydrates, proteins, lipids, and eventually LPS, and can modulate the secretion of GI hormones, the epithelial transport of water and electrolytes, and the GI transit. In the colon, PAC could act reducing the risk of colorectal cancer. Their degradation by the gut microbiota generates several metabolites with protective properties for the colonic epithelium and, when absorbed, for the extra-intestinal tissues. The numerous properties of PACs in the GI tract probably represent a large part of their overall effects on human health and contribute to explain the impact of the consumption of fruits and vegetables against the non-communicable chronic diseases. Due to these properties, they could eventually be used for the dietary management of several GI diseases, or as complementary treatment to attenuate adverse effects associated with the administration of certain drugs. However, further investigations are necessary to fully understand the mechanisms and effects of their use, as well as the doses and formulations necessary to generate the desired effects. Finally, since the beneficial effects of PACs are not only limited to humans, as shown by their positive effects in ruminant nutrition; accordingly, PACs could also be used to improve the productivity in livestock breeding and the healthy properties of animals products.
MC contributed to Sections “Introduction,” “Effect on GI Hormones Secretion and Gastric Emptying,” “Antiemetic Properties,” “Effects of PACs in the Digestion and Absorption of Nutrients,” “Effects of PACs in Diarrhea and GI Motility,” “The PACs in the Colon,” “Effect on the Intestinal Microbiota,” “Protective Effect of PACs on Colorectal Cancer,” and “Con-clusion.” XW contributed to Sections “Absorption of Flavan-3-ols and PACs,” “Intestinal Metabolism and Bioavailability of Flavan-3-ols and PACs,” and “Transformation of PACs by Gut Microbiota.” CC-P contributed to Sections “PACs in the Stomach,” “Protective Effect against Helicobacter pylori (H. pylori),” “Protective Effect against Gastric Inflammation,” and “Protective Effect of PACs in Colonic Inflammation.” MG contributed to Sections “PACs in the Mouth,” “Effect on GI Hormones Secretion and Gastric Emptying,” “Antiemetic Properties,” “Absorption of Flavan-3-ols and PACs,” “Intestinal Metabolism and Bioavailability of Flavan-3-ols and PACs,” “Effects of PACs in Diarrhea and GI Motility,” “Protective Effect of PACs on Colorectal Cancer,” and “Conclusion.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supported by Fondecyt 1120290 and Fondecyt Initiation 11130232 from Conicyt, Chile. MC was recipient of a doctoral fellowship (21120806) from Conicyt Chile.
PACs, proanthocyanidins; DP, degree of polymerization; GI, gastrointestinal; Helicobater pylori, H. pylori; VacA, vacuolating cytotoxin A; HMW, high molecular weight; LMW, low molecular weight; NSAIDs, non-steroidal antiinflammatory drugs; NOS, nitric oxide synthase; iNOS, inducible nitric oxide synthase; SH, sulfhydryl compounds; EGF, epidermal growth factor; PCNA, proliferating cell nuclear antigen; NO, nitric oxide; GLP-1, glucagon-like peptide-1; SGLT1, sodium–glucose cotransporter 1; GLUT, glucose transporter; TG, triglycerides; CS, cholesterol; FFAs, free fatty acids; IBAT, ileal bile acid transporter; AUC, area under the curve; NPC1L1, Niemann–Pick C1-like protein 1; LPS, lipopolysaccharide; IM, intestinal microbiota; SCFAs, short chain fatty acids; IBD, inflammatory bowel diseases; OR, odds ratio.
1. Rodrigo R, Libuy M, Feliu F, Hasson D. Polyphenols in disease: from diet to supplements. Curr Pharm Biotechnol (2014) 15(4):304–17. doi: 10.2174/138920101504140825113815
2. Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl (2011) 50(3):586–621. doi:10.1002/anie.201000044
3. Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal (2013) 18(14):1818–92. doi:10.1089/ars.2012.4581
4. Shoji T. Chemical properties, bioavailability, and metabolomics of fruit proanthocyanidins. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in Human Health and Disease. San Diego: Academic Press (2014). p. 339–51.
5. Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric (2000) 80(7):1094–117. doi:10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1
6. Clifford MN, Crozier A. Phytochemicals in Teas and Tisanes and Their Bioavailability. Teas, Cocoa and Coffee. Oxford: Wiley-Blackwell (2011). p. 45–98.
7. Monagas M, Urpi-Sarda M, Sanchez-Patan F, Llorach R, Garrido I, Gomez-Cordoves C, et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct (2010) 1(3):233–53. doi:10.1039/c0fo00132e
8. Wang W, Bostic TR, Gu L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem (2010) 122(4):1193–8. doi:10.1016/j.foodchem.2010.03.114
9. Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry (2005) 66(18):2281–91. doi:10.1016/j.phytochem.2005.05.022
10. Foo LY, Lu YR, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod (2000) 63(9):1225–8. doi:10.1021/np000128u
11. Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett (2008) 269(2):378–87. doi:10.1016/j.canlet.2008.03.049
12. de la Iglesia R, Milagro FI, Campión J, Boqué N, Martínez JA. Healthy properties of proanthocyanidins. Biofactors (2010) 36(3):159–68. doi:10.1002/biof.79
13. Bladé C, Arola L, Salvadó M-J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res (2010) 54(1):37–59. doi:10.1002/mnfr.200900476
14. Rasmussen SE, Frederiksen H, Struntze Krogholm K, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol Nutr Food Res (2005) 49(2):159–74. doi:10.1002/mnfr.200400082
15. Hanhineva K, Torronen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkanan H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci (2010) 11(4):1365–402. doi:10.3390/ijms11041365
16. Gonzalez-Abuin N, Pinent M, Casanova-Marti A, Arola L, Blay M, Ardevol A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr Med Chem (2015) 22(1):39–50. doi:10.2174/0929867321666140916115519
17. Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr (2005) 81(1 Suppl):230S–42S.
18. Deprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C, et al. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr (2000) 130(11):2733–8.
19. Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med (2005) 38(6):763–72. doi:10.1016/j.freeradbiomed.2004.11.020
20. Rinaldi A, Jourdes M, Teissedre PL, Moio L. A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem (2014) 164:142–9. doi:10.1016/j.foodchem.2014.05.050
21. Tsuchiya H, Sato M, Kato H, Okubo T, Juneja LR, Kim M. Simultaneous determination of catechins in human saliva by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl (1997) 703(1–2):253–8. doi:10.1016/S0378-4347(97)00412-X
22. Weiss E, Lev-Dor R, Sharon N, Ofek I. Inhibitory effect of a high-molecular-weight constituent of cranberry on adhesion of oral bacteria. Crit Rev Food Sci Nutr (2002) 42(3):285–92. doi:10.1080/10408390209351917
23. Kim D, Hwang G, Liu Y, Wang Y, Singh A, Vorsa N, et al. Cranberry flavonoids modulate cariogenic properties of mixed-species biofilm through exopolysaccharides-matrix disruption. PLoS One (2015) 10(12):e0145844. doi:10.1371/journal.pone.0145844
24. Phansalkar R, Nam J, Chen S, McAlpine J, Napolitano J, Leme A, et al. A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia (2015) 101:169–78. doi:10.1016/j.fitote.2014.12.006
25. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med (2001) 345(11):784–9. doi:10.1056/NEJMoa001999
26. Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev (2006) 19(3):449–90. doi:10.1128/CMR.00054-05
27. Kalali B, Mejias-Luque R, Javaheri A, Gerhard M. H. pylori virulence factors: influence on immune system and pathology. Mediators Inflamm (2014) 426309(10):21. doi:10.1155/2014/426309
28. Bauerfeind P, Garner R, Dunn BE, Mobley HL. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut (1997) 40(1):25–30. doi:10.1136/gut.40.1.25
29. Stein M, Rappuoli R, Covacci A. The cag pathogenicity island. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Washington, DC: ASM Press (2001). p. 345–54.
30. Zhang L, Ma J, Pan K, Go VL, Chen J, You WC. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter (2005) 10(2):139–45. doi:10.1111/j.1523-5378.2005.00301.x
31. Cote J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr (2010) 50(7):666–79. doi:10.1080/10408390903044107
32. Gotteland M, Andrews M, Toledo M, Munoz L, Caceres P, Anziani A, et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition (2008) 24(5):421–6. doi:10.1016/j.nut.2008.01.007
33. Shmuely H, Burger O, Neeman I, Yahav J, Samra Z, Niv Y, et al. Susceptibility of Helicobacter pylori isolates to the antiadhesion activity of a high-molecular-weight constituent of cranberry. Diagn Microbiol Infect Dis (2004) 50(4):231–5. doi:10.1016/j.diagmicrobio.2004.08.011
34. Burger O, Ofek I, Tabak M, Weiss EI, Sharon N, Neeman I. A high molecular mass constituent of cranberry juice inhibits helicobacter pylori adhesion to human gastric mucus. FEMS Immunol Med Microbiol (2000) 29(4):295–301. doi:10.1111/j.1574-695X.2000.tb01537.x
35. Burger O, Weiss E, Sharon N, Tabak M, Neeman I, Ofek I. Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit Rev Food Sci Nutr (2002) 42(3 Suppl):279–84. doi:10.1080/10408390209351916
36. Federico F, Francisco AT-B. Flavonoids. Handbook of Analysis of Active Compounds in Functional Foods. Boca Raton, FL: CRC Press (2012). p. 289–316.
37. Rohdewald PJ. Pycnogenol®, French Maritime Pine Bark Extract. Encyclopedia of Dietary Supplements. New York: Taylor & Francis (2005). p. 545–53.
38. Rohdewald P, Beil W. In vitro inhibition of Helicobacter pylori growth and adherence to gastric mucosal cells by Pycnogenol. Phytother Res (2008) 22(5):685–8. doi:10.1002/ptr.2409
39. Kayser O, Kolodziej H. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med (1997) 63(6):508–10. doi:10.1055/s-2006-957752
40. Wittschier N, Lengsfeld C, Vorthems S, Stratmann U, Ernst JF, Verspohl EJ, et al. Large molecules as anti-adhesive compounds against pathogens. J Pharm Pharmacol (2007) 59(6):777–86. doi:10.1211/jpp.59.6.0004
41. Beil W, Kilian P. EPs 7630, an extract from Pelargonium sidoides roots inhibits adherence of Helicobacter pylori to gastric epithelial cells. Phytomedicine (2007) 6:5–8. doi:10.1016/j.phymed.2006.11.024
42. Wittschier N, Faller G, Hensel A. An extract of Pelargonium sidoides (EPs 7630) inhibits in situ adhesion of Helicobacter pylori to human stomach. Phytomedicine (2007) 14(4):285–8. doi:10.1016/j.phymed.2006.12.008
43. Adeniyi BA, Onwubuche BC, Anyiam FM, Ekundayo O, Mahady GB. Anti-Helicobacter pylori activities of Eucalyptus grandis: effects on susceptibility, urease activity and cell surface hydrophobicity. Pharm Biol (2009) 47(1):13–7. doi:10.1080/13880200802397988
44. Pastene E, Troncoso M, Figueroa G, Alarcon J, Speisky H. Association between polymerization degree of apple peel polyphenols and inhibition of Helicobacter pylori urease. J Agric Food Chem (2009) 57(2):416–24. doi:10.1021/jf8025698
45. Pastene E, Parada V, Avello M, Ruiz A, Garcia A. Catechin-based procyanidins from Peumus boldus Mol. aqueous extract inhibit Helicobacter pylori urease and adherence to adenocarcinoma gastric cells. Phytother Res (2014) 28(11):1637–45. doi:10.1002/ptr.5176
46. Yahiro K, Shirasaka D, Tagashira M, Wada A, Morinaga N, Kuroda F, et al. Inhibitory effects of polyphenols on gastric injury by Helicobacter pylori VacA toxin. Helicobacter (2005) 10(3):231–9. doi:10.1111/j.1523-5378.2005.00315.x
47. Ruggiero P, Rossi G, Tombola F, Pancotto L, Lauretti L, Del Giudice G, et al. Red wine and green tea reduce H. pylori- or VacA-induced gastritis in a mouse model. World J Gastroenterol (2007) 13(3):349–54. doi:10.3748/wjg.v13.i3.349
48. Hribova P, Khazneh E, Zemlicka M, Svajdlenka E, Ghoneim MM, Elokely KM, et al. Antiurease activity of plants growing in the Czech Republic. Nat Prod Res (2014) 28(12):868–73. doi:10.1080/14786419.2014.888553
49. Saito M, Hosoyama H, Ariga T, Kataoka S, Yamaji N. Antiulcer activity of grape seed extract and procyanidins. J Agric Food Chem (1998) 46(4):1460–4. doi:10.1021/jf9709156
50. Tadic VM, Dobric S, Markovic GM, Dordevic SM, Arsic IA, Menkovic NR, et al. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem (2008) 56(17):7700–9. doi:10.1021/jf801668c
51. Chai WM, Chen CM, Gao YS, Feng HL, Ding YM, Shi Y, et al. Structural analysis of proanthocyanidins isolated from fruit stone of Chinese hawthorn with potent antityrosinase and antioxidant activity. J Agric Food Chem (2014) 62(1):123–9. doi:10.1021/jf405385j
52. Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am (2010) 39(3):433–64. doi:10.1016/j.gtc.2010.08.010
53. Marlicz W, Loniewski I, Grimes DS, Quigley EM. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: contrasting interactions in the stomach and small intestine. Mayo Clin Proc (2014) 89(12):1699–709. doi:10.1016/j.mayocp.2014.07.015
54. Berenguer B, Trabadela C, Sanchez-Fidalgo S, Quilez A, Mino P, De la Puerta R, et al. The aerial parts of Guazuma ulmifolia Lam. protect against NSAID-induced gastric lesions. J Ethnopharmacol (2007) 114(2):153–60. doi:10.1016/j.jep.2007.07.019
55. Kim TH, Jeon EJ, Cheung DY, Kim CW, Kim SS, Park SH, et al. Gastroprotective effects of grape seed proanthocyanidin extracts against nonsteroid anti-inflammatory drug-induced gastric injury in rats. Gut Liver (2013) 7(3):282–9. doi:10.5009/gnl.2013.7.3.282
56. Gonzalez-Gomez JC, Ayala-Burgos A, Gutierrez-Vazquez E. Total phenols and condensed tannins in tree species with potential as forage sources in the tropics. Livest Res Rural Dev (2006) 18(11):1–5.
57. Moraes Tde M, Rodrigues CM, Kushima H, Bauab TM, Villegas W, Pellizzon CH, et al. Hancornia speciosa: indications of gastroprotective, healing and anti-Helicobacter pylori actions. J Ethnopharmacol (2008) 120(2):161–8. doi:10.1016/j.jep.2008.08.001
58. Hiruma-Lima CA, Rodrigues CM, Kushima H, Moraes TM, Lolis Sde F, Feitosa SB, et al. The anti-ulcerogenic effects of Curatella americana L. J Ethnopharmacol (2009) 121(3):425–32. doi:10.1016/j.jep.2008.10.017
59. Tanae MM, Lima-Landman MT, De Lima TC, Souccar C, Lapa AJ. Chemical standardization of the aqueous extract of Cecropia glaziovii Sneth endowed with antihypertensive, bronchodilator, antiacid secretion and antidepressant-like activities. Phytomedicine (2007) 14(5):309–13. doi:10.1016/j.phymed.2007.03.002
60. Souccar C, Cysneiros RM, Tanae MM, Torres LM, Lima-Landman MT, Lapa AJ. Inhibition of gastric acid secretion by a standardized aqueous extract of Cecropia glaziovii Sneth and underlying mechanism. Phytomedicine (2008) 15(6–7):462–9. doi:10.1016/j.phymed.2008.02.006
61. Santos RC, Kushima H, Rodrigues CM, Sannomiya M, Rocha LR, Bauab TM, et al. Byrsonima intermedia A. Juss.: gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J Ethnopharmacol (2012) 140(2):203–12. doi:10.1016/j.jep.2011.12.008
62. Pierre JF, Heneghan AF, Feliciano RP, Shanmuganayagam D, Roenneburg DA, Krueger CG, et al. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN J Parenter Enteral Nutr (2013) 37(3):401–9. doi:10.1177/0148607112463076
63. Xu X, Xie B, Pan S, Liu L, Wang Y, Chen C. Effects of sea buckthorn procyanidins on healing of acetic acid-induced lesions in the rat stomach. Asia Pac J Clin Nutr (2007) 16(Suppl 1):234–8.
64. Iwasaki Y, Matsui T, Arakawa Y. The protective and hormonal effects of proanthocyanidin against gastric mucosal injury in Wistar rats. J Gastroenterol (2004) 39(9):831–7. doi:10.1007/s00535-004-1399-5
65. Zayachkivska OS, Gzhegotsky MR, Terletska OI, Lutsyk DA, Yaschenko AM, Dzhura OR. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J Physiol Pharmacol (2006) 57(Suppl 5):155–67.
66. González-Abuín N, Martínez-Micaelo N, Blay M, Ardévol A, Pinent M. Grape-seed procyanidins prevent the cafeteria-diet-induced decrease of glucagon-like peptide-1 production. J Agric Food Chem (2014) 62(5):1066–72. doi:10.1021/jf405239p
67. Serrano J, Casanova-Marti A, Gil-Cardoso K, Blay MT, Terra X, Pinent M, et al. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct (2016) 7(1):483–90. doi:10.1039/c5fo00892a
68. Ko JL, Tsai CH, Liu TC, Lin MY, Lin HL, Ou CC. Differential effects of grape juice on gastric emptying and renal function from cisplatin-induced acute adverse toxicity. Hum Exp Toxicol (2016) 35(8):808–17. doi:10.1177/0960327115607079
69. Miller MJ, Reuter BK, Wallace JL, Sharkey KA. A unique therapeutic approach to emesis and itch with a proanthocyanidin-rich genonutrient. J Transl Med (2008) 6:3. doi:10.1186/1479-5876-6-3
70. Li QZ, Cho HS, Jeun SH, Kim KJ, Choi SJ, Sung KW. Effects of grape seed proanthocyanidin on 5-hydroxytryptamine3 receptors in NCB-20 neuroblastoma cells. Biol Pharm Bull (2011) 34(7):1109–15. doi:10.1248/bpb.34.1109
71. Spencer JP, Chaudry F, Pannala AS, Srai SK, Debnam E, Rice-Evans C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun (2000) 272(1):236–41. doi:10.1006/bbrc.2000.2749
72. Rios LY, Bennett RN, Lazarus SA, Remesy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr (2002) 76(5):1106–10.
73. Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signal (2001) 3(6):957–67. doi:10.1089/152308601317203503
74. Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med (2003) 35(8):837–44. doi:10.1016/S0891-5849(03)00394-0
75. Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (-)-epicatechin and their mixture in orally administered rats. J Nutr (2001) 131(11):2885–91.
76. Donovan JL, Crespy V, Manach C, Morand C, Besson C, Scalbert A, et al. Catechin is metabolized by both the small intestine and liver of rats. J Nutr (2001) 131(6):1753–7.
77. Kivits GAA, van der Sman FJP, Tijburg LBM. Analysis of catechins from green and black tea in humans: a specific and sensitive colorimetric assay of total catechins in biological fluids. Int J Food Sci Nutr (1997) 48(6):387–92. doi:10.3109/09637489709028587
78. Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr (2008) 138(8):1535S–42S.
79. Donovan JL, Manach C, Rios L, Morand C, Scalbert A, Remesy C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr (2002) 87(4):299–306. doi:10.1079/BJN2001517
80. Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, et al. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr (2005) 94(2):170–81. doi:10.1079/BJN20051480
81. Shoji T, Masumoto S, Moriichi N, Akiyama H, Kanda T, Ohtake Y, et al. Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem (2006) 54(3):884–92. doi:10.1021/jf052260b
82. Choy YY, Jaggers GK, Oteiza PI, Waterhouse AL. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J Agric Food Chem (2013) 61:121–7. doi:10.1021/jf301939e
83. Jimenez-Ramsey LM, Rogler JC, Housley TL, Butler LG, Elkin RG. Absorption and distribution of 14C-labeled condensed tannins and related sorghum phenolics in chickens. J Agric Food Chem (1994) 42(4):963–7. doi:10.1021/jf00040a024
84. Terrill TH, Waghorn GC, Woolley DJ, McNabb WC, Barry TN. Assay and digestion of 14C-labelled condensed tannins in the gastrointestinal tract of sheep. Br J Nutr (1994) 72(3):467–77. doi:10.1079/BJN19940048
85. Choy YY, Quifer-Rada P, Holstege DM, Frese SA, Calvert CC, Mills DA, et al. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct (2014) 5(9):2298–308. doi:10.1039/c4fo00325j
86. Kahle K, Huemmer W, Kempf M, Scheppach W, Erk T, Richling E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J Agric Food Chem (2007) 55(26):10605–14. doi:10.1021/jf071942r
87. Van Amelsvoort JM, Van Hof KH, Mathot JN, Mulder TP, Wiersma A, Tijburg LB. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica (2001) 31(12):891–901. doi:10.1080/00498250110079149
88. Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr (2000) 130(8):2073S–85S.
89. Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, et al. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr (2003) 42(1):29–42. doi:10.1007/s00394-003-0397-3
90. Henry-Vitrac C, Desmouliere A, Girard D, Merillon JM, Krisa S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur J Nutr (2006) 45(7):376–82. doi:10.1007/s00394-006-0609-8
91. Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition (2002) 18(1):75–81. doi:10.1016/S0899-9007(01)00695-5
92. Ahmed Nasef N, Mehta S, Ferguson LR. Dietary interactions with the bacterial sensing machinery in the intestine: the plant polyphenol case. Front Genet (2014) 5:64. doi:10.3389/fgene.2014.00064
93. Cardona F, Andrés-Lacueva C, Tulipania S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem (2013) 24(8):1415–22. doi:10.1016/j.jnutbio.2013.05.001
94. Stalmach A, Mullen W, Steiling H, Williamson G, Lean ME, Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res (2010) 54(3):323–34. doi:10.1002/mnfr.200900194
95. Hollman PCH, Arts ICW. Flavonols, flavones and flavanols – nature, occurrence and dietary burden. J Sci Food Agric (2000) 80(7):1081–93. doi:10.1002/(SICI)1097-0010(20000515)80:7<1081::AID-JSFA566>3.0.CO;2-G
96. García-Ramírez B, Fernández-Larrea J, Salvadó MJ, Ardèvol A, Arola L, Bladé C. Tetramethylated dimeric procyanidins are detected in rat plasma and liver early after oral administration of synthetic oligomeric procyanidins. J Agric Food Chem (2006) 54(7):2543–51. doi:10.1021/jf0527753
97. Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev (1995) 4(4):393–9.
98. Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med (2004) 70(12):1103–14. doi:10.1055/s-2004-835835
99. Aura AM. Colon-derived microbial metabolites of dietary phenolic compounds. In: Crozier A, editor. Flavonoids and Related Compounds: Bioavailability and Function. CRC Press (2012). doi:10.1201/b11872-11
100. Scalbert A, Deprez S, Mila I, Albrecht AM, Huneau JF, Rabot S. Proanthocyanidins and human health: systemic effects and local effects in the gut. Biofactors (2000) 13(1–4):115–20. doi:10.1002/biof.5520130119
101. Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem (1981) 256(9):4494–7.
102. Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res (2013) 57(1):48–57. doi:10.1002/mnfr.201200511
103. Barrett A, Ndou T, Hughey CA, Straut C, Howell A, Dai Z, et al. Inhibition of alpha-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J Agric Food Chem (2013) 61(7):1477–86. doi:10.1021/jf304876g
104. Guzmán-Maldonado H, Paredes-López O, Biliaderis CG. Amylolytic enzymes and products derived from starch: a review. Crit Rev Food Sci Nutr (1995) 35(5):373–403. doi:10.1080/10408399509527706
105. Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol (2007) 53(3):287–92. doi:10.3177/jnsv.53.287
106. Hassan HMM. Inhibitory effects of red grape seed extracts on pancreatic α-amylase and lipase. Glob J Biotechnol Biochem (2014) 9(4):130–6.
107. Adisakwattana S, Lerdsuwankij O, Poputtachai U, Minipun A, Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal alpha-glucosidase and pancreatic alpha-amylase. Plant Food Hum Nutr (2011) 66(2):143–8. doi:10.1007/s11130-011-0226-4
108. Schäfer A, Högger P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol®) effectively inhibit α-glucosidase. Diabetes Res Clin Pract (2007) 77(1):41–6. doi:10.1016/j.diabres.2006.10.011
109. Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol®), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther (2002) 40(4):158–68. doi:10.5414/CPP40158
110. Zhang HW, Yerigui YY, Ma CM. Structures and antioxidant and intestinal disaccharidase inhibitory activities of A-type proanthocyanidins from peanut skin. J Agric Food Chem (2013) 61(37):8814–20. doi:10.1021/jf402518k
111. Tsujita T, Shintani T, Sato H. Preparation and characterisation of peanut seed skin polyphenols. Food Chem (2014) 151:15–20. doi:10.1016/j.foodchem.2013.11.072
112. Tsujita T, Shintani T, Sato H. alpha-Amylase inhibitory activity from nut seed skin polyphenols. 1. Purification and characterization of almond seed skin polyphenols. J Agric Food Chem (2013) 61(19):4570–6. doi:10.1021/jf400691q
113. Naz S, Siddiqi R, Dew TP, Williamson G. Epigallocatechin-3-gallate inhibits lactase but is alleviated by salivary proline-rich proteins. J Agric Food Chem (2011) 59(6):2734–8. doi:10.1021/jf103072z
114. Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, et al. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition (2007) 23(4):351–5. doi:10.1016/j.nut.2007.01.007
115. Kawakami K, Aketa S, Nakanami M, Iizuka S, Hirayama M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their alpha-amylase inhibitory activity. Biosci Biotechnol Biochem (2010) 74(7):1380–5. doi:10.1271/bbb.100056
116. Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr (2003) 89(1):3–9. doi:10.1079/BJN2002763
117. Thorens B. Facilitated glucose transporters in epithelial cells. Annu Rev Physiol (1993) 55:591–608. doi:10.1146/annurev.ph.55.030193.003111
118. Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab (2009) 296(5):E985–92. doi:10.1152/ajpendo.00004.2009
119. Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J (2000) 350(Pt 1):155–62. doi:10.1042/bj3500155
120. Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J (2000) 350(Pt 1):149–54. doi:10.1042/bj3500149
121. Farrell TL, Ellam SL, Forrelli T, Williamson G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: interactions with SGLT1 and GLUT2 transporters. Biofactors (2013) 39(4):448–56. doi:10.1002/biof.1090
122. Alzaid F, Cheung HM, Preedy VR, Sharp PA. Regulation of glucose transporter expression in human intestinal caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS One (2013) 8(11):e78932. doi:10.1371/journal.pone.0078932
123. Oliveira DM, Freitas HS, Souza MF, Arcari DP, Ribeiro ML, Carvalho PO, et al. Yerba mate (Ilex paraguariensis) aqueous extract decreases intestinal SGLT1 gene expression but does not affect other biochemical parameters in alloxan-diabetic Wistar rats. J Agric Food Chem (2008) 56(22):10527–32. doi:10.1021/jf8021404
124. Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, et al. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res (2014) 58(9):1795–808. doi:10.1002/mnfr.201400016
125. de la Garza AL, Etxeberria U, Lostao MP, San Roman B, Barrenetxe J, Martinez JA, et al. Helichrysum and grapefruit extracts inhibit carbohydrate digestion and absorption, improving postprandial glucose levels and hyperinsulinemia in rats. J Agric Food Chem (2013) 61(49):12012–9. doi:10.1021/jf4021569
126. Cermak R, Landgraf S, Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br J Nutr (2004) 91(6):849–55. doi:10.1079/BJN20041128
127. Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res (2010) 54:1773–80. doi:10.1002/mnfr.201000019
128. Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, et al. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem (2000) 48(11):5618–23. doi:10.1021/jf0006832
129. Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett (2005) 579(7):1653–7. doi:10.1016/j.febslet.2004.12.099
130. Constanzo LS. Gastrointestinal physiology. 4 ed. In: Constanzo LS, editor. Physiology. Philadelphia: Saunders Elsevier (2010). p. 327–78.
131. Masson CJ, Plat J, Mensink RP, Namiot A, Kisielewski W, Namiot Z, et al. Fatty acid- and cholesterol transporter protein expression along the human intestinal tract. PLoS One (2010) 5(4):e10380. doi:10.1371/journal.pone.0010380
132. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab (2009) 296(6):E1183–94. doi:10.1152/ajpendo.90899.2008
133. Redinger RN. Nuclear receptors in cholesterol catabolism: molecular biology of the enterohepatic circulation of bile salts and its role in cholesterol homeostasis. J Lab Clin Med (2003) 142(1):7–20. doi:10.1016/S0022-2143(03)00088-X
134. Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, et al. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem (2007) 55(11):4604–9. doi:10.1021/jf078004b
135. Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, et al. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and tri-glyceride absorption. J Agric Food Chem (2007) 55(14):5906. doi:10.1021/jf078004b
136. Gu Y, Hurst WJ, Stuart DA, Lambert JD. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem (2011) 59(10):5305–11. doi:10.1021/jf200180n
137. Wong X, Madrid AM, Tralma K, Castillo R, Carrasco-Pozo C, Navarrete P, et al. Polyphenol extracts interfere with bacterial lipopolysaccharide in vitro and decrease postprandial endotoxemia in human volunteers. J Funct Foods (2016) 26:406–17. doi:10.1016/j.jff.2016.08.011
138. Matsumoto K, Kadowaki A, Ozaki N, Takenaka M, Ono H, Yokoyama S, et al. Bile acid-binding ability of kaki-tannin from young fruits of persimmon (Diospyros kaki) in vitro and in vivo. Phytother Res (2011) 25(4):624–8. doi:10.1002/ptr.3306
139. Adisakwattana S, Moonrat J, Srichairat S, Chanasit C, Tirapongporn H, Chanathong B, et al. Lipid-Lowering mechanisms of grape seed extract (Vitis vinifera L) and its antihyperlidemic activity. J Med Plants Res (2010) 4(20):2113–20.
140. Qin BL, Dawson HD, Schoene NW, Polansky MM, Anderson RA. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition (2012) 28(11–12):1172–9. doi:10.1016/j.nut.2012.03.020
141. Quesada H, Diaz S, Pajuelo D, Fernandez-Iglesias A, Garcia-Vallve S, Pujadas G, et al. The lipid-lowering effect of dietary proanthocyanidins in rats involves both chylomicron-rich and VLDL-rich fractions. Br J Nutr (2012) 108(2):208–17. doi:10.1017/S0007114511005472
142. Delehanty JB, Johnson BJ, Hickey TE, Pons T, Ligler FS. Binding and neutralization of lipopolysaccharides by plant proanthocyanidins. J Nat Prod (2007) 70(11):1718–24. doi:10.1021/np0703601
143. Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res (2009) 50(1):90–7. doi:10.1194/jlr.M800156-JLR200
144. Cani PD, Bibiloni R, Knauf C, Neyrinck AM, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes (2008) 57(6):1470–81. doi:10.2337/db07-1403
146. Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev (2008) 88(1):249–86. doi:10.1152/physrev.00018.2006
147. Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology (1997) 113(1):332–40. doi:10.1016/S0016-5085(97)70112-4
148. Goncalves R, Soares S, Mateus N, de Freitas V. Inhibition of trypsin by condensed tannins and wine. J Agric Food Chem (2007) 55(18):7596–601. doi:10.1021/jf071490i
149. Gonçalves R, Mateus N, Pianet I, Laguerre M, de Freitas V. Mechanisms of tannin-induced trypsin inhibition: a molecular approach. Langmuir (2011) 27(21):13122–9. doi:10.1021/la202280c
150. Horigome T, Kumar R, Okamoto K. Effects of condensed tannins prepared from leaves of fodder plants on digestive enzymes in vitro and in the intestine of rats. Br J Nutr (1988) 60(2):275–85. doi:10.1079/BJN19880099
151. Brás NF, Goncalves R, Mateus N, Fernandes PA, Ramos MJ, de Freitas V. Inhibition of pancreatic elastase by polyphenolic compounds. J Agric Food Chem (2010) 58(19):10668–76. doi:10.1021/jf1017934
152. Brás NF, Goncalves R, Fernandes PA, Mateus N, Ramos MJ, de Freitas V. Understanding the binding of procyanidins to pancreatic elastase by experimental and computational methods. Biochemistry (2010) 49(25):5097–108. doi:10.1021/bi100410q
153. Uchida S, Ikari N, Ohta H, Niwa M, Nonaka G, Nishioka I, et al. Inhibitory effects of condensed tannins on angiotensin converting enzyme. Jpn J Pharmacol (1987) 43(2):242–6. doi:10.1254/jjp.43.242
154. Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res (2013) 69(1):95–107. doi:10.1016/j.phrs.2013.01.003
155. Patra AK, Saxena J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J Sci Food Agric (2011) 91(1):24–37. doi:10.1002/jsfa.4152
156. Cássia Santos R, Kushima H, Martins Rodrigues C, Sannomiya M, Machado Rocha LR, Bauab TM, et al. Byrsonima intermedia A. Juss.: gastric and duodenal anti-ulcer, antimicrobial and antidiarrheal effects in experimental rodent models. J Ethnopharmacol (2012) 140:203–12. doi:10.1016/j.jep.2011.12.008
157. Velazquez C, Calzada F, Esquivel B, Barbosa E, Calzada S. Antisecretory activity from the flowers of Chiranthodendron pentadactylon and its flavonoids on intestinal fluid accumulation induced by Vibrio cholerae toxin in rats. J Ethnopharmacol (2009) 126(3):455–8. doi:10.1016/j.jep.2009.09.016
158. Valcheva-Kuzmanova S, Kuzmanov K. Inhibitory effect of Aronia melanocarpa fruit juice on intestinal transit rate in rats. Acta Aliment (2011) 40(3):396–9. doi:10.1556/AAlim.2010.0009
159. Rozhon EJ, Khandwala AS, Sabouni A, Balwani GP, Chan JW-H, Sesin DF. Method of Treating Secretory Diarrhea with Enteric Formulations of Proanthocyanidin Polymer. US Patent No 7341744 B1 (2008).
160. Etxeberria U, Fernandez-Quintela A, Milagro FI, Aguirre L, Martinez JA, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem (2013) 61(40):9517–33. doi:10.1021/jf402506c
161. Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res (2010) 61(3):219–25. doi:10.1016/j.phrs.2009.11.001
162. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr (1995) 125(6):1401–12.
163. Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol (2012) 302(1):G1–9. doi:10.1152/ajpgi.00048.2011
164. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr (2015) 54(3):325–41. doi:10.1007/s00394-015-0852-y
165. Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut (2016) 65(2):330–9. doi:10.1136/gutjnl-2015-309990
166. Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr (2007) 137(3 Suppl 2):751S–5S.
167. Appeldoorn MM, Vincken JP, Aura AM, Hollman PC, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites. J Agric Food Chem (2009) 57(3):1084–92. doi:10.1021/jf803059z
168. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther (2008) 27(2):104–19. doi:10.1111/j.1365-2036.2007.03562.x
169. Blachier F, Mariotti F, Huneau JF, Tome D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids (2007) 33(4):547–62. doi:10.1007/s00726-006-0477-9
170. Brunser O, Gotteland M. Probiotics and prebiotics in human health: an overview. In: Watson R, Preedy V, editors. Bioactive Foods in Promoting Health: Probiotics and Prebiotics. Kidlington: Academic Press (2010). p. 73–93.
171. Cardona F, Andres-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuno MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem (2013) 24(8):1415–22. doi:10.1016/j.jnutbio.2013.05.001
172. Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, et al. Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr (2008) 99(4):782–92. doi:10.1017/S0007114507853384
173. Cueva C, Sanchez-Patan F, Monagas M, Walton GE, Gibson GR, Martin-Alvarez PJ, et al. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: changes in microbial groups and phenolic metabolites. FEMS Microbiol Ecol (2013) 83(3):792–805. doi:10.1111/1574-6941.12037
174. Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr (2011) 93(1):62–72. doi:10.3945/ajcn.110.000075
175. Yamakoshi J, Tokutake S, Kikuchi M, Konishi H, Mitsuoka T. Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and fecal odor. Microb Ecol Health Dis (2001) 13:25–31.
176. Walle TW, Walgren RA, Walle K, Galijatovic A, Vaidyanathan J. Understanding the bioavailability of flavonoids through studies in caco-2 cells. In: Rice-Evans CA, Packer L, editors. Flavonoids in Health and Disease. New York, NY: Marcel Dekker, Inc (2003). p. 349–61.
177. Aura A-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem Rev (2008) 7(3):407–29. doi:10.1007/s11101-008-9095-3
178. Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem (2009) 57(15):6485–501. doi:10.1021/jf902107d
179. Roowi S, Stalmach A, Mullen W, Lean ME, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem (2010) 58(2):1296–304. doi:10.1021/jf9032975
180. Das NP. Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man. Biochem Pharmacol (1971) 20(12):3435–45. doi:10.1016/0006-2952(71)90449-7
181. Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol (2000) 13(3):177–84. doi:10.1021/tx9901837
182. Ottaviani JI, Kwik-Uribe C, Keen CL, Schroeter H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am J Clin Nutr (2012) 95(4):851–8. doi:10.3945/ajcn.111.028340
183. Aura A-M, Mattila I, Seppänen-Laakso T, Miettinen J, Oksman-Caldentey K-M, Orešič M. Microbial metabolism of catechin stereoisomers by human faecal microbiota: comparison of targeted analysis and a non-targeted metabolomics method. Phytochem Lett (2008) 1(1):18–22. doi:10.1016/j.phytol.2007.12.001
184. Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, Mila I, et al. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr (2003) 133(2):461–7.
185. van’t Slot G, Humpf HU. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J Agric Food Chem (2009) 57(17):8041–8. doi:10.1021/jf900458e
186. Engemann A, Hubner F, Rzeppa S, Humpf HU. Intestinal metabolism of two A-type procyanidins using the pig cecum model: detailed structure elucidation of unknown catabolites with Fourier transform mass spectrometry (FTMS). J Agric Food Chem (2012) 60(3):749–57. doi:10.1021/jf203927g
187. Meselhy MR, Nakamura N, Hattori M. Biotransformation of (-)-epicatechin 3-O-gallate by human intestinal bacteria. Chem Pharm Bull (Tokyo) (1997) 45(5):888–93. doi:10.1248/cpb.45.888
188. Rios LY, Gonthier MP, Remesy C, Mila I, Lapierre C, Lazarus SA, et al. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr (2003) 77(4):912–8.
189. Urpi-Sarda M, Monagas M, Khan N, Lamuela-Raventos RM, Santos-Buelga C, Sacanella E, et al. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem (2009) 394(6):1545–56. doi:10.1007/s00216-009-2676-1
190. Ward NC, Croft KD, Puddey IB, Hodgson JM. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic Acid, an important metabolite of proanthocyanidins in humans. J Agric Food Chem (2004) 52(17):5545–9. doi:10.1021/jf049404r
191. van Dorsten FA, Peters S, Gross G, Gomez-Roldan V, Klinkenberg M, de Vos RC, et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J Agric Food Chem (2012) 60(45):11331–42. doi:10.1021/jf303165w
192. Bazzocco S, Mattila I, Guyot S, Renard CM, Aura AM. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur J Nutr (2008) 47(8):442–52. doi:10.1007/s00394-008-0747-2
193. Ou K, Sarnoski P, Schneider KR, Song K, Khoo C, Gu L. Microbial catabolism of procyanidins by human gut microbiota. Mol Nutr Food Res (2014) 58(11):2196–205. doi:10.1002/mnfr.201400243
194. Carrasco-Pozo C, Gotteland M, Castillo RL, Chen C. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic beta-cells dysfunction induced by high cholesterol. Exp Cell Res (2015) 334(2):270–82. doi:10.1016/j.yexcr.2015.03.021
195. Amiot A, Peyrin-Biroulet L. Current, new and future biological agents on the horizon for the treatment of inflammatory bowel diseases. Therap Adv Gastroenterol (2015) 8(2):66–82. doi:10.1177/1756283X14558193
196. Yoshioka Y, Akiyama H, Nakano M, Shoji T, Kanda T, Ohtake Y, et al. Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int Immunopharmacol (2008) 8(13–14):1802–7. doi:10.1016/j.intimp.2008.08.021
197. Cheah KY, Bastian SE, Acott TM, Abimosleh SM, Lymn KA, Howarth GS. Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Dig Dis Sci (2013) 58(4):970–7. doi:10.1007/s10620-012-2464-1
198. Shoji T, Mutsuga M, Nakamura T, Kanda T, Akiyama H, Goda Y. Isolation and structural elucidation of some procyanidins from apple by low-temperature nuclear magnetic resonance. J Agric Food Chem (2003) 51(13):3806–13. doi:10.1021/jf0300184
199. Yang T, Li X, Zhu W, Chen C, Sun Z, Tan Z, et al. Alteration of antioxidant enzymes and associated genes induced by grape seed extracts in the primary muscle cells of goats in vitro. PLoS One (2014) 9(9):e107670. doi:10.1371/journal.pone.0107670
200. Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A (2002) 99(22):14338–43. doi:10.1073/pnas.212290499
201. Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol (2008) 86(12):841–9. doi:10.1139/Y08-089
202. Wang YH, Yang XL, Wang L, Cui MX, Cai YQ, Li XL, et al. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can J Physiol Pharmacol (2010) 88(9):888–98. doi:10.1139/Y10-071
203. Wang YH, Ge B, Yang XL, Zhai J, Yang LN, Wang XX, et al. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol (2011) 11(10):1620–7. doi:10.1016/j.intimp.2011.05.024
204. Li X, Yang X, Cai Y, Qin H, Wang L, Wang Y, et al. Proanthocyanidins from grape seeds modulate the NF-kappaB signal transduction pathways in rats with TNBS-induced ulcerative colitis. Molecules (2011) 16(8):6721–31. doi:10.3390/molecules16086721
205. Gentile C, Perrone A, Attanzio A, Tesoriere L, Livrea MA. Sicilian pistachio (Pistacia vera L.) nut inhibits expression and release of inflammatory mediators and reverts the increase of paracellular permeability in IL-1beta-exposed human intestinal epithelial cells. Eur J Nutr (2015) 54(5):811–21. doi:10.1007/s00394-014-0760-6
206. Jung M, Triebel S, Anke T, Richling E, Erkel G. Influence of apple polyphenols on inflammatory gene expression. Mol Nutr Food Res (2009) 53(10):1263–80. doi:10.1002/mnfr.200800575
207. Wong X, Carrasco-Pozo C, Escobar E, Navarrete P, Blachier F, Andriamihaja M, et al. Deleterious effect of p-cresol on human colonic epithelial cells prevented by proanthocyanidin-containing polyphenol extracts from fruits and proanthocyanidin bacterial metabolites. J Agric Food Chem (2016) 64(18):3574–83. doi:10.1021/acs.jafc.6b00656
208. WHO Organization. Key Facts About Cancer. (2016). Available from: http://www.who.int/cancer/about/facts/en/
209. Katiyar SK, Athar M. Grape seeds: ripe for cancer chemoprevention. Cancer Prev Res (Phila) (2013) 6(7):617–21. doi:10.1158/1940-6207.CAPR-13-0193
210. Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr (2009) 139(9):1806S–12S. doi:10.3945/jn.109.106864
211. Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res (2011) 55(1):32–45. doi:10.1002/mnfr.201000412
212. Woo HD, Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J Gastroenterol (2013) 19(7):1011–9. doi:10.3748/wjg.v19.i7.1011
213. Hsu C, Lin Y, Chou C, Zhou S, Hsu Y, Liu C, et al. Mechanisms of grape seed procyanidin-induced apoptosis in colorectal carcinoma cells. Anticancer Res (2009) 29(1):283–9.
214. Yi W, Fischer J, Krewer G, CC A. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J Agric Food Chem (2005) 53:7320–9. doi:10.1021/jf051333o
215. Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res (2006) 12(20 Pt 1):6194–202. doi:10.1158/1078-0432.CCR-06-1465
216. Kim YJ, Park HJ, Yoon SH, Kim MJ, Leem KH, Chung JH, et al. Anticancer effects of oligomeric proanthocyanidins on human colorectal cancer cell line, SNU-C4. World J Gastroenterol (2005) 11(30):4674–8. doi:10.3748/wjg.v11.i30.4674
217. Minker C, Duban L, Karas D, Jarvinen P, Lobstein A, Muller CD. Impact of procyanidins from different berries on caspase 8 activation in colon cancer. Oxid Med Cell Longev (2015) 2015:154164. doi:10.1155/2015/154164
218. Huang S, Yang N, Liu Y, Gao J, Huang T, Hu L, et al. Grape seed proanthocyanidins inhibit colon cancer-induced angiogenesis through suppressing the expression of VEGF and Ang1. Int J Mol Med (2012) 30(6):1410–6. doi:10.3892/ijmm.2012.1147
219. Singletary KW, Meline B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer (2001) 39(2):252–8. doi:10.1207/S15327914nc392_15
220. Nomoto H, Iigo M, Hamada H, Kojima S, Tsuda H. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr Cancer (2004) 49(1):81–8. doi:10.1207/s15327914nc4901_11
221. Rossi M, Negri E, Parpinel M, Lagiou P, Bosetti C, Talamini R, et al. Proanthocyanidins and the risk of colorectal cancer in Italy. Cancer Causes Control (2010) 21(2):243–50. doi:10.1007/s10552-009-9455-3
222. Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, et al. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev (2007) 16(4):684–93. doi:10.1158/1055-9965.EPI-06-0785
223. Zamora-Ros R, Not C, Guinó E, Luján-Barroso L, García RM, Biondo S, et al. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case–control study (the Bellvitge Colorectal Cancer Study). Cancer Causes Control (2013) 24:549–57. doi:10.1007/s10552-012-9992-z
224. Kyle JA, Sharp L, Little J, Duthie GG, McNeill G. Dietary flavonoid intake and colorectal cancer: a case-control study. Br J Nutr (2010) 103(3):429–36. doi:10.1017/S0007114509991784
225. Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR Jr, Scrafford CG, et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women’s Health Study. Int J Cancer (2008) 123(3):664–71. doi:10.1002/ijc.23564
Keywords: proanthocyanidins, gastrointestinal tract, Helicobacter pylori, intestinal microbiota, digestive enzymes
Citation: Cires MJ, Wong X, Carrasco-Pozo C and Gotteland M (2017) The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front. Nutr. 3:57. doi: 10.3389/fnut.2016.00057
Received: 16 October 2016; Accepted: 13 December 2016;
Published: 03 January 2017
Edited by:
Marco Falasca, Curtin University, AustraliaReviewed by:
Marcello Iriti, University of Milan, ItalyCopyright: © 2017 Cires, Wong, Carrasco-Pozo and Gotteland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María José Cires, bWFyaWFqb3NlY2lyZXNAZ21haWwuY29t;
Catalina Carrasco-Pozo, Y2F0YWxpbmFjYXJyYXNjb0BtZWQudWNoaWxlLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.