- 1Institute of Biosciences of Botucatu, Campus Botucatu, São Paulo State University (UNESP), Botucatu, SP, Brazil
- 2School of Sciences, Campus Bauru, São Paulo State University (UNESP), Bauru, SP, Brazil
- 3Harvard T. Chan School of Public Health, Harvard University, Boston, MA, United States
Research indicates that by 2050, more than 150 million people will be living with Alzheimer's disease (AD), a condition associated with neurodegeneration due to the accumulation of amyloid-beta and tau proteins. In addition to genetic background, endocrine disruption, and cellular senescence, management of the gut microbiota has emerged as a key element in the diagnosis, progression, and treatment of AD, as certain bacterial metabolites can travel through the bloodstream and cross the blood-brain barrier. This mini-review explores the relationship between tau protein accumulation and gut dysbiosis in Drosophila melanogaster. This model facilitates the investigation of how gut-derived metabolites contribute to neurocognitive impairment and dementia. Understanding the role of direct and indirect bacterial by-products, such as lactate and acetate, in glial cell activation and tau protein dynamics may provide insights into the mechanisms of AD progression and contribute to more effective treatments. Here we discuss how the simplicity and extensive genetic tools of Drosophila make it a valuable model for studying these interactions and testing potential therapeutics, including probiotics. Integrating Drosophila studies with other established models may reveal conserved pathways and accelerate the translation of findings into clinical applications.
1 Introduction
The incidence of Alzheimer's disease (AD) has notably increased in recent years. The number of patients is projected to triple by 2050 (Scheltens et al., 2021), causing not only suffering to family, friends and caregivers (Beata et al., 2023), but deeply consequences to health systems. The condition is strongly associated to the accumulation of amyloid-beta (Aβ) and tau proteins (Palmqvist et al., 2021; Hou et al., 2019; Rydbom et al., 2021), but a secondary approach leveraging the influence of the intestinal tract on the brain has been established (Vogt et al., 2017; Pluta et al., 2020). The modulation of the microorganisms found in the gut showed a new path to treat the disease, since the molecules produced by the microbiota can reach neurons and glial cells and influence them in several ways (Huang et al., 2023; Mayneris-Perxachs et al., 2022).
In addition to the traditional murine models, Drosophila melanogaster is an inexpensive, genetically modulable and easily reproducible model (Jennings, 2011) that is currently used in AD and microbiota-related research (Rydbom et al., 2021; Kong et al., 2018; Tan et al., 2020). In addition, the molecular and cellular conserved aspects of Drosophila support its use in intestinal epithelium (Apidianakis and Rahme, 2011), and brain-gut communication research (Makdissi et al., 2023; Kitani-Morii et al., 2021), encouraging new AD diagnosis insights from an interorgan perspective.
Aspects of Drosophila gut microbiota are common with humans, including bacterial genera such as Acetobacter and Lactobacillus (Simhadri et al., 2017), which produce acetic acid and lactic acid, respectively. The undissociated form of acetic acid, acetate, presents anti-neuroinflammatory properties (Liu et al., 2020), while lactate modulates aging in flies (Long et al., 2020) and is transported from glial cells to neurons, where it is utilized in the tricarboxylic acid cycle (Xu et al., 2023; Volkenhoff et al., 2015). These organisms play a fundamental role in disrupted energy metabolism associated with AD.
Glial cell types such as microglia participates in tau protein engulfment and neuroprotection in both zebrafish (Hassan-Abdi et al., 2019) and mammals (Freeman and Doherty, 2006; Yildirim et al., 2019). Since its Drosophila flies' counterparts—neuropil, cortex, and ensheathing glia (Freeman and Doherty, 2006; Doherty et al., 2009)—also play a role in tau phagocytosis, the activation of ensheathing glia is believed to be crucial for elucidating the pathways involved in tau protein generation (Figure 1A).
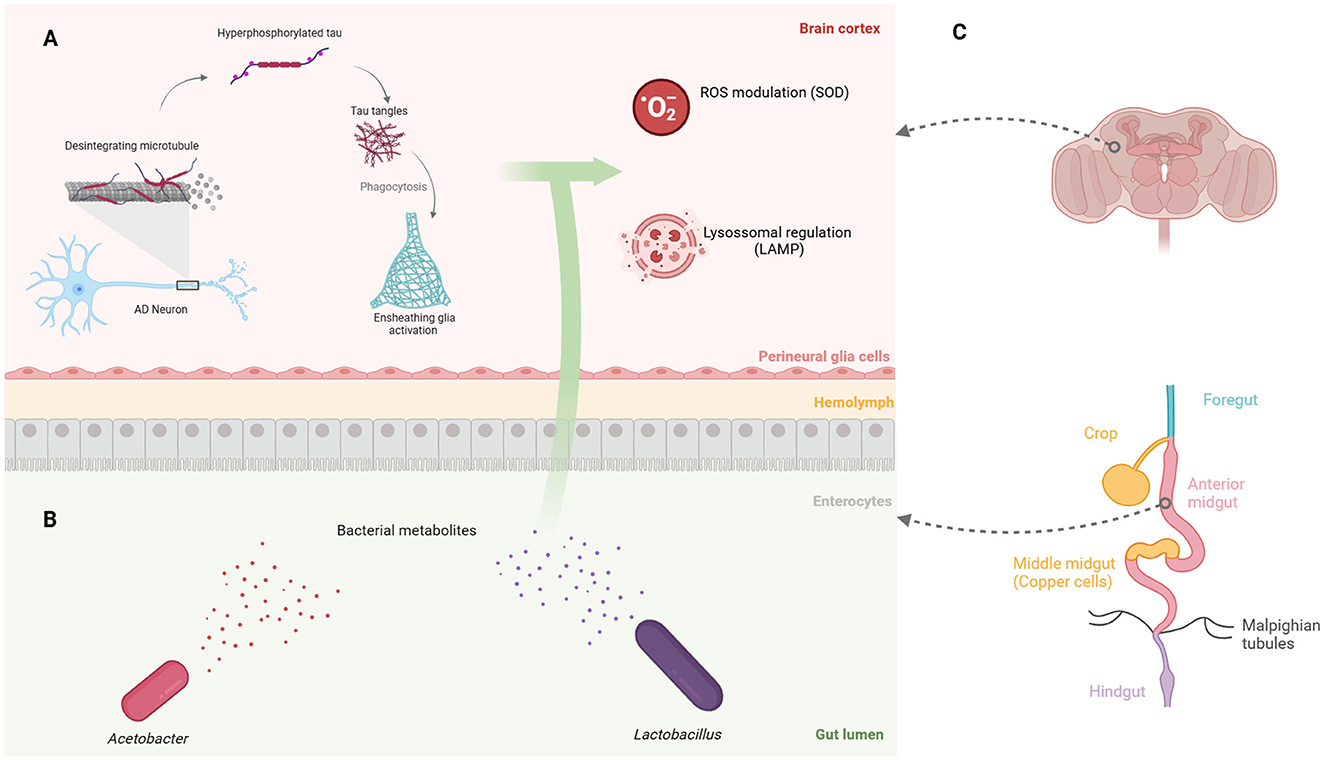
Figure 1. Gut bacterial metabolites in Drosophila affect brain processes associated with Alzheimer's disease. (A) Engulfment of tau fragments by Drosophila ensheathing glia is linked to ROS production and lysosomal activity, both of which may be modulated by gut bacterial metabolites. (B) The two primary gut bacteria genera shared between flies and humans: gram-negative Acetobacter and gram-positive Lactobacillus. (C) The gastrointestinal regions of Drosophila, highlighting the low pH region of copper cells in the middle midgut, which influences the microbiota composition. Created in https://BioRender.com.
Exploring the impact of gut-derived lactate and acetate on AD progression and glial activation in fly models can provide valuable insights into how bacterial by-products modulate neuro-cognitive and homeostatic functions, ultimately guiding more effective treatments for the disease. This mini-review highlights the potential of Drosophila as a robust model for investigating the associations between the flies' gut microbiota and the human microbiota, a connection that helps uncover the mechanisms linking bacterial balance to AD progression and inform future therapeutics.
2 Proteins associated with Alzheimer's disease
Aβ and tau proteins are the molecules more frequently associated to Alzheimer's disease progression (Lei et al., 2021; Scheltens et al., 2021; Panza et al., 2019). Tau protein is found in neuronal cells of the central nervous system (CNS), mainly in dendrites and axons regions (Rawat et al., 2022), and a diversity of post-translational modifications can cause its abnormal function (Giong et al., 2021). The excessive phosphorylation of tau protein by enzymes known as kinases destabilizes it, making it prone to detaching from microtubules, organelles essential for transporting vesicles and molecules throughout neurons.
Specific regions of tau can be abnormally phosphorylated or, more precisely, hyperphosphorylated. Serine, tyrosine and threonine are the amino acids where this addition occurs, and depending on the position of the phosphate attachment, numerous variants are formed. For instance, tau hyperphosphorylated on threonine 181 is found in blood, which optimize its use as an easy-to-collect biomarker in AD diagnosis (Thijssen et al., 2020), whereas the deficiency of super oxide dismutase 2 (SOD2) exacerbate the levels of tau hyperphosphorylated on serine 396 in mice (Flynn and Melov, 2013; Melov et al., 2007).
Tau phosphorylation introduces a negatively charged phosphate group to the peptide, changing its electrostatics and making it more hydrophilic (Alquezar et al., 2021). The pathological phosphorylation along with the diminished clearance of tau fragments by glial cells and neuroinflammation trigger the formation of insoluble paired helical filaments (Rawat et al., 2022).
The expression of the Lamp1 gene (lysosomal-associated membrane protein) is decreased in Drosophila fruit flies expressing proteins related to Parkinson's, indicating that lysosomal degradative activity plays a crucial role in protecting against oxidative stress and locomotor deficits (Rahmani et al., 2022). Additionally, Lamp1 is down-regulated in flies expressing Aβ while being up-regulated in models of amyloid- β precursor protein (AβPP) (Bergkvist et al., 2020). This suggests that these vesicles regulate the degradation and toxicity of Aβ oligomers, with significant implications for tau pathology. In contrast, Lamp2 mutant mice are more severely affected by vacuole formation compared to Lamp1 (Chaudhry et al., 2022; Rahmani et al., 2022), indicating that their respective alleles operate through different mechanisms across species. Nevertheless, both isoforms are recognized as equally significant biomarkers in the context of neurodegenerative research.
3 Gut microbiota and Alzheimer's disease
The interconnection between diet, microbiota, and the intestinal epithelium offers valuable insights into brain health. The gastrointestinal tract engages in a complex bidirectional communication with the nervous system through a sophisticated network of signaling pathways (Makdissi et al., 2023). In mammals, the gut microbiota influences the development of the newborn immune system (Donald and Finlay, 2023), the differentiation of anti-inflammatory Treg cells (Arpaia et al., 2013), hormone levels, neurotransmitter metabolism, neuronal signaling (Morais et al., 2021), and the integrity of blood-brain barrier (Fung et al., 2017). However, the mechanisms through which the intestinal host-microbiota interactions remotely alter brain physiology remain an area of ongoing research (Fung et al., 2017), especially in invertebrate models.
A wide range of bacterial genera perform gut-related functions. Lactobacillus rhamnosus modulates the levels of the inhibitory neurotransmitter γ-aminobutyric acid, also known as GABA (Barrett et al., 2012), leading to the regulation of anxiety and depression both in mice (Bravo et al., 2011; Tsai et al., 2023) and humans (Slykerman et al., 2017). Moreover, psychological stress increases the abundance of the gut commensal L. murinus in mice, a producer of indole-3-acetate (IAA), which contributes to the loss of intestinal secretory cells (Wei et al., 2024). In addition, Lactobacillus shows an intrinsic positive metabolic interplay with Acetobacter strains (Dodge et al., 2023), that are equally reduced in neurodegenerative diseases (Liu et al., 2023). The interaction between these groups encourages further studies on how bacterial metabolites may influence neurological diseases (Figure 1B), especially given the diversity of these molecules, which tends to decline with age and the progression of AD (Kong et al., 2018; Lynn et al., 2022).
Some studies have shown that the microbiota can be modified or improved to protect patients against the neurocognitive decline. Instead of administering isolated species such as Lactobacillus (Kleerebezem et al., 2010), the solution may lie in fostering an optimal gut—and external—environment that promotes the growth of beneficial bacteria, while also considering their key metabolites. Even social interactions seem to play a role in microbiome-associated diseases (Valles-Colomer et al., 2023). In this context, transplantation of feces from human with AD to germ-free mice decreases the abundance of nervous system mediators, including GABA, taurine, and valine (Fujii et al., 2019). Additionally, the fecal microbiome of patients with the disease exhibits increased levels of Bacteroidetes, and decreased levels of Firmicutes and Bifidobacterium (Vogt et al., 2017), reinforcing the synergy between microbiota diversity and neuronal processes.
Although more studies on brain-gut-microbiota communication are necessary for establishing effective therapies for CNS disorders, multidisciplinary approaches provide valuable insights and sustain the development of future treatments (Grenham et al., 2011). Furthermore, the specific bacterial species most significantly altered during AD progression remain uncertain, highlighting the need for continued research to effectively utilize bacterial groups as biomarkers in early diagnosis. Investigating the correlation between bacterial metabolites, such as acetate, and taxonomic composition data (Ferreiro et al., 2023) could clarify the role of specific gut taxa in AD.
4 Drosophila as a gut-brain axis model
Drosophila is frequently used in genetic research, and its tractable microbiome makes it a valuable axenic and gnotobiotic model (Brummel et al., 2004; Steven et al., 2021). This allows controlled interactions between the host and known microorganisms, which can be useful in assessing aggressive behaviors (Jia et al., 2021) and locomotion patterns (Schretter et al., 2018). With a relatively simple microbiota (Marra et al., 2021), D. melanogaster holds microbial communities of 2 to 30 species, that are represented by two phyla: Proteobacteria and Firmicutes. The most consistently associated species across different studies are lactic and acetic acid bacteria that reflects the fermentative substrates on which flies feed (Arias-Rojas and Iatsenko, 2022).
The intestine of Drosophila exhibits well-conserved molecular aspects with humans (Apidianakis and Rahme, 2011) and distinct pH zones (Sapre et al., 2020), making it a widely used model in gut-related studies (Iatsenko et al., 2018; Dodge et al., 2023; Silva et al., 2020). The gastrointestinal tract is divided into the foregut, midgut and hindgut, with the midgut harboring the gastric acid-producing copper cells (Miguel-Aliaga et al., 2018; Broderick and Lemaitre, 2012) (Figure 1C), which, similarly to the human stomach, may affect pH-sensitive bacteria and influence the microbiota composition (Storelli et al., 2018). The Drosophila gut is altered by the ingestion of Pseudomonas entomophila (Vodovar et al., 2005) and Erwinia carotovora (Buchon et al., 2009), which influence the cytoskeleton composition of gut epithelial cells and promote intestinal stem cell proliferation, respectively. Additionally, the gut epithelium secretes a mucus layer and the chitin-based peritrophic matrix, which act as filters for pathogenic microorganisms (Vodovar et al., 2005; Apidianakis and Rahme, 2011).
Species such as Acetobacter fabarum and Lactobacillus brevis assist in Drosophila nutrition (Sommer and Newell, 2019), while microbiota-derived acetate activates intestinal innate immunity (Jugder et al., 2021). Furthermore, Lactobacillus plantarum, a bacterium found in the Drosophila intestine, influences larval growth through a nutrient-sensing system (Storelli et al., 2011), and the gut microbiome prevents rapid fluctuations in the circadian cycle of flies (Zhang et al., 2023), reinforcing the communication between the two organs.
In fly models of both AD and Parkinson's disease, the proportion of Acetobacter and Lactobacillus is lower than in healthy controls (Kong et al., 2018; Liu et al., 2023). Lactic acid is the main metabolite of Lactobacillus and stimulates the production of reactive oxygen species (ROS) via the intestinal NADPH oxidase Nox (Iatsenko et al., 2018), a process strongly associated with neurodegeneration. Moreover, the Drosophila's metabolism is highly adaptive; when the glycolytic pathway is insufficient, its glial cells can switch to using fatty acids to fuel neuronal metabolism (McMullen et al., 2023), suggesting that these cells contribute to the gut-brain axis as either intermediaries in neurodegeneration or nutrient processing. In summary, both microbial metabolites and the composition of microbial species are strong candidates for contributing to AD progression.
The Drosophila gut-brain axis is also reflected in its anatomy, where nerve fibers are regulated by cells in the digestive tract. Serotonergic enterochromaffin cells, a type of cell found in the human gut epithelium, were shown to modulate sensory nerves via serotonin receptors and synaptic connections (Bellono et al., 2017). Some subtypes of these enterochromaffin cells are also found in Drosophila (Guo et al., 2022), suggesting that flies, like humans, experience environmental, metabolic, and homeostatic signals from the gut directly to their nervous system.
5 The CNS glial cells of Drosophila
Fruit flies are extensively used as animal models in neurocognitive and physiological experiments (Kitani-Morii et al., 2021). These studies employ various assays, including negative geotaxis (Rahmani et al., 2022; Ferreiro et al., 2018), gastric motility (Rydbom et al., 2021), and memory-related behavior (Gil-Martí et al., 2023). Physiological and behavioral alterations associated with AD can be assessed through multiple methods, such as monitoring sleep (Vaccaro et al., 2020), profiling the transcriptome (Marsh et al., 2016; Zhang et al., 2023; Liu et al., 2023), assessing lifespan (Vaccaro et al., 2020; McMullen et al., 2023), quantifying bacteria (Zhang et al., 2023; Trébuchet et al., 2019), evaluating microglial metabolic alterations (Marsh et al., 2016; Huang et al., 2023), and assessing glial development (Stork et al., 2012).
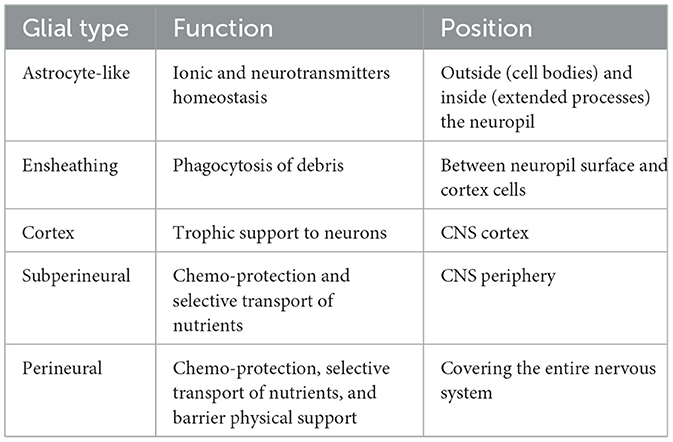
The Drosophila nervous system exhibits a significant level of complexity, sharing cellular, genetic, and functional characteristics with its mammalian counterparts (Salazar et al., 2022). Some authors categorize glial cells into four categories: cortical glia, neuropil glia, peripheral glia, and surface glia (Freeman and Doherty, 2006; Yildirim et al., 2019), but the classification may vary depending on characteristics such as cell body position and form. A comprehensive classification is presented in Table 1, considering the morphological and functional similarities of glial subtypes.
The evolution of the nervous system has resulted in a higher proportion of glial cells compared to neurons, with estimates of 15% in flies, 50% in mice, and 90% in humans, indicating an increasing contribution of glia according to complexity (Kremer et al., 2017). Similar to mammalian microglia, the surface and neuropil glia of Drosophila—specifically, the ensheathing glia—perform macrophage-like functions (Freeman and Doherty, 2006), suggesting their involvement in the engulfment of Aβ and tau fragments.
The perineural and subperineural glia perform a blood-brain barrier role in Drosophila, controlling the passage of bacterial metabolites to the brain. Furthermore, glial cells, such as astrocytes, supply lactate to neurons (Hascup et al., 2022), a function of cellular cooperation that is also conserved in Drosophila (Volkenhoff et al., 2015), but is abnormally altered in energy-demanding neurons affected by AD. The variety of transgenic lineages and the ease of using flies as axenic and gnotobiotic models make this organism useful in researching neurological conditions as diverse as AD and autism (Salim et al., 2021).
Similar to the mammalian vagus nerve, Drosophila gut-brain communication is mediated by serotonergic neurons that innervate its intestine (Schoofs et al., 2014). This enteric nervous system of the invertebrate model—including both neurons and glial cells—connects to the central nervous system via the antennal nerve (Salim et al., 2021; Schoofs et al., 2014), a crucial pathway for transporting bacterial metabolites across the gut-brain axis. The relatively small number of glial cells in flies, compared to mammals, may offer a unique opportunity to better understand glial communication with the neuronal microenvironment.
Intriguingly, when AD disrupts the gut microbiota of mammals, Lactobacillus produces such high levels of GABA that the mucin layer is compromised, allowing the movement of solutes and metabolites out of the intestine (Conn et al., 2024). Furthermore, enteric glial cells of mice express GABA signaling receptors (Deng et al., 2023), raising the question of whether a similar host-bacteria communication could occur in Drosophila, a model organism with well-characterized genome and largely mapped neuronal connectome.
In mammals, the neuroinflammation related to AD is intricately associated with microglial activation (Johnson et al., 2020), which causes the cell to undergo a morphological transformation from a slender, ramified form to a more rounded shape with fewer extensions (Loh et al., 2024; Colombo et al., 2021). In addition, the gut microbiome of mice has been shown to modulate the expression of AD-related genes, such as Apoe and Trem2 (Huang et al., 2023). In flies, the interaction of neurons with their support cells and the expression of genes involved in Aβ clearance in glial cells (Yang et al., 2017) warrant a deeper investigation into the molecular mechanisms underlying gut-brain axis.
6 Discussion
The indirect mechanisms linking intestinal dysbiosis to the progression of Alzheimer's disease remain poorly understood, with most current studies primarily focusing on direct correlations. The complexity of inter-organ communication and the impact of environmental factors such as stress, sleep, and social interactions on neurocognitive impairment are still in discussion. Employing an in vivo system for such investigations could better reflect the complexity of signals that CNS cells receive and process, producing a more representative output that facilitates the development of new therapies.
The well-characterized genome and genetic tools of Drosophila, along with its simpler microbiota and low maintenance requirements, serve as motivating reasons to use this model for evaluating the influence of extraneural events on the progression of Alzheimer's disease. The behaviors exhibited by the flies and their metabolic pathways are highly mappable, facilitating the analysis of the interplay among comprehensive and robust hypotheses.
Fruit flies are also an excellent model for developing and testing drugs, though they are still timidly utilized in biotechnology research. Considering the development of probiotics, the modifiable microbiome of Drosophila could accelerate the creation of new medications and improve safety before clinical trials. When used in pioneering research and in association with complementary models, Drosophila can foster new discoveries in the gut-brain axis field, translating evolutionarily conserved associations into theragnostic solutions, from bench to bedside.
Author contributions
SA: Conceptualization, Writing – original draft, Writing – review & editing, Methodology. PL-F: Funding acquisition, Supervision, Writing – review & editing. CZ: Funding acquisition, Writing – review & editing. MP-S: Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was financially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES no. 88887.980293/2024-00), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP no. 2022/02636-9).
Acknowledgments
The grammatical revision of the text was made using ChatGPT-3.5 and DeepL Write.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Only for grammatical revision and text cohesion.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alquezar, C., Arya, S., and Kao, A. W. (2021). Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 11:595532. doi: 10.3389/fneur.2020.595532
Apidianakis, Y., and Rahme, L. G. (2011). Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Models Mech. 4, 21–30. doi: 10.1242/dmm.003970
Arias-Rojas, A., and Iatsenko, I. (2022). The role of microbiota in Drosophila melanogaster aging. Front. Aging 3, 909509. doi: 10.3389/fragi.2022.909509
Arpaia, N., Campbell, C., Fan, X., Dikiy, S., Veeken, J. V. D., deRoos, P., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Barrett, E., Ross, R. P., O'Toole, P. W., Fitzgerald, G. F., and Stanton, C. (2012). γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 113, 411–17. doi: 10.1111/j.1365-2672.2012.05344.x
Beata, B. K., Wojciech, J., Johannes, K., Piotr, L., and Barbara, M. (2023). Alzheimer's disease—biochemical and psychological background for diagnosis and treatment. Int. J. Mol. Sci. 24, 1059. doi: 10.3390/ijms24021059
Bellono, N. W., Bayrer, J. R., Leitch, D. B., Castro, J., Zhang, C., O'Donnell, T. A., et al. (2017). Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198.e16. doi: 10.1016/j.cell.05.034
Bergkvist, L., Du, Z., Elovsson, G., Appelqvist, H., Itzhaki, L. S., Kumita, J. R., et al. (2020). Mapping pathogenic processes contributing to neurodegeneration in Drosophila models of Alzheimer's disease. FEBS Open Bio. 10, 338–350. doi: 10.1002/2211-5463.12773
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Nat. Acad. Sci. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Broderick, N. A., and Lemaitre, B. (2012). Gut-associated microbes of Drosophila melanogaster. Gut Microbes. 3, 307–321. doi: 10.4161/gmic.19896
Brummel, T., Ching, A., Seroude, L., Simon, A. F., and Benzer, S. (2004). Drosophila lifespan enhancement by exogenous bacteria. Proc. Nat. Acad. Sci. 101, 12974–12979. doi: 10.1073/pnas.0405207101
Buchon, N., Broderick, N. A., Poidevin, M., Pradervand, S., and Lemaitre, B. (2009). (2009). Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211. doi: 10.1016/j.chom.01.003
Chaudhry, N., Sica, M., Surabhi, S., Hernandez, D. S., Mesquita, A., Selimovic, A., et al. (2022). Lamp1 Mediates Lipid Transport, but Is Dispensable for Autophagy in Drosophila. Autophagy 18, 2443–58. doi: 10.1080/15548627.2022.2038999
Colombo, A. V., Sadler, R. K., Llovera, G., Singh, V., Roth, S., Heindl, S., et al. (2021). Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. eLife 10, e59826. doi: 10.7554/eLife.59826
Conn, K. A., Borsom, E. M., and Cope, E. K. (2024). Implications of microbe-derived ɤ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer's disease. Gut Microbes 16, 2371950. doi: 10.1080/19490976.2024.2371950
Deng, Z., Li, D., Yan, X., Lan, J., Han, D., Fan, K., et al. (2023). Activation of GABA receptor attenuates intestinal inflammation by modulating enteric glial cells function through inhibiting NF-κB pathway. Life Sci. 329, 121984. doi: 10.1016/j.lfs.2023.121984
Dodge, R., Jones, E. W., Zhu, H., Obadia, B., Martinez, D. J., Wang, C., et al. (2023). A symbiotic physical niche in Drosophila melanogaster regulates stable association of a multi-species gut microbiota. Nat. Commun. 14, 1557. doi: 10.1038/s41467-023-36942-x
Doherty, J., Logan, M. A., Taşdemir, O. E., and Freeman, M. R. (2009). Ensheathing glia function as phagocytes in the adult drosophila brain. J. Neurosci. 29, 4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009
Donald, K., and Finlay, B. B. (2023). Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat. Rev. Immunol. 23, 735–748. doi: 10.1038/s41577-023-00874-w
Ferreiro, A. L., Choi, J., Ryou, J., Newcomer, E. P., Thompson, R., Bollinger, R. M., et al. (2023). Gut microbiome composition may be an indicator of preclinical Alzheimer's disease. Sci.Transl. Med. 15:eabo2984. doi: 10.1126/scitranslmed.abo2984
Ferreiro, M. J., Pérez, C., Marchesano, M., Ruiz, S., Caputi, A., Aguilera, P., et al. (2018). Drosophila melanogaster white mutant W1118 undergo retinal degeneration. Front. Neurosci. 11, 732. doi: 10.3389/fnins.2017.00732
Flynn, J. M., and Melov, S. (2013). (2013). SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radical Biol. Med. 62:4–12. doi: 10.1016/j.freeradbiomed.05.027
Freeman, M. R., and Doherty, J. (2006). Glial cell biology in drosophila and vertebrates. Trends NeuroSci. 29, 82–90. doi: 10.1016/j.tins.12.002
Fujii, Y., Nguyen, T. T. T., Fujimura, Y., Kameya, N., Nakamura, S., Arakawa, K., et al. (2019). Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer's disease. Biosci. Biotechnol. Biochem. 83, 2144–52. doi: 10.1080/09168451.2019.1644149
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–55. doi: 10.1038/nn.4476
Gil-Martí, B., Barredo, C. G., Pina-Flores, S., Poza-Rodriguez, A., Treves, G., Rodriguez-Navas, C., et al. (2023). A simplified courtship conditioning protocol to test learning and memory in drosophila. STAR Protocols 4, 101572. doi: 10.1016/j.xpro.2022.101572
Giong, H. K., Subramanian, M., Yu, K., and Lee, J-. S. (2021). Non-rodent genetic animal models for studying tauopathy: review of Drosophila, Zebrafish, and C. Elegans models. Int. J. Mol. Sci. 22, 8465. doi: 10.3390/ijms22168465
Grenham, S., Clarke, G., Cryan, J. F., and Dinan, T. G. (2011). Brain? Gut? Microbe communication in health and disease. Front. Physiol. 2, 94. doi: 10.3389/fphys.2011.00094
Guo, X., Lv, J., and Xi, R. (2022). The specification and function of enteroendocrine cells in Drosophila and mammals: a comparative review. FEBS J. 289, 4773–4796. doi: 10.1111/febs.16067
Hascup, E. R., Sime, L. N., Peck, M. R., and Hascup, K. N. (2022). Amyloid-?42 stimulated hippocampal lactate release is coupled to glutamate uptake. Sci. Rep. 12, 2775. doi: 10.1038/s41598-022-06637-2
Hassan-Abdi, R., Brenet, A., Bennis, M., Yanicostas, C., and Soussi-Yanicostas, N. (2019). Neurons expressing pathological tau protein trigger dramatic changes in microglial morphology and dynamics. Front. Neurosci. 13, 1199. doi: 10.3389/fnins.2019.01199
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7
Huang, Y., Wu, J., Zhang, H., Li, Y., Wen, L., Tan, X., et al. (2023). The gut microbiome modulates the transformation of microglial subtypes. Mol. Psychiatry 28, 1611–1621. doi: 10.1038/s41380-023-02017-y
Iatsenko, I., Boquete, J-. P., and Lemaitre, B. (2018). Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens drosophila lifespan. Immunity 49, 929–942.e5. doi: 10.1016/j.immuni.09.017
Jennings, B. H. (2011). Drosophila—a versatile model in biology and medicine. Mat. Today 14, 190–195. doi: 10.1016/S1369-7021(11)70113-4
Jia, Y., Jin, J., Hu, S., Geng, K., Han, L., Kang, C. R., et al. (2021). Gut microbiome modulates drosophila aggression through octopamine signaling. Nat. Commun. 12, 2698. doi: 10.1038/s41467-021-23041-y
Johnson, E. C., Dammer, E. B., Duong, D. M., Ping, L., Zhou, M., Yin, L., et al. (2020). Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 26, 769–780. doi: 10.1038/s41591-020-0815-6
Jugder, B. E., Kamareddine, L., and Watnick, P. I. (2021). Microbiota-derived acetate activates intestinal innate immunity via the Tip60 histone acetyltransferase complex. Immunity 54, 1683–1697.e3. doi: 10.1016/j.immuni.05.017
Kitani-Morii, F., Friedland, R. P., Yoshida, H., and Mizuno, T. (2021). Drosophila as a model for microbiota studies of neurodegeneration. J. Alzheimer's Dis. 84, 479–90. doi: 10.3233/JAD-215031
Kleerebezem, M., Hols, P., Bernard, E., Rolain, T., Zhou, M., Siezen, R. J., et al. (2010). The extracellular biology of the Lactobacilli. FEMS Microbiol. Rev. 34, 199–230. doi: 10.1111/j.1574-6976.2009.00208.x
Kong, Y., Jiang, B., and Luo, X. (2018). Gut microbiota influences Alzheimer's disease pathogenesis by regulating acetate in Drosophila Model. Future Microbiol. 13, 1117–1128. doi: 10.2217/fmb-2018-0185
Kremer, M. C., Jung, C., Batelli, S., Rubin, G. M., and Gaul, U. (2017). The Glia of the Adult D Rosophila nervous system. Glia 65, 606–638. doi: 10.1002/glia.23115
Lei, P., Ayton, S., and Bush, A. I. (2021). The essential elements of Alzheimer's disease. J. Biol. Chem. 296, 100105. doi: 10.1074/jbc.REV120.008207
Liu, J., Li, H., Gong, T., Chen, W., Mao, S., Kong, Y., et al. (2020). Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer's disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agricult. Food Chem. 68, 7152–7161. doi: 10.1021/acs.jafc.0c02807
Liu, X., Yang, M., Liu, R., Zhou, F., Zhu, H., Wang, X., et al. (2023). The Impact of Parkinson's Disease-Associated Gut Microbiota on the Transcriptome in Drosophila. Edited by John M. Chaston. Microbiol. Spectrum 11, e00176–23. doi: 10.1128/spectrum.00176-23
Loh, J. S., Mak, W. Q., Tan, L. K. S., Ng, C. X., Chan, H. H., Yeow, S. H., et al. (2024). Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 9:37. doi: 10.1038/s41392-024-01743-1
Long, D. M., Frame, A. K., Reardon, P. N., Cumming, R. C., Hendrix, D. A., Kretzschmar, D., et al. (2020). Lactate dehydrogenase expression modulates longevity and neurodegeneration in Drosophila melanogaster. Aging 12, 10041–10058. doi: 10.18632/aging.103373
Lynn, D. J., Benson, S. C., Lynn, M. A., and Pulendran, B. (2022). Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol. 22, 33–46. doi: 10.1038/s41577-021-00554-7
Makdissi, S., Parsons, B. D., and Di Cara, F. (2023). Towards early detection of neurodegenerative diseases: a gut feeling. Front. Cell Dev. Biol. 11:1087091. doi: 10.3389/fcell.2023.1087091
Marra, A., Hanson, M. A., Kondo, S., Erkosar, B., and Lemaitre, B. (2021). Drosophila antimicrobial peptides and lysozymes regulate gut microbiota composition and abundance. mBio 12:e0082421. doi: 10.1128/mBio.00824-21
Marsh, S. E., Abud, E. M., Lakatos, A., Karimzadeh, A., Yeung, S. T., Davtyan, H., et al. (2016). The adaptive immune system restrains alzheimer's disease pathogenesis by modulating microglial function. Proc. Nat. Acad. Sci. 113:E1316–25. doi: 10.1073/pnas.1525466113
Mayneris-Perxachs, J., Castells-Nobau, A., Arnoriaga-Rodríguez, M., Martin, M., Vega-Correa, L. D. L., Zapata, C., et al. (2022). Microbiota alterations in proline metabolism impact depression. Cell Metabol. 34, 681–701.e10. doi: 10.1016/j.cmet.04.001
McMullen, E., Hertenstein, H., Strassburger, K., Deharde, L., Brankatschk, M., Schirmeier, S., et al. (2023). Glycolytically impaired drosophila glial cells fuel neural metabolism via β-oxidation. Nat. Commun. 14:2996. doi: 10.1038/s41467-023-38813-x
Melov, S., Adlard, P. A., Morten, K., Johnson, F., Golden, T. R., Hinerfeld, D., et al. (2007). Mitochondrial oxidative stress causes hyperphosphorylation of Tau. Edited by Joseph El Khoury. PLoS ONE 2:e536. doi: 10.1371/journal.pone.0000536
Miguel-Aliaga, I., Jasper, H., and Lemaitre, B. (2018). Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 210, 357–396. doi: 10.1534/genetics.118.300224
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Palmqvist, S., Tideman, P., Cullen, N., Zetterberg, H., Blennow, K., Initiative, t. A. D. N., et al. (2021). Prediction of future Alzheimer's disease dementia using plasma Phospho-Tau combined with other accessible measures. Nat. Med. 27, 1034–1042. doi: 10.1038/s41591-021-01348-z
Panza, F., Lozupone, M., Logroscino, G., and Imbimbo, B. P. (2019). A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88. doi: 10.1038/s41582-018-0116-6
Pluta, R., Ułamek-Kozioł, M., Januszewski, S., and Czuczwar, S. J. (2020). Gut microbiota and pro/prebiotics in Alzheimer's disease. Aging 12, 5539–5550. doi: 10.18632/aging.102930
Rahmani, Z., Surabhi, S., Rojo-Cortés, F., Dulac, A., Jenny, A., Birman, S., et al. (2022). Lamp1 deficiency enhances sensitivity to α-synuclein and oxidative stress in drosophila models of Parkinson disease. Int. J. Mol. Sci. 23:13078. doi: 10.3390/ijms232113078
Rawat, P., Sehar, U., Bisht, J., Selman, A., Culberson, J., Reddy, P. H., et al. (2022). Phosphorylated Tau in Alzheimer's disease and other tauopathies. Int. J. Mol. Sci. 23:12841. doi: 10.3390/ijms232112841
Rydbom, J., Kohl, H., Hyde, V. R., and Lohr, K. M. (2021). Altered gut microbial load and immune activation in a drosophila model of human tauopathy. Front. Neurosci. 15:731602. doi: 10.3389/fnins.2021.731602
Salazar, G., Ross, G., Maserejian, A. E., and Coutinho-Budd, J. (2022). Quantifying glial-glial tiling using automated image analysis in drosophila. Front. Cell. Neurosci. 16:826483. doi: 10.3389/fncel.2022.826483
Salim, S., Banu, A., Alwa, A., Gowda, S. B. M., and Mohammad, F. (2021). The gut-microbiota-brain axis in autism: what drosophila models can offer? J. Neurodevelop. Disord. 13, 37. doi: 10.1186/s11689-021-09378-x
Sapre, N., Chakraborty, R., Purohit, P., Bhat, S., Das, G., Bajpe, S. R., et al. (2020). Enteric pH Responsive cargo release from PDA and PEG coated mesoporous silica nanoparticles: a comparative study in Drosophila melanogaster. RSC Adv. 10, 11716–11726. doi: 10.1039/C9RA11019D
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397, 1577–1590. doi: 10.1016/S0140-6736(20)32205-4
Schoofs, A., Hückesfeld, S., Surendran, S., and Pankratz, M. J. (2014). Serotonergic pathways in the drosophila larval enteric nervous system. J. Insect Physiol. 69:118–125. doi: 10.1016/j.jinsphys.05.022
Schretter, C. E., Vielmetter, J., Bartos, I., Marka, Z., Marka, S., Argade, S., et al. (2018). A gut microbial factor modulates locomotor behaviour in drosophila. Nature 563, 402–406. doi: 10.1038/s41586-018-0634-9
Silva, V., Palacios-Muñoz, A., Okray, Z., Adair, K. L., Waddell, S., Douglas, A. E., et al. (2020). The impact of the gut microbiome on memory and sleep in Drosophila. J. Exp. Biol. 224:233619. doi: 10.1242/jeb.233619
Simhadri, R. K., Fast, E. M., Guo, R., Schultz, M. J., Vaisman, N., Ortiz, L., et al. (2017). The gut commensal microbiome of Drosophila melanogaster is modified by the endosymbiont Wolbachia, Edited by Karen L. Visick. mSphere 2, e00287–17. doi: 10.1128/mSphere.00287-17
Slykerman, R. F., Hood, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMed. 24:159–165. doi: 10.1016/j.ebiom.09.013
Sommer, A. J., and Newell, P. D. (2019). Metabolic basis for mutualism between gut bacteria and its impact on the Drosophila melanogaster host. Edited by Shuang-Jiang Liu. Appl. Environ. Microbiol. 85, e01882–18. doi: 10.1128/AEM.01882-18
Steven, B., Hyde, J., LaReau, J. C., and Brackney, D. E. (2021). The axenic and gnotobiotic mosquito: emerging models for microbiome host interactions. Front. Microbiol. 12:714222. doi: 10.3389/fmicb.2021.714222
Storelli, G., Defaye, A., Erkosar, B., Hols, P., Royet, J., Leulier, F., et al. (2011). Lactobacillus plantarum promotes drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 14, 403–414. doi: 10.1016/j.cmet.07.012
Storelli, G., Strigini, M., Grenier, T., Bozonnet, L., Schwarzer, M., Daniel, C., et al. (2018). Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont Lactobacillus plantarum. Cell Metabol. 27, 362–377.e8. doi: 10.1016/j.cmet.11.011
Stork, T., Bernardos, R., and Freeman, M. R. (2012). Analysis of glial cell development and function in Drosophila. Cold Spring Harbor Protocols 2012, 1–17. doi: 10.1101/pdb.top067587
Tan, F. H. P., Liu, G., Lau, S-. Y. A., Jaafar, M. H., Park, Y-. H., Azzam, G., et al. (2020). Lactobacillus probiotics improved the gut microbiota profile of a Drosophila melanogaster Alzheimer's disease model and alleviated neurodegeneration in the eye. Benef. Microbes 11, 79–90. doi: 10.3920/BM2019.0086
Thijssen, E. H., La Joie, R., Wolf, A., Strom, A., Wang, P., Iaccarino, L., et al. (2020). Diagnostic value of plasma phosphorylated Tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nature Med. 26, 387–397. doi: 10.1038/s41591-020-0762-2
Trébuchet, G., Cattenoz, P. B., Zsámboki, J., Mazaud, D., Siekhaus, D. E., Fanto, M., et al. (2019). The repo homeodomain transcription factor suppresses hematopoiesis in Drosophila and preserves the glial fate. J. Neurosci. 39, 238–255. doi: 10.1523/JNEUROSCI.1059-18.2018
Tsai, W. H., Yeh, W-. L., Chou, C-. H., Wu, C-. L., Lai, C-. H., Yeh, Y-. T., et al. (2023). Suppressive effects of lactobacillus on depression through regulating the gut microbiota and metabolites in C57BL/6J mice induced by ampicillin. BioMed. 11:1068. doi: 10.3390/biomedicines11041068
Vaccaro, A., Dor, Y. K., Nambara, K., Pollina, E. A., Lin, C., Greenberg, M. E., et al. (2020). Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307–1328.e15. doi: 10.1016/j.cell.04.049
Valles-Colomer, M., Blanco-Míguez, A., Manghi, P., Asnicar, F., Dubois, L., Golzato, D., et al. (2023). The person-to-person transmission landscape of the gut and oral microbiomes. Nature 614, 125–135. doi: 10.1038/s41586-022-05620-1
Vodovar, N., Vinals, M., Liehl, P., Basset, A., Degrouard, J., Spellman, P., et al. (2005). Drosophila host defense after oral infection by an entomopathogenic pseudomonas species. Proc. Nat. Acad. Sci. USA. 102, 11414–19. doi: 10.1073/pnas.0502240102
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer's disease. Sci. Rep. 7:13537. doi: 10.1038/s41598-017-13601-y
Volkenhoff, A., Weiler, A., Letzel, M., Stehling, M., Klämbt, C., Schirmeier, S., et al. (2015). Glial glycolysis is essential for neuronal survival in drosophila. Cell Metab. 22, 437–447. doi: 10.1016/j.cmet.07.006
Wei, W., Liu, Y., Hou, Y., Cao, S., Chen, Z., Zhang, Y., et al. (2024). Psychological stress-induced microbial metabolite indole-3-acetate disrupts intestinal cell lineage commitment. Cell Metabol. 36, 466–483.e7 doi: 10.1016/j.cmet.12
Xu, D., Vincent, A., González-Gutiérrez, A., Aleyakpo, B., Anoar, S., Giblin, A., et al. (2023). A monocarboxylate transporter rescues frontotemporal dementia and Alzheimer's disease models. Edited by Giovanni Bosco. PLOS Genet. 19:e1010893. doi: 10.1371/journal.pgen.1010893
Yang, C. N., Wu, M-. F., Liu, C-. C., Jung, W-. H., Chang, Y-. C., Lee, W-.P., et al. (2017). Differential protective effects of connective tissue growth factor against Aβ neurotoxicity on neurons and glia. Hum. Mol. Genet. 26, 3909–3921. doi: 10.1093/hmg/ddx278
Yildirim, K., Petri, J., Kottmeier, R., and Klämbt, C. (2019). Drosophila glia: few cell types and many conserved functions. Glia 67, 5–26. doi: 10.1002/glia.23459
Keywords: Alzheimer's disease, Drosophila melanogaster, gut-brain axis, microbiota, neurodegeneration
Citation: Alves SdM, Lisboa-Filho PN, Zilli Vieira CL and Piacenti-Silva M (2025) Alzheimer's disease and gut-brain axis: Drosophila melanogaster as a model. Front. Neurosci. 19:1543826. doi: 10.3389/fnins.2025.1543826
Received: 11 December 2024; Accepted: 15 January 2025;
Published: 04 February 2025.
Edited by:
Deiziane Viana da Silva Costa, University of Virginia, United StatesReviewed by:
Ilias Kounatidis, The Open University, United KingdomCopyright © 2025 Alves, Lisboa-Filho, Zilli Vieira and Piacenti-Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel de Mattos Alves, samuel.alves@unesp.br
†ORCID: Samuel de Mattos Alves orcid.org/0000-0001-5876-9507
Paulo Noronha Lisboa-Filho orcid.org/0000-0002-7734-4069
Carolina Letícia Zilli Vieira orcid.org/0000-0002-8763-3331
Marina Piacenti-Silva orcid.org/0000-0001-7096-3652
 Samuel de Mattos Alves
Samuel de Mattos Alves