- 1Department of Biomedical Engineering, Tehran University of Medical Sciences, Tehran, Iran
- 2Research Center for Biomedical Technologies and Robotics (RCBTR), Advanced Medical Technologies and Equipment Institute (AMTEI), Tehran University of Medical Science (TUMS), Tehran, Iran
This paper illustrates the development of two efficient source localization algorithms for electroencephalography (EEG) data, aimed at enhancing real-time brain signal reconstruction while addressing the computational challenges of traditional methods. Accurate EEG source localization is crucial for applications in cognitive neuroscience, neurorehabilitation, and brain-computer interfaces (BCIs). To make significant progress toward precise source orientation detection and improved signal reconstruction, we introduce the Accelerated Linear Constrained Minimum Variance (ALCMV) beamforming toolbox and the Accelerated Brain Source Orientation Detection (AORI) toolbox. The ALCMV algorithm speeds up EEG source reconstruction by utilizing recursive covariance matrix calculations, while AORI simplifies source orientation detection from three dimensions to one, reducing computational load by 66% compared to conventional methods. Using both simulated and real EEG data, we demonstrate that these algorithms maintain high accuracy, with orientation errors below 0.2% and signal reconstruction accuracy within 2%. These findings suggest that the proposed toolboxes represent a substantial advancement in the efficiency and speed of EEG source localization, making them well-suited for real-time neurotechnological applications.
1 Introduction
Accurate source localization in electroencephalography (EEG) is fundamental for understanding brain function and neural dynamics, making it essential in cognitive neuroscience, neurorehabilitation, and brain-computer interface (BCI) applications. Despite the critical role of EEG in these fields, high speed processing remains a significant challenge due to the high computational demands of current source localization algorithms. As applications continue to demand both speed and precision, the development of accelerated algorithms for source orientation detection and beamforming has become increasingly important.
Traditionally, Linearly Constrained Minimum Variance (LCMV) beamforming has been the go-to technique for EEG source localization. This approach is favored for its ability to spatially filter signals, enhancing the signal-to-noise ratio (SNR) by suppressing noise and interference. However, LCMV beamforming methods can be computationally intensive, especially when applied to high-density EEG systems with numerous channels (Ilmoniemi and Sarvas, 2024). The computational complexity of covariance matrix calculations and matrix inversions, coupled with the need for accurate estimation of brain source orientation, makes fast processing difficult.
To address these challenges, we propose a set of accelerated algorithms aimed at improving the efficiency of both LCMV beamforming and source orientation detection. Specifically, we have developed two MATLAB toolboxes (Nan and Yulin, 2011): ALCMV and AORI. The ALCMV toolbox expedites the LCMV beamforming by initiating recursive calculations as soon as we have acquired EEG samples equivalent to the number of electrode channels—necessary for achieving a full-rank matrix—we can significantly enhance processing speed. This approach enables faster source localization while maintaining accuracy (Jonmohamadi et al., 2014).
In the LCMV algorithm, electrical signals generated by brain sources in specific directions propagate through various layers of the brain and ultimately reach the EEG recording electrodes. If the source activity is denoted by S(t) and the estimated source activity by , we employ beamforming techniques to isolate and reconstruct the source signals from the noisy background (Sekihara and Nagarajan, 2015).
Existing EEG source localization methods, such as minimum norm estimation and traditional LCMV beamforming, have demonstrated their effectiveness in various applications. Automatic localization of seizure events from EEG signals has been explored through machine learning, frequency transforms, and nonlinear association analysis. Although these approaches offer valuable insights, accurately pinpointing the seizure onset zone (SOZ) remains difficult due to the low signal-to-noise ratio (SNR) of scalp EEG recordings (Myers et al., 2020). Beamformer techniques and the LORETA method are widely applied for source localization, but these solutions are computationally intensive and often yield non-unique or ambiguous source estimates (Myers et al., 2020). Recent studies have focused on analyzing the phase and energy of EEG signals to reliably localize seizure foci on the scalp, achieving 93.3% precision and accuracy and 100% sensitivity in detecting true seizure activity, even in the presence of common EEG artifacts (Myers et al., 2020). However, these methods often struggle with fast processing due to their reliance on extensive matrix computations.
For example, minimum norm estimation techniques tend to require significant computational resources, making them less suitable for fast applications (Huang et al., 2014). Beamforming methods, such as LCMV, have demonstrated potential in localizing rhythmic ictal activity and offer several advantages over traditional ECD models, particularly in the context of temporal lobe epilepsy. The next step would involve further validating these methods with larger patient datasets while integrating accelerated algorithms to improve computational efficiency. This would make real-time application in clinical environments more feasible (Garcia Dominguez et al., 2023). The beamforming method can be reliably applied to both mesial and neocortical temporal lobe epilepsy, providing significant improvements in pre-surgical evaluation but they have lot. Similarly, conventional LCMV beamforming can become computationally prohibitive as the number of EEG channels increases, leading to longer processing times and potential delays in source localization (Jaiswal et al., 2020). Our approach addresses these limitations by introducing novel algorithms that reduce the computational load without compromising accuracy (Masoud et al., 2025). The recursive calculation method implemented in the ALCMV toolbox and the dimensionality reduction provided by the AORI toolbox offer significant improvements over traditional methods (Oostenveld et al., 2011). By building on the strengths of LCMV beamforming while mitigating its weaknesses, our work represents a meaningful advancement in the field of EEG source localization (Sekihara and Nagarajan, 2015).
The primary innovation of this study lies in the combination of recursive calculations and dimensionality reduction to accelerate source localization in EEG. Unlike previous approaches that treat the problem in a static manner, our methods dynamically adapt to the incoming EEG data, enabling faster calculations. The ALCMV toolbox optimizes the LCMV beamforming process, reducing the time required to achieve full-rank matrix conditions. At the same time, the AORI toolbox transforms the complex three-dimensional orientation problem into a more manageable one-dimensional problem, further enhancing computational efficiency. These innovations are not only theoretical but have been implemented in MATLAB toolboxes designed for practical use in real-time applications. By validating our algorithms with simulated EEG signals using the EEGg1 toolbox (Vaziri et al., 2024), we have demonstrated that these methods can achieve substantial gains in processing speed while maintaining the precision necessary for accurate source localization.
The accelerated algorithms developed in this study hold significant potential for a wide range of EEG applications. In neurorehabilitation, epilepsy detection, emotion recognition, for instance, fast and accurate source localization is crucial for real-time feedback in brain-computer interface systems. Similarly, in clinical diagnostics, such as seizure detection and monitoring, the ability to quickly identify the origin of abnormal brain activity can be life-saving. Our toolboxes provide the better spatial resolution without sacrificing accuracy, making them valuable assets for both research and clinical practice.
This study introduces novel algorithms—ALCMV and AORI—that address long-standing gaps in brain mapping by significantly improving the efficiency of EEG source localization. Through recursive covariance calculations and dimensionality reduction, these toolboxes reduce computational load while maintaining high accuracy. These innovations enable real-time brain signal processing, overcoming delays associated with traditional methods.
2 Method
The present study employs the Linearly Constrained Minimum Variance (LCMV) beamformer as a cornerstone for advanced EEG signal analysis, focusing on source localization and signal reconstruction. The LCMV beamformer leverages spatial filtering to minimize output variance while maintaining signal integrity, enabling precise localization of brain activity. This method incorporates mathematical constructs, such as the lead field matrix derived from MRI imaging and forward problem solutions, alongside efficient computational techniques for covariance matrix estimation and inversion. Through the development and integration of novel algorithms, including the Accelerated Brain Source Orientation Detection (AORI) toolbox and enhancements to covariance matrix computations, we aim to address the computational challenges typically associated with high-dimensional EEG data. These advancements are implemented and validated using simulated and experimental EEG datasets, ensuring robust and efficient application to real-world scenarios.
The LCMV beamformer is formulated as a spatial adaptive filter that minimizes the output variance, enhancing its ability to localize sources. Mathematically, this is expressed in Equation 1.
where w(r) is the beamforming weight vector, R is the covariance matrix of the transposed EEG data, and 𝐿 is the lead field matrix, typically obtained through MRI imaging and forward problem solutions (Cho et al., 2015). The source estimate (t) is given by Equation 2.
where y(t) is the recorded EEG signal (Van Veen et al., 1997). is the transpose of w(r) from Equation 1.
2.1 Covariance calculation and efficiency
The covariance matrix is a key computational component when working with EEG data, particularly in the context of LCMV beamforming. For k channels and N recorded samples, the EEG data matrix typically has dimensions k × N. However, the covariance matrix derived from the transposed EEG data is a much smaller k × k matrix, reducing the computational burden and providing substantial efficiency. The covariance between EEG channels j and k can be computed in Equation 3 (Wu and Xiao, 2012).
where and are the means of the 𝑗-th row and k-th column, respectively. This computational efficiency is one of the primary advantages of the LCMV beamformer, allowing for precise source localization without excessive computational load.
2.2 Source orientation estimation
Estimating the orientation of brain sources often involves utilizing 3D MRI images of the individual. However, this process can be computationally demanding. To improve efficiency, we developed the AORI toolbox, which reduces the dimensionality of the orientation problem from three to one by leveraging the lead field matrix and the inverse covariance matrix. The optimal direction is calculated by maximizing the output power. Mathematically, the optimal orientation is given in Equation 4.
where represents the eigenvector corresponding to the smallest eigenvalue. The reduction in dimensionality can lead to faster calculations and more efficient source localization.
2.3 Covariance calculation in ALCMV beamforming
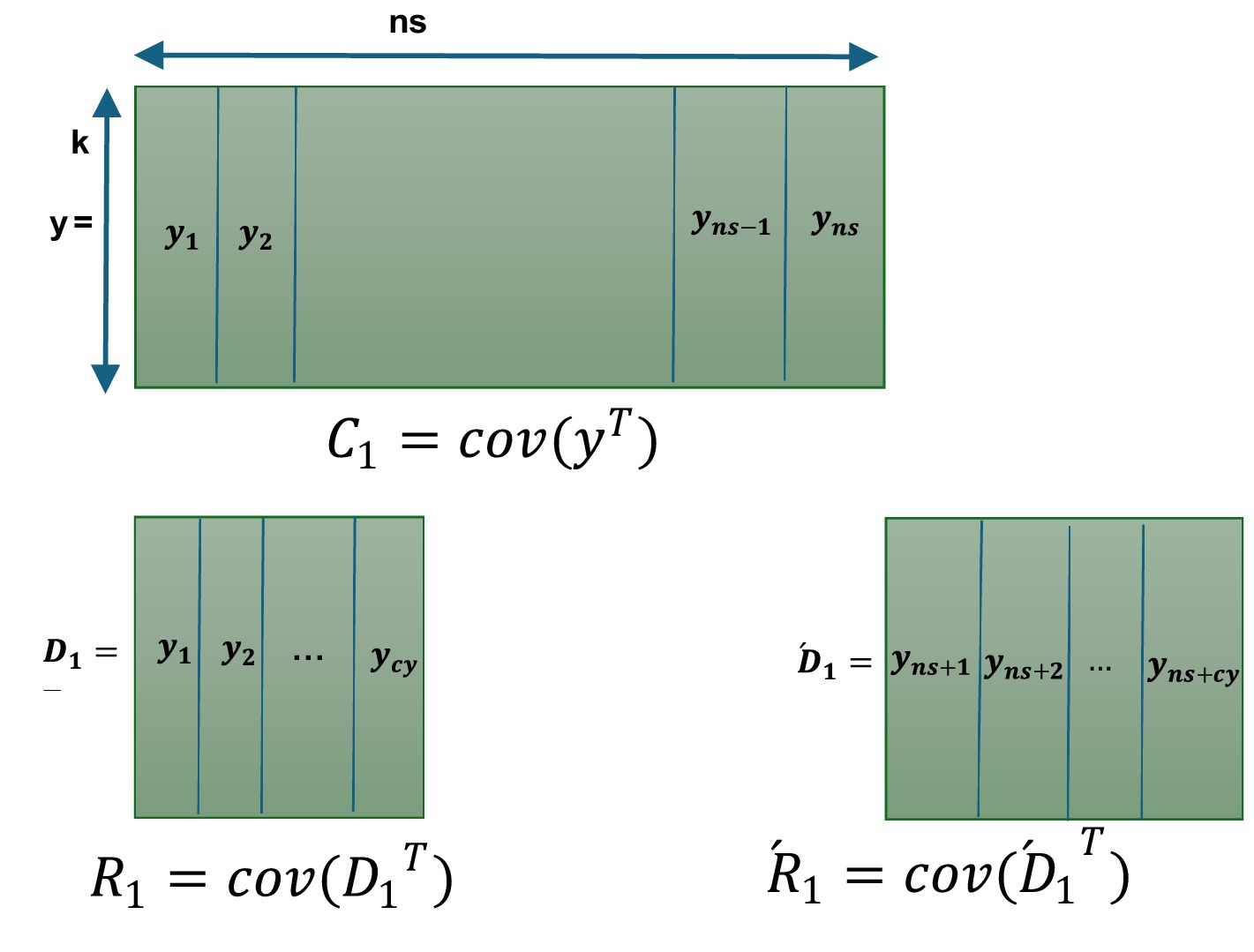
To address inverse covariance requirement reliably, we adopted the Miller computational method for recursively calculating the inverse covariance. In the ALCMV method, covariance was initially calculated using the addition and subtraction technique. Subsequently, inversion was performed using the general inverse method, implemented through Miller’s approach. In Figure 1, covariance using the addition and subtraction method contains first samples (ns) that are equal to the number of channels (k) and calculate the covariance using the standard method, which gives us the initial covariance ( ). After recording another cy (cycle) samples now we have ns + cy samples, we separate the first cy samples of the EEG data and the last cy samples of the EEG data and name them and ( , respectively. Then, we calculate the covariance of the transposed and and name them and . Finally, we use Equation 3 to calculate the second updated covariance The Equation 3 can be extended in Equation 5 (Wu and Xiao, 2012).
It is important to note that the number of columns used in the initial calculations (ns) must ensure that the matrix is full rank. In other words, ns should be at least equal to the number of electrode signal channels (k). For the remaining data, however, the covariance can be updated incrementally (cy = 1), vector by vector or any value less than (ns/2).
2.4 Inverse covariance calculation with General Miller (GM) in ALCMV beamforming
To calculate the inverse covariance matrix efficiently, we employed the General Miller (GM) method, which is known for its robustness in handling high-dimensional data. The GM approach in Equation 7 leverages iterative refinement to achieve accurate estimates, making it particularly suitable for cases where standard inversion techniques may fail due to numerical instability (Oliver, 1998).
2.4.1 Miller algorithm
Each time covariance is calculated using the addition and subtraction method, before the final addition and subtraction, in Miller method the inversion is performed specifically for the addition and subtraction. By combining the two methods, we proceed step by step for recursive and accelerated calculations.
2.4.2 Miller’s equation
To apply the Miller method to Equation 6, we first denote the current covariance ( ) matrix as G (Cn = G) (Equation 8). Then, we define as (Equation 9). Thus, the updated covariance matrix ( ) is expressed as (Equation 10).
Equation 8 step one in Miller equation
Equation 9 step two in Miller equation
Equation 10 step three in Miller equation
2.5 General Miller equation
In the general Miller method, the matrix 𝐻 is transformed into a sum of rank-one matrices. This involves decomposing 𝐻 into a summation of rank-one components, where each component is a product of single column vectors and their corresponding row vectors (Equation 8). This allows 𝐻 to be expressed as a sum of these rank-one matrices ( ).
Given that H was the transposed covariance of a part of the EEG, the maximum number of sums is equal to the number of channels (k). This decomposition method does not yield unique solutions. However, in ALCMV, to construct , we kept the i-th column of matrix H and set the other elements to zero. The updated inverse covariance is calculated by Equation 7.
Equation 11 step four in Miller equation
Equation 12 step five in Miller equation
Equation 13 step six in Miller equation.
: “tr” function sums the elements on the main diagonal of the square matrix, resulting in a one-dimensional (coefficient).
Substituting Equation 10 into Equation 7 results in Equation 14.
This method is capable of solving the inverse covariance recursively. We will have a recursive inverse covariance, which provides us with flexibility for quick responses. The covariance method, utilizing addition and subtraction with a function named “ICOV” in MATLAB, the general Miller method with a function titled “GMINVSUM,” and the general miller inverse covariance with a function called “RECUR_INVICOV,” have been implemented in MATLAB (Miller, 1981).
2.6 Accelerated brain source orientation detection (AORI)
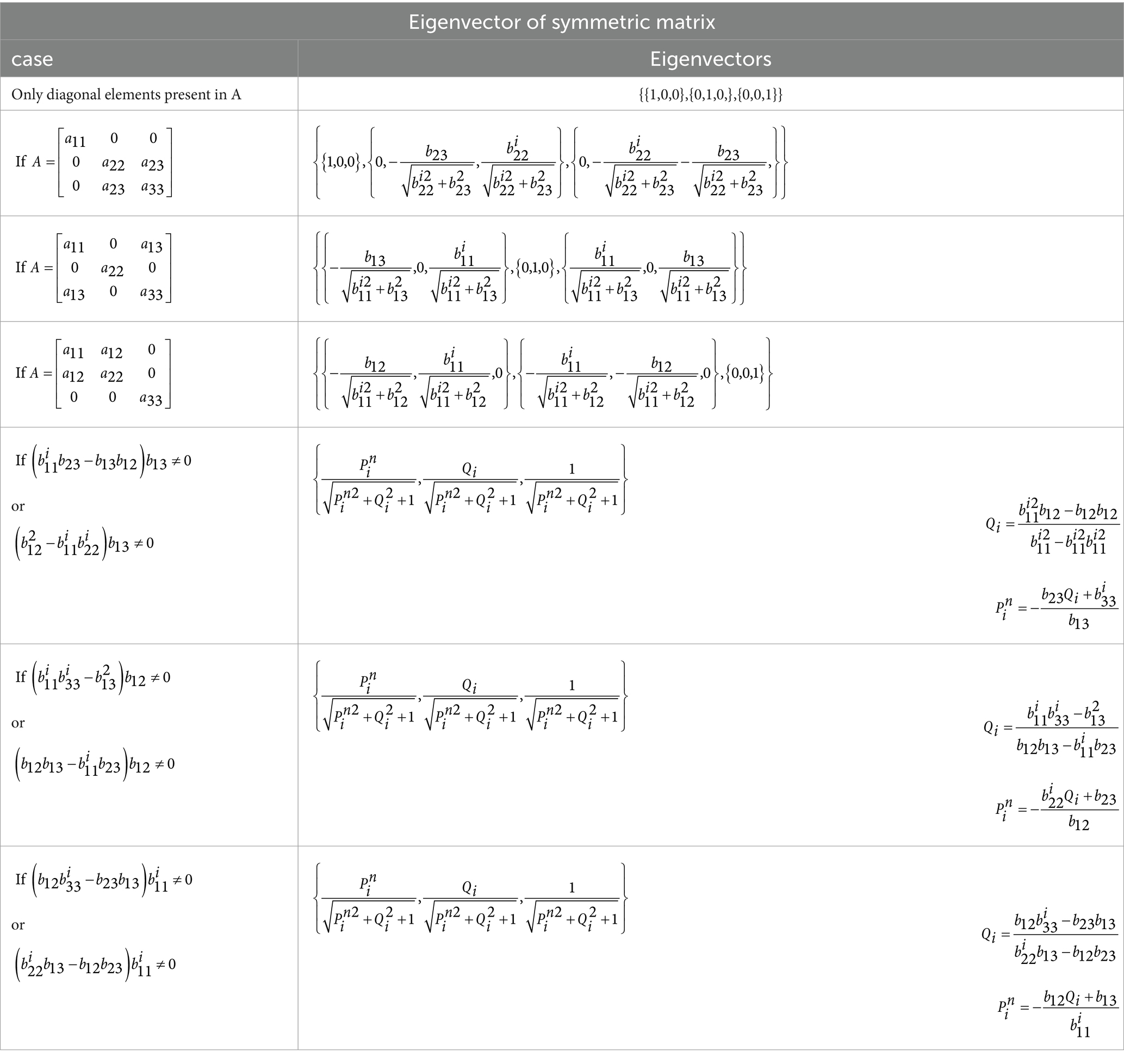
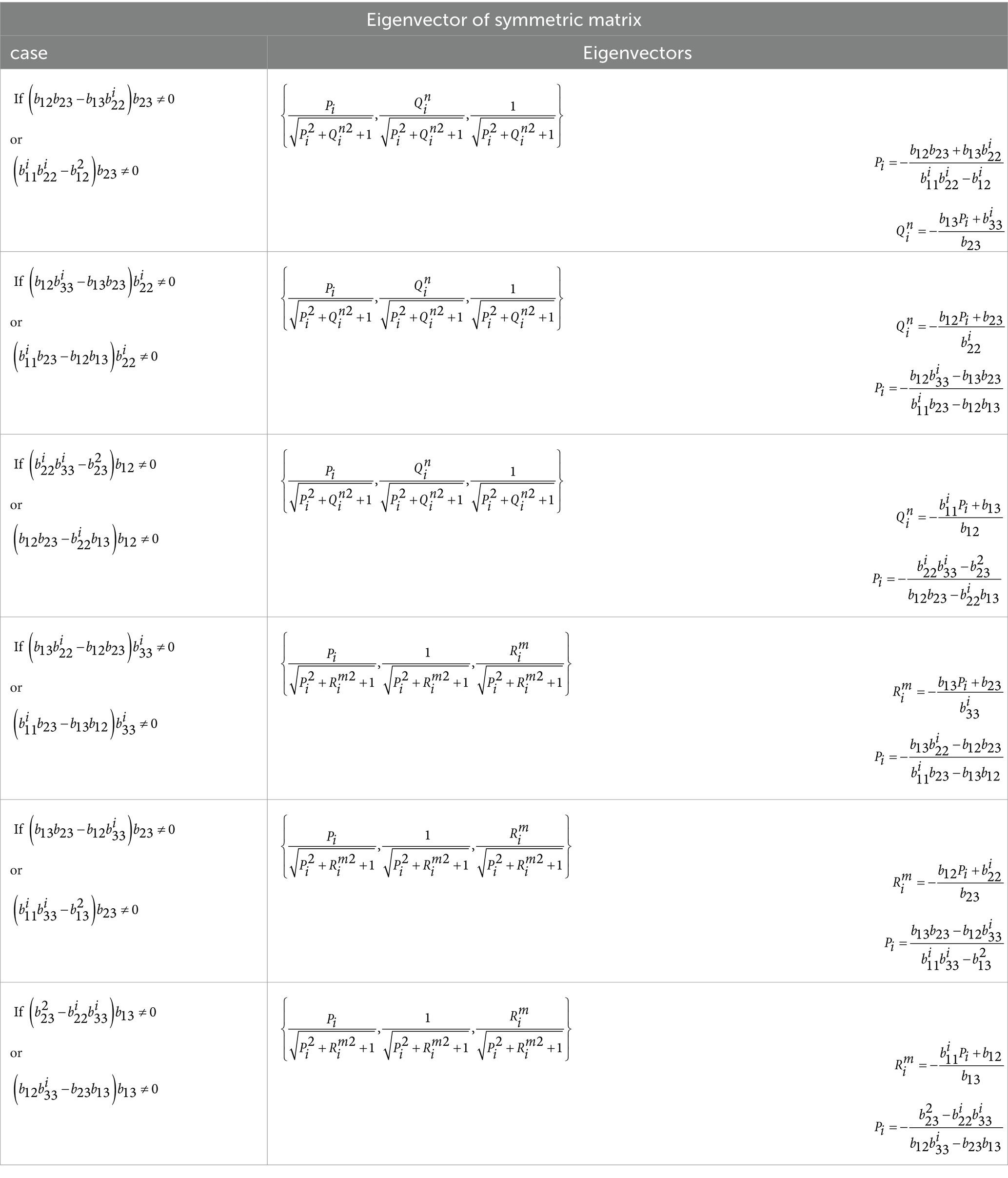
By examining the characteristics of the expression , specifically, we discovered that the result of is always a 3 × 3 symmetric matrix. We used an optimal, reliable, fast, and dedicated method to calculate the eigenvector of this matrix, which is noteworthy for its minimal computations and ease of implementation. A custom function has been written for this purpose, available under the name “smlegnVec” (Siddique and Khraishi, 2020). The smlegnVec function has been implemented in MATLAB 2020a and achieves an accuracy equivalent to MATLAB commands with fewer computations. Fast orientation detection takes another step toward accelerated signal reconstruction of sources, as it reduces dimensionality by multiplying the lead field matrix, with dimensions (number of channels × 3), by the orientation matrix, with dimensions (3 × 1). This results in the involvement of a vector with dimensions (number of channels × 1) instead of a multidimensional matrix in future computations, significantly contributing to speed enhancement. In Equation 4, the maximum rank of the matrix A= (number of independent rows or columns) will be 3. As a result, we will have a maximum of 3 eigenvalues (where i = 1,2,3).
Equation 15 ‘A’ Matrix with elements.
In AORI, the rapid detection of brain source orientation was achieved through the use of Tables 1, 2, and switch-case commands.
2.7 Data acquisition
In addition to simulated EEG data, obtaining T1 MRI images and asynchronous 24-channel EEG from 4 healthy individuals aged 20 to 55 years. To generate EEG source signals, pre-prepared EEG data for voluntary movements of hands and feet available on the internet and publicly accessible have been used. Using Equation 16, we calculate the error for the number of brain sources in the x, y, and z directions relative to the maximum power method, and then normalize it by dividing by the maximum difference values. Where [ ] is the result of “eig” function and [ ] as a result of “smlegnVec” function. We used absolute value cause both vector [ ] and its opposite, [ ], are the vectors corresponding to the smallest eigenvalue. Therefore, both of them are correct solutions (Fa-Long and Yan-Da, 1994).
With the presence of the direction vector, our signal has been reduced from 3 dimensions to 1 dimension. Therefore, for error analysis, we use Equation 17. In Equation 17, we calculate the absolute difference between the reconstructed signals of the accelerated and traditional LCMV methods and then normalized it with maximum value in each signal. N is the total number of elements in the reconstructed matrix, where r denotes the number of rows and c denotes the number of columns, as shown in Equation 18 (Ryynänen et al., 2004; Dalal et al., 2011).
3 Results
The following sections present the results from applying the ALCMV, AORI, ORI, and LCMV methods to simulated EEG data with EEGg and real EEG with moving thumb movement task (Alomari et al., 2013). The orientation and signal reconstruction results are compared across the different approaches.
3.1 ORI and LCMV method results
The traditional ORI method was applied to the same EEGg data to obtain orientation results, which were then compared with those from the AORI method. Additionally, the conventional LCMV (Linearly Constrained Minimum Variance) beamforming method was used for signal reconstruction. The results from LCMV were compared with the source reconstruction outputs from the ALCMV method. Errors for the orientation results were calculated using Equation 16, while errors for signal reconstruction were computed using Equation 16 and Equation 17.
3.2 AORI, ALCMV, and comparative results
EEGg (generated EEG) data were first processed using the AORI (Accelerated Brain Source Orientation Detection) method, which provided the initial orientation results. Following this, the ALCMV (Accelerated Linear Constrained Minimum Variance) method was applied to the EEGg data to perform source signal reconstruction. The reconstruction error was then calculated using Equation 16 and Equation 17.
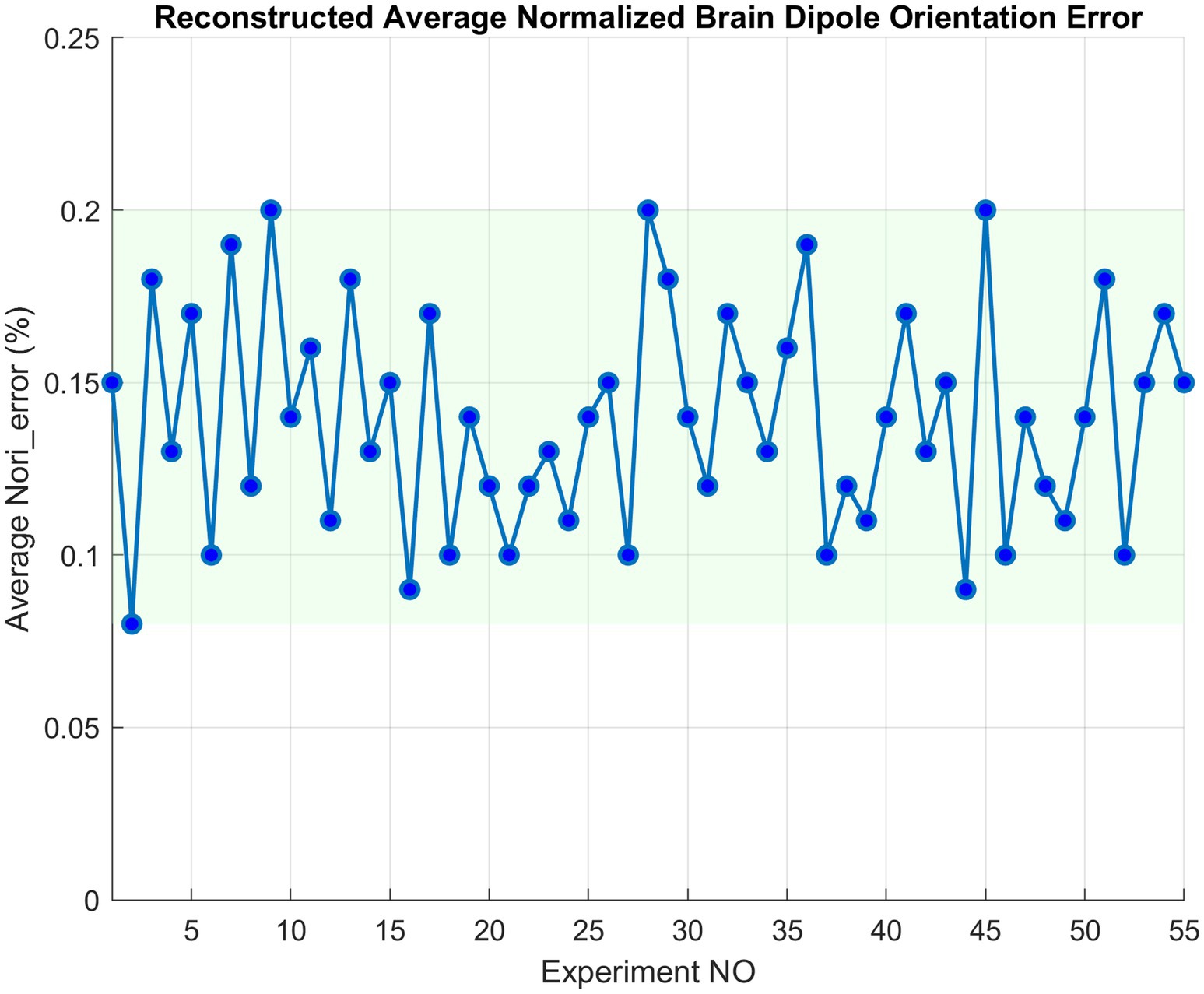
On average, ALCMV combined with AORI reduces computational load by 66% compared to LCMV and ORI. Additionally, the were consistently below 0.2%, indicating an accuracy of approximately 99.8% (Figure 2). The average was 2%.
3.3 Real EEG validation
The real EEG data underwent signal reconstruction using ALCMV, and the results were compared to the real EEG sources. The comparison clearly illustrated the spike detection; however, the amplitude decreased significantly. To address this, the normalized version of each segment was recursively calculated, achieving 98% accuracy (Figure 3).
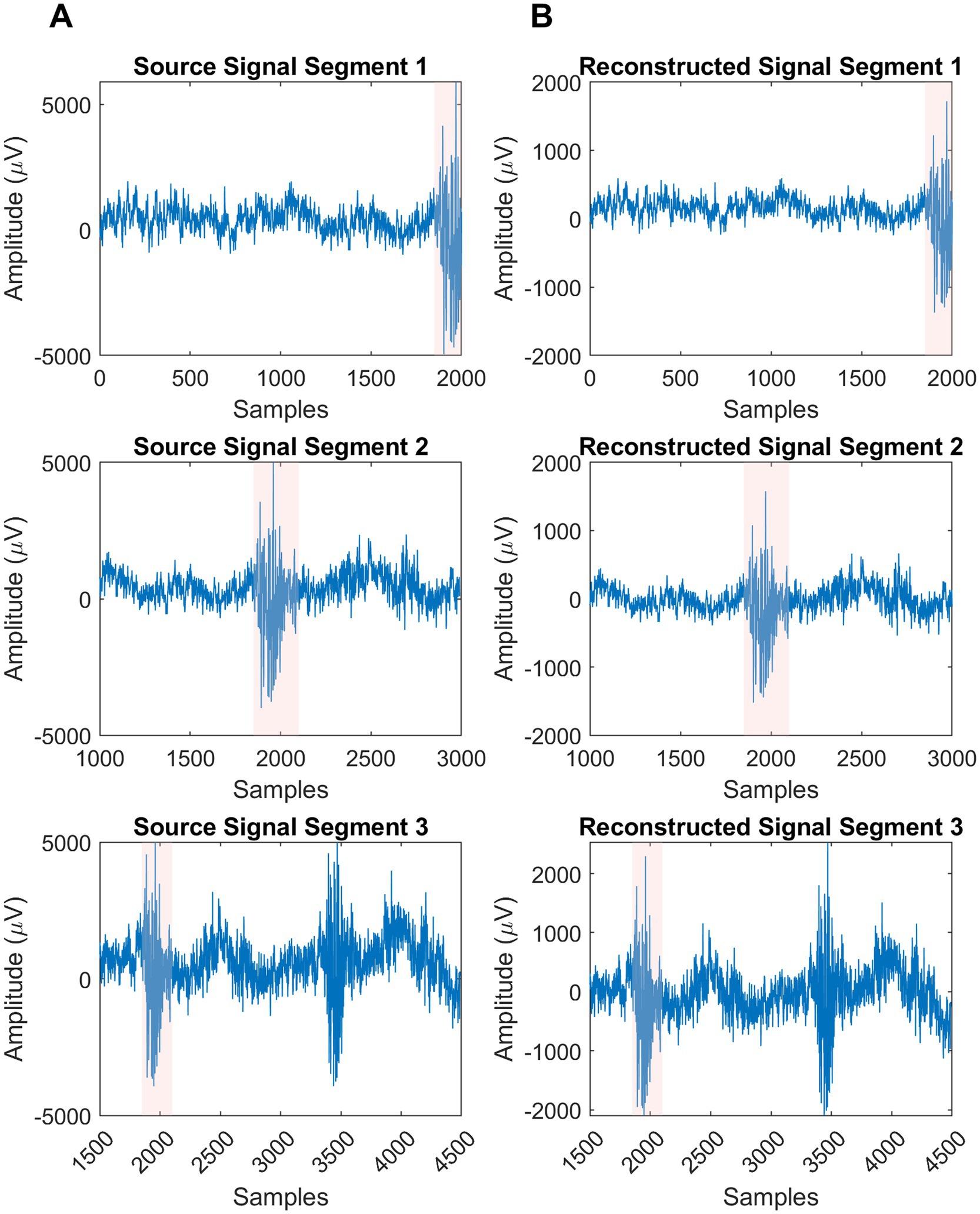
Figure 3. Visualization of real sources and their reconstructions segments. (A) Column is the produced sources in EEGg. (B) Column is the result of ALCMV and AORI signal reconstruction algorithm.
The most active source locations in the sensory motor cortex, identified by summing the absolute values of source signals, were detected using the ORI and LCMV methods, as well as their adaptive versions (AORI and ALCMV) (Idowu et al., 2020). These methods were validated using real EEG data. The detection error for the most active and second most active sources in 3D source localization was 3.2% (Figure 4), corresponding to an accuracy of 96.8%, with a 66% reduction in computational cost.
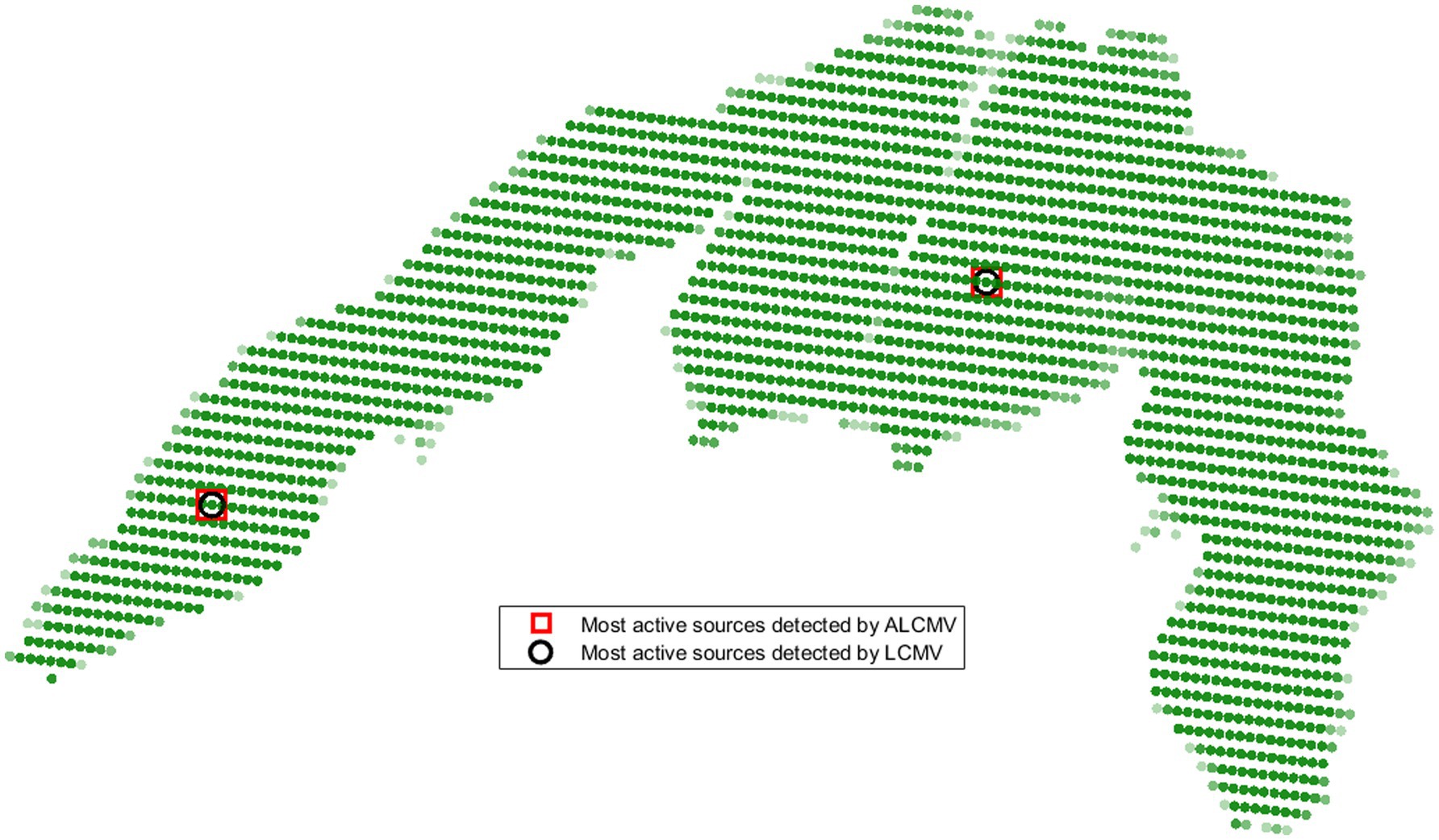
Figure 4. Locations of the most active sources detected by ALCMV and LCMV in the sensory motor cortex.
In summary, the average reduction in computational load was 66%. The accuracy of detecting active sources from non-active sources was 99.92%, meaning that out of 10,000 sources, 9,992 were correctly classified as active or non-active. The average normalized correlation of signal reconstruction with EEG data was 0.98, while for the LCMV, ORI, ALCMV, and AORI methods, the average normalized correlation was 0.95. The average localization error for detecting the most active source using ALCMV and LCMV was 2.6 mm.
4 Discussion
The results of this study highlight the significant performance improvements achieved through the proposed ALCMV and AORI algorithms in the context of EEG source localization. The primary advantage of these methods lies in their ability to reduce computational load while maintaining a high degree of accuracy. This is particularly important for real-time applications, such as neurorehabilitation and brain-computer interface (BCI) systems, where fast and accurate source localization is crucial.
The recursive covariance calculation in the ALCMV toolbox is a notable innovation, allowing the beamforming process to begin before the full set of data is available. This approach, combined with the General Miller method for inverse covariance calculation, significantly reduces the time required for source signal reconstruction. Similarly, the AORI toolbox simplifies the traditionally complex task of source orientation detection by reducing the problem from three dimensions to one. This not only accelerates the orientation estimation process but also maintains the accuracy of source localization.
The validation results from both simulated and real EEG data further reinforce the effectiveness of these methods. The 66% reduction in computational load is a substantial improvement over traditional techniques, such as LCMV and ORI, which often struggle with processing delays, especially when dealing with large datasets. Additionally, the low orientation error (below 0.2%) and signal reconstruction error (approximately 2%) demonstrate that these accelerated algorithms do not compromise the precision of EEG source localization.
In real-world applications, the enhanced speed and accuracy of the ALCMV and AORI toolboxes have significant implications. For example, in clinical diagnostics, fast and precise localization of abnormal brain activity could aid in early detection and intervention for neurological disorders, such as epilepsy. In BCI applications, the ability to localize brain signals in real time could improve the responsiveness and reliability of the system, thereby enhancing the overall user experience.
While this study focuses on EEG data, the principles behind the ALCMV and AORI algorithms could potentially be adapted for other types of neuroimaging data, such as magnetoencephalography (MEG). Future work could explore the application of these accelerated algorithms in other domains and further optimize the toolboxes for use in different EEG system configurations. EEG source localization is an ill-posed problem due to the mismatch between the number of electrodes and activated neurons. This aligns with the need for advanced algorithms in your paper to address this challenge by improving the accuracy and efficiency of source detection through LCMV beamforming and other accelerated techniques (Zorzos et al., 2024).
While the integration of EEG and MRI data through ALCMV for source localization has shown promising results in terms of accuracy and spike detection, several hardware and setup limitations must be considered, particularly in real-world applications. One significant challenge arises from the use of specialized software such as MATLAB to create masks that combine MRI with EEG channel data. Although this approach facilitates a more efficient mapping of brain activity, it requires a high level of computational resources, which can be a barrier for widespread clinical use. In practice, clinicians may face difficulties with the high setup time and the complexity of these tools, which could hinder their adoption in fast-paced clinical environments where quick decision-making is crucial. Furthermore, the reliance on the precise alignment of EEG and MRI data can present difficulties in patients with certain anatomical variations or movement artifacts, leading to potential inaccuracies in localization and reduced reliability in real-time feedback.
Moreover, while the feedback system provided by the integrated EEG and MRI processing aids in the speed and accuracy of brain-computer interface (BCI) systems, these systems are not yet fully optimized for use in rehabilitation settings outside of controlled environments. In real-world scenarios, such as home-based rehabilitation or in-field applications, hardware limitations (e.g., portability, sensor calibration) and setup requirements (e.g., lengthy preparatory time) could hinder the effectiveness and scalability of the system. Ensuring robust performance in diverse, less-controlled settings, where subjects may not be as compliant or may experience physical discomfort, remains an area for improvement. Lastly, while this system offers a higher degree of personalization and real-time feedback for users, future research will need to address the cost-effectiveness and accessibility of such technologies to facilitate broader clinical implementation, especially for individuals with limited resources or those in remote areas.
In conclusion, the ALCMV and AORI toolboxes represent a significant advancement in EEG source localization, providing a solution that balances speed and accuracy. These toolboxes offer practical benefits for both research and clinical settings, where real-time data processing is increasingly becoming a necessity.
4.1 Limitations
The ALCMV system remains highly sensitive to the precise placement of EEG electrodes and their alignment with MRI images, making it dependent on laboratory conditions and requiring stricter standards for accurate results. It is also reliant on MRI data and the leadfield matrix (Wolters et al., 2004), preventing it from functioning independently of these. Additionally, ALCMV is unable to reconstruct multiple source signals simultaneously, requiring significant computational resources for parallel processing. Its accuracy diminishes in the presence of background noise correlated with the dominant signal (Kobald et al., 2016), and while higher spatial resolution is possible with more sources, it demands more powerful hardware and careful lab setups.
4.2 Future work
Future work could involve expanding non-invasive experiments to include more active sources, multiple tasks, and specialized cases such as brain diseases or tumors, enabling real-time tracking of brain activity. Simplifying the preparation of large datasets for deep learning through ALCMV and AORI could lead to real-time algorithmic diagnostic systems for patient classification. There is also potential for applying ALCMV in detecting readiness potential (RP) signals in intelligent machines (Yousefzadeh et al., 2022) with BCIs, as well as integrating these algorithms for simultaneous fMRI and EEG recordings. Additionally, enhancing BCI systems and robotics with ALCMV and AORI for fast brain activity tracking could improve real-time control and responsiveness.
5 Conclusion
In this study, we developed and evaluated two accelerated algorithms, ALCMV and AORI, aimed at enhancing the efficiency of EEG source localization. The results demonstrated that these algorithms significantly reduce computational load by 66% while maintaining high accuracy, with orientation and signal reconstruction errors below 0.2 and 2%, respectively. These improvements make the algorithms suitable for real-time applications, such as neurorehabilitation and brain-computer interfaces. Future work could focus on extending these methods to other neuroimaging techniques and optimizing them for broader clinical use.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study is part of an MSc thesis supported by the Tehran University of Medical sciences [code of ethics: IR.TUMS.MEDICINE.REC.1400.1102]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AY: Methodology, Software, Writing – original draft, Writing – review & editing. BM: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This study is part of an MSc thesis supported by the Tehran University of Medical Sciences [project code: 1403-4-101-76170 and the code of ethics: IR.TUMS.MEDICINE.REC.1400.1102]. The codes are available at: https://github.com/Avayekta/ALCMV-AORI.git.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI was used to enhance the clarity and quality of the writing. I would like to inform you that a draft of my research has been uploaded to the arXiv website.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^EEGg is a toolbox designed for generating synthetic EEG data using both real and simulated EEG signals (Vaziri et al., 2024).
References
Alomari, M. H., Samaha, A., and AlKamha, K. (2013). Automated classification of L/R hand movement EEG signals using advanced feature extraction and machine learning. Int. J. Adv. Comput. Sci. Appl. 4. doi: 10.14569/IJACSA.2013.040628
Cho, J.-H., Vorwerk, J., Wolters, C. H., and Knösche, T. R. (2015). Influence of the head model on EEG and MEG source connectivity analyses. NeuroImage 110, 60–77. doi: 10.1016/j.neuroimage.2015.01.043
Dalal, S. S., Zumer, J. M., Guggisberg, A. G., Trumpis, M., Wong, D. D. E., Sekihara, K., et al. (2011). MEG/EEG source reconstruction, statistical evaluation, and visualization with NUTMEG. Comput Intell Neurosci 2011:758973. doi: 10.1155/2011/758973
Fa-Long, L., and Yan-Da, L. (1994). Real-time computation of the eigenvector corresponding to the smallest eigenvalue of a positive definite matrix. IEEE Trans. Circuits Syst. Fundam. Theory Appl. 41, 550–553. doi: 10.1109/81.311545
Garcia Dominguez, L., Tarazi, A., Valiante, T., and Wennberg, R. (2023). Beamforming seizures from the temporal lobe using magnetoencephalography. Can. J. Neurol. Sci. 50, 201–213. doi: 10.1017/cjn.2022.1
Huang, M.-X., Huang, C. W., Robb, A., Angeles, A. M., Nichols, S. L., Baker, D. G., et al. (2014). MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. NeuroImage 84, 585–604. doi: 10.1016/j.neuroimage.2013.09.022
Idowu, O. P., Fang, P., and Li, G. (2020). Bio-inspired algorithms for optimal feature selection in motor imagery-based brain-computer Interface. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 519–522. doi: 10.1109/EMBC44109.2020.9176244
Ilmoniemi, R. J., and Sarvas, J. Brain signals: physics and mathematics of MEG and EEG. Available online at: https://direct.mit.edu/books/monograph/4319/Brain-SignalsPhysics-and-Mathematics-of-MEG-and (Accessed September 17, 2024)
Jaiswal, A., Nenonen, J., Stenroos, M., Gramfort, A., Dalal, S. S., Westner, B. U., et al. (2020). Comparison of beamformer implementations for MEG source localization. NeuroImage 216:116797. doi: 10.1016/j.neuroimage.2020.116797
Jonmohamadi, Y., Poudel, G., Innes, C., Weiss, D., Krueger, R., and Jones, R. (2014). Comparison of beamformers for EEG source signal reconstruction. Biomed Signal Proces Control 14, 175–188. doi: 10.1016/j.bspc.2014.07.014
Kobald, S. O., Getzmann, S., Beste, C., and Wascher, E. (2016). The impact of simulated MRI scanner background noise on visual attention processes as measured by the EEG. Sci. Rep. 6:28371. doi: 10.1038/srep28371
Masoud, S., al-Nashash, H., and Mir, H. (2025). Inverse problem for M/EEG source localization: a review. IEEE Sensors J. 25, 2103–2124. doi: 10.1109/JSEN.2024.3502917
Miller, K. S. (1981). On the inverse of the sum of matrices. Math. Mag. 54, 67–72. doi: 10.2307/2690437
Myers, M. H., Padmanabha, A., Bidelman, G. M., and Wheless, J. W. (2020). Seizure localization using EEG analytical signals. Clin. Neurophysiol. 131, 2131–2139. doi: 10.1016/j.clinph.2020.05.034
Nan, H., and Yulin, H. (2011). “Research and comparison of anti-jamming performances of LMS and LCMV algorithm based on MATLAB simulation” in IEEE 10th International Conference on Electronic Measurement & Instruments.
Oliver, D. (1998). Calculation of the inverse of the covariance. Math. Geol. 30, 911–933. doi: 10.1023/A:1021734811230
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J.-M. (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011:156869. doi: 10.1155/2011/156869
Ryynänen, O., Hyttinen, J., and Malmivuo, J. (2004). Study on the spatial resolution of EEG--effect of electrode density and measurement noise. Conf. Proc. Annu. Int. 2004, 4409–4412. doi: 10.1109/IEMBS.2004.1404226
Sekihara, K., and Nagarajan, S. S. (2015). Electromagnetic brain imaging: A Bayesian perspective. Cham: Springer International Publishing.
Siddique, A. B., and Khraishi, T. (2020). Eigenvalues and eigenvectors for 3×3 symmetric matrices: an analytical approach. J. Adv. Math. Comput. Sci. 35, 106–118. doi: 10.9734/JAMCS/2020/v35i730308
Van Veen, B. D., van Drongelen, W., Yuchtman, M., and Suzuki, A. (1997). Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. I.E.E.E. Trans. Biomed. Eng. 44, 867–880. doi: 10.1109/10.623056
Vaziri, A. Y., Makkiabadi, B., and Samadzadehaghdam, N. EEGg: Generating Synthetic EEG Signals in Matlab Environment. Front. Biomed. Technol. :13165. doi: 10.18502/fbt.v10i3.13165
Wolters, C. H., Grasedyck, L., and Hackbusch, W. (2004). Efficient computation of lead field bases and influence matrix for the FEM-based EEG and MEG inverse problem. Inverse Problems 20, 1099–1116. doi: 10.1088/0266-5611/20/4/007
Wu, W. B., and Xiao, H. (2012). “Covariance matrix estimation in time series” in Handbook of statistics (Amsterdam: Elsevier), 187–209.
Yousefzadeh, M., Hasanpour, M., Zolghadri, M., Salimi, F., Yektaeian Vaziri, A., Mahmoudi Aqeel Abadi, A., et al. (2022). Deep learning framework for prediction of infection severity of COVID-19. Front. Med. 9:940960. doi: 10.3389/fmed.2022.940960
Keywords: EEG source localization, beamforming, neural signal processing, LCMV, accelerated algorithms, source orientation detection, recursive calculations, brain-computer interface
Citation: Yektaeian Vaziri A and Makkiabadi B (2025) Accelerated algorithms for source orientation detection and spatiotemporal LCMV beamforming in EEG source localization. Front. Neurosci. 18:1505017. doi: 10.3389/fnins.2024.1505017
Edited by:
Jürgen Dammers, Helmholtz Association of German Research Centres (HZ), GermanyReviewed by:
Mohammad Pooyan, Shahed University, IranKeivan Kaboutari, University of Aveiro, Portugal
Jaspreet Kaur, University of Glasgow, United Kingdom
Copyright © 2025 Yektaeian Vaziri and Makkiabadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bahador Makkiabadi, Yi1tYWtraWFiYWRpQHR1bXMuYWMuaXI=
 Ava Yektaeian Vaziri
Ava Yektaeian Vaziri Bahador Makkiabadi
Bahador Makkiabadi