
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Neurol., 24 March 2025
Sec. Neuro-Otology
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1560787
This article is part of the Research TopicThe Vestibular System: A tribute to Professor Dr. Hans StrakaView all 14 articles
 Jun Maruta1,2*
Jun Maruta1,2*Mal de débarquement syndrome (MdDS) is an under-recognized and poorly understood illness. Typically triggered by prolonged exposure to passive motion during a voyage on a cruise ship or airplane, MdDS is primarily characterized by a continuous phantom perception of oscillatory self-motion such as rocking, swaying, or bobbing, or a sensation of gravitational pull (collectively identified as non-spinning vertigo) and associated sensations of imbalance (1–3). The self-motion symptoms of MdDS are generally accompanied by somatic complaints (e.g., headaches and visually induced dizziness), reduced cognitive functions (e.g., decreased attention and short-term memory), and affective problems (e.g., depression and anxiety). These symptoms can be severe enough to often lead to long-term disability (4–6) and for some patients to develop suicidal thoughts (7, 8).
Transient mal de débarquement (commonly known as “sea legs”), representing the common after-sensation that mimics the exposed physical motion and associated postural instability, has been recognized for centuries (9, 10). Although MdDS, a chronic manifestation of mal de débarquement, has gained increasing recognition following the 1987 publication of a six-patient case series (1), the illness has yet to permeate the awareness of clinicians and is often misdiagnosed as a mental disorder, vestibular migraine, or dizziness caused by peripheral vestibular dysfunction. Patients are said to typically make 2–5 visits to healthcare professionals before their MdDS diagnosis, but those who undergo 20 or more such visits are not uncommon (2, 4, 5, 11). As such, the actual prevalence of the illness cannot be determined presently, but at least a small percentage of patients seen at large clinical centers specializing in balance and dizziness are identified as having MdDS (12, 13) and ~80% of reported cases are women (4, 6, 14, 15).
The pathogenesis of MdDS is poorly understood, but the experience of mal de débarquement suggests some form of motion-induced entrainment in the central vestibular pathways (16–19). MdDS is not associated with an injury or overt structural change in the peripheral or central nervous system, but is rather thought to be a disorder generated from synaptic changes that can be reversed (14, 19–22). Most people spontaneously recover from transient mal de débarquement within hours to several days (16, 21). Why the condition persists into a chronic form in some people, or how accompanying symptoms develop is not clear.
The likelihood of spontaneous remission is said to decrease with time (2, 23). Further, once diagnosed with MdDS, patients face limited treatment options. Although MdDS is believed to hinge on central vestibular processing, conventional vestibular physical therapy generally offers little benefit (6, 19, 24). Some patients experience partial symptom relief from benzodiazepines, a class of GABA-A agonists (6, 19, 21), but the site of their pharmacological action is not understood, and harmful effects including dependence must be considered (25, 26). Other patients may experience improved quality of life with vestibular migraine medications, but symptom improvement appears domain-specific, and patients' sensitivity to medications may require a greater degree of dose management than typical (27, 28).
It was against this backdrop that a possible link was discovered between MdDS and the velocity storage mechanism of the central vestibular system (20, 29). This discovery opened opportunities for positive long-term outcomes of MdDS through approaches that target the neural malleability of velocity storage, thereby addressing the root cause of the illness (14, 20, 30–32).
Velocity storage is closely associated with the vestibulo-ocular reflex (VOR) as it was first examined as a stored eye movement drive that generally prolongs the vestibular and optokinetic responses beyond the input activity through slow charging and discharging (33–36), but it can also be activated by proprioceptive cues for continuous rotation (37–39). Indeed, velocity storage is a center of multimodal sensory integration. In addition to ocular reflexes, velocity storage is thought to contribute to postural control (31, 40) and the perception of self-motion (34, 35, 37, 41, 42). Ocular, postural, and perceptual responses are often conceptualized as the sum of the outputs of the velocity storage and non-velocity storage pathways, with the latter directly relaying peripheral sensory activity (Figure 1) (31, 33–35, 40, 42). Sectioning vestibular commissural fibers caudal to the abducens nucleus selectively abolishes the sluggish VOR components attributed to velocity storage while sparing fast direct responses, supporting the presence of the separate neural pathways (43, 44).
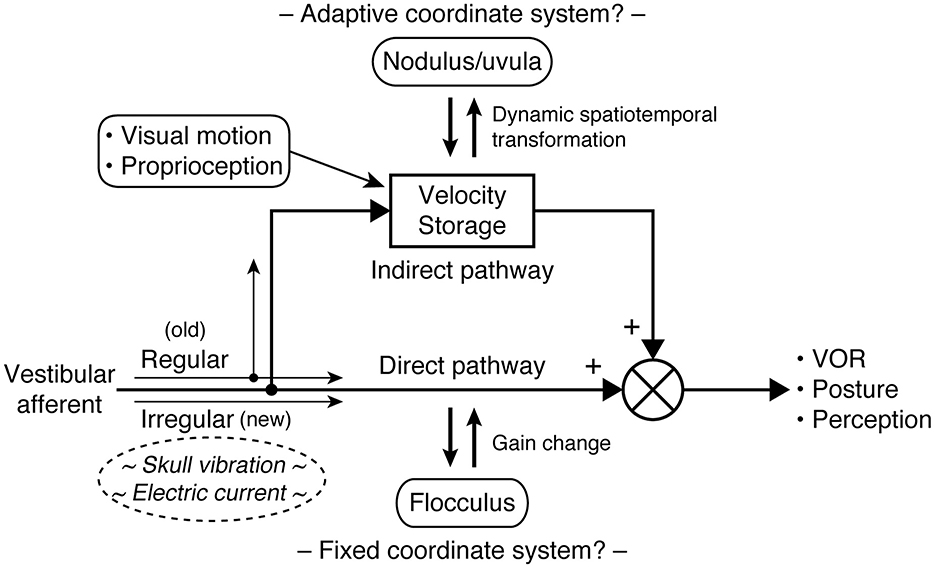
Figure 1. Central vestibular processing conceptualized with parallel direct and indirect pathways. In the VOR, the direct pathway corresponds to the three-neuron arc of the reflex and interacts with the cerebellar flocculus to calibrate its output. The indirect (velocity storage) pathway involves multiple sensory integration and interacts with the cerebellar nodulus and uvula to support a unified sense of self-motion and stability. Type I and type II hair cells in the vestibular organs are respectively synapsed by calyceal and bouton endings of afferent fibers, which in turn are associated with irregular and regular patterns of signaling. Phylogenetically, velocity storage and type II vestibular hair cells antecede type I vestibular hair cells. As it is hypothesized that irregular fibers bypass velocity storage, the difference in the responsiveness of regular and irregular fibers to vibration or galvanic stimuli may be utilized to elucidate pathological vestibular conditions, including MdDS.
Velocity storage does not merely prolong the signals received from peripheral sensors, but actively reconstructs and dynamically reshapes information about self-motion, embodying a more dynamic working-memory like quality than short-term memory (45). For example, off-vertical axis rotation (OVAR) in darkness induces continuous compensatory nystagmus by activating the otolith organs, which are not rotation sensors in a normal sense (46). Similarly, stepping in place on a circular treadmill in darkness induces nystagmus that compensates for the apparent circling (38, 39). Further, per-rotatory nystagmus in response to off-center rotation while facing in or away from the direction of motion develops an out-of-plane, vertical component as the centripetal acceleration tilts the gravito-inertial field sideways (47). Critically, in order for velocity storage to interpret the incoming information and perform coordinate transformations in these manners, it needs to maintain its own referential representation of three-dimensional space.
Studies of the ocular and postural reflexes have revealed that sensorimotor transformation in the brain may be facilitated by the use of a common coordinate frame consistent with the orthogonal arrangement of the semicircular canals (48–52). Such transformation is aided by external cues related to the constant presence of gravity in the terrestrial environment (46, 53–56). However, this arrangement is established through motion exposure-dependent neuroplasticity and can undergo changes in an unusual acceleration environment (29, 52, 55, 57, 58). Damage to the cerebellar caudal vermis (nodulus and uvula) compromises the ability to generate nystagmus during OVAR or reorient eye velocity to the gravito-inertial field (59–62). Therefore, the coordinate transformations in the indirect (velocity storage) pathway are shaped through brainstem-cerebellar interactions and are also subject to the flexibility of the internal reference frame. In contrast, the direct pathway appears to operate on a fixed coordinate system whose outcome is determined by gain calibration and vector summation of separate channels (Figure 1) (63–65).
MdDS is thought to result from a failure in velocity storage to readapt to a normal acceleration environment after adapting to passive motion (29, 58). A treatment approach designed to correct the presumably maladapted spatial properties of velocity storage with a combination of visual and vestibular stimuli has significantly improved the overall outcomes of MdDS, with a success rate of ~80% and ~50% in short and long terms, respectively (14, 15, 20, 30–32, 66–70). Still, substantially many patients do not benefit from the treatment, and the neural basis of the illness is far from clear.
Physical signs associated with MdDS such as postural imbalance and direction-changing nystagmus are not present in all patients nor unique to MdDS, nor do they indicate the subjective severity of the illness (3, 20). Instead, the diagnosis of MdDS is based solely on clinical history and subjective reports (3, 71). The symptoms of MdDS greatly overlap with those of another chronic vestibular disorder known as persistent postural perceptual dizziness (PPPD) (72), which with MdDS may represent a large spectrum of non-spinning vertigo (3, 6). However, exposure to passive motion typically worsens symptoms of PPPD while temporarily alleviates those of MdDS (2, 3, 71). Thus, the effect of passive motion may be a critical difference between MdDS and PPPD (3).
The effect of passive motion also reveals the ability of inner-ear-driven signals to modulate symptoms of MdDS. The mechanism for this phenomenon is not known. However, in experimental animals, some neurons in the vestibulo-olivo-cerebellar pathway of the caudal vermis were found with oscillatory entrainment in their spontaneous activity after exposure to minutes of cyclic tilting motion, but this oscillatory activity was transiently reset by, rather than superimpose with, vestibular signals consisting of other frequencies, only to resume when the stimulus stopped (73). The direct relevance of this finding to MdDS is not clear, but caudal vermal representation of adaptation to a moving environment as well as transient cessation of such activity by new motion exposure seem significant given the area's close relevance to velocity storage.
Galvanic vestibular stimulation (GVS) affords stimulation of vestibular primary afferents without moving the head, by instead passing low electrical current through the skin over the mastoids. GVS is becoming widely applied to both experimental and clinical testing of balance functions in health and neurological conditions (74, 75), but not yet in MdDS. GVS is also used to generate head motion cues during interactions in virtual/simulated environments (76–78).
Cathodal and anodal current respectively activates and silences vestibular afferent fibers, with lower threshold for irregular than regular afferents (74, 75, 79–81). By vector summation, directionally different responses can be elicited. For example, bilateral bipolar stimulation with the anode on the right side induces a rightward postural sway (or an overall illusory leftward rotational perception in immobilized subjects), and bilateral monopolar anodal stimulation with a distant reference electrode induces a backward postural response (74, 77, 78). Under certain conditions, blindfolded human subjects can be steered by remotely-controlled GVS while walking (82). Bilateral bipolar injection of noise current increases the activity of afferent fibers in a stochastic manner (75) to induce a non-specific self-motion perception in normal subjects, reported as “weird” but generally more comfortable and less irritating or nauseating than GVS with square-wave pulses of equivalent current amplitude (83).
Bone conducted vibration (BCV) applied to the skull at ~100 Hz is another means to stimulate vestibular primary afferents without moving the head, other than the vibration motion itself, typically with an amplitude of 1 mm or less at an intensity of a body massager (84–86). BCV reportedly highly selectively activates irregular afferents (87–89), but is otherwise a global vestibular stimulus because skull vibration stimulates afferent nerves from both ears. Normally, the effects of bilaterally activated vestibular nerves are said to be negated centrally because of the push-pull organization of the vestibular system; however, unilateral dysfunction creates a response asymmetry, forming the basis of the skull vibration-induced nystagmus (SVIN) test (84, 86). The safety and sensitivity of the SVIN test are well established (84, 85).
SVIN in individuals with unilateral vestibular loss appears and disappears abruptly at the onset and cessation of BCV, respectively, without a progressive buildup in intensity or slow-decaying after-nystagmus (90, 91). This characteristic is different from the charging and discharging behavior of velocity storage with a time constant of at least several seconds even with unilateral vestibular loss (92, 93). Further, interference by BCV to the nystagmic response to cold caloric stimulation to the intact ear is similarly abrupt and in accordance with the amplitude and duration of SVIN, indicating separately sustained velocity storage activity during BCV (91). Therefore, a possible “tilt dump” effect (94) from the concurrent stimulation of otolithic afferents during BCV may be ruled out as an explanation for the lack of after-nystagmus associated with SVIN.
From such unique characteristics and mechanism underlying SVIN, it is hypothesized that irregular afferents bypass the velocity storage mechanism (Figure 1) (95, 96). By contrast, regular afferents' contribution to velocity storage is supported by the existing knowledge. Vestibular type I and type II hair cells, respectively contact calyceal and bouton endings of afferent fibers, which are roughly associated with irregular and regular firing patterns (96, 97). Vestibular type I hair cells are phylogenetically new, as anamniotes (fishes and amphibians) only have type II vestibular hair cells, but some such species demonstrate well-functioning velocity storage (98–101). Further, the VOR during sinusoidal rotation or OVAR in genetically manipulated mice with deficient type I hair cell development does not suggest impaired velocity storage (97). A note of possible significance, however, is that GVS and BCV may have different selectivity for irregular afferents, as modest after-nystagmus has been observed to accompany GVS-induced nystagmus (102, 103). Indeed, the intensity of GVS, thus the level of recruitment of more regular afferent fibers, that is required to induce eye movement is known to be higher than that for inducing postural or perceptual responses (74, 80, 81, 104).
Although the neurological etiology and factors that contribute to the development of MdDS are not clear, several mechanisms have been postulated, including maladaptation of velocity storage (20, 29), entrainment in the cerebral networks (105), vestibular migraine (27, 28), and hormonal dysregulation (106, 107). Since MdDS is not associated with peripheral damage, the SVIN test may at first glance seem to have little to inform the pathophysiology of the illness. However, it is not yet known if patients with MdDS will experience temporary alleviation of their symptoms with BCV or low-intensity GVS. As these stimuli selectively or preferentially activate irregular afferents, a negative result would indicate a specific role of regular afferents in modulating symptoms of MdDS during passive motion as well as indirectly support the velocity storage-basis of MdDS pathogenesis. A positive result may indicate a different pathogenic mechanism and necessitate a revision to the current understanding of MdDS, but would also indicate the potential utility of these stimuli in the diagnosis or treatment of the illness. The velocity storage mechanism may still be accessed with high-intensity GVS, which may find its own utility. Lastly, premorbid asymmetry in the vestibular sensitivity may contribute to the activation of velocity storage while naturally interacting in the environment, possibly making the individual more susceptible to MdDS during exposure to passive motion (58). BCV or GVS may also be useful in probing such a possibility.
MdDS is a debilitating vestibular disorder whose pathophysiology is presently little understood. An animal-based model of MdDS would be useful but has not been established yet (29, 58, 108). BCV and GVS are well-studied artificial vestibular stimuli that may be safely tested on patients. Since these stimuli selectively or preferentially activate irregular vestibular afferents and may, perhaps to a different degree, bypass the velocity storage mechanism, studying their effects may fill a piece of the puzzle of MdDS pathophysiology.
JM: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BCV, bone conducted vibration; GVS, galvanic vestibular stimulation; MdDS, mal de débarquement syndrome; OVAR, off-vertical axis rotation; PPPD, persistent postural perceptual dizziness; SVIN, skull vibration-induced nystagmus; VOR, vestibulo-ocular reflex.
1. Brown JJ, Baloh RW. Persistent mal de debarquement syndrome: a motion-induced subjective disorder of balance. Am J Otolaryngol. (1987) 8:219–22. doi: 10.1016/S0196-0709(87)80007-8
2. Cha Y-H. Mal de debarquement syndrome: new insights. Ann NY Acad Sci. (2015) 1343:63–8. doi: 10.1111/nyas.12701
3. Cha Y-H, Baloh RW, Cho C, Magnusson M, Song J-J, Strupp M, et al. Mal de débarquement syndrome diagnostic criteria: consensus document of the classification committee of the Bárány society. J Vestib Res. (2020) 30:285–93. doi: 10.3233/VES-200714
4. Mucci V, Canceri JM, Brown R, Dai M, Yakushin S, Watson S, et al. Mal de debarquement syndrome: a survey on subtypes, misdiagnoses, onset and associated psychological features. J Neurol. (2018) 265:486–99. doi: 10.1007/s00415-017-8725-3
5. Macke A, LePorte A, Clark BC. Social, societal, and economic burden of mal de debarquement syndrome. J Neurol. (2012) 259:1326–30. doi: 10.1007/s00415-011-6349-6
6. Cha Y-H, Cui YY, Baloh RW. Comprehensive clinical profile of mal de debarquement syndrome. Front Neurol. (2018) 9:261. doi: 10.3389/fneur.2018.00261
7. Hughes CK, Eliason MJ, Matsuoka AJ. The hidden enemy: mal de débarquement syndrome and its impact on military operations. Mil Med. (2023) 189:usad449. doi: 10.1093/milmed/usad449
8. Cohen B, Dai M, Smouha E, Cho C. Mal de débarquement syndrome. Neurol Clin Pract. (2015) 5:369–70. doi: 10.1212/01.CPJ.0000472925.22196.a2
10. Irwin JA. The pathology of sea-sickness. Lancet. (1881) 2:907–9. doi: 10.1016/S0140-6736(02)38129-7
11. Saha KC, Fife TD. Mal de débarquement syndrome: review and proposed diagnostic criteria. Neurol Clin Pract. (2015) 5:209–15. doi: 10.1212/CPJ.0000000000000116
12. Cha Y-H. Less common neuro-otologic disorders. Continuum. (2012) 18:1142–57. doi: 10.1212/01.CON.0000421623.56525.11
13. Formeister EJ, Chae R, Wong E, Chiao W, Pasquesi L, Sharon JD. Episodic vs. chronic dizziness: an analysis of predictive factors. Ann Otol Rhinol Laryngol. (2022) 131:403–11. doi: 10.1177/00034894211025416
14. Dai M, Cohen B, Cho C, Shin S, Yakushin SB. Treatment of the mal de debarquement syndrome: a 1-year follow-up. Front Neurol. (2017) 8:175. doi: 10.3389/fneur.2017.00175
15. Schoenmaekers C, Jillings S, De Laet C, Zarowski A, Wuyts FL. Guideline for standardized approach in the treatment of the mal de debarquement syndrome. Front Neurol. (2024) 15:1359116. doi: 10.3389/fneur.2024.1359116
16. Cohen H. Mild mal de debarquement after sailing. Ann NY Acad Sci. (1996) 781:598–600. doi: 10.1111/j.1749-6632.1996.tb15734.x
17. Nachum Z, Shupak A, Letichevsky V, Ben-David J, Tal D, Tamir A, et al. Mal de debarquement and posture: reduced reliance on vestibular and visual cues. Laryngoscope. (2004) 114:581–6. doi: 10.1097/00005537-200403000-00036
18. Bailey CF, Cagle GK, Grozier CD, Lehtola KN, Weaver JF, Wilson SJ, et al. Gathering your ‘sea legs': extended durations in an offshore environment increases postural sway excursions. Gait Posture. (2021) 86:45–50. doi: 10.1016/j.gaitpost.2021.02.014
19. Hain TC, Cherchi M. “Mal de débarquement syndrome.” In:Furman JM, Lempert T, , editors. Handbook of Clinical Neurology. Amsterdam: Elsevier (2016). p. 391–5. doi: 10.1016/B978-0-444-63437-5.00028-5
20. Dai M, Cohen B, Smouha E, Cho C. Readaptation of the vestibulo-ocular reflex relieves the mal de debarquement syndrome. Front Neurol. (2014) 5:124. doi: 10.3389/fneur.2014.00124
22. Cha Y-H, Gleghorn D, Doudican B. Occipital and cerebellar theta burst stimulation for mal de debarquement syndrome. Otol Neurotol. (2019) 40:e928–37. doi: 10.1097/MAO.0000000000002341
23. Cha Y-H, Brodsky J, Ishiyama G, Sabatti C, Baloh RW. Clinical features and associated syndromes of mal de debarquement. J Neurol. (2008) 255:1038–44. doi: 10.1007/s00415-008-0837-3
24. Cedras AM, Moin-Darbari K, Foisy K, Auger S, Nguyen D, Champoux F, et al. Questioning the impact of vestibular rehabilitation in mal de debarquement syndrome. Audiol Neurootol. (2023) 29:1–7. doi: 10.1159/000533684
25. Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. (2007) 369:1047–53. doi: 10.1016/S0140-6736(07)60464-4
26. Guina J, Merrill B. Benzodiazepines I: upping the care on downers: the evidence of risks, benefits and alternatives. J Clin Med. (2018) 7:17. doi: 10.3390/jcm7020017
27. Ghavami Y, Haidar YM, Ziai KN, Moshtaghi O, Bhatt J, Lin HW, et al. Management of mal de debarquement syndrome as vestibular migraines. Laryngoscope. (2017) 127:1670–5. doi: 10.1002/lary.26299
28. Beh SC, Chiang H-S, Sanderson C. The interconnections of mal de débarquement syndrome and vestibular migraine. Laryngoscope. (2021) 131:E1653–61. doi: 10.1002/lary.29475
29. Dai M, Raphan T, Cohen B. Adaptation of the angular vestibulo-ocular reflex to head movements in rotating frames of reference. Exp Brain Res. (2009) 195:553–67. doi: 10.1007/s00221-009-1825-2
30. Maruta J, Cho C, Raphan T, Yakushin SB. Symptom reduction in mal de débarquement syndrome with attenuation of the velocity storage contribution in the central vestibular pathways. Front Rehabil Sci. (2024) 5:1331135. doi: 10.3389/fresc.2024.1331135
31. Yakushin SB, Raphan T, Cho C. Treatment of gravitational pulling sensation in patients with mal de debarquement syndrome (MdDS): a model-based approach. Front Integr Neurosci. (2022) 16:801817. doi: 10.3389/fnint.2022.801817
32. Yakushin SB, Zink R, Clark BC, Liu C. Readaptation treatment of mal de debarquement syndrome with a virtual reality app: a pilot study. Front Neurol. (2020) 11:814. doi: 10.3389/fneur.2020.00814
33. Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. (1977) 270:321–44. doi: 10.1113/jphysiol.1977.sp011955
34. Raphan T, Cohen B, Matsuo V. “A velocity-storage mechanism responsible for optokinetic nystagmus (OKN), optokinetic after-nystagmus (OKAN) and vestibular nystagmus.” In:Baker R, Berthoz A, , editors. Control of Gaze by Brain Stem Neurons. Developments in Neuroscience. Amsterdam: Elsevier (1977). p. 37–47.
35. Robinson DA. “Vestibular and optokinetic symbiosis: an example of explaining by modelling.” In:Baker R, Berthoz A, , editors. Control of Gaze by Brain Stem Neurons. Developments in Neuroscience. Amsterdam: Elsevier (1977). p. 49–58.
36. ter Braak J. Untersuchungen über optokinetischen Nystagmus. Arch Neerl Physiol Homme Anim. (1936) 21:309–376.
37. Brandt Th, Büchele W, Arnold F. Arthrokinetic nystagmus and ego-motion sensation. Exp Brain Res. (1977) 30:331–8. doi: 10.1007/BF00237260
38. Bles W, Jong JMV, Wit GD. Somatosensory compensation for loss of labyrinthine function. Acta Otolaryngol. (1984) 97:213–21. doi: 10.3109/00016488409130982
39. Solomon D, Cohen B. Stabilization of gaze during circular locomotion in darkness. II Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J Neurophysiol. (1992) 67:1158–70. doi: 10.1152/jn.1992.67.5.1158
40. Haggerty SE, Wu AR, Sienko KH, Kuo AD. A shared neural integrator for human posture control. J Neurophysiol. (2017) 118:894–903. doi: 10.1152/jn.00428.2016
41. Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Broussard DM, Buttner-Ennever J, et al. The Vestibular System: A Sixth Sense. New York, NY: Oxford University Press (2012). doi: 10.1093/acprof:oso/9780195167085.001.0001
42. Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, et al. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. (2011) 105:209–23. doi: 10.1152/jn.00154.2010
43. Katz E, de Jong JMV, Buettner-Ennever J, Cohen B. Effects of midline medullary lesions on velocity storage and the vestibulo-ocular reflex. Exp Brain Res. (1991) 87:505–20. doi: 10.1007/BF00227076
44. Wearne S, Raphan T, Cohen B. Contribution of vestibular commissural pathways to spatial orientation of the angular vestibuloocular reflex. J Neurophysiol. (1997) 78:1193–7. doi: 10.1152/jn.1997.78.2.1193
45. Maruta J. On labyrinthine function loss, motion sickness immunity, and velocity storage. Front Neurol. (2024) 15:1426213. doi: 10.3389/fneur.2024.1426213
46. Cohen B, Suzuki JI, Raphan T. Role of the otolith organs in generation of horizontal nystagmus: effects of selective labyrinthine lesions. Brain Res. (1983) 276:159–64. doi: 10.1016/0006-8993(83)90558-9
47. Merfeld DM, Young LR, Tomko DL, Paige GD. Spatial orientation of VOR to combined vestibular stimuli in squirrel monkeys. Acta Otolaryngol Suppl. (1991) 481:287–92. doi: 10.3109/00016489109131403
48. Simpson JI, Graf W. Eye-muscle geometry and compensatory eye movements in lateral-eyed and frontal-eyed animals. Ann N Y Acad Sci. (1981) 374:20–30. doi: 10.1111/j.1749-6632.1981.tb30856.x
49. Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit's cerebellar flocculus. II Complex and simple spike responses of Purkinje cells. J Neurophysiol. (1988) 60:2091–121. doi: 10.1152/jn.1988.60.6.2091
50. Masino T, Knudsen EI. Horizontal and vertical components of head movement are controlled by distinct neural circuits in the barn owl. Nature. (1990) 345:434–7. doi: 10.1038/345434a0
51. Angelaki DE, Yakusheva TA, Green AM, Dickman JD, Blazquez PM. Computation of egomotion in the macaque cerebellar vermis. Cerebellum. (2010) 9:174–82. doi: 10.1007/s12311-009-0147-z
52. Branoner F, Straka H. Semicircular canal influences on the developmental tuning of the translational vestibulo-ocular reflex. Front Neurol. (2018) 9:404. doi: 10.3389/fneur.2018.00404
53. Dai MJ, Raphan T, Cohen B. Spatial orientation of the vestibular system: dependence of optokinetic after-nystagmus on gravity. J Neurophysiol. (1991) 66:1422–39. doi: 10.1152/jn.1991.66.4.1422
54. Raphan T, Dai M, Maruta J, Waespe W, Henn V, Suzuki J-I, et al. Canal and otolith afferent activity underlying eye velocity responses to pitching while rotating. Annals NY Acad Sci. (1999) 871:181–94. doi: 10.1111/j.1749-6632.1999.tb09184.x
55. Dai M, Raphan T, Kozlovskaya I, Cohen B. Vestibular adaptation to space in monkeys. Otolaryngol Head Neck Surg. (1998) 119:65–77. doi: 10.1016/S0194-5998(98)70175-5
56. Lackner JR. The importance of being in touch. Front Neurol. (2021) 12:646640. doi: 10.3389/fneur.2021.646640
57. Eron JN, Cohen B, Raphan T, Yakushin SB. Adaptation of orientation vectors of otolith-related central vestibular neurons to gravity. J Neurophysiol. (2008) 100:1686–90. doi: 10.1152/jn.90289.2008
58. Maruta J. Lasting alteration of spatial orientation induced by passive motion in rabbits and its possible relevance to mal de débarquement syndrome. Front Neurol. (2023) 14:1110298. doi: 10.3389/fneur.2023.1110298
59. Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. (1998) 79:2690–715. doi: 10.1152/jn.1998.79.5.2690
60. Angelaki DE, Hess BJ. Lesion of the nodulus and ventral uvula abolish steady-state off-vertical axis otolith response. J Neurophysiol. (1995) 73:1716–20. doi: 10.1152/jn.1995.73.4.1716
61. Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. (1995) 73:1729–51. doi: 10.1152/jn.1995.73.5.1729
62. Cohen B, John P, Yakushin SB, Buettner-Ennever J, Raphan T. The nodulus and uvula: source of cerebellar control of spatial orientation of the angular vestibulo-ocular reflex. Ann N Y Acad Sci. (2002) 978:28–45. doi: 10.1111/j.1749-6632.2002.tb07553.x
63. van der Steen J, Simpson JI, Tan J. Functional and anatomic organization of three-dimensional eye movements in rabbit cerebellar flocculus. J Neurophysiol. (1994) 72:31–46. doi: 10.1152/jn.1994.72.1.31
64. Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. (1992) 90:526–38. doi: 10.1007/BF00230935
65. Ito M, Nisimaru N, Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. (1977) 265:833–54. doi: 10.1113/jphysiol.1977.sp011747
66. Schenk SM, Wagner JM, Miller JA, Lyons-White TM, Venn EC, April MD, et al. Treatment of mal de debarquement syndrome in a deployed environment. Mil Med. (2018) 183:e775–8. doi: 10.1093/milmed/usy108
67. Hoppes CW, Vernon M, Morrell RL, Whitney SL. Treatment of mal de debarquement syndrome in a computer-assisted rehabilitation environment. Mil Med. (2022) 187:e1011–5. doi: 10.1093/milmed/usab077
68. Hojnacki M. Treatment of mal de debarquement syndrome in an audiology-vestibular clinic. J Am Acad Audiol. (2022) 33:364–70. doi: 10.1055/s-0042-1757769
69. Mucci V, Perkisas T, Jillings SD, Van Rompaey V, Van Ombergen A, Fransen E, et al. Sham-controlled study of optokinetic stimuli as treatment for mal de debarquement syndrome. Front Neurol. (2018) 9:887. doi: 10.3389/fneur.2018.00887
70. Schoenmaekers C, De Smet D, Deblieck C, Van Riel J, Zarowski A, Wuyts FL. Virtual reality application matches the most established treatment for mal de debarquement syndrome: a non-inferiority, randomized, open clinical trial. Neurotherapeutics. (2024) 21:e00390. doi: 10.1016/j.neurot.2024.e00390
71. Ampomah KK, Clark BC, Arnold WD, Burwell D. An uncommon cause of headache and dizziness after cruise travel: case report of mal de debarquement syndrome. J Osteopath Med. (2021) 121:471–4. doi: 10.1515/jom-2020-0224
72. Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the Bárány society. J Vestib Res. (2017) 27:191–208. doi: 10.3233/VES-170622
73. Barmack NH, Shojaku H. Vestibularly induced slow oscillations in climbing fiber responses of Purkinje cells in the cerebellar nodulus of the rabbit. Neuroscience. (1992) 50:1–5. doi: 10.1016/0306-4522(92)90376-D
74. Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. (1985) 96:2301–16. doi: 10.1152/japplphysiol.00008.2004
75. Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation: from basic concepts to clinical applications. J Neurophysiol. (2019) 121:2237–55. doi: 10.1152/jn.00035.2019
76. Reed-Jones RJ, Reed-Jones JG, Trick LM, Vallis LA. “Can galvanic vestibular stimulation reduce simulator adaptation syndrome?” In: Proceedings of the 4th International Driving Symposium on Human Factors in Driver Assessment, Training, and Vehicle. Stevenson, Washington, USA: University of Iowa (2007). p. 534–40. doi: 10.17077/drivingassessment.1288
77. Groth C, Tauscher J-P, Heesen N, Hattenbach M, Castillo S, Magnor M. Omnidirectional galvanic vestibular stimulation in virtual reality. IEEE Trans Vis Comput Graph. (2022) 28:2234–44. doi: 10.1109/TVCG.2022.3150506
78. Aoyama K, Iizuka H, Ando H, Maeda T. Four-pole galvanic vestibular stimulation causes body sway about three axes. Sci Rep. (2015) 5:10168. doi: 10.1038/srep10168
79. Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. (1984) 51:1236–56. doi: 10.1152/jn.1984.51.6.1236
80. Ezure K, Cohen MS, Wilson VJ. Response of cat semicircular canal afferents to sinusoidal polarizing currents: implications for input-output properties of second-order neurons. J Neurophysiol. (1983) 49:639–48. doi: 10.1152/jn.1983.49.3.639
81. Brontë-Stewart HM, Lisberger SG. Physiological properties of vestibular primary afferents that mediate motor learning and normal performance of the vestibulo-ocular reflex in monkeys. J Neurosci. (1994) 14:1290–308. doi: 10.1523/JNEUROSCI.14-03-01290.1994
82. Fitzpatrick RC, Butler JE, Day BL. Resolving head rotation for human bipedalism. Curr Biol. (2006) 16:1509–14. doi: 10.1016/j.cub.2006.05.063
83. Dakin CJ, Son GML, Inglis JT, Blouin J-S. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. (2007) 583:1117–27. doi: 10.1113/jphysiol.2007.133264
84. Dumas G, Curthoys IS, Lion A, Perrin P, Schmerber S. The skull vibration-induced nystagmus test of vestibular function-a review. Front Neurol. (2017) 8:41. doi: 10.3389/fneur.2017.00041
85. Dumas G, Quatre R, Schmerber S. How to do and why perform the skull vibration-induced nystagmus test. Eur Ann Otorhinolaryngol Head Neck Dis. (2021) 138:287–90. doi: 10.1016/j.anorl.2020.11.014
86. Curthoys IS. The neural basis of skull vibration induced nystagmus (SVIN). Audiol Res. (2021) 11:557–66. doi: 10.3390/audiolres11040050
87. Dlugaiczyk J, Burgess AM, Curthoys IS. Activation of guinea pig irregular semicircular canal afferents by 100 hz vibration: clinical implications for vibration-induced nystagmus and vestibular-evoked myogenic potentials. Otol Neurotol. (2020) 41:e961–70. doi: 10.1097/MAO.0000000000002791
88. Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp Brain Res. (2011) 210:347–52. doi: 10.1007/s00221-010-2499-5
89. Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. (2006) 175:256–67. doi: 10.1007/s00221-006-0544-1
90. Ohki M, Murofushi T, Nakahara H, Sugasawa K. Vibration-induced nystagmus in patients with vestibular disorders. Otolaryngol Head Neck Surg. (2003) 129:255–8. doi: 10.1016/S0194-5998(03)00529-1
91. Dumas G, Perrin P, Schmerber S. Nystagmus induced by high frequency vibrations of the skull in total unilateral peripheral vestibular lesions. Acta Otolaryngol. (2008) 128:255–62. doi: 10.1080/00016480701477677
92. Wade SW, Halmagyi GM, Black FO, McGarvie LA. Time constant of nystagmus slow-phase velocity to yaw-axis rotation as a function of the severity of unilateral caloric paresis. Am J Otol. (1999) 20:471–8.
93. Hain TC, Zee DS. Velocity storage in labyrinthine disorders. Ann N Y Acad Sci. (1992) 656:297–304. doi: 10.1111/j.1749-6632.1992.tb25216.x
94. Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. (1985) 228:199–202. doi: 10.1126/science.3871968
95. Shemesh AA, Kattah JC, Zee DS, Zuma E, Maia F, Otero-Millan J. Amplification of vibration induced nystagmus in patients with peripheral vestibular loss by head tilt. Front Neurol. (2024) 15:1420699. doi: 10.3389/fneur.2024.1420699
96. Curthoys IS, Zee DS, Dumas G, Pastras CJ, Dlugaiczyk J. Skull vibration induced nystagmus, velocity storage and self-stability. Front Neurol. (2025) 16:1533842. doi: 10.3389/fneur.2025.1533842
97. Ono K, Keller J, López Ramírez O, González Garrido A, Zobeiri OA, Chang HHV, et al. Retinoic acid degradation shapes zonal development of vestibular organs and sensitivity to transient linear accelerations. Nat Commun. (2020) 11:63. doi: 10.1038/s41467-019-13710-4
98. Dieringer N, Reichenberger I, Graf W. Differences in optokinetic and vestibular ocular reflex performance in teleosts and their relationship to different life styles. Brain Behav Evol. (1992) 39:289–304. doi: 10.1159/000114126
99. Manteuffel G, Kopp J, Himstedt W. Amphibian optokinetic after nystagmus: properties and comparative analysis in various species. Brain Behav Evol. (1986) 28:186–97. doi: 10.1159/000118702
100. Miki S, Urase K, Baker R, Hirata Y. Velocity storage mechanism drives a cerebellar clock for predictive eye velocity control. Sci Rep. (2020) 10:6944. doi: 10.1038/s41598-020-63641-0
101. Mukhopadhyay M, Pangrsic T. Synaptic transmission at the vestibular hair cells of amniotes. Mol Cell Neurosci. (2022) 121:103749. doi: 10.1016/j.mcn.2022.103749
102. Angelaki DE, Perachio AA, Mustari MJ, Strunk CL. Role of irregular otolith afferents in the steady-state nystagmus during off-vertical axis rotation. J Neurophysiol. (1992) 68:1895–900. doi: 10.1152/jn.1992.68.5.1895
103. Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. (1991) 11:1636–48. doi: 10.1523/JNEUROSCI.11-06-01636.1991
104. Highstein SM, Goldberg JM, Moschovakis AK, Fernández C. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. II Correlation with output pathways of secondary neurons. J Neurophysiol. (1987) 58:719–38. doi: 10.1152/jn.1987.58.4.719
105. Cha YH, Ding L, Yuan H. Neuroimaging markers of mal de débarquement syndrome. Front Neurol. (2021) 12:636224. doi: 10.3389/fneur.2021.636224
106. Murphy TP. Mal de debarquement syndrome: a forgotten entity? Otolaryngol Head Neck Surg. (1993) 109:10–3. doi: 10.1177/019459989310900103
107. Mucci V, Jacquemyn Y, Van Ombergen A, Van de Heyning PH, Browne CJ. A new theory on GABA and calcitonin gene-related peptide involvement in mal de debarquement syndrome predisposition factors and pathophysiology. Med Hypotheses. (2018) 120:128–34. doi: 10.1016/j.mehy.2018.08.024
Keywords: bone conducted vibration, galvanic vestibular stimulation (GVS), irregular vestibular afferents, motion adaptation, nystagmus, spatial orientation, velocity storage, vestibulo-ocular reflex (VOR)
Citation: Maruta J (2025) The utility of artificial vestibular stimulation in decoding the pathophysiology of mal de débarquement syndrome. Front. Neurol. 16:1560787. doi: 10.3389/fneur.2025.1560787
Received: 14 January 2025; Accepted: 07 March 2025;
Published: 24 March 2025.
Edited by:
Mathieu Beraneck, Université Paris Cité, FranceReviewed by:
Viviana Mucci, Western Sydney University, AustraliaCopyright © 2025 Maruta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Maruta, anVuLm1hcnV0YUBtc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.