- 1Department of Neurology, Korea University Medical Center, Seoul, Republic of Korea
- 2Department of Radiology, Korea University Medical Center, Seoul, Republic of Korea
- 3Neurotology and Neuro-ophthalmology Laboratory, Korea University Medical Center, Seoul, Republic of Korea
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Korea University Medical Center, Seoul, Republic of Korea
- 5Dizziness Center, Clinical Neuroscience Center, Seoul National University Bundang Hospital, Seongnam, Republic of Korea
- 6Department of Neurology, Seoul National University College of Medicine, Seoul, Republic of Korea
Objective: Acute unilateral peripheral vestibulopathy or vestibular neuritis (AUPV/VN) manifests as acute onset vertigo, often accompanied by nausea, vomiting, and moderate gait instability. It is suspected when vestibular hypofunction is documented on video-head impulse (video-HITs) and caloric tests in the presence of contralesionally beating horizontal-torsional nystagmus. Herein, we report patients presenting with acute vestibular syndrome (AVS) showing selective otolithic dysfunction in the presence of normal caloric and video-HITs and abnormal enhancement of the peripheral vestibular structures on MRI.
Methods: We retrospectively reviewed the medical records of patients presenting with AVS between September 2019 and April 2024 at a tertiary referral hospital in South Korea. All patients underwent extensive neurotologic evaluation, including cervical and ocular vestibular-evoked myogenic potentials (cVEMP and oVEMP, respectively), subjective visual vertical, video-oculography, video-HITs, caloric tests, and audiometry. Patients also underwent MRI according to a standard protocol for the inner ear and internal acoustic canal with an additional 3D-fluid attenuated inversion recovery sequence acquired 4 h after intravenous gadolinium injection.
Results: We identified four patients with selective otolith dysfunction. Video-HITs and caloric test results were normal in all patients, except one with a canal paresis on the opposite side of otolithic dysfunction. Patients usually showed abnormal oVEMP (n = 3) and cVEMP (n = 2) or subjective visual vertical (n = 3). Gadolinium enhancements were found in the vestibule (n = 3), inferior (n = 2) or superior (n = 1) vestibular nerves on dedicated inner ear MRI.
Discussion: Selective otolithic dysfunction can present with AVS, which can be easily overlooked. A thorough neurotologic evaluation and MRI dedicated to the inner ear can help detect selective otolithic dysfunction, expanding the clinical spectrum of AVS.
Introduction
The diagnosis of vestibular neuritis (VN) or acute unilateral peripheral vestibulopathy (AUPV) is based on clinical features and neurotologic findings in the absence of other causes (1). AUPV/VN manifests as acute onset vertigo, often accompanied by nausea, vomiting, and moderate gait instability (1, 2). In addition to these hallmark symptoms, video-head impulse (video-HITs) and caloric tests can identify peripheral vestibular hypofunction in the presence of contralesionally beating horizontal-torsional nystagmus obeying Alexander’s law (1).
Selective otolith dysfunction has been identified as a cause of vertigo in prior studies (3–5). Patients can present with benign paroxysmal positional vertigo or Ménière’s disease (MD), although 37% of patients could not be categorized into any of the established clinical entities (6, 7). Patients can also present with acute spontaneous vertigo (i.e., acute vestibular syndrome; AVS), while showing normal results on tests for angular vestibular-ocular reflex (VOR) (8, 9). In such cases, patients exhibit spontaneous nystagmus with horizontal-torsional components indistinguishable from AUPV/VN (9, 10).
The primary vestibular afferent or inner ear can be visualized using various imaging techniques (11–13). Recent application of the 4-h delayed imaging technique has aided in visualizing vestibular damage (13–18). In contrast to conventional MRI (12, 19), the 4-h delayed 3D imaging technique reportedly detected positive results on the labyrinth or nerve of approximately 50% of patients with AUPV/VN (13, 14, 18). Although confounded by other factors, 4-h delayed 3D fluid-attenuated inversion recovery (3D-FLAIR) images can reliably quantify vestibular damage in patients with AUPV/VN (13, 14, 20). Meanwhile, MRI results of isolated otolith dysfunction have not been reported for now.
Herein, we report the cases of four patients with selective otolith dysfunction presenting with acute spontaneous vertigo. Selective deficits were documented solely on cervical and ocular vestibular-evoked myogenic potentials (cVEMP and oVEMP, respectively) or subjective visual vertical (SVV), while showing normal results on caloric and video-HITs. Patients also showed positive results on the inner ear or primary vestibular afferents on 3D-FLAIR sequences. Our findings may provide more diagnostic and localization information on the causes of acute spontaneous vertigo that are often overlooked.
Materials and methods
Patients
We retrospectively analyzed the medical records of 77 patients who presented with first-ever spontaneous vertigo/dizziness and underwent inner ear MRI between September 2019 and November 2024 at Korea University Medical Center. Patients with a posterior circulatory stroke were excluded from the study. We further excluded 62 patients whose neurotologic findings were consistent with AUPV/VN (1), those whose MRI scans revealed endolymphatic hydrops on either side of the ear with gadolinium enhancement (n = 8), and those with miscellaneous neurotologic findings or negative MRI results (n = 3). Finally, we identified four patients with positive MRI findings who did not fully meet the established criteria for AUPV/VN (1).
All patients were followed up at the outpatient clinic every other month for 6 months since symptom onset. Each patient was queried regarding dizziness symptoms through phone calls every 3 months as part of a routine protocol of the AVS registry.
Neurotologic evaluation
In addition to a standard neurologic examination, all patients underwent bedside evaluation and video-oculographic recording of spontaneous (SN), gaze-evoked, and head-shaking nystagmus (HSN; SLVNG, SLMED, Seoul, South Korea) (21). All patients underwent bedside HITs and video-HITs. Detailed methods for video-HITs have been previously described (22).
Patients also underwent bithermal caloric and SVV (NDI-150, M2S, Seoul, South Korea) tests, as well as cVEMP and oVEMP tests, as previously described. Briefly, oVEMPs were elicited by tapping the hairline at the AFz using an electric reflex hammer (Tendon hammer, VIASYS Healthcare, Conshohocken, PA, USA). Bilateral responses were recorded simultaneously following the application of the tapping stimuli. Up to 60 tapping stimuli were applied at a frequency of 2 Hz and approximately 0.45 g of force. The responses were averaged for each test, and the average latencies of the initial negative peak (n1) and n1–p1 amplitudes were determined. oVEMP responses were obtained at least twice, from which the mean was calculated. The interaural difference (IAD, %) of the oVEMP amplitudes was calculated as follows: IAD = [100 × (ARight − ALeft)/(ARight + ALeft); A = n1–p1 amplitude].
cVEMPs were recorded with the patient lying supine on a bed with the head raised by approximately 30° and rotated to one side to contract the sternocleidomastoid muscle (SCM). A short burst of alternating tone (110 dB nHL, 123.5 dB SPL, 500 Hz, rise time = 2 ms, plateau = 3 ms, and fall time = 2 ms) was applied monoaurally at a frequency of 2.1 Hz via headphones. The signal was sampled (48 kHz), amplified, and bandpass-filtered at 30–1500 Hz. cVEMP responses were recorded without performing rectification or smoothing. cVEMP responses to up to 80 stimuli were averaged for each test. Responses were obtained at least twice for each ear, from which the mean values were calculated.
Absolute cVEMP amplitudes were normalized and divided by the mean tonic activation of the SCM during the recording. To compare the normalized p13–n23 amplitudes between the right and left sides, the IAD (%) was calculated. The p13 peak latency was also calculated. To determine the reference ranges, oVEMP and cVEMP responses of 16 healthy participants (nine men, mean age ± standard deviation = 65 ± 9 years) with no history of auditory or vestibular disorders (reference range for oVEMP: n1 latency <8.32 ms, IAD < 23.9%; reference range for cVEMP: p13 latency <19.4 ms, normalized p13–n23 amplitude >1.1 μV, IAD < 31.0%) were used (22).
MRI
MRI was performed using 3-T MRI scanners (Magnetum Skyra, Magnetum Prisma, and Magnetum Vida units, Siemens, Erlangen, Germany) with a receive-only 64-channel phased array coil, as previously described (14, 15). Patients underwent a standard MRI protocol for the internal acoustic canal (IAC) with an additional axial FLAIR sequence, acquired 4 h after intravenous injection of a standard dose of gadoterate meglumine (0.1 mmol/kg, 0.2 mL/kg; Dotarem®, Guerbet, Roissy, France) (15). Patients also underwent diffusion-weighted imaging spaced 48 h either before or after conducting IAC MRI to rule out central vestibulopathy.
Six freehand round or polygonal regions of interest (ROIs) were manually assigned to each neural structure, including the canalicular segment of the superior (4.60 mm2) and inferior vestibular nerves (4.60 mm2); the vestibule (20.40 mm2); and each semicircular canal for the horizontal (HC; 6.90–9.39 mm2), anterior (AC; 3.22–3.68 mm2), and posterior canals (PC; 6.90–9.39 mm2). The signal intensity of the medulla was measured in the same manner as that for normalization. The normalized signal intensity on the 4-h delayed 3D-FLAIR of the enhancing lesion was defined as the signal intensity of the enhanced portion divided by that of the medulla. The normalized intensities of each organ on the healthy side were used as controls. When the normalized signal intensity of the affected side exceeded the mean + 2 standard deviations of the signal intensity of each neural structure derived from the healthy side in patients with AUPV/VN (upper normal limit <1.49 and < 1.62 for the superior and inferior vestibular nerves, respectively; <0.69 for the vestibule; <0.40, <0.61, and < 0.63 for the HC, AC, and PC, respectively), this was defined as abnormal enhancement (14).
Results
Clinical characteristics
Table 1 presents the detailed clinical profiles of patients. Among 77 patients with AUPV with inner ear imaging, four patients (4/77, 5%) were included in the analyses (age range, 32–74 years, two male). No intravenous or oral corticosteroid treatment was administered to any patient. All patients presented with acute spontaneous dizziness/vertigo associated with nausea and vomiting. Postural instability when standing or walking was reported, with truncal ataxia grade 1 in two patients and grade 2 in the other two patients. Patients also described a true whirling sensation (n = 2), boarding a rocking boat (n = 2), and to-and-fro sensation (n = 1). Patients showed no focal neurological deficits at presentation or during the follow-up period of at least 6 months. None of the patients reported new-onset headache, tinnitus, ear fullness, or hearing loss during the 1-year follow-up. Following treatment, symptoms resolved within 1 week, with no residual dizziness or recurrence.
Neurotologic findings
Neurotologic findings are summarized in Table 1. All patients showed spontaneous nystagmus without visual fixation, including horizontal-torsional nystagmus with (n = 2) or without (n = 2) vertical components. The nystagmus was mainly horizontal. The slow-phase velocity of spontaneous nystagmus ranged from 1.3 to 4.1°/s. Spontaneous nystagmus was mostly suppressed or disappeared during visual fixation. None of the patients had gaze-evoked nystagmus during lateral gaze. The results of bedside HITs were negative in all patients. Horizontal head shaking elicited nystagmus in three patients, following the horizontal direction of spontaneous nystagmus. Otoscopic findings were normal in all patients.
None of the patients showed decreased VOR gain in any canal during video-HITs. One patient (Patient 4) showed canal paresis contralateral to the side of VEMP and MRI abnormalities (Figures 1, 2); otherwise, none of the other three patients exhibited canal paresis. Patient 4 was included in the analysis because canal paresis was toward the direction of nystagmus, which is not typical of peripheral vestibulopathy. oVEMP responses were abnormal in all patients, with three of them (Patients 1, 2, and 4) also showing decreased cVEMP responses on the affected side on MRI (Figure 2). The SVV was tilted in two patients, always on the ipsilesional side, as depicted on MRI. Pure tone and speech audiometry measurements were normal, except mild symmetric high-tone hearing impairment.
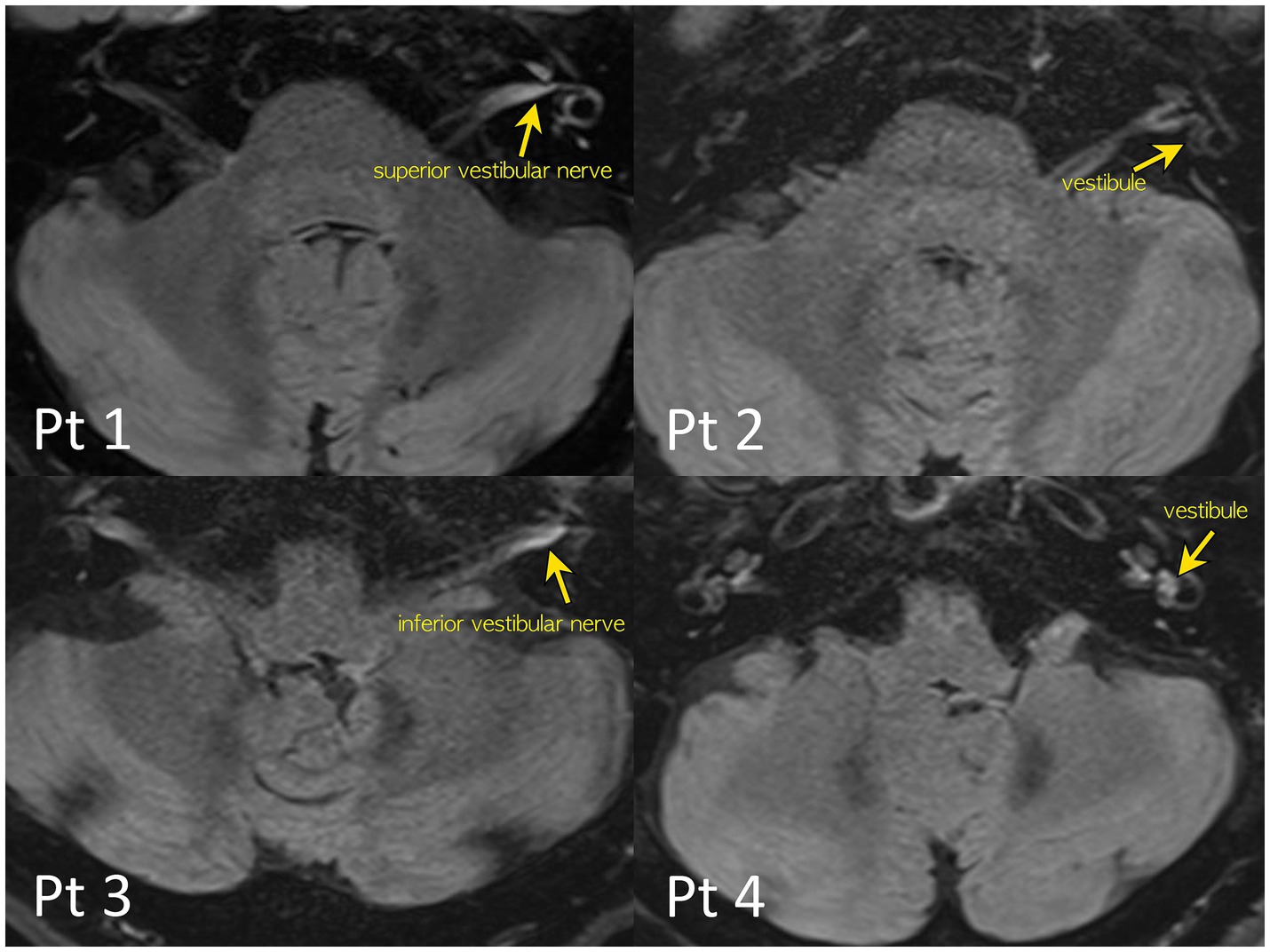
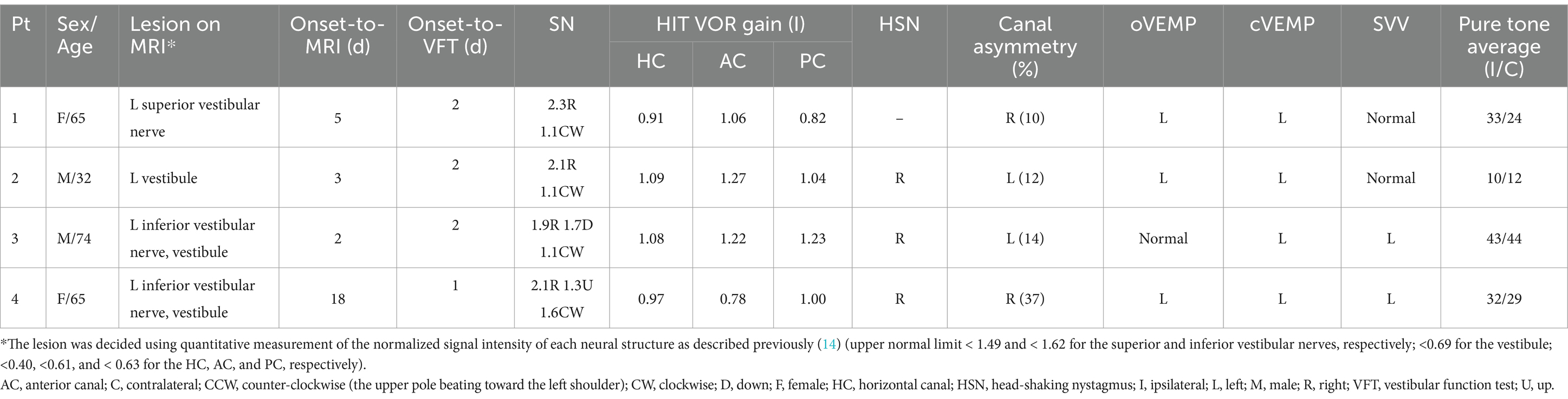
Figure 1. 4-h delayed 3D-FLAIR images of the patients. Quantitative evaluation of a degree of the perilymphatic enhancement. The signal intensity ratios of the vestibular nerves and inner ear structure to that of the signal intensity of the medulla were calculated to avoid bias from patient-related artifacts.
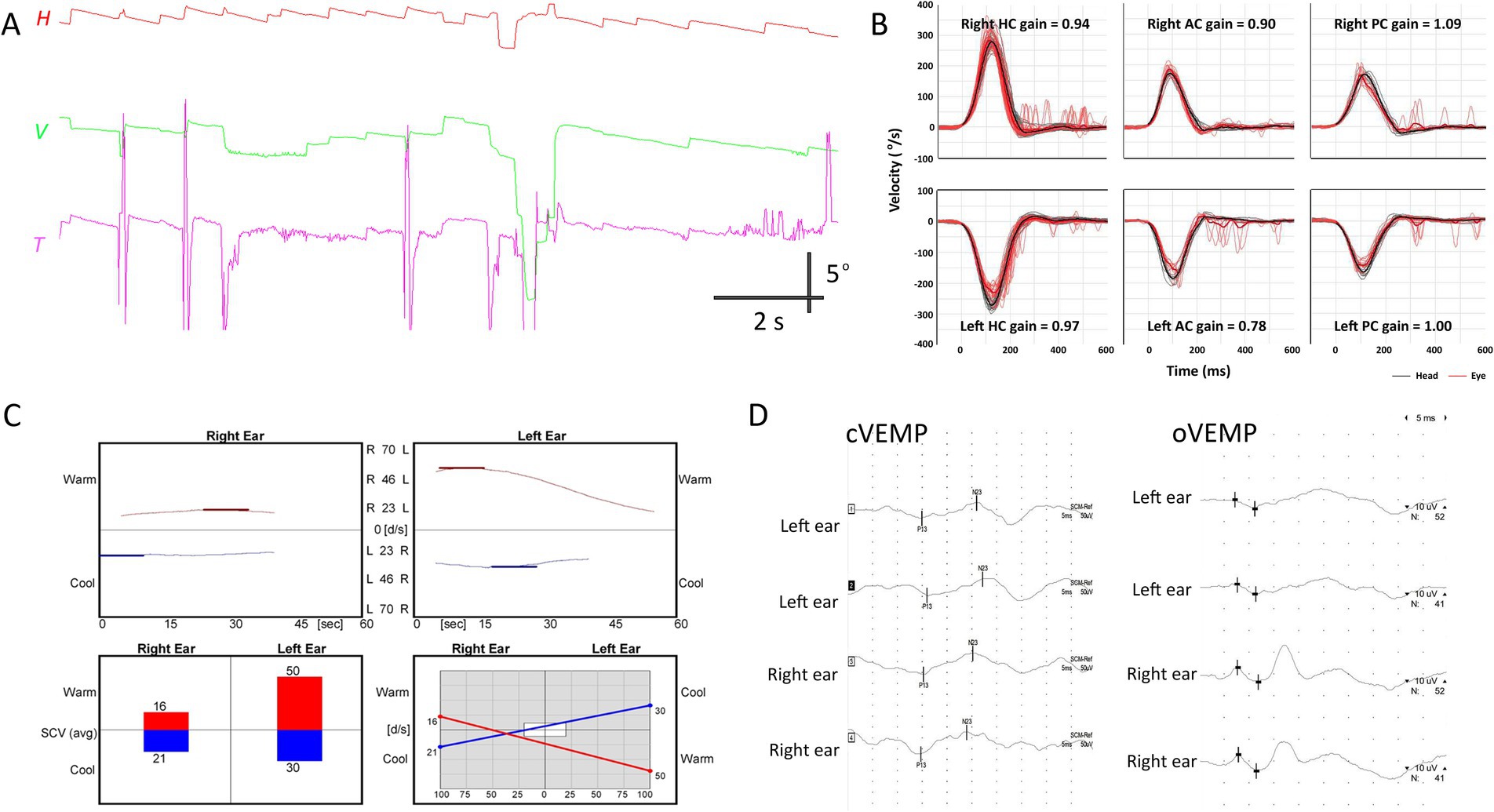
Figure 2. Neurotologic findings in patient 4. (A) Video-oculography shows spontaneous nystagmus beating rightward, upward with a clockwise component. (B) Video head-impulse tests are normal. (C) Bithermal caloric tests reveal canal paresis of 33% in the right ear. (D) cVEMP and oVEMP show relatively decreased response during left ear stimulation, with 25.7 and 33.3% interaural differences, respectively. AC, anterior canal; cVEMP, cervical vestibular-evoked myogenic potential; H, horizontal position of the right eye; HC, horizontal canal; oVEMP, ocular VEMP; PC, posterior canal; T, torsional position of the right eye; V, vertical position of the right eye.
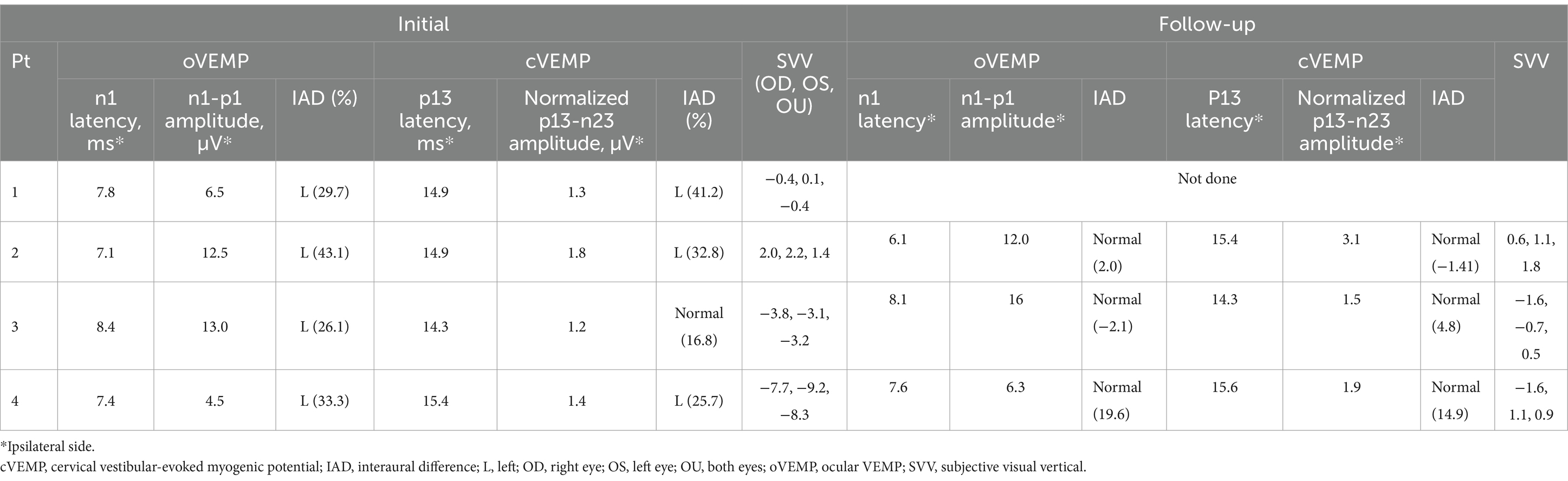
Three patients (Patients 2–4) underwent follow-up evaluation 2 months later, showing no changes in video-HITs. Following treatment, spontaneous nystagmus disappeared in all patients. Canal paresis in Patient 4 was resolved, and all patients showed normal caloric test results on follow-up examination. cVEMP, oVEMP, and SVV results were normal (Figure 3 and Table 2).
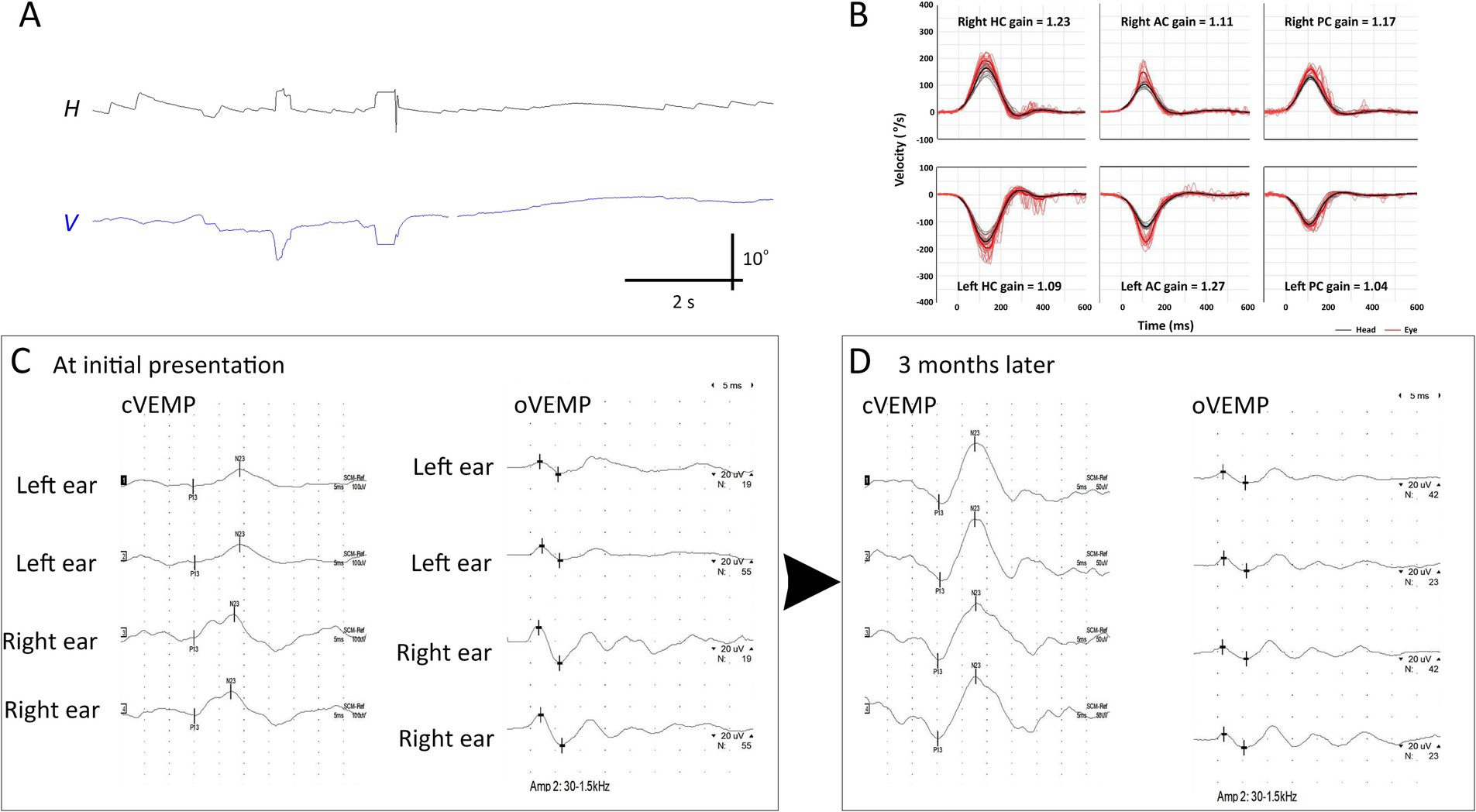
Figure 3. Initial and follow-up neurotologic evaluation of patient 2. (A) Initially, video-oculography showed spontaneous nystagmus beating right and clockwise (the torsional graph is omitted since artifacts). (B) Video head-impulse tests are normal. (C) Initially, oVEMP and cVEMP responses are decreased during left ear stimulation. (D) These decreased cVEMP and oVEMP responses become normal 3 months later. AC, anterior canal; cVEMP, cervical vestibular-evoked myogenic potential; H, horizontal position of the left eye; HC, horizontal canal; oVEMP, ocular VEMP; PC, posterior canal; V, vertical position of the left eye.
MRI findings
Notably, 4-h delayed 3D FLAIR MRI revealed gadolinium enhancement in the vestibule (n = 3), followed by the inferior (n = 2) or superior (n = 1) vestibular nerves (Figure 1).
Discussion
The main findings of our study can be summarized as follows: (1) Selective otolith dysfunction abnormality can be found in 5% of patients presenting with AVS. (2) Patients showed impaired cVEMP, oVEMP, and SVV responses while showing mostly insignificant caloric and video-HIT findings, thereby not fulfilling the diagnostic criteria for AUPV/VN. (3) MRI dedicated to the inner ear can aid in detecting selective otolith dysfunction by showing gadolinium enhancement in the vestibule and vestibular nerves.
Possible etiology for acute vestibular impairment in study patients
Apart from inflammation, gadolinium enhancement may be attributed to other etiologies that damage the primary vestibular afferent or labyrinth, including MD, labyrinthitis, and vestibular schwannoma (20, 23–25). However, our extensive neurotologic evaluations excluded the possibilities of other vestibular disorders in our patients.
The acute symptom onset and prominent spontaneous nystagmus, which resolved thereafter, clearly indicated that our patients experienced acute and symptomatic vestibular impairment. The presence of perilymphatic gadolinium enhancement also supports the presence of acute vestibular damage, causing a breakdown of the blood–nerve or blood–labyrinthine barriers (26). Clinical characteristics and prognosis mostly resembled AUPV/VN, suggesting a possible inflammatory or microvascular etiology in the vestibular organ (27, 28).
One plausible explanation for the negative caloric and video-HIT results is that vestibular damage was too subtle to be detected using neurotologic tests. However, this cannot explain the robust gadolinium enhancement observed on MRI, given that MRI positivity correlates with the degree of vestibular deficits (13, 14). As VOR may change over time owing to peripheral recovery or central adaptation (29), video-HITs and caloric tests may have failed to detect these vestibular deficits. However, both bithermal caloric and video-HIT findings remained normal during the follow-up evaluation. Given that canal paresis lasts for 1 year in most patients with AUPV/VN (30–32), its resolution in Patient 4 at the 2-month follow-up implied that the angular VOR was not affected in the first place. Hence, how can these findings be explained?
Selective loss of otolith function as a culprit of acute spontaneous vertigo
Owing to advances in neurotologic tests, the function of each semicircular canal and otolithic organ (utricle and saccule) can be thoroughly assessed. In this context, an inflammatory etiology can selectively damage the otolithic organs while sparing the semicircular canals (8). Selective otolithic dysfunction can be encountered in the clinical setting, presenting with acute vertigo/dizziness (9, 33–35). The clinical presentation can be acute spontaneous (monophasic) or recurrent spontaneous vertigo (i.e., polyphasic) (6, 36). In the latter case, it is usually regarded as a limited form of MD (36–38). These patients frequently exhibit selective cVEMP abnormalities, explained by endolymphatic hydrops preferentially involving the saccule and apical turn of the cochlea in the earlier stages of MD (36, 39).
The clinical characteristics are indistinguishable from those of typical AUPV/VN affecting the angular VOR system (8). Accordingly, the patient presents with horizontal nystagmus, which obeys Alexander’s law. However, the function of the semicircular canal is preserved, and no discernible results are observed on conventional MRI. In such cases, the only abnormality might be decreased n1–p1 amplitude on the opposite side of the direction of nystagmus, suggesting a selective utricular dysfunction origin (9). Selective otolith dysfunction accounts for approximately 2.7% of patients presenting with AVS (35), a rate similar to the 5% observed in our cohort. This condition is often overlooked unless a thorough neurotologic evaluation is accomplished. The otolithic involvement is usually unilateral but also can occur bilaterally (40).
Otolith dysfunction and spontaneous nystagmus
Can selective otolith dysfunction induce spontaneous nystagmus? Earlier animal studies have shown discrepant results. Electrical stimulation of the utricular nerve results in tonic deviation of the eyes but may not generate spontaneous nystagmus in rabbits (41). In contrast, horizontal nystagmus can be evoked following severance of the utricular nerve in cats (42). Alternatively, vigorous nystagmus can be elicited when the utricular macula is mechanically stimulated (41). The nystagmus usually beats toward the intact side, consistent with our findings (41). Other than horizontal nystagmus, otolith dysfunction may result in various patterns of nystagmus, given that vertical ocular drift can also be generated depending on the stimulus intensity or level of anesthesia in cats (43, 44). Horizontal nystagmus beating to the intact ear is frequently reported in clinical studies, while downbeat nystagmus has been rarely observed (40). These spontaneous nystagmus can be explained by the disrupted balance of neural activity between the vestibular nuclei on both sides (45). This is also theoretically plausible, as compensatory eye movement can be elicited in the yaw, pitch, and roll plane, depending on the gravito-inertial acceleration estimated in part by the utricle.
Possible etiology causing selective otolith dysfunction
Otolithic dysfunction can result from inflammation that selectively affects the otolithic organs, similar to the mechanism observed in AUPV/VN, which is explained by the reactivation of type 1 herpes simplex virus (27). Alternatively, transient ischemia, as a vascular etiology, may also be considered. Ischemic damage can occur at the microvascular level, resulting from occlusion of the end arterioles and hypoperfusion in the vestibular organ due to the formation of platelet–monocyte aggregates. A bioinformatic analysis has shown neutrophil activation in the sera of patients with AUPV/VN, which can damage endothelial cells and induce thrombosis (46). While MRI dedicated to the inner ear can help localize the lesion, it cannot definitively determine the etiology, as both vascular and inflammatory etiologies may present similarly (47).
Additionally, vascular compromise in the inner ear can arise from macrovascular occlusion. The labyrinth is susceptible to ischemia due to its high metabolic demands, and the internal auditory artery is an end artery with minimal collateral circulation from the otic capsule (48). The superior part of the vestibular labyrinth is particularly vulnerable to ischemia, probably due to the small caliber of the anterior vestibular artery and lack of collateral supply (49). Notably, VEMP impairment can be the sole heralding sign of labyrinthine ischemia preceding a full-blown anterior inferior cerebellar artery stroke (50). In this context, Patient 4 showed spontaneous nystagmus beating toward the direction of the canal paresis, a finding associated with central vestibulopathy (21).
MRI as an ancillary test for detecting vestibular damage
In addition to VOR gain measurement or documentation of canal paresis, alternative methods have been adopted for detecting vestibular damage. Anecdotal reports of corrective saccadic analyses have suggested a compatible or even higher chance of differentiating AUPV/VN from its mimickers (21, 51, 52). Calculating the gain asymmetry between the sides can also aid in differentiating posterior circulation stroke from AUPV/VN (53, 54). However, video-HITs alone cannot inherently detect all peripheral vestibulopathies, and caloric tests can complement in this context (55, 56). Our findings suggest that, combined with neurotologic tests, inner-ear imaging allows for the visual assessment of the damage in the primary vestibular afferents and labyrinth. MRI can supplement neurotologic evaluation by visually replicating the abnormality, although not perfectly, thereby potentiating the expansion of the clinical spectrum of AUPV/VN.
Nevertheless, discrepancies were observed between the imaging and functional studies in our patients. This inconsistency poses challenges in correlating imaging with clinical findings and suggests the need for further validation of the imaging protocol. Thus, our results should be interpreted as preliminary, requiring further validation in larger cohorts.
Interpretation of VEMP
Various stimuli can elicit VEMP responses, including short, intense auditory stimuli (e.g., tone bursts or clicks), bone-conduced vibration, forehead taps, or galvanic stimulation. We adopted forehead tapping and tone-burst sounds to elicit oVEMP and cVEMP, respectively. The advantage of applying forehead tapping or vibration is that these methods are less likely to be influenced by aging. As sound stimulation can often fail to evoke oVEMP responses bilaterally, it can show false positive responses in older patients (57). When oVEMP responses are recorded simultaneously in both eyes while tapping the forehead, IAD is estimated with reasonable test–retest reliability and inter-rater variation (57, 58). However, n1–p1 amplitude in both sides can vary depending on the exposure of inferior oblique, non-central stimulus location, or asymmetric convergence (58). This may explain the decreased n1–p1 amplitude in the contralesional side in Patient 4 on the follow-up test. Alternatively, it can be ascribed to vestibular compensation balancing the neural activity on both sides (59). Meanwhile, forehead tapping is not optimal for cVEMP since tapping the forehead midline can be technically difficult while the patient is rotating and flexing the neck.
MRI issues that should not be neglected
MRI may aid in the detection of selective otolith dysfunction. However, as diagnosing AUPV/VN requires the assessment of conspicuous neurotologic signs, MRI alone cannot be used for detecting AUPV/VN. In our study, MRI was performed because the patients had spontaneous nystagmus associated with normal video-HITs, possibly indicating central vestibulopathy. We propose that inner-ear MRI may offer valuable insights into the etiology of an AVS of miscellaneous origin and its underlying mechanism when readily stratified. However, our results should be interpreted with caution. In general, MRI is not mandatory if neurotologic findings are conspicuous for AUPV/VN (1).
Dissociation of neurotologic and MRI findings
Despite the abnormal oVEMP, cVEMP, and SVV findings, gadolinium enhancement was not confined to the vestibule but was also detected in the inferior or superior vestibular nerves of our patients. Notably, in Patient 4, the direction of canal paresis was opposite to that of the lesion documented on MRI. The oVEMP, cVEMP, and SVV findings did not correspond with the side of canal paresis in that patient, which cannot be fully explained by false lateralization of the caloric test resulting from overexcitation of the vestibular afferent or labyrinth (60). Due to the small sample size, whether this electrophysiologic-imaging dissociation is common remains unclear.
Clinical implication and caveats for future studies
By conducting thorough neurotologic evaluations and utilizing supporting imaging findings, our study confirms the presence of selective otolith dysfunction as a possible cause of acute vertigo. However, this study had some limitations. Most importantly, the sample size was too small to observe significant trends. As mentioned above, we did not observe any correlation between the imaging findings and the oVEMP, cVEMP, or SVV parameters. It remains unclear whether any effect is present as a group of acts in a larger group of patients. Additionally, VEMP findings can vary depending on the clinical setting and cut-off values. The sensitivity and specificity of VEMP testing could be critical when interpreting the results. VEMP results can be influenced by factors such as individual muscle tone and the testing environment, potentially leading to variability and inconsistencies in outcomes (61). The small sample size and retrospective design also limit the generalization of the findings. Further evaluations are warranted to provide convincing evidence of an otolith dysfunction origin. For instance, conducting the head heave test or observing nystagmus changes during back-and-forth linear movements in prone or supine positions could offer additional insights.
In conclusion, selective otolith dysfunction can give rise to AVS. Extensive neurotologic evaluation and imaging can help broaden the clinical spectrum of AUPV/VN. These findings may help inform the development of new protocols for patients with selective otolith dysfunction.
Data availability statement
Anonymized data will be made available upon reasonable request from any qualified investigator.
Ethics statement
This study was performed in accordance with the guidelines of the Institutional Review Board of Korea University Anam Hospital (2023AN0297) and followed the tenets of the Declaration of Helsinki. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
K-TK: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SP: Formal analysis, Writing – review & editing. S-UL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EP: Supervision, Writing – review & editing. BK: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. J-SK: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Basic Research Program through the National Research Foundation of Korea (NRF), funded by the MSIT (2022R1A4A1018869 and 2017R1C1B5017922).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Strupp, M, Bisdorff, A, Furman, J, Hornibrook, J, Jahn, K, Maire, R, et al. Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J Vestib Res. (2022) 32:389–406. doi: 10.3233/VES-220201
3. Brandt, T. Otolithic vertigo. Adv Otorhinolaryngol. (2001) 58:34–47. Available at: https://books.google.co.kr/books?hl=en&lr=&id=UjjoxDKygXAC&oi=fnd&pg=PA34&ots=L9Ri4yA1Ye&sig=g_i4QRAXdiNVtsPMamRIZF_vygA&redir_esc=y#v=onepage&q&f=false
4. Murofushi, T, Suh, M-W, and Manzari, L. Isolated otolith dysfunction and vertigo. Front Neurol. (2022) 13:1030513. doi: 10.3389/fneur.2022.1030513
5. Murofushi, T, Komiyama, S, Suizu, R, and Yoshimura, E. Otolithic vertigo in children: report of 3 cases. Acta Oto Laryngol Case Rep. (2016) 1:71–4. doi: 10.1080/23772484.2016.1235466
6. Fujimoto, C, Suzuki, S, Kinoshita, M, Egami, N, Sugasawa, K, and Iwasaki, S. Clinical features of otolith organ-specific vestibular dysfunction. Clin Neurophysiol. (2018) 129:238–45. doi: 10.1016/j.clinph.2017.11.006
7. De Chua, KW, Yuen, HW, Low, DYM, and Kamath, SH. The prevalence of isolated otolith dysfunction in a local tertiary hospital. J Otol. (2022) 17:5–12. doi: 10.1016/j.joto.2021.06.003
8. Curthoys, IS, Burgess, AM, and Manzari, L. The evidence for selective loss of otolithic function. Semin Neurol. (2020) 40:033–9. doi: 10.1055/s-0039-3402064
9. Manzari, L, Burgess, A, and Curthoys, I. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur Arch Otorrinolaringol. (2012) 269:2441–5. doi: 10.1007/s00405-012-2127-z
10. Barber, H, and Morrison, M. Clinical manifestations of otolith dysfunction. Adv Otorhinolaryngol. (1973) 20:396–404.
11. Park, KM, Shin, KJ, Ha, SY, Park, JS, and Kim, SE. A case of acute vestibular neuritis visualized by three-dimensional FLAIR-VISTA magnetic resonance imaging. Neuro Ophthalmology. (2014) 38:60–1. doi: 10.3109/01658107.2013.874454
12. Karlberg, M, Annertz, M, and Magnusson, M. Acute vestibular neuritis visualized by 3-T magnetic resonance imaging with high-dose gadolinium. Arch Otolaryngol Head Neck Surg. (2004) 130:229–32. doi: 10.1001/archotol.130.2.229
13. Byun, H, Chung, JH, Lee, SH, Park, CW, Park, DW, and Kim, TY. Clinical value of 4-hour delayed gadolinium-enhanced 3D FLAIR MR images in acute vestibular neuritis. Laryngoscope. (2018) 128:1946–51. doi: 10.1002/lary.27084
14. Kim, KT, Park, S, Lee, SU, Park, E, Kim, B, Kim, BJ, et al. Four-hour-delayed 3D-FLAIR MRIs in patients with acute unilateral peripheral vestibulopathy. Ann Clin Trans Neurol. (2024) 11:2030–9. doi: 10.1002/acn3.52123
15. Kim, K-T, Park, E, Lee, S-U, Kim, B, Kim, B-J, and Kim, J-S. Clinical features and neurotological findings in patients with acute unilateral peripheral vestibulopathy associated with antiganglioside antibody. Neurology. (2023) 101:e1913–21. doi: 10.1212/WNL.0000000000207814
16. Boegle, R, Gerb, J, Kierig, E, Becker-Bense, S, Dieterich, M, and Kirsch, V. Intravenous delayed gadolinium-enhanced MR imaging of the endolymphatic space: a methodological comparative study. Front Neurol. (2021) 12:647296. doi: 10.3389/fneur.2021.647296
17. Venkatasamy, A, Huynh, TT, Wohlhuter, N, Vuong, H, Rohmer, D, Charpiot, A, et al. Superior vestibular neuritis: improved detection using FLAIR sequence with delayed enhancement (1 h). Eur Arch Otorrinolaringol. (2019) 276:3309–16. doi: 10.1007/s00405-019-05639-7
18. Eliezer, M, Maquet, C, Horion, J, Gillibert, A, Toupet, M, Bolognini, B, et al. Detection of intralabyrinthine abnormalities using post-contrast delayed 3D-FLAIR MRI sequences in patients with acute vestibular syndrome. Eur Radiol. (2019) 29:2760–9. doi: 10.1007/s00330-018-5825-0
19. Strupp, M, Jäger, L, Müller-Lisse, U, Arbusow, V, Reiser, M, and Brandt, T. High resolution Gd-DTPA MR imaging of the inner ear in 60 patients with idiopathic vestibular neuritis: no evidence for contrast enhancement of the labyrinth or vestibular nerve. J Vestib Res. (1998) 8:427–33. doi: 10.3233/VES-1998-8603
20. Song, CI, Pogson, JM, Andresen, NS, and Ward, BK. Mri with gadolinium as a measure of blood-labyrinth barrier integrity in patients with inner ear symptoms: a scoping review. Front Neurol. (2021) 12:662264. doi: 10.3389/fneur.2021.662264
21. Kim, S-H, Lee, S-U, Cho, B-H, Cho, K-H, Yu, S, Kim, B-J, et al. Analyses of head-impulse tests in patients with posterior circulation stroke and vestibular neuritis. Neurology. (2023) 100:e2374–85. doi: 10.1212/WNL.0000000000207299
22. Kim, KT, Baik, K, Lee, SU, Park, E, Lee, CN, Woo, D, et al. Ocular vestibular-evoked myogenic potential assists in differentiation of multiple system atrophy from Parkinson's disease. J Mov Disord. (2024) 17:398–407. doi: 10.14802/jmd.24120
23. Bowen, AJ, Carlson, ML, and Lane, JI. Inner ear enhancement with delayed 3D-FLAIR MRI imaging in vestibular schwannoma. Otol Neurotol. (2020) 41:1274–9. doi: 10.1097/MAO.0000000000002768
24. Lee, IH, Kim, H-J, Chung, WH, Kim, E, Moon, JW, Kim, ST, et al. Signal intensity change of the labyrinth in patients with surgically confirmed or radiologically diagnosed vestibular schwannoma on isotropic 3D fluid-attenuated inversion recovery MR imaging at 3 T. Euro Radiol. (2010) 20:949–57. doi: 10.1007/s00330-009-1626-9
25. Bernaerts, A, Vanspauwen, R, Blaivie, C, van Dinther, J, Zarowski, A, Wuyts, FL, et al. The value of four stage vestibular hydrops grading and asymmetric perilymphatic enhancement in the diagnosis of Menière’s disease on MRI. Neuroradiology. (2019) 61:421–9. doi: 10.1007/s00234-019-02155-7
26. Naganawa, S, and Nakashima, T. Cutting edge of inner ear MRI. Acta Otolaryngol. (2009) 129:15–21. doi: 10.1080/00016480902729819
27. Arbusow, V, Schulz, P, Strupp, M, Dieterich, M, Von Reinhardstoettner, A, Rauch, E, et al. Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol. (1999) 46:416–9. doi: 10.1002/1531-8249(199909)46:3<416::AID-ANA20>3.0.CO;2-W
28. Kassner, SS, Schöttler, S, Bonaterra, GA, Stern-Straeter, J, Hormann, K, Kinscherf, R, et al. Proinflammatory activation of peripheral blood mononuclear cells in patients with vestibular neuritis. Audiol Neurootol. (2011) 16:242–7. doi: 10.1159/000320839
29. Mantokoudis, G, Schubert, MC, Tehrani, ASS, Wong, AL, and Agrawal, Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol Neurotol. (2014) 35:148–54. doi: 10.1097/MAO.0b013e3182956196
30. Choi, KD, Oh, SY, Kim, HJ, Koo, JW, Cho, BM, and Kim, JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. (2007) 117:1307–12. doi: 10.1097/MLG.0b013e31805c08ac
31. Zellhuber, S, Mahringer, A, and Rambold, HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorrinolaringol. (2014) 271:2375–83. doi: 10.1007/s00405-013-2723-6
32. Halmagyi, G, Weber, K, and Curthoys, I. Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci. (2010) 28:37–46. doi: 10.3233/RNN-2010-0533
33. Park, HG, Lee, JH, Oh, SH, Park, MK, and Suh, M-W. Proposal on the diagnostic criteria of definite isolated otolith dysfunction. J Audiol Otol. (2019) 23:103–11. doi: 10.7874/jao.2018.00374
34. Curthoys, IS, and Manzari, L. Otolithic disease: clinical features and the role of vestibular evoked myogenic potentials. Semin Neurol. (2013) 33:231–7. doi: 10.1055/s-0033-1354595
35. Manzari, L, Koch, G, and Tramontano, M. Selective asymmetry of ocular vestibular-evoked myogenic potential in patients with acute utricular macula loss. J Int Adv Otol. (2021) 17:58–63. doi: 10.5152/iao.2020.18012020
36. Murofushi, T, Komiyama, S, Hayashi, Y, and Yoshimura, E. Frequency preference in cervical vestibular evoked myogenic potential of idiopathic otolithic vertigo patients. Does it reflect otolithic endolymphatic hydrops? Acta Otolaryngol. (2015) 135:995–9. doi: 10.3109/00016489.2015.1022834
37. Lee, SU, Kim, HJ, Choi, JY, Koo, JW, and Kim, JS. Abnormal cervical vestibular-evoked myogenic potentials predict evolution of isolated recurrent vertigo into Meniere’s disease. Front Neurol. (2017) 8:463. doi: 10.3389/fneur.2017.00463
38. Murofushi, T, Komiyama, S, Hayashi, Y, and Yoshimura, E. Is otolithic vertigo accompanied by hearing loss caused by sacculocochlear endolymphatic hydrops? Acta Otolaryngol. (2016) 136:38–42. doi: 10.3109/00016489.2015.1081277
39. Pender, D. Endolymphatic hydrops and Ménière's disease: a lesion meta-analysis. J Laryngol Otol. (2014) 128:859–65. doi: 10.1017/S0022215114001972
40. Manzari, L, MacDougall, HG, Burgess, AM, and Curthoys, IS. Selective otolith dysfunctions objectively verified. J Vestib Res. (2014) 24:365–73. doi: 10.3233/VES-140537
41. Janeke, J, Jongkees, L, and Qosterveld, W. Relationship between otoliths and nystagmus. Acta Otolaryngol. (1970) 69:1–6. doi: 10.3109/00016487009123330
42. Fluur, E, and Siegborn, J. The otolith organs and the nystagmus problem. Acta Otolaryngol. (1973) 76:438–42. doi: 10.3109/00016487309121533
43. Goto, F, Meng, H, Bai, R, Sato, H, Imagawa, M, Sasaki, M, et al. Eye movements evoked by selective saccular nerve stimulation in cats. Auris Nasus Larynx. (2004) 31:220–5. doi: 10.1016/j.anl.2004.03.002
44. Goto, F, Meng, H, Bai, R, Sato, H, Imagawa, M, Sasaki, M, et al. Eye movements evoked by the selective stimulation of the utricular nerve in cats. Auris Nasus Larynx. (2003) 30:341–8. doi: 10.1016/j.anl.2003.07.003
45. Curthoys, IS, and Halmagyi, GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. (1995) 5:67–107. doi: 10.3233/VES-1995-5201
46. Oh, EH, Rhee, JK, Shin, JH, Cho, JW, Kim, DS, Park, JY, et al. Neutrophil-mediated immune response as a possible mechanism of acute unilateral vestibulopathy. J Vestib Res. (2020) 30:363–74. doi: 10.3233/VES-200044
47. Eliezer, M, Verillaud, B, Guichard, J-P, Kania, R, Toupet, M, Herman, P, et al. Labyrinthine infarction caused by vertebral artery dissection: consideration based on MRI. J Neurol. (2019) 266:2575–7. doi: 10.1007/s00415-019-09456-0
48. Perlman, H, Kimura, R, and Fernandez, C. Experiments on temporary obstruction of the internal auditory artery. Laryngoscope. (1959) 69:591–613. doi: 10.1288/00005537-195906000-00001
49. Kim, J, Lopez, I, DiPatre, P, Liu, F, Ishiyama, A, and Baloh, R. Internal auditory artery infarction Clinicopathologic correlation. Neurology. (1999) 52:40–4. doi: 10.1212/WNL.52.1.40
50. Lee, S-U, Kim, H-J, Choi, J-Y, and Kim, J-S. Abnormal vestibular-evoked myogenic potentials as an isolated finding of probable transient labyrinthine ischemia. J Neurol. (2017) 264:1523–5. doi: 10.1007/s00415-017-8511-2
51. Chen, L, Todd, M, Halmagyi, GM, and Aw, S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. (2014) 83:1513–22. doi: 10.1212/WNL.0000000000000906
52. Mantokoudis, G, Tehrani, ASS, Wozniak, A, Eibenberger, K, Kattah, JC, Guede, CI, et al. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol Neurotol. (2015) 36:457–65. doi: 10.1097/MAO.0000000000000638
53. Celebisoy, N. Acute vestibular syndrome: clinical head impulse test versus video head impulse test. J Neurol. (2018) 265:44–7. doi: 10.1007/s00415-018-8804-0
54. Guler, A, Karbek Akarca, F, Eraslan, C, Tarhan, C, Bilgen, C, Kirazli, T, et al. Clinical and video head impulse test in the diagnosis of posterior circulation stroke presenting as acute vestibular syndrome in the emergency department. J Vestib Res. (2017) 27:233–42. doi: 10.3233/VES-170620
55. Perez, N, and Rama-Lopez, J. Head-impulse and caloric tests in patients with dizziness. Otol Neurotol. (2003) 24:913–7. doi: 10.1097/00129492-200311000-00016
56. Lee, JY, Kwon, E, Kim, H-J, Choi, J-Y, Oh, HJ, Koo, W, et al. Dissociated results between caloric and video head impulse tests in dizziness: prevalence, pattern, lesion location, and etiology. J Clin Neurol. (2020) 16:277–84. doi: 10.3988/jcn.2020.16.2.277
57. Nguyen, KD, Welgampola, MS, and Carey, JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. (2010) 31:793–802. doi: 10.1097/MAO.0b013e3181e3d60e
58. Iwasaki, S, Smulders, Y, Burgess, A, McGarvie, L, Macdougall, H, Halmagyi, G, et al. Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at FzA new indicator of unilateral otolithic loss. Audiol Neurootol. (2008) 13:396–404. doi: 10.1159/000148203
59. Curthoys, IS. Vestibular compensation and substitution. Curr Opin Neurol. (2000) 13:27–30. doi: 10.1097/00019052-200002000-00006
60. Lee, S-U, Kim, H-J, Choi, J-Y, Koo, J-W, and Kim, J-S. Evolution of caloric responses during and between the attacks of Meniere’s disease. J Neurol. (2021) 268:2913–21. doi: 10.1007/s00415-021-10470-4
Keywords: vestibular neuritis, otolith, dizziness, vertigo, magnetic resonance imaging
Citation: Kim K-T, Park S, Lee S-U, Park E, Kim B and Kim J-S (2024) Selective otolithic dysfunction in patients presenting with acute spontaneous vertigo: consideration based on MRI. Front. Neurol. 15:1517112. doi: 10.3389/fneur.2024.1517112
Edited by:
Andrea Castellucci, Azienda USL – IRCCS di Reggio Emilia, ItalyReviewed by:
Leonardo Manzari, MSA ENT Academy Center, ItalyJae Ho Chung, Hanyang University, Republic of Korea
Copyright © 2024 Kim, Park, Lee, Park, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sun-Uk Lee, c3VsZWU3MTZAZ21haWwuY29t
 Keun-Tae Kim
Keun-Tae Kim Sangeun Park2
Sangeun Park2 Sun-Uk Lee
Sun-Uk Lee Euyhyun Park
Euyhyun Park Ji-Soo Kim
Ji-Soo Kim