
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 24 October 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1487808
This article is part of the Research Topic Cerebral cavernous malformations View all 3 articles
Purpose of review: Cavernous malformations (CM) are vascular lesions in the brain and spinal cord, characterized by clusters of endothelial-lined caverns lacking proper tight junctions. These malformations may be discovered incidentally or present with symptoms such as headaches, focal neurologic deficits, or seizures, with or without hemorrhage. This review focuses on non-surgical management considerations important for women with CM, who face challenges related to pregnancy, exogenous hormone use, anticonvulsive therapy, bone health, and mental health.
Recent findings: Emerging evidence suggests that both estrogen and progesterone may influence CM lesion behavior. Exogenous hormones, including those in oral contraceptives and oral hormone replacement therapy, indicate an elevated risk of symptomatic hemorrhage (SH) and may also influence seizure frequency and severity, particularly in women taking antiseizure medications (ASMs). Data suggest that the risk of CM hemorrhage during pregnancy is similar to the risk when not pregnant, although limitations to these studies will be reviewed.
Summary: This review synthesizes the current literature on the interplay between estrogen and progesterone and CM lesion behavior, highlighting the importance of gender- and sex-specific factors in clinical decision-making. Special attention is given to the implications of exogenous hormone use, seizure management, and the psychological well-being of women with CM, underscoring the need for a multidisciplinary approach tailored to the unique needs of this patient population.
• The clinical presentation, size, and location of CM in female patients are similar to men, but sex-specific hormonal influences may impact outcomes and management strategies.
• The risk of CM-associated hemorrhage or seizure during pregnancy is generally low; however, careful preparation, planning, and monitoring are essential to ensure optimal outcomes for both mother and infant.
• Caution is advised when considering oral exogenous estrogen and progesterone in women with CM, as these may increase the risk of SH and influence seizure activity.
• Women with CM and seizure disorders should be informed about the long-term effects of ASMs, including their impact on bone health, contraceptive interactions, and potential effects on a fetus or breastfed infant.
• Addressing mental health is as important as managing physical health in women with CM, requiring comprehensive care.
Cavernous malformations (CM) are vascular lesions primarily in the brain and spinal cord that affect approximately 0.5% of the population (1, 2). These lesions are composed of clusters of dilated capillaries that lack properly formed tight junctions, making them prone to hemorrhage (3–5). CM can be identified incidentally or manifest with seizures, focal neurological deficits, and headaches with or without radiologic evidence of hemorrhage (6). CMs can be classified as either familial or sporadic (7, 8). The familial cerebral cavernous malformation syndrome (FCCM), is typically characterized by multiple lesions and is associated with mutations in the CCM1 (KRIT 1), CCM2, and CCM3 (PDCD10) genes (9–11). Conversely, the sporadic form generally involves a single CM, which is frequently associated with a developmental venous anomaly (DVA) (12–14).
The risk of symptomatic hemorrhage (SH), particularly recurrent events, is a major concern for patients with CM, with morbidity increasing after each hemorrhage. Key risk factors include prior SH and brainstem location (15). The risk of recurrent hemorrhage is highest within the first year and tends to plateau after about 2.5 years, underscoring the need for effective management strategies to prevent hemorrhage and reduce associated morbidity (16, 17). The clinical management of CM primarily aims to prevent SH, which is often addressed through surgical excision when appropriate (18–20). In women, however, the management of concurrent conditions including epilepsy, pregnancy planning and pregnancy, and use of oral estrogen and progesterone may be equally as important to consider in the conservative management of CMs (18).
This review aims to provide a comprehensive overview of the clinical management considerations in women with CM. A multidisciplinary approach is important to address the specific needs of this population (Figure 1).

Figure 1. Key consideration for female patients with cavernous malformations (AED, antiepileptic drug). Created in BioRender. Bektas, D. (2024) BioRender.com/u04p136.
Cavernous malformations (CMs) often present in female patients during their reproductive years, with many cases first identified between the second and fifth decades of life, similar to the age group presenting in males (21). Over the past 20 years, numerous cohort descriptions and natural history studies have detailed the initial clinical presentation, and outcomes in patients with CMs (16, 22, 23).
While some studies have suggested female sex as a risk factor for SH, others have not. Aiba et al. followed 110 patients over 4.7 years, finding that females more frequently presented with SH during the second to fifth decades of life, while males more commonly presented with incidental findings (21). Similarly, Robinson observed a significant difference in SH presentation between male and female patients (24). The Scottish Intracranial Vascular Malformation Study (SIVMS) cohort, a population-based study, reported a higher proportion of female patients presenting initially with SH or focal neurologic deficit compared to male patients, although this difference did not reach statistical significance (17). In a study of 68 patients over a 5.2-year period, Moriarty et al. found that female patients experienced a significantly higher hemorrhage rate of 4.2% per patient-year, compared to just 0.9% per patient-year in males, despite having similar initial clinical presentations (25). However, other studies, such as the individual meta-analysis by Horne and colleagues, have identified SH at diagnosis and brainstem location as the primary factors that increase the risk of prospective hemorrhage (15). Whether the differences noted in these cohorts was due to the influence of exogenous or endogenous hormones is not certain.
Endogenous hormones, particularly estrogen and progesterone, have been hypothesized to influence CM behavior (26–28). Early studies suggested that women are more likely to present with SH during periods of significant hormonal change, such as pregnancy (21, 24), due to factors such as hyperdynamic circulation, increased turbulent blood flow, and higher estrogen levels leading to endothelial cell degeneration. Also, placental production of growth factors such as VEGF, bFGF, and placental growth factors were hypothesized to contribute to proliferation and angiogenesis, potentially exacerbating CM lesional activity (29) Hormonal changes during pregnancy have also been proposed to cause intralesional CM or DVA thrombosis, leading to venous hypertension and subsequent CM hemorrhage (29, 30). More recent studies, including those by Kalani, Witiw, and Joseph, suggest that the risk of hemorrhage during pregnancy may not differ significantly from the non-pregnant state, but have limitations (31–33). These studies are discussed in more detail under the section “Pregnancy and CM Management.” Although the literature has not specifically addressed the effects of endogenous estrogen on CMs, it has been suggested that estrogen plays a crucial role in maintaining blood–brain barrier integrity and supporting vascular repair. Estrogen may enhance the repair of endothelial cells and help restore the tight junctions within the blood–brain barrier (34, 35), which could potentially mitigate the risk of hemorrhage. However, this protective effect might decrease with aging (36, 37), leading to greater permeability of the blood–brain barrier and an increased susceptibility to hemorrhagic stroke.
Zhang et al. suggest that progesterone may also negatively affect the cerebral cavernous malformation (CCM) complex, which stabilizes the endothelium (26, 28, 38). Recent research has further elucidated the role of progesterone receptors in CCMs, indicating that activation of progesterone receptors can disrupt the blood–brain barrier and exacerbate CM pathology by promoting vascular permeability and inflammation (38). Additionally, the absence or dysregulation of progesterone receptor signaling has been associated with structural changes in the blood–brain barrier, further contributing to the progression of CMs (27).
These data limitations underscore the need for more robust, prospective studies to better understand the relationship between endogenous hormonal fluctuations and CM behavior. Moreover, there remains a need for further investigation into how other hormonal changes, such as those during the menstrual cycle and menopause, may influence CM behavior, as the current literature has predominantly focused on pregnancy and hormone use.
Concerns have been raised about the impact of exogenous estrogen and progesterone, such as those found in oral contraceptives and oral hormone replacement therapy (HRT) used for perimenopausal symptoms on CM behavior (39).
A prospective cohort study from the Mayo Clinic found that brainstem location and estrogen use in female patients were associated with an initial presentation of SH, although the study had a small sample size and did not differentiate between hormone delivery methods (40). A larger study by Zuurbier and colleagues analyzed 722 female patients, 137 of whom had taken exogenous estrogen and/or progesterone for contraception or HRT. The study found that 33.6% of patients taking hormones experienced a prospective CM hemorrhage, compared to 15.6% of those not taking hormones. The increased hemorrhage risk was particularly significant for those using oral contraceptives, especially when combined with smoking. In the HRT group, oral hormone replacement, but not other hormone delivery methods were associated with an increased risk of SH. While estrogen-containing contraceptives were associated with an increased risk, the study grouped oral progesterone-only contraceptives together with combined estrogen-progesterone options, rather than evaluating them separately. Additionally, progesterone-only methods delivered via intrauterine devices (IUDs) were not evaluated due to differences in the ascertainment of data between included cohorts. This grouping approach presents a limitation, as it does not allow for a clear assessment of the risks associated with progesterone-only contraceptives.
While the above study raises concerns about exogenous oral estrogen and progesterone in patients with CM, many questions remain. If estrogen increases SH risk by its influence on intralesional thrombosis, then the effect should be dose dependent, dependent on which generation progesterone it is combined with and may be dependent on the mode of delivery (transdermal versus vaginal versus oral). The potential negative effects of progesterone on the CCM complex should still be considered given the work by Zhang and colleagues, but further research is necessary to accurately distinguish the risks of progesterone separated from the estrogen effect (39). A women’s health specialist should be consulted to discuss alternative options such as copper IUDs for pregnancy prevention or consider the lowest risk estrogen/progesterone combination in patients with medical conditions requiring hormones.
For transgender patients undergoing hormone therapy, similar considerations apply. Counseling for transgender patients should address both the need for gender-affirming care and the risks associated with hormone therapy in the context of CM location, symptoms, and treatment options.
In summary, estrogen-containing contraceptives and oral hormone replacement therapies should generally be avoided in women with CM due to the increased risk of thrombosis and CM hemorrhage associated with estrogen-containing therapies. In addition, given the potential effects of progesterone on the CCM complex, these options should be chosen carefully and monitored closely (26, 28). However, given the many uncertainties of the current data and the need for hormonal therapy in certain conditions, a personalized approach should be emphasized in clinical practice. An individual with CM considering hormone therapy should be evaluated to determine their individual risk of SH and the morbidity associated with potential hemorrhage and compare that to the risks, benefits and alternatives to hormone therapy. For example, there are familial CM patients who inherit the gene, but have no lesions or have only lesions that appear on hemosiderin sensitive sequences. The risk in such patients may not be similar to those with a recently symptomatic hemorrhagic event, brainstem lesion, or enlarging CM lesion. Hormone therapy use should be carefully monitored, with individualized care plans to minimize risk while effectively addressing the patient’s symptoms (39).
Seizure management in women with CM involves additional complexities due to the interactions between antiseizure medications (ASMs) and hormonal therapies as well as the influence of pregnancy, which is further discussed in the paragraph about considerations relevant to pregnancy. In addition to the potential negative consequences of estrogen and progesterone on SH risk, there are additional considerations in CM patients with seizures. Many ASMs, particularly enzyme-inducing medications like carbamazepine, phenytoin, and phenobarbital, can reduce the effectiveness of hormonal contraceptives, potentially leading to contraceptive failure. This reduction in contraceptive effectiveness can lead to unintended pregnancies, which is concerning because many ASMs have teratogenic potential, posing risks to the developing fetus (41–43) (Table 1). Conversely, hormonal therapies can decrease serum levels of ASMs like lamotrigine, which may reduce seizure control and necessitate close monitoring and dose adjustments (42, 44, 45). HRT used for perimenopausal symptoms can also influence seizure control. A small randomized controlled study found that oral HRT can significantly worsen seizure control, though this effect was not specifically tested in patients with CMs, necessitating careful consideration when prescribing these therapies (46). Additionally, endogenous hormones, particularly estrogen, are known to be “pro-convulsant,” with some patients experiencing an increase in seizure frequency during periods of high estrogen levels, such as during pregnancy or certain phases of the menstrual cycle (41).
These interactions underscore the importance of a personalized approach to managing seizures in women with CM, considering both the effectiveness of seizure control and the potential risks associated with hormonal therapies. Proper counseling on the choice and use of contraception is vital to ensure that neither the efficacy of the contraceptive nor the ASM is compromised, thereby reducing the risk of unplanned pregnancies and ensuring optimal management of epilepsy (42, 47).
Female patients are generally at a higher risk for osteopenia and osteoporosis compared to males (48), and this risk is further elevated in patients with cavernous malformations (CM) who are on enzyme-inducing ASMs. The enzyme-inducing properties of these ASMs can lead to decreased bone density, exacerbating the risk of bone health issues (49–51) (Table 2). Additional risk factors for osteopenia include advancing age, low body weight, and a history of glucocorticoid therapy (52). To manage these risks, regular bone density screening is recommended for female patients with CM. Supplementation with vitamin D and calcium is also advised to help mitigate the risk of osteopenia and osteoporosis (53). In addition to the value of vitamin D supplementation for bone density, recent studies suggest that vitamin D supplementation may also play a role in influencing CM behavior, possibly through its effects on oxidative stress and the gut microbiome (54–56). Therefore, maintaining adequate vitamin D levels is crucial not only for bone health but also for potentially beneficial effects on CM management.
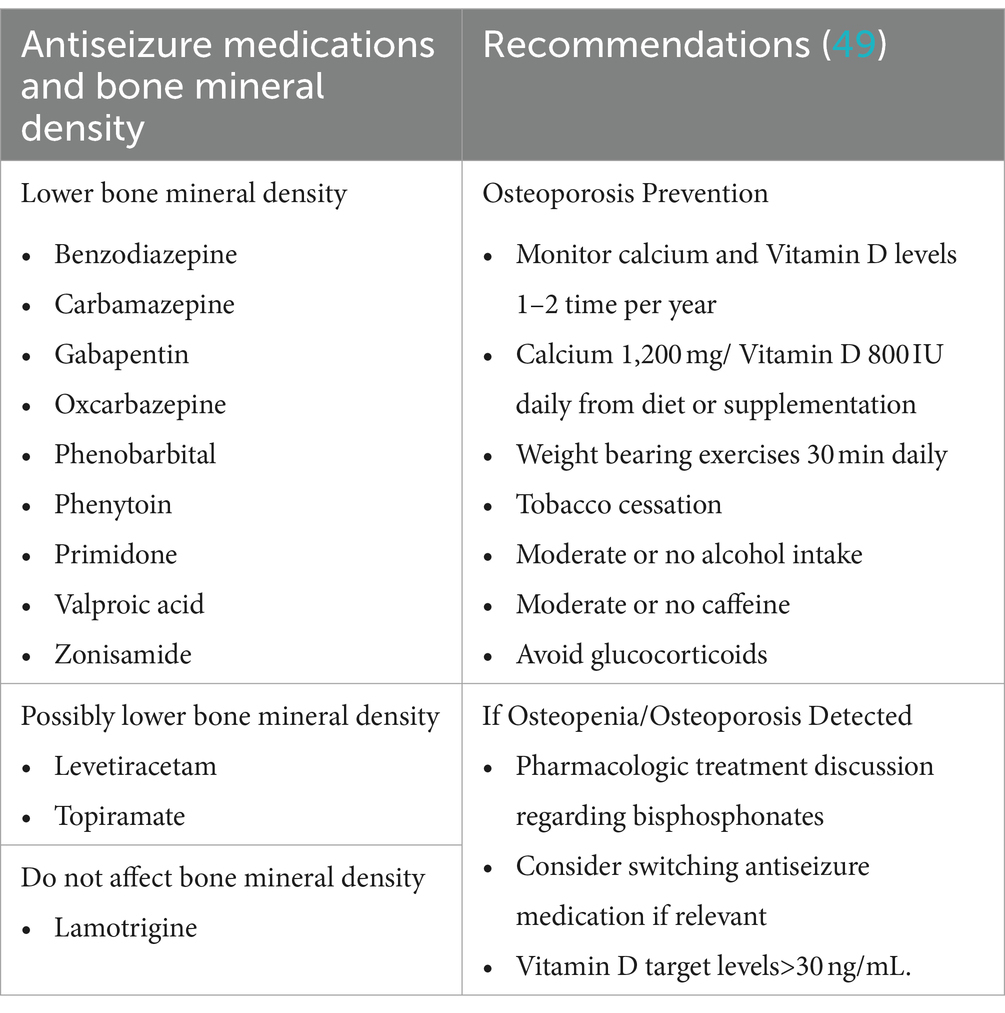
Table 2. Effects of antiseizure medications on bone mineral density and osteoporosis prevention recommendations.
While the absolute risk of SH and seizures during pregnancy are generally low for most patients with CM, there are several factors that require careful consideration (Figure 2).
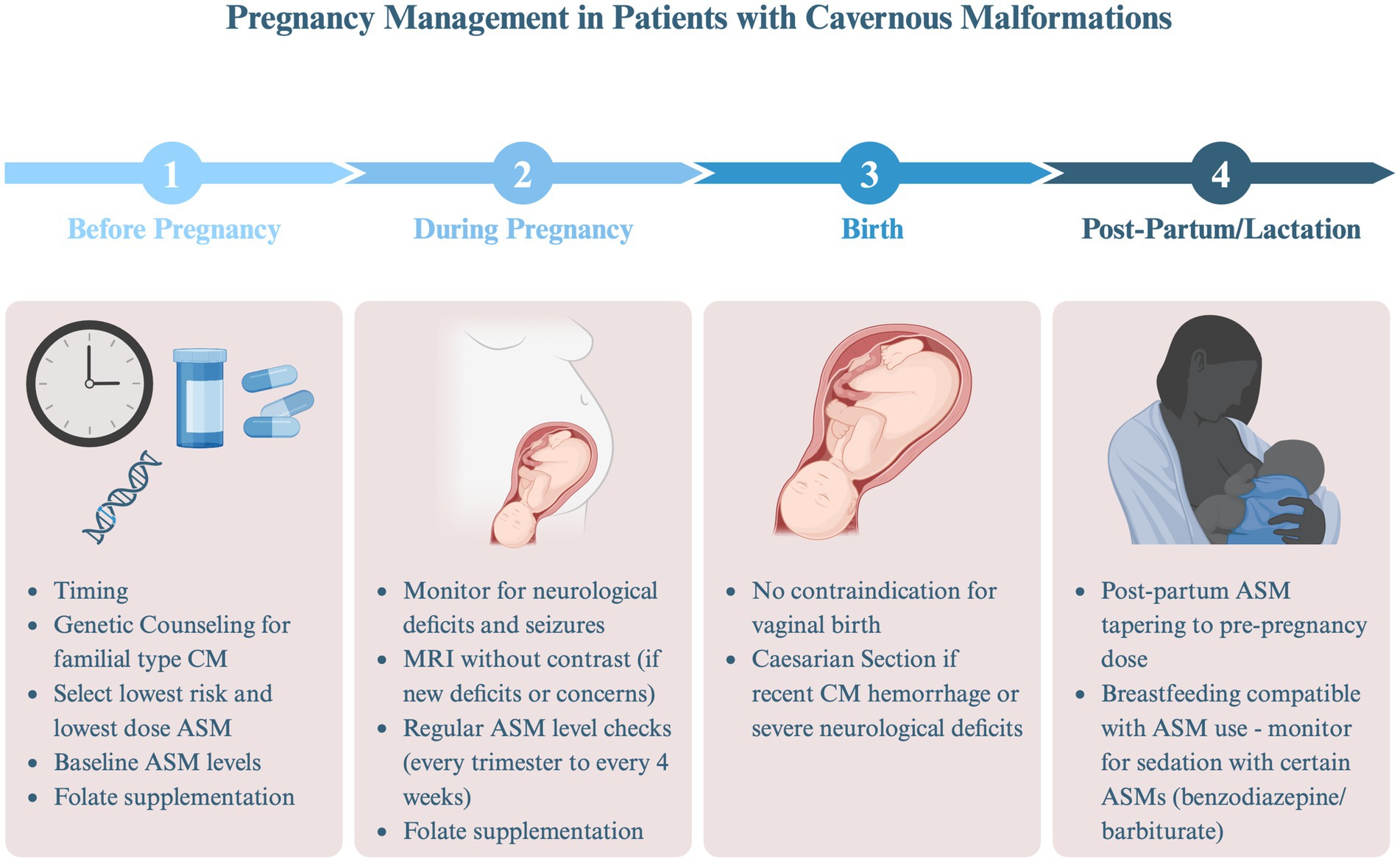
Figure 2. Pregnancy management in patients with cavernous malformations (AED, antiepileptic drug). Created in BioRender. Bektas, D. (2024) BioRender.com/e32g825.
Pre-planning and preparation are important for patients with CM who are considering pregnancy. Discussions should include optimal timing of conception relative to the individuals SH risk and seizure frequency, safety of select medication (e.g., ASMs), and specific considerations for patients with FCCM.
A neurologist or obstetrician should guide patients on the optimal timing to consider conceiving. Ideally, pregnancy should be planned when the risk of CM hemorrhage is lowest, and if the patient has seizures, they should be well-controlled. Natural history studies indicate that the risk of recurrent hemorrhage is highest within the first year following an initial event and begins to plateau around 2.5 years thereafter (16). Consequently, patients who have recently experienced SH are generally advised to wait at least 1 year before conceiving, if feasible, considering maternal age and other factors. Similarly, since frequent and/or convulsive seizures during pregnancy can negatively affect both the mother and fetus, achieving adequate seizure control prior to conception is ideal.
For female patients with a history of seizures, managing ASMs involves important considerations, especially when planning for pregnancy. First, ASMs may have teratogenic potential, posing risk to the developing fetus. The fetus is most vulnerable to congenital malformations early in development, often before the pregnancy is recognized (41–43). To mitigate these risks, folate supplementation in addition to discussions on whether the ASM should be continued, held or changed to an alternative should take place. It is recommended that women of childbearing age on ASMs take at least 0.4 mg of folic acid daily, as this supplementation may help reduce the risk of neural tube defects associated with ASM use (47). Prospective registry studies indicate that valproic acid has the highest teratogenic potential, while lamotrigine and levetiracetam are associated with the lowest risk. The teratogenic effects of newer ASMs remain less well understood (57–62). Additionally, achieving optimal seizure control before pregnancy is crucial, as poorly controlled seizures can increase the risk of preterm labor and pose significant risks to the fetus. Therefore, baseline ASM levels should be obtained and maintained at effective therapeutic levels to ensure both maternal and fetal safety during pregnancy (44, 63–65).
Patients with FCCM should receive genetic counseling regarding the risk of passing the mutated gene to their offspring. The genes CCM1, CCM2, and CCM3 are inherited in an autosomal dominant manner. However, not all individuals with the gene develop CM lesions, and among those who do, symptoms may vary significantly (66, 67). Data suggests that by age 80, approximately 60.4% of patients with FCCM will experience at least one seizure (68), and up to 64% will experience at least one symptomatic cerebral or spinal hemorrhage within the first 10 years after diagnosis. It is also important to note that as many as 20% of patients may remain asymptomatic for many years (69).
For patients with familial CCM, reproductive decisions can be complex and may require input from genetic counselors, fertility experts, and obstetricians (69). Patients may choose to proceed with natural conception, consider using donor sperm or eggs, explore in vitro fertilization (IVF) with or without embryo selection (70), or consider adoption. While not studied in CM patients, there is an increased risk of venous thromboembolism in patients undergoing IVF (71). Given that CM hemorrhage may be the result of intralesional thrombosis, concern has been raised about the potential for IVF medications to cause CM hemorrhage. Thus, the decision to pursue IVF should be made with careful consideration of the individual’s CM hemorrhage risk and in consultation with a healthcare provider experienced in managing CM. Genetic testing of embryos and chorionic villus sample require prior knowledge of the parental mutation. Chorionic villus sampling (CVS) for genetic testing can be performed between 10 and 12 weeks of pregnancy (72, 73).
Early case reports and series initially raised concerns that pregnancy might increase the risk of CM hemorrhage. However, more recent studies from large tertiary care centers suggest that the risk of CM hemorrhage during pregnancy may be comparable to the non-pregnant state (31–33). For example, Kalani et al. followed 64 female patients with 168 pregnancies and found that four patients experienced five hemorrhages during pregnancy, resulting in an annual hemorrhage rate of 3.4% per year pregnant. The study concluded that this rate was similar to the natural history of CM hemorrhage in non-pregnant patients, although the average age of diagnosis was not specified (31).
In a study from Toronto, Witiw and colleagues assessed 186 female patients with 349 pregnancies. They estimated the risk of CM hemorrhage by assuming the patient was at risk between the ages of 15 and 45 (childbearing years). The rate of hemorrhage during pregnancy was calculated as 1.15% per patient-year, compared to 1.01% per patient-year during the non-pregnant state. However, this methodology assumed that CM lesions were present throughout the reproductive years, despite the unclear timing of cavernoma development, potentially underestimating the risk during pregnancy, particularly given that the average age of diagnosis in the Toronto study was 42.7 years (32).
Joseph and colleagues addressed this potential confounder by assessing the risk of CM hemorrhage in female patients diagnosed with CM prior to age 46. Of the 90 patients, 136 pregnancies occurred before CM diagnosis, with four cases of CM hemorrhage during pregnancy leading to the initial diagnosis. Of 21 patients with 32 pregnancies after the CM diagnosis, no hemorrhages occurred during subsequent pregnancies. The rate of hemorrhage during the non-pregnant state in the same group was 10.4% per patient-year. The authors concluded that the risk of CM hemorrhage was similar in both the pregnant and non-pregnant states. However, they acknowledged the small sample size and the possibility of selection bias, as patients who experienced SH might have deferred future pregnancies, further complicating the interpretation of results (33).
Patients should be counseled that the absolute risk of complications during pregnancy is low, but close monitoring for clinical symptoms is important. Patients should be educated about their individual potential symptoms to be concerned about, which can vary depending on CM location—whether in the spine, brainstem, or cortex. Symptoms of concern, such as persistent, severe headaches or focal neurological deficits, may necessitate further investigation to rule out conditions like venous thrombosis, pre-eclampsia, or CM-related complications. MRI without contrast is considered safe during pregnancy if such concerns arise.
In the non-pregnant state, surgery is generally favored for CM patients with recurrent seizures unresponsive to medication or SH with mass effect in non-eloquent regions, as well as in cases of repeated hemorrhages and increasing disability in eloquent regions. When symptomatic or hemorrhagic CMs present with acute neurological decline, urgent surgical intervention is typically warranted (74). Most experts agree that surgical resection during pregnancy should be reserved for those with rapidly progressive symptoms and that it should be performed by a multidisciplinary team at a specialized center (29, 75–77).
Most patients with well-controlled seizures before pregnancy will remain seizure-free during pregnancy (64). However, 20–30% of patients may experience increased seizure frequency during pregnancy. This is often due to a decline in ASM levels, which can drop significantly during pregnancy, particularly with medications like lamotrigine, levetiracetam, oxcarbazepine, topiramate, and zonisamide. This decline is attributed to changes in maternal renal and hepatic metabolism, volume of distribution, and interference by placental enzymes. Therefore, it is recommended to establish baseline ASM levels prior to pregnancy and to monitor levels throughout pregnancy, adjusting doses as necessary to maintain pre-pregnancy levels. Depending on the ASM, levels may need to be checked every 4 weeks (47), although no standardized testing frequency protocols have been published. Other factors contributing to seizure exacerbation during pregnancy may include stress, sleep deprivation, and infection. If a previously well-controlled seizure disorder worsens without an apparent cause, MRI might be considered to assess for CM hemorrhage.
Pushing during childbirth has raised concerns about the potential for intracerebral hemorrhage in patients with vascular malformations. However, there have been only rare reports of hemorrhage from a CM during delivery (58). Vaginal delivery is generally considered safe and reasonable for stable patients with CM (29). In cases where the patient has a recent neurologic issue, such as a significant focal neurologic deficit or a recent CM hemorrhage, a Cesarean section may be considered.
The postpartum period, typically defined as the first 4–6 weeks after birth, requires ongoing monitoring of neurological symptoms and ASM levels, as hormonal fluctuations can persist for up to 6 months. For women whose ASM dose was increased during pregnancy, serum levels may rise shortly after delivery, necessitating dose reductions to avoid toxicity. In ASMs cleared by glucuronidation, such as lamotrigine, tapering should begin as early as 3 days postpartum, while ASMs with renal clearance may return to pre-pregnancy metabolism within 2–3 weeks postpartum (41, 44).
Breastfeeding offers numerous health benefits, but there are specific considerations for patients with CM. If an MRI is needed postpartum, it should be performed without contrast, or the patient should pump and discard breast milk for 24–48 h after the procedure. ASMs can be present in breast milk, though at lower concentrations than a fetus is exposed to during pregnancy. Studies have not demonstrated adverse effects of maternal ASM use during breastfeeding on the IQ, social or motor skills, or behavior of breastfed children (78, 79). Therefore, women on ASMs can breastfeed but should monitor their babies for signs of sedation, particularly if taking benzodiazepines or barbiturates (41).
Mental health concerns, including anxiety, depression, and fatigue, are prevalent among patients with CM, regardless of sex. These issues often arise from the uncertainty of living with a potentially hemorrhagic lesion, the impact of seizure disorders, and the burden of chronic neurological symptoms. Studies have shown that anxiety, fatigue, and impaired social and physical function are common complaints among CM patients, though these issues are not statistically different between male and female patients (80–83). Anxiety is common in patients with CM. Anxiety may relate to a new diagnosis or symptoms or can be induced by the location of the lesion. Patients may experience additional anxiety related to the risk of hemorrhage, or concerns about passing on genetic mutations in familial cases. Those with seizure disorders may also worry about the side effects of ASMs, including potential cognitive or mood changes. The influence of hormonal fluctuations on CM-related symptoms and the challenges of managing CM during pregnancy may also play a role. Counseling for patients should address these sex-and gender-specific concerns, providing strategies for managing these mental health challenges effectively. Rehabilitation programs, particularly those adapted from stroke rehabilitation protocols, may offer both physical and mental health support tailored to the needs of CM patients (84). These programs can be particularly beneficial for patients with ongoing physical dysfunction and fatigue, helping them cope with both the physical and emotional challenges associated with the condition, and ultimately improving their overall quality of life.
Managing women with CM requires a comprehensive, multidisciplinary approach that addresses the unique challenges posed by hormonal influences, pregnancy, seizures, and mental health. Neurologists should take a central role in coordinating care, ensuring that all aspects of the patient’s health are considered in the management plan. This includes facilitating communication between different specialists and ensuring comprehensive management that anticipates and addresses the various challenges these patients face. Further research is needed to better understand the interactions between hormones—including estrogen, progesterone, and other sex hormones—and CM, as well as the long-term outcomes for women with these lesions in both clinical and experimental settings. Specifically, studies assessing which patient characteristics, and which hormonal therapies raise risk of SH will be impactful. In addition, further study on the influence of endogenous hormones and pregnancy could provide further insight on clinical management strategies.
DB: Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Investigation. GL: Supervision, Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing. KF: Formal analysis, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
KF has consulted for Ovid therapeutics, Blueprint Orphan, and Recursion pharmaceutical. KS is a site primary investigator for a study funded by UCB. GL has consulted for Nested Knowledge and Superior Medical Editors.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Flemming, KD, Graff-Radford, J, Aakre, J, Kantarci, K, Lanzino, G, Brown, RD, et al. Population-based prevalence of cerebral cavernous malformations in older adults: Mayo clinic study of aging. JAMA Neurol. (2017) 74:801–5. doi: 10.1001/jamaneurol.2017.0439
2. Ren, J, Jiang, N, Bian, L, Dmytriw, AA, Zeng, G, He, C, et al. Natural history of spinal cord cavernous malformations: a Multicenter cohort study. Neurosurgery. (2022) 90:390–8. doi: 10.1227/NEU.0000000000001842
3. Kondziolka, D, Lunsford, LD, and Kestle, JRW. The natural history of cerebral cavernous malformations. J Neurosurg. (1995) 83:820–4. doi: 10.3171/jns.1995.83.5.0820
4. Schneider, H, Errede, M, Ulrich, NH, Virgintino, D, Frei, K, and Bertalanffy, H. Impairment of tight junctions and glucose transport in endothelial cells of human cerebral cavernous malformations. J Neuropathol Exp Neurol. (2011) 70:417–29. doi: 10.1097/NEN.0b013e31821bc40e
5. Wong, JH, Awad, IA, and Kim, JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. (2000) 46:1454–9. doi: 10.1097/00006123-200006000-00027
6. Flemming, KD . Incidence, prevalence, and clinical presentation of cerebral cavernous malformations. Methods Mol Biol. (2020). doi: 10.1007/978-1-0716-0640-7_2
7. Zabramski, JM, Wascher, TM, Spetzler, RF, Johnson, B, Golfinos, J, Drayer, BP, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. (1994) 80:422–32. doi: 10.3171/jns.1994.80.3.0422
8. Zeineddine, HA, Girard, R, Saadat, L, Shen, L, Lightle, R, Moore, T, et al. Phenotypic characterization of murine models of cerebral cavernous malformations. Lab Investig. (2019) 99:319–30. doi: 10.1038/s41374-018-0030-y
9. Bergametti, F, Denier, C, Labauge, P, Arnoult, M, Boetto, S, Clanet, M, et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. (2005) 76:42–51. doi: 10.1086/426952
10. Liquori, CL, Berg, MJ, Squitieri, F, Leedom, TP, Ptacek, L, Johnson, EW, et al. Deletions in CCM2 are a common cause of cerebral cavernous malformations. Am J Hum Genet. (2007) 80:69–75. doi: 10.1086/510439
11. Stahl, S, Gaetzner, S, Voss, K, Brackertz, B, Schleider, E, Sürücü, O, et al. Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: in-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum Mutat. (2008) 29:709–17. doi: 10.1002/humu.20712
12. Yu, T, Liu, X, Lin, X, Bai, C, Zhao, J, Zhang, J, et al. The relation between angioarchitectural factors of developmental venous anomaly and concomitant sporadic cavernous malformation. BMC Neurol. (2016) 16:183. doi: 10.1186/s12883-016-0691-3
13. Das, K, Rangari, K, Singh, S, Bhaisora, K, Jaiswal, A, and Behari, S. Coexistent cerebral cavernous malformation and developmental venous anomaly: does an aggressive natural history always call for surgical intervention? Asian. J Neurosurg. (2019) 14:318–21. doi: 10.4103/ajns.AJNS_196_18
14. Idiculla, PS, Gurala, D, Philipose, J, Rajdev, K, and Patibandla, P. Cerebral cavernous malformations, developmental venous anomaly, and its coexistence: a review. Eur Neurol. (2020) 83:360–8. doi: 10.1159/000508748
15. Horne, MA, Flemming, KD, Su, IC, Stapf, C, Brown, RD, Christianson, TJ, et al. Abstract 193: cerebral cavernous malformation location and mode of presentation predict the risk of Hemorrhage during their untreated clinical course: individual patient data Meta-analysis. Stroke. (2015) 46:193. doi: 10.1161/str.46.suppl_1.193
16. Horne, MA, Flemming, KD, Su, IC, Stapf, C, Jeon, JP, Li, D, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. (2016) 15:166–73. doi: 10.1016/S1474-4422(15)00303-8
17. Salman, RAS, Hall, JM, Horne, MA, Moultrie, F, Josephson, CB, Bhattacharya, JJ, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. (2012) 11:217–24. doi: 10.1016/S1474-4422(12)70004-2
18. Gross, BA, and Du, R. Cerebral cavernous malformations: natural history and clinical management. Expert Rev Neurother. (2015) 15:771–7. doi: 10.1586/14737175.2015.1055323
19. Hoffman, JE, Wittenberg, B, Morel, B, Youssef, S, Seinfeld, J, Folzenlogen, Z, et al. Tailored treatment options for cerebral cavernous malformations. J Personalized Med. (2022) 12:831. doi: 10.3390/jpm12050831
20. Petr, O, and Lanzino, G. Brainstem cavernous malformations. J Neurosurg Sci. (2015) 59:271–82. doi: 10.1055/s-0038-1625933
21. Aiba, T, Tanaka, R, Koike, T, Kameyama, S, Takeda, N, and Komata, T. Natural history of intracranial cavernous malformations. J Neurosurg. (1995) 83:56–9. doi: 10.3171/jns.1995.83.1.0056
22. Taslimi, S, Modabbernia, A, Amin-Hanjani, S, Barker, FG, and Macdonald, RL. Natural history of cavernous malformation: systematic review and meta-analysis of 25 studies. Neurology. (2016) 86:1984–91. doi: 10.1212/WNL.0000000000002701
23. Gross, BA, and Du, R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. (2013) 118:437–43. doi: 10.3171/2012.10.JNS121280
24. Robinson, JR, Awad, IA, and Little, JR. Natural history of the cavernous angioma. J Neurosurg. (1991) 75:709–14. doi: 10.3171/jns.1991.75.5.0709
25. Moriarity, J, Clatterbuck, R, and Rigamonti, D. The natural history of cavernous malformations. Neurosurg Clin N Am. (1999) 10:411–7. doi: 10.1016/S1042-3680(18)30175-X
26. Zhang, J, and Abou-Fadel, JS. Calm the raging hormone - a new therapeutic strategy involving progesterone-signaling for hemorrhagic CCMs, vol. 5. Los Angeles, USA: OAE Publishing Inc. (2021).
27. Abou-Fadel, J, Jiang, X, Padarti, A, Goswami, DG, Smith, M, Grajeda, B, et al. mPR-specific actions influence maintenance of the blood–brain barrier (BBB). Int J Mol Sci. (2022) 23:684. doi: 10.3390/ijms23179684
28. Abou-Fadel, J, Jiang, X, Grajeda, B, Padarti, A, Ellis, CC, Flores, E, et al. CCM signaling complex (CSC) couples both classic and non-classic progesterone receptor signaling. Cell Communication and Signaling. (2022) 20:120. doi: 10.1186/s12964-022-00926-z
29. Merlino, L, Del Prete, F, Titi, L, and Piccioni, MG. Cerebral cavernous malformation: management and outcome during pregnancy and puerperium. A systematic review of literature. J Gynecol Obstetrics and Human Reprod. (2021) 50:101927. doi: 10.1016/j.jogoh.2020.101927
30. Gazzaz, M, Sichez, J, Capelle, L, and Fohanno, D. Recurrent bleeding of thalamic cavernous angioma under hormonal treatment. A case report. Neurochirurgie. (1999) 45:413–6.
31. Kalani, MYS, and Zabramski, JM. Risk for symptomatic hemorrhage of cerebral cavernous malformations during pregnancy: clinical article. J Neurosurg. (2013) 118:50–5. doi: 10.3171/2012.8.JNS12241
32. Witiw, CD, Abou-Hamden, A, Kulkarni, AV, Silvaggio, JA, Schneider, C, and Wallace, MC. Cerebral cavernous malformations and pregnancy: Hemorrhage risk and influence on obstetrical management. Neurosurgery. (2012) 71:626–31. doi: 10.1227/NEU.0b013e31825fd0dc
33. Joseph, NK, Kumar, S, Brown, RD, Lanzino, G, and Flemming, KD. Influence of pregnancy on Hemorrhage risk in women with cerebral and spinal cavernous malformations. Stroke. (2021) 52:434–41. doi: 10.1161/STROKEAHA.120.031761
34. Maggioli, E, McArthur, S, Mauro, C, Kieswich, J, Kusters, DHM, Reutelingsperger, CPM, et al. Estrogen protects the blood–brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. (2016) 51:212–22. doi: 10.1016/j.bbi.2015.08.020
35. Kuruca, SE, Karadenizli, S, Akgun-Dar, K, Kapucu, A, Kaptan, Z, and Uzum, G. The effects of 17β-estradiol on blood brain barrier integrity in the absence of the estrogen receptor alpha; an in-vitro model. Acta Histochem. (2017) 119:638–47. doi: 10.1016/j.acthis.2017.07.005
36. Sohrabji, F, Bake, S, and Lewis, DK. Age-related changes in brain support cells: implications for stroke severity. Neurochem Int. (2013) 63:291–301. doi: 10.1016/j.neuint.2013.06.013
37. Zahreddine, R, Davezac, M, Buscato, M, Smirnova, N, Laffargue, M, Henrion, D, et al. A historical view of estrogen effect on arterial endothelial healing: from animal models to medical implication. Atherosclerosis. (2021) 338:30–8. doi: 10.1016/j.atherosclerosis.2021.10.013
38. Gnanasekaran, R, Aickareth, J, Hawwar, M, Sanchez, N, Croft, J, and Zhang, J. CmPn/CmP Signaling networks in the maintenance of the blood vessel barrier. J Personalized Med. (2023) 13:751. doi: 10.3390/jpm13050751
39. Zuurbier, SM, Santos, AN, Flemming, KD, Schmidt, B, Jabbarli, R, Lanzino, G, et al. Female hormone therapy and risk of intracranial Hemorrhage from cerebral cavernous malformations: a Multicenter observational cohort study. Neurology. (2023) 100:6888. doi: 10.1212/WNL.0000000000206888
40. Flemming, KD, Kumar, S, Brown, RD, and Lanzino, G. Predictors of initial presentation with Hemorrhage in patients with cavernous malformations. World Neurosurg. (2020) 133:e767–73. doi: 10.1016/j.wneu.2019.09.161
41. Bui, E . Women’s issues in epilepsy. CONTINUUM Lifelong Learn Neurol. (2022) 28:399–427. doi: 10.1212/CON.0000000000001126
42. Muschett, MR, Ewig, C, Morris, E, Adkins, LE, Goodin, A, and Brown, J. A review of concordance and quality in clinical guidelines for hormonal contraceptives to mitigate drug-drug interactions in women with epilepsy. Pharmacoepidemiol Drug Saf. (2024) 33:e5861. doi: 10.1002/pds.5861
43. Herzog, AG, Mandle, HB, Cahill, KE, Fowler, KM, and Hauser, WA. Predictors of unintended pregnancy in women with epilepsy. Neurology. (2017) 88:728–33. doi: 10.1212/WNL.0000000000003637
44. Du, Y, Fang, W, Huang, W, Xu, Q, Gong, J, Xia, N, et al. Changes in seizure frequency and anti-seizure medication therapy during pregnancy and one year postpregnancy. Epilepsy Behav. (2023) 144:109256. doi: 10.1016/j.yebeh.2023.109256
45. Reimers, A, Helde, G, and Brodtkorb, E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. (2005) 46:1414–7. doi: 10.1111/j.1528-1167.2005.10105.x
46. Harden, CL, Herzog, AG, Nikolov, BG, Koppel, BS, Christos, PJ, Fowler, K, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia. (2006) 47:1447–51. doi: 10.1111/j.1528-1167.2006.00507.x
47. Tomson, T, Battino, D, Bromley, R, Kochen, S, Meador, K, Pennell, P, et al. Management of epilepsy in pregnancy: a report from the international league against epilepsy task force on women and pregnancy. Epileptic Disord. (2019) 21:497–517. doi: 10.1684/epd.2019.1105
48. Muzzi Camargos, B . Epidemiology and risk factors for osteoporosis in women. Osteoporos Int. (2018) 29:S122. doi: 10.1007/s00198-018-4439-3
49. Sazgar, M . Treatment of women with epilepsy. CONTINUUM Lifelong Learn Neurol. (2019) 25:408–30. doi: 10.1212/CON.0000000000000713
50. Valsamis, HA, Arora, SK, Labban, B, and McFarlane, SI. Antiepileptic drugs and bone metabolism. Nutr Metab. (2006) 3:36. doi: 10.1186/1743-7075-3-36
51. Miziak, B, Błaszczyk, B, Chrościńska-Krawczyk, M, Danilkiewicz, G, Jagiełło-Wójtowicz, E, and Czuczwar, SJ. The problem of osteoporosis in epileptic patients taking antiepileptic drugs. Expert Opin Drug Saf. (2014) 13:935–46. doi: 10.1517/14740338.2014.919255
52. Dargent-Molina, P . Epidemiology and risk factors of osteoporosis. Rev Med Interne. (2004) 25:S517–25. doi: 10.1016/S0248-8663(04)80049-3
53. Lazzari, AA, Dussault, PM, Thakore-James, M, Gagnon, D, Baker, E, Davis, SA, et al. Prevention of bone loss and vertebral fractures in patients with chronic epilepsy - antiepileptic drug and osteoporosis prevention trial. Epilepsia. (2013) 54:1997–2004. doi: 10.1111/epi.12351
54. Girard, R, Khanna, O, Shenkar, R, Zhang, L, Wu, M, Jesselson, M, et al. Peripheral plasma vitamin D and non-HDL cholesterol reflect the severity of cerebral cavernous malformation disease. Biomark Med. (2016) 10:255–64. doi: 10.2217/bmm.15.118
55. Gibson, CC, Zhu, W, Davis, CT, Bowman-Kirigin, JA, Chan, AC, Ling, J, et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation. (2015) 131:289–99. doi: 10.1161/CIRCULATIONAHA.114.010403
56. Flemming, KD, Kumar, S, Brown, RD, Singh, RJ, Whitehead, K, McCreath, L, et al. Cavernous malformation hemorrhagic presentation at diagnosis associated with low 25-hydroxy-vitamin D level. Cerebrovasc Dis. (2020) 49:216–22. doi: 10.1159/000507789
57. Campbell, E, Kennedy, F, Russell, A, Smithson, WH, Parsons, L, Morrison, PJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry. (2014) 85:1029–34. doi: 10.1136/jnnp-2013-306318
58. De Jong, A, Benayoun, L, Bekrar, Y, Forget, S, and Wernet, A. Obstetric anaesthesia in two patients with cerebral cavernomas: about two cases. Ann Fr Anesth Reanim. (2012) 31:635. doi: 10.1016/j.annfar.2012.02.020
59. Hernández-Díaz, S, Smith, CR, Shen, A, Mittendorf, R, Hauser, WA, Yerby, M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. (2012) 78:1692–9. doi: 10.1212/WNL.0b013e3182574f39
60. Mawhinney, E, Craig, J, Morrow, J, Russell, A, Smithson, WH, Parsons, L, et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. (2013) 80:400–5. doi: 10.1212/WNL.0b013e31827f0874
61. Hunt, S, Russell, A, Smithson, WH, Parsons, L, Robertson, I, Waddell, R, et al. Topiramate in pregnancy: preliminary experience from the UK epilepsy and pregnancy register. Neurology. (2008) 71:272–6. doi: 10.1212/01.wnl.0000318293.28278.33
62. Tomson, T, Battino, D, Bonizzoni, E, Craig, J, Lindhout, D, Perucca, E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. (2018) 17:530–8. doi: 10.1016/S1474-4422(18)30107-8
63. Li, J, Toffa, DH, and Nguyen, DK. Epilepsy and pregnancy: an audit of specialized care. Can J Neurol Sci. (2022) 49:678–87. doi: 10.1017/cjn.2021.190
64. Pennell, PB, French, JA, May, RC, Gerard, E, Kalayjian, L, Penovich, P, et al. Changes in seizure frequency and antiepileptic therapy during pregnancy. N Engl J Med. (2020) 383:2547–56. doi: 10.1056/NEJMoa2008663
65. Arfman, IJ, Wammes-van der Heijden, EA, ter Horst, PGJ, Lambrechts, DA, Wegner, I, and Touw, DJ. Therapeutic drug monitoring of antiepileptic drugs in women with epilepsy before, during, and after pregnancy. Clin Pharmacokinet. (2020) 59:427–45. doi: 10.1007/s40262-019-00845-2
66. Zafar, A, Quadri, SA, Farooqui, M, Ikram, A, Robinson, M, Hart, BL, et al. Familial cerebral cavernous malformations. Stroke. (2019) 50:1294–301. doi: 10.1161/STROKEAHA.118.022314
67. Flemming, KD, Smith, E, Marchuk, D, and Derry, WB. (2003). GeneReviews®. Familial Cerebral Cavernous Malformations. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1293/ (Accessed on 2024 Oct 6).
68. Fox, CK, Nelson, J, McCulloch, CE, Weinsheimer, S, Pawlikowska, L, Hart, B, et al. Seizure incidence rates in children and adults with familial cerebral cavernous malformations. Neurology. (2021) 97:e1210–6. doi: 10.1212/WNL.0000000000012569
69. Alalfi, MO, Lanzino, G, and Flemming, KD. Clinical presentation, hemorrhage risk, and outcome in patients with familial cavernous malformations: a pragmatic prospective analysis of 75 patients. J Neurosurg. (2023) 139:1018–24. doi: 10.3171/2023.1.JNS222434
70. Practice Committee and Genetic Counseling Professional Group of the American Society for Reproductive Medicine, American Society for Reproductive Medicine, Washington, D.C. Indications and management of preimplantation genetic testing for monogenic conditions: a committee opinion. Fertil Steril. (2023) 120:61–71. doi: 10.1016/j.fertnstert.2023.03.003
71. Lattová, V, Dostál, J, Vodička, J, and Procházka, M. The risk of thromboembolism in relation to in vitro fertilization. Ceska Gynekol. (2019) 84:229–32.
72. Heckerling, PS, and Verp, MS. Amniocentesis or chorionic villus sampling for prenatal genetic testing: a decision analysis. J Clin Epidemiol. (1991) 44:657–70. doi: 10.1016/0895-4356(91)90027-7
73. Antsaklis, A, Souka, AP, Daskalakis, G, Kavalakis, Y, and Michalas, S. Second-trimester amniocentesis vs. chorionic villus sampling for prenatal diagnosis in multiple gestations. Ultrasound Obstet Gynecol. (2002) 20:476–81. doi: 10.1046/j.1469-0705.2002.00826.x
74. Akers, A, Al-Shahi Salman, R, Awad, IA, Dahlem, K, Flemming, K, Hart, B, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Clin Neurosurg. (2017) 80:665–80. doi: 10.1093/neuros/nyx091
75. Burkhardt, JK, Bozinov, O, Nürnberg, J, Shin, B, Woernle, CM, Ulrich, NH, et al. Neurosurgical considerations on highly eloquent brainstem cavernomas during pregnancy. Clin Neurol Neurosurg. (2012) 114:1172–6. doi: 10.1016/j.clineuro.2012.02.040
76. Cohen-Gadol, AA, Friedman, JA, Friedman, JD, Tubbs, RS, Munis, JR, and Meyer, FB. Neurosurgical management of intracranial lesions in the pregnant patient: a 36-year institutional experience and review of the literature - clinical article. J Neurosurg. (2009) 111:1150–7. doi: 10.3171/2009.3.JNS081160
77. Yamada, S, Nakase, H, Nakagawa, I, Nishimura, F, Motoyama, Y, and Park, YS. Cavernous malformations in pregnancy. Neurol Med Chir (Tokyo). (2013) 53:555–60. doi: 10.2176/nmc.53.555
78. Veiby, G, Engelsen, BA, and Gilhus, NE. Early child development and exposure to antiepileptic drugs prenatally and through breastfeeding: a prospective cohort study on children of women with epilepsy. JAMA Neurol. (2013) 70:1367–74. doi: 10.1001/jamaneurol.2013.4290
79. Meador, KJ, Baker, GA, Browning, N, Cohen, MJ, Bromley, RL, Clayton-Smith, J, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. (2014) 168:729–36. doi: 10.1001/jamapediatrics.2014.118
80. Kim, H, Flemming, KD, Nelson, JA, Lui, A, Majersik, JJ, Dela, CM, et al. Baseline characteristics of patients with cavernous angiomas with symptomatic hemorrhage in multisite trial readiness project. Stroke. (2021) 52:3829–38. doi: 10.1161/STROKEAHA.120.033487
81. Kumar, S, Lanzino, G, and Flemming, KD. Affected health domains in patients with brainstem cavernous malformations. Acta Neurochir. (2019) 161:2521–6. doi: 10.1007/s00701-019-04075-0
82. Herten, A, Chen, B, Saban, D, Santos, A, Wrede, K, Jabbarli, R, et al. Health-related quality of life in patients with untreated cavernous malformations of the central nervous system. Eur J Neurol. (2021) 28:491–9. doi: 10.1111/ene.14546
83. Bicalho, VC, Bergmann, A, Domingues, F, Frossard, JT, and De Souza, JPBM. Cerebral cavernous malformations: patient-reported outcome validates conservative management. Cerebrovasc Dis. (2017) 44:313. doi: 10.1159/000480125
Keywords: cavernous malformation, female, women, seizure, epilepsy, estrogen, progesterone, management
Citation: Bektas D, Lanzino G, Smith KM and Flemming KD (2024) Tailored management of cavernous malformations in women: considerations and strategies—a review. Front. Neurol. 15:1487808. doi: 10.3389/fneur.2024.1487808
Received: 28 August 2024; Accepted: 11 October 2024;
Published: 24 October 2024.
Edited by:
Maurizio Acampa, Siena University Hospital, ItalyReviewed by:
Jaesung Peter Choi, University of Technology Sydney, AustraliaCopyright © 2024 Bektas, Lanzino, Smith and Flemming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly D. Flemming, Flemming.Kelly@mayo.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.