- 1Henan University of Chinese Medicine, Zhengzhou, China
- 2Nanyang Hospital of Traditional Chinese Medicine, Nanyang, China
- 3Shenzhen Pingle Orthopedic Hospital (Shenzhen Pingshan Traditional Chinese Medicine Hospital), Shenzhen, China
In recent years, substantial advancements have been made in understanding the pathophysiology of ischemic stroke. Despite these developments, therapeutic options for cerebral ischemia remain limited due to stringent time windows and various contraindications. Consequently, there has been a concentrated effort to elucidate the underlying mechanisms of cerebral ischemic injury. Emerging research indicates that neutrophil extracellular traps (NETs) exacerbate inflammation and damage in ischemic brain tissue, contributing to neuronal cell death. The inhibition of NETs has shown potential in preventing thrombosis and the infiltration of immune cells. Central to the formation of NETs are P-selectin and its ligand, P-selectin glycoprotein ligand-1 (PSGL-1), which represent promising therapeutic targets. This review explores the detrimental impact of P-selectin, PSGL-1, and NETs on cerebral ischemia. Additionally, it delineates the processes by which P-selectin and PSGL-1 stimulate NETs production and provides evidence that blocking these molecules reduces NETs formation. This novel insight highlights a potential therapeutic avenue that warrants further investigation by researchers in the field.
1 Introduction
Stroke is the leading cause of mortality worldwide (1, 2) and significantly impacts the daily lives of survivors due to its high incidence of disability. Ischemic stroke, which accounts for approximately 87% of all stroke occurrences (3), is primarily caused by the obstruction of blood supply to the brain. This condition arises from a combination of etiological and related factors. Ischemia triggers a cascade of biochemical events which includes energy depletion, ionic imbalances and excitotoxicity, oxidative stress, cellular death, activation of the complement system, initiation of inflammatory and immune responses, induction of the expression of adhesion molecules on activated endothelial cells, resulting in the rolling of blood-borne inflammatory cells, adhesion and extravasation (4–12). This series of processes ultimately results in permanent damage to the brain (8, 13).
Over the past decade, substantial advancements have been made in understanding the pathophysiology of cerebral ischemia. Despite these advancements, therapeutic options for stroke remain insufficient (11). Pharmacologic thrombolysis or mechanical thrombectomy is recommended for only a small subset of stroke patients, primarily due to time constraints and other contraindications (14, 15). Furthermore, the outcomes of thrombolysis or thrombectomy procedures are not always favorable (16, 17). Consequently, there has been considerable scholarly focus on exploring the pathophysiological mechanisms underlying cerebral ischemic injury, as well as on developing interventions aimed at reducing the incidence of disability and mortality associated with ischemic stroke. Recent evidence suggests that the interplay between inflammation and thrombosis is critical in the pathogenesis of cerebral ischemic injury (18, 19). However, traditional anti-inflammatory and antithrombotic therapies do not adequately address these interactions. This underscores the necessity for the development of innovative therapeutic strategies that specifically target the inflammatory and thrombotic processes involved in cerebral ischemia (18).
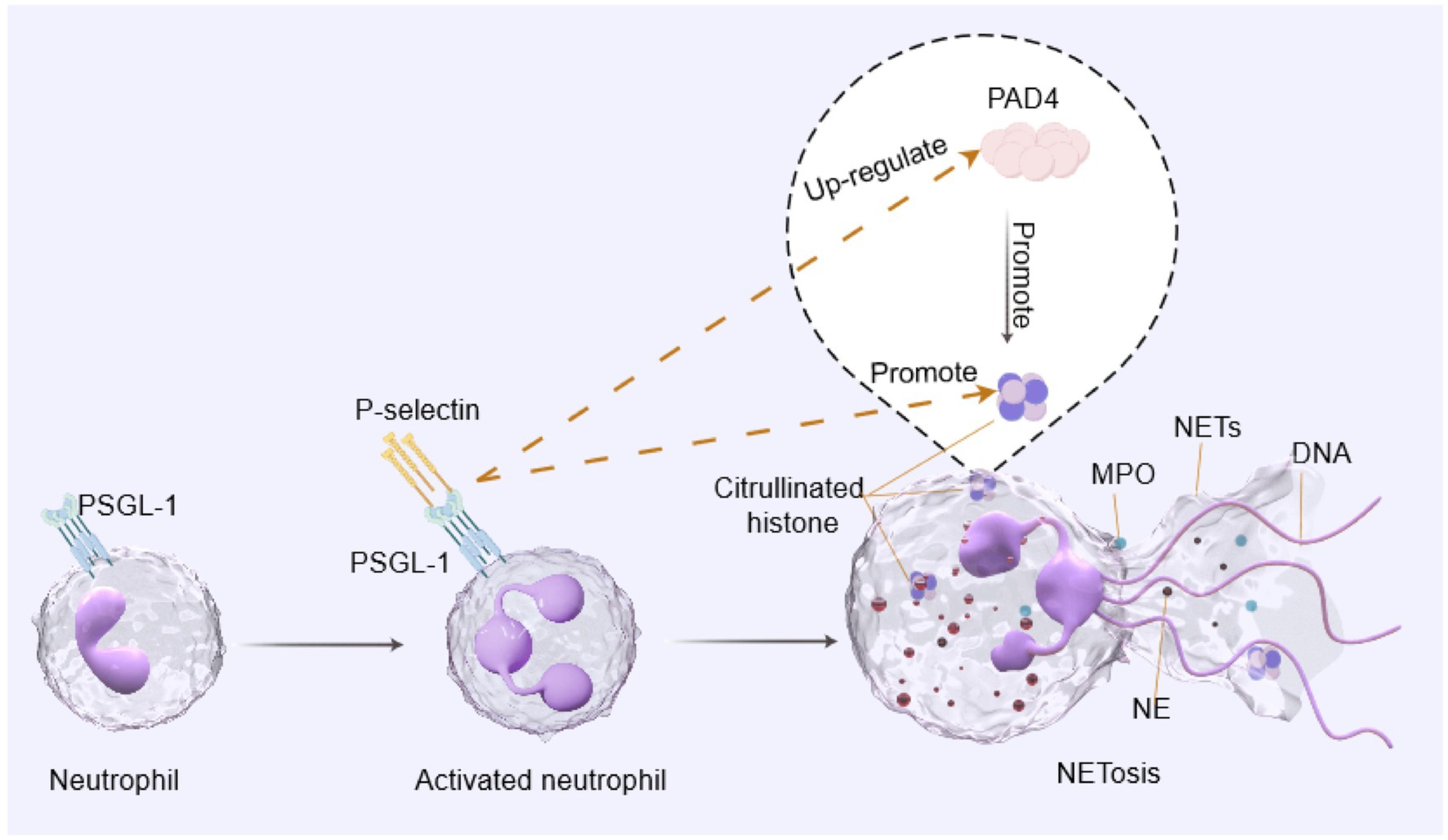
It is widely recognized that platelet activation plays a crucial role in the development and progression of thrombosis and inflammation in stroke. P-selectin (CD62p, Selp) is a highly sensitive and specific marker of platelet activation. It facilitates the adhesion functions of platelets, neutrophils, and endothelial cells, thereby initiating thrombosis and promoting inflammatory signaling (20). PSGL-1 serves as the primary ligand for P-selectin and is integral to its various functions (21). P-selectin and PSGL-1 playing pivotal roles in driving NETs formation (14, 22). Recent studies have demonstrated that the formation of neutrophil extracellular traps (NETs) exacerbates inflammation and thrombosis (22), which can have adverse long-term functional consequences after stroke in mice (23). A further study has shown that the presence of NETs in the brains of ischemic stroke patients and the inhibition of NETs represents a potential therapeutic strategy for ischemic stroke (24). Consequently, a more profound comprehension of the role of NETs in brain injury in ischemic stroke, along with the clarification of the underlying mechanisms, may facilitate the identification of novel and promising therapeutic targets. This review will explore how P-selectin and PSGL-1 promote NETs formation, leading to inflammation and thrombosis in patients with ischemic stroke.
2 P-selectin/PSGL-1 and NETs
2.1 P-selectin and PSGL-1
P-selectin, a 140 kDa granule membrane protein, is part of the selectin family of adhesion molecules (25). Its expression is markedly elevated in the venous blood of patients with progressive ischemic stroke and strongly correlates with the time of disease onset (26). P-selectin is expressed in platelets and stored on the membranes of alpha granules. Upon cell activation, P-selectin translocates to the platelet surface, playing a critical role in the adhesion of activated platelets and monocytes (27, 28). It facilitates leukocyte rolling on post-capillary microvessels, a prerequisite for subsequent leukocyte recruitment to sites of inflammation or infection (20). Consequently, a deficiency in P-selectin results in a delayed recruitment of leukocytes to sites of inflammation. Additionally, the secretion of CCL2 and IL-8 by monocytes critically depends on P-selectin, with CCL2 expression linked to stroke severity (1). CCL2 promotes monocyte mobilization from the bone marrow into the bloodstream by binding to CCR2, implicating the CCL2/CCR2 axis in monocyte recruitment into ischemic brain tissue (1, 29, 30). P-selectin regulates CCL2 expression to control monocyte migration (1).
PSGL-1, a 210 kDa dimeric glycoprotein, binds to P-, E-, and L-selectin (21, 31), facilitating neutrophil aggregation and rolling on endothelial surfaces (32). Its expression is upregulated during inflammation to enhance cell migration into inflamed tissues (21, 33, 34). Upon platelet activation, P-selectin is phosphorylated and translocated to the membrane, where it binds with PSGL-1 (1) (PSGL-1 acts as the primary receptor for P-selectin (35)). This interaction promotes neutrophil recruitment and creates a proinflammatory environment, exacerbating cerebral ischemic injury.
2.2 Neutrophil extracellular traps
Neutrophils constitute the primary host defence line against infection and participate in the earliest host defence responses during infection or injury. Neutrophils use various strategies to kill microbes, including phagocytosis, degranulation, production of reactive oxygen species (ROS), production of chemokines and cytokines, and the release of NETs to enhance their anti-microbial properties (36, 37). NETs were first discovered in 1996 (38), Brinkmann et al. (39–42) provide further details of the process and named it NETosis. The release of NETs depends on activation of the NADPH oxidase complex (NOX), which is initiated by the protein kinase C (PKC)-Raf/MERK/ERK cascade (40). PSGL-1 (the primary receptor for P-selectin) has the capacity to activate the ERK pathway (43), thereby facilitating the release of NETs. Furthermore, this complex triggers the activation of enzymes such as myeloperoxidase (MPO), neutrophil elastase (NE), and protein-arginine deaminase type 4 (PAD4) (44–47). PAD4 catalyses histones’ citrullination promoting chromatin depolymerisation and cell lysis. As a result, DNA, citrullinated histones (citH3) and other intracellular particles are released, forming NETs (48–51). A further study has indicated that reducing PAD4 and mitigating the release of NETs may be feasible by suppressing the ERK1/2 signaling pathway (52).
3 NETs as a novel mechanism of cerebral ischemic injury
3.1 NETs induce thromboinflammation
NETs have the capability to bind to microorganisms, leading to their death or immobilization (41, 53). Additionally, NETs can promote phagocytosis by other neutrophils and phagocytes, contributing to the innate immune response (48, 54, 55). NETs exhibit both beneficial and pathological effects (40, 42, 44, 48). While NETs are believed to primarily capture bacteria and pathogens to combat infections, they exhibit neurotoxicity when formed in the brain parenchyma (56, 57). Excessive NETosis can be detrimental, resulting in uncontrolled inflammatory responses and tissue lesions (40). This process directly causes cell damage and subsequently recruits proinflammatory cells and proteins, forms immune complexes, induces autoantibody production, and causes further tissue damage (40, 58, 59). Moreover, NETs formation can also be stimulated by factors such as cytokines (60) and activated platelets (39). The interaction between neutrophils and platelets leads to the generation of high mobility group protein B1 (HMGB1) (61), which is released by platelets and triggers NETs production. NETs are also thought to stimulate thrombosis (62–64) by cleaving clotting factors, activating platelets, and enhancing thrombin generation, which can result in decreased blood flow and worsen tissue ischemia. This process can cause organ damage by promoting thrombosis and vascular occlusion.
In the context of cerebral ischemia, a complex pathophysiological cascade is initiated, involving both thrombotic and inflammatory pathways, which act as key contributors to ischemic damage (65). Thrombotic and inflammatory processes are highly intertwined factors contributing to cerebral vessel occlusion and stroke, which, in turn, elicits local and systemic inflammatory responses (66). Consequently, novel therapeutic options within the thromboinflammatory field are currently emerging (65). A recent study has demonstrated that post-stroke thromboinflammation may be mediated by pyruvate kinase muscle 2 (PKM2). NETosis exacerbates inflammation and associated damage in ischemic brain tissue (67), and triggers neuronal death (68). Inhibition of NETosis in MCAO animals using PAD inhibitors markedly decreased the infiltration of immune cells and vascular damage (14, 69). In addition, NETs dissolution via DNase substantially decreases BBB injury, augments the coverage of microvascular cells, and improves the formation of new functional blood vessels, consequently reducing thrombosis during ischemic stroke (67, 69, 70). These results indicate that NETs may have a deleterious impact on ischemic stroke. Therefore, Denorme and colleagues concluded that innovative therapeutic interventions targeting the formation of NETs, hold promise as safe treatments for ischemic stroke and should be further investigated (14).
3.2 P-selectin/PSGL-1 regulates NETs as a novel mechanism of cerebral ischemic injury
When adhering to leukocytes, activated platelets prompt leukocytes to undergo inflammatory processes (71). Research indicates that activated platelets can induce the formation of neutrophil extracellular traps (NETs) even in the absence of infection, potentially presenting high mobility group box 1 (HMGB1) to neutrophils to facilitate this process (72, 73). HMGB1 plays a critical role in enhancing the production of P-selectin (74), which is essential for the activation of platelets to induce NETs formation (22). P-selectin activates leukocytes through signaling via PSGL-1 (22, 75), promoting the expression of PAD4 and activating the histone citrullination pathway (14, 76), thereby triggering NETosis (22).
In vitro (77), P-selectin activates inflammation-related genes in neutrophils via PSGL-1, and the signaling of PSGL-1 promotes the formation of NETs. In vivo experiments showed (78) that Antiphospholipid Syndrome (APS) IgG significantly increased thrombosis in WT mice (which did not have PSGL-1 knocked out), while it had no significant impact on PSGL-1 knockouts. Furthermore, the thrombotic phenotype was restored in PSGL-1-deficient mice following the infusion of WT neutrophils, the anti-PSGL-1 monoclonal antibody also inhibited APSIgG-induced thrombosis in WT mice. Further research has demonstrated that the suppression of PSGL-1 leads to a decrease in plasma-based NETs biomarkers, such as myeloperoxidase-DNA, in acute lung inflammation and sepsis animal models (22, 79). Blocking neutrophil PSGL-1 entirely suppressed citrullination of histone H3, as induced by activated platelets. Citrullination of histone is believed to be the most reliable biochemical marker of NETs (80). Thus, these findings propose that the interaction between P-selectin and PSGL-1 activates signals for histone citrullination. This evidence indicates that P-selectin and PSGL-1 have potential as a therapeutic approach to inhibit the formation of NETs and to reduce pathological thrombosis and inflammation (22, 78).
4 Conclusion and future directions
In recent years, a growing number of researchers have investigated the role and mechanisms of NETs in disease. NETs have been detected in numerous organ tissues and inflammatory diseases. The approach to managing different ailments is increasingly focused on regulating NETs as a therapeutic objective. The pathogenesis and treatment strategies of neuroinflammation and thrombosis in cerebral ischemia are important areas of research. However, the molecular mechanisms underlying these conditions are not yet fully comprehended by researchers.
Following an episode of cerebral ischemia, it is possible that NETs act as the initial trigger for neuroinflammation and thrombosis. This process is regulated by P-selectin/PSGL-1 and presents a potentially effective therapeutic target for the treatment of cerebral ischemia, using techniques for inhibiting P-selectin or PSGL-1. Nevertheless, the evidence from a murine model of lupus indicates that PSGL-1 deficiency is associated with a reduction in stroke size. However, this is accompanied by an exacerbation of nephritis (81). In light of this limitation, further studies are necessary to assess the mode of action of these inhibitory methods and their impact on the immune system, to select effective treatments devoid of harmful effects.
Our team has long been dedicated to investigating treatments for cerebral ischemic injury (82, 83). Whole transcriptome gene sequencing studies have revealed that numerous inflammatory factors, chemokines, and selectins exhibit differential expression in the brain tissue of MCAO rats compared to control rats. Notably, electroacupuncture treatment significantly modulated the expression of these differentially expressed genes in the brain tissue of MCAO rats (82). Our next objective is to elucidate the biological processes by which P-selectin/PSGL-1 regulates NETs and to conduct a comprehensive investigation into the role and mechanisms of electroacupuncture in mitigating cerebral ischemic damage. We anticipate that in the future, targeting P-selectin/PSGL-1 or NETs will have broad applicability in the treatment of cerebral ischemia.
Author contributions
XL: Conceptualization, Writing – original draft. YM: Writing – review & editing. DW: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The graphical abstract was created using Figdraw, for which we would like to express our gratitude.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bai, M, Sun, R, Cao, B, Feng, J, and Wang, J. Monocyte-related cytokines/chemokines in cerebral ischemic stroke. CNS Neurosci Ther. (2023) 29:3693–712. doi: 10.1111/cns.14368
2. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
3. Tsao, CW, Aday, AW, Almarzooq, ZI, Anderson, CAM, Arora, P, Avery, CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
4. Karaszewski, B, Wardlaw, JM, Marshall, I, Cvoro, V, Wartolowska, K, Haga, K, et al. Early brain temperature elevation and anaerobic metabolism in human acute ischaemic stroke. Brain. (2009) 132:955–64. doi: 10.1093/brain/awp010
5. Castillo, J, Loza, MI, Mirelman, D, Brea, J, Blanco, M, Sobrino, T, et al. A novel mechanism of neuroprotection: blood glutamate grabber. J Cereb Blood Flow Metab. (2016) 36:292–301. doi: 10.1177/0271678X15606721
6. Du, S, Wang, H, Li, J, Huang, W, Jiang, X, Cui, E, et al. Design and synthesis of 9-phenanthranilamide derivatives and the study of anti-inflammatory, antioxidant and neuroprotective activities. Bioorg Chem. (2023) 141:106861. doi: 10.1016/j.bioorg.2023.106861
7. Peerschke, EI, Yin, W, and Ghebrehiwet, B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. (2010) 47:2170–5. doi: 10.1016/j.molimm.2010.05.009
8. Li, C, Sun, T, and Jiang, C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm Sin B. (2021) 11:1767–88. doi: 10.1016/j.apsb.2020.11.019
9. Brown, GC, and Neher, JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. (2014) 15:209–16. doi: 10.1038/nrn3710
10. Winneberger, J, Schöls, S, Lessmann, K, Rández-Garbayo, J, Bauer, AT, Mohamud Yusuf, A, et al. Platelet endothelial cell adhesion molecule-1 is a gatekeeper of neutrophil transendothelial migration in ischemic stroke. Brain Behav Immun. (2021) 93:277–87. doi: 10.1016/j.bbi.2020.12.026
11. Yu, H, Luo, H, Chang, L, Wang, S, Geng, X, Kang, L, et al. The NEDD8-activating enzyme inhibitor MLN4924 reduces ischemic brain injury in mice. Proc Natl Acad Sci U S A. (2022) 119:e2111896119. doi: 10.1073/pnas.2111896119
12. Gelderblom, M, Leypoldt, F, Steinbach, K, Behrens, D, Choe, CU, Siler, DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. (2009) 40:1849–57. doi: 10.1161/STROKEAHA.108.534503
13. Iadecola, C, and Anrather, J. The immunology of stroke: from mechanisms to translation. Nat Med. (2011) 17:796–808. doi: 10.1038/nm.2399
14. Denorme, F, Rustad, JL, and Campbell, RA. Brothers in arms: platelets and neutrophils in ischemic stroke. Curr Opin Hematol. (2021) 28:301–7. doi: 10.1097/MOH.0000000000000665
15. Vanacker, P, Lambrou, D, Eskandari, A, Mosimann, PJ, Maghraoui, A, and Michel, P. Eligibility and predictors for acute revascularization procedures in a stroke center. Stroke. (2016) 47:1844–9. doi: 10.1161/STROKEAHA.115.012577
16. Badhiwala, JH, Nassiri, F, Alhazzani, W, Selim, MH, Farrokhyar, F, Spears, J, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA. (2015) 314:1832–43. doi: 10.1001/jama.2015.13767
17. González, RG, Furie, KL, Goldmacher, GV, Smith, WS, Kamalian, S, Payabvash, S, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke. (2013) 44:3109–13. doi: 10.1161/STROKEAHA.113.001938
18. Stoll, G, and Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke – implications for treatment. Nat Rev Neurol. (2019) 15:473–81. doi: 10.1038/s41582-019-0221-1
19. Dhanesha, N, Patel, RB, Doddapattar, P, Ghatge, M, Flora, GD, Jain, M, et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. (2022) 139:1234–45. doi: 10.1182/blood.2021012322
20. Ludwig, RJ, Schön, MP, and Boehncke, WH. P-selectin: a common therapeutic target for cardiovascular disorders, inflammation and tumour metastasis. Expert Opin Ther Targets. (2007) 11:1103–17. doi: 10.1517/14728222.11.8.1103
21. Fu, Y, He, S, Waheed, AA, Dabbagh, D, Zhou, Z, Trinité, B, et al. PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells. Proc Natl Acad Sci U S A. (2020) 117:9537–45. doi: 10.1073/pnas.1916054117
22. Etulain, J, Martinod, K, Wong, SL, Cifuni, SM, Schattner, M, and Wagner, DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. (2015) 126:242–6. doi: 10.1182/blood-2015-01-624023
23. Patel, RB, Dhanesha, N, Sutariya, B, Ghatge, M, Doddapattar, P, Barbhuyan, T, et al. Targeting neutrophil α9 improves functional outcomes after stroke in mice with obesity-induced hyperglycemia. Stroke. (2023) 54:2409–19. doi: 10.1161/STROKEAHA.123.042714
24. Denorme, F, Portier, I, Rustad, JL, Cody, MJ, de Araujo, CV, Hoki, C, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. (2022) 132:132 (10). doi: 10.1172/JCI154225
25. Dunlop, LC, Skinner, MP, Bendall, LJ, Favaloro, EJ, Castaldi, PA, Gorman, JJ, et al. Characterization of GMP-140 (P-selectin) as a circulating plasma protein. J Exp Med. (1992) 175:1147–50. doi: 10.1084/jem.175.4.1147
26. Wang, Q, Zhao, W, and Bai, S. Association between plasma soluble P-selectin elements and progressive ischemic stroke. Exp Ther Med. (2013) 5:1427–33. doi: 10.3892/etm.2013.985
27. Liu, Y, Fu, Y, Wang, Q, Li, M, Zhou, Z, Dabbagh, D, et al. Proteomic profiling of HIV-1 infection of human CD4(+) T cells identifies PSGL-1 as an HIV restriction factor. Nat Microbiol. (2019) 4:813–25. doi: 10.1038/s41564-019-0372-2
28. Tinoco, R, Carrette, F, Barraza, ML, Otero, DC, Magaña, J, Bosenberg, MW, et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. (2016) 44:1190–203. doi: 10.1016/j.immuni.2016.04.015
29. Yang, J, Zhang, L, Yu, C, Yang, XF, and Wang, H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. (2014) 2:1. doi: 10.1186/2050-7771-2-1
30. Schuette-Nuetgen, K, Strecker, JK, Minnerup, J, Ringelstein, EB, and Schilling, M. MCP-1/CCR-2-double-deficiency severely impairs the migration of hematogenous inflammatory cells following transient cerebral ischemia in mice. Exp Neurol. (2012) 233:849–58. doi: 10.1016/j.expneurol.2011.12.011
31. Laszik, Z, Jansen, PJ, Cummings, RD, Tedder, TF, McEver, RP, and Moore, KL. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. (1996) 88:3010–21. doi: 10.1182/blood.V88.8.3010.bloodjournal8883010
32. Stadtmann, A, Germena, G, Block, H, Boras, M, Rossaint, J, Sundd, P, et al. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J Exp Med. (2013) 210:2171–80. doi: 10.1084/jem.20130664
33. Nishimura, Y, Shimojima, M, Tano, Y, Miyamura, T, Wakita, T, and Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat Med. (2009) 15:794–7. doi: 10.1038/nm.1961
34. Somers, WS, Tang, J, Shaw, GD, and Camphausen, RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. (2000) 103:467–79. doi: 10.1016/S0092-8674(00)00138-0
35. Abadier, M, and Ley, K. P-selectin glycoprotein ligand-1 in T cells. Curr Opin Hematol. (2017) 24:265–73. doi: 10.1097/MOH.0000000000000331
36. Tan, C, Aziz, M, and Wang, P. The vitals of NETs. J Leukoc Biol. (2021) 110:797–808. doi: 10.1002/JLB.3RU0620-375R
37. Kaplan, MJ, and Radic, M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. (2012) 189:2689–95. doi: 10.4049/jimmunol.1201719
38. Takei, H, Araki, A, Watanabe, H, Ichinose, A, and Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. (1996) 59:229–40. doi: 10.1002/jlb.59.2.229
39. Clark, SR, Ma, AC, Tavener, SA, McDonald, B, Goodarzi, Z, Kelly, MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. (2007) 13:463–9. doi: 10.1038/nm1565
40. Mutua, V, and Gershwin, LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. (2021) 61:194–211. doi: 10.1007/s12016-020-08804-7
41. Brinkmann, V, and Zychlinsky, A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. (2007) 5:577–82. doi: 10.1038/nrmicro1710
42. Brinkmann, V, and Zychlinsky, A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. (2012) 198:773–83. doi: 10.1083/jcb.201203170
43. Spertini, C, Baïsse, B, and Spertini, O. Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases. J Biol Chem. (2012) 287:10693–702. doi: 10.1074/jbc.M111.318022
44. Papayannopoulos, V, Metzler, KD, Hakkim, A, and Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. (2010) 191:677–91. doi: 10.1083/jcb.201006052
45. Hawez, A, Al-Haidari, A, Madhi, R, Rahman, M, and Thorlacius, H. MiR-155 regulates PAD4-dependent formation of neutrophil extracellular traps. Front Immunol. (2019) 10:2462. doi: 10.3389/fimmu.2019.02462
46. Smith, CK, Vivekanandan-Giri, A, Tang, C, Knight, JS, Mathew, A, Padilla, RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. (2014) 66:2532–44. doi: 10.1002/art.38703
47. Parker, H, and Winterbourn, CC. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front Immunol. (2012) 3:424. doi: 10.3389/fimmu.2012.00424
48. Brinkmann, V, Reichard, U, Goosmann, C, Fauler, B, Uhlemann, Y, Weiss, DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
49. Reeves, EP, Lu, H, Jacobs, HL, Messina, CG, Bolsover, S, Gabella, G, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. (2002) 416:291–7. doi: 10.1038/416291a
50. Lewis, HD, Liddle, J, Coote, JE, Atkinson, SJ, Barker, MD, Bax, BD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. (2015) 11:189–91. doi: 10.1038/nchembio.1735
51. Martinod, K, Demers, M, Fuchs, TA, Wong, SL, Brill, A, Gallant, M, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. (2013) 110:8674–9. doi: 10.1073/pnas.1301059110
52. Ma, H, Yao, W, Peng, B, Liu, X, Chen, J, Lin, Y, et al. Mercury-containing preparations attenuate neutrophil extracellular trap formation in mice and humans through inhibiting the ERK1/2 pathway. J Ethnopharmacol. (2024) 321:117421. doi: 10.1016/j.jep.2023.117421
53. Branzk, N, Lubojemska, A, Hardison, SE, Wang, Q, Gutierrez, MG, Brown, GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. (2014) 15:1017–25. doi: 10.1038/ni.2987
54. Fuchs, TA, Abed, U, Goosmann, C, Hurwitz, R, Schulze, I, Wahn, V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. (2007) 176:231–41. doi: 10.1083/jcb.200606027
55. Wang, Y, Li, M, Stadler, S, Correll, S, Li, P, Wang, D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. (2009) 184:205–13. doi: 10.1083/jcb.200806072
56. Allen, C, Thornton, P, Denes, A, McColl, BW, Pierozynski, A, Monestier, M, et al. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol. (2012) 189:381–92. doi: 10.4049/jimmunol.1200409
57. Euler, M, and Hoffmann, MH. The double-edged role of neutrophil extracellular traps in inflammation. Biochem Soc Trans. (2019) 47:1921–30. doi: 10.1042/BST20190629
58. Petretto, A, Bruschi, M, Pratesi, F, Croia, C, Candiano, G, Ghiggeri, G, et al. Neutrophil extracellular traps (NET) induced by different stimuli: a comparative proteomic analysis. PLoS One. (2019) 14:e0218946. doi: 10.1371/journal.pone.0218946
59. Bruschi, M, Bonanni, A, Petretto, A, Vaglio, A, Pratesi, F, Santucci, L, et al. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J Rheumatol. (2020) 47:377–86. doi: 10.3899/jrheum.181232
60. Gupta, AK, Hasler, P, Holzgreve, W, Gebhardt, S, and Hahn, S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. (2005) 66:1146–54. doi: 10.1016/j.humimm.2005.11.003
61. Vogel, S, Bodenstein, R, Chen, Q, Feil, S, Feil, R, Rheinlaender, J, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. (2015) 125:4638–54. doi: 10.1172/JCI81660
62. Fuchs, TA, Brill, A, Duerschmied, D, Schatzberg, D, Monestier, M, Myers, DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. (2010) 107:15880–5. doi: 10.1073/pnas.1005743107
63. von Brühl, ML, Stark, K, Steinhart, A, Chandraratne, S, Konrad, I, Lorenz, M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. (2012) 209:819–35. doi: 10.1084/jem.20112322
64. Gould, TJ, Vu, TT, Swystun, LL, Dwivedi, DJ, Mai, SH, Weitz, JI, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. (2014) 34:1977–84. doi: 10.1161/ATVBAHA.114.304114
65. Szepanowski, RD, Haupeltshofer, S, Vonhof, SE, Frank, B, Kleinschnitz, C, and Casas, AI. Thromboinflammatory challenges in stroke pathophysiology. Semin Immunopathol. (2023) 45:389–410. doi: 10.1007/s00281-023-00994-4
66. Kehrel, BE, and Fender, AC. Resolving thromboinflammation in the brain after ischemic stroke? Circulation. (2016) 133:2128–31. doi: 10.1161/CIRCULATIONAHA.116.022858
67. Kang, L, Yu, H, Yang, X, Zhu, Y, Bai, X, Wang, R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. (2020) 11:2488. doi: 10.1038/s41467-020-16191-y
68. Kim, SW, Lee, H, Lee, HK, Kim, ID, and Lee, JK. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. (2019) 7:94. doi: 10.1186/s40478-019-0747-x
69. Chen, R, Zhang, X, Gu, L, Zhu, H, Zhong, Y, Ye, Y, et al. New insight into neutrophils: a potential therapeutic target for cerebral ischemia. Front Immunol. (2021) 12:692061. doi: 10.3389/fimmu.2021.692061
70. De Meyer, SF, Suidan, GL, Fuchs, TA, Monestier, M, and Wagner, DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. (2012) 32:1884–91. doi: 10.1161/ATVBAHA.112.250993
71. Wagner, DD, and Frenette, PS. The vessel wall and its interactions. Blood. (2008) 111:5271–81. doi: 10.1182/blood-2008-01-078204
72. Caudrillier, A, Kessenbrock, K, Gilliss, BM, Nguyen, JX, Marques, MB, Monestier, M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. (2012) 122:2661–71. doi: 10.1172/JCI61303
73. Maugeri, N, Campana, L, Gavina, M, Covino, C, De Metrio, M, Panciroli, C, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost. (2014) 12:2074–88. doi: 10.1111/jth.12710
74. Luo, Y, Li, SJ, Yang, J, Qiu, YZ, and Chen, FP. HMGB1 induces an inflammatory response in endothelial cells via the RAGE-dependent endoplasmic reticulum stress pathway. Biochem Biophys Res Commun. (2013) 438:732–8. doi: 10.1016/j.bbrc.2013.07.098
75. Jurk, K, and Kehrel, BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. (2005) 31:381–92. doi: 10.1055/s-2005-916671
76. Xu, Q, Shi, M, Ding, L, Xia, Y, Luo, L, Lu, X, et al. High expression of P-selectin induces neutrophil extracellular traps via the PSGL-1/Syk/Ca2+/PAD4 pathway to exacerbate acute pancreatitis. Front Immunol. (2023) 14:1265344. doi: 10.3389/fimmu.2023.1265344
77. Yago, T, Liu, Z, Ahamed, J, and McEver, RP. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood. (2018) 132:1426–37. doi: 10.1182/blood-2018-05-850859
78. Knight, JS, Meng, H, Coit, P, Yalavarthi, S, Sule, G, Gandhi, AA, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight. (2017) 2:e93897. doi: 10.1172/jci.insight.93897
79. Sreeramkumar, V, Adrover, JM, Ballesteros, I, Cuartero, MI, Rossaint, J, Bilbao, I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. (2014) 346:1234–8. doi: 10.1126/science.1256478
80. Zhu, D, Zhang, Y, and Wang, S. Histone citrullination: a new target for tumors. Mol Cancer. (2021) 20:90. doi: 10.1186/s12943-021-01373-z
81. Wang, H, Knight, JS, Hodgin, JB, Wang, J, Guo, C, Kleiman, K, et al. Psgl-1 deficiency is protective against stroke in a murine model of lupus. Sci Rep. (2016) 6:28997. doi: 10.1038/srep28997
82. Li, X. Brain protection mechanism of electroacupuncture at Shuigou acupoint on cerebral ischemia rats based on high-throughput sequencing technology and inflammation regulation. Shanghai University of Traditional Chinese Medicine (2022).
Keywords: neutrophil extracellular traps, NETs, P-selectin, PSGL-1, cerebral ischemic
Citation: Li X, Ma Y and Wang D (2024) The role of P-selectin/PSGL-1 in regulating NETs as a novel mechanism in cerebral ischemic injury. Front. Neurol. 15:1442613. doi: 10.3389/fneur.2024.1442613
Edited by:
Eduardo Candelario-Jalil, University of Florida, United StatesReviewed by:
Rakesh B. Patel, The University of Iowa, United StatesCopyright © 2024 Li, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongbin Wang, NTcwNjMwMDA5QHFxLmNvbQ==
 Xiao Li
Xiao Li Yamin Ma2
Yamin Ma2