
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 11 November 2024
Sec. Headache and Neurogenic Pain
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1430288
Pathological neuropathic pain is a common complication following spinal cord injury. Due to its high incidence, prolonged duration, tenacity, and limited therapeutic efficacy, it has garnered increasing attention from both basic researchers and clinicians. The pathogenesis of neuropathic pain after spinal cord injury is multifaceted, involving factors such as structural and functional alterations of the central nervous system, pain signal transduction, and inflammatory effects, posing significant challenges to clinical management. Currently, drugs commonly employed in treating spinal cord injury induced neuropathic pain include analgesics, anticonvulsants, antidepressants, and antiepileptics. However, a subset of patients often experiences suboptimal therapeutic responses or severe adverse reactions. Therefore, emerging treatments are emphasizing a combination of pharmacological and non-pharmacological approaches to enhance neuropathic pain management. We provide a comprehensive review of past literature, which aims to aim both the mechanisms and clinical interventions for pathological neuropathic pain following spinal cord injury, offering novel insights for basic science research and clinical practice in spinal cord injury treatment.
Traumatic Spinal cord injury (SCI) is a prevalent critical clinical condition, with an estimated incidence ranging from 15 to 40 cases per million, attributed to various factors such as traffic accidents, falls from high altitudes, and violent impacts (1, 2). Patients afflicted with spinal cord impairment often experience subsequent sensory and motor dysfunctions, leading to a cascade of complications including secondary pulmonary infections, deep venous thrombosis, urinary retention, pressure ulcers, pain, and psychological distress (3). Neuropathic pain (NP) stands out as one of the most prevalent complications following SCI, affecting approximately 53 to 80% of individuals (4). This condition manifests as spontaneous pain, paresthesia, or hyperalgesia below the level of injury, predominantly in the lower limbs, with pain intensity typically higher at night than during the day (5, 6). Spontaneous pain can be further categorized into continuous and intermittent forms; the former is characterized by sensations of burning or squeezing, while the latter presents as electric shock-like pain or tingling sensations. Hyperalgesia involves heightened responses to noxious stimuli, while allodynia refers to various abnormally evoked responses to innocuous stimuli (7, 8). These symptoms significantly diminish patients’ quality of life and impose substantial personal and socioeconomic burdens (4, 9, 10). Moreover, NP can precipitate anxiety disorders, depression, substance abuse, and other mental health conditions, and in severe cases, suicidal ideation (11, 12).
The pathogenesis of NP following SCI is intricate and arises from a combination of multiple pathological reactions (13). The development and maintenance of pain involve the peripheral nervous system, the spinal cord, and the higher central nervous system structures above the spinal cord. Due to the complex pathogenesis of neuralgia after SCI, its treatment has always posed a significant challenge in clinical practice. Currently, drugs primarily used for NP treatment are opioid analgesics, anticonvulsants (14), such as Tramadol (15), gabapentin (16, 17), pregabalin (18), lamotrigine (19), and amitriptyline (20), which aim to alleviate pain. However, many patients remain dissatisfied with pain relief (21). Simultaneously, these medications are associated with numerous side effects, including fever, nausea, dizziness, rash, weakness, drowsiness, and other psychiatric disorders (14, 21, 22). Additionally, various other therapeutic strategies exist, such as natural compounds (23, 24), electroacupuncture stimulation (25), repetitive transcranial magnetic stimulation therapy (26), transcranial direct current stimulation (27), and spinal cord stimulation (28). In this review, we discuss and analyze the mechanisms and therapeutic measures of NP after SCI in hopes of aiding for better treatment.
NP following SCI is characterized by alterations in normal sensory signals at various levels, including peripheral structures, the spinal cord, and supraspinal regions. These changes occur over weeks or months, leading to an amplification of nociceptive information and resulting in central sensitization of pain perception. NP following SCI is generally characterized by widespread and multifaceted sensory loss and/or chronic pain (29, 30). Pain was observed not only at the site of the injury but also in regions below the level of the injury, and it did not diminish over time. The underlying mechanisms are complex. To elucidate the pathophysiology of neuropathic pain following SCI, this section will be divided into three parts: the peripheral nervous system, the spinal cord itself, and the supraspinal structures. Figure 1 illustrates the schematic representation of the pathophysiology of NP following SCI.
Current mechanistic studies on NP after SCI have primarily focused on neuronal changes within pain pathways at both spinal and supraspinal levels, particularly those associated with inflammation and glial activation. However, chronic hyperexcitability also occurs in primary nociceptors following SCI, leading to altered function and spontaneous activity of peripheral receptors throughout the neural pathway (31). Neuronal alterations in spinal and supraspinal pain pathways affect central processes in primary sensory neurons, triggering hyperexcitable states and spontaneous activity in nociceptors, which consequently drive hypersensitivity and pain (31). Increasing preclinical evidence indicates that mitigating heightened activity in primary sensory neurons by selectively targeting receptors in peripheral nervous system effectively alleviates peripheral nervous system pain (32). Ritter et al. (33) reported on the downregulation of Kv3.4 potassium channels in dorsal root ganglion neurons post-SCI, highlighting its dysregulation in SCI-NP models. This downregulation contributes to the hyperexcitability of nociceptors, forming the basis for persistent pain after SCI. Yang et al. (34) showed that downregulation of Nav1.8 channels was able to reduce SCI-induced spontaneous nociceptor activity, thereby further reducing SCI-NP. Two weeks post-cervical hemicontusion injury, contralateral nociceptor hyperexcitability was observed, attributed to diminished Kv3.4 channel membrane expression (35). In 2017, the group elucidated in a novel publication that spinal cord injury (SCI)-induced Kv3.4 channel dysfunction is mediated through the inhibition of the calcineurin phosphatase (36).
After a SCI, numerous pathological changes occur within the spinal cord, leading to a heightened focus on NP pathogenesis research. Key areas of study include alterations in neuronal excitability (37), glial cell activation (38, 39), upregulation of calcium channel expression (7, 40, 41), immune-inflammatory responses (42), disrupted neurotransmitter secretion (43), imbalances in neurotrophic factors (44), and the roles of non-coding RNAs (2).
Neuronal hyperactivity, characterized by heightened spontaneous excitability or abnormal increases in neuronal activity in response to thermal, chemical, and mechanical stimuli, is a notable phenomenon. In the context of SCI, ischemia and upregulated neurotrophic factor levels contribute to the structural atrophy of the spinal cord and synaptic circuit alterations. These changes induce spontaneous and secondary hyperexcitability in spinal cord sensory neurons, leading to a decreased threshold potential, enlarged receptive fields, and heightened nociception to similar stimuli (37, 45–47). The hallmark of this state is the shift of the resting membrane potential toward less negative values, potentially precipitating spontaneous depolarization and the subsequent activation of neurons, a phenomenon termed “central sensitization.”
Glial cells, including astrocytes, microglia, and oligodendrocytes, are widespread in the nervous system. SCI triggers the activation of astrocytes and microglia, resulting in a notable increase in cell size and the thickening of their processes (48). This activation leads to the release of bioactive substances and cytokines, which heighten the sensitivity of spinal dorsal horn nerves to pain sensation and contribute to the maintenance of pathological pain (38, 39, 49, 50). The time course of astrocyte activation appears to coincide with both the transition from acute to chronic pain and the maintenance phase of chronic pain (51). Astrocyte activation following SCI is dependent on the secretion of several proteins, including connexin 43, calbindin S100B, and aquaporin-4. In numerous NP models, there is a notable increase in the number of gap junction channels and an upregulation of connexin 43 (Cx43) expression (52). This upregulation contributes to the secretion of various cytokines involved in NP development. Cx43 is predominantly expressed in astrocytes, and gap junctions formed by Cx43 play a critical role in the pathogenesis of NP following SCI. Post-injury, there is a substantial increase in gap junction channels both between neurons and glial cells, as well as among glial cells themselves. Additionally, glial cells exhibit enhanced sensitivity to pain mediators such as ATP. Prolonged exposure to ATP leads to the release of a cascade of cytokines from these cells, which further activates glial cells and exacerbates neuronal damage (52). Research indicates that Cx43 is rapidly and persistently upregulated in astrocytes following SCI. Furthermore, the use of Cx43 inhibitors has been linked to relief from NP (52, 53). S100β, a calcium-binding protein of the EF-hand family, is predominantly expressed by astrocytes within the central nervous system. Its production and secretion are markedly elevated in reactive astrocytes. At high extracellular concentrations, S100β exhibits neurotoxic effects and its secretion into the extracellular space further stimulates astrocyte activation, leading to S100β autocrine signaling (54). Inhibiting S100β production by astrocytes may provide an effective strategy to suppress astrocyte activation. In a rat model of incomplete SCI, the administration of an S100β inhibitor was found to alleviate NP following SCI. Histological analyses support the conclusion that inhibiting S100β production and astrocytic activation contributes to the reduction of NP. Moreover, there is a strong correlation between the intensity of S100β expression at the injury sites and the severity of NP (55). Aquaporins are a family of small, integral membrane proteins, with Aquaporin-4 (AQP-4) being a prominent member and one of the most extensively studied aquaporins (56). Evidence from both in vitro and in vivo studies suggests that AQP-4 plays a critical role in astrocyte activation. In wound healing and scratch assays, impaired migration was observed in astrocyte cultures derived from AQP-4 deficient mice compared to wild-type mice, with no significant differences noted among the wild-type samples (57). Auguste et al. employed an adult mouse astrocyte migration assay to investigate the involvement of AQP-4 in astrocyte migration in vivo. Their findings revealed that AQP-4 +/+ astrocytes exhibited preferential migration toward the wound site, whereas AQP-4 −/− astrocytes demonstrated significantly reduced migratory ability (56, 58). Additionally, Yu et al. utilized a rat T13 spinal cord hemi-section model and found that AQP-4 expression was significantly upregulated in in L4/5 spinal cord segments, primarily in astrocytes within the spinal dorsal horn (SDH).
Microglia play both deleterious and protective roles in NP following SCI (59). Activation of microglia results in the release of proinflammatory cytokines and chemokines that enhance neuronal reactivity, thereby exacerbating pain (60–62). The formation of a glial scar is a typical response to SCI. This scar comprises astrocytic processes and a significant quantity of glial fibers. Although the mechanical strength of a glial scar is inferior to that of a collagenous scar, it plays a crucial role in providing structural support to the injured tissue and in preventing further damage (63). However, glial scars can also contribute to the emergence of NP, with microglial activation considered necessary for glial scarring (64–66). Additionally, microglia have functions such as removing cell debris, tissue repair, promoting the release of anti-inflammatory cytokines, and aiding axonal regeneration, which can have a positive impact on pathological NP after SCI (67–69). The role of oligodendrocytes in the pathogenesis of NP has been less explored, but given their abundance as a type of glia, their role cannot be disregarded. Many multiple sclerosis patients experience pain alongside oligodendrocyte loss, suggesting a potential interaction between oligodendrocyte damage and human pain (70). Furthermore, studies have shown that oligodendrocyte ablation using diphtheria toxin in adult mice leads to the development of NP behaviors (71). As research on oligodendrocytes continues, the involvement of activated glial cells in pain transmission after SCI will be further elucidated.
Voltage-gated calcium channels depolarize the cell membrane, allowing calcium to enter the cell. This process has various physiological effects, including secretion, contraction, neurotransmission, and gene expression (72). The alpha-2-delta-1 (Cavaα2δ-1) subunit protein plays a crucial role in the functional assembly of voltage-gated calcium channels, regulating calcium channel current density and synaptogenesis (73–75). Experimental evidence has shown that excessive up-regulation of the calcium channel Cavaα2δ-1 subunit protein in the spinal dorsal horn leads to the development of tactile allodynia in Peripheral nerve injury model (76). Conversely, blocking the up-regulation of the Cavaα2δ-1 subunit protein, such as through knockdown of Cavaα2δ-1, Cavaα2δ-1 antisense oligodeoxynucleotide, or drugs inhibiting Cavaα2δ-1 subunit protein, can prevent and treat the development of pathological NP (7, 77, 78).
The immune-inflammatory responses triggered by SCI play a crucial role in the development of NP. Pro-inflammatory factors exacerbate local tissue damage, while anti-inflammatory factors aid in repair, depending on the timing and activation state of immune cells (79–82). After SCI, the permeability of the blood-spinal cord barrier (BSCB) increases, allowing macrophages, granulocytes, and lymphocytes from the bloodstream to infiltrate the injured spinal cord (83). These cells, along with other inflammatory factors, induce inflammatory responses in the tissues surrounding the injury site, leading to neuronal apoptosis, scarring, and dysfunction of peripheral nerves within the injured spinal cord (84–87). In the context of SCI, M1 macrophages exert detrimental effects on nerves, whereas M2 macrophages promote neuronal and axonal regeneration and mitigate local inflammatory responses (88, 89). Following SCI, M1 macrophages are rapidly and persistently upregulated, while M2 macrophages are expressed more slowly and transiently. The imbalance in M1/M2 macrophage expression results in a robust inflammatory response that contributes to pain induction (90).
Moreover, the migration of T and B lymphocytes to the injury site after SCI initiates a multifaceted adaptive immune response. This response is characterized by increased expression of pro-inflammatory cytokines such as IL-1α, IL-1β, and TNF-α, which transmit noxious information to the central nervous system and exacerbate secondary injury in patients with acute or chronic SCI (91). These pro-inflammatory factors may sensitize injured spinal dorsal horn neurons by promoting the release of excitatory amino acids and substance P, thereby inducing pain (92, 93).
Serotonin (5-HT) is an endogenous neurotransmitter widely distributed throughout the nervous system, exerting dual effects of both causing and relieving pain (94, 95). While 5-HT1 and 5-HT2 receptor subtypes generally have analgesic effects, models of persistent pain suggest that activation of the 5-HT3 receptor plays a role in sustaining pain (94). In the central nervous system, serotonergic neurons are primarily located in the raphe nucleus of the brainstem and play a crucial role in the descending analgesic system; intrathecal injection of 5-HT demonstrates a significant analgesic effect after SCI (96). Following SCI, 5-HT within the peripheral nervous system can transmit nociceptive signals either directly through second messengers or indirectly by modulating ion channel activity (97). Glutamate (Glu) in the central nervous system is implicated in the transmission of nociceptive information and neurotoxicity, closely associated with the onset of pain following SCI (98). Gamma-aminobutyric acid (GABA) serves as an inhibitory neurotransmitter, whose expression decreases post-SCI, consequently disrupting the “GABA-glutamate-glutamine cycle” in vivo (99, 100). Studies have shown that the administration of GABA receptor agonists significantly alleviates pain in rats with SCI (99). Substance P, a neuropeptide closely linked to pain, also participates in the regulation of NP following SCI. Inhibiting the release of substance P delays the onset of NP by 1 to 4 days (101). Additionally, substance P-fragment 1–7, a metabolite of substance P, demonstrates significant pain-alleviating effects when administered peripherally to alleviate SCI-induced pain (102).
The neurotrophic family includes neurotrophin-3 (NT-3), ciliary neurotrophic factor (CNTF), basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF), glial cell-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF). These factors are crucial for promoting the survival, proliferation, and axonal regeneration of various cell types following SCI. Neurotrophin-3 (NT3) is a potent neurotrophic factor that plays a crucial role in neuronal regeneration and functional recovery following SCI. Besides sustaining the survival of sympathetic neurons, sensory neurons, basal forebrain cholinergic neurons, and motor neurons, NT3 also supports the differentiation of dopaminergic neurons and promotes the sprouting of lateral branches of the corticospinal tract (CST) (103). However, spinal cord function markedly improves with elevated NT3 concentrations (104). Ciliary neurotrophic factor (CNTF), a polypeptide, activates signaling cascades including JAK/STAT, MAPK, ERK1/2, AMPK, mTOR, and AKT via its receptors (105–108). CNTF exerts pivotal roles in neuronal development and nervous system homeostasis by enhancing survival and differentiation in sensory, sympathetic, and motor neurons through the modulation of gene expression (109). Fibroblast growth factors (FGFs) are classified into acidic FGF (aFGF) and basic FGF (bFGF) based on their isoelectric properties. Both aFGF (acidic Fgf1) and bFGF can enhance the regeneration of spinal cord and dorsal root ganglion neurons in both human and animal models following SCI (110–112). aFGF exhibits strong neurotrophic effects and promotes neuronal growth (113). The mechanism of action of bFGF includes inhibiting apoptosis and c-fos gene expression at the injury site, stabilizing calcium and magnesium ion levels to prevent toxicity, regulating glial cell responses, and reducing glial scar formation (103). Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor beta (TGF-β) superfamily, exhibits strong neurotrophic effects on motor, sensory, and dopaminergic neurons. It has been shown to effectively stimulate axonal regeneration and promote myelin repair in central nervous system (CNS) injuries (114). Transplantation of lentivirus-mediated GDNF-secreting cells into the SCI site significantly increased nerve fiber density at the injury site and improved motor function recovery (115). The truncated isoform of the tyrosine receptor kinase (BTrkB), a primary receptor for brain-derived neurotrophic factor (BDNF), mediates BDNF signaling through various classical pathways (44, 116, 117). BDNF binds to the tropomyosin receptor kinase (Trk) B receptor, promoting the development, differentiation, and regeneration of sensory neurons, cholinergic neurons, dopaminergic neurons, and GABAergic neurons (114, 118). Additionally, BDNF facilitates myelination, regulates synaptic plasticity, and influences synaptic transmission (119). Notably, BDNF, as a member of the neurotrophic family, also modulates NP following SCI (44, 120, 121). Post-SCI upregulation of BDNF in the spinal dorsal horn is implicated in hyperalgesia and tactile allodynia, with TrkB-specific knockdown in mice markedly attenuating pain responses (44).
Noncoding RNAs (ncRNAs) typically do not encode proteins but rather regulate protein expression and numerous cellular, biochemical, and physiological processes. They are generally classified into micro RNAs (miRNAs), circular RNAs (circRNAs), and long ncRNAs (lncRNAs) (2). MiRNAs, composed of approximately 22 nucleotides, are involved in secondary injury and repair processes following SCI. For example, reduced expression of miRNA-139-5p in the spinal cord of mice post-SCI was associated with pain hypersensitivity, whereas intrathecal administration of miRNA-139-5p agonist mitigated pain hypersensitivity, enhanced survival of damaged spinal cord neurons, and promoted motor function recovery (122). Moreover, miRNA-139-5p was found to reduce pain sensitivity and facilitate functional recovery in SCI mice by targeting 20-like kinase 1 in mammalian sterile lines (122). Additionally, intrathecal injection of miRNA-132-3p mimics in rats mimicked hyperalgesia attributed to spinal α-aminomethyloxazolopropionate receptor 1 expression, implicating miRNA-132-3p in pain information processing (123). In a study utilizing peripheral blood samples from SCI patients for sequencing, two lncRNAs (LINC01119 and LINC02447) were directly implicated in the NP pathway (124). Zhao et al. (125) discovered a conserved lncRNA, Kcna2 antisense RNA, correlated with Kcna2 in rat DRG sensory neurons, which is significantly upregulated by peripheral nerve injury, resulting in Kcna2 repression and neuropathic pain onset. Conversely, inhibition of Kcna2 antisense RNA expression effectively reverses the neural injury-induced downregulation of Kcna2 in the DRG, thereby mitigating both the development and maintenance of neuropathic pain. This suggests a potential significant role for these lncRNAs as biomarkers in SCI-induced NP pathways. Circular RNAs, a type of abundant lncRNAs characterized by a closed continuous loop structure, exhibit high stability. These circRNAs, shown to be neuron-specific, modulate miRNA expression during NP pathology (2). The mechanisms and roles of non-coding RNAs in regulating NP after SCI are still in early stages and necessitate further in-depth investigation in the future.
The spinal cord, as a central component of the nervous system, not only causes localized pathological changes after injury but also transmits pain signals through its ascending pathways to the brainstem and thalamus, eventually projecting to the cerebral cortex. Following SCI, bidirectional signal transduction between the spinal cord and cerebral cortex is disrupted. This destruction of sensory and motor conduction pathways leads to plastic changes in the structure of the cerebral cortex. Such remodeling affects not only sensory and motor functions but also the regulation of nociceptive information. In the early stages of SCI, atrophy occurs in the primary sensory cortex (S1) and primary motor cortex (M1), with the degree of atrophy correlating positively with the severity of the injury (126). Functional magnetic resonance imaging (fMRI) studies have confirmed dynamic reorganization in the sensory and motor cortices following SCI in patients with complete cervical SCI, revealing decreased functional connectivity between these regions and highlighting the plastic changes in the brain after SCI (127). Patients experiencing pain post-SCI exhibit reduced gray matter volume in the paracentral lobule of the S1 region. Incomplete sensory afferent and efferent information may lead to maladaptive remodeling of the S1 region, which can contribute to pain development. Additionally, a robust correlation exists between the extent of S1 region reorganization post-SCI and the severity of persistent neuropathic pain (128). The subcortical thalamus, serving as a relay station for sensory information, receives various types of sensory input (excluding olfactory information) from across the body. It plays a crucial role in sensing and regulating nociceptive information and is considered a key site for endogenous pain modulation (129). Seminowicz et al. (130) demonstrated that, 7 days after SCI, functional connectivity between the ventral posterolateral nucleus (VPL) and the S1 region of the thalamus decreased, while connectivity between the S1 region and other nociceptive processing cortical areas (such as the insula and anterior cingulate cortex) increased. By day 14 post-injury, connectivity between the VPL and the contralateral thalamus had increased. The temporal correlation between the enhanced functional connectivity within thalamic and cortical regions and the development of mechanical hyperalgesia in SCI rats suggests that abnormal pain perception after SCI may result from dysregulation in functional connectivity between the thalamus and pain perception cortical regions.
Metabolic changes in the brain after SCI also significantly impact NP. Magnetic resonance spectroscopy (MRS) can detect alterations in brain metabolism in NP patients following SCI, providing further insight into pain mechanisms. Studies have found changes in cingulate metabolism in NP patients post-SCI, including decreased levels of N-acetylaspartate (NAA) and γ-aminobutyric acid (GABA), and increased levels of inositol (Ins) (131, 132). These metabolic changes correlate with pain severity, with the NAA/Ins and glutamate (Glu)/Ins ratios significantly affected. Additionally, GABA aggregation has been associated with changes in functional connectivity, with thalamic GABA content negatively correlating with connectivity in thalamocortical tracts. Specifically, greater loss of thalamic GABA corresponds to closer connections between the VPL and other brain regions such as S1, S2, and the Insular lobe (131).
Clinically, various drug treatments are available for NP following SCI. However, some patients may experience poor therapeutic outcomes or serious adverse reactions (21, 133). Anticonvulsants are commonly utilized in clinical practice, with gabapentin drugs (pregabalin, gabapentin) being particularly prevalent. These drugs do not directly act on GABA receptors but enhance inhibitory neuronal activity through interactions with N-type voltage-gated calcium channels or indirectly on NMDA receptors. Additionally, they reduce glutamate release and inhibit nociceptive information transmission in chronic constriction injury model (134), but have not been validated in SCI models. Numerous studies have demonstrated the pain-relieving effects of pregabalin and gabapentin in SCI-induced NP, with common adverse effects including mild to moderate transient drowsiness, dizziness, and edema (133). Amitriptyline, a commonly used tricyclic antidepressant in clinical practice, has demonstrated some analgesic efficacy for NP following SCI in randomized controlled trials (135). Tricyclic antidepressants exert their effects through multiple mechanisms, primarily by inhibiting the reuptake of norepinephrine and serotonin, thus inhibiting nociceptive information transmission (136, 137). Common side effects of amitriptyline at an average maximum daily dose of 50 mg include dry mouth, drowsiness, fatigue, constipation, increased cramps, urinary retention, and sweating (133, 138). Anticonvulsants like lamotrigine reduce neuronal hyperexcitability by inhibiting voltage-sensitive sodium channels (Nav) and inhibiting the pathological release of glutamate (19). However, lamotrigine is indicated only for NP caused by incomplete SCI and has side effects such as vertigo, nausea, visual impairment, and rash (19, 139). Opioid analgesics are also clinically utilized to manage neuralgia post-spinal cord injury. Tramadol’s analgesic effect results from weak opioid-like mechanisms and monoaminergic actions, as well as a synergistic effect of the two. A randomized, double-blind, controlled study demonstrated that tramadol reduced pain intensity scores and injury severity in 35 SCI patients after 4 weeks of treatment, significantly alleviating pain below the injury level and improving sleep, although it did not significantly improve depression (15). Therefore, the pursuit of newer, more effective, and safer treatment modalities for post-SCI neuralgia continues.
The development of a novel drug entails a substantial investment of time and financial resources (140, 141). Therefore, natural compounds have garnered attention in the treatment of various diseases in recent years due to their minimal side effects and cost-effectiveness (142). Han et al. developed a rat model of thoracic SCI to demonstrate that intrathecal administration of resveratrol effectively alleviated pain post-SCI in rats, possibly by inhibiting neuroinflammation via the JAK2/STAT3 signaling pathway in the lumbar spinal dorsal horns (143). Additionally, several other natural compounds, including salidroside, betulinic acid, and quercetin, have shown promising results in the function recovery of post-spinal cord injury, but more evidence is needed to confirm these role in treating neuropathic pain (144–146).
Antisense oligonucleotides represent a class of drugs designed to modulate expression levels by targeting both coding and non-coding RNAs, showing considerable potential across diverse fields including genetic diseases, cancer, neurodegenerative diseases, and NP (147). These oligonucleotides exhibit specificity based on their sequence, thereby offering the capability to target virtually any known RNA sequence. Their customizable sequences and chemistry render them highly versatile and adaptable (147). This therapeutic approach has garnered approval from the US Food and Drug Administration (FDA) for clinical treatment (148). However, the necessity for high-quality randomized controlled clinical trials persists to validate its efficacy and assess associated risks in future applications. Pharmacotherapy remains an important integral part of the treatment of SCI-induced NP due to its high compliance and relatively small financial burden (Figure 2).
Acupuncture, a traditional Chinese medicine treatment method with over three thousand years of practical experience, has been utilized in the management of various diseases (149). Electroacupuncture (EA) is a specific acupuncture technique that has demonstrated efficacy in treating numerous SCI-related conditions, such as dyskinesia, NP, and spasticity (150). However, the precise mechanism underlying electroacupuncture’s effectiveness in alleviating NP remains unclear and warrants further investigation. Liu et al. explore the effect that electroacupuncture treatment in SCI rats activating miR-214,which targeted pain related protein Nav1.3, may reduce NP after SCI Given its non-invasive nature, safety profile, affordability, and minimal side effects, electroacupuncture holds significant promise in managing NP post-spinal cord injury.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive neuromodulation technique that utilizes electromagnetic coils to generate magnetic fields, forming pulse stimuli in the cerebral cortex and even deep brain regions, thereby regulating cortical excitability and promoting functional remodeling (151). In published studies, the most common stimulation site for NP following SCI with rTMS is the M1 region, and the most commonly used dose is 5–10 Hz, which has been shown to produce the best analgesic effects after 5–10 treatments (152). In a randomized double-blind controlled study of rTMS for acute NP (pain duration ≤3 weeks) after SCI, 48 patients were randomly divided into a high-frequency rTMS group (rTMS stimulation frequency of 10 Hz, stimulation site of M1 contralateral to the affected hand, once a day, for a total of 18 sessions) and a sham stimulation group. No analgesic drugs (such as gabapentin, pregabalin, etc.) were taken during the treatment period, and the results showed that the high-frequency rTMS group had significantly reduced pain after treatment compared with the sham stimulation group (153). Other studies have also investigated the effects of 10 Hz rTMS over the dorsolateral left prefrontal cortex (DLPFC) region (once a day for a total of 10 treatments over a 2-week period) on NP following SCI (154). Results showed a significant decrease in daily pain scores during stimulation in the high-frequency rTMS group but not in the sham group. Although the target areas stimulated in the rTMS studies were different, they all resulted in significant pain reduction for the patients. Regarding the mechanism of action, transcranial magnetic stimulation seem reduce pro-inflammatory cytokines, such as IL-1b, IL-6, and TNF-a, while increasing anti-inflammatory cytokines, including IL-10 and brain-derived neurotrophic factor (BDNF), in cortical and subcortical tissues (152); additional in vitro and in vivo studies and clinical trials are required to investigate these mechanism. This inhibits neuroinflammation after SCI, consequently reducing NP.
Transcranial direct current stimulation (tDCS) is a non-invasive technique that employs a constant, low-intensity direct current (typically ranging from 1 to 2 mA) to modulate neuronal activity in the cerebral cortex (155). Research has explored the application of tDCS, specifically with a single current intensity of 2 mA, on NP patients following SCI. Assessment was conducted after 20 min of continuous treatment, revealing significantly lower pain scores in the tDCS group compared to the sham stimulation group immediately post-treatment and 24 h later. This suggests that the analgesic effect of single tDCS extends beyond the stimulation period, manifesting as an aftereffect well beyond the duration of stimulation (156). In a randomized controlled trial, the efficacy of tDCS on NP post-SCI was evaluated in two treatment phases separated by a 3-month interval. In the initial stage, 33 patients were randomly assigned to either the tDCS group (n = 16) or the sham stimulation group (n = 17). The treatment involved administering tDCS at a 2 mA DC intensity, once every 20 min, once daily, for a total of 5 days. In the subsequent phase (comprising 9 patients in total, 6 in the tDCS group and 3 in the sham stimulation group), a further 10 tDCS sessions (one session per day lasting 20 min) were administered. Results demonstrated a significant reduction in pain scores in the tDCS group compared to the sham group at the 1-week follow-up in the first phase and at the 4-week follow-up in the second phase (157). The most widely proposed mechanism underlying the effectiveness of tDCS for NP following SCI involves the modulation of spontaneous cortical neuronal activity through polarized resting membranes. This modulation affects various pain-related structures, including the anterior cingulate gyrus and periaqueductal gray, ultimately modulating the affective components of pain perception and experience (27). In summary, both rTMS and tDCS have demonstrated analgesic effects on NP following SCI. However, limited clinical data and studies exist, necessitating further research to elucidate their effects and mechanisms of action.
Spinal cord stimulation (SCS) was initially proposed based on Melzack and Wall’s gating theory, which posits the existence of a “gate action” mechanism within the spinal cord and brain’s pain conduction pathways. According to this theory, afferent impulses from Aδ and C fibers open the gate, allowing pain signals to be transmitted to the central nervous system and produce the sensation of pain. Conversely, when SCS stimulates Aβ fibers, the gate closes, resulting in retrograde inhibition of nociceptive signals entering the spinal cord, thus achieving analgesia (158). Subsequent research has revealed that SCS not only activates brainstem nuclei and the rostral ventromedial medulla oblongata but also modulates nociceptive signals at the spinal cord level through descending fiber projections from supraspinal cell regions (159). Furthermore, SCS can activate the frontal gyrus, limbic system, and thalamus via the pain ascending conduction pathway, thereby exerting analgesic effects and improving cognitive function (160). Spinal cord electrical stimulation has been widely utilized for various refractory pain conditions. This procedure involves identifying the corresponding spinal cord segments of pain under local anesthesia and implanting electrodes into the spinal epidural space to deliver pulse currents and stimulate the spinal cord nerves (28). The post-operative spinal cord electrical stimulation typically occurs in two phases: a test phase and a permanent implant phase. During the test phase, a trial system is implanted for approximately one week to assess its effectiveness. If there is a 50% or greater improvement in pain baseline values, the permanent implant system is then implanted (161). SCS has been found to be more effective in improving pain in patients with incomplete SCI compared to those with complete SCI. This efficacy may be influenced by factors such as the distance between the injury site and the implanted SCS electrode, as well as the number of residual intact nerve fibers (28). With advancements in technology, various new modes of SCS have emerged. One such mode is burst SCS, which is characterized by low-energy delivery. Burst SCS applies five continuous wave pulse sequences at specific internal frequencies (500 Hz) and pulse widths (1 ms, with intervals of 1 ms), occurring 40 times per second. Burst SCS has been shown to effectively inhibit pain below the level of injury in patients with complete paraplegia. A case report demonstrates a significant reduction in the frequency and intensity of pain, with therapeutic effects lasting over three months (162). However, extensive clinical trials are still required to substantiate these findings. The other is high-frequency SCS therapy, which provides electrical stimulation pulses of short duration (30 μs) and high frequency (10,000 Hz) without paresthesia compared with traditional spinal cord stimulation. A sham-controlled study showed that both high-frequency SCS and burst SCS could reduce levels of SCI-related NP (163). In summary, for patients with NP after spinal cord injury, non-pharmacological treatment has different characteristics (Figure 3), but all of them have great potential in reducing pain and improving quality of life.
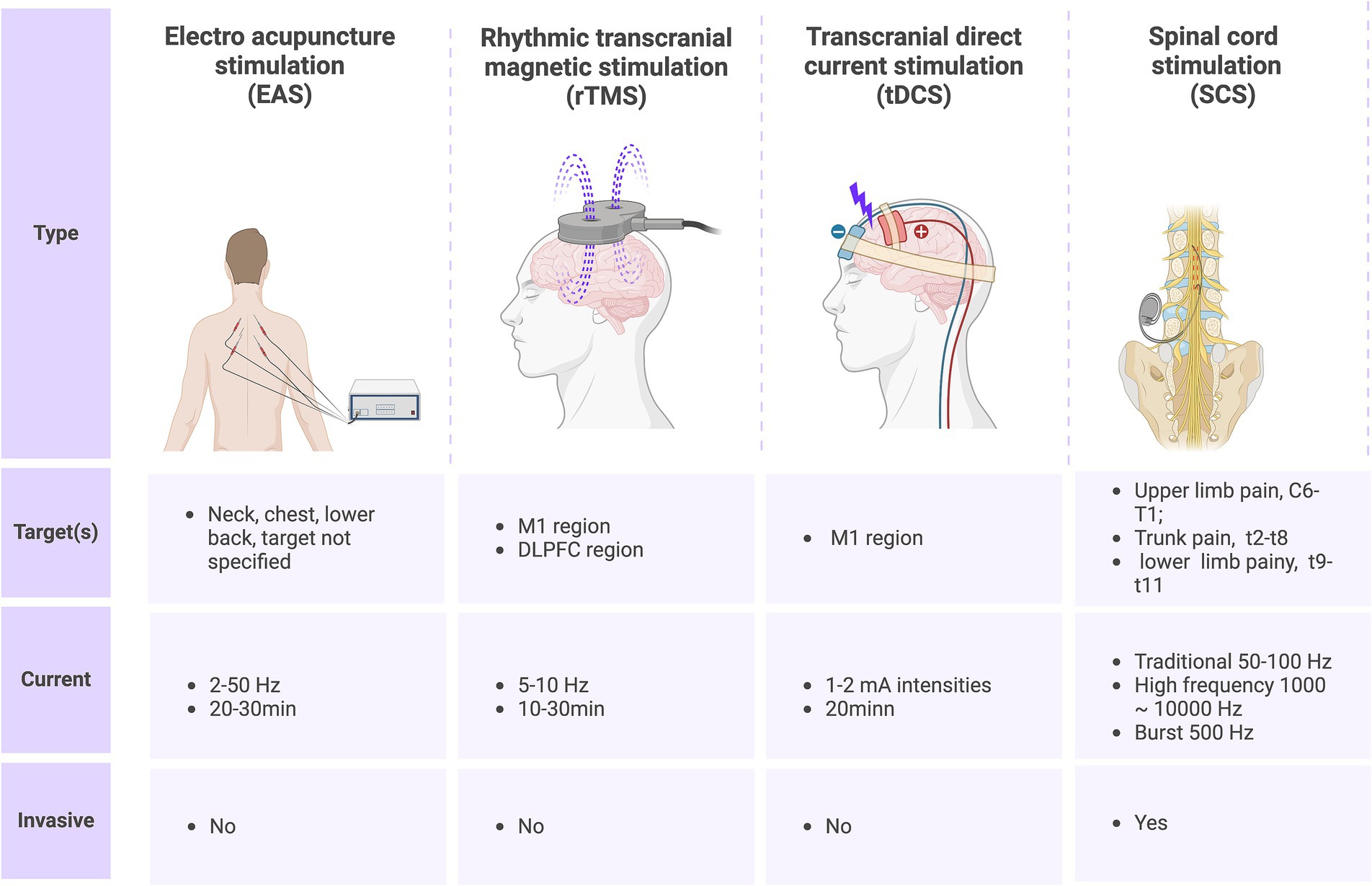
Figure 3. Current nonpharmacological treatments for SCI-induced neuropathic pain and their characteristics.
Despite the variety of treatments available for SCI, current options remain insufficient to fully address the associated clinical problems, and many patients continue to experience persistent pain. Recent research has increasingly focused on the role of gut microbiota in SCI. Chen et al. analyzed the relationship between gut microbiota, inflammatory markers in serum, and pain behavior parameters. Their findings revealed a significant increase in the abundance of Helicobacter pylori, Bacillus spp., Streptococcus spp., Roche spp., and Lactobacillus spp., while genera such as Ignacillus spp., Butyric monas spp., and Escherichia spp. showed reduced abundance. Another study highlighted that oral antibiotics could induce changes in gut microbiota that mitigate the development of NP, accompanied by improvements in inflammatory parameters. This suggests that gut microbiota might influence the development and progression of NP, potentially through the modulation of pro-inflammatory and anti-inflammatory T cells (164). These findings could offer new avenues for studying NP following SCI. Additionally, NP after SCI is often linked with psychological factors such as fatigue, anxiety, and depression. In this context, mental imagery (MI) therapy has garnered increasing interest. Research indicates that MI can effectively alleviate NP in SCI patients (165). This method is noted for its simplicity, safety, and reproducibility; however, its efficacy can vary among patients. While most studies report that MI significantly relieves NP after SCI (166, 167), some research suggests that MI may be less effective or even exacerbate pain in certain cases (168, 169). These discrepancies may stem from differences in SCI severity, timing, and location of injury, or variations in MI treatment protocols. Therefore, further investigation is necessary to clarify its efficacy and underlying mechanisms. Nanomedicine represents an emerging field within nanotechnology, characterized by unique biological properties such as a high surface-to-volume ratio, distinctive structural attributes, capacity for surface modification, ability to permeate biological barriers, and extended circulation time in the bloodstream (170). The utilization of nanomedicines can significantly enhance the pharmacokinetic and pharmacodynamic profiles of drugs, enable prolonged release, and achieve targeted delivery to specific sites through labeling with selective ligands. In the realm of cell therapy, nanomedicine plays a pivotal role by efficiently guiding cell differentiation and trans differentiation, and mitigating immunogenic responses associated with cell therapy through the encapsulation of various proteins and polymers. In gene therapy, nanomaterials serve as proficient vectors for delivering different genes, thereby addressing the limitations inherent in both viral and non-viral vectors (171). Hence, nanomedicine enables targeted drug delivery for neuropathic pain (NP) following spinal cord injury (SCI), enhancing therapeutic efficacy and minimizing side effects. Furthermore, it facilitates the orientation of reparative cells, biological factors, and genes, thereby advancing spinal cord regeneration and alleviating pain. Nanomaterials offer a novel and promising vehicle for the treatment of NP following SCI, demonstrating substantial potential for future development. In clinical practice, it is essential to differentiate patients based on factors such as symptomatology, pain levels, complications, gender, and age. Tailoring treatment plans to these individual differences, and employing a combination of therapeutic approaches, may lead to optimal outcomes.
Neuropathic pain is a prevalent complication of SCI, described by patients as an intolerable condition that significantly impacts their long-term quality of life. It emerges as a consequence of neuronal remodeling subsequent to SCI, representing a pathological response triggered by neurological injury induced by local mechanical compression, ischemia, and inflammation of the spinal cord. The pathogenic mechanisms underlying NP following SCI are intricate, with most studies focusing on rodent models. This review comprehensively summarizes current research on the mechanisms underlying spinal cord injury (SCI)-related neuropathic pain (NP). At the peripheral level, it discusses nociceptor hyperexcitability and spontaneous activity. At the spinal cord level, it addresses changes in neuronal excitability, glial cell activation, calcium channel expression, immune-inflammatory responses, neurotransmitter secretion disorders, neurotrophic factor imbalances, and the role of non-coding RNAs. Furthermore, at the supraspinal level, it explores structural plasticity changes in brain regions and alterations in brain metabolism.
In clinical practice, the primary treatments for SCI-related NP typically involve analgesic, anticonvulsant, antidepressant, and antispasmodic medications. However, their effectiveness is often limited, and they may elicit adverse reactions that do not fully address the needs of patients. Emerging therapies for SCI-NP include novel drugs such as natural compounds and antisense oligonucleotides, which offer advantages such as minimal side effects, easy access to raw materials, and adaptability. Nonetheless, their established therapeutic efficacy requires validation through additional clinical trials.
Electroacupuncture, a therapeutic modality in traditional Chinese medicine, plays a significant role in the management of SCI-NP. Particularly beneficial for patients unable to undergo conventional medical treatment, electroacupuncture offers fewer side effects and enhanced therapeutic outcomes. Furthermore, it can be integrated with other treatments to potentially reduce dosage requirements and achieve a synergistic therapeutic effect.
Both rTMS and tDCS are non-invasive brain stimulation techniques that have shown promise in reducing NP following SCI, thereby improving patients’ quality of life. However, the lack of a definitive and standardized treatment regimen for either rTMS or tDCS, along with the heterogeneity of existing studies in terms of intervention cycles and outcome measures, complicates comparisons between interventions and anticipated outcomes. This lack of consensus in clinical application results in variable treatment outcomes. While spinal cord stimulation holds promise for treating NP after SCI, research in this area is still in its developmental stages. Practical limitations such as cost, intervention protocols, treatment frequency, and technical variability, as well as ethical considerations regarding the use of sham interventions, hinder its widespread adoption. Further research is warranted to establish its precise efficacy. In cases where patients exhibit poor responsiveness to conventional and non-invasive treatments, early consideration of spinal cord stimulation intervention may be beneficial in delaying pain progression and improving overall symptoms and quality of life.
In conclusion, the intricate mechanism underlying NP after SCI underscores the need for individualized treatment plans tailored to each patient’s unique condition. Optimal outcomes are likely to be achieved through a combination of various treatment modalities.
JL: Investigation, Writing – original draft. WK: Writing – review & editing. XW: Supervision, Writing – review & editing. FP: Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Johansson, E, Luoto, TM, Vainionpää, A, Kauppila, A-M, Kallinen, M, Väärälä, E, et al. Epidemiology of traumatic spinal cord injury in Finland. Spinal Cord. (2020) 59:761–8. doi: 10.1038/s41393-020-00575-4
2. Mazzone, GL, Coronel, MF, Mladinic, M, and Samano, C. An update to pain management after spinal cord injury: from pharmacology to circRNAs. Rev Neurosci. (2023) 34:599–611. doi: 10.1515/revneuro-2022-0089
3. Ge, L, Arul, K, Ikpeze, T, Baldwin, A, Nickels, JL, and Mesfin, A. Traumatic and nontraumatic spinal cord injuries. World Neurosurg. (2018) 111:e142–8. doi: 10.1016/j.wneu.2017.12.008
4. Burke, D, Fullen, BM, Stokes, D, and Lennon, O. Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur J Pain. (2016) 21:29–44. doi: 10.1002/ejp.905
5. Finnerup, NB. Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord. (2017) 55:1046–50. doi: 10.1038/sc.2017.70
6. Celik, EC, Erhan, B, and Lakse, E. The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord. (2012) 50:585–9. doi: 10.1038/sc.2012.26
7. Boroujerdi, A, Zeng, J, Sharp, K, Kim, D, Steward, O, and Luo, DZ. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain. (2011) 152:649–55. doi: 10.1016/j.pain.2010.12.014
8. Mills, CD, Johnson, KM, and Hulsebosch, CE. Group I metabotropic glutamate receptors in spinal cord injury: roles in neuroprotection and the development of chronic central pain. J Neurotrauma. (2002) 19:23–42. doi: 10.1089/089771502753460213
9. Finnerup, NB. Pain in patients with spinal cord injury. Pain. (2013) 154:S71–6. doi: 10.1016/j.pain.2012.12.007
10. Chi, B, Chau, B, Yeo, E, and Ta, P. Virtual reality for spinal cord injury-associated neuropathic pain: systematic review. Ann Phys Rehabil Med. (2019) 62:49–57. doi: 10.1016/j.rehab.2018.09.006
11. Cairns, DM, Adkins, RH, and Scott, MD. Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain? Arch Phys Med Rehabil. (1996) 77:329–35. doi: 10.1016/S0003-9993(96)90079-9
12. Finnerup, NB, Haroutounian, S, Kamerman, P, Baron, R, Bennett, DLH, Bouhassira, D, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. (2016) 157:1599–606. doi: 10.1097/j.pain.0000000000000492
13. Colloca, L, Ludman, T, Bouhassira, D, Baron, R, Dickenson, AH, Yarnitsky, D, et al. Neuropathic pain. Nat Rev Dis Prim. (2017) 3:17002. doi: 10.1038/nrdp.2017.2
14. Attal, N. Spinal cord injury pain. Rev Neurol (Paris). (2021) 177:606–12. doi: 10.1016/j.neurol.2020.07.003
15. Norrbrink, C, and Lundeberg, T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. (2009) 25:177–84. doi: 10.1097/AJP.0b013e31818a744d
16. Guy, S, Mehta, S, Leff, L, Teasell, R, and Loh, E. Anticonvulsant medication use for the management of pain following spinal cord injury: systematic review and effectiveness analysis. Spinal Cord. (2014) 52:89–96. doi: 10.1038/sc.2013.146
17. Tai, Q, Kirshblum, S, Chen, B, Millis, S, Johnston, M, and DeLisa, JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. (2016) 25:100–5. doi: 10.1080/10790268.2002.11753609
18. Dalal, KL, Felix, ER, and Cardenas, DD. Pregabalin for the management of neuropathic pain in spinal cord injury. Pain Manag. (2013) 3:359–67. doi: 10.2217/pmt.13.35
19. Finnerup, NB, Sindrup, SH, Bach, FW, Johannesen, IL, and Jensen, TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. (2002) 96:375–83. doi: 10.1016/S0304-3959(01)00484-5
20. Rintala, DH, Holmes, SA, Courtade, D, Fiess, RN, Tastard, LV, and Loubser, PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. (2007) 88:1547–60. doi: 10.1016/j.apmr.2007.07.038
21. Warms, CA, Turner, JA, Marshall, HM, and Cardenas, DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. (2002) 18:154–63. doi: 10.1097/00002508-200205000-00004
22. Rowbotham, MC, Duan, RW, Thomas, J, Nothaft, W, and Backonja, M-M. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. (2009) 146:245–52. doi: 10.1016/j.pain.2009.06.013
23. Tian, M-M, Li, Y-X, Liu, S, Zhu, C-H, Lan, X-B, Du, J, et al. Glycosides for peripheral neuropathic pain: a potential medicinal components. Molecules. (2022) 27:255. doi: 10.3390/molecules27010255
24. Badshah, I, Qazi, NG, Ali, F, Minhas, AM, Alvi, AM, Kandeel, M, et al. Emodin alleviates chronic constriction injury-induced neuropathic pain and inflammation via modulating PPAR-gamma pathway. PLoS One. (2023) 18:e0287517. doi: 10.1371/journal.pone.0287517
25. Wei, J-a, Hu, X, Zhang, B, Liu, L, Chen, K, So, K-F, et al. Electroacupuncture activates inhibitory neural circuits in the somatosensory cortex to relieve neuropathic pain. iScience. (2021) 24:102066. doi: 10.1016/j.isci.2021.102066
26. Gao, F, Chu, H, Li, J, Yang, M, Du, L, Li, J, et al. Repetitive transcranial magnetic stimulation for pain after spinal cord injury: a systematic review and meta-analysis. J Neurosurg Sci. (2017) 61:514–22. doi: 10.23736/S0390-5616.16.03809-1
27. Li, C, Jirachaipitak, S, Wrigley, P, Xu, H, and Euasobhon, P. Transcranial direct current stimulation for spinal cord injury-associated neuropathic pain. Korean J Pain. (2021) 34:156–64. doi: 10.3344/kjp.2021.34.2.156
28. Huang, Q, Duan, W, Sivanesan, E, Liu, S, Yang, F, Chen, Z, et al. Spinal cord stimulation for pain treatment after spinal cord injury. Neurosci Bull. (2018) 35:527–39. doi: 10.1007/s12264-018-0320-9
29. Alles, SRA, and Smith, PA. Etiology and pharmacology of neuropathic pain. Pharmacol Rev. (2018) 70:315–47. doi: 10.1124/pr.117.014399
30. Shiao, R, and Lee-Kubli, CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics. (2018) 15:635–53. doi: 10.1007/s13311-018-0633-4
31. Bedi, SS, Yang, Q, Crook, RJ, Du, J, Wu, Z, Fishman, HM, et al. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci. (2010) 30:14870–82. doi: 10.1523/JNEUROSCI.2428-10.2010
32. Uniyal, A, Tiwari, V, Tsukamoto, T, Dong, X, Guan, Y, and Raja, SN. Targeting sensory neuron GPCRs for peripheral neuropathic pain. Trends Pharmacol Sci. (2023) 44:1009–27. doi: 10.1016/j.tips.2023.10.003
33. Ritter, DM, Zemel, BM, Lepore, AC, and Covarrubias, M. Kv3.4 channel function and dysfunction in nociceptors. Channels (Austin). (2015) 9:209–17. doi: 10.1080/19336950.2015.1056949
34. Yang, Q, Crook, RJ, and Walters, ET. Knockdown of Nav1.8 blocks both spontaneous activity in small DRG neurons and reflex hypersensitivity after spinal cord injury. Soc Neurosci Abstr. (2012). doi: 10.1523/JNEUROSCI.5316-13.2014
35. Ritter, DM, Zemel, BM, Hala, TJ, O'Leary, ME, Lepore, AC, and Covarrubias, M. Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J Neurosci. (2015) 35:1260–73. doi: 10.1523/JNEUROSCI.1594-14.2015
36. Zemel, BM, Muqeem, T, Brown, EV, Goulao, M, Urban, MW, Tymanskyj, SR, et al. Calcineurin dysregulation underlies spinal cord injury-induced K(+) channel dysfunction in DRG neurons. J Neurosci. (2017) 37:8256–72. doi: 10.1523/JNEUROSCI.0434-17.2017
37. Odem, MA, Bavencoffe, AG, Cassidy, RM, Lopez, ER, Tian, J, Dessauer, CW, et al. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain. (2018) 159:2347–62. doi: 10.1097/j.pain.0000000000001341
38. Krenz, NR, and Weaver, LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem. (2000) 74:730–9. doi: 10.1046/j.1471-4159.2000.740730.x
39. Gwak, YS, Hulsebosch, CE, and Leem, JW. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. (2017) 2017:1–14. doi: 10.1155/2017/2480689
40. Kusuyama, K, Tachibana, T, Yamanaka, H, Okubo, M, Yoshiya, S, and Noguchi, K. Upregulation of calcium channel alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord injury-induced tactile allodynia. Spine J. (2018) 18:1062–9. doi: 10.1016/j.spinee.2018.01.010
41. Park, J, and Luo, ZD. Calcium channel functions in pain processing. Channels. (2014) 4:510–7. doi: 10.4161/chan.4.6.12869
42. Huang, W, Kabbani, N, Brannan, TK, Lin, MK, Theiss, MM, Hamilton, JF, et al. Association of a Functional Polymorphism in the CHRFAM7A gene with inflammatory response mediators and neuropathic pain after spinal cord injury. J Neurotrauma. (2019) 36:3026–33. doi: 10.1089/neu.2018.6200
43. Gwak, YS, Kang, J, Unabia, GC, and Hulsebosch, CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. (2012) 234:362–72. doi: 10.1016/j.expneurol.2011.10.010
44. Cao, T, Matyas, JJ, Renn, CL, Faden, AI, Dorsey, SG, and Wu, J. Function and mechanisms of truncated BDNF receptor TrkB.T1 in neuropathic pain. Cells. (2020) 9:1194. doi: 10.3390/cells9051194
45. Hains, BC, Johnson, KM, Eaton, MJ, Willis, WD, and Hulsebosch, CE. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience. (2003) 116:1097–110. doi: 10.1016/S0306-4522(02)00729-7
46. Bavencoffe, A, Li, Y, Wu, Z, Yang, Q, Herrera, J, Kennedy, EJ, et al. Persistent electrical activity in primary nociceptors after spinal cord injury is maintained by Scaffolded adenylyl cyclase and protein kinase a and is associated with altered adenylyl cyclase regulation. J Neurosci. (2016) 36:1660–8. doi: 10.1523/JNEUROSCI.0895-15.2016
47. Crown, ED, Gwak, YS, Ye, Z, Johnson, KM, and Hulsebosch, CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. (2008) 213:257–67. doi: 10.1016/j.expneurol.2008.05.025
48. Watson, JL, Hala, TJ, Putatunda, R, Sannie, D, and Lepore, AC. Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury. PLoS One. (2014) 9:e109099. doi: 10.1371/journal.pone.0109099
49. Ji, R-R, Berta, T, and Nedergaard, M. Glia and pain: is chronic pain a gliopathy? Pain. (2013) 154:S10–28. doi: 10.1016/j.pain.2013.06.022
50. Michael, BD, Bricio-Moreno, L, Sorensen, EW, Miyabe, Y, Lian, J, Solomon, T, et al. Astrocyte- and neuron-derived CXCL1 drives neutrophil transmigration and blood-brain barrier permeability in viral encephalitis. Cell Rep. (2020) 32:108150. doi: 10.1016/j.celrep.2020.108150
51. Donnelly, CR, Andriessen, AS, Chen, G, Wang, K, Jiang, C, Maixner, W, et al. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics. (2020) 17:846–60. doi: 10.1007/s13311-020-00905-7
52. Wang, A, and Xu, C. The role of connexin43 in neuropathic pain induced by spinal cord injury. Acta Biochim Biophys Sin. (2019) 51:554–60. doi: 10.1093/abbs/gmz038
53. Chen, MJ, Kress, B, Han, X, Moll, K, Peng, W, Ji, RR, et al. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. (2012) 60:1660–70. doi: 10.1002/glia.22384
54. Michetti, F, D'Ambrosi, N, Toesca, A, Puglisi, MA, Serrano, A, Marchese, E, et al. The S100B story: from biomarker to active factor in neural injury. J Neurochem. (2018) 148:168–87. doi: 10.1111/jnc.14574
55. Ishiguro, H, Kaito, T, Hashimoto, K, Kushioka, J, Okada, R, Tsukazaki, H, et al. Administration of ONO-2506 suppresses neuropathic pain after spinal cord injury by inhibition of astrocytic activation. Spine J. (2019) 19:1434–42. doi: 10.1016/j.spinee.2019.04.006
56. Pan, Q-L, Lin, F-X, Liu, N, and Chen, R-C. The role of aquaporin 4 (AQP4) in spinal cord injury. Biomed Pharmacother. (2022) 145:112384. doi: 10.1016/j.biopha.2021.112384
57. Saadoun, S, Papadopoulos, MC, Watanabe, H, Yan, D, Manley, GT, and Verkman, AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. (2005) 118:5691–8. doi: 10.1242/jcs.02680
58. Auguste, KI, Jin, S, Uchida, K, Yan, D, Manley, GT, Papadopoulos, MC, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. (2007) 21:108–16. doi: 10.1096/fj.06-6848com
59. Sun, C, Deng, J, Ma, Y, Meng, F, Cui, X, Li, M, et al. The dual role of microglia in neuropathic pain after spinal cord injury: detrimental and protective effects. Exp Neurol. (2023) 370:114570. doi: 10.1016/j.expneurol.2023.114570
60. Giuliani, C. The flavonoid quercetin induces AP-1 activation in FRTL-5 thyroid cells. Antioxidants. (2019) 8:112. doi: 10.3390/antiox8050112
61. Kwok, CHT, Learoyd, AE, Canet-Pons, J, Trang, T, and Fitzgerald, M. Spinal interleukin-6 contributes to central sensitisation and persistent pain hypersensitivity in a model of juvenile idiopathic arthritis. Brain Behav Immun. (2020) 90:145–54. doi: 10.1016/j.bbi.2020.08.004
62. Tiwari, V, Guan, Y, and Raja, SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev. (2014) 45:19–27. doi: 10.1016/j.neubiorev.2014.05.002
63. Bradbury, EJ, and Burnside, ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. (2019) 10:3879. doi: 10.1038/s41467-019-11707-7
64. Kumar, H, Choi, H, Jo, M-J, Joshi, HP, Muttigi, M, Bonanomi, D, et al. Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol Commun. (2018) 6:73. doi: 10.1186/s40478-018-0576-3
65. Zhang, H, Zhou, Z-L, Xie, H, Tian, X-B, Xu, H-L, Li, W, et al. Microglial depletion impairs glial scar formation and aggravates inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural Regen Res. (2023) 18:1325–31. doi: 10.4103/1673-5374.357912
66. Yoshizaki, S, Tamaru, T, Hara, M, Kijima, K, Tanaka, M, Konno, DJ, et al. Microglial inflammation after chronic spinal cord injury is enhanced by reactive astrocytes via the fibronectin/β1 integrin pathway. J Neuroinflammation. (2021) 18:12. doi: 10.1186/s12974-020-02059-x
67. Poppell, M, Hammel, G, and Ren, Y. Immune regulatory functions of macrophages and microglia in central nervous system diseases. Int J Mol Sci. (2023) 24:925. doi: 10.3390/ijms24065925
68. Atta, AA, Ibrahim, WW, Mohamed, AF, and Abdelkader, NF. Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology. (2023) 31:1053–67. doi: 10.1007/s10787-023-01216-x
69. Ahmad, KA, Shoaib, RM, Ahsan, MZ, Deng, M-Y, Ma, L, Apryani, E, et al. Microglial IL-10 and β-endorphin expression mediates gabapentinoids antineuropathic pain. Brain Behav Immun. (2021) 95:344–61. doi: 10.1016/j.bbi.2021.04.007
70. Urits, I, Adamian, L, Fiocchi, J, Hoyt, D, Ernst, C, Kaye, AD, et al. Advances in the understanding and Management of Chronic Pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep. (2019) 23:800. doi: 10.1007/s11916-019-0800-2
71. Gritsch, S, Lu, J, Thilemann, S, Wörtge, S, Möbius, W, Bruttger, J, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun. (2014) 5:5472. doi: 10.1038/ncomms6472
72. Nanou, E, and Catterall, WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. (2018) 98:466–81. doi: 10.1016/j.neuron.2018.03.017
73. Hendrich, J, Van Minh, AT, Heblich, F, Nieto-Rostro, M, Watschinger, K, Striessnig, J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. (2008) 105:3628–33. doi: 10.1073/pnas.0708930105
74. Eroglu, Ç, Allen, NJ, Susman, MW, O'Rourke, NA, Park, CY, Özkan, E, et al. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. (2009) 139:380–92. doi: 10.1016/j.cell.2009.09.025
75. Li, C-Y, Zhang, X-L, Matthews, EA, Li, K-W, Kurwa, A, Boroujerdi, A, et al. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain. (2006) 125:20–34. doi: 10.1016/j.pain.2006.04.022
76. Li, C-Y, Song, Y-H, Higuera, ES, and Luo, ZD. Spinal dorsal horn Calcium Channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. (2004) 24:8494–9. doi: 10.1523/JNEUROSCI.2982-04.2004
77. Levendoglu, F, Ogün, CO, Ozerbil, O, Ogün, TC, and Ugurlu, H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. (2004) 29:743–51. doi: 10.1097/01.BRS.0000112068.16108.3A
78. Raffaeli, W, Felzani, G, Tenti, M, Greco, L, D’Eramo, MP, Proietti, S, et al. A nutritional supplement as adjuvant of Gabapentinoids for adults with neuropathic pain following spinal cord injury and stroke: preliminary results. Healthcare. (2023) 11:2563. doi: 10.3390/healthcare11182563
79. Carpenter, RS, Jiang, RR, Brennan, FH, Hall, JCE, Gottipati, MK, Niewiesk, S, et al. Human immune cells infiltrate the spinal cord and impair recovery after spinal cord injury in humanized mice. Sci Rep. (2019) 9:19105. doi: 10.1038/s41598-019-55729-z
80. Miron, VE, and Franklin, RJM. Macrophages and CNS remyelination. J Neurochem. (2014) 130:165–71. doi: 10.1111/jnc.12705
81. Vazquez, MI, Catalan-Dibene, J, and Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. (2015) 74:318–26. doi: 10.1016/j.cyto.2015.02.007
82. Hu, X, Leak, RK, Thomson, AW, Yu, F, Xia, Y, Wechsler, LR, et al. Promises and limitations of immune cell-based therapies in neurological disorders. Nat Rev Neurol. (2018) 14:559–68. doi: 10.1038/s41582-018-0028-5
83. Yao, Y, Xu, J, Yu, T, Chen, Z, Xiao, Z, Wang, J, et al. Flufenamic acid inhibits secondary hemorrhage and BSCB disruption after spinal cord injury. Theranostics. (2018) 8:4181–98. doi: 10.7150/thno.25707
84. Pineau, I, and Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. (2006) 500:267–85. doi: 10.1002/cne.21149
85. Zhou, X, He, X, and Ren, Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. (2014) 9:1787–95. doi: 10.4103/1673-5374.143423
86. Orr, MB, and Gensel, JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. (2018) 15:541–53. doi: 10.1007/s13311-018-0631-6
87. Beck, KD, Nguyen, HX, Galvan, MD, Salazar, DL, Woodruff, TM, and Anderson, AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. (2010) 133:433–47. doi: 10.1093/brain/awp322
88. Kroner, A, Greenhalgh, AD, Zarruk, JG, Passos dos Santos, R, Gaestel, M, and David, S. TNF and increased intracellular Iron Alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. (2014) 83:1098–116. doi: 10.1016/j.neuron.2014.07.027
89. Thompson, CD, Zurko, JC, Hanna, BF, Hellenbrand, DJ, and Hanna, A. The therapeutic role of Interleukin-10 after spinal cord injury. J Neurotrauma. (2013) 30:1311–24. doi: 10.1089/neu.2012.2651
90. Kigerl, KA, Gensel, JC, Ankeny, DP, Alexander, JK, Donnelly, DJ, and Popovich, PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. (2009) 29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009
91. Jones, TB. Lymphocytes and autoimmunity after spinal cord injury. Exp Neurol. (2014) 258:78–90. doi: 10.1016/j.expneurol.2014.03.003
92. Li, H, Kong, W, Chambers, CR, Yu, D, Ganea, D, Tuma, RF, et al. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell Immunol. (2018) 329:1–9. doi: 10.1016/j.cellimm.2018.02.016
93. Morioka, N, Takeda, K, Kumagai, K, Hanada, T, Ikoma, K, Hide, I, et al. Interleukin‐1β‐induced substance P release from rat cultured primary afferent neurons driven by two phospholipase A2 enzymes: secretory type IIA and cytosolic type IV. J Neurochem. (2002) 80:989–97. doi: 10.1046/j.0022-3042.2002.00722.x
94. Oatway, MA, Chen, Y, and Weaver, LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. (2004) 110:259–68. doi: 10.1016/j.pain.2004.03.040
95. You, H-J, Colpaert, FC, and Arendt-Nielsen, L. The novel analgesic and high-efficacy 5-HT1A receptor agonist F 13640 inhibits nociceptive responses, wind-up, and after-discharges in spinal neurons and withdrawal reflexes. Exp Neurol. (2005) 191:174–83. doi: 10.1016/j.expneurol.2004.08.031
96. Jeong, CY, Choi, JI, and Yoon, MH. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur J Pharmacol. (2004) 502:205–11. doi: 10.1016/j.ejphar.2004.08.048
97. Sánchez-Brualla, I, Boulenguez, P, Brocard, C, Liabeuf, S, Viallat-Lieutaud, A, Navarro, X, et al. Activation of 5-HT2A receptors restores KCC2 function and reduces neuropathic pain after spinal cord injury. Neuroscience. (2018) 387:48–57. doi: 10.1016/j.neuroscience.2017.08.033
98. Naderi, A, Asgari, AR, Zahed, R, Ghanbari, A, Samandari, R, and Jorjani, M. Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats. Metab Brain Dis. (2014) 29:763–70. doi: 10.1007/s11011-014-9570-z
99. Gwak, YS, Tan, HY, Nam, TS, Paik, KS, Hulsebosch, CE, and Leem, JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. (2006) 23:1111–24. doi: 10.1089/neu.2006.23.1111
100. Gwak, YS, and Hulsebosch, CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. (2011) 60:799–808. doi: 10.1016/j.neuropharm.2010.12.030
101. Lee, SE, and Kim, J-H. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res. (2007) 58:245–9. doi: 10.1016/j.neures.2007.03.004
102. Carlsson-Jonsson, A, Gao, T, Hao, J-X, Fransson, R, Sandström, A, Nyberg, F, et al. N-terminal truncations of substance P1–7 amide affect its action on spinal cord injury-induced mechanical allodynia in rats. Eur J Pharmacol. (2014) 738:319–25. doi: 10.1016/j.ejphar.2014.05.060
103. Muheremu, A, Shu, L, Liang, J, Aili, A, and Jiang, K. Sustained delivery of neurotrophic factors to treat spinal cord injury. Transl Neurosci. (2021) 12:494–511. doi: 10.1515/tnsci-2020-0200
104. Gu, Y-L, Yin, L-W, Zhang, Z, Liu, J, Liu, S-J, Zhang, L-F, et al. Neurotrophin expressions in neural stem cells grafted acutely to transected spinal cord of adult rats linked to functional improvement. Cell Mol Neurobiol. (2012) 32:1089–97. doi: 10.1007/s10571-012-9832-4
105. Boulton, TG, Stahl, N, and Yancopoulos, GD. Ciliary neurotrophic factor leukemia inhibitory factor Interleukin-6 Oncostatin-M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth-factors. J Biol Chem. (1994) 269:11648–55. doi: 10.1016/S0021-9258(19)78174-5
106. Schiemann, WP, Graves, LM, Baumann, H, Morella, KK, Gearing, DP, Nielsen, MD, et al. Phosphorylation of the human leukemia inhibitory factor (Lif) receptor by mitogen-activated protein-kinase and the regulation of Lif receptor function by heterologous receptor activation. P Natl Acad Sci USA. (1995) 92:5361–5. doi: 10.1073/pnas.92.12.5361
107. Leibinger, M, Andreadaki, A, and Fischer, D. Role of mTOR in neuroprotection and axon regeneration after inflammatory stimulation. Neurobiol Dis. (2012) 46:314–24. doi: 10.1016/j.nbd.2012.01.004
108. Askvig, JM, and Watt, JA. The MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic cultures. J Cell Commun Signal. (2015) 9:217–31. doi: 10.1007/s12079-015-0268-8
109. Pasquin, S, Sharma, M, and Gauchat, J-F. Ciliary neurotrophic factor (CNTF): new facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev. (2015) 26:507–15. doi: 10.1016/j.cytogfr.2015.07.007
110. Cheng, H, Liao, KK, Liao, SF, Chuang, TY, and Shih, YH. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine. (2004) 29:E284–8. doi: 10.1097/01.BRS.0000131217.61390.2C
111. Teng, YD, Mocchetti, I, and Wrathall, JR. Basic and acidic fibroblast growth factors protect spinal motor neuronesin vivoafter experimental spinal cord injury. Eur J Neurosci. (1998) 10:798–802. doi: 10.1046/j.1460-9568.1998.00100.x
112. Tsai, MC, Shen, LF, Kuo, HS, Cheng, HR, and Chak, KF. Involvement of acidic fibroblast growth factor in spinal cord injury repair processes revealed by a proteomics approach. Mol Cell Proteomics. (2008) 7:1668–87. doi: 10.1074/mcp.M800076-MCP200
113. Ying, Y, Zhang, Y, Tu, Y, Chen, M, Huang, Z, Ying, W, et al. Hypoxia response element-directed expression of aFGF in neural stem cells promotes the recovery of spinal cord injury and attenuates SCI-induced apoptosis. Front Cell Dev Biol. (2021) 9:693694. doi: 10.3389/fcell.2021.693694
114. Rosich, K, Hanna, BF, Ibrahim, RK, Hellenbrand, DJ, and Hanna, A. The effects of glial cell line-derived neurotrophic factor after spinal cord injury. J Neurotrauma. (2017) 34:3311–25. doi: 10.1089/neu.2017.5175
115. Hwang, K, Jung, K, Kim, IS, Kim, M, Han, J, Lim, J, et al. Glial cell line-derived neurotrophic factor-overexpressing human neural stem/progenitor cells enhance therapeutic efficiency in rat with traumatic spinal cord injury. Exp Neurobiol. (2019) 28:679–96. doi: 10.5607/en.2019.28.6.679
116. Renn, CL, Leitch, CC, and Dorsey, SG. In vivo evidence that truncated Trkb.T1 participates in nociception. Mol Pain. (2009) 5:61. doi: 10.1186/1744-8069-5-61
117. Matyas, JJ, O'Driscoll, CM, Yu, L, Coll-Miro, M, Daugherty, S, Renn, CL, et al. Truncated TrkB.T1-mediated astrocyte dysfunction contributes to impaired motor function and neuropathic pain after spinal cord injury. J Neurosci. (2017) 37:3956–71. doi: 10.1523/JNEUROSCI.3353-16.2017
118. Vaz, SH, Jorgensen, TN, Cristovao-Ferreira, S, Duflot, S, Ribeiro, JA, Gether, U, et al. Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J Biol Chem. (2011) 286:40464–76. doi: 10.1074/jbc.M111.232009
119. Arvanian, VL, and Mendell, LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. (2001) 14:1800–8. doi: 10.1046/j.0953-816x.2001.01811.x
120. Garraway, SM, Turtle, JD, Huie, JR, Lee, KH, Hook, MA, Woller, SA, et al. Intermittent noxious stimulation following spinal cord contusion injury impairs locomotor recovery and reduces spinal brain-derived neurotrophic factor-tropomyosin-receptor kinase signaling in adult rats. Neuroscience. (2011) 199:86–102. doi: 10.1016/j.neuroscience.2011.10.007
121. Garraway, SM, Woller, SA, Huie, JR, Hartman, JJ, Hook, MA, Miranda, RC, et al. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain. (2014) 155:2344–59. doi: 10.1016/j.pain.2014.08.034
122. Wang, P, Zhang, Y, Xia, Y, Xu, D, Wang, H, Liu, D, et al. MicroRNA-139-5p promotes functional recovery and reduces pain hypersensitivity in mice with spinal cord injury by targeting mammalian sterile 20-like kinase 1. Neurochem Res. (2020) 46:349–57. doi: 10.1007/s11064-020-03170-4
123. Leinders, M, Üçeyler, N, Pritchard, RA, Sommer, C, and Sorkin, LS. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp Neurol. (2016) 283:276–86. doi: 10.1016/j.expneurol.2016.06.025
124. Zhao, J, Yang, L, Huang, L, and Li, Z. Screening of disease-related biomarkers related to neuropathic pain (NP) after spinal cord injury (SCI). Hum Genomics. (2021) 15:5. doi: 10.1186/s40246-021-00303-w
125. Zhao, X, Tang, Z, Zhang, H, Atianjoh, FE, Zhao, JY, Liang, L, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. (2013) 16:1024–31. doi: 10.1038/nn.3438
126. Hou, JM, Yan, RB, Xiang, ZM, Zhang, H, Liu, J, Wu, YT, et al. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. (2014) 266:208–15. doi: 10.1016/j.neuroscience.2014.02.013
127. Oni-Orisan, A, Kaushal, M, Li, W, Leschke, J, Ward, BD, Vedantam, A, et al. Alterations in cortical sensorimotor connectivity following complete cervical spinal cord injury: a prospective resting-state fMRI study. PLoS One. (2016) 11:e0150351. doi: 10.1371/journal.pone.0150351
128. Wrigley, PJ, Press, SR, Gustin, SM, Macefield, VG, Gandevia, SC, Cousins, MJ, et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. (2009) 141:52–9. doi: 10.1016/j.pain.2008.10.007
129. You, HJ, Lei, J, and Pertovaara, A. Thalamus: the 'promoter' of endogenous modulation of pain and potential therapeutic target in pathological pain. Neurosci Biobehav Rev. (2022) 139:104745. doi: 10.1016/j.neubiorev.2022.104745
130. Seminowicz, DA, Jiang, L, Ji, Y, Xu, S, Gullapalli, RP, and Masri, R. Thalamocortical asynchrony in conditions of spinal cord injury pain in rats. J Neurosci. (2012) 32:15843–8. doi: 10.1523/JNEUROSCI.2927-12.2012
131. Gustin, SM, Wrigley, PJ, Youssef, AM, McIndoe, L, Wilcox, SL, Rae, CD, et al. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain. (2014) 155:1027–36. doi: 10.1016/j.pain.2014.02.008
132. Pradip, RPY, Pattany, M, Eva, G, Noga, W, Bowen, BC, Arizala, A-M, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. (2002) 23:901–5.
133. Widerström-Noga, E. Neuropathic pain and spinal cord injury: management, phenotypes, and biomarkers. Drugs. (2023) 83:1001–25. doi: 10.1007/s40265-023-01903-7
134. Kumar, N, Laferriere, A, Yu, JSC, Leavitt, A, and Coderre, TJ. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. (2010) 113:552–61. doi: 10.1111/j.1471-4159.2010.06625.x
135. Agarwal, N, and Joshi, M. Effectiveness of amitriptyline and lamotrigine in traumatic spinal cord injury-induced neuropathic pain: a randomized longitudinal comparative study. Spinal Cord. (2016) 55:126–30. doi: 10.1038/sc.2016.123
136. Choucair-Jaafar, N, Salvat, E, Freund-Mercier, M-J, and Barrot, M. The antiallodynic action of nortriptyline and terbutaline is mediated by β2 adrenoceptors and δ opioid receptors in the Ob/Ob model of diabetic polyneuropathy. Brain Res. (2014) 1546:18–26. doi: 10.1016/j.brainres.2013.12.016
137. Huang, Y, Chen, H, Chen, S-R, and Pan, H-L. Duloxetine and amitriptyline reduce neuropathic pain by inhibiting primary sensory input to spinal dorsal horn neurons via α1- and α2-adrenergic receptors. ACS Chem Neurosci. (2023) 14:1261–77. doi: 10.1021/acschemneuro.2c00780
138. Cardenas, DD, Warms, CA, Turner, JA, Marshall, H, Brooke, MM, and Loeser, JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. (2002) 96:365–73. doi: 10.1016/S0304-3959(01)00483-3
139. Rana, MH, Khan, AAG, Khalid, I, Ishfaq, M, Javali, MA, Baig, FAH, et al. Therapeutic approach for trigeminal neuralgia: a systematic review. Biomedicines. (2023) 11:2606. doi: 10.3390/biomedicines11102606
140. DiMasi, JA, Hansen, RW, and Grabowski, HG. The price of innovation: new estimates of drug development costs. J Health Econ. (2003) 22:151–85. doi: 10.1016/S0167-6296(02)00126-1
141. Aronson, JK. Drug development: more science, more education. Br J Clin Pharmacol. (2005) 59:377–8. doi: 10.1111/j.0306-5251.2005.02420.x
142. Aronson, JK. Old drugs – new uses. Br J Clin Pharmacol. (2007) 64:563–5. doi: 10.1111/j.1365-2125.2007.03058.x
143. Han, J, Hua, Z, Yang, WJ, Wang, S, Yan, F, Wang, JN, et al. Resveratrol suppresses neuroinflammation to alleviate mechanical allodynia by inhibiting Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway in a rat model of spinal cord injury. Front Mol Neurosci. (2023) 16:1116679. doi: 10.3389/fnmol.2023.1116679
144. Wu, C, Chen, H, Zhuang, R, Zhang, H, Wang, Y, Hu, X, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. (2021) 17:1138–52. doi: 10.7150/ijbs.57825
145. Wang, YY, Xiong, M, Wang, MS, Chen, HD, Li, WJ, and Zhou, XZ. Quercetin promotes locomotor function recovery and axonal regeneration through induction of autophagy after spinal cord injury. Clin Exp Pharma Physio. (2021) 48:1642–52. doi: 10.1111/1440-1681.13573
146. Wang, CG, Wang, QQ, Lou, YT, Xu, JX, Feng, ZH, Chen, Y, et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med. (2018) 22:1148–66. doi: 10.1111/jcmm.13368
147. Quemener, AM, Centomo, ML, Sax, SL, and Panella, R. Small drugs, huge impact: the extraordinary impact of antisense oligonucleotides in research and drug development. Molecules. (2022) 27:536. doi: 10.3390/molecules27020536
148. Wurster, CD, and Ludolph, AC. Antisense oligonucleotides in neurological disorders. Ther Adv Neurol Disord. (2018) 11:1756286418776932. doi: 10.1177/1756286418776932
149. Wang, M, Liu, W, Ge, J, and Liu, S. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. (2023) 14:1147718. doi: 10.3389/fimmu.2023.1147718
150. Liu, J, and Wu, Y. Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed Pharmacother. (2017) 89:1125–35. doi: 10.1016/j.biopha.2017.02.077
151. Li, H, Wang, J, Li, C, and Xiao, ZCochrane Common Mental Disorders Group. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Rev. (2014) 2014:CD009083. doi: 10.1002/14651858.CD009083.pub2
152. Bai, Y-W, Yang, Q-H, Chen, P-J, and Wang, X-Q. Repetitive transcranial magnetic stimulation regulates neuroinflammation in neuropathic pain. Front Immunol. (2023) 14:1172293. doi: 10.3389/fimmu.2023.1172293
153. Yuan, H, Mou, X, Sun, X-L, Wang, H, Ju, F, Sun, W, et al. Analgesic effects of directed repetitive transcranial magnetic stimulation in acute neuropathic pain after spinal cord injury. Pain Med. (2020) 21:1216–23. doi: 10.1093/pm/pnz290
154. Nardone, R, Höller, Y, Langthaler, PB, Lochner, P, Golaszewski, S, Schwenker, K, et al. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord. (2016) 55:20–5. doi: 10.1038/sc.2016.87
155. Kesikburun, S. Non-invasive brain stimulation in rehabilitation. Turkish J Phys Med Rehabil. (2022) 68:1–8. doi: 10.5606/tftrd.2022.10608
156. Ngernyam, N, Jensen, MP, Arayawichanon, P, Auvichayapat, N, Tiamkao, S, Janjarasjitt, S, et al. The effects of transcranial direct current stimulation in patients with neuropathic pain from spinal cord injury. Clin Neurophysiol. (2015) 126:382–90. doi: 10.1016/j.clinph.2014.05.034
157. Thibaut, A, Carvalho, S, Morse, LR, Zafonte, R, and Fregni, F. Delayed pain decrease following M1 tDCS in spinal cord injury: a randomized controlled clinical trial. Neurosci Lett. (2017) 658:19–26. doi: 10.1016/j.neulet.2017.08.024
158. Melzack, R, and Wall, PD. Pain mechanisms: a new theory. Science. (1965) 150:971–9. doi: 10.1126/science.150.3699.971
159. Joosten, EA, and Franken, G. Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain. (2020) 161:S104–13. doi: 10.1097/j.pain.0000000000001854
160. Meuwissen, KPV, van der Toorn, A, Gu, JW, Zhang, TC, Dijkhuizen, RM, and Joosten, EAJ. Active recharge burst and tonic spinal cord stimulation engage different Supraspinal mechanisms: a functional magnetic resonance imaging study in peripherally injured chronic neuropathic rats. Pain Pract. (2020) 20:510–21. doi: 10.1111/papr.12879
161. Hong, A, Varshney, V, Hare, GMT, and Mazer, CD. Spinal cord stimulation: a nonopioid alternative for chronic pain management. Can Med Assoc J. (2020) 192:E1264–7. doi: 10.1503/cmaj.200229
162. Reck, TA, and Landmann, G. Successful spinal cord stimulation for neuropathic below-level spinal cord injury pain following complete paraplegia: a case report. Spinal Cord Ser Cases. (2017) 3:17049. doi: 10.1038/scsandc.2017.49
163. Kim, JK, You, J, Son, S, Suh, I, and Lim, JY. Comparison of intermittent theta burst stimulation and high-frequency repetitive transcranial magnetic stimulation on spinal cord injury-related neuropathic pain: a sham-controlled study. J Spinal Cord Med. (2023):1–7. doi: 10.1080/10790268.2023.2277964
164. Ding, W, You, Z, Chen, Q, Yang, L, Doheny, J, Zhou, X, et al. Gut microbiota influences neuropathic pain through modulating Proinflammatory and anti-inflammatory T cells. Anesth Analg. (2020) 132:1146–55. doi: 10.1213/ANE.0000000000005155
165. Kaur, J, Ghosh, S, Sahani, AK, and Sinha, JK. Mental imagery as a rehabilitative therapy for neuropathic pain in people with spinal cord injury: a randomized controlled trial. Neurorehab Neural Re. (2020) 34:1038–49. doi: 10.1177/1545968320962498
166. Trost, Z, Anam, M, Seward, J, Shum, C, Rumble, D, Sturgeon, J, et al. Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: findings from a preliminary investigation of feasibility and clinical efficacy. Pain. (2022) 163:350–61. doi: 10.1097/j.pain.0000000000002348
167. Putrino, D, Tabacof, L, Breyman, E, Revis, J, Soomro, Z, Chopra, D, et al. Pain reduction after short exposure to virtual reality environments in people with spinal cord injury. Int J Environ Res Public Health. (2021) 18:8923. doi: 10.3390/ijerph18178923
168. Roosink, M, Robitaille, N, Jackson, PL, Bouyer, LJ, and Mercier, C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: an exploratory study. Restor Neurol Neurosci. (2016) 34:227–35. doi: 10.3233/RNN-150563
169. Gustin, SM, Wrigley, PJ, Henderson, LA, and Siddall, PJ. Brain circuitry underlying pain in response to imagined movement in people with spinal cord injury. Pain. (2010) 148:438–45. doi: 10.1016/j.pain.2009.12.001
170. Mendes, BB, Sousa, DP, Conniot, J, and Conde, J. Nanomedicine-based strategies to target and modulate the tumor microenvironment. Trends Cancer. (2021) 7:847–62. doi: 10.1016/j.trecan.2021.05.001
Keywords: spinal cord injury, neuropathic pain, glial cell, electroacupuncture, spinal cord stimulation
Citation: Li J, Kang W, Wang X and Pan F (2024) Progress in treatment of pathological neuropathic pain after spinal cord injury. Front. Neurol. 15:1430288. doi: 10.3389/fneur.2024.1430288
Received: 09 May 2024; Accepted: 23 October 2024;
Published: 11 November 2024.
Edited by:
Jacqueline Sagen, University of Miami, United StatesReviewed by:
Vinod Tiwari, Indian Institute of Technology (BHU), IndiaCopyright © 2024 Li, Kang, Wang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Pan, cGFuZmFuZzM3MEBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.