- 1The Changzhou Clinical College of Xuzhou Medical University, Changzhou, Jiangsu, China
- 2Department of Otorhinolaryngology, Changzhou Third People’s Hospital, Changzhou, Jiangsu, China
- 3Department of Otorhinolaryngology, The First People’s Hospital of Changzhou, Changzhou, Jiangsu, China
Objective: This study aims to assess the potential efficacy of cochlear implantation as a treatment for patients with Waardenburg syndrome (WS) and to guide clinical work by comparing the effect of auditory and speech recovery after cochlear implantation in patients with WS and non-WS.
Methods: PubMed, the Cochrane Library, CNKI, and Wanfang Data were sources for retrieving literature on cochlear implantation in WS, and clinical data meeting the inclusion criteria were meta-analyzed using RevMan5.41.
Results: A total of nine articles were included in this study, including 132 patients with WS and 815 patients in the control group. Meta-analysis showed that there are no significant differences in the scores for categories of audit performance (CAP), speech intelligibility rating (SIR), and parents’ evaluation of aural/oral performance of children (PEACH) between the WS group and the control group.
Conclusion: Cochlear implantation demonstrates comparable auditory and speech recovery outcomes for WS patients and non-WS patients.
1 Introduction
Waardenburg syndrome (WS), discovered and named by Dutch physician Waardenburg in 1951 (1), is an autosomal dominant genetic disorder primarily characterized by auditory pigmentary abnormalities. Its key manifestations include inner canthus heterotopia, iris heterochrony, white hair on the forehead, and hereditary sensorineural deafness (2). WS is closely related to the abnormal migration and differentiation of melanocytes. In the inner ear, melanocytes differentiate into intermediate cells within the stria vascularis of the cochlea (3, 4). When gene mutations affect melanocyte differentiation and migration, they may influence the cochlea’s internal environment, resulting in sensorineural hearing loss.
Cochlear implantation (CI) stands out as the primary approach for auditory and speech therapy (5). Currently, there exists a scarcity of research samples and variations in evaluation criteria for assessing the effectiveness of CI in WS patients. Therefore, this paper aims to conduct a comprehensive literature review, identify common evaluation indicators, and evaluate the therapeutic impact of CI on patients with WS.
2 Materials and methods
This meta-analysis was performed in line with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (6). This review was also registered on PROSPERO (Registration ID: CRD42022356957).
2.1 Literature search
A comprehensive search strategy was implemented by combining subject words with free words across multiple databases, including PubMed, the Cochrane Library, CNKI, and Wanfang. Both English and Chinese languages were utilized for the search. The exploration period extended from the establishment time of each database to December 31, 2023. The keywords employed in the search encompassed “Waardenburg syndrome,” “cochlear,” “cochlear implant,” and “cochlear implantation.”
2.2 Literature inclusion and exclusion criteria
2.2.1 Literature inclusion criteria
1. The inclusion criteria encompass randomized controlled trials (RCTs), cohort studies, case–control studies, or comparative studies.
2. The study population should consist of individuals undergoing auditory rehabilitation through CI.
3. The evaluation should focus on the assessment of auditory and/or speech skills in patients who have undergone CI.
2.2.2 Literature exclusion criteria
1. Articles lacking comparable auditory and speech outcomes between WS and other CI patients.
2. Studies that did not specifically explore auditory rehabilitation through CI.
3. Articles with a high risk of bias.
2.3 Data collection and extraction
Three authors independently conducted data extraction from the full texts of eligible articles. The following data were recorded: the first author’s name, publication year, the number of patients enrolled in each study, postoperative evaluation indicators, and recovery rates. Discrepancies were resolved through discussions among the authors.
2.4 Quality assessment
The risk of bias was assessed using the Newcastle Ottawa Scale (NOS). Two evaluators conducted independent assessments of the literature, and a final evaluation was performed by a third party. This third party, a senior chief physician, possessed extensive clinical research experience. A score of ≥6 is considered high-quality literature, and < 6 is not included.
2.5 Statistical methods
RevMan5.41 software was used for the analysis. A Q-test was used for the heterogeneity test. If the heterogeneity is low (p > 0.1, I2 < 50%), the fixed-effect model was selected; if the heterogeneity is high (p ≤ 0.1, I2 ≥ 50%), the random effect model was used to carry out sensitivity analysis on the source of heterogeneity.
3 Results
3.1 Literature search results
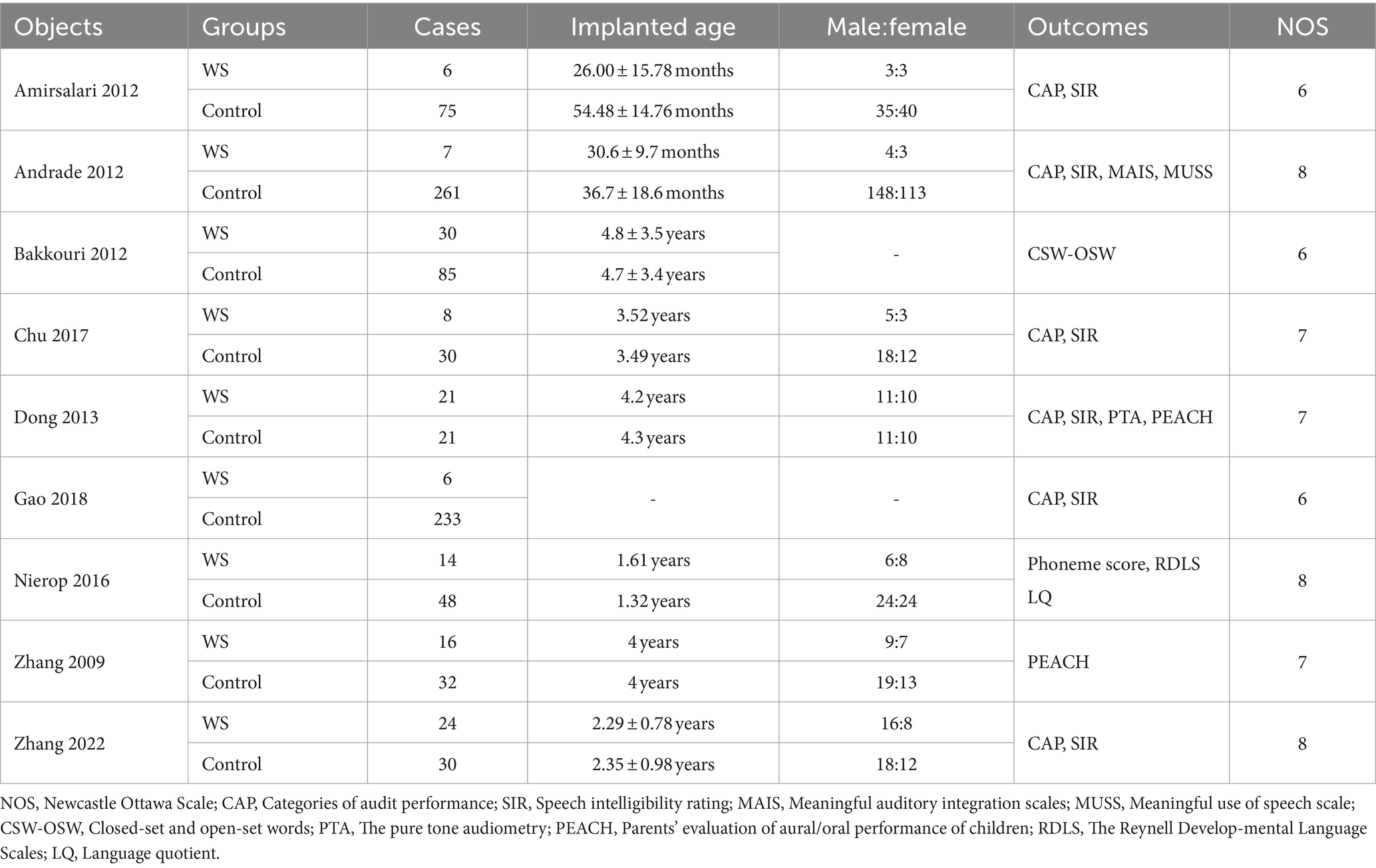
A total of 228 documents were retrieved, finally, nine qualified documents were selected for analysis (7–15), as shown in Table 1, The literature screening process is shown in Figure 1.
3.2 Literature heterogeneity test
Heterogeneity tests were conducted for each test, and fixed-effects model analysis was performed on categories of audit performance (CAP), speech intelligibility rating (SIR), and parents’ evaluation of aural/oral performance of children (PEACH) scores (p > 0.05, I2 < 50%).
3.3 Comparison of postoperative CAP scores
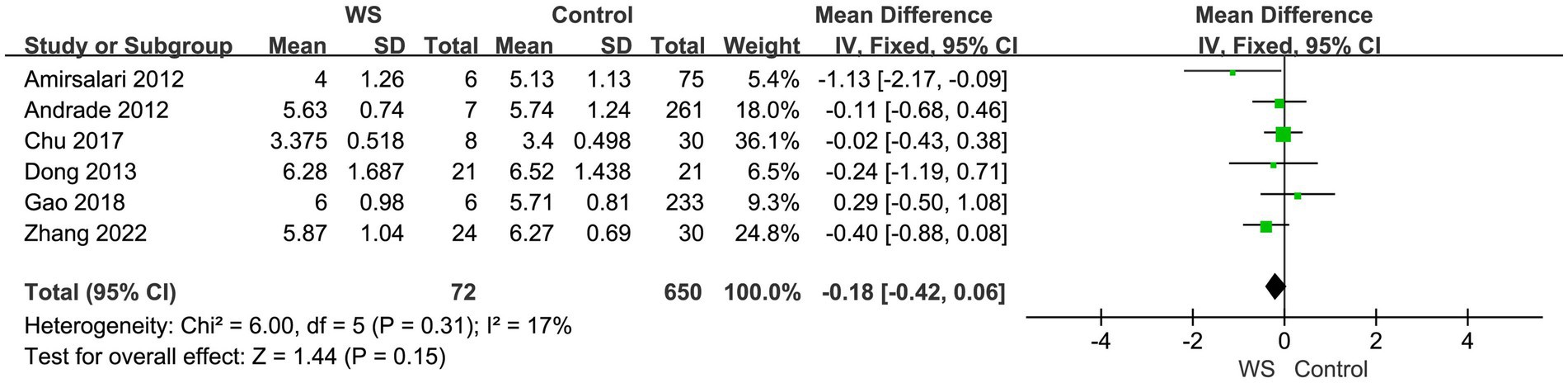
Six studies, involving a total of 722 cases (72 cases in the WS group and 650 cases in the control group), compared the CAP scores of the WS group and the control group after CI. The fixed-effect model was used for analysis, and the combined-effect test result yielded Z = 1.44, p = 0.15. This suggests that there is no statistical difference between the WS group and the control group in terms of CAP scores, as shown in Figure 2.
3.4 Comparison of postoperative SIR scores
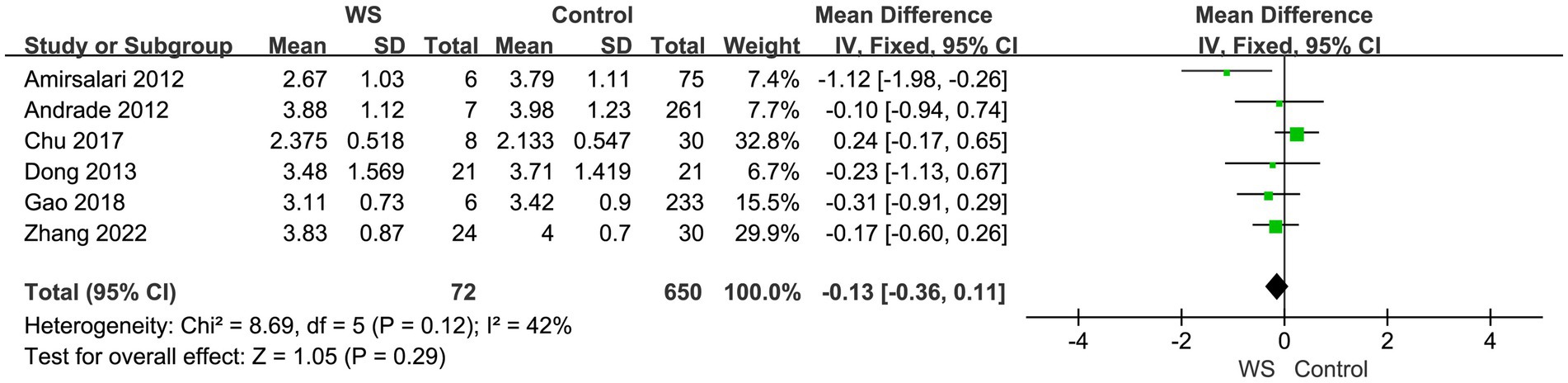
Six studies, involving a total of 722 cases (72 cases in the WS group and 650 cases in the control group), compared the SIR scores of the WS group and the control group after CI. The fixed-effect model was used for analysis, and the combined-effect test result yielded Z = 1.05, p = 0.29. This indicates that there is no statistical difference between the WS group and the control group in terms of SIR scores, as shown in Figure 3.
3.5 Comparison of postoperative PEACH scores
3.5.1 Comparison of telephone scores
Two studies, involving a total of 90 cases (37 cases in the WS group and 53 cases in the control group), compared the telephone scores of the WS group and the control group after CI. The fixed-effect model was used for analysis, and the combined-effect scale test result yielded Z = 0.42, p = 0.68. This reveals that there is no statistical difference between the WS group and the control group in terms of the comparison of telephone scores, as shown in Figure 4.
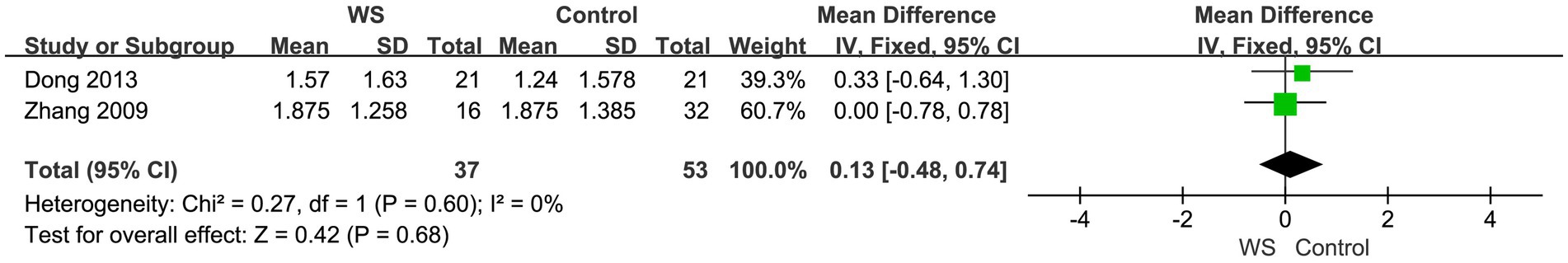
Figure 4. Forest map of telephone communication score comparison between the WS group and the control group.
3.5.2 Comparison of quiet environment scores
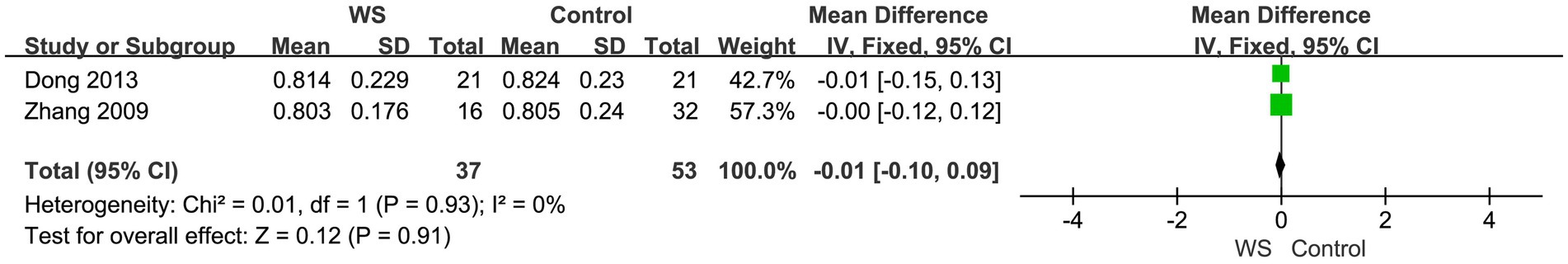
Two studies, involving a total of 90 cases (37 cases in the WS group and 53 cases in the control group), compared the quiet environment scores of the WS group and the control group after CI. The fixed-effect model was used for analysis, and the combined-effect scale test result yielded Z = 0.12, p = 0.91. This indicates that there is no statistical difference between the WS group and the control group in terms of quiet environment scores, as shown in Figure 5.
3.5.3 Comparison of noise environment scores
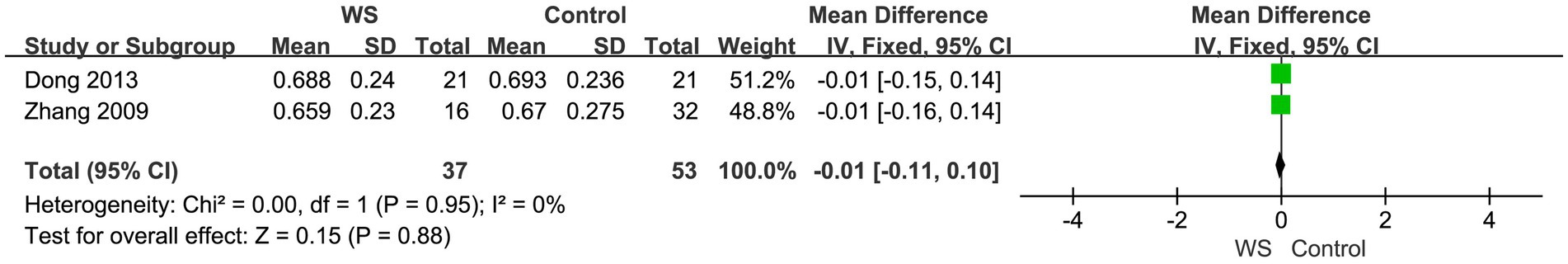
Two studies, involving a total of 90 cases (37 cases in the WS group and 53 cases in the control group), compared the noise environment scores of the WS group and the control group after CI. The fixed-effect model was used for analysis, and the combined-effect test result yielded Z = 0.15, p = 0.88. This suggests that there is no statistical difference between the WS group and the control group in terms of noise environment scores, as shown in Figure 6.
3.6 Publication bias analysis
The literature exhibits inconsistency in the included evaluation indicators. A funnel plot was generated for bias analysis of each study, and the results showed that the literature was symmetrically distributed on both sides, indicating an absence of publication bias. However, during SIR analysis, one literature source exhibited high heterogeneity.
4 Discussion
Waardenburg syndrome can be categorized into four types based on its clinical manifestations. Type 1 presents with ectopic inner canthus, sensorineural deafness, heterochromatic iris, white frontal hair, hypopigmentation, and straight eyebrows. Type 2 lacks ectopic inner canthus but is otherwise similar to Type 1. Type 3, also known as Klein Waardenburg syndrome, is characterized by the features of Type 1 along with muscular dysplasia and upper limb contracture, Type 4, identified as Waardenburg Shah syndrome or Waardenburg Hirschsprung disease, corresponds to Type 2 and is accompanied by Hirschsprung disease. Types 1 and 2 collectively constitute a significant proportion (16, 17). About 60% of individuals with WS types I and III suffer from sensorineural hearing loss, while 90% of those with types II and IV experience sensorineural hearing loss (18).
Some genes are believed to be related to the onset of WS. According to current research, PAX3 is related to the pathogenesis of WS1 and WS3 (19, 20), MITF and SNAI2 play a role in the pathogenesis of WS2 (21), SOX10 is related to the pathogenesis of WS2 and WS4 (22, 23), EDNRB and EDN3 are related to the pathogenesis of WS4 (24, 25). Some WS patients are accompanied by semicircular canal dysplasia, cochlear dysplasia, and large vestibular aqueduct. Structural malformations of the cochlear and labyrinth have not been reported (17). At present, most studies show that patients with WS recover well after CI, but some studies still report that the postoperative effect on patients is not good (7). Lovett et al. (2) compared the hearing and speech outcomes of WS patients before and after CI. The results showed that CI can be an effective way for improving the hearing and speech ability of WS patients. But we still want to find out whether WS and non-WS have a similar prognosis.
Both CAP and SIR were proposed by Nikolopoulos et al. of Nottingham University and filled in by patients’ relatives (26, 27). These assessments provide straightforward information about children’s hearing levels and speech abilities following surgery, making them widely used for evaluating the postoperative rehabilitation outcomes of cochlear implants (7, 8). In this study, six sets of investigations utilized CAP and SIR scores to assess auditory and speech abilities in the two groups. The results indicated no significant difference in postoperative CAP and SIR scores between the WS group and the control group.
Parents’ evaluation of aural/oral performance of children was developed by the National Acoustic Laboratory (NAL) (28) and is used for the evaluation of the auditory speech effect after cochlear implantation. Trained professionals administer the evaluation by prompting parents with questions, and responses involve describing specific cases, offering evidence of auditory speech recovery. This approach helps avoid the potential bias of direct “yes” or “no” responses, contributing to a more objective assessment (29). In this analysis, two studies utilized the PEACH score, and the results indicated no significant differences in postoperative telephone scores, quiet environment scores, and noise environment scores.
5 Limitations
This study has some limitations. First, the analysis is constrained by the inclusion of only a small number of studies. Second, the absence of detailed information regarding WS types and underlying genotypes hinders further discussion and exploration. Third, some of the included research samples have small populations, potentially impacting the generalizability of the findings. Fourth, in some studies, the operation time of patients was not consistently reported, introducing variability in the data. Finally, the majority of studies are from China, suggesting a geographical bias. The study calls for more diverse data from other countries to enhance the breadth and applicability of the findings.
6 Conclusion
There was no obvious difference in the auditory and speech recovery effects between WS patients after cochlear implantation and individuals undergoing other cochlear implant procedures. Cochlear implantation emerges as an effective method for auditory and speech therapy in WS patients, demonstrating favorable postoperative recovery effects. However, substantiating this conclusion requires a large number of high-quality disease control studies to provide robust evidence and validation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FQ: Writing – review & editing, Writing – original draft. SG: Writing – original draft. XY: Writing – original draft. XL: Writing – review & editing. JM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful for the support of the patients and colleagues in the department.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waardenburg, PJ. A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am J Hum Genet. (1951) 3:195–253.
2. Lovett, A, Eastwood, M, Metcalfe, C, Muzaffar, J, Monksfield, P, and Bance, M. Outcomes of cochlear implantation in early-deafened patients with Waardenburg syndrome: a systematic review and narrative synthesis. Laryngoscope Investig Otolaryngol. (2023) 8:1094–107. doi: 10.1002/lio2.1110
3. Vandamme, N, and Berx, G. From neural crest cells to melanocytes: cellular plasticity during development and beyond. Cell Mol Life Sci. (2019) 76:1919–34. doi: 10.1007/s00018-019-03049-w
4. Eckhard, A, Gleiser, C, Rask-Andersen, H, Arnold, H, Liu, W, Mack, A, et al. Co-localisation of K(ir)4.1 and AQP4 in rat and human cochleae reveals a gap in water channel expression at the transduction sites of endocochlear K(+) recycling routes. Cell Tissue Res. (2012) 350:27–43. doi: 10.1007/s00441-012-1456-y
5. Roudbari, F, Dallal Amandi, AR, Bonyadi, M, Sadeghi, L, and Jabbarpour, N. SOX10Identification of a de novo, Novel Pathogenic Variant in the Splice Region of the Gene in an Iranian Azeri Turkish Family with Waardenburg Syndrome. Mol Syndromol. (2023) 14:516–22. doi: 10.1159/000531566
6. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
7. Amirsalari, S, Ajallouyean, M, Saburi, A, Haddadi Fard, A, Abed, M, and Ghazavi, Y. Cochlear implantation outcomes in children with Waardenburg syndrome. Eur Arch Otorrinolaringol. (2012) 269:2179–83. doi: 10.1007/s00405-011-1877-3
8. de Sousa Andrade, SM, Monteiro, ART, Martins, JHF, Alves, MC, Santos Silva, LF, Quadros, JMC, et al. Cochlear implant rehabilitation outcomes in Waardenburg syndrome children. Int J Pediatr Otorhinolaryngol. (2012) 76:1375–8. doi: 10.1016/j.ijporl.2012.06.010
9. El Bakkouri, W, Loundon, N, Thierry, B, Nevoux, J, Marlin, S, Rouillon, I, et al. Cochlear implantation and congenital deafness: perceptive and lexical results in 2 genetically pediatric identified population. Otol Neurotol. (2012) 33:539–44. doi: 10.1097/MAO.0b013e31824bae35
10. Chu, YH, Zhang, ZF, Chen, CL, and Lan, HX. Therapeutic effect of cochlear implantation in children with Waardenburg syndrome. Henan J Surg. (2017) 23:5–6. doi: 10.16193/j.cnki.hnwk.2017.04.003
11. Dong, SQ, Li, JN, Jiao, QS, Sun, L, Liu, RY, Hao, QQ, et al. The effectiveness analysis of the cochlear implantation in patients with Waardenburg syndrome. Chin Sci J Hear Speech Rehab. (2013) 5:350–3. doi: 10.3969/j.issn.1672-4933.2013.05.006
12. Gao, FF, Wang, QR, Yu, SD, Gu, LT, Shi, YH, and Geng, B. Clinical analysis of language rehabilitation in 295 cases of cochlear implantation. J Otolaryngol Ophthalmol Shandong Univ. (2018) 32:62–5.
13. van Nierop, JWI, Snabel, RR, Langereis, M, Pennings, RJE, Admiraal, RJC, Mylanus, EAM, et al. Paediatric cochlear implantation in patients with Waardenburg syndrome. Audiol Neurotol. (2016) 21:187–94. doi: 10.1159/000444120
14. Zhang, ZL, Cao, KL, Wei, CG, Luan, L, and Li, H. The outcomes measures of the cochlear implantation in patients with Waardenburg syndrome. J Audiol Speech Pathol. (2009) 17:372–5.
15. Zhang, XY, Lin, Y, Xu, Z, Zhang, YK, Ren, CC, Zhao, ZMY, et al. Outcomes of cochlear implantation in children with Waardenburg syndrome and influencing factors. Chin J Otol. (2022) 20:720–5.
16. Wang, G, Li, X, Gao, X, Su, Y, Han, M, Gao, B, et al. Analysis of genotype-phenotype relationships in 90 Chinese probands with Waardenburg syndrome. Hum Genet. (2022) 141:839–52. doi: 10.1007/s00439-021-02301-3
17. Lee, CY, Lo, MY, Chen, YM, Lin, PH, Hsu, CJ, Chen, PL, et al. Identification of nine novel variants across PAX3, SOX10, EDNRB, and MITF genes in Waardenburg syndrome with next-generation sequencing. Mol Genet Genomic Med. (2022) 10:e2082. doi: 10.1002/mgg3.2082
18. Pingault, V, Ente, D, Dastot-Le Moal, F, Goossens, M, Marlin, S, and Bondurand, N. Review and update of mutations causing Waardenburg syndrome. Hum Mutat. (2010) 31:391–406. doi: 10.1002/humu.21211
19. Yang, SZ, Hou, L, Qi, X, Wang, GJ, Huang, SS, Zhang, SS, et al. A gross deletion of the PAX3 gene in a large Chinese family with Waardenburg syndrome type I. World J Pediatr. (2023) 19:1203–7. doi: 10.1007/s12519-023-00746-2
20. Huang, S, Song, J, He, C, Cai, X, Yuan, K, Mei, L, et al. Genetic insights, disease mechanisms, and biological therapeutics for Waardenburg syndrome. Gene Ther. (2022) 29:479–97. doi: 10.1038/s41434-021-00240-2
21. Wen, J, Song, J, Chen, J, Feng, Z, Jing, Q, Gong, W, et al. Modeling of pigmentation disorders associated with MITF mutation in Waardenburg syndrome revealed an impaired melanogenesis pathway in iPS-derived melanocytes. Pigm Cell Melanoma Res. (2023) 37:21–35. doi: 10.1111/pcmr.13118
22. Wang, Y, Chai, Y, Zhang, P, and Zang, W. A novel variant of the SOX10 gene associated with Waardenburg syndrome type IV. BMC Med Genet. (2023) 16:147. doi: 10.1186/s12920-023-01572-1
23. Guo, M, Li, Q, Jiang, C, Li, S, and Ruan, B. A de novo mutation in SOX10 in a Chinese boy with Waardenburg syndrome type 2. J Int Adv Otol. (2023) 19:255–9. doi: 10.5152/iao.2023.22745
24. Zhang, L, Wan, Y, and Wang, N. Waardenburg syndrome type 4 coexisting with open-angle glaucoma: a case report. J Med Case Rep. (2022) 16:264. doi: 10.1186/s13256-022-03460-1
25. Chandra Mohan, SLN. Case of Waardenburg Shah syndrome in a family with review of literature. J Otolaryngol. (2018) 13:105–10. doi: 10.1016/j.joto.2018.05.005
26. Archbold, S, Lutman, ME, and Nikolopoulos, T. Categories of auditory performance: inter-user reliability. Br J Audiol. (1998) 32:7–12. doi: 10.3109/03005364000000045
27. Allen, MC, Nikolopoulos, TP, and O’Donoghue, GM. Speech intelligibility in children after cochlear implantation. Am J Otolaryngol. (1998) 19:742–6.
28. Ching, TY, and Hill, M. The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) scale: normative data. J Am Acad Audiol. (2007) 18:220–35. doi: 10.3766/jaaa.18.3.4
Keywords: Waardenburg syndrome, cochlear implantation, auditory, speech, meta-analysis
Citation: Qin F, Guo S, Yin X, Lu X and Ma J (2024) Auditory and speech outcomes of cochlear implantation in patients with Waardenburg syndrome: a meta-analysis. Front. Neurol. 15:1372736. doi: 10.3389/fneur.2024.1372736
Edited by:
Philippe Perrin, Université de Lorraine, FranceReviewed by:
Jorge Humberto Ferreira Martins, Polytechnic Institute of Porto, PortugalErika Celis-Aguilar, Autonomous University of Sinaloa, Mexico
Copyright © 2024 Qin, Guo, Yin, Lu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Ma, Njc1MDkwODk3QHFxLmNvbQ==
 Feng Qin
Feng Qin Siquan Guo3
Siquan Guo3