- Center for Rehabilitation Medicine, Department of Neurology, Zhejiang Provincial Peoples Hospital (Affiliated People's Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, China
Objective: The study aimed to investigate whether branch-site occlusion (BSO) sign could predict the etiology of acute intracranial large artery occlusion (ILVO) and the stentriever (SR) response.
Methods: We systematically reviewed studies that evaluated the predictive role of BSO for the etiology of ILVO-AIS or EVT outcome between 1 January 2000 and 31 August 2022 from PubMed, Embase, and Web of Science.
Results: The sensitivity and specificity of BSO sign predicting etiology of ILVO-AIS were 0.87 (95% CI 0.81–0.91) and 0.64 (95% CI 0.33–0.87), respectively. The sensitivity and specificity of BSO sign predicting stentriever response were 0.84 (95% CI 0.63–0.94) and 0.61 (95% CI 0.18–0.92), respectively.
Conclusion: The BSO sign could be a valid and precise imaging marker to predict embolism caused ILVO-AIS and recanalization success by SR without rescue therapy.
1. Introduction
Acute ischemic stroke (AIS) caused by intracranial large vessel occlusion (ILVO) is a severe and life-threatening disease (1, 2). Endovascular therapy (EVT) is an effective treatment for patients who fail intravenous thrombolysis that may improve prognosis in patients with ILVO-AIS (3, 4). Nevertheless, EVT is not successful in all patients. The possible causes of recanalization failure are different etiologies of ILVO, such as intracranial atherosclerosis (ICAS) and embolism (5). Therefore, it is helpful for identifying the cause of occlusion before performing EVT (3, 5, 6). However, in clinical practice, it is difficult to distinguish between ICAS-AIS and embolism-AIS. Along with the evolution of EVT techniques, intra-procedural angiographic signs have been demonstrated in previous studies and may help to understand the potential etiologies of ILVO-AIS (7). Thus, for accurate and timely selection of EVT strategies, focus should be given to intra-procedural angiographic signs.
Recently, several studies have shown that the branching-site occlusion (BSO) sign in large intracranial arteries can be used as an intra-procedural angiographic sign for predicting embolic occlusion (5, 8, 9). The BSO sign is identified as ILVO with at least one of the following three angiographic signs: (1) anterior communicating artery collateral flow that could not advance to the contralateral internal or middle cerebral artery because the internal cerebral artery bifurcation site was involved (T-occlusion); (2) direct visualization of a Y-shaped or T-shaped filling defect involving a branching site (Y- or T-shaped clot); and (3) another branch could not be seen or was only partially seen when the stentriever was deployed up to one branch across the occlusion site (9). Furthermore, previous studies also showed that the BSO sign can predict the procedural success of the stentriever (SR) without rescue therapy (RT), which has been an effective EVT strategy for ILVO-AIS (9, 10). However, results regarding the predictive value of the BSO sign for the procedural success of the SR without RT varied across studies. Therefore, we performed this meta-analysis to estimate the predictive value of the BSO sign for embolic-related ILVO or AIS (embolic-ILVO/AIS) and the procedural success of the SR without RT in this study.
2. Methods
2.1. Search strategy
We implemented the meta-analysis according to PRISMA guidelines (11). Eligible studies must have a clear definition of the BSO sign and ILVO-AIS. A BSO sign was identified as the direct visualization of a Y-shaped or T-shaped filling defect involving a branching site (9). ILVO-AIS was defined as a stroke caused by occlusion of the internal carotid artery, or middle cerebral arteries, M1 and M2, verified by vascular exams (12).
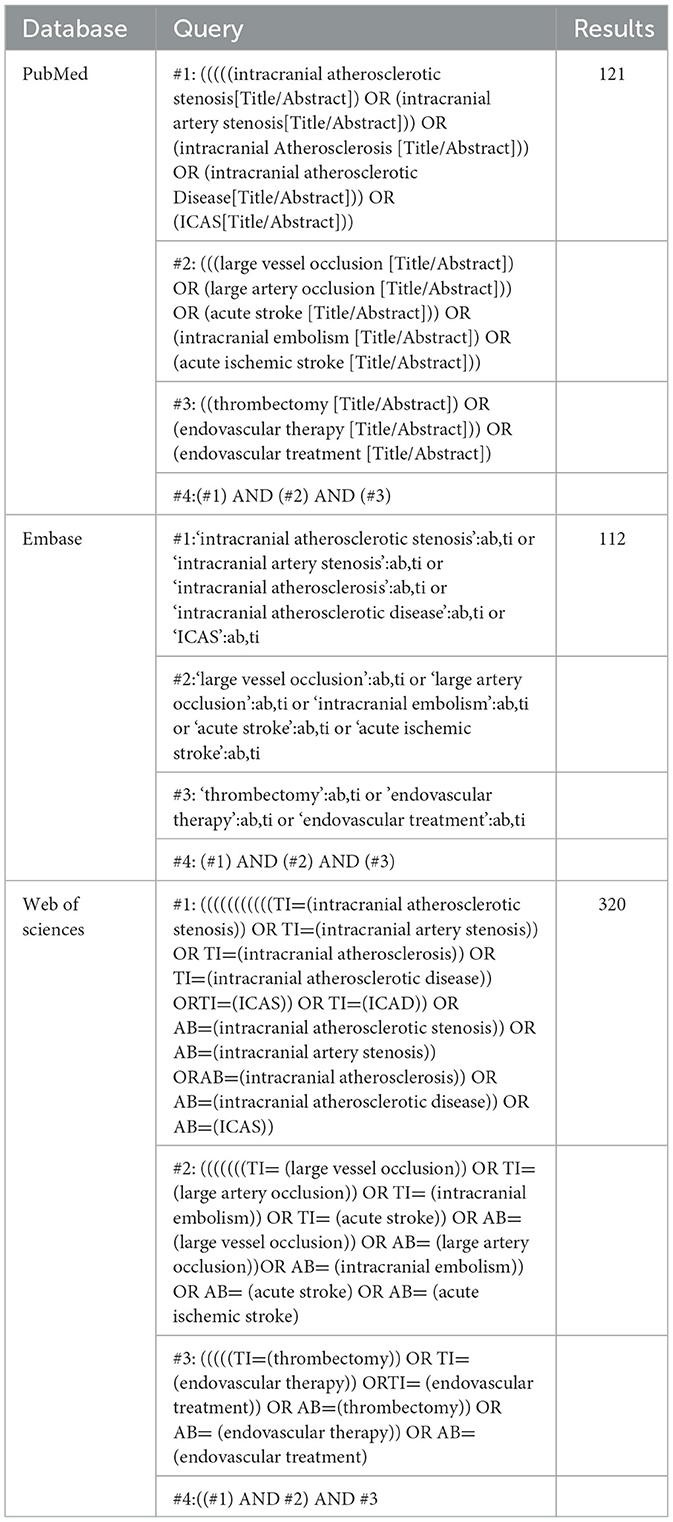
We systematically searched PubMed, Embase, and Web of Science for studies that assessed the BSO for predicting the etiology of ILVO-AIS or EVT outcome between 1 January 2000 and 31 August 2022. The search language was limited to English. The search strategy is available in Table 1.
2.2. Selection criteria
The inclusion criteria of this study were as follows: (1) studies investigating the association between the BSO sign and outcomes; (2) etiology of LVO-AISs treated with EVT; and (3) studies that provided sufficient information for the construction of the 2 × 2 contingency table (i.e., true and false positives and negatives were provided). Reviews, correspondences, case reports, expert opinions, editorials, and studies with incomplete data were all excluded from the analysis.
2.3. Endpoints
(1) Etiology: Stroke etiologies were defined as ICAS or embolism according to the criteria used among the included studies (3, 9, 13).
(2) Therapeutic outcomes: They are defined as recanalization success (RS) by SR without RT. RS was defined as mTICI 2b-3 (9, 10, 14).
2.4. Data extraction and quality assessment
Two investigators independently assessed the included studies and extracted data. Data for the etiology and therapeutic outcomes were extracted to calculate true and false positive and negative rates. We assessed the quality of included studies according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) by RevMan 5.4 software. The QUADAS-2 consists of four parts, each containing multiple items. The response options for each item were yes, no, and unclear (15).
2.5. Statistical analysis
2 × 2 tables were tabulated through true- and false-positives and true- and false-negatives for evaluating the predictive value of the BSO sign for the etiology of ILVO-AIS. We used the numbers to calculate sensitivity and specificity with corresponding 95% confidence intervals (CI). Data were synthesized using a bivariate meta-analysis with a random-effects model developed for synthesis. The meta-analysis was performed by calculating the pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and incidence of RS after SR. To graphically present the results, we plotted the hierarchical summary receiver operating characteristic curves and calculated the area under the curve with its 95% CI by the MIDAS module for STATA (version 15.1) (16). Statistical significance was defined as a p-value of ≤ 0.05. We calculated I2 to assess the degree of heterogeneity across studies. Heterogeneity was assessed by meta-regression. Publication bias was assessed by funnel plot.
3. Results
3.1. Search results
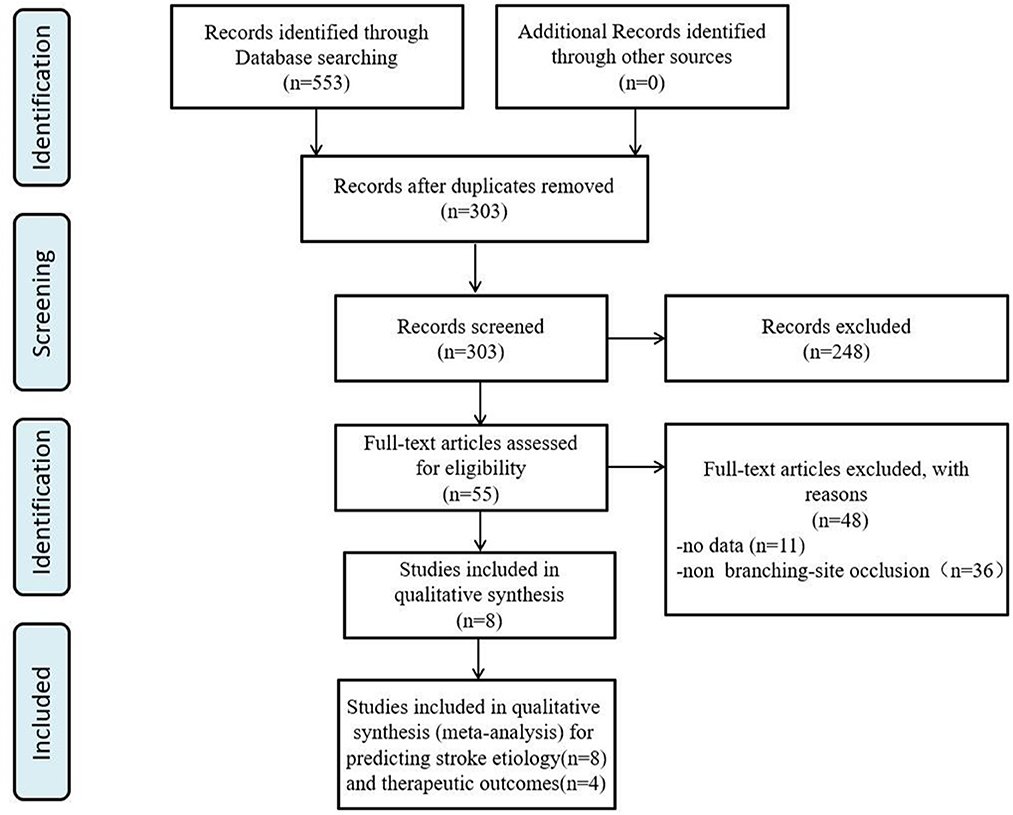
We identified 553 articles through database searches. After removing duplicates (250) and studies that did not examine the association between imaging signs with ILVO-AIS (n = 248), 55 articles remained for further analysis. Of these, 48 articles were excluded: 12 studies provided insufficient raw data, and the other 36 studies assessed imaging signs other than the BSO sign. We finally obtained a total of seven studies for analysis (Figure 1).
3.2. Study characteristics
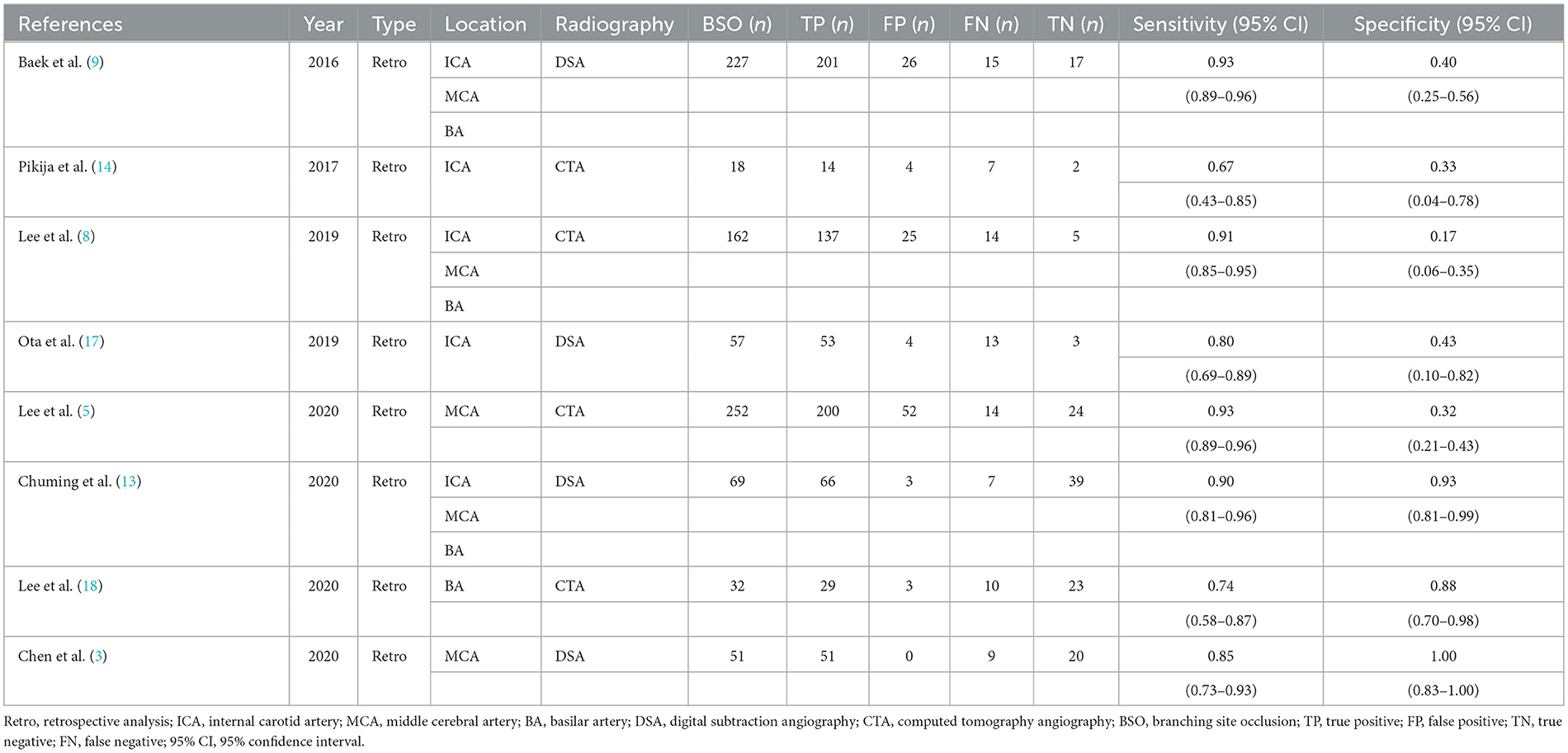
A total of seven studies (1,017 patients) were included. All results, except one, demonstrated the relationship between the BSO sign and the etiology of ILVO-AIS. The main characteristics of each study are shown in Table 2. All studies were retrospective analyses and provided specific inclusion and/or exclusion criteria. The number of studies using DSA to demonstrate the BSO sign (3) was approximately equal to that of CTA (4).
3.3. Quality assessment
The overall quality was moderate (Figure 2). None of the included studies fulfilled all the items of the QUADAS-2 checklist. Some studies did not report whether a consecutive or random sample of patients was enrolled (8, 13, 18); two studies did not mention whether the BSO sign was interpreted without knowledge of the results of the reference standard, and it was unclear if reviewers of the reference standard were blinded to the results of TTO sign (Figure 2) (8, 14).
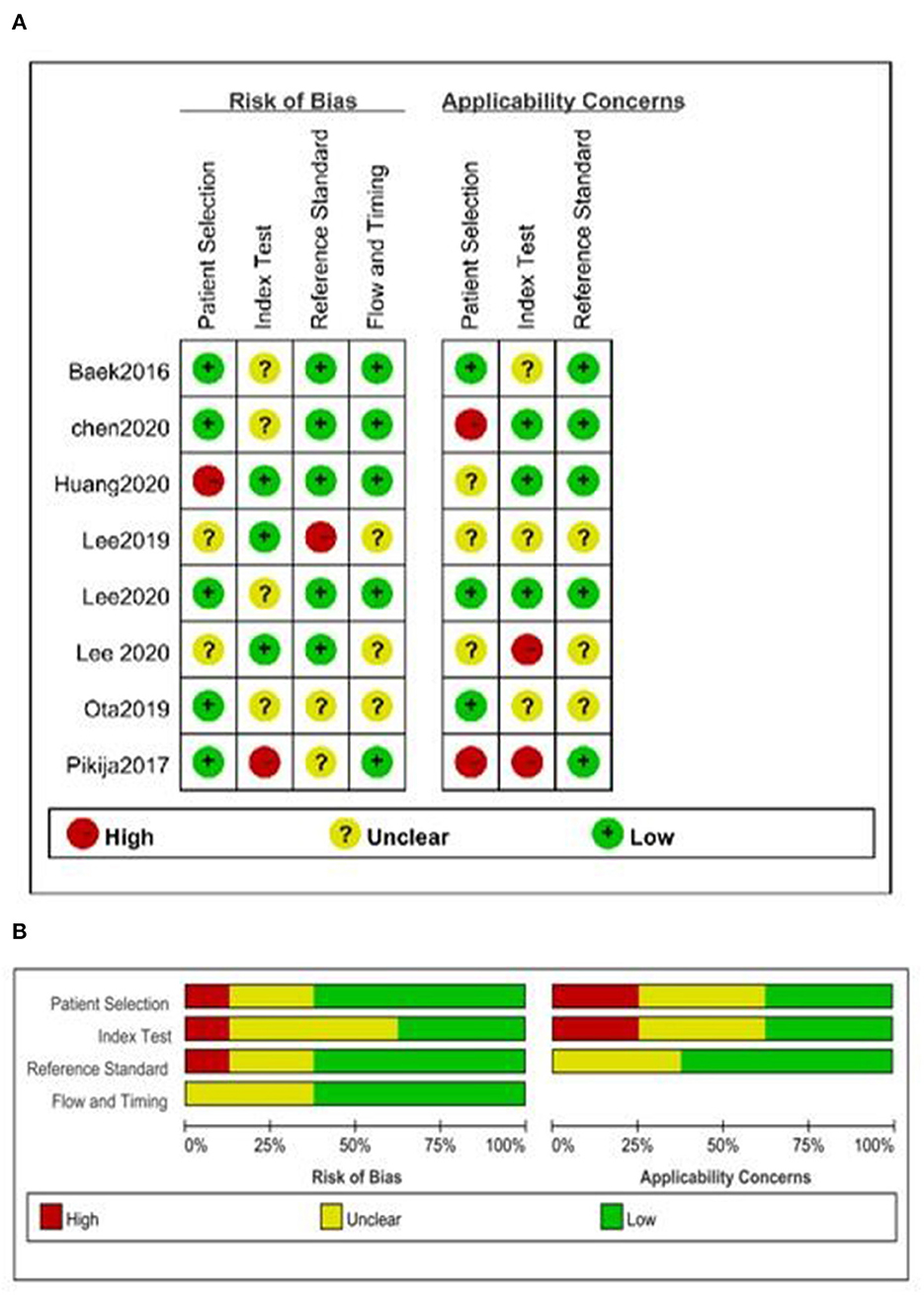
Figure 2. Quality assessment of included studies. (A) Summary of risk of bias and applicability concerns and (B) risk of bias and applicability concerns graph.
3.4. Meta-analysis
3.4.1. The predictive value of the BSO sign for predicting ILVO-AIS etiology
A total of 1,090 patients were included in the meta-analysis for predicting the value of the BSO sign for embolism-AIS. Of these, 868 patients had a BSO sign evident by DSA or CTA, 627 patients of whom were finally diagnosed with embolism-AIS; the remaining cases were diagnosed as ICAS-AIS. Summary estimates of sensitivity and specificity by random-effects were 0.87 (95% CI 0.81–0.91) and 0.64 (95% CI 0.33–0.87), respectively (Figure 3A). The area under the curve was 0.88 (95% CI 0.85–0.91) (Figure 3E). The positive likelihood ratio was 2.5 (95% CI 1.1–5.7) (a person diagnosed with embolism-AIS by BSO sign is 2.5 times more likely than non-embolism), the negative likelihood ratio was 0.20 (95% CI 0.12–0.33), and the diagnostic odds ratio was 12 (95% CI 3–44). In patients with suspected AIS, in the case of 50% pre-test probability, the post-test probability of a positive test result was 71% (Figure 3C).
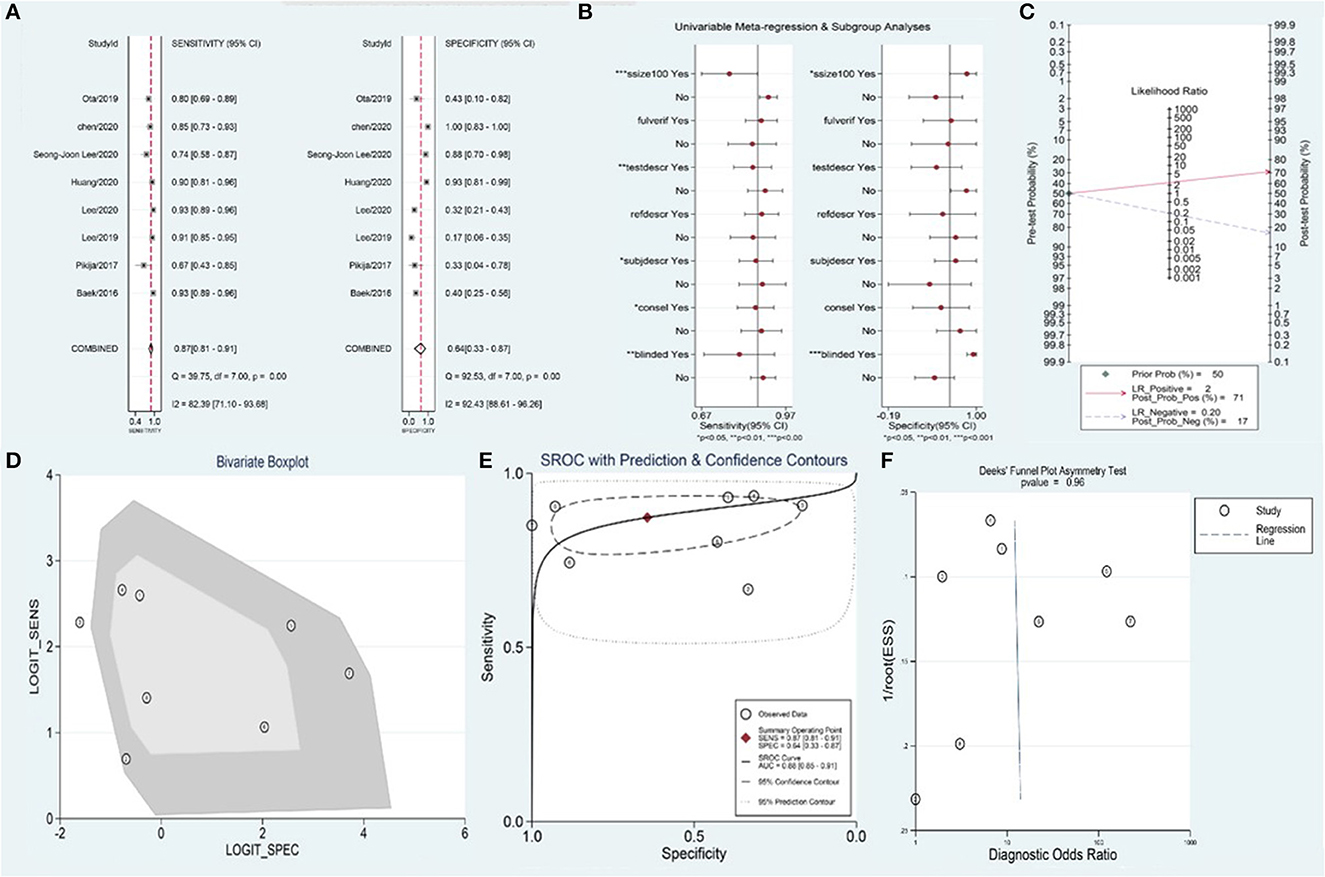
Figure 3. Meta-analysis of BSO for diagnosis of embolism-AIS. (A) Sensitivity and specificity of BSO for the diagnosis of embolism-AIS, (B) meta-regression of the BSO for diagnosis of embolism-AIS, (C) Fagan nomogram of the BSO for diagnosis of embolism-AIS, (D) bivariate boxplot of the BSO for diagnosis of embolism-AIS, (E) summary receiver operating characteristic (ROC) curve of BSO for diagnosis of embolism-AIS, and (F) funnel plot of publication bias about BSO diagnosis of embolism-AIS.
The overall I2 for the bivariate model was 97% (95% CI 0.95–0.99%), suggesting there was substantial heterogeneity between studies (Figure 3D). Meta-regression indicated that the heterogeneity of sensitivity mainly came from the sample size, blinding, and the consistency of index test standard and reference standard of included studies. Additionally, the heterogeneity of specificity mainly came from the sample size and blinding of included studies (Figure 3B). No significant publication bias was observed in the included studies (p = 0.96) (Figure 3F).
3.4.2. Therapeutic outcomes
A total of 517 patients were included in the meta-analysis evaluating the predictive value of the BSO sign for RS after SR without RT. RS after SR without RT was achieved in 495 patients, and the BSO sign was found in 295 of these cases.
The pooled sensitivity and specificity were 0.84 (95% CI 0.63–0.94) and 0.61 (95% CI 0.18–0.92), respectively (Figure 4A). The area under the curve was 0.83 (95% CI 0.80–0.86) (Figure 4B). The positive likelihood ratio was 2.2 (95% CI 0.7–6.6), the negative likelihood ratio was 0.26 (95% CI 0.12–0.58), and the diagnostic odds ratio was 8 (95% CI 2.0–4.0). In patients with suspected RF after primary thrombectomy, with a pre-test probability of 50%, the post-test probability of positive test results was 69% (Figure 4C).

Figure 4. Meta-analysis of BSO for the prediction of RS after SR without rescue therapy. (A) Sensitivity and specificity of BSO for the prediction of RS after SR without RT, (B) summary ROC curve of BSO for the prediction of RS after SR without RT, (C) Fagan nomogram of the BSO for the prediction of RS after SR without RT, (D) bivariate boxplot of BSO for the prediction of RS after SR without RT, and (E) funnel plot of publication bias about BSO for the prediction of RS after SR without RT.
The overall I2 for the bivariate model was 97% (95% CI 95–99), suggesting there was substantial heterogeneity between studies (Figure 4D). To further determine the source of heterogeneity, we planned to perform a meta-regression analysis, but it could not be achieved due to the small number of included studies. No significant publication bias was observed in the included studies (p = 0.94) (Figure 4E).
4. Discussion
Stentriever thrombectomy is the main EVT technique used for ILVO-AIS (19). However, it is ineffective in ~20% of patients (20, 21). It is thought that the success of SR is associated with the pathogenesis of ILVO-AIS (10). Non-embolic ILVO-AIS resulting from intracranial atherosclerotic stenosis tends to get less benefit from SR. Therefore, the pre-procedural identification of ILVO-AIS etiology may predict the effect of SR and help operators choose the optimal EVT strategy to increase RS rates and shorten puncture-to-recanalization times (9).
Several methods, including the hyperdense artery sign, susceptibility vessel sign, fixed focal stenosis on follow-up imaging, histological examination of extracted clots, comprehensive cardiological evaluation after the lapse of the hyperacute stage, and intra-procedural angiographic signs (cut-off, tapered, and meniscus) could predict the pathogenesis of ILVO-AIS and response to first-line endovascular strategies (7). However, several studies have reported the relationship between the BSO sign and the etiology of ILVO-AIS (9, 14). It was thought that the BSO sign could be an independent predictor of the underlying stroke mechanism and SR response. In actuality, the concept of the BSO sign is related to the generation of embolic-ILVO-AIS. An embolus or floating clot has a greater tendency to obstruct an arterial branching site, while on the contrary, ICAS seems to predominantly involve arterial trunks before bifurcation (22). In this study, we conducted a meta-analysis to evaluate the sensitivity and specificity of the BSO sign as an imaging marker of embolic ILVO-AIS. We found that the BSO sign could be a valid and precise marker to predict embolic ILVO-AIS, with a sensitivity of 87% and a specificity of 64%.
Patients with ILVO-AIS from different causes responded differently to SR treatment. In cases resulting from ICAS, very high re-occlusion rates were present after initial recanalization by RS. This resulted in prolonged puncture-to-recanalization time and a worse prognosis. The use of an SR might cause atheromatous surface injury, thus increasing platelet activation and leading to re-occlusion and even dissection. On the contrary, AIS caused by embolism was highly responsive to SR-based EVT and was associated with a very low re-occlusion rate. Previous studies have shown that patients with embolic occlusion treated with SR presented with both higher recanalization rates and better prognoses (23–25). Embolism-AIS is commonly caused by atrial fibrillation, which usually consists of red thrombi that are rich in erythrocytes (26). The characteristics of the red thrombi were higher clot burden and higher density than white thrombi (6, 27). These physiological characteristics may be associated with the successful SR (6, 9). However, on the contrary, previous studies showed that thrombi from a large atherosclerotic artery had the highest volume fraction of RBCs, while cardioembolic thrombi had the lowest (28). Further studies are needed for revealing the association between the etiology and the pathogenesis of cerebral thrombi to improve the efficacy of choosing first-line endovascular strategies and clinical outcomes (29).
In this context, the identification of ILVO-AIS etiology is critical for EVT strategy choice, which might influence the recanalization success rate and patient prognosis. Our results showed that patients with embolic-AIS treated by SR had favorable clinical outcomes. Additionally, the BSO sign could be a valid and precise imaging marker to predict RS by SR without RT with a sensitivity of 84% and a specificity of 61%.
4.1. Conclusion
Our meta-analysis found that the BSO sign could be a valid and precise imaging marker to predict embolic ILVO-AIS and RS by SR without RT. This may provide potential evidence for choosing a first-line EVT strategy to increase the RS rate and shorten puncture-to-recanalization time.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XJ drafting and revision of the manuscript for content, including medical writing for content, study concept or design, and analysis or interpretation of data. ZS drafting and revision of the manuscript for content, including medical writing for content, major role in the acquisition of data, and analysis or interpretation of data. PW major role in the acquisition of data. XW and FL drafting and revision of the manuscript for content, including medical writing for content, major role in the acquisition of data, study concept or design, and analysis or interpretation of data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ospel JM, Holodinsky JK, Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1832–43. doi: 10.1016/j.jacc.2019.10.034
3. Chen WH, Yi TY, Zhan AL, Wu YM, Lu YY, Li YM, et al. Stent-unsheathed effect predicts acute distal middle cerebral artery atherosclerotic disease-related occlusion. J Neurol Sci. (2020) 416:116957. doi: 10.1016/j.jns.2020.116957
4. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
5. Lee SJ, Park SY, Hong JM, Choi JW, Kang DH, Kim YW, et al. Etiological approach to understanding recanalization failure in intracranial large vessel occlusion and thrombectomy: close to embolism but distant from atherosclerosis. Front Neurol. (2020) 11:598216. doi: 10.3389/fneur.2020.598216
6. Yi TY, Chen WH, Wu YM, Zhang MF, Zhan AL, Chen YH, et al. Microcatheter “first-pass effect” predicts acute intracranial artery atherosclerotic disease-related occlusion. Neurosurgery. (2019) 84:1296–305. doi: 10.1093/neuros/nyy183
7. Liang W, Wang Y, Du Z, Mang J, Wang J. Intraprocedural angiographic signs observed during endovascular thrombectomy in patients with acute ischemic stroke: a systematic review. Neurology. (2021) 96:1080–90. doi: 10.1212/WNL.0000000000012069
8. Lee SJ, Hong JM, Choi JW, Kang DH, Kim YW, Kim YS, et al. CTA-based truncal-type occlusion is best matched with postprocedural fixed focal stenosis in vertebrobasilar occlusions. Front Neurol. (2018) 9:1195. doi: 10.3389/fneur.2018.01195
9. Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Song D, et al. Importance of truncal-type occlusion in stentriever-based thrombectomy for acute stroke. Neurology. (2016) 87:1542–50. doi: 10.1212/WNL.0000000000003202
10. Baek JH, Kim BM, Yoo J, Nam HS, Kim YD, Kim DJ, et al. Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke. (2017) 48:2746–52. doi: 10.1161/STROKEAHA.117.018096
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
13. Chuming H, Yifan H, Ke X, Chukai X, Weijie Z, Hui L, et al. Association between occlusion type and etiology of acute intracranial large artery occlusion. Front Neurol. (2020) 11:582388. doi: 10.3389/fneur.2020.582388
14. Pikija S, Magdic J, Killer-Oberpfalzer M, Florea C, Hauer L, Novak HF, et al. Proximal flow to middle cerebral artery is associated with higher thrombus density in terminal internal carotid artery occlusion. Ann Clin Transl Neurol. (2017) 4:517–21. doi: 10.1002/acn3.429
15. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
16. Dwamena B. MIDAS: Stata module for Meta-Analytical Integration of Diagnostic Test Accuracy Studies. Statistical Software Components (2007).
17. Ota T, Shigeta K, Amano T, Kaneko J. Endovascular treatment for acute carotid T and carotid non-T occlusion: a retrospective multicentre study of 81 patients. Interv Neuroradiol. (2019) 25:497–501. doi: 10.1177/1591019919846568
18. Lee SJ, Hong JM, Choi JW, Park JH, Park B, Kang DH, et al. Predicting Endovascular treatment outcomes in acute vertebrobasilar artery occlusion: a model to aid patient selection from the ASIAN KR registry. Radiology. (2020) 294:628–37. doi: 10.1148/radiol.2020191227
19. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association Focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:3020–35. doi: 10.1161/STR.0000000000000074
20. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
21. Song D, Cho AH. Previous and recent evidence of endovascular therapy in acute ischemic stroke. Neurointervention. (2015) 10:51–9. doi: 10.5469/neuroint.2015.10.2.51
22. Jiang WJ, Wang YJ, Du B, Wang SX, Wang GH, Jin M, et al. Stenting of symptomatic M1 stenosis of middle cerebral artery: an initial experience of 40 patients. Stroke. (2004) 35:1375–80. doi: 10.1161/01.STR.0000128018.57526.3a
23. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
24. Lee JS, Lee SJ, Hong JM, Choi JW, Yoo J, Hong JH, et al. Solitaire thrombectomy for acute stroke due to intracranial atherosclerosis-related occlusion: ROSE ASSIST study. Front Neurol. (2018) 9:1064. doi: 10.3389/fneur.2018.01064
25. Lee JS, Lee SJ, Yoo JS, Hong JH, Kim CH, Kim YW, et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J Stroke. (2018) 20:394–403. doi: 10.5853/jos.2018.01627
26. Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. (2005) 36:2379–83. doi: 10.1161/01.STR.0000185932.73486.7a
27. Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. (2013) 44:243–5. doi: 10.1161/STROKEAHA.112.674127
28. Weisel JW, Litvinov RI. Visualizing thrombosis to improve thrombus resolution. Research and practice in thrombosis and haemostasis. (2021) 5:38–50. doi: 10.1002/rth2.12469
Keywords: branch-site occlusion sign, ILVO-AIS, endovascular therapy, stentriever, neurology
Citation: Jiang X, Shi Z, Wang P, Wang X and Liu F (2023) Branch-site occlusion sign predicts the embolic origin of acute ischemic stroke: a meta-analysis. Front. Neurol. 14:1139756. doi: 10.3389/fneur.2023.1139756
Received: 07 January 2023; Accepted: 16 May 2023;
Published: 07 June 2023.
Edited by:
Slaven Pikija, University Hospital Salzburg, AustriaReviewed by:
Takeshi Yoshimoto, National Cerebral and Cardiovascular Center, JapanAndrei Filioglo, Emergency Medicine Institute, Moldova
Copyright © 2023 Jiang, Shi, Wang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, ZmVpaGFpMTAyM0BzaW5hLmNu; Xu Wang, MTQ1MDAxMzA3MkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Xinzhao Jiang
Xinzhao Jiang Zongjie Shi
Zongjie Shi Peng Wang
Peng Wang Xu Wang
Xu Wang