
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nanotechnol., 19 March 2025
Sec. Biomedical Nanotechnology
Volume 7 - 2025 | https://doi.org/10.3389/fnano.2025.1475969
 Alireza Nomani1*
Alireza Nomani1* Aishwarya Saraswat1
Aishwarya Saraswat1 Yu Zhang2
Yu Zhang2 Ashwin C. Parenky1†
Ashwin C. Parenky1† Chun-Tien Jimmy Kuo2
Chun-Tien Jimmy Kuo2 Heather Brown2
Heather Brown2 Suzanne Hartford2†
Suzanne Hartford2† Bindhu Rayaprolu1
Bindhu Rayaprolu1 Amardeep Singh Bhupender Bhalla1
Amardeep Singh Bhupender Bhalla1 Mohammed Shameem1
Mohammed Shameem1Ribonucleic acid-lipid nanoparticle (RNA-LNP) therapeutics, a powerful nanomedicine platform, have already demonstrated their efficacy in diverse applications. Their improved stability and efficacy are exemplified by successful and rapid launch of mRNA vaccines, as well as marketed siRNA drug product. Beyond infectious diseases, RNA-LNPs show promise in addressing unmet needs in women’s health, for instance, gynecologic cancers (e.g., ovarian, cervical) and novel treatments for conditions such as osteoporosis, endometriosis, and congenital disorders. However, important challenges persist, including off-target effects, immunogenicity, and potential risks and ethical issues in their application for pregnant or lactating women. This review summarizes current key preclinical and clinical progress, discusses targeting strategies of LNPs (e.g., active and passive delivery), and presents current knowledge on RNA-LNP safety in pregnant and non-pregnant women and neonates as vulnerable populations. As RNA-LNP technologies evolve – with relevant preclinical animal models, next-generation RNA platforms and improved lipid chemistries – they can hold significant potential for transforming care in women’s health through safer, effective, personalized, and innovative curative interventions.
RNA-LNP therapeutics is an advancing field with substantial potential for disease prevention or treatment (Hou et al., 2021; Kulkarni et al., 2019). Lipid nanoparticles (LNPs) are efficient delivery systems that can facilitate the delivery of therapeutic RNA or DNA molecules to specific cells or tissues, improving the stability, biodistribution, and intracellular uptake of these delicate macromolecules. The success of mRNA-LNPs in vaccine development, exemplified by coronavirus disease 2019 (COVID-19) and respiratory syncytial virus (RSV) vaccines, as well as the success of commercially available patisiran, a siRNA-based lipid nanoparticle, has opened ample research and development opportunities in the RNA-LNP therapeutics. Notably, RNA therapeutics show promising applications in women’s health, helping to address unmet needs in gynecological and obstetric conditions, such as endometriosis, gynecologic cancers, and viral infections (Zhang et al., 2021; Gildiz and Minko, 2023). Moreover, by conjugating cell-specific ligands or antigens to the LNP surfaces, RNA-LNPs can achieve explicit tissue targeting while minimizing systemic exposure. Despite the clear benefits, important challenges persist, including off-target effects, unwanted immune system activation due to the types of lipids or RNAs used in the formulations, and potential impacts on the female reproductive systems, motherhood, or neonatal developmental course in pregnant women.
RNA therapeutics offers unique opportunities for gene expression (e.g., therapeutic proteins, antigen-based vaccines) and gene manipulation/editing (e.g., knockdown, knockout) (Damase et al., 2021; Zhu et al., 2022). Advances in LNP-based delivery system have paved the way for more effective and scalable RNA medicines (Paunovska et al., 2022). The gradually increasing number of RNA-based medications on the market, coupled with the growing body of preclinical and clinical studies, underscores the rising significance of these gene therapy modalities. Among these, mRNA has seen a surge in clinical trials and development due to its improved safety and in vivo efficiency over the past few decades (Sahin et al., 2014; Xiong et al., 2018). mRNA has been used in a wide range of applications, such as vaccine and immunotherapy (Pardi et al., 2018; Buschmann et al., 2021; Pozharov and Minko, 2023), protein or enzyme replacement therapy (Qin et al., 2022), and transient cellular reprogramming (Warren and Lin, 2019).
Beyond mRNA, RNA interferences (RNAi) platforms (siRNAs, miRNA, and lncRNA-coding RNAs) enable sequence specific silencing of aberrant genes (Friedrich and Aigner, 2022; Cieśla and Pandey, 2020; Hu et al., 2020). siRNAs, for instance, can transiently reduce gene expression by destroying their target mRNA to treat a range of diseases (Sajid et al., 2021; Ngamcherdtrakul and Yantasee, 2019). Although miRNAs and lncRNAs function slightly differently from siRNA they likewise show therapeutic potential in modulating cellular processes, including inflammation and tumorigenesis (Cieśla and Pandey, 2020). Meanwhile, CRISPR/Cas systems can permanently edit the genome guided by small sequence of accompanying gRNAs in their formulations, thus making this gene editing approach a possibility for heritable conditions (Cai et al., 2016). Yet, across these approaches, one of the greatest challenges remains safely and efficiently delivering RNA to the correct intracellular compartment at sufficient dose (Paunovska et al., 2022; Buschmann et al., 2021). Presently, lipids and lipid-like materials, such as LNPs, stand out as the most effective, synthetic, non-viral agents for delivering RNAs. LNPs have achieved clinical success in mRNA delivery, especially demonstrated by their application in mRNA-LNP vaccines against COVID-19 and RSV (Eygeris et al., 2022; Skerritt et al., 2024; Wilson et al., 2023).
LNPs exhibit a uniform spherical morphology within their self-assembled structures in aqueous environments (Figure 1). They have gained popularity as a delivery system for nucleic acids due to their ability to effectively shield and protect the payload in vivo and enhance intracellular delivery. This payload protection is especially critical for mRNAs as they are inherently unstable macromolecular structures and susceptible to rapid degradation by environmental stresses and nucleases during storage post-manufacturing and when administered to patients.
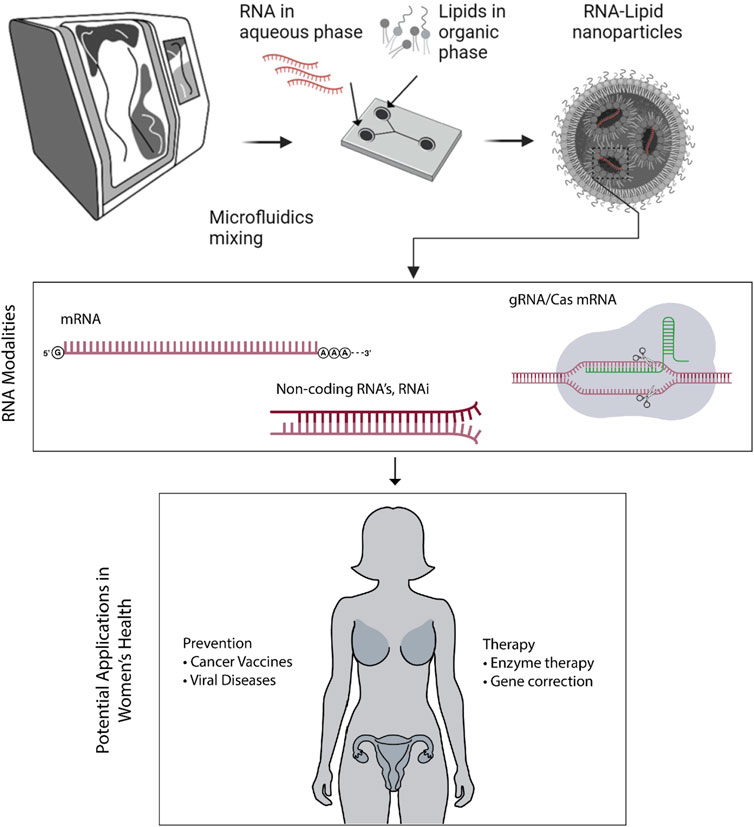
Figure 1. LNPs can be manufactured through robust microfluidics mixing method, carrying different RNA modalities. These modalities have potential applications in women’s health through preventative or therapeutic approaches.
The versatile potential of RNA-LNP therapy also extends to the critical areas of women’s health. Specifically, RNA-LNP therapy presents two key applications in this field: (i) as a standalone or in combination with orthodox protocols of therapy in gynecological and obstetric conditions; and (ii) as prophylactic vaccines, such as mRNA-based immunizations (Figure 1). While administration routes play a significant role in the clinical efficacy, this review focuses on how RNA-LNP systems are being applied in women’s disease. For a deeper discussion of administration strategies, readers may refer to prior reviews (e.g., (Swingle et al., 2023a) which is an informative article on nanoparticle delivery in this regard).
Additionally, this review provides a comprehensive overview of research, preclinical and clinical studies on RNA therapy in women’s diseases, particularly focusing on therapeutic and vaccine applications. We also delve into targeting strategies that can enhance RNA-LNP applications in women’s health. Moreover, we offer a brief overview of relevant low-cost small animal models and highlight safety considerations, including immunogenicity and possible reproductive system impacts in women.
Therapeutic modalities such as small molecule drugs, monoclonal antibodies (mAbs), and RNA-based therapies employ distinct mechanisms to silence or activate certain pathways through the target proteins with each modality having their own advantages and challenges (Figure 2). Small molecule drugs bind to the active site of proteins to inhibit or activate their function. Their low molecular weight and simpler structure enhance diffusion across membranes, enabling oral bioavailability and sometimes crossing the membrane barriers such as blood-brain barrier. They often show predictable pharmacokinetics (PK) and lower costs, though challenges like drug resistance, poor solubility, dose limitation, short half-life, and off-target toxicity remain (Liu G. H. et al., 2020). In comparison, mAbs can act by multiple ways including blocking of cellular signaling pathways, modulation of the immune system, immune-mediated cell toxicity, and/or vascular disruption. By that, mAbs represent higher sensitivity, better specificity, and less cross-reactivity and toxicity compared to small molecules. Yet, their large molecular size, high manufacturing cost, immunogenicity, complex PK, short to moderate half-life and poor stability pose major hurdles for their successful clinical translation (Rodriguez-Nava et al., 2023).
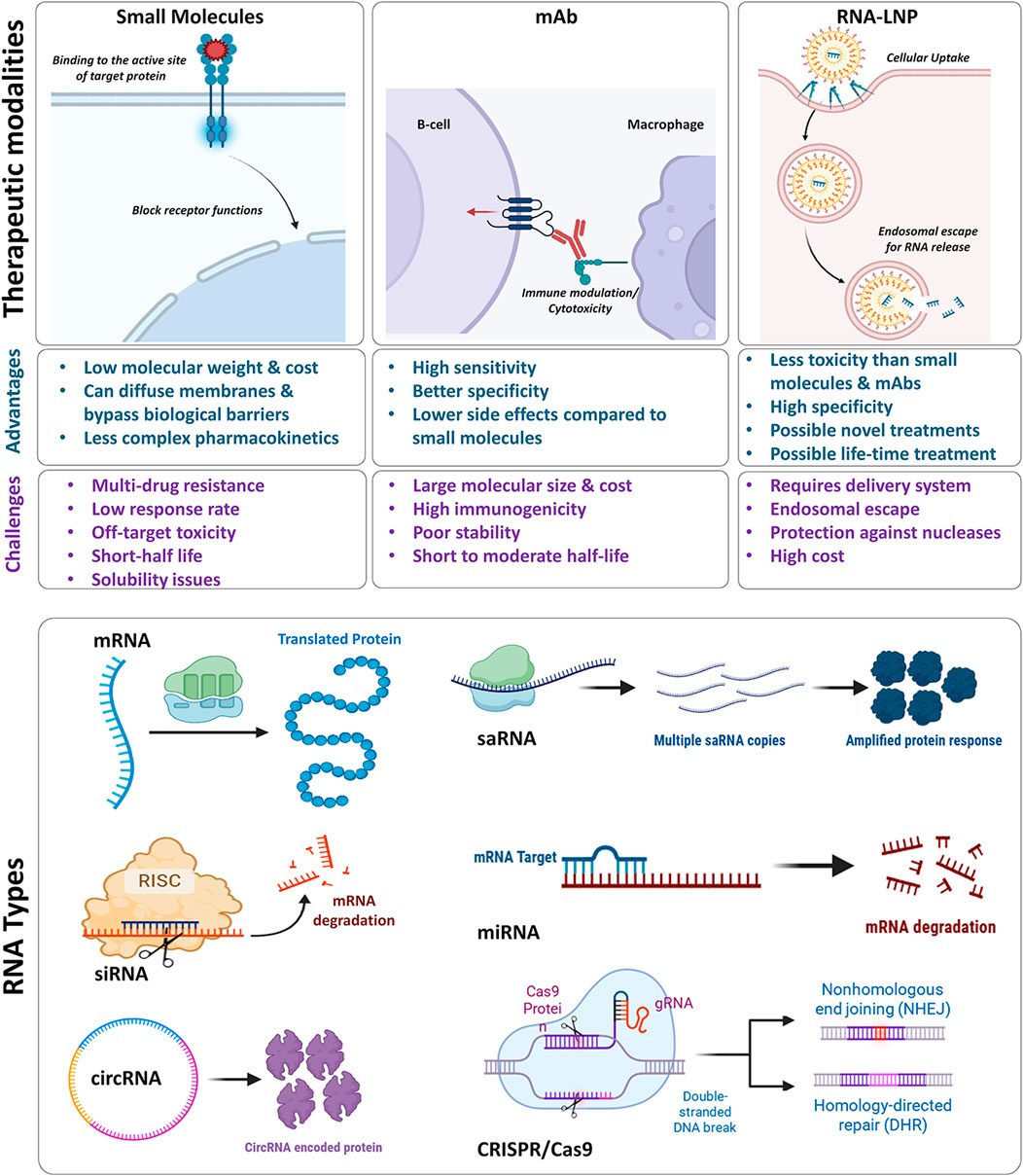
Figure 2. Upper panel represents comparison of different therapeutic modalities including small molecules, monoclonal antibodies, and RNA-LNPs. It compares their mechanism of actions, advantages, and challenges. Bottom panel shows different RNA types and their molecular mode of actions. The figure has been made using icons from BioRender.com
RNA therapeutics can overcome some of the challenges associated with traditional small molecules and mAbs, encompassing a variety of RNA types (mRNA, siRNA, circular RNA, saRNA, microRNA, and CRISPR/Cas9) (Figure 2, bottom panel). For example, mRNA, once delivered to the cells by the appropriate delivery systems (e.g., LNPs), it will translate into the target protein to elicit an adequate and very specific response. RNA interference (RNAi) strategy using siRNA generally involves a gene silencing mechanism that is induced by targeting complementary mRNA for degradation (Dana et al., 2017). Circular RNAs act as RNA-bonding proteins to modulate gene expression and translation of target proteins. While self-amplifying RNA is engineered to replicate itself into host cells for enhanced protein expression and immune response (Zhou et al., 2020). Genome editing via CRISPR/Cas9 involves recognition of the target sequence, its cleavage, and finally repair. For this, the single guide RNA recognizes the target sequence in the gene of interest through a complementary base pair on the target DNA sequence while Cas9 nuclease makes double-stranded DNA breaks which is then repaired by either non-homologous end joining or homology-directed repair cellular mechanisms (Asmamaw and Zawdie, 2021). By these mechanisms, RNA therapeutics provide a vast opportunity to design novel treatments for diseases currently without effective medicines. RNAs are less toxic than small molecules and technically have a good solubility.
Crucially, each RNA modality offers a unique duration of therapeutic effect – ranging from short-lived (days to weeks) for mRNA-based protein expression, to medium-term (weeks to months) for siRNA or saRNA interventions, and even long-lasting (potentially years) for CRISPR/Cas9-mediated gene editing. This versatility allows clinicians to tailor treatment regimens to the disease’s severity and duration. At the same time, some RNA molecules are inherently unstable and readily degraded by nucleases, necessitating effective delivery systems to preserve their structure and functionality. Given this, various delivery systems have been developed including LNPs to successfully transport these RNA molecules to their target site of action while preserving their therapeutic benefits.
LNPs are non-viral gene delivery systems that are formed by self-assembly of its lipid components to deliver the encapsulated RNA molecules to their target site. They possess several advantages including biocompatibility, non-immunogenic nature, higher in vivo stability and targeting capabilities. Typically, LNPs contain four main lipid components: ionizable lipid that binds to the negatively charged RNA and assists in endosomal escape; phospholipids to provide structural integrity; cholesterol for structural stability and mediating cellular uptake; as well as PEGylated lipids for higher LNP stability in the systemic circulation. LNPs are most commonly manufactured using the microfluidic mixing technology in which the four lipid components (in organic phase) are rapidly mixed with the RNA solution (in aqueous phase) in the microfluidic mixer to form the resultant LNPs via self-assembly (Figure 1).
Following administration, LNPs undergo cellular uptake commonly through endocytosis following which they are entrapped into endosomal compartments. In endosomes, the ionizable lipid becomes protonated to fuse with the endosomal membrane and release the encapsulated RNA into the cytoplasm for successful translation (in case of mRNA delivery) (Wang J. et al., 2024; Haque et al., 2024). These RNA-LNPs have multiple therapeutic applications and the ensuing sections detail how these approaches specifically target women’s health conditions, highlighting recent progress and remaining obstacles.
Table 1 shows some of the clinical trials investigating RNA-LNP therapies in gynecologic cancers. Of these, four of the active studies focus on breast cancer - three specifically on triple-negative breast cancer (TNBC), a subtype that currently lacks effective therapeutic options (Bianchini et al., 2022). Ovarian cancer follows breast cancers in trial frequencies, with three studies evaluating RNA-LNPs. Improper and late-stage diagnosis in ovarian cancer is common due to subtle early symptoms, and peritoneal metastasis further complicates eradication (Nomani et al., 2021; Malekshah et al., 2019; Sarkar et al., 2018). Consequently, more effective treatments are urgently needed. Here, RNA-LNP vaccines have shown promise, offering precise targeting and robust immune activation. The following subsections highlight research, preclinical developments, and ongoing clinical trials that demonstrate the potential of RNA-LNPs strategies in gynecological oncology.
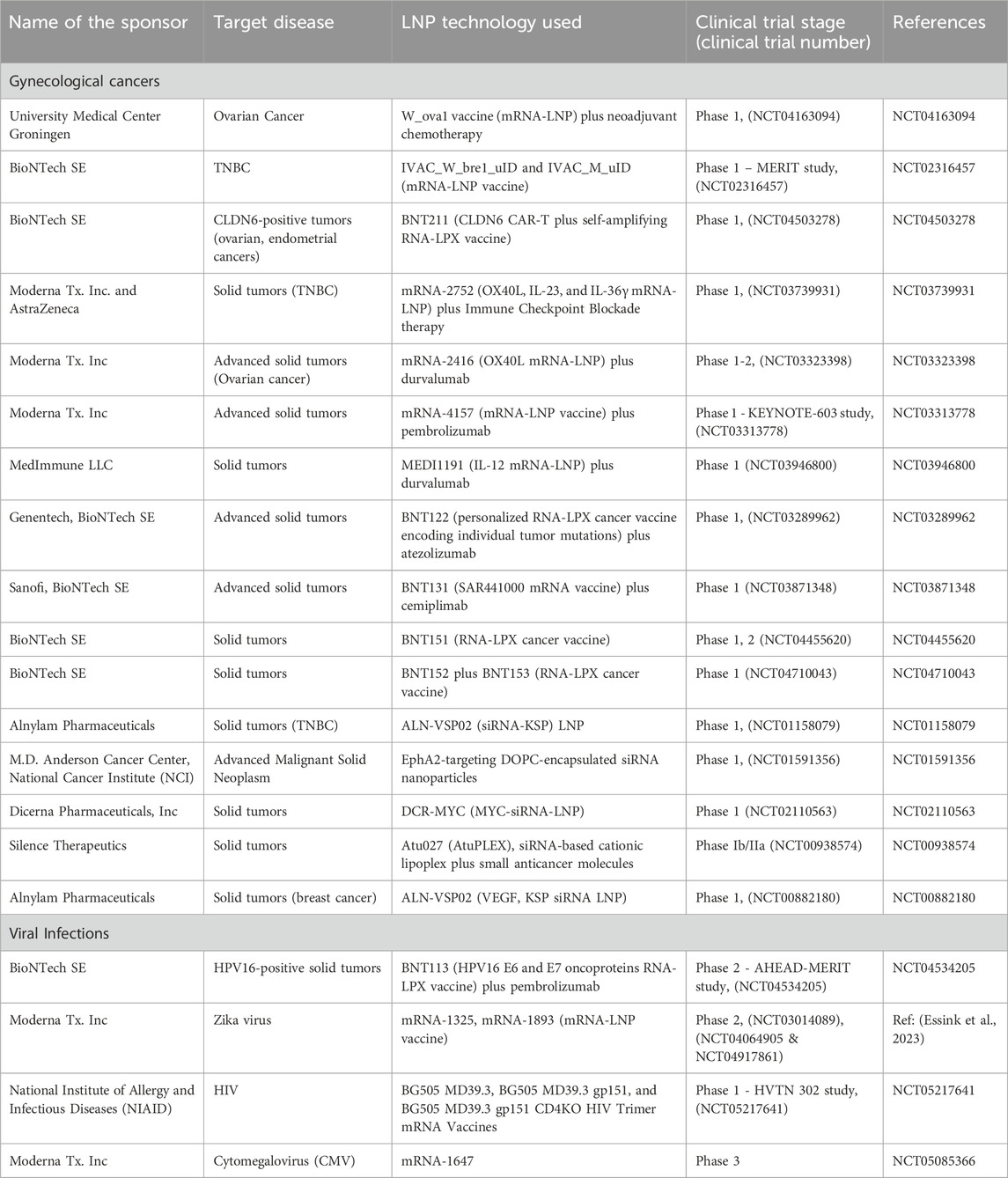
Table 1. Selected ongoing clinical trials of RNA-LNP therapies for women’s diseases including gynecological cancers and viral infections.
Nucleic acid vaccines present a compelling alternative to conventional vaccines. Unlike traditional vaccines, nucleic acid vaccines enable the delivery of multiple antigens simultaneously, and thus encoding various antigens to stimulate a broader T-cell response in antigen-presenting cells (APCs). This approach not only enhances immune recognition but also reduces the risk of protein or virus-related contaminations during production, thereby minimizing patient complications and side effects (Ho et al., 2021; Kulkarni et al., 2021; Pardi et al., 2020). mRNA vaccines, in particular, demonstrate unique advantages over DNA vaccines, including higher protein expression, reduced toxicity, and streamlined, scalable manufacturing (Pardi et al., 2018; Jahanafrooz et al., 2020; Park et al., 2021; Chaudhary et al., 2021). Although mRNA is inherently unstable, chemical modifications (Kim S. C. et al., 2022) and advanced delivery vehicles such as LNPs help regulated its in vivo half-life and mitigate degradation.
Furthermore, unlike DNA or viral vaccines, mRNA does not integrate into the genome, a key safety feature that significantly lowers the risk of insertional mutagenesis (Campillo-Davo et al., 2018; Mancianti et al., 2021). Additionally, mRNA is a minimal genetic vector, which can help evade anti-vector immunity and therefore, enable repeated administration (McKinlay et al., 2018). These attributes coupled with LNPs’ proven efficiency in cellular internalization and endosomal escape (Hou et al., 2021; Kon et al., 2022; Tenchov et al., 2021) makes mRNA-LNP platforms particularly appealing for gynecologic malignancies such as ovarian and breat cancers.
Tumor-specific immune responses can be stimulated by exploiting surface antigen differences between tumor cells and normal cells. Unlike infectious diseases, cancer vaccines are used therapeutically to present specific antigens to the host cells (Yaddanapudi et al., 2013; Nahas et al., 2018). While traditional protein/peptide antigens for cancer vaccines have not been successful, mRNA vaccines have shown promise in anti-cancer treatments. mRNA vaccines can express tumor-associated antigens in antigen-presenting cells (APCs) during vaccination, therefore activating APCs and triggering innate/adaptive immune responses to inhibit tumor development and progression. As mentioned, Table 1 depicts some of the current clinical trials targeting gynecological malignancies using RNA-LNP therapies. It is worth noting that although these therapy strategies are not limited to the vaccine approach, we can observe that the prevention and immune potentiation through RNA vaccines are among the commonly used and promising methods. Below, we review a few of the preclinical and clinical studies for the most prevalent gynecological cancers using RNA therapies.
Ovarian and uterine cancers are among the most prevalent gynecologic malignancies in the United States (Giaquinto et al., 2022). Earlier clinical trials of ovarian cancer vaccines showed limited efficacy often linked to inadequate immune responses (Nishida et al., 2022; Chow et al., 2020). However, recent preclinical and early-phase clinical studies have shown promising results for mRNA vaccines in ovarian cancer. For instance, a first-in-human phase I trial (NCT04163094) is testing an LNP-based mRNA vaccine in combination with neoadjuvant chemotherapy in ovarian cancer patients. The designed mRNA encodes three tumor-associated antigens specific to ovarian cancer. The outcome of this clinical trial study is being evaluated by comparing the patients’ intratumoral T cell expansion specific to the triple antigens before and after chemotherapy and five rounds of 100 µg mRNA vaccines. Although detailed data remain sparse, the design reflects the growing emphasis on ovarian markers such as CA125 (Mucin 16), overexpressed in more than 80% of ovarian tumors (Singh et al., 2008; Karlan et al., 1988). In another study, Korzun et al. used LNPs-delivered follistatin (FST) mRNA to ovarian cancer cells to reduce elevated activin A levels and associated cachexia (Korzun et al., 2022). In this study, combination therapy with cisplatin further demonstrated improved survival in mice and counteracted muscle atrophy and cancer-associated cachexia in vivo.
Another gynecological cancer with the highest occurrence rate in women is breast cancer, which is responsible for one in every six cancer deaths among women worldwide (Kim et al., 2009; Arnold et al., 2022). Within this group, TNBC – characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression – comprises approximately 10%–20% of all breast cancers cases. TNBC exhibits aggressive progression and features high metastatic rate and poor prognosis (Bianchini et al., 2022; Ismail-Khan and Bui, 2010; Huang M. et al., 2022). Unlike other breast cancer subtypes, TNBC lacks therapeutic targets and is unresponsive to typical hormonal and HER2-based therapies. However, the emergence of mRNA vaccines offers a promising solution for TNBC treatment by leveraging the tumor-specific immune response. In particular, MUC1, a tumor-associated antigen often overexpressed in various carcinomas, including breast, ovary, and pancreas (Zhou et al., 2019; Yamamoto et al., 2019), is a focal point for these vaccines.
Researchers at Huang and Liu labs have developed mannose-modified LNPs to deliver MUC1 mRNA directly to dendritic cells (DCs) in the lymph nodes (Lin et al., 2022; Liu et al., 2018). Vaccination with these mRNA-loaded LNPs has demonstrated potent stimulation of immune responses against TNBC tumor models. Combining MUC1 mRNA vaccine with an anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody further augmented APC activation, enhanced innate/adaptive immunity, and inhibited T regulatory pathways (Lin et al., 2022; Liu et al., 2018). To further improve the efficacy of cancer vaccines, researchers are exploring combinations with adjuvants, co-stimulatory therapeutics, immunotherapies, and conventional therapies. In an ongoing three-arm phase 1 clinical trial named Mutanome Engineered RNA Immuno-Therapy (TNBC-MERIT), LNPs serve as the basis for an individualized RNA vaccine (IVAC™) encoding tumor-associated antigens against TNBC (NCT02316457). This trial evaluates the safety, tolerability, and induced T-cell responses and attempts to treat each patient with the relevant and immunogenic RNA vaccines based on the individual patient’s tumor characteristics. The flexibility and ease of design and manufacturing of customized RNA-LNP vaccines can potentially provide transforming care for highly aggressive breast cancers subtypes and increase the success rate in women’s malignancies more broadly.
In addition to mRNA-based vaccines, LNPs have also been explored for delivering siRNA in several gynecologic cancers. Several lipid nanocarrier systems are undergoing or have completed clinical trials to assess their safety, efficacy, and translation potential. For instance, ALN-VSP02 (Alnylam Pharmaceuticals) formulation combines kinesin spindle protein (KSP) and vascular epithelial growth factor (VEGF)-siRNA encapsulated in LNP system. A phase I trial enrolled 30 patients with advanced solid tumors, administering intravenous doses of ALN-VSP02 in dose-escalation (0.1–1.5 mg/kg). No major safety concerns were observed, and notably, one patient with endometrial cancer and hepatic metastasis achieved a complete response after 50 doses of ALN-VSP02 (Tabernero et al., 2013a). A dose of 1.25 mg/kg every 2 weeks was recommended for subsequent Phase II evaluations [NCT00882180 (Cervantes et al., 2011; Tabernero et al., 2013b)].
Atu027 (Silence Therapeutics) is yet another siRNA-based LNP formulation designed to knockdown protein kinase N3 (PKN3). In a Phase Ib/IIa (AtuPLEX) involving patients with advanced solid tumors (Strumberg et al., 2012), Atu027 was administered as a single agent and in combination with other anticancer therapies. It was proven to be well-tolerated up to 0.180 mg/kg, with dose-dependent increases in siRNA antisense strand concentrations in plasma. Dose escalation is ongoing for this study.
DCR-MYC or DCR-M1711 (Dicerna Pharmaceuticals), is a Dicer substrate siRNA (DsiRNA) encapsulated in EnCore™ LNP to downregulate c-Myc across multiple solid tumors. Phase Ib/II trial demonstrated a favorable safety profile at various doses, with some showing promising tumor shrinkage after one cycle of 0.1 mg/kg dose (Tolcher et al., 2015). Despite encouraging initial results, the trial was terminated due to dose-limiting toxicities and sponsor decisions (Harrington et al., 2021).
EPHARNA, also referred to as EphA2-siRNA-DOPC (NCI) formulated by encapsulation of EphA2-siRNA within dioleoylphosphatidylcholine (DOPC) liposoms to reduce EphA2 expression in tumors such as breast and ovarian cancers. When tested in mice, single-dose EPHARNA administration showed minimal toxicity or any pathologic or dose-related microscopic findings in acute or recovery phases. Similarly, minimal infiltration of mononuclear cells was found in major organs when tested in Rhesus macaques. This formulation entered a phase I trial to be tested intravenously twice weekly in recruited patients with advanced metastatic solid cancers [NCT01591356, (Landen et al., 2005)].
MicroRNAs (miRNAs) are key regulators of pathophysiological processes in gynecologic malignancies, acting as either oncogenes (oncomiRs) or tumor suppressors. LNPs, in turn, can effectively deliver miRNA therapeutics – alone or in combination – to treat solid tumors, including TNBC. For instance, Hayward et al. developed LNPs with hyaluronic acid (HA)-modified LNPs to deliver tumor suppressor MicroRNA125a-5p in HER2 positive metastatic breast cancer (Hayward et al., 2016). These HA-LNPs could cause the knockdown of the HER2 proto-oncogene in patient-derived metastatic breast cancer cells (21MT-1), thereby impairing essential signaling pathways involving phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase (MAPK) and inhibiting tumor growth.
Similarly, Dinami et al. signified that LNPs incorporating miR-182-3p could reduce telomeric repeat-binding factor 2 (TRF2) expression in patient-derived TNBC xenograft models, significantly impairing tumor progression (Dinami et al., 2023). Additionally, this LNP formulation could cross the blood-brain barrier to address metastatic brain lesions. In another study, Nevskaya and colleagues demonstrated that delivering a combination of three miRNAs; miR-195-5p, miR-520a, and miR-630, using LNPs, could result in downregulation of SOX2, MYC, TERT, and FZD9 genes in human breast cells and inhibit stemness, thus reducing lung metastasis in vivo C57BL/6 mouse model (Nevskaya et al., 2024). Given this, the development of nanotechnology, especially LNPs, that deliver miRNA therapeutics have shown growing promise of miRNA-LNP technology for future clinical applications in gynecologic oncology.
There is broad ongoing research to identify the potential of CRISPR-Cas9 technology in gynecological cancers, thereby laying grounds for its further clinical application. For instance, Tang et al. used phenylboronic acid (PBA)-derived LNPs to achieve cell-selective genome editing in sialic acid (SA)-overexpressing cervical cancer cells (Tang et al., 2019). Delivery of p53 mRNA and CRISPR components successfully knocked out the HPV18E6 gene, significantly inhibiting tumor growth in vitro and in vivo.
Antibody-conjugated LNPs provide an additional route to targeted genome editing using CRISPR/Cas approach. Rosenblum et al. functionalized CRISPR LNPs with an epidermal growth factor receptor (EGFR) antibody, enabling selective uptake into disseminated ovarian tumors. This strategy yielded ∼80% gene editing and extended survival by 80% in an ovarian cancer mouse (OV8-bearing) model (Rosenblum et al., 2020). Zhang et al. advanced the platform by fabricating a multiplexed dendrimer LNP (siFAK + CRISPR PD-L1-LNPs) co-delivering focal adhesion kinase (FAK) siRNA, Cas9 mRNA, and sgRNA to human ovarian cancer cells (IGROV1) (Zhang et al., 2022). In an ovarian cancer xenograft model (ID8-Luc), PD-L1 knockdown and FAK inhibition together significantly reduced metastasis. This may highlight the potential synergy between CRISPR-based gene editing and RNA interference in gynecologic oncology.
Table 1 also summarizes clinical trials investigating RNA-LNP therapies for viral infections. The breadth of preclinical and clinical research spans multiple pathogens that disproportionately affect women, including human papillomavirus (HPV)–related cancers (e.g., NCT04534205 by BioNTech), human immunodeficiency virus (HIV; NCT05217641 by NIH), Zika virus (NCT04917861 by Moderna), cytomegalovirus (CMV; NCT05085366 by Moderna), and genital herpes simplex virus (HSV; NCT06033261 by Moderna and NCT05432583 by BioNTech). Information regarding the details of clinical trials and the status of ongoing studies on these topics can be found using the search engine at (www.clinicaltrials.gov). In the following two subsections, we will review a few of these studies but with more emphasis on the additional preclinical and research studies found in the literature related to RNA-LNP for HPV and HIV infection and highlighting their relevance to women’s health.
Human papillomavirus (HPV) is a non-enveloped DNA virus with over 200 identified serotypes, about 40 of which can infect the anogenital and mucosal lining through sexual contact. The Centers for Disease Control and Prevention (CDC) reports that more than 42 million Americans are infected with high-risk pathogenic subtypes of HPV, with an annual detection of 13 million new infections (About Human Papillomavirus, 2025). Fifteen high-risk HPV subtypes have been identified to cause cervical intraepithelial neoplasia and squamous cell carcinomas (Quinlan, 2021; Balasubramaniam et al., 2019). These high-risk subtypes contribute to various cancers, including cervical, oropharyngeal, anal, penile, vaginal, and vulvar, the majority of these through the action of the E6 and E7 oncoproteins, which alter host cellular functions leading to the development of cancerous cells (Balasubramaniam et al., 2019; Longworth and Laimins, 2004). For instance, cervical cancer, which affects nearly 500,000 women annually and leads to 300,000 deaths worldwide (Cohen et al., 2019), is predominantly caused by HPV and can be prevented through routine screening, enabling healthcare providers to identify and remove pre-cancerous lesions. Additionally, most vaginal and vulvar cancers are caused by HPV. Furthermore, over 90% of anal cancers are HPV-induced and more common in women than men (Cohen et al., 2019).
Extensive research has shown that HPV-positive tumors exhibit high levels of lymphocyte infiltration. This, in turn, provides a strong rationale for immunotherapy to modulate HPV-related carcinogenesis. Various strategies have been developed to activate the adaptive immune system against HPV-infected tumors. These strategies include immune checkpoint blockade, viral antigen recognition through therapeutic vaccines, adaptive cell therapies, and their combinations with radiotherapy or chemotherapeutics. Unfortunately, there are currently no specific therapies available for HPV-induced cancers, such as cervical cancers. Nevertheless, nanoparticle formulations such as gold, polymeric, or lipid nanoparticles have been investigated to eliminate HPV infection or provide curative measures for these cancers (Medina-Alarcon et al., 2017; Rupar et al., 2019).
Targeting the HPV E6/E7 oncoproteins is a promising therapeutic approach for HPV-related cancers. This includes inducing immune cell populations against E6/E7, silencing oncoprotein expression through RNA interference (RNAi), or utilizing genome editing tools like the CRISPR/Cas system to knock down or knock out the E6 and E7 oncogenes. These gene editing approaches can be combined with LNPs to enhance therapeutic efficiency (Xiong et al., 2021; Aghamiri et al., 2020). Kampel et al. demonstrated that anti-EGFR-targeted LNPs carrying anti-E6/E7 siRNAs led to approximately 50% greater cancer regression than the untargeted siRNA-LNPs, thus providing a promising pathway for cancer treatment through oncoprotein elimination (Kampel et al., 2021). Moreover, E6/E7 oncoprotein-encoded mRNA LNPs showed enhanced antitumor efficacy when co-administered with the stimulator of interferon genes (STING) adjuvant systemically (Tse et al., 2021). Additionally, mRNA-LNPs have been developed to deliver mRNA encoding HPV antigens to cervical cancer cells (Sahin et al., 2020; Kranz et al., 2016; Kreiter et al., 2008). This approach induces significant proliferation of cytotoxic lymphocytes and stimulates immune responses against HPV-positive tumors. Also, Da Silva et al. demonstrated that a single low-dose immunization with any of the three developed LNP-based herpes simplex virus type 1 glycoprotein D (gDE7) mRNA vaccines (self-amplifying mRNA, unmodified mRNA, and nucleoside-modified non-replicating mRNA vaccines) resulted in the activation of E7-specific CD8+ T cells, generated memory T cell responses to prevent tumor relapses, and destroyed cervical tumors (Ramos da Silva et al., 2023a). Furthermore, combining mRNA vaccines encapsulated in lipid nanocarriers with immune checkpoint inhibition shows potential for improving the clinical efficacy in cervical cancer (Grunwitz et al., 2019).
HIV affects women worldwide, with approximately 53% of the 39.0 million people living with HIV in 2022 being women over 15 years of age (Grunwitz et al., 2019). In addition to medical challenges, women face significant psychosocial burdens, particularly related to preventing mother-to-child vertical transmission (Fauk et al., 2022). HIV is a retrovirus that enters the host by targeting the cluster of differentiation 4 (CD4) receptor on immune cells and binds to co-receptors like C-C chemokine receptor type 5 (CCR5) and C-X-C chemokine receptor type 4 (CXCR4). The virus reverse transcribes its RNA, integrates into the host DNA, and consequently produces viral particles infecting new host cells. Dendritic cells infected with the virus can transmit it to T cells, leading to further virus dissemination. Studies suggest that the R5 strain of HIV exhibits preferential amplification and dissemination (Deeks et al., 2015; Morrow et al., 2007). Transmission of HIV can occur through mucosal surfaces such as rectal, genital, and, less commonly, oral. While vaginal mucus provides some protection against viral entry, the thin mucus lining of the rectum allows direct access to leukocytes (Morrow et al., 2007).
Current HIV therapeutics include five classes of antivirals: entry inhibitors, reverse transcription inhibitors (RTIs; including both nucleoside RTIs and non-nucleoside RTIs), integrase/transfer inhibitors (INSTIs), and protease inhibitors. Standard first-line regimens often combine an INSTI with RTIs, sometimes augmented with CYP3A inhibitors for longevity and efficacy (Gunthard et al., 2016; HIV/AIDS. In, 2023). In cases of HIV with multiple drug resistance, two new antibody-based therapeutics, ibalizumab (a CD4 post-attachment inhibitor) and leronlimab (a CCR5 inhibitor), can be considered. These antibodies are administered intravenously every 7–14 days to complement existing antiviral therapies. FDA-approved antiretroviral drugs disrupt virion replication by targeting different stages of the virus life cycle, including viral genome replication, protein cleavage, packaging, and virus release from cells. Although these regimens suppress viral loads below detectable limits, they cannot eliminate integrated viral genomes in immune cells. To address the issue of the integrated viral genomes, LNPs with gene editing capabilities are being developed (Herskovitz et al., 2021). These LNPs, co-encapsulating Cas9 mRNA and TatDE guide RNAs (gRNAs), can cleave the HIV genome within infected cells through tumor necrosis factor alpha (TNF-α) stimulation, effectively preventing viral outgrowth (Herskovitz et al., 2021). In parallel, mRNA-LNPs conjugated to anti-CD4 antibodies are being explored to selectively target HIV-infected CD4+ T cells (Tombacz et al., 2021), while others focus on delivering HIV-1 gp160–encoding mRNA or anti-CCR5 siRNA to prevent viral entry (Saunders et al., 2021), (Mu et al., 2022; Traore et al., 2022). For instance, Pardi et al. showed that following 6 weeks of a single immunization with mRNA-LNP encoding Env gp120, rabbits and rhesus macaques produced anti-gp120 IgG titers, which were further boosted by subsequent immunization (Pardi et al., 2019). Beyond preventive strategies, Pardons et al. illustrated the application of LNPs where the reactivation capacity of Tat mRNA-LNP was demonstrated in CD4+ T cells from HIV patients. Combined with panobinostat, it also caused potent latency reversal, enabling multi-omic analysis of the HIV-1 reservoir for future applications (Pardons et al., 2023).
In another example, a novel approach of inducing “broadly neutralizing antibodies” (bnAbs) against the conserved sites of the HIV-1 spike created a significant hope for a long-lasting prevention and treatment of this disease, specifically by using germline targeting method (Liu Y. et al., 2020; Xie et al., 2024). In this strategy, naïve B cells are recruited to the germinal center and by a guided maturation and sequential exposures to the B cells to the designed antigens, they will be activated and the related memory B cells will be generated. These memory B cells can induce the required immune response (bnAbs) when the protection is needed against the virus infection. In a clinical trial, the germline targeting method was evaluated as a proof of the concept through inducing VCR01-class of bnAbs for engineered outer domain germline targeting version 8 (eOD-GT8) 60mer [(Cohen et al., 2023), NCT03547245]. Given the ease of design, scale up, and adaptability of the mRNA to variants, mRNA-LNP can also be a promising candidate to induce the immunogens in germline targets. For instance, Xie et al. reported the efficacy of a mRNA-LNP delivering germline-targeting N332-GT5 and two other booster immunogens (B11 and B16) to immunize mice against HIV-1 virus in BG18gH mouse model. They found that their prime-boost strategy using mRNA-LNPs could derive affinity maturation in the target B cells and elicit precursors to bnAbs against HIV-1 variants. They concluded that this strategy may resolve some of the major HIV-related vaccine developments challenges (Xie et al., 2024).
Though maternal antibodies provide infants with crucial early immune protection, they can also interfere with de novo infant antibody responses. This challenge is especially pertinent for HIV-1 vertical transmission. To address maternal antibodies-mediated suppression of immune response, mRNA-LNPs vaccines are being explored in preclinical models. Willis et al. (Willis et al., 2020) reported a nucleoside-modified mRNA-LNP vaccine that could establish prolonged germinal center reaction and partially overcome maternal antibody neutralization in mice pups to induce de novo immune response against influenza virus. They hypothesized that this prolonged immune response could be due to the prolonged antigen availability by mRNA-LNPs leading to stronger germinal center responses.
Despite efforts in antiviral development, no definitive HIV cure or universal prophylaxis exists. Emerging RNA-LNP platforms, bnAbs, and combination therapies may improve patient outcomes by targeting multiple steps in viral pathogenesis, with particular relevance to maternal and infant health (Landovitz et al., 2023).
Although gynecologic cancers and viral infections are among the main concerns in women’s health, additional conditions, such as osteoporosis, endometriosis, uterine fibroids, and various congenital disorders, also significantly affect the well-being of many women. In this section, we discuss the potential of RNA-LNP technologies to address these conditions by offering novel RNA-based therapeutic strategies that go beyond the scope of traditional interventions.
Osteoporosis is a common disorder characterized by low bone mass, deterioration of bone microarchitecture, and increased risk of brittle fracture (NIH, 2001). The imbalance between bone formation and resorption is more prevalent in postmenopausal women, leading to heightened susceptibility to fractures. Current anti-osteoporosis treatments primarily include hormonal therapies and bisphosphonates, both of which aim to prevent bone resorption or enhance restoration.
Gene therapy offers a promising route for novel osteoporosis treatments. For instance, studies targeting bone marrow mesenchymal stem cells (MSCs) have demonstrated the potential of manipulating endogenous bone morphogenetic proteins (BMPs) and growth differentiation factors (GDFs) to promote osteo-induction (Vhora et al., 2019). Vhora et al. (2019) employed ionizable LNPs bearing bone-homing peptides to deliver plasmid-encoded BMP-9, thereby stimulating osteoblastic lineage cells. In another study, Basha et al. (2022) used LNPs encapsulating siRNA to silence the suppressor gene GNAS, facilitating bone restoration. Also, Xue and colleagues built upon these concepts by developing a series of bisphosphonate (BP) lipid-like materials to identify an BP-LNP formulation 490BP-C14. This formulation improved mRNA expression and localization in the in vivo bone microenvironment when tested in mice (Xue et al., 2022). Intravenous administration of 490BP-C14 resulted in therapeutic bone morphogenetic protein-2 secretion from the bone microenvironment. Such targeted LNP systems could complement current therapies by preventing bone loss and enhancing new bone formation in women diagnosed with osteoporosis.
Non-coding RNAs – such as RNA interference (RNAi), circular RNAs (circRNAs), and long non-coding RNAs (lncRNAs) – have also demonstrated therapeutic potential for managing osteoporosis (Ko et al., 2020; Silva et al., 2019). CircRNAs are particularly interesting because of their unique structure, which makes them less prone to degradation than non-circular RNAs (Enuka et al., 2016). They have been shown to serve as biomarkers for the treatment of osteoporosis by promoting osteogenesis (Huang et al., 2019; Zhao et al., 2018; Yu and Liu, 2019; Hansen et al., 2013; Chen et al., 2019; Gu et al., 2017). In this regard, LNPs, as the delivery vehicle, can improve RNA therapy for osteoporosis by enhancing its efficacy by successfully delivering the RNA cargo to the desired cell lineages (Basha et al., 2016; Hallan et al., 2022).
Endometriosis is caused by the ectopic endometrial tissue dispersion and growth outside the endometrium (Kabani et al., 2022). It affects roughly 10% of women of reproductive age, causing pain and infertility with a considerable impact on quality of life (Giudice and Kao, 2004; Culley et al., 2013). The currently available treatments include surgical interventions and medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and hormonal therapy using progestins (Vercellini et al., 2011; Becker et al., 2017). However, these treatments have several side effects and are not always effective in women who plan to conceive (Vercellini et al., 2011). Therefore, there is a growing need for nonhormonal, safe, and effective therapies that can target endometriosis by inhibiting the development of new lesions (Adams et al., 2017).
Non-coding RNAs (ncRNAs), such as miRNAs and lncRNAs, are among the non-conventional therapeutic tools for gene regulation and potential treatment of endometriosis (Adams et al., 2017; Evans et al., 2016). Many studies have demonstrated that miRNAs significantly regulate gene expression in endometriosis (Panir et al., 2018; Teague et al., 2010). Preclinical studies in murine models have shown that the therapeutic delivery of miRNAs, such as Let-7b and miRNA-142-3p, can effectively suppress endometriotic lesions via downregulation of various genes that have a role in endometriosis, including Kruppel-like factor 9 (KLF9) and vascular endothelial growth factor A (VEGF-A) signaling (Sahin et al., 2018; Ma et al., 2019). Moreover, miRNAs can modulate endometrial inflammation by regulating pro-inflammatory cytokines, such as TNF-α, interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) in patients with endometriosis (Nematian et al., 2018).
LncRNAs likewise influence transcription and translation, with aberrant expression linked to endometriosis onset (Yan et al., 2020). For example, downregulation of the lncRNA H19 reduces endometrial cell dissemination via the H19/let-7/insulin-like growth factor 1 receptor (Igf1r) pathway (Ghazal et al., 2015). Another study demonstrated that a lncRNA (LINC00261) could inhibit the growth and proliferation of endometriotic cells (Sha et al., 2017).
In parallel, nanoparticle-based delivery strategies have shown promise for targeting endometriotic cells. Bedin et al. combined a low-density lipoprotein (LDL)-like nanoemulsion loaded lipid nanoparticles (LDE) with a chemotherapeutic agent to target endometrial cells and prevent significant systemic side effects of chemotherapy (Bedin et al., 2019). A significant uptake of LDE was observed in the endometriotic foci to enhance the consumption of LDL by these endometrial cells. Building on these findings, RNA-LNP systems designed to modulate key regulatory RNAs may offer an alternative therapeutic approach for endometriosis.
Uterine fibroids (leiomyomas) are the most common benign uterine tumors in reproductive-age women, impacting up to 70% over their lifetimes (Baird et al., 2003; Wise and Laughlin-Tommaso, 2016; Al-Hendy et al., 2017; Yang et al., 2022). Although benign, fibroids can cause extensive symptoms, including heavy menstrual bleeding, pelvic discomfort, and infertility. Standard treatments – ranging from surgery and hormonal therapies (e.g., gonadotropin-releasing hormone or GnRH, agonists) to uterine artery embolization – often carry side effects or risks that necessitate the search for safer alternatives (Yang et al., 2022). While published research on RNA-LNPs for fibroid treatment is limited, other nanoparticle approaches have demonstrated feasibility for delivering therapeutic nucleic acids. For instance, a peptide-based nanoparticle targeting αvβ3 integrin transported a suicide gene (HSV-TK) to uterine leiomyoma cells, increasing cytotoxicity with ganciclovir (Egorova et al., 2022). Under a similar strategy, the researchers demonstrated that a peptide-based magnetic nanoparticle formulation could promote the delivery of plasmid DNA to uterine leiomyoma cells and induce cell death after sequential GCV treatments (Shtykalova et al., 2022). Given LNPs’ favorable safety profiles and proven clinical success, their application in uterine fibroid therapy – especially for gene-based or immunotherapeutic approaches – holds potential. Indeed, immunotherapy is an emerging area of investigation, as evidenced by a Phase II trial using inactivated myoma tissue and blood samples for oral vaccination (NCT03550703). By leveraging tumor-specific or personalized antigenic targets, mRNA-LNP vaccines akin to those developed for certain gynecologic cancers may represent a future direction for fibroid management.
Congenital disorders involve structural or functional abnormalities that arise prenatally, often causing significant morbidity and mortality. Existing interventions – such as fetal surgery, in utero gene therapy, and in utero stem cell transplantation (IUSCT) – frequently pose high risks (Swingle et al., 2023a; Palanki et al., 2021). Amongst these, in utero gene therapy offers advantages, including being minimally invasive and achieving sustained phenotype correction for many genetic diseases. Recent advances have explored in utero mRNA-LNP delivery to treat or prevent congenital conditions. Swingle and co-workers developed stable mRNA-LNPs for intra-amniotic delivery to show potent mRNA delivery in vitro and in utero in a murine model (Swingle et al., 2022). They used the orthogonal design of experiments (DOE) to screen a space of 256 possible LNP formulations. They identified that more stable mRNA-LNPs have higher in utero mRNA delivery than unstable LNPs. Therefore, developing this stable ionizable RNA-LNP formulation in amniotic fluid paved the way for screening potential LNPs designed for prenatal delivery to treat congenital diseases in pregnant women. This group also formulated VEGF-A mRNA-LNPs for delivery to the placental cells, including trophoblasts, endothelial cells, and immune cells, to achieve placental vasodilation that would benefit maternal and fetal health (Swingle et al., 2023b).
Another research group also built a library of ionizable LNPs for in utero mRNA delivery to mouse fetuses that resulted in the accumulation with the livers, lungs, and intestines with higher efficiency and safety as opposed to DLin-MC3-DMA and jetPEI®-based delivery systems (Riley et al., 2021). There have also been attempts to target organs like the heart, kidneys, lungs, and gastrointestinal tract via in utero delivery of LNPs. Gao et al. demonstrated that using mRNA-LNP complexes, 0.64%–12.4% of the cells in the lungs, heart, liver, kidneys, brain, and gastrointestinal tract were transfected with low toxicity. Additionally, LNPs complexed with Cas9 mRNA and sgRNA could edit 0.2%–0.8% of cells in the liver, heart, gastrointestinal tract, and kidneys of Ai9 mice following in utero delivery (Gao et al., 2023). This extrahepatic targeting could be leveraged to address various congenital diseases in pregnant women.
Furthermore, hereditary angioedema (HAE), a rare genetic disorder with a strong female predominance, exemplifies how RNA-LNP delivery might also combat rare pregnancy-aggravated conditions (Caballero et al., 2014). For example, NTLA-2002, a CRISPR/Cas9 LNP platform, was developed by Intellia Therapeutics® to target the kallikrein B1 (KLKB1) gene. Its single administration resulted in ∼70% KLKB1 gene editing and >90% reduction of kallikrein protein when tested in mice and monkeys. NTLA-2002 is undergoing a Phase II clinical trial in recruited HAE patients (NCT05120830).
The diverse applications of RNA-LNP therapeutics in women’s health, from gynecologic cancers and viral infections to osteoporosis and congenital disorders, underscore the immense potential of this platform. Nevertheless, the successful translation of RNA-LNPs into clinical practice hinges on overcoming multiple challenges – particularly those related to targeted delivery, safety, and long-term efficacy. The following section explores these obstacles and discusses emerging strategies to optimize RNA-LNPs for women’s health applications.
LNPs are a powerful system for delivering nucleic acids, which offers numerous advantages such as efficient packaging, high cargo capacity, low immunogenicity, scalable manufacturing, long-term physical stability, and adjustable physicochemical properties (Cullis and Hope, 2017; Kenjo et al., 2021). Despite these strengths, conventional LNPs often accumulate in and are extensively cleared by the liver for two main reasons: 1) anatomical and physiological features that foster LNP uptake (Tsoi et al., 2016) and 2) the formation of a protein corona that intensifies hepatic clearance (Dilliard and Siegwart, 2023; Loughrey and Dahlman, 2022; Akinc et al., 2019). Hence, to unlock the full potential of RNA-LNPs in women’s health, it is crucial to achieve non-liver-targeted LNP delivery. Several strategies have been proposed to mitigate liver uptake and reroute LNPs to other organs, tissues, and cells – many of which are directly applicable to female conditions. Below, we outline three broad approaches for optimizing RNA-LNP delivery: local administration, passive targeting, and active targeting. Select examples then illustrate how each strategy can be harnessed for women’s diseases.
A straightforward way to bypass hepatic accumulation of LNPs is to change the administration route from intravenous delivery to other administration routes, which could shorten the delivery path and enhance accessibility to the target cells (Figure 3) (Chenthamara et al., 2019; Kim J. et al., 2022; Zimmermann et al., 2022; Leong and Ge, 2022; Patel et al., 2019; Kumbhar et al., 2022; Bardoliwala et al., 2021). For example, intra-tumoral injection has been utilized to deliver LNPs and could slow down tumor progression or even eliminate established tumors (Liu et al., 2022; Li et al., 2020; Hsu et al., 2013). In one study on a TNBC model, a tumor acidity-responsive nanoparticle released chemokine C-C motif ligand 25 (CCL25) protein and cluster of differentiation 47 (CD47) siRNA sequentially after intra-tumoral administration, and therefore enhanced immunotherapy against the cancer cells by promoting CCR9+ CD8+ T cell tumor infiltration (Chen et al., 2020).
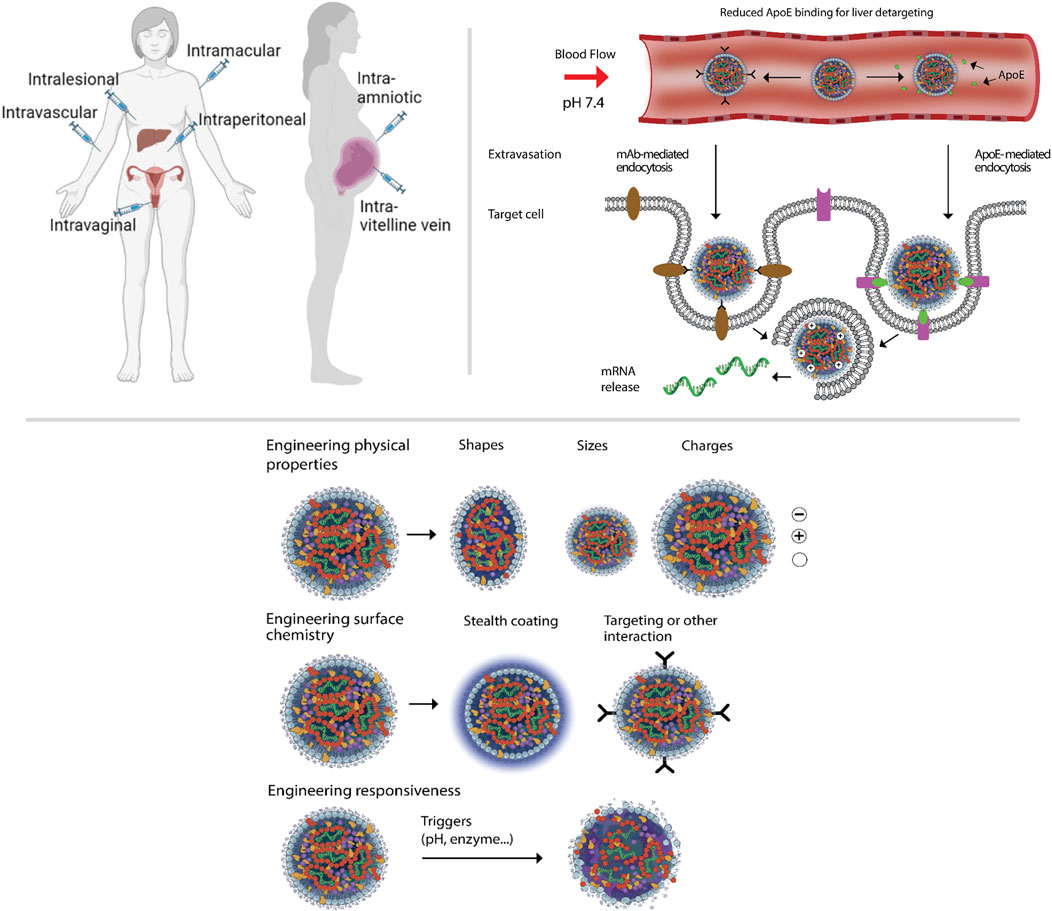
Figure 3. Top left panel shows schematic representation of the strategies for RNA-LNP therapeutics targeting through different routes of administration in non-pregnant and pregnant women. Top right panel emphasizes the crucial features and components (cell receptors, ligands) for the apoprotein E (ApoE)-mediated versus monoclonal antibody-targeted LNPs endocytosis after systemic administration. Bottom panel shows the strategies for engineering physicochemical properties of RNA-LNPs. The figure has been made using icons from BioRender.com
Additionally, intramuscular (IM) administration has been chosen for mRNA vaccine development. This approach can shorten the route for LNPs to lymph node clusters and enhance immune responses (Miao et al., 2021; Hassett et al., 2019). Zika virus is of particular concern to women’s health because it affects them at nearly twice the rate of men and can be transmitted from a pregnant woman to her fetus. Richner et al. developed the modified mRNA vaccines with LNP, successfully preventing Zika virus disease through IM delivery. One concern for Zika virus mRNA therapy is that the produced antibody against Zika may cross-react with the Dengue virus and enhance its infection in cells. However, Richner et al. have shown that the mRNA-LNP approach reduced cross-reaction risk and sensitized individuals to subsequent exposure to Dengue virus in mice (Richner et al., 2017). Furthermore, clinical trials for mRNA-1893 and mRNA-1325 from Moderna are underway to combat the Zika virus (Essink et al., 2023).
The intraperitoneal (IP) route of administration has also been explored for targeted gene editing using LNPs as delivery systems in gynecological cancers such as ovarian cancer. For instance, Rosenblum and Gutkin et al. developed EGFR-targeted CRISPR-LNPs against PLK1 (sgPLK1-cLNPs) that led to selective uptake into disseminated ovarian cancer cells and facilitated up to ∼80% gene editing in vivo, significantly inhibiting tumor growth and improving survival by 80% in a mouse model (Rosenblum et al., 2020).
Another method of the local delivery in female disorders is the intravaginal administration. Using vaginal delivery, the cargos avoid liver first-pass clearance and systemic dilution effects and can be used for uterovaginal viral infections and gynecologic cancers. Upon vaginal administration, the first uterine pass effect (Campana-Seoane et al., 2019; De Ziegler et al., 1997) allows preferential drug accumulation in the uterus and vagina through counter-current drug exchange between the blood in the vaginal veins and the uterine artery. Vagina tissues have a high surface area with high blood flow in the muscular layers, which helps improved drug absorbance at this route. Also, it has been reported that adding polyethylene glycol to the surface of liposomes can enhance tissue penetration of intravaginal delivery against HPV infection in the ex vivo sheep vaginal epithelium model (Joraholmen et al., 2017). However, unlike the successful application of intravaginal delivery of small molecules, for macromolecules such as RNAs, the thick mucosal layer of vagina and fast clearance of the mucous layer limit the delivery. Additionally, the delivery of RNA molecules through this route faces another challenge due to the low pH (3.5–4.5) of the vagina’s surface (Kim et al., 2018). Since RNA-LNPs are a self-assembled and pH-dependent structure, the low pH of the vaginal environment may significantly affect the stability of the particles and their efficacy.
In fetal congenital disorders, in utero delivery can be a valuable option for gene delivery (Swingle et al., 2022; Riley et al., 2021; Ullrich et al., 2021). It can be accomplished either by injection in the vitelline vein (which provides direct access to the fetal liver) or through intra-amniotic fluid (Palanki et al., 2021; Swingle et al., 2022; Ullrich et al., 2021). For example, in a study by Riley et al., different LNPs were screened for luciferase mRNA delivery via mouse vitelline vein injection (Riley et al., 2021). They identified formulations that efficiently could accumulate within fetal livers, lungs, and intestines compared to DLin-MC3-DMA and jetPEI. They observed that the selected LNPs could deliver mRNAs and induced significant expression of the target protein in the liver. Also, from the same scientific group, Swingle et al. engineered a library of LNPs using orthogonal design of experiments to evaluate the effect of LNP structure on their stability in amniotic fluid and to enable intra-amniotic mRNA delivery in utero (Swingle et al., 2022). Their study found multiple lead LNPs that were stable in ex utero amniotic fluids of different animal species and in humans. Also, they showed that these ex-vivo screened LNPs enabled effective mRNA delivery in primary fetal lung fibroblast cell culture and in utero by intra-amniotic injection in a murine model.
Table 2 depicts selected studies on actively targeted RNA-LNPs for women’s health. Although local delivery bypasses many systemic barriers, IV administration remains vital for widespread treatment of women’s diseases. Under these conditions, targeted LNPs must traverse the bloodstream and overcome organ-specific barriers en route to non-liver tissues (Figure 3, and (Mitchell et al., 2021; Whitehead et al., 2009; Yin et al., 2014). There are some challenges and opportunities that targeted delivery in female patients need to be considered when applying the targeted RNA-LNP therapy. Below, we first discuss targeted RNA-LNP delivery as a general term. Then, we give a few brief examples of delivery for women’s diseases by RNA-LNPs.
As a general term, the targeted LNP delivery system must: 1) prevent glomerular filtration, phagocytosis, and degradation of nucleic acid therapeutics in the kidney, liver, and bloodstream; 2) cross the vascular endothelial barrier and extracellular matrix; and 3) enter targeted cells, escape endosomes, and release loaded nucleic acids. Successful retargeting of LNPs to non-liver sites first requires de-targeting from the liver, and thus extending the blood circulation time. Immune-invisible coatings like PEGylation (Suk et al., 2016), inert polymeric coatings (Debayle et al., 2019; Chen et al., 2021), cell membrane coating (Fang et al., 2018; Luk and Zhang, 2015), and “self” peptides (Khan et al., 2022; Oltra et al., 2014) resist serum protein binding, reducing clearance by the reticuloendothelial system (RES). Elongated circulation time increases the likelihood of LNPs encountering targeted cells. Saunders et al. (Saunders et al., 2020) developed an effective method to minimize LNP capture by liver cells. By pretreating with a “nanoprimer” before LNPs loaded with RNA therapeutics, the bioavailability of RNA-loaded LNPs significantly improved (Saunders et al., 2020). Ouyang et al. (Ouyang et al., 2020) discovered a threshold dose that overwhelms Kupffer cell uptake rates. Above this threshold, liver clearance of nanoparticles nonlinearly decreases, extending circulation time (Ouyang et al., 2020).
Researchers have made significant advancements in re-targeting strategies by exploring the potential of passive and active targeting techniques/engineering (Figure 3) for redirecting LNPs toward desired targets (Dilliard and Siegwart, 2023).
Passive targeting leverages the physical properties of LNPs, such as size, shape, and charge, to facilitate their accumulation at specific sites (Mitchell et al., 2021; Truong et al., 2015). Notably, an in vivo systemic investigation revealed that nanoparticles with a diameter of 50 nm exhibited superior tumor accumulation and penetration compared to counterparts measuring 20 nm or 200 nm in diameter (Tang et al., 2014). Adjusting the net charge of RNA lipoplex nanoparticles allowed precise and effective targeting in vivo, specifically to the lung or spleen (Kranz et al., 2016).
Furthermore, recent studies have shed light on the relationship between passive targeting factors and the formation of a protein corona on the nanoparticle surface. In a study examining the impact of nanoparticle shape on protein corona formation, researchers observed a significantly higher protein deposition on rod-like particles compared to spheres, potentially influencing the fate of the nanoparticles in vivo (Madathiparambil Visalakshan et al., 2020). Another intriguing approach, known as selective organ targeting (SORT), has gained considerable attention (Wang et al., 2023). Incorporating SORT molecules with altered charges enables precise modulation of LNP biodistribution, therefore facilitating non-liver tissue-specific gene editing (Wang et al., 2023). Subsequent research uncovered that the inclusion of SORT components influenced the apparent pKa and serum protein adsorption of LNPs. The targeting mechanism was also elucidated, revealing that the disassociation of sheddable PEG-lipids resulted in distinct protein bindings to the exposed SORT molecules. This mechanism facilitated the delivery of LNPs to specific tissues expressing high levels of cognate receptors (Dilliard et al., 2021). Thus, akin to ApoE mediating Onpattro’s targeting of the liver, other serum proteins could mediate these LNPs to different organs or tissues (Akinc et al., 2019).
In their research, another group of scientists achieved the absorption of different serum proteins with different compositions on the surface of LNPs by modifying the chemical structure of ionizable cationic lipids (Qiu et al., 2022; Chen et al., 2022). This finding holds significant potential for influencing the biodistribution of LNPs. Specifically, their N-series LNPs effectively delivered mouse tuberous sclerosis complex-2 mRNA to the lungs, consequently resulting in reduced tumor burden in a preclinical model of lymphangio-leiomyomatosis (Qiu et al., 2022). Furthermore, using a novel ionizable cationic lipid and optimized formulation, they successfully developed LNPs that displayed lymph node-specific targeting capabilities and enhanced CD8+ T cell response to the encoded full-length ovalbumin model antigen (Chen et al., 2022).
The tuning of physicochemical properties in LNPs shows promise for achieving targeted delivery to several major organs and tissues. However, further improvements are necessary to direct LNPs to additional organs and specific cell subgroups while minimizing delivery to undesired cells and tissues, particularly the liver. Notably, successful passive targeting methods mentioned above involve the formation of distinct protein coronas. It can be challenging to isolate and determine the contributions of passive targeting and serum protein-mediated targeted delivery. Moreover, the adsorption of serum proteins onto LNPs is difficult to control and determine precisely, as protein binding is dynamic and exchangeable. Additionally, variations in serum protein compositions and cell receptor levels across different species further complicate the situation in the research and preclinical vs clinical studies.
Active targeting strategies have effectively enhanced targeting precision and efficiency by incorporating ligands into LNPs. These ligands can include aptamers, sugars, antibodies, antibody fragments, and vitamins, which can be modified on the LNP surface to facilitate ligand-mediated targeted delivery (Mitchell et al., 2021; Liang et al., 2015; Rurik et al., 2022; Katakowski et al., 2016). Various techniques, such as direct conjugation, post-insertion, and biological approaches, can fabricate ligand-modified nanoparticles (Heath et al., 1980; Torchilin et al., 1979; Huang et al., 1980; Marcos-Contreras et al., 2020; Kedmi et al., 2018). Notably, this active targeting approach has been recently used in research to direct and preferentially accumulate RNA-LNPs for women’s disorders such as gynecological cancers (Tang et al., 2019; Rosenblum et al., 2020; Okamoto et al., 2018).
Directly targeting cancer cells is an effective and straightforward approach to cancer treatment. Okamoto et al. decorated siRNA-loaded LNPs with Fab’ against heparin-binding epidermal growth factor (EGF)-like growth factor (Okamoto et al., 2018). The targeted LNPs successfully delivered siRNA to MDA-MB-231 human triple-negative breast cancer cells in vivo and suppressed polo-like kinase 1 (PLK1), thus leading to significant inhibition of tumor growth (Okamoto et al., 2018). For targeted gene editing in cancer treatment, Rosenblum et al. encapsulated gene editing tools (Cas9 mRNA and sgRNA) in LNPs and engineered the LNPs for targeted delivery by modifying EGFR antibodies on the surface (Rosenblum et al., 2020). In the ovarian tumor model, the targeted LNPs achieved selective uptake mediated by the targeting ligands, achieved up to 80% gene editing in vivo, inhibited tumor growth, and improved survival by 80% (Rosenblum et al., 2020).
In another study, Tang and co-workers also achieved cervical cancer cell-selective mRNA delivery and CRISPR/Cas9 genome editing by designing phenylboronic acid (PBA) derived LNPs (PBA-BADP/p53 mRNA NPs) to prohibit cancer cell growth and show higher gene expression in HeLa cells over non-cancerous cells (Tang et al., 2019). They concluded that this active targeting of LNPs was achieved by the high affinity of PBA-modified LNPs to the cell surface sialic acid which is significantly over-expressed in cancer cells than normal cells.
Bioconjugation targeting ligands onto LNPs provides a more accurate and predictable approach for targeted delivery outside the liver. The targeted LNPs can benefit from over 3 decades of historical efforts in learning about immunoliposomes (antibody-coupled liposomes) using similar strategies for antibody conjugations and designs. Clinical trials such as on MM-302 and C225-Ils-dox for breast cancer (NCT01702129, NCT03603379, NCT01304797) and other lipid-based delivery clinical studies can be useful to pave the correct path for RNA-LNP therapy in women’s health. However, extensive works are needed for successful clinical translation of these modalities.
Although mRNA vaccines can generate rapid protein expression, their short half-life may limit applications requiring longer-term protein production. Self-amplifying RNA (saRNA) was developed to address this challenge (Figure 2). By incorporating a viral replicon encoding nonstructural genes, saRNA constructs can amplify themselves intracellularly, prolonging protein expression and enhancing immunogenicity compared to conventional mRNA (Blakney et al., 2021; Bloom et al., 2021). Lower doses of saRNA-LNPs can thus achieve higher immune responses, offering potential in both prophylactic vaccines and therapeutic applications demanding substantial protein expression.
Nevertheless, presence of a viral replicon in the saRNA structure significantly increases its size by thousands of bases to pose stability issues. Also, these nucleotides that are incorporated into the saRNA structure must be compatible with the T7 RNA polymerase and cellular translation machinery, as well as the viral nonstructural genes that are necessary for RNA amplification and subsequent protein expression (Maruggi et al., 2019). To resolve this issue, Aziz et al. utilized noncanonical nucleotides, such as 5-methylcytidine (m5C) and 5-methyluridine (m5U), that were found to be compatible with standard mRNA translation and reduced the immune stimulation and recognition of RNA molecules by Toll-like receptors (TLRs) (Azizi et al., 2024). Hence, the use of such modified nucleotides with saRNA-based LNPs displayed great potential for a variety of therapeutic applications. Similarly, another group of researchers developed an saRNA-LNP vaccine to demonstrate substantially increased immunogenicity in comparison to the unformulated RNA (Geall et al., 2012). Ramos da Silva et al. explored the potential of saRNA-LNP as a cancer vaccine for human papillomavirus-associated cancers. It was shown that a single dose of an saRNA-LNP vaccine encoding a fusion protein of the type 1 herpes simplex virus (HSV-1) glycoprotein D (gD) and the HPV-16 E7 oncoprotein (E7) could induce robust immune response to control advanced tumor progression in preclinical settings (Ramos da Silva et al., 2023b).
Despite these advances, concerns remain regarding the replicative nature of such vaccines, particularly in pregnant women where viral replicons could theoretically affect fetal development (Comes et al., 2023). However, studies on attenuated YFV17D vector vaccines suggest that, while replicons can cross the placenta, they may not necessarily harm embryonic growth (da Silva et al., 2020). Additional preclinical and clinical studies are still required to implement saRNA-LNP based vaccines in vulnerable individuals.
Circular RNAs (circRNAs) are a novel vaccine platform in which the RNA is covalently closed without free ends, increasing resistance to enzymatic degradation (Figure 2). Translation of circRNAs relies on internal ribosome entry sites (IRES) or m6A modifications, leading to higher protein expression and a longer half-life than linear mRNA counterparts (Bai et al., 2022; Wesselhoeft et al., 2018). Even low doses of circRNA formulations can generate robust immune responses, making them attractive candidates for next-generation vaccine development. For instance, CircRNARBD and VFLIP-X have shown potent immunogenicity against SARS-CoV-2 in preclinical models (Qu et al., 2022; Seephetdee et al., 2022). Moreover, a circRNAOVA-luc-LNP (OVA[257-264]-luciferase-coding circRNA) vaccine was also constructed by a group of researchers to show anti-tumor immune response in a wide range of difficult-to-treat malignancies (Li et al., 2022).
Despite these promising results, the clinical translation of circRNA-LNP formulations is yet limited by various challenges encountered in their development, manufacturing, quality control, and safety. Designing circRNA-LNP vaccines to optimize self-adjuvant effects, improve circularization efficiency, and refine fragment length remains a key step for maximum efficacy (Chen et al., 2023). Additional safety concerns center on that fact that exogenous circRNAs may disrupt the biological functions of naturally present circRNAs in the body following administration. Noticeably, it has been shown that circRNAs are present in granulosa cells in ovarian follicles, placenta, several different fetal tissues, and maternal blood in pregnant women (Arthurs et al., 2022). It has also been proven that circRNAs are involved in a variety of biological processes that are related to the pathogenesis of pre-eclampsia during pregnancy (Liao et al., 2024). Given this, administration of circRNA-LNP formulations in pregnant women can also result in unnecessary complications. Also, circRNA-LNP vaccines contain residual dsRNA content, which could result in unwanted immunogenicity or myocarditis after vaccination (Han et al., 2021; Singh et al., 2021).
Although little research exists specifically for circRNA-LNPs in gynecologic cancers, expanding these studies could illuminate their therapeutic potential while addressing the unique safety requirements of pregnant women. Technological advancements that address current delivery and formulation challenges will be necessary to fully exploit circRNA vaccines and therapeutics against infections and malignancies.
A more comprehensive understanding is needed regarding the safety and efficacy of RNA-LNPs before these modalities can be widely used for gynecological conditions in clinics. As of late 2024, we could find three clinical trials directly mentioned pregnant women, neonates, or lactation when searching “mRNA vaccine” plus “pregnancy” terms on the search engine www.clinicaltrials.gov website (NCT06143046 on RSV vaccination and NCT06503900 and NCT05618548 on COVID vaccine). Current data – especially from mRNA-LNP vaccines – suggest comparable safety profiles in pregnant and nonpregnant women, with no elevated risk of severe adverse effects in infants (Swingle et al., 2023a; Ellington and Olson, 2022; Prahl et al., 2022; Ogata et al., 2022; Yeo et al., 2021). Nonetheless, broader studies with diverse populations are essential to confirm the safety of repeated RNA-LNP administration during and after pregnancy. Historically, many early non-viral vectors (e.g., lipoplexes and polyplexes) failed in vivo due to permanently charged cationic or non-biodegradable lipids/polymers in their formulations. Today’s LNPs typically feature lower surface charge, improved biocompatibility, and less tissue accumulation (Liao et al., 2024; Moghimi and Simberg, 2022; Dolgin, 2021). However, mRNA-LNPs still trigger innate immune responses via Toll-like receptors (TLRs), melanoma differentiation-associated protein 5 (MDA5), and NOD-like receptor protein 3 (NLRP3), leading to the production of interferon-gamma (IFN-γ), IL 1β, and IL 6. Moreover, mRNA-LNP platforms induce CD8+ T cells, T follicular helper (Tfh) cells, and germinal center (GC) B cells, potentially heightening immunogenicity.
The adverse effects of mRNA-LNP can be caused by three currently known mechanisms, including i) IgE-mediated anaphylaxis ii) IgM-mediated complement activation-related pseudoallergy (CARPA) and iii) autoimmune reaction (Khalid and Frischmeyer-Guerrerio, 2023; Lee et al., 2023). PEG-lipid components are reported as the underlying reason for the side effects through mechanisms i and ii (Khalid and Frischmeyer-Guerrerio, 2023), while mechanism iii is mainly caused by mRNA-LNP itself when it is recognized as a self-antigen that can initiate autoimmune diseases. To modulate the immune response, several strategies are suggested such as adjusting the size and sequence chemistry of the mRNA and nucleotides, modifying the charge of the LNP by changing the lipid composition and chemistry, addition of adjuvants in case of vaccines, or exploring different routes of administration.
Having said that, concerns initially raised about the current LNP formulations due to the need for more clarity about the potential direct or indirect toxic or immune adverse effects on mothers and fetuses. These concerns were regarding the induction of a systemic inflammatory immune response against the nucleic acid cargo or synthetic lipids (PEGylated lipids and ionizable lipids) in humans, especially in susceptible and already immune-compromised conditions such as pregnancy. These concerns and uncertainty resulted in excluding pregnant women in the initial clinical trials of the COVID-19 vaccine, and by far, we still have a limited understanding of the mRNA-LNP vaccines on pregnant women and fetuses. In a study by Prahl et al., they found that COVID mRNA-LNP products were detectable for up to 1 month in the serum of pregnant or lactating women after the first vaccine shot and up to 1 week after the second shot (Prahl et al., 2022). The possibility of bypassing the placenta barrier by these nanoparticles and entering the fetal bodies was among the primary reasons for excluding pregnant women from the clinical trials.
Despite these concerns, real-world data from pregnant women receiving mRNA-LNP COVID-19 vaccines suggest that LNPs at the used composition and dose for vaccination cannot pass through the placenta (Prahl et al., 2022; Ogata et al., 2022). While anti-COVID IgG is detectable in both fetuses and neonates, conferring passive immunity, no mRNA-LNP particle has been identified in neonates (Prahl et al., 2022). Given that currently there is no approved COVID-19 vaccines for children younger than 6 months, this vertically transmitted immunity would be life-saving for the newborn at this stage.
The mRNA used in the COVID vaccine is N1-methyl-pseudouridine-modified (N1m). Although N1m-mRNA is less detectable by the toll-like receptors (TLR) 7 and 8 and considered a safer modality, it requires continued surveillance to address uncertainties around maternal-fetal impacts. The ionizable lipids are of primary concern in interleukin (mainly, IL-1) induction and, thus, subsequent activation of the pro-inflammatory cytokines, leading to a general immune activation with the general manifestation of pain, swelling, fever, and in some cases, severe inflammation reaction in patients (Tahtinen et al., 2022).
Additionally, recent studies emphasize that both the chemical structure of ionizable lipids and the administration route significantly influence toxicity, transfection efficiency, and immunogenicity in pregnant women. For instance, Chaudhary et al. (Chaudhary et al., 2024) reported that the ionizable lipid amine headgroup defines the delivery and expression of LNPs to placental BeWo b30 human trophoblasts. They observed that structures similar to naturally occurring polyamine such as spermidine and spermine showed the highest delivery and efficacy. However, with concern to the safety, the lipid tail imposed the most effect with branched ionizable lipids eliciting highest hemolytic activity compared to the linear lipid tails. Furthermore, they evaluated the cytokine and chemokine profiles, and they demonstrated the organ tropism in pregnant mice correlated with the immunogenic routes and structures of the screened ionizable lipids. After intravenous administration, some of the screened LNPs suppressed IL-1B in pregnant mice. Patrolling myeloid cells are among the abundantly transfected cells by the administered LNPs and IL-1B is a factor that promotes infiltration of these myeloid cells in spleen and lymph nodes. They concluded that this IL-1B suppression by certain intravenous LNPs might indirectly cause the reduced spleen and lymph node mRNA-LNP transfection through suppressed myeloid cells infiltration into these organs. They also observed that LNPs with high impact on the immune homeostasis could significantly impact pup development. The elevated B and T cell infiltration in the placenta was correlated with the impeded pup growth which was supposed to be due to the elevated inflammatory cytokines (Chaudhary et al., 2024; Graham et al., 2021).
Such findings underscore the complexity of selecting suitable LNPs and necessitate extensive preclinical safety testing, especially given the proliferation of next-generation lipid moieties [e.g., see (Han et al., 2024; Liu et al., 2021)]. Overall, although current evidence indicates that RNA-LNPs can be relatively safe and efficacious, especially in nonpregnant adults, more comprehensive preclinical and clinical investigations are required. This is particularly true for vulnerable populations (e.g., pregnant women) and for conditions needing repeated dosing. Low-cost, validated animal models for women’s diseases remain essential for early-stage screening, as discussed below.
Preclinical models are indispensable for understanding the therapeutic mechanisms, safety, and efficacy of RNA-LNP interventions in women’s diseases. Larger species such as bovine, porcine, and ovine models can mimic human physiology in specific contexts, but mice remain the most common due to their availability, cost-effectiveness, and well-characterized genetics. For the purposes and scope of this review, we are focusing on the latter category. Table 3 briefly overviews some mouse models used to study various women’s diseases. Murine models fall into two main categories: (i) genetically modified mice that replicate molecular pathways relevant to human disease, and (ii) chemically or surgically induced models. While these systems afford invaluable mechanistic insights, they do not always recapitulate the complexity of human pathology. For instance, the keratinized mouse vaginal epithelium – with thickness fluctuating across estrous phases – limits translational relevance for human vaginal drug delivery (McCracken et al., 2021). Some gynecological conditions are challenging to mimic in mice models mainly because of their short duration and lack of spontaneous incidence in mice. For instance, endometriosis at the late stages requires that mice be evaluated for months to a year, which makes it very hard to keep the animal alive for such a duration. Additionally, only non-human primate species have spontaneous endometriosis, and other animals, including mice, lack this capability (Lagana et al., 2018). Similarly, most murine models of ovarian and cervical cancers cannot accurately capture recurrent malignancies or drug resistance. Moreover, placental differences (haemotrichorial in mice vs. haemomonochorial in humans) complicate direct extrapolation to pregnancy-related conditions (Furukawa et al., 2014).
Despite these constraints, innovations in genetic engineering and immunocompromised “humanized” mice can bring preclinical studies closer to clinical reality. Nevertheless, investigators must remain cautious when inferring therapeutic outcomes for women’s health. Mouse data, while foundational, should be integrated with complementary in vitro and in silico studies, as well as larger, more physiologically analogous animal models, to robustly predict clinical responses.
Recent research has explored the possible benefits of using nanoparticles (including LNPs) in gynecological and pregnancy-related disorders (Bedin et al., 2019; Riley et al., 2021; Michy et al., 2019; Liu et al., 2017; Zhai et al., 2018). Such systems could be used for targeted drug delivery to the female reproductive system in both pregnant and non-pregnant women, improving treatment outcomes (Riley et al., 2021; Sharma et al., 2021; Young et al., 2022) and even serve as a theranostic platform in gynecological disorders (Moses et al., 2020). Despite these benefits, detailed knowledge of nanoparticle risk/benefit profiles in gynecology, pregnancy, and lactation remains limited, largely due to the complexity of female reproductive physiology and ethical restrictions on clinical testing (Sharma et al., 2021).
The female reproductive system is dynamic and undergoes significant changes during pregnancy and lactation, making it essential to understand the interactions between nanoparticles and the maternal-fetal interface, as well as their potential impact on fetal and neonatal health (Sharma et al., 2021; de Araujo et al., 2020; Grafmuller et al., 2013). Ethical considerations further restrict data availability, particularly regarding potential risks to pregnant women and their fetuses. Current knowledge gaps include whether nanoparticle exposure affects ovulation, implantation, embryo development, and hormonal balance (Ajdary et al., 2021; Ahmad, 2022), and how placental accumulation might impair fetal growth or health (Ahmad, 2022). Having said that, this direct impact of NPs is not always the only issue in pregnancy applications. The other deriving fetal toxicity may come through the maternal toxic response to the administered dose that can be vertically transmitted through the placenta and impact the fetus with toxicity and abnormal growth or teratogenicity (Dugershaw et al., 2020). These concerns largely explain why pregnant women are typically excluded from early drug discovery trials (Mazer-Amirshahi et al., 2014), as witnessed during initial COVID-19 vaccine testing (Prahl et al., 2022; Dangel et al., 2022; Couzin-Frankel, 2022). Recent studies eventually shed some light on the safety of mRNA-LNP vaccines in pregnancy. The available data show that LNP products can be detected in serum for a month after the first dose of the COVID vaccine and a week after the second dose administration. Prahl et al. found that LNPs cannot pass the placenta and were not detected in the fetus, though IgGs against SARS-CoV-2 antigens were detectable in the fetal blood, indicating passive immunization of the fetus (Prahl et al., 2022). While small molecules and certain antibodies (such as IgG but not IgM) generally traverse the placental barrier and reach the neonatal organs, nanoparticles, such as conventional LNP composition (used in mRNA-LNP COVID vaccines), do not unless specially engineered to passively or actively target in utero delivery (Figueroa-Espada et al., 2020).
Another challenge of LNPs is the unclear acute and chronic effects of LNPs and RNA molecules on women’s health. As the treatment with gene therapy modalities, such as mRNA in enzyme replacement therapy, is chronic, accumulating LNPs in tissues over repeated administration may result in adverse health effects. Nevertheless, this may be less crucial for the limited-dosing frequency of gene correction methods, such as CRISPR/Cas, etc. Our knowledge of chronic immune system interaction with LNPs or in vitro-transcribed RNA molecules has yet to mature fully. This potential chronic immunogenicity could be a concern for vulnerable populations such as immune-compromised elderly women or pregnant women. Our understanding of COVID-19 vaccines and RNA delivery by lipids may help to elucidate some of the other individual and sex-related influences and allergic reactions to the LNP components, especially for the maternal and perinatal situation where the mother’s physiology is already at a relatively immune-compromised state (Sharma et al., 2021).
It has been shown that some of the administered lipids in RNA-LNP products can enhance the adjuvant effects of the carrying components and induction of efficient immune response in a useful way for vaccine purposes (Luan et al., 2022). Nonetheless, there is a possibility that an uncontrolled immune response may also lead to unintended consequences, such as inflammation due to reactive oxygen species production (ROS) in the placenta of pregnant women or tissue/organ damage. Furthermore, the potential impact of LNPs and RNA molecules on the reproductive system, particularly post-partum and lactation, requires careful scrutiny. In a survey of lactating mothers who received the COVID vaccine, all participants showed detectable levels of neutralizing antibodies in breastmilk after the second dose of vaccine, and only 2% of the mothers had detectable mRNA-vaccine particles or components in their breastmilk over 1 week post-vaccination (Yeo et al., 2021). This study did not find any sensitivity in newborns after maternal vaccination, and the authors concluded that nursing post-vaccination confers temporary passive immunity (Yeo et al., 2021). Further studies are needed to fully uncover the safety of these therapeutics for both the mother and the developing fetus or breastmilk-fed infant to avoid any potential harm.
RNA-LNP therapeutics present a promising modality in women’s health, potentially addressing a host of gynecological and obstetric conditions, including endometriosis, gynecologic cancers, and viral infections. Nonetheless, several challenges remain, particularly regarding targeting efficiency, safety in pregnancy, and long-term effects on reproductive organs and maternal-fetal and nursing health. Followings are some suggested future directions to drive this field forward.
Continued innovation and screening in biocompatible and biodegradable ionizable lipid chemistries and substitutes to the PEG-lipid can reduce toxicity and immunogenicity, limit selective tissue accumulation, and extend circulation time. Similarly, newer generations of RNAs, such as circRNA, and saRNA, can improve the efficacy of RNAs while reducing impurities (like double stranded RNAs) and address the short-half-life of mRNA in vivo. Systematic and high throughput screening in preclinical models, including DNA and RNA barcoding (Xue et al., 2024; Dahlman et al., 2017) will facilitate the development of safer, more potent RNA-LNP formulations, paving the way for repeated or long-term applications in women’s health.
New approaches are needed to enhance extra-hepatic delivery of RNA-LNPs. The efforts should not only cover the physicochemical properties, such as optimal particle size and surface engineering, but it also requires improving the active targeting approach via ligand conjugation for precise accumulation in female reproductive tissues. Improved targeting can be achieved through conjugation of bispecific mAbs or smaller fragments of mAbs (e.g., antibody Fabs, VHHs, Affibodies, Nanobodies, etc.) (Wang S. et al., 2024; Amabile et al., 2024) with higher affinity so that the developed LNPs retain their size ideal for enhanced extra-hepatic delivery and target organs cellular uptake.
Integrating RNA-LNP therapies with other modalities – such as existing hormonal and small molecule treatments – can harness synergistic effects. Furthermore, individualized cancer vaccine designs, tailored to patient-specific biomarkers hold potential for more effective targeted treatments. Several of these individualized cancer vaccines are under development and the preliminary results indicate the potential of using RNA-LNPs for inducing long-term prevention of recurrence with improved prognosis for the female-related cancer patients (Huang T. et al., 2022; Jacob et al., 2024).
In conclusion, although the field of RNA-LNP therapeutics for women’s health remains relatively nascent, advances in high throughput screening, targeted LNP design, next-generation RNA and lipid chemistries, and gene-editing technologies such as CRISPR/Cas systems, signal a rapidly evolving landscape. This platform’s capacity for high specificity, adaptable treatment durations, potential synergy with other therapies, and ease of manufacturing/scale up highlights its transformative promise. Nevertheless, to ensure long-term success, safety must be thoroughly established through extensive research and clinical validation – especially in vulnerable populations such as pregnant and lactating women. By addressing these challenges and building upon current achievements, RNA-LNP therapeutics can realize their full potential in delivering personalized, highly effective treatments for conditions that profoundly affect women’s lives.
AN: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing–original draft, Writing–review and editing. AS: Writing–original draft, Writing–review and editing. YZ: Writing–original draft. AP: Writing–original draft. C-TK: Writing–original draft. HB: Writing–original draft. SH: Writing–review and editing. BR: Writing–original draft, Writing–review and editing. AS: Project administration, Writing–original draft, Writing–review and editing. MS: Funding acquisition, Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors are current or previous employees of Regeneron Pharmaceuticals.
Authors would like to thank Arash Hatefi (Rutgers University), Paul Panipinto (Washington State University), Alexander Josowitz, and Ying Wang (Merck Pharmaceuticals) for their contributions on providing insights and comments on the manuscript.
Authors AN, AS, YZ, AP, C-TK, HB, SH, BR, AB, and MS were employed byRegeneron Pharmaceuticals.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, B. D., Parsons, C., Walker, L., Zhang, W. C., and Slack, F. J. (2017). Targeting noncoding RNAs in disease. J. Clin. Invest. 127 (3), 761–771. doi:10.1172/JCI84424
Aghamiri, S., Talaei, S., Roshanzamiri, S., Zandsalimi, F., Fazeli, E., Aliyu, M., et al. (2020). Delivery of genome editing tools: a promising strategy for HPV-related cervical malignancy therapy. Expert Opin. Drug Deliv. 17 (6), 753–766. doi:10.1080/17425247.2020.1747429
Ahmad, A. (2022). Safety and toxicity implications of multifunctional drug delivery nanocarriers on reproductive systems in vitro and in vivo. Front. Toxicol. 4, 895667. doi:10.3389/ftox.2022.895667
Ajdary, M., Keyhanfar, F., Moosavi, M. A., Shabani, R., Mehdizadeh, M., and Varma, R. S. (2021). Potential toxicity of nanoparticles on the reproductive system animal models: a review. J. Reprod. Immunol. 148, 103384. doi:10.1016/j.jri.2021.103384
Akinc, A., Maier, M. A., Manoharan, M., Fitzgerald, K., Jayaraman, M., Barros, S., et al. (2019). The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14 (12), 1084–1087. doi:10.1038/s41565-019-0591-y
Al-Hendy, A., Myers, E. R., and Stewart, E. (2017). Uterine fibroids: burden and unmet medical need. Semin. Reprod. Med. 35 (6), 473–480. doi:10.1055/s-0037-1607264
Aliota, M. T., Caine, E. A., Walker, E. C., Larkin, K. E., Camacho, E., and Osorio, J. E. (2016). Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 10 (4), e0004682. doi:10.1371/journal.pntd.0004682
Allam, A., Majji, S., Peachman, K., Jagodzinski, L., Kim, J., Ratto-Kim, S., et al. (2015). TFH cells accumulate in mucosal tissues of humanized-DRAG mice and are highly permissive to HIV-1. Sci. Rep. 5, 10443. doi:10.1038/srep10443
Amabile, A., Phelan, M., Yu, Z., Silva, P., Marks, A., Morla-Folch, J., et al. (2024). Bispecific antibody targeting of lipid nanoparticles. bioRxiv, 2024.12.20.629467. doi:10.1101/2024.12.20.629467
Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M., et al. (2022). Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66, 15–23. doi:10.1016/j.breast.2022.08.010
Arthurs, A. L., Jankovic-Karasoulos, T., Smith, M. D., and Roberts, C. T. (2022). Circular RNAs in pregnancy and the placenta. Int. J. Mol. Sci. 23 (9), 4551. doi:10.3390/ijms23094551
Asmamaw, M., and Zawdie, B. (2021). Mechanism and applications of CRISPR/Cas-9-Mediated genome editing. Biologics 15, 353–361. doi:10.2147/BTT.S326422
Attalla, S., Taifour, T., Bui, T., and Muller, W. (2021). Insights from transgenic mouse models of PyMT-induced breast cancer: recapitulating human breast cancer progression in vivo. Oncogene 40 (3), 475–491. doi:10.1038/s41388-020-01560-0
Azizi, H., Renner, T. M., Agbayani, G., Simard, B., Dudani, R., Harrison, B. A., et al. (2024). Self-amplifying RNAs generated with the modified nucleotides 5-methylcytidine and 5-methyluridine mediate strong expression and immunogenicity in vivo. Nar. Mol. Med. 1 (2). doi:10.1093/narmme/ugae004
Bai, Y., Liu, D., He, Q., Liu, J., Mao, Q., and Liang, Z. (2022). Research progress on circular RNA vaccines. Front. Immunol. 13, 1091797. doi:10.3389/fimmu.2022.1091797
Baird, D. D., Dunson, D. B., Hill, M. C., Cousins, D., and Schectman, J. M. (2003). High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am. J. Obstet. Gynecol. 188 (1), 100–107. doi:10.1067/mob.2003.99
Balasubramaniam, S. D., Balakrishnan, V., Oon, C. E., and Kaur, G. (2019). Key molecular events in cervical cancer development. Med. Kaunas. 55 (7), 384. doi:10.3390/medicina55070384
Bardoliwala, D., Javia, A., Ghosh, S., Misra, A., and Sawant, K. (2021). Formulation and clinical perspectives of inhalation-based nanocarrier delivery: a new archetype in lung cancer treatment. Ther. Deliv. 12 (5), 397–418. doi:10.4155/tde-2020-0101
Basha, G., Cottle, A. G., Pretheeban, T., Chan, K. Y., Witzigmann, D., Young, R. N., et al. (2022). Lipid nanoparticle-mediated silencing of osteogenic suppressor GNAS leads to osteogenic differentiation of mesenchymal stem cells in vivo. Mol. Ther. 30 (9), 3034–3051. doi:10.1016/j.ymthe.2022.06.012
Basha, G., Ordobadi, M., Scott, W. R., Cottle, A., Liu, Y., Wang, H., et al. (2016). Lipid nanoparticle delivery of siRNA to osteocytes leads to effective silencing of SOST and inhibition of sclerostin in vivo. Mol. Ther. Nucleic Acids 5 (9), e363. doi:10.1038/mtna.2016.68
Becker, C. M., Gattrell, W. T., Gude, K., and Singh, S. S. (2017). Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil. Steril. 108 (1), 125–136. doi:10.1016/j.fertnstert.2017.05.004
Bedin, A., Maranhao, R. C., Tavares, E. R., Carvalho, P. O., Baracat, E. C., and Podgaec, S. (2019). Nanotechnology for the treatment of deep endometriosis: uptake of lipid core nanoparticles by LDL receptors in endometriotic foci. Clin. (Sao Paulo) 74, e989. doi:10.6061/clinics/2019/e989
Bianchini, G., De Angelis, C., Licata, L., and Gianni, L. (2022). Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat. Rev. Clin. Oncol. 19 (2), 91–113. doi:10.1038/s41571-021-00565-2
Blakney, A. K., Ip, S., and Geall, A. J. (2021). An update on self-amplifying mRNA vaccine development. Vaccines (Basel) 9 (2), 97. doi:10.3390/vaccines9020097
Bloom, K., van den Berg, F., and Arbuthnot, P. (2021). Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28 (3-4), 117–129. doi:10.1038/s41434-020-00204-y
Buschmann, M. D., Carrasco, M. J., Alishetty, S., Paige, M., Alameh, M. G., and Weissman, D. (2021). Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 9 (1), 65. doi:10.3390/vaccines9010065
Caballero, T., Canabal, J., Rivero-Paparoni, D., and Cabanas, R. (2014). Management of hereditary angioedema in pregnant women: a review. Int. J. Womens Health 6, 839–848. doi:10.2147/IJWH.S46460
Cai, L., Fisher, A. L., Huang, H., and Xie, Z. (2016). CRISPR-mediated genome editing and human diseases. Genes. Dis. 3 (4), 244–251. doi:10.1016/j.gendis.2016.07.003
Campana-Seoane, M., Perez-Gago, A., Vazquez, G., Conde, N., Gonzalez, P., Martinez, A., et al. (2019). Vaginal residence and pharmacokinetic preclinical study of topical vaginal mucoadhesive W/S emulsions containing ciprofloxacin. Int. J. Pharm. 554, 276–283. doi:10.1016/j.ijpharm.2018.11.022
Campillo-Davo, D., Fujiki, F., Van den Bergh, J. M. J., De Reu, H., Smits, E., Goossens, H., et al. (2018). Efficient and non-genotoxic RNA-based engineering of human T cells using tumor-specific T cell receptors with minimal TCR mispairing. Front. Immunol. 9, 2503. doi:10.3389/fimmu.2018.02503
Cervantes, A., Alsina, M., Tabernero, J., Infante, J. R., LoRusso, P., Shapiro, G., et al. (2011). Phase I dose-escalation study of ALN-VSP02, a novel RNAi therapeutic for solid tumors with liver involvement. J. Clin. Oncol. 29 (15_Suppl. l), 3025. doi:10.1200/jco.2011.29.15_suppl.3025
Chaudhary, N., Newby, A. N., Arral, M. L., Yerneni, S. S., LoPresti, S. T., Doerfler, R., et al. (2024). Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes. Proc. Natl. Acad. Sci. U. S. A. 121 (11), e2307810121. doi:10.1073/pnas.2307810121
Chaudhary, N., Weissman, D., and Whitehead, K. A. (2021). mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20 (11), 817–838. doi:10.1038/s41573-021-00283-5
Chen, H., Cong, X., Wu, C., Wu, X., Wang, J., Mao, K., et al. (2020). Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9(+) T cells. Sci. Adv. 6 (5), eaax4690. doi:10.1126/sciadv.aax4690
Chen, J., Ye, Z., Huang, C., Qiu, M., Song, D., Li, Y., et al. (2022). Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl. Acad. Sci. U. S. A. 119 (34), e2207841119. doi:10.1073/pnas.2207841119
Chen, R., Wang, S. K., Belk, J. A., Amaya, L., Li, Z., Cardenas, A., et al. (2023). Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 41 (2), 262–272. doi:10.1038/s41587-022-01393-0
Chen, S., Zhong, Y., Fan, W., Xiang, J., Wang, G., Zhou, Q., et al. (2021). Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat. Biomed. Eng. 5 (9), 1019–1037. doi:10.1038/s41551-021-00701-4
Chen, X., Ouyang, Z., Shen, Y., Liu, B., Zhang, Q., Wan, L., et al. (2019). CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. 16 (9), 1249–1262. doi:10.1080/15476286.2019.1624470
Chenthamara, D., Subramaniam, S., Ramakrishnan, S. G., Krishnaswamy, S., Essa, M. M., Lin, F. H., et al. (2019). Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 23, 20. doi:10.1186/s40824-019-0166-x
Chow, S., Berek, J. S., and Dorigo, O. (2020). Development of therapeutic vaccines for ovarian cancer. Vaccines (Basel) 8 (4), 657. doi:10.3390/vaccines8040657
Cieśla, M. (2020). “Chapter 11 - RNA in cancer,” in Rna-based regulation in human health and disease. Editors R. Pandey (Academic Press). doi:10.1016/B978-0-12-817193-6.00011-X
Cohen, K. W., De Rosa, S. C., Fulp, W. J., deCamp, A. C., Fiore-Gartland, A., Mahoney, C. R., et al. (2023). A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses. Sci. Transl. Med. 15 (697), eadf3309. doi:10.1126/scitranslmed.adf3309Available online at: Cancer.gov.In
Cohen, P. A., Jhingran, A., Oaknin, A., and Denny, L. (2019). Cervical cancer. Lancet 393 (10167), 169–182. doi:10.1016/S0140-6736(18)32470-X
Comes, J. D. G., Pijlman, G. P., and Hick, T. A. H. (2023). Rise of the RNA machines - self-amplification in mRNA vaccine design. Trends Biotechnol. 41 (11), 1417–1429. doi:10.1016/j.tibtech.2023.05.007
Couzin-Frankel, J. (2022). The pregnancy gap. Science 375 (6586), 1216–1220. doi:10.1126/science.adb2029
Cuevas, I. C., Sahoo, S. S., Kumar, A., Zhang, H., Westcott, J., Aguilar, M., et al. (2019). Fbxw7 is a driver of uterine carcinosarcoma by promoting epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. U. S. A. 116 (51), 25880–25890. doi:10.1073/pnas.1911310116
Culley, L., Law, C., Hudson, N., Denny, E., Mitchell, H., Baumgarten, M., et al. (2013). The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum. Reprod. Update 19 (6), 625–639. doi:10.1093/humupd/dmt027
Cullis, P. R., and Hope, M. J. (2017). Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25 (7), 1467–1475. doi:10.1016/j.ymthe.2017.03.013
Dahlman, J. E., Kauffman, K. J., Xing, Y., Shaw, T. E., Mir, F. F., Dlott, C. C., et al. (2017). Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl. Acad. Sci. U. S. A. 114 (8), 2060–2065. doi:10.1073/pnas.1620874114
Damase, T. R., Sukhovershin, R., Boada, C., Taraballi, F., Pettigrew, R. I., and Cooke, J. P. (2021). The limitless future of RNA therapeutics. Front. Bioeng. Biotechnol. 9, 628137. doi:10.3389/fbioe.2021.628137
Dana, H., Chalbatani, G. M., Mahmoodzadeh, H., Karimloo, R., Rezaiean, O., Moradzadeh, A., et al. (2017). Molecular mechanisms and biological functions of siRNA. Int. J. Biomed. Sci. 13 (2), 48–57. doi:10.59566/ijbs.2017.13048
Dangel, A., Yu, V., Liang, C., and Beninger, P. (2022). Mind the gap: COVID-19 highlights the research void in pregnancy. Am. J. Obstet. Gynecol. MFM 4 (3), 100566. doi:10.1016/j.ajogmf.2022.100566
da Silva, F. C., Magaldi, F. M., Sato, H. K., and Bevilacqua, E. (2020). Yellow fever vaccination in a mouse model is associated with uninterrupted pregnancies and viable neonates except when administered at implantation period. Front. Microbiol. 11, 245. doi:10.3389/fmicb.2020.00245
de Araujo, T. E., Milian, I. C. B., de Souza, G., da Silva, R. J., Rosini, A. M., Guirelli, P. M., et al. (2020). Experimental models of maternal-fetal interface and their potential use for nanotechnology applications. Cell Biol. Int. 44 (1), 36–50. doi:10.1002/cbin.11222
Debayle, M., Balloul, E., Dembele, F., Xu, X., Hanafi, M., Ribot, F., et al. (2019). Zwitterionic polymer ligands: an ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials 219, 119357. doi:10.1016/j.biomaterials.2019.119357
Deeks, S. G., Overbaugh, J., Phillips, A., and Buchbinder, S. (2015). HIV infection. Nat. Rev. Dis. Prim. 1, 15035. doi:10.1038/nrdp.2015.35
De Ziegler, D., Bulletti, C., De Monstier, B., and Jaaskelainen, A. S. (1997). The first uterine pass effect. Ann. N. Y. Acad. Sci. 828, 291–299. doi:10.1111/j.1749-6632.1997.tb48550.x
Dilliard, S. A., Cheng, Q., and Siegwart, D. J. (2021). On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 118 (52), e2109256118. doi:10.1073/pnas.2109256118
Dilliard, S. A., and Siegwart, D. J. (2023). Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater 8 (4), 282–300. doi:10.1038/s41578-022-00529-7
Dinami, R., Pompili, L., Petti, E., Porru, M., D'Angelo, C., Di Vito, S., et al. (2023). MiR-182-3p targets TRF2 and impairs tumor growth of triple-negative breast cancer. EMBO Mol. Med. 15 (1), e16033. doi:10.15252/emmm.202216033
Dolgin, E. (2021). The tangled history of mRNA vaccines. Nature 597 (7876), 318–324. doi:10.1038/d41586-021-02483-w
Dugershaw, B. B., Aengenheister, L., Hansen, S. S. K., Hougaard, K. S., and Buerki-Thurnherr, T. (2020). Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part Fibre Toxicol. 17 (1), 31. doi:10.1186/s12989-020-00359-x
Egorova, A., Selutin, A., Maretina, M., Selkov, S., and Kiselev, A. (2022). Peptide-based nanoparticles for αvβ3 integrin-targeted DNA delivery to cancer and uterine leiomyoma cells. Molecules 27 (23), 8363. doi:10.3390/molecules27238363
Ellington, S., and Olson, C. K. (2022). Safety of mRNA COVID-19 vaccines during pregnancy. Lancet Infect. Dis. 22 (11), 1514–1515. doi:10.1016/S1473-3099(22)00443-1
Enuka, Y., Lauriola, M., Feldman, M. E., Sas-Chen, A., Ulitsky, I., and Yarden, Y. (2016). Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44 (3), 1370–1383. doi:10.1093/nar/gkv1367
Essink, B., Chu, L., Seger, W., Barranco, E., Le Cam, N., Bennett, H., et al. (2023). The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 23 (5), 621–633. doi:10.1016/S1473-3099(22)00764-2
Evans, J. R., Feng, F. Y., and Chinnaiyan, A. M. (2016). The bright side of dark matter: lncRNAs in cancer. J. Clin. Invest. 126 (8), 2775–2782. doi:10.1172/JCI84421
Eygeris, Y., Gupta, M., Kim, J., and Sahay, G. (2022). Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 55 (1), 2–12. doi:10.1021/acs.accounts.1c00544
Fang, R. H., Kroll, A. V., Gao, W., and Zhang, L. (2018). Cell membrane coating nanotechnology. Adv. Mater 30 (23), e1706759. doi:10.1002/adma.201706759
Fauk, N. K., Mwanri, L., Hawke, K., Mohammadi, L., and Ward, P. R. (2022). Psychological and social impact of HIV on women living with HIV and their families in low- and middle-income asian countries: a systematic search and critical review. Int. J. Environ. Res. Public Health 19 (11), 6668. doi:10.3390/ijerph19116668
Figueroa-Espada, C. G., Hofbauer, S., Mitchell, M. J., and Riley, R. S. (2020). Exploiting the placenta for nanoparticle-mediated drug delivery during pregnancy. Adv. Drug Deliv. Rev. 160, 244–261. doi:10.1016/j.addr.2020.09.006
Friedrich, M., and Aigner, A. (2022). Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs 36 (5), 549–571. doi:10.1007/s40259-022-00549-3
Furukawa, S., Kuroda, Y., and Sugiyama, A. (2014). A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 27 (1), 11–18. doi:10.1293/tox.2013-0060
Gao, K., Li, J., Song, H., Han, H., Wang, Y., Yin, B., et al. (2023). In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles. Bioact. Mater 25, 387–398. doi:10.1016/j.bioactmat.2023.02.011
Garson, K., Gamwell, L. F., Pitre, E. M., and Vanderhyden, B. C. (2012). Technical challenges and limitations of current mouse models of ovarian cancer. J. Ovarian Res. 5 (1), 39. doi:10.1186/1757-2215-5-39
Geall, A. J., Verma, A., Otten, G. R., Shaw, C. A., Hekele, A., Banerjee, K., et al. (2012). Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci., 109(36), 14604–14609. doi:10.1073/pnas.1209367109
Ghazal, S., McKinnon, B., Zhou, J., Mueller, M., Men, Y., Yang, L., et al. (2015). H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 7 (8), 996–1003. doi:10.15252/emmm.201505245
Giaquinto, A. N., Broaddus, R. R., Jemal, A., and Siegel, R. L. (2022). The changing landscape of gynecologic cancer mortality in the United States. Obstet. Gynecol. 139 (3), 440–442. doi:10.1097/AOG.0000000000004676
Gildiz, S., and Minko, T. (2023). Nanotechnology-based nucleic acid vaccines for treatment of ovarian cancer. Pharm. Res. 40 (1), 123–144. doi:10.1007/s11095-022-03434-4
Giudice, L. C., and Kao, L. C. (2004). Endometriosis. Lancet 364 (9447), 1789–1799. doi:10.1016/s0140-6736(04)17403-5
Grafmuller, S., Manser, P., Krug, H. F., Wick, P., and von Mandach, U. (2013). Determination of the transport rate of xenobiotics and nanomaterials across the placenta using the ex vivo human placental perfusion model. J. Vis. Exp. 76, 50401. doi:10.3791/50401
Graham, J. J., Longhi, M. S., and Heneghan, M. A. (2021). T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 121, 102651. doi:10.1016/j.jaut.2021.102651
Grunwitz, C., Salomon, N., Vascotto, F., Selmi, A., Bukur, T., Diken, M., et al. (2019). HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology 8 (9), e1629259. doi:10.1080/2162402X.2019.1629259Available online at: https://www.unaids.org/en/resources/documents/2020/in-danger-global-aids-update
Gu, X., Li, M., Jin, Y., Liu, D., and Wei, F. (2017). Identification and integrated analysis of differentially expressed lncRNAs and circRNAs reveal the potential ceRNA networks during PDLSC osteogenic differentiation. BMC Genet. 18 (1), 100. doi:10.1186/s12863-017-0569-4
Gunthard, H. F., Saag, M. S., Benson, C. A., del Rio, C., Eron, J. J., Gallant, J. E., et al. (2016). Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA 316 (2), 191–210. doi:10.1001/jama.2016.8900
Hallan, S. S., Amirian, J., Brangule, A., and Bandere, D. (2022). Lipid-based nano-sized cargos as a promising strategy in bone complications: a review. Nanomater. (Basel) 12 (7), 1146. doi:10.3390/nano12071146
Han, J., Li, S., Feng, Y., He, Y., Hong, W., and Ye, Z. (2021). A novel circular RNA (hsa_circ_0059930)-mediated miRNA-mRNA axis in the lipopolysaccharide-induced acute lung injury model of MRC-5 cells. Bioengineered 12 (1), 1739–1751. doi:10.1080/21655979.2021.1916276
Han, X., Xu, J., Xu, Y., Alameh, M. G., Xue, L., Gong, N., et al. (2024). In situ combinatorial synthesis of degradable branched lipidoids for systemic delivery of mRNA therapeutics and gene editors. Nat. Commun. 15 (1), 1762. doi:10.1038/s41467-024-45537-z
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495 (7441), 384–388. doi:10.1038/nature11993
Haque, M. A., Shrestha, A., Mikelis, C. M., and Mattheolabakis, G. (2024). Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery. Int. J. Pharm. X 8, 100283. doi:10.1016/j.ijpx.2024.100283
Harrington, C. T., Sotillo, E., Dang, C. V., and Thomas-Tikhonenko, A. (2021). Tilting MYC toward cancer cell death. Trends Cancer 7 (11), 982–994. doi:10.1016/j.trecan.2021.08.002
Hassett, K. J., Benenato, K. E., Jacquinet, E., Lee, A., Woods, A., Yuzhakov, O., et al. (2019). Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11. doi:10.1016/j.omtn.2019.01.013
Hayward, S. L., Francis, D. M., Kholmatov, P., and Kidambi, S. (2016). Targeted delivery of MicroRNA125a-5p by engineered lipid nanoparticles for the treatment of HER2 positive metastatic breast cancer. J. Biomed. Nanotechnol. 12 (3), 554–568. doi:10.1166/jbn.2016.2194
Heath, T. D., Fraley, R. T., and Papahdjopoulos, D. (1980). Antibody targeting of liposomes: cell specificity obtained by conjugation of F(ab')2 to vesicle surface. Science 210 (4469), 539–541. doi:10.1126/science.7423203
Herskovitz, J., Hasan, M., Patel, M., Blomberg, W. R., Cohen, J. D., Machhi, J., et al. (2021). CRISPR-Cas9 mediated exonic disruption for HIV-1 elimination. EBioMedicine 73, 103678. doi:10.1016/j.ebiom.2021.103678
Ho, W., Gao, M., Li, F., Li, Z., Zhang, X. Q., and Xu, X. (2021). Next-generation vaccines: nanoparticle-mediated DNA and mRNA delivery. Adv. Healthc. Mater 10 (8), e2001812. doi:10.1002/adhm.202001812
Hou, X., Zaks, T., Langer, R., and Dong, Y. (2021). Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater 6 (12), 1078–1094. doi:10.1038/s41578-021-00358-0
Hsu, S. H., Yu, B., Wang, X., Lu, Y., Schmidt, C. R., Lee, R. J., et al. (2013). Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine 9 (8), 1169–1180. doi:10.1016/j.nano.2013.05.007
Hu, B., Zhong, L., Weng, Y., Peng, L., Huang, Y., Zhao, Y., et al. (2020). Therapeutic siRNA: state of the art. Signal Transduct. Target Ther. 5 (1), 101. doi:10.1038/s41392-020-0207-x
Hu, Z., Ding, W., Zhu, D., Yu, L., Jiang, X., Wang, X., et al. (2015). TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J. Clin. Invest. 125 (1), 425–436. doi:10.1172/JCI78206
Huang, A., Huang, L., and Kennel, S. J. (1980). Monoclonal antibody covalently coupled with fatty acid. A reagent for in vitro liposome targeting. J. Biol. Chem. 255 (17), 8015–8018. doi:10.1016/s0021-9258(19)70595-x
Huang, M., Haiderali, A., Fox, G. E., Frederickson, A., Cortes, J., Fasching, P. A., et al. (2022a). Economic and humanistic burden of triple-negative breast cancer: a systematic literature review. Pharmacoeconomics 40 (5), 519–558. doi:10.1007/s40273-021-01121-7
Huang, T., Peng, L., Han, Y., Wang, D., He, X., Wang, J., et al. (2022b). Lipid nanoparticle-based mRNA vaccines in cancers: current advances and future prospects. Front. Immunol. 13, 922301. doi:10.3389/fimmu.2022.922301
Huang, Y., Xie, J., and Li, E. (2019). Comprehensive circular RNA profiling reveals circ_0002060 as a potential diagnostic biomarkers for osteoporosis. J. Cell. Biochem. 120 (9), 15688–15694. doi:10.1002/jcb.28838
Ismail-Khan, R., and Bui, M. M. (2010). A review of triple-negative breast cancer. Cancer control. 17 (3), 173–176. doi:10.1177/107327481001700305
Jacob, E. M., Huang, J., and Chen, M. (2024). Lipid nanoparticle-based mRNA vaccines: a new frontier in precision oncology. Precis. Clin. Med. 7 (3), pbae017. doi:10.1093/pcmedi/pbae017
Jahanafrooz, Z., Baradaran, B., Mosafer, J., Hashemzaei, M., Rezaei, T., Mokhtarzadeh, A., et al. (2020). Comparison of DNA and mRNA vaccines against cancer. Drug Discov. Today 25 (3), 552–560. doi:10.1016/j.drudis.2019.12.003
Jiang, P., Wang, L., Hou, B., Zhu, J., Zhou, M., Jiang, J., et al. (2018). A novel HPV16 E7-affitoxin for targeted therapy of HPV16-induced human cervical cancer. Theranostics 8 (13), 3544–3558. doi:10.7150/thno.24607
Joraholmen, M. W., Basnet, P., Acharya, G., and Skalko-Basnet, N. (2017). PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 113, 132–139. doi:10.1016/j.ejpb.2016.12.029
Kabani, Z., Ramos-Nino, M. E., and Ramdass, P. (2022). Endometriosis and COVID-19: a systematic review and meta-analysis. Int. J. Mol. Sci. 23 (21), 12951. doi:10.3390/ijms232112951
Kampel, L., Goldsmith, M., Ramishetti, S., Veiga, N., Rosenblum, D., Gutkin, A., et al. (2021). Therapeutic inhibitory RNA in head and neck cancer via functional targeted lipid nanoparticles. J. Control Release 337, 378–389. doi:10.1016/j.jconrel.2021.07.034
Karlan, B. Y., Amin, W., Casper, S. E., and Littlefield, B. A. (1988). Hormonal regulation of CA125 tumor marker expression in human ovarian carcinoma cells: inhibition by glucocorticoids. Cancer Res. 48 (12), 3502–3506.
Katakowski, J. A., Mukherjee, G., Wilner, S. E., Maier, K. E., Harrison, M. T., DiLorenzo, T. P., et al. (2016). Delivery of siRNAs to dendritic cells using dec205-targeted lipid nanoparticles to inhibit immune responses. Mol. Ther. 24 (1), 146–155. doi:10.1038/mt.2015.175
Kedmi, R., Veiga, N., Ramishetti, S., Goldsmith, M., Rosenblum, D., Dammes, N., et al. (2018). A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13 (3), 214–219. doi:10.1038/s41565-017-0043-5
Kenjo, E., Hozumi, H., Makita, Y., Iwabuchi, K. A., Fujimoto, N., Matsumoto, S., et al. (2021). Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 12 (1), 7101. doi:10.1038/s41467-021-26714-w
Khalid, M. B., and Frischmeyer-Guerrerio, P. A. (2023). The conundrum of COVID-19 mRNA vaccine-induced anaphylaxis. J. Allergy Clin. Immunol. Glob. 2 (1), 1–13. doi:10.1016/j.jacig.2022.10.003
Khan, S., Sharifi, M., Gleghorn, J. P., Babadaei, M. M. N., Bloukh, S. H., Edis, Z., et al. (2022). Artificial engineering of the protein corona at bio-nano interfaces for improved cancer-targeted nanotherapy. J. Control Release 348, 127–147. doi:10.1016/j.jconrel.2022.05.055
Kim, J., Jozic, A., Lin, Y., Eygeris, Y., Bloom, E., Tan, X., et al. (2022b). Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano 16 (9), 14792–14806. doi:10.1021/acsnano.2c05647
Kim, S., Traore, Y. L., Ho, E. A., Shafiq, M., Kim, S. H., and Liu, S. (2018). Design and development of pH-responsive polyurethane membranes for intravaginal release of nanomedicines. Acta Biomater. 82, 12–23. doi:10.1016/j.actbio.2018.10.003
Kim, S. C., Sekhon, S. S., Shin, W. R., Ahn, G., Cho, B. K., Ahn, J. Y., et al. (2022a). Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell Toxicol. 18 (1), 1–8. doi:10.1007/s13273-021-00171-4
Kim, S. H., Castro, F., Paterson, Y., and Gravekamp, C. (2009). High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 69 (14), 5860–5866. doi:10.1158/0008-5472.CAN-08-4855
Ko, N. Y., Chen, L. R., and Chen, K. H. (2020). The role of micro RNA and long-non-coding RNA in osteoporosis. Int. J. Mol. Sci. 21 (14), 4886. doi:10.3390/ijms21144886
Kon, E., Elia, U., and Peer, D. (2022). Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 73, 329–336. doi:10.1016/j.copbio.2021.09.016
Korzun, T., Moses, A. S., Kim, J., Patel, S., Schumann, C., Levasseur, P. R., et al. (2022). Nanoparticle-based follistatin messenger RNA therapy for reprogramming metastatic ovarian cancer and ameliorating cancer-associated cachexia. Small 18 (44), e2204436. doi:10.1002/smll.202204436
Kranz, L. M., Diken, M., Haas, H., Kreiter, S., Loquai, C., Reuter, K. C., et al. (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534 (7607), 396–401. doi:10.1038/nature18300
Kreiter, S., Selmi, A., Diken, M., Sebastian, M., Osterloh, P., Schild, H., et al. (2008). Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 180 (1), 309–318. doi:10.4049/jimmunol.180.1.309
Kulkarni, J. A., Witzigmann, D., Chen, S., Cullis, P. R., and van der Meel, R. (2019). Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 52 (9), 2435–2444. doi:10.1021/acs.accounts.9b00368
Kulkarni, J. A., Witzigmann, D., Thomson, S. B., Chen, S., Leavitt, B. R., Cullis, P. R., et al. (2021). The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16 (6), 630–643. doi:10.1038/s41565-021-00898-0
Kumbhar, P., Manjappa, A., Shah, R., Jha, N. K., Singh, S. K., Dua, K., et al. (2022). Inhalation delivery of repurposed drugs for lung cancer: approaches, benefits and challenges. J. Control Release 341, 1–15. doi:10.1016/j.jconrel.2021.11.015
Lagana, A. S., Garzon, S., Franchi, M., Casarin, J., Gullo, G., and Ghezzi, F. (2018). Translational animal models for endometriosis research: a long and windy road. Ann. Transl. Med. 6 (22), 431. doi:10.21037/atm.2018.08.24
Landen, C. N., Chavez-Reyes, A., Bucana, C., Schmandt, R., Deavers, M. T., Lopez-Berestein, G., et al. (2005). Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 65 (15), 6910–6918. doi:10.1158/0008-5472.CAN-05-0530
Landovitz, R. J., Scott, H., and Deeks, S. G. (2023). Prevention, treatment and cure of HIV infection. Nat. Rev. Microbiol. 21, 657–670. doi:10.1038/s41579-023-00914-1
Lee, Y., Jeong, M., Park, J., Jung, H., and Lee, H. (2023). Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. & Mol. Med. 55 (10), 2085–2096. doi:10.1038/s12276-023-01086-x
Leong, E. W. X., and Ge, R. (2022). Lipid nanoparticles as delivery vehicles for inhaled therapeutics. Biomedicines 10 (9), 2179. doi:10.3390/biomedicines10092179
Li, H., Peng, K., Yang, K., Ma, W., Qi, S., Yu, X., et al. (2022). Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 12 (14), 6422–6436. doi:10.7150/thno.77350
Li, Y., Su, Z., Zhao, W., Zhang, X., Momin, N., Zhang, C., et al. (2020). Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1 (9), 882–893. doi:10.1038/s43018-020-0095-6
Liang, C., Guo, B., Wu, H., Shao, N., Li, D., Liu, J., et al. (2015). Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 21 (3), 288–294. doi:10.1038/nm.3791
Liao, Q., Zheng, L., Xu, L., Jiang, L., Luo, J., and Yan, J. (2024). A nested case-control study of circular ribonucleic acid expression profiles in the peripheral blood of pregnant women with pre-eclampsia. Pak J. Med. Sci. 40 (11), 2658–2664. doi:10.12669/pjms.40.11.10317
Lin, X., Chen, H., Xie, Y., Zhou, X., Wang, Y., Zhou, J., et al. (2022). Combination of CTLA-4 blockade with MUC1 mRNA nanovaccine induces enhanced anti-tumor CTL activity by modulating tumor microenvironment of triple negative breast cancer. Transl. Oncol. 15 (1), 101298. doi:10.1016/j.tranon.2021.101298
Liu, B., Han, L., Liu, J., Han, S., Chen, Z., and Jiang, L. (2017). Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int. J. Nanomedicine 12, 955–968. doi:10.2147/IJN.S115136
Liu, G. H., Chen, T., Zhang, X., Ma, X. L., and Shi, H. S. (2020a). Small molecule inhibitors targeting the cancers. MedComm 3 (4), e181. doi:10.1002/mco2.181
Liu, J. Q., Zhang, C., Zhang, X., Yan, J., Zeng, C., Talebian, F., et al. (2022). Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control Release 345, 306–313. doi:10.1016/j.jconrel.2022.03.021
Liu, L., Wang, Y., Miao, L., Liu, Q., Musetti, S., Li, J., et al. (2018). Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol. Ther. 26 (1), 45–55. doi:10.1016/j.ymthe.2017.10.020
Liu, S., Cheng, Q., Wei, T., Yu, X., Johnson, L. T., Farbiak, L., et al. (2021). Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater 20 (5), 701–710. doi:10.1038/s41563-020-00886-0
Liu, Y., Cao, W., Sun, M., and Li, T. (2020b). Broadly neutralizing antibodies for HIV-1: efficacies, challenges and opportunities. Emerg. Microbes Infect. 9 (1), 194–206. doi:10.1080/22221751.2020.1713707
Longworth, M. S., and Laimins, L. A. (2004). Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68 (2), 362–372. doi:10.1128/MMBR.68.2.362-372.2004
Lorentzen, C. L., Haanen, J. B., Met, O., and Svane, I. M. (2022). Clinical advances and ongoing trials of mRNA vaccines for cancer treatment. Lancet Oncol. 23 (10), e450–e458. doi:10.1016/S1470-2045(22)00372-2
Loughrey, D., and Dahlman, J. E. (2022). Non-liver mRNA delivery. Acc. Chem. Res. 55 (1), 13–23. doi:10.1021/acs.accounts.1c00601
Luan, N., Cao, H., Wang, Y., Lin, K., and Liu, C. (2022). Ionizable lipid nanoparticles enhanced the synergistic adjuvant effect of CpG ODNs and QS21 in a varicella zoster virus glycoprotein E subunit vaccine. Pharmaceutics 14 (5), 973. doi:10.3390/pharmaceutics14050973
Luk, B. T., and Zhang, L. (2015). Cell membrane-camouflaged nanoparticles for drug delivery. J. Control Release 220 (Pt B), 600–607. doi:10.1016/j.jconrel.2015.07.019
Ma, L., Li, Z., Li, W., Ai, J., and Chen, X. (2019). MicroRNA-142-3p suppresses endometriosis by regulating KLF9-mediated autophagy in vitro and in vivo. RNA Biol. 16 (12), 1733–1748. doi:10.1080/15476286.2019.1657352
Madathiparambil Visalakshan, R., Gonzalez Garcia, L. E., Benzigar, M. R., Ghazaryan, A., Simon, J., Mierczynska-Vasilev, A., et al. (2020). The influence of nanoparticle shape on protein corona formation. Small 16 (25), e2000285. doi:10.1002/smll.202000285
Malekshah, O. M., Sarkar, S., Nomani, A., Patel, N., Javidian, P., Goedken, M., et al. (2019). Bioengineered adipose-derived stem cells for targeted enzyme-prodrug therapy of ovarian cancer intraperitoneal metastasis. J. Control Release 311-312, 273–287. doi:10.1016/j.jconrel.2019.09.006
Mancianti, N., Guarnieri, A., Tripodi, S., Salvo, D. P., and Garosi, G. (2021). Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 34 (4), 1039–1040. doi:10.1007/s40620-021-01091-1
Marcos-Contreras, O. A., Greineder, C. F., Kiseleva, R. Y., Parhiz, H., Walsh, L. R., Zuluaga-Ramirez, V., et al. (2020). Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 117 (7), 3405–3414. doi:10.1073/pnas.1912012117
Marion, S. L., Watson, J., Sen, N., Brewer, M. A., Barton, J. K., and Hoyer, P. B. (2013). 7,12-dimethylbenz[a]anthracene-induced malignancies in a mouse model of menopause. Comp. Med. 63 (1), 6–12.
Maruggi, G., Zhang, C., Li, J., Ulmer, J. B., and Yu, D. (2019). mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 27 (4), 757–772. doi:10.1016/j.ymthe.2019.01.020
Mazer-Amirshahi, M., Samiee-Zafarghandy, S., Gray, G., and van den Anker, J. N. (2014). Trends in pregnancy labeling and data quality for US-approved pharmaceuticals. Am. J. Obstet. Gynecol. 211 (6), 690 e1–e11. doi:10.1016/j.ajog.2014.06.013
McCracken, J. M., Calderon, G. A., Robinson, A. J., Sullivan, C. N., Cosgriff-Hernandez, E., and Hakim, J. C. E. (2021). Animal models and alternatives in vaginal research: a comparative review. Reprod. Sci. 28 (6), 1759–1773. doi:10.1007/s43032-021-00529-y
McKinlay, C. J., Benner, N. L., Haabeth, O. A., Waymouth, R. M., and Wender, P. A. (2018). Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl. Acad. Sci., 115(26), E5859–E5866. doi:10.1073/pnas.1805358115
Medina-Alarcon, K. P., Voltan, A. R., Fonseca-Santos, B., Moro, I. J., de Oliveira Souza, F., Chorilli, M., et al. (2017). Highlights in nanocarriers for the treatment against cervical cancer. Mater Sci. Eng. C Mater Biol. Appl. 80, 748–759. doi:10.1016/j.msec.2017.07.021
Miao, L., Zhang, Y., and Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Mol. Cancer 20 (1), 41. doi:10.1186/s12943-021-01335-5
Michy, T., Massias, T., Bernard, C., Vanwonterghem, L., Henry, M., Guidetti, M., et al. (2019). Verteporfin-loaded lipid nanoparticles improve ovarian cancer photodynamic therapy in vitro and in vivo. Cancers (Basel) 11 (11), 1760. doi:10.3390/cancers11111760
Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., and Langer, R. (2021). Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20 (2), 101–124. doi:10.1038/s41573-020-0090-8
Moghimi, S. M., and Simberg, D. (2022). Pro-inflammatory concerns with lipid nanoparticles. Mol. Ther. 30 (6), 2109–2110. doi:10.1016/j.ymthe.2022.04.011
Morrow, G., Vachot, L., Vagenas, P., and Robbiani, M. (2007). Current concepts of HIV transmission. Curr. HIV/AIDS Rep. 4 (1), 29–35. doi:10.1007/s11904-007-0005-x
Moses, A. S., Taratula, O. R., Lee, H., Luo, F., Grenz, T., Korzun, T., et al. (2020). Nanoparticle-based platform for activatable fluorescence imaging and photothermal ablation of endometriosis. Small 16 (18), e1906936. doi:10.1002/smll.201906936
Mu, Z., Wiehe, K., Saunders, K. O., Henderson, R., Cain, D. W., Parks, R., et al. (2022). mRNA-encoded HIV-1 Env trimer ferritin nanoparticles induce monoclonal antibodies that neutralize heterologous HIV-1 isolates in mice. Cell Rep. 38 (11), 110514. doi:10.1016/j.celrep.2022.110514
Nagao, N., Wakabayashi, H., Miyamura, G., Kato, S., Naito, Y., and Sudo, A. (2020). CTLA-4Ig improves hyperalgesia in a mouse model of osteoporosis. Int. J. Mol. Sci. 21 (24), 9479. doi:10.3390/ijms21249479
Nahas, M. R., Rosenblatt, J., Lazarus, H. M., and Avigan, D. (2018). Anti-cancer vaccine therapy for hematologic malignancies: an evolving era. Blood Rev. 32 (4), 312–325. doi:10.1016/j.blre.2018.02.002
Nematian, S. E., Mamillapalli, R., Kadakia, T. S., Majidi Zolbin, M., Moustafa, S., and Taylor, H. S. (2018). Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b-5p and let-7b-5p regulate macrophage cytokine production. J. Clin. Endocrinol. Metab. 103 (1), 64–74. doi:10.1210/jc.2017-01199
Nevskaya, K. V., Pershina, A. G., Hmelevskaya, E. S., Efimova, L. V., Ibragimova, M. K., Dolgasheva, D. S., et al. (2024). Prevention of metastasis by suppression of stemness genes using a combination of microRNAs. J. Med. Chem. 67 (7), 5591–5602. doi:10.1021/acs.jmedchem.3c02199
Ngamcherdtrakul, W., and Yantasee, W. (2019). siRNA therapeutics for breast cancer: recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. 214, 105–120. doi:10.1016/j.trsl.2019.08.005
Nih, D. (2001). Consensus development panel on osteoporosis prevention and therapy: osteoporosis prevention, diagnosis, and therapy. JAMA 285 (6), 785–795. doi:10.1001/jama.285.6.785
Nishida, S., Morimoto, S., Oji, Y., Morita, S., Shirakata, T., Enomoto, T., et al. (2022). Cellular and humoral immune responses induced by an HLA class I-restricted peptide cancer vaccine targeting WT1 are associated with favorable clinical outcomes in advanced ovarian cancer. J. Immunother. 45 (1), 56–66. doi:10.1097/CJI.0000000000000405
Nomani, A., Li, G., Yousefi, S., Wu, S., Malekshah, O. M., Nikkhoi, S. K., et al. (2021). Gadolinium-labeled affibody-XTEN recombinant vector for detection of HER2+ lesions of ovarian cancer lung metastasis using quantitative MRI. J. Control Release 337, 132–143. doi:10.1016/j.jconrel.2021.07.022
Ogata, A. F., Cheng, C. A., Desjardins, M., Senussi, Y., Sherman, A. C., Powell, M., et al. (2022). Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. 74 (4), 715–718. doi:10.1093/cid/ciab465
Okamoto, A., Asai, T., Hirai, Y., Shimizu, K., Koide, H., Minamino, T., et al. (2018). Systemic administration of siRNA with anti-HB-EGF antibody-modified lipid nanoparticles for the treatment of triple-negative breast cancer. Mol. Pharm. 15 (4), 1495–1504. doi:10.1021/acs.molpharmaceut.7b01055
Oltra, N. S., Nair, P., and Discher, D. E. (2014). From stealthy polymersomes and filomicelles to “self” Peptide-nanoparticles for cancer therapy. Annu. Rev. Chem. Biomol. Eng. 5, 281–299. doi:10.1146/annurev-chembioeng-060713-040447
Ouyang, B., Poon, W., Zhang, Y. N., Lin, Z. P., Kingston, B. R., Tavares, A. J., et al. (2020). The dose threshold for nanoparticle tumour delivery. Nat. Mater 19 (12), 1362–1371. doi:10.1038/s41563-020-0755-z
Palanki, R., Peranteau, W. H., and Mitchell, M. J. (2021). Delivery technologies for in utero gene therapy. Adv. Drug Deliv. Rev. 169, 51–62. doi:10.1016/j.addr.2020.11.002
Panir, K., Schjenken, J. E., Robertson, S. A., and Hull, M. L. (2018). Non-coding RNAs in endometriosis: a narrative review. Hum. Reprod. Update 24 (4), 497–515. doi:10.1093/humupd/dmy014
Pardi, N., Hogan, M. J., Porter, F. W., and Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17 (4), 261–279. doi:10.1038/nrd.2017.243
Pardi, N., Hogan, M. J., and Weissman, D. (2020). Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 65, 14–20. doi:10.1016/j.coi.2020.01.008
Pardi, N., LaBranche, C. C., Ferrari, G., Cain, D. W., Tombacz, I., Parks, R. J., et al. (2019). Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques. Mol. Ther. Nucleic Acids 15, 36–47. doi:10.1016/j.omtn.2019.03.003
Pardons, M., Cole, B., Lambrechts, L., van Snippenberg, W., Rutsaert, S., Noppe, Y., et al. (2023). Potent latency reversal by Tat RNA-containing nanoparticle enables multi-omic analysis of the HIV-1 reservoir. Nat. Commun. 14 (1), 8397. doi:10.1038/s41467-023-44020-5
Park, J. W., Lagniton, P. N. P., Liu, Y., and Xu, R. H. (2021). mRNA vaccines for COVID-19: what, why and how. Int. J. Biol. Sci. 17 (6), 1446–1460. doi:10.7150/ijbs.59233
Patel, A. K., Kaczmarek, J. C., Bose, S., Kauffman, K. J., Mir, F., Heartlein, M. W., et al. (2019). Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater 31 (8), e1805116. doi:10.1002/adma.201805116
Paunovska, K., Loughrey, D., and Dahlman, J. E. (2022). Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23 (5), 265–280. doi:10.1038/s41576-021-00439-4
Pelch, K. E., Sharpe-Timms, K. L., and Nagel, S. C. (2012). Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J. Vis. Exp. (59), e3396. doi:10.3791/3396
Pozharov, V. P., and Minko, T. (2023). Nanotechnology-based RNA vaccines: fundamentals, advantages and challenges. Pharmaceutics 15 (1), 194. doi:10.3390/pharmaceutics15010194
Prahl, M., Golan, Y., Cassidy, A. G., Matsui, Y., Li, L., Alvarenga, B., et al. (2022). Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat. Commun. 13 (1), 4422. doi:10.1038/s41467-022-32188-1
Qin, S., Tang, X., Chen, Y., Chen, K., Fan, N., Xiao, W., et al. (2022). mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target Ther. 7 (1), 166. doi:10.1038/s41392-022-01007-w
Qiu, M., Tang, Y., Chen, J., Muriph, R., Ye, Z., Huang, C., et al. (2022). Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. U. S. A. 119 (8). doi:10.1073/pnas.2116271119
Qu, L., Yi, Z., Shen, Y., Lin, L., Chen, F., Xu, Y., et al. (2022). Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185 (10), 1728–1744 e16. doi:10.1016/j.cell.2022.03.044
Quinlan, J. D. (2021). Human papillomavirus: screening, testing, and prevention. Am. Fam. Physician 104 (2), 152–159.
Ramos da Silva, J., Bitencourt Rodrigues, K., Formoso Pelegrin, G., Silva Sales, N., Muramatsu, H., de Oliveira Silva, M., et al. (2023b). Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med., 15(686), eabn3464. doi:10.1126/scitranslmed.abn3464
Ramos da Silva, J., Bitencourt Rodrigues, K., Formoso Pelegrin, G., Silva Sales, N., Muramatsu, H., de Oliveira Silva, M., et al. (2023a). Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 15 (686), eabn3464. doi:10.1126/scitranslmed.abn3464
Richner, J. M., Himansu, S., Dowd, K. A., Butler, S. L., Salazar, V., Fox, J. M., et al. (2017). Modified mRNA vaccines protect against Zika virus infection. Cell 168 (6), 1114–1125 e10. doi:10.1016/j.cell.2017.02.017
Riley, R. S., Kashyap, M. V., Billingsley, M. M., White, B., Alameh, M. G., Bose, S. K., et al. (2021). Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7 (3), eaba1028. doi:10.1126/sciadv.aba1028
Rodriguez-Nava, C., Ortuno-Pineda, C., Illades-Aguiar, B., Flores-Alfaro, E., Leyva-Vazquez, M. A., Parra-Rojas, I., et al. (2023). Mechanisms of action and limitations of monoclonal antibodies and single chain fragment variable (scFv) in the treatment of cancer. Biomedicines 11 (6), 1610. doi:10.3390/biomedicines11061610
Rosenblum, D., Gutkin, A., Kedmi, R., Ramishetti, S., Veiga, N., Jacobi, A. M., et al. (2020). CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6 (47), eabc9450. doi:10.1126/sciadv.abc9450
Rupar, M. J., Golusinski, P., Golusinski, W., and Masternak, M. M. (2019). Human Papillomavirus and the use of nanoparticles for immunotherapy in HPV-related cancer: a review. Rep. Pract. Oncol. Radiother. 24 (6), 544–550. doi:10.1016/j.rpor.2019.08.006
Rurik, J. G., Tombacz, I., Yadegari, A., Mendez Fernandez, P. O., Shewale, S. V., Li, L., et al. (2022). CAR T cells produced in vivo to treat cardiac injury. Science 375 (6576), 91–96. doi:10.1126/science.abm0594
Sahin, C., Mamillapalli, R., Yi, K. W., and Taylor, H. S. (2018). microRNA Let-7b: a Novel treatment for endometriosis. J. Cell Mol. Med. 22 (11), 5346–5353. doi:10.1111/jcmm.13807
Sahin, U., Kariko, K., and Tureci, O. (2014). mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 13 (10), 759–780. doi:10.1038/nrd4278
Sahin, U., Oehm, P., Derhovanessian, E., Jabulowsky, R. A., Vormehr, M., Gold, M., et al. (2020). An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585 (7823), 107–112. doi:10.1038/s41586-020-2537-9
Sajid, M. I., Moazzam, M., Cho, Y., Kato, S., Xu, A., Way, J. J., et al. (2021). siRNA therapeutics for the therapy of COVID-19 and other coronaviruses. Mol. Pharm. 18 (6), 2105–2121. doi:10.1021/acs.molpharmaceut.0c01239
Santos, C., Vilanova, M., Medeiros, R., and Gil da Costa, R. M. (2017). HPV-transgenic mouse models: tools for studying the cancer-associated immune response. Virus Res. 235, 49–57. doi:10.1016/j.virusres.2017.04.001
Sarkar, S., Malekshah, O. M., Nomani, A., Patel, N., and Hatefi, A. (2018). A novel chemotherapeutic protocol for peritoneal metastasis and inhibition of relapse in drug resistant ovarian cancer. Cancer Med. 7 (8), 3630–3641. doi:10.1002/cam4.1631
Saunders, K. O., Pardi, N., Parks, R., Santra, S., Mu, Z., Sutherland, L., et al. (2021). Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines 6 (1), 50. doi:10.1038/s41541-021-00307-6
Saunders, N. R. M., Paolini, M. S., Fenton, O. S., Poul, L., Devalliere, J., Mpambani, F., et al. (2020). A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 20 (6), 4264–4269. doi:10.1021/acs.nanolett.0c00752
Seephetdee, C., Bhukhai, K., Buasri, N., Leelukkanaveera, P., Lerdwattanasombat, P., Manopwisedjaroen, S., et al. (2022). A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera. Antivir. Res. 204, 105370. doi:10.1016/j.antiviral.2022.105370
Sha, L., Huang, L., Luo, X., Bao, J., Gao, L., Pan, Q., et al. (2017). Long non-coding RNA LINC00261 inhibits cell growth and migration in endometriosis. J. Obstet. Gynaecol. Res. 43 (10), 1563–1569. doi:10.1111/jog.13427
Sharma, A., Sah, N., Kannan, S., and Kannan, R. M. (2021). Targeted drug delivery for maternal and perinatal health: challenges and opportunities. Adv. Drug Deliv. Rev. 177, 113950. doi:10.1016/j.addr.2021.113950
Shtykalova, S., Egorova, A., Maretina, M., Baranov, V., and Kiselev, A. (2022). Magnetic nanoparticles as a component of peptide-based DNA delivery system for suicide gene therapy of uterine leiomyoma. Bioeng. (Basel) 9 (3), 112. doi:10.3390/bioengineering9030112
Silva, A. M., Moura, S. R., Teixeira, J. H., Barbosa, M. A., Santos, S. G., and Almeida, M. I. (2019). Long noncoding RNAs: a missing link in osteoporosis. Bone Res. 7, 10. doi:10.1038/s41413-019-0048-9
Singh, A. P., Senapati, S., Ponnusamy, M. P., Jain, M., Lele, S. M., Davis, J. S., et al. (2008). Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 9 (11), 1076–1085. doi:10.1016/S1470-2045(08)70277-8
Singh, B., Kaur, P., Cedeno, L., Brahimi, T., Patel, P., Virk, H., et al. (2021). COVID-19 mRNA vaccine and myocarditis. Eur. J. Case Rep. Intern Med. 8 (7), 002681. doi:10.12890/2021_002681
Skerritt, J. H., Tucek-Szabo, C., Sutton, B., and Nolan, T. (2024). The platform technology approach to mRNA product development and regulation. Vaccines (Basel) 12 (5), 528. doi:10.3390/vaccines12050528
Strumberg, D., Schultheis, B., Traugott, U., Vank, C., Santel, A., Keil, O., et al. (2012). Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int. J. Clin. Pharmacol. Ther. 50 (1), 76–78. doi:10.5414/cpp50076
Suk, J. S., Xu, Q., Kim, N., Hanes, J., and Ensign, L. M. (2016). PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99 (Pt A), 28–51. doi:10.1016/j.addr.2015.09.012
Swingle, K. L., Billingsley, M. M., Bose, S. K., White, B., Palanki, R., Dave, A., et al. (2022). Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J. Control Release 341, 616–633. doi:10.1016/j.jconrel.2021.10.031
Swingle, K. L., Ricciardi, A. S., Peranteau, W. H., and Mitchell, M. J. (2023a). Delivery technologies for women’s health applications. Nat. Rev. Bioeng. 1 (6), 408–425. doi:10.1038/s44222-023-00040-w
Swingle, K. L., Safford, H. C., Geisler, H. C., Hamilton, A. G., Thatte, A. S., Billingsley, M. M., et al. (2023b). Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy. J. Am. Chem. Soc. 145 (8), 4691–4706. doi:10.1021/jacs.2c12893
Tabernero, J., Shapiro, G. I., LoRusso, P. M., Cervantes, A., Schwartz, G. K., Weiss, G. J., et al. (2013a). 3rd: first-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3 (4), 406–417. doi:10.1158/2159-8290.CD-12-0429
Tabernero, J., Shapiro, G. I., LoRusso, P. M., Cervantes, A., Schwartz, G. K., Weiss, G. J., et al. (2013b). First-in-Humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3 (4), 406–417. doi:10.1158/2159-8290.Cd-12-0429
Tahtinen, S., Tong, A. J., Himmels, P., Oh, J., Paler-Martinez, A., Kim, L., et al. (2022). IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23 (4), 532–542. doi:10.1038/s41590-022-01160-y
Tang, L., Yang, X., Yin, Q., Cai, K., Wang, H., Chaudhury, I., et al. (2014). Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. U. S. A. 111 (43), 15344–15349. doi:10.1073/pnas.1411499111
Tang, Q., Liu, J., Jiang, Y., Zhang, M., Mao, L., and Wang, M. (2019). Cell-selective messenger RNA delivery and CRISPR/Cas9 genome editing by modulating the interface of phenylboronic acid-derived lipid nanoparticles and cellular surface sialic acid. ACS Appl. Mater Interfaces 11 (50), 46585–46590. doi:10.1021/acsami.9b17749
Teague, E. M., Print, C. G., and Hull, M. L. (2010). The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update 16 (2), 142–165. doi:10.1093/humupd/dmp034
Tenchov, R., Bird, R., Curtze, A. E., and Zhou, Q. (2021). Lipid Nanoparticles─From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15 (11), 16982–17015. doi:10.1021/acsnano.1c04996
Tolcher, A. W., Papadopoulos, K. P., Patnaik, A., Rasco, D. W., Martinez, D., Wood, D. L., et al. (2015). Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 33 (15_Suppl. l), 11006. doi:10.1200/jco.2015.33.15_suppl.11006
Tombacz, I., Laczko, D., Shahnawaz, H., Muramatsu, H., Natesan, A., Yadegari, A., et al. (2021). Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 29 (11), 3293–3304. doi:10.1016/j.ymthe.2021.06.004
Torchilin, V. P., Khaw, B. A., Smirnov, V. N., and Haber, E. (1979). Preservation of antimyosin antibody activity after covalent coupling to liposomes. Biochem. Biophys. Res. Commun. 89 (4), 1114–1119. doi:10.1016/0006-291x(79)92123-5
Traore, Y. L., Chen, Y., Padilla, F., and Ho, E. A. (2022). Segmented intravaginal ring for the combination delivery of hydroxychloroquine and anti-CCR5 siRNA nanoparticles as a potential strategy for preventing HIV infection. Drug Deliv. Transl. Res. 12 (4), 816–825. doi:10.1007/s13346-021-00983-w
Truong, N. P., Whittaker, M. R., Mak, C. W., and Davis, T. P. (2015). The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 12 (1), 129–142. doi:10.1517/17425247.2014.950564
Tse, S. W., McKinney, K., Walker, W., Nguyen, M., Iacovelli, J., Small, C., et al. (2021). mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 29 (7), 2227–2238. doi:10.1016/j.ymthe.2021.03.002
Tsoi, K. M., MacParland, S. A., Ma, X. Z., Spetzler, V. N., Echeverri, J., Ouyang, B., et al. (2016). Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater 15 (11), 1212–1221. doi:10.1038/nmat4718
Ullrich, S. J., Freedman-Weiss, M., Ahle, S., Mandl, H. K., Piotrowski-Daspit, A. S., Roberts, K., et al. (2021). Nanoparticles for delivery of agents to fetal lungs. Acta Biomater. 123, 346–353. doi:10.1016/j.actbio.2021.01.024
Vercellini, P., Crosignani, P., Somigliana, E., Vigano, P., Frattaruolo, M. P., and Fedele, L. (2011). Waiting for Godot': a commonsense approach to the medical treatment of endometriosis. Hum. Reprod. 26 (1), 3–13. doi:10.1093/humrep/deq302
Vhora, I., Lalani, R., Bhatt, P., Patil, S., and Misra, A. (2019). Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int. J. Pharm. 563, 324–336. doi:10.1016/j.ijpharm.2019.04.006
Wang, J., Ding, Y., Chong, K., Cui, M., Cao, Z., Tang, C., et al. (2024a). Recent advances in lipid nanoparticles and their safety concerns for mRNA delivery. Vaccines (Basel) 12 (10), 1148. doi:10.3390/vaccines12101148
Wang, S., Wang, H., Drabek, A., Smith, W. S., Liang, F., and Huang, Z. R. (2024b). Unleashing the potential: designing antibody-targeted lipid nanoparticles for industrial applications with CMC considerations and clinical outlook. Mol. Pharm. 21 (1), 4–17. doi:10.1021/acs.molpharmaceut.3c00735
Wang, X., Liu, S., Sun, Y., Yu, X., Lee, S. M., Cheng, Q., et al. (2023). Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18 (1), 265–291. doi:10.1038/s41596-022-00755-x
Warren, L., and Lin, C. (2019). mRNA-based genetic reprogramming. Mol. Ther. 27 (4), 729–734. doi:10.1016/j.ymthe.2018.12.009
Wesselhoeft, R. A., Kowalski, P. S., and Anderson, D. G. (2018). Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9 (1), 2629. doi:10.1038/s41467-018-05096-6
Whitehead, K. A., Langer, R., and Anderson, D. G. (2009). Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8 (2), 129–138. doi:10.1038/nrd2742
Willis, E., Pardi, N., Parkhouse, K., Mui, B. L., Tam, Y. K., Weissman, D., et al. (2020). Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 12 (525), eaav5701. doi:10.1126/scitranslmed.aav5701
Wilson, E., Goswami, J., Baqui, A. H., Doreski, P. A., Perez-Marc, G., Zaman, K., et al. (2023). Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N. Engl. J. Med. 389 (24), 2233–2244. doi:10.1056/NEJMoa2307079
Wise, L. A., and Laughlin-Tommaso, S. K. (2016). Epidemiology of uterine fibroids: from menarche to menopause. Clin. Obstet. Gynecol. 59 (1), 2–24. doi:10.1097/GRF.0000000000000164
Xie, Z., Lin, Y. C., Steichen, J. M., Ozorowski, G., Kratochvil, S., Ray, R., et al. (2024). mRNA-LNP HIV-1 trimer boosters elicit precursors to broad neutralizing antibodies. Science 384 (6697), eadk0582. doi:10.1126/science.adk0582
Xiong, J., Tan, S., Yu, L., Shen, H., Qu, S., Zhang, C., et al. (2021). E7-Targeted nanotherapeutics for key HPV afflicted cervical lesions by employing CRISPR/Cas9 and poly (Beta-Amino ester). Int. J. Nanomedicine 16, 7609–7622. doi:10.2147/IJN.S335277
Xiong, Q., Lee, G. Y., Ding, J., Li, W., and Shi, J. (2018). Biomedical applications of mRNA nanomedicine. Nano Res. 11 (10), 5281–5309. doi:10.1007/s12274-018-2146-1
Xue, L., Gong, N., Shepherd, S. J., Xiong, X., Liao, X., Han, X., et al. (2022). Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J. Am. Chem. Soc. 144 (22), 9926–9937. doi:10.1021/jacs.2c02706
Xue, L., Hamilton, A. G., Zhao, G., Xiao, Z., El-Mayta, R., Han, X., et al. (2024). High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models. Nat. Commun. 15 (1), 1884. doi:10.1038/s41467-024-45422-9
Yaddanapudi, K., Mitchell, R. A., and Eaton, J. W. (2013). Cancer vaccines: looking to the future. Oncoimmunology 2 (3), e23403. doi:10.4161/onci.23403
Yamamoto, M., Jin, C., Hata, T., Yasumizu, Y., Zhang, Y., Hong, D., et al. (2019). MUC1-C integrates chromatin remodeling and PARP1 activity in the DNA damage response of triple-negative breast cancer cells. Cancer Res. 79 (8), 2031–2041. doi:10.1158/0008-5472.CAN-18-3259
Yan, W., Hu, H., and Tang, B. (2020). Progress in understanding the relationship between long noncoding RNA and endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 5, 100067. doi:10.1016/j.eurox.2019.100067
Yang, Q., Ciebiera, M., Bariani, M. V., Ali, M., Elkafas, H., Boyer, T. G., et al. (2022). Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr. Rev. 43 (4), 678–719. doi:10.1210/endrev/bnab039
Yeo, K. T., Chia, W. N., Tan, C. W., Ong, C., Yeo, J. G., Zhang, J., et al. (2021). Neutralizing activity and SARS-CoV-2 vaccine mRNA persistence in serum and breastmilk after BNT162b2 vaccination in lactating women. Front. Immunol. 12, 783975. doi:10.3389/fimmu.2021.783975
Yin, H., Kanasty, R. L., Eltoukhy, A. A., Vegas, A. J., Dorkin, J. R., and Anderson, D. G. (2014). Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15 (8), 541–555. doi:10.1038/nrg3763
Young, R. E., Nelson, K. M., Hofbauer, S. I., Vijayakumar, T., Alameh, M. G., Weissman, D., et al. (2022). Lipid nanoparticle composition drives mRNA delivery to the placenta. bioRxiv, 2022.12.22.521490. doi:10.1101/2022.12.22.521490
Yu, L., and Liu, Y. (2019). circRNA_0016624 could sponge miR-98 to regulate BMP2 expression in postmenopausal osteoporosis. Biochem. Biophys. Res. Commun. 516 (2), 546–550. doi:10.1016/j.bbrc.2019.06.087
Zhai, J., Luwor, R. B., Ahmed, N., Escalona, R., Tan, F. H., Fong, C., et al. (2018). Paclitaxel-loaded self-assembled lipid nanoparticles as targeted drug delivery systems for the treatment of aggressive ovarian cancer. ACS Appl. Mater Interfaces 10 (30), 25174–25185. doi:10.1021/acsami.8b08125
Zhang, D., Wang, G., Yu, X., Wei, T., Farbiak, L., Johnson, L. T., et al. (2022). Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17 (7), 777–787. doi:10.1038/s41565-022-01122-3
Zhang, M., Bai, X., Zeng, X., Liu, J., Liu, F., and Zhang, Z. (2021). circRNA-miRNA-mRNA in breast cancer. Clin. Chim. Acta 523, 120–130. doi:10.1016/j.cca.2021.09.013
Zhao, K., Zhao, Q., Guo, Z., Chen, Z., Hu, Y., Su, J., et al. (2018). Hsa_Circ_0001275: a potential novel diagnostic biomarker for postmenopausal osteoporosis. Cell Physiol. Biochem. 46 (6), 2508–2516. doi:10.1159/000489657
Zhou, R., Yazdanifar, M., Roy, L. D., Whilding, L. M., Gavrill, A., Maher, J., et al. (2019). CAR T cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front. Immunol. 10, 1149. doi:10.3389/fimmu.2019.01149
Zhou, W. Y., Cai, Z. R., Liu, J., Wang, D. S., Ju, H. Q., and Xu, R. H. (2020). Circular RNA: metabolism, functions and interactions with proteins. Mol. Cancer 19 (1), 172. doi:10.1186/s12943-020-01286-3
Zhu, D., Shen, H., Tan, S., Hu, Z., Wang, L., Yu, L., et al. (2018). Nanoparticles based on poly (β-Amino ester) and HPV16-targeting CRISPR/shRNA as potential drugs for HPV16-related cervical malignancy. Mol. Ther. 26 (10), 2443–2455. doi:10.1016/j.ymthe.2018.07.019
Zhu, Y., Zhu, L., Wang, X., and Jin, H. (2022). RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 13 (7), 644. doi:10.1038/s41419-022-05075-2
Keywords: lipid nanoparticles, RNA therapeutics, women’s health, gynecological cancers, targeted delivery, gene therapy, safety
Citation: Nomani A, Saraswat A, Zhang Y, Parenky AC, Kuo C-TJ, Brown H, Hartford S, Rayaprolu B, Singh Bhupender Bhalla A and Shameem M (2025) RNA-lipid nanoparticle therapeutics for women’s health. Front. Nanotechnol. 7:1475969. doi: 10.3389/fnano.2025.1475969
Received: 04 August 2024; Accepted: 27 February 2025;
Published: 19 March 2025.
Edited by:
Majid Jabir, University of Technology, IraqReviewed by:
Parviz Vahedi, Maragheh University of Medical Sciences, IranCopyright © 2025 Nomani, Saraswat, Zhang, Parenky, Kuo, Brown, Hartford, Rayaprolu, Singh Bhupender Bhalla and Shameem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alireza Nomani, YWxpcmV6YS5ub21hbmlAcmVnZW5lcm9uLmNvbQ==
†Former Regeneron Pharmaceuticals employees
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.