- 1College of Business, King Khalid University, Abha, Saudi Arabia
- 2Department of Mathematics and Computer Science, Faculty of Science, Suez University, Suez, Egypt
- 3Department of Mathematics and Statistics, College of Science, Taif University, Taif, Saudi Arabia
- 4Department of mathematics, Applied College at Khulis, University Of Jeddah, Jeddah, Saudi Arabia
- 5Mathematics Department, Faculty of Science, Al-Azhar University (Assiut Branch), Assiut, Egypt
- 6Basic Science, Faculty of Engineering, The British University in Egypt, Al-Shorouk City, Cairo, Egypt
- 7Instituto de Ciencias Matemáticas ICMAT, CSIC, UAM, UCM, UC3M, Madrid, Spain
In this study, we investigated the dynamics of unsteady electroosmotic pulsatile flow involving a hybrid nanofluid within a curved artery, influenced by both stenosis and an embedded catheter. The hybrid nanofluid, a mixture of silver
1 Introduction
Hybrid nanofluids, particularly those with non-Newtonian properties, have attracted significant interest lately due to their broad applications in biomedical engineering and fluid transportation. When combined with electroosmotic flow, these hybrid nanofluids exhibit additional complexities in behavior, making them well suited for use in microscale and nanoscale fluid manipulation scenarios (Sundar et al., 2013). Nanofluids and hybrid nanofluids are utilized in various disciplines, demonstrating their flexibility and potential impact. There are a variety of applications for nanofluids and hybrid nanofluids in fields such as biomedical applications, the energy sector, manufacturing, and engineering (El Kot and Abd Elmaboud, 2021; El-Masry et al., 2020; Ali et al., 2020). Abdal et al. (2021) investigated the effects of bio-convection and activation energy on the Reiner–Rivlin nanofluid flow over a rotating disk with partial slips. By deriving a system of coupled nonlinear differential equations based on the Reiner–Rivlin fluid relationships, the study explores a variety of non-Newtonian fluid models and slip coefficients for numerical analysis. Ali et al. (2022) delved into the impact of volume fraction and Coriolis and Lorentz forces on the behavior of water-based silver (Ag) nanoparticle flow toward a continuously stretching sheet.
Electroosmotic flow, influenced by applied electric fields, offers precise control over fluid movement, which is highly desirable in medical contexts such as targeted drug delivery and microfluidic systems. Wang et al. (2009) demonstrated the ability of electroosmotic pumps to precisely regulate microflow in microfluidic applications. Hybrid nanofluids, typically composed of metal-based nanoparticles dispersed in base fluids, provide enhanced thermal and flow properties, making them ideal for improving the fluid efficiency in medical systems. Sarkar et al. (2015) and Huminic and Huminic 2018() reviewed hybrid nanofluids and their improved thermal properties, emphasizing their potential in various heat transfer applications. Bhatti et al. (2024) investigated third-grade fluid motion between vertical parallel walls utilizing an electromagnetic hydrodynamic approach. The walls, oriented vertically, contain a nanofluid with sodium alginate infused with gold and iron oxide nanoparticles in a porous structure. The Darcy–Brinkman–Forchheimer model is applied, particularly in scenarios with non-Darcy media. Numerical solutions are obtained using a shooting approach for the nonlinear differential equations. The results are analyzed through graphs and tables, revealing the favorable industrial application potential of the nanoparticle combination studied. Recent research has shown that these fluids exhibit superior heat transfer capabilities, which is crucial for biomedical use cases. Sundar et al. (2014) demonstrated that hybrid nanofluids containing multiwalled carbon nanotubes (MWCNTs) and
The study of pulsatile flow in curved arteries with stenosis has gained importance in understanding cardiovascular health. Long et al. (2001) conducted numerical investigations on physiologically realistic pulsatile flow through arterial stenosis, providing key insights into flow patterns and wall shear stress distributions essential for understanding atherosclerosis. Pulsatile flow, which mimics the heartbeat’s rhythm, is greatly influenced by arterial curvature and stenosis (Perktold et al., 1991). Perktold et al. (1991) performed three-dimensional numerical analyses of pulsatile flow in carotid artery bifurcations, revealing that arterial geometry significantly affects flow patterns and wall shear stress, both critical factors in atherosclerosis.
Stenosis, or the narrowing of arteries, presents challenges to blood flow, increasing wall shear stress and pressure gradients, potentially leading to adverse cardiovascular events (Johnston et al., 2004). Curved arteries add to the complexity by introducing secondary flow patterns that further influence flow dynamics. Studying such systems is crucial for advancing treatments for vascular diseases (Berger et al., 1983). Berger et al. (1983) reviewed the flow in curved pipes, showing how curvature induces secondary flows that alter the shear stress distribution, which is critical in understanding vascular pathologies. Recent research underscores the importance of considering realistic arterial geometries for accurate predictions of hemodynamic forces (Morris et al., 2016) (Cheng et al., 2006). Morris et al. (2016) discussed how computational fluid dynamics modeling using patient-specific geometries improves our understanding of cardiovascular hemodynamics, aiding in diagnosis and treatment. Cheng et al. (2006) used in vivo MR angiography to quantify deformations of the superficial femoral artery. Numerical studies indicate that stenosed arteries under pulsatile flow behave differently from healthy arteries, highlighting the need for specialized treatments (Gijsen et al., 1999). Huo and Kassab (2006) developed a theoretical and experimental model of pulsatile blood flow in the coronary arterial tree, demonstrating significant differences in hemodynamic parameters in stenosed arteries compared to healthy arteries, emphasizing the need for tailored therapeutic strategies.
The finite-difference technique is widely used for numerically solving complex fluid flow problems, particularly in biomedical applications like cardiovascular treatments (Quarteroni et al., 2000). Quarteroni et al. (2000) discussed computational methods in vascular fluid dynamics and how numerical techniques like the finite difference method solve fluid–structure interaction problems in blood flow simulations. This method is highly beneficial for solving nonlinear partial differential equations, which arise in blood flow modeling, especially in stenosed and catheterized arteries. Basha et al. (2022) used finite volume methods to study the behavior of gold and copper biomagnetic blood flow in an inclined stenosed artery with varying viscosities. Haghighi et al. (2006) numerically investigated pulsatile blood flow through a stenosed elastic artery. The finite-difference method provides a stable and efficient framework for understanding the intricate interactions between blood, vessel walls, and medical devices such as stents or catheters (LaDisa et al., 2005). LaDisa et al. (2005) used computational models to predict the sites of neointimal hyperplasia after stent implantation, demonstrating the role of numerical methods in improving cardiovascular device design and patient outcomes. Studies have shown that the finite-difference method can efficiently model heat transfer and predict flow behavior in biomedical systems, leading to better clinical outcomes (Xu et al., 2009). Xu et al. (2009) reviewed mathematical modeling of skin bioheat transfer, emphasizing the role of numerical methods like the finite-difference technique in predicting thermal responses in tissues.
Mathematical modeling and analysis are essential for understanding the complex behaviors of non-Newtonian hybrid nanofluids in biomedical applications. The Carreau non-Newtonian model is a foundational mathematical framework for characterizing shear-thinning fluids, like blood, especially under pulsatile flow in stenosed and curved arteries (Apostolidis and Beris, 2014). Apostolidis and Beris (2014) provided a detailed mathematical analysis of blood rheology using the Carreau model, advancing the understanding of non-Newtonian fluid dynamics under physiological conditions. Recent studies have also explored the impact of magnetic fields on the flow of non-Newtonian fluids in stenosed arteries. The mathematical analysis of pulsatile flow in curved arteries has advanced through the use of numerical methods like the finite-difference method (Tzirtzilakis, 2005). Tzirtzilakis (2005) developed a mathematical model to study the effects of magnetic fields on blood flow using finite-difference techniques. Additionally, studies on thermophoretic effects in non-Newtonian fluid flow have provided new perspectives on heat transfer in biological systems. Pal and Mondal (2011) analyzed thermophoresis and Brownian motion effects in non-Newtonian nanofluid flow over a stretching sheet, offering mathematical models that can be applied in biomedical engineering. Hassan et al. (2024) discussed a viscoelastic three-element viscous model, comprising a spring in parallel with one dashpot and a second dashpot in series, and used it to analyze the heat and mass flow properties of the fluid across a variable-thickness sheet. Transport equations incorporating this model are solved using the numerical Runge–Kutta (RK) technique. The research highlights that viscosity exerts a more substantial influence on the outcomes than other parameters. Notably, a significant enhancement of 22
The main objective of this research is to analyze the unsteady electroosmotic pulsatile flow of a hybrid nanofluid through a stenosed, curved, catheterized artery using the Carreau non-Newtonian model. The study uses a numerical approach based on the finite-difference method to solve the governing equations of fluid motion, temperature, and concentration. This research is significant because it offers new insights into the behavior of hybrid nanofluids under electroosmotic forces in complex arterial geometries, which could help design more effective cardiovascular treatments and medical devices. The study results contribute to a broader understanding of hemodynamics in diseased arteries, providing valuable implications for improving patient outcomes in cardiovascular care.
2 Problem description and mathematical model
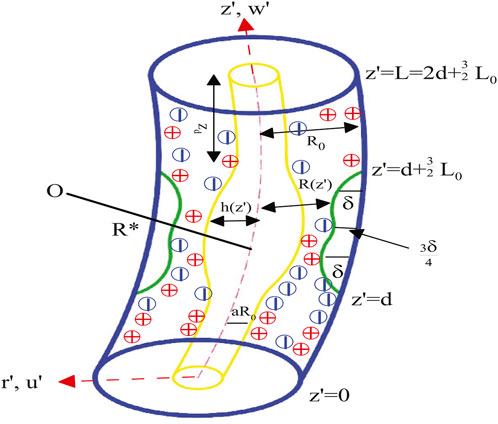
In our paper, we examine the unsteady laminar electroosmotic pulsatile flow of an incompressible Carreau non-Newtonian hybrid nanofluid (
where
where
The conservative continuity, momentum, energy, and concentration governing equations with thermophoresis and Brownian motion are as follows (Ramanamurthy et al., 2013; El Kot and Abd Elmaboud, 2024; El-Dabe and Mostapha, 2020; Tawade et al., 2022; El Kot and Abd Elmaboud, 2023):
Continuity equation:
Momentum equations:
Energy equation:
Concentration equation:
where
Considering that the electrolyte combination
where
Ionic energy density is described as
Here,
By substituting
The extra stress tensor for the Carreau hybrid nanofluid according to the shear rate is formulated as (Akbar and Nadeem, 2014; Rana and Liao, 2019; Wajihah and Sankar, 2021; Zaman and Khan, 2021)
where
Except for the fluid’s thermal conductivity and viscosity, all of its physical properties should remain constant. It is hypothesized that the viscosity varies with temperature in addition to shear rate dependence and presuming
Here, the initial and boundary constraints are
Non-dimensional variables and parameters are defined as [(El Kot and Abd Elmaboud, 2024) (El Kot and Abd Elmaboud, 2023)]
where
Based on the suppositions and simplifications outlined by Young (1968) for mild stenosis
The corresponding initial and boundary constraints are
where Equations 23, 24 are the non-dimensional walls and
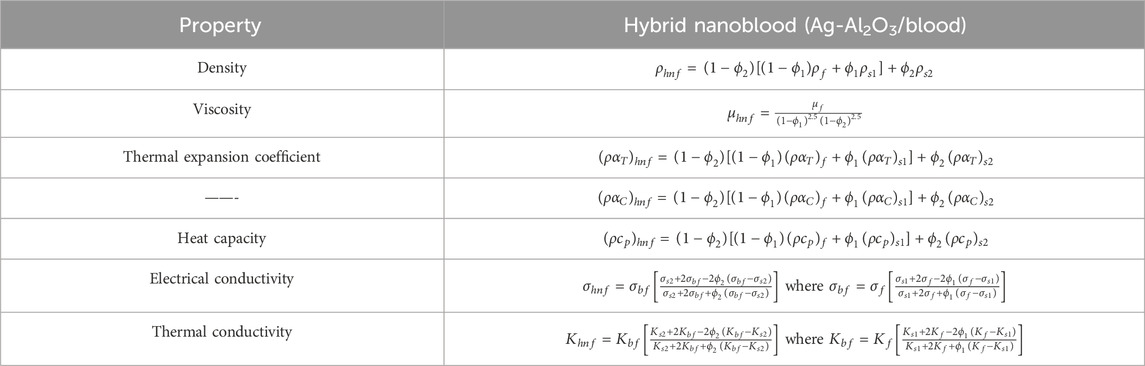
Comparing hybrid nanofluids to simple nanofluids, the former have shown better stability and thermal properties. In many facets of human endeavor, such as electronics, medical, power, and chemical engineering equipment, tiny concentrations of hybrid nanoparticles of metal or metal oxides are used. Tables 1, 2 list the thermal and physical characteristics of blood, silver nanoparticles, and aluminum oxide nanoparticles. In this case, the volume fraction for silver and aluminum oxide nanoparticles is denoted by
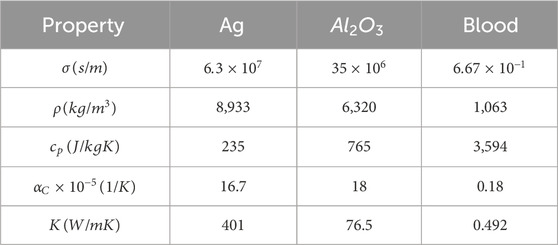
Table 2. Thermal physical properties of silver (Ag) and aluminum oxide
3 Numerical solution technique
The unsteady electroosmotic pulsatile flow-governing equations of the hybrid nanofluid are nonlinear partial differential equations governed by continuity, momentum, energy, and species concentration. To solve these equations, the finite-difference method was used due to its effectiveness in handling complex boundary conditions typically encountered in arterial geometries. More specifically, the Crank–Nicolson scheme was chosen as it provides a good balance between computational cost and accuracy. The solution domain was discretized using a uniform grid, and a semi-implicit formulation was used to ensure convergence. The resulting algebraic equations were solved iteratively until the desired level of accuracy was achieved, ensuring reliable simulations of the flow dynamics within the stenosed, curved, and catheterized artery. We used the finite-difference method to solve the coupled nonlinear partial differential Equations 17–21 with initial and boundary conditions given by Equation 22. To proceed, the physical domain is transformed into a regular uniform domain using the transformation:
Using Equation 25 in Equations 17–24, we obtain:
The associated initial and boundary conditions are
The finite-difference approach is used by the Crank–Nicolson semi-implicit discretization because it is unconditionally stable. Let us consider a uniform grid with spacing
The associated initial and boundary conditions are
The function FindRoot in Mathematica software is used to solve the produced system of nonlinear algebraic equations (Equations 31–35) and obtain the consecutive solutions. After several cycles of this procedure, a steady state is achieved. The steady-state solution is assumed to be obtained when the absolute differences between the values of
4 Results and discussions
In this section, we discuss the effects of various physical parameters on the flow, heat transfer, and mass transfer characteristics of the hybrid nanofluid through a stenosed curved artery. Specifically, the influences of electroosmotic forces, pulsatility, curvature, and nanoparticle concentration on velocity, temperature, and concentration profiles are examined. The interaction of these parameters shows significant variations in hemodynamic parameters, which may hold relevance for medical applications involving catheters. The following sections present the results in relation to the figures provided, highlighting trends and comparing the effects of different governing parameters on velocity, temperature, and concentration.
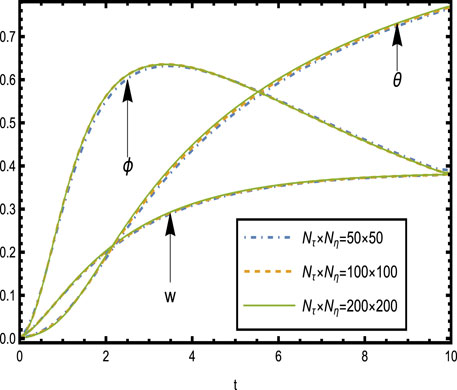
4.1 Grid independence test
The grid independence test was carried out to ensure that the solution was not dependent on the mesh size used in the numerical solution, guaranteeing credible and ensuring computational efficiency for the velocity, temperature, and concentration profiles without sacrificing accuracy. The model was solved by using grids with dimensions of
4.2 Velocity
Figures 3, 4 show the behavior of the electric potential
Figure 3. Potential electricity
The velocity profiles for the hybrid nanofluid are shown in Figures 5–9, illustrating the complex interaction of various physical parameters. Figure 5 shows the velocity distribution across the artery for different values of wall zeta potential
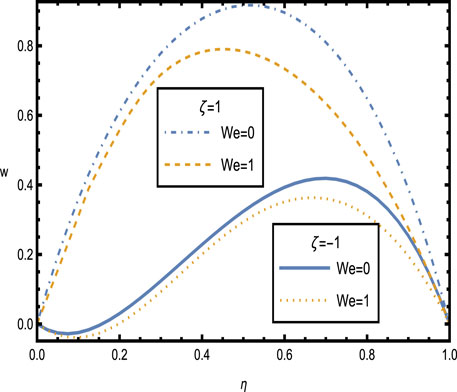
Figure 5. Velocity distributions
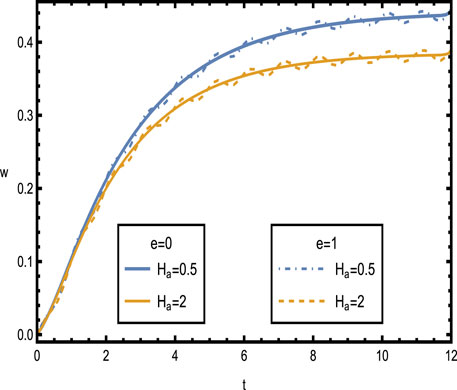
Figure 6. Velocity distributions
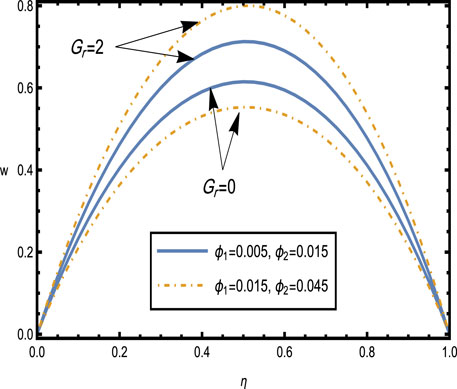
Figure 7. Velocity distributions
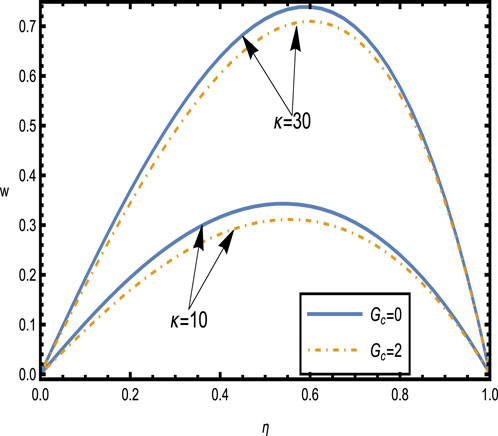
Figure 8. Velocity distributions
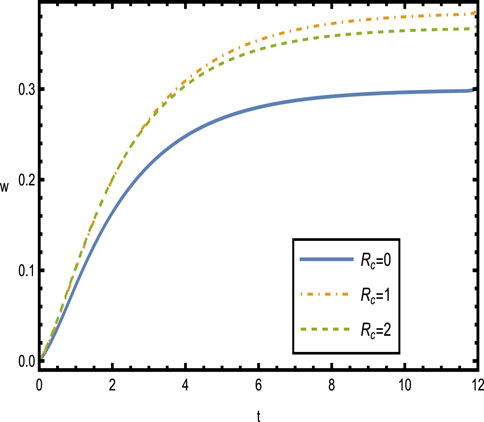
Figure 6 shows the velocity distributions over time
Figure 7 shows the velocity distributions for different values of the Grashof number
Figure 8 shows the velocity distributions for different values of the electroosmotic parameter
Figure 9 presents the velocity distributions over time
4.3 Heat and concentration distribution
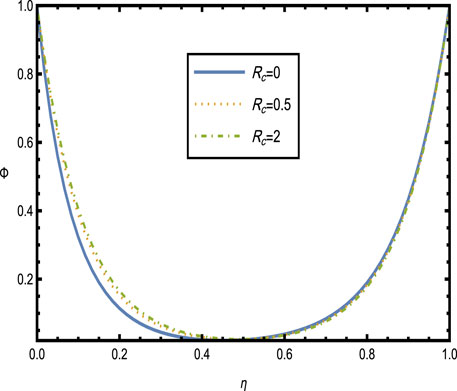
Figure 10 shows the distribution of temperature and concentration for different values of the curvature parameter
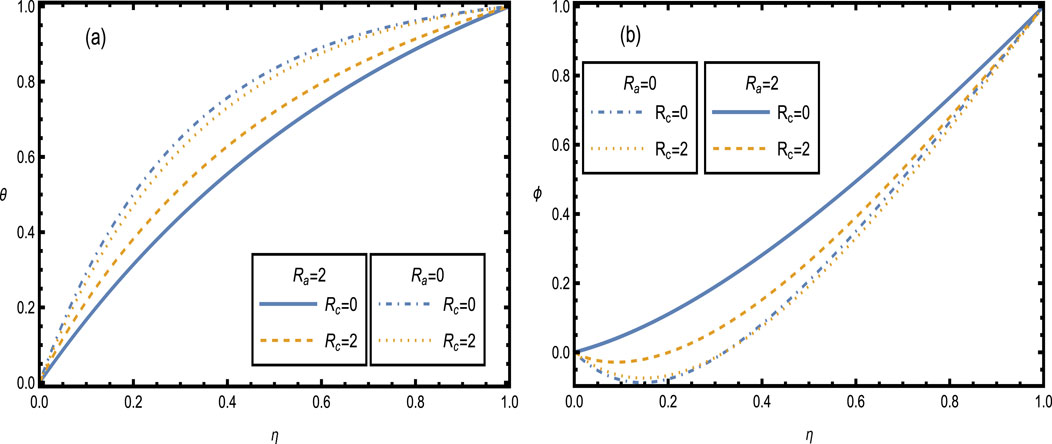
Figure 10. Heat and concentration distributions [Panels (A, B)] for different values of the curvature parameter
Figure 11 shows the heat and concentration distributions for different values of the thermophoresis parameter
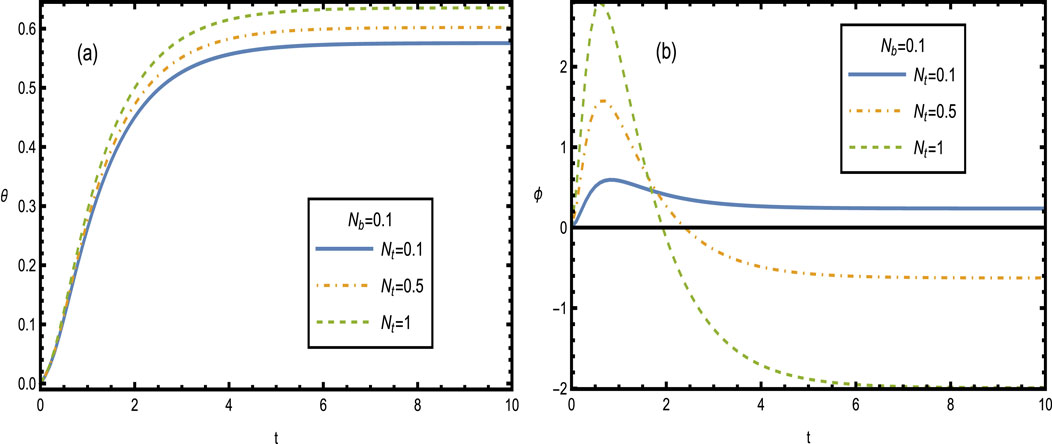
Figure 11. Heat and concentration distributions [Panels (A, B)] for different values of the thermophoresis parameter
Figure 12 shows the heat and concentration distributions for different values of the Brownian motion parameter
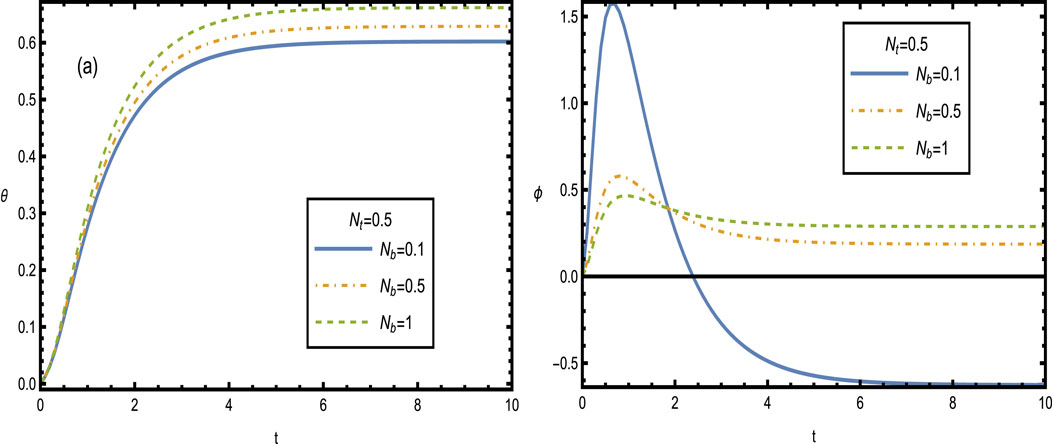
Figure 12. Heat and concentration distributions [Panels (A, B)] for different values of the Brownian motion parameter
Figure 13 shows the impact of the Schmidt number
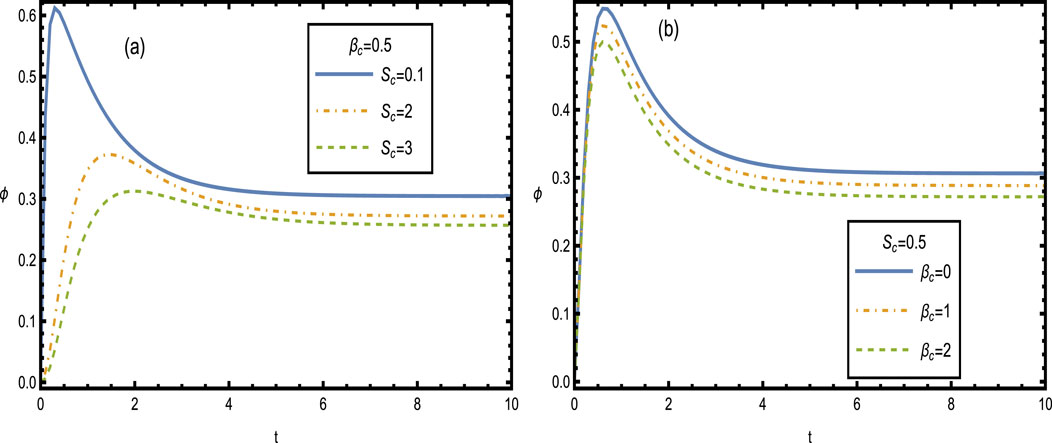
Figure 13. Concentration distributions
5 Conclusion
The flow of hybrid nanofluid through a stenosed, curved, and catheterized artery was treated as unsteady electroosmotic pulsatile flow using the Carreau non-Newtonian model. The characteristics of the velocity, temperature, and concentration distributions were analyzed for various representative physical parameters. The main findings are as follows.
• The results of the dimensionless potential distribution shown in Figure 2B match the results obtained by Wang et al. (2020).
• Numerical analysis of the hybrid nanofluid flow within a stenosed, curved, and catheterized artery showed significant influences of electroosmotic forces, curvature, and pulsatility on velocity, temperature, and concentration profiles.
• Increases in the electroosmotic and Weissenberg parameters substantially accelerated fluid velocity by reducing viscous drag while improving mass transport.
• The curvature parameter and radiation effects played a crucial role in creating asymmetric heat and concentration distributions, optimizing thermal and mass transport.
• The thermophoresis parameter influenced temperature and concentration distributions by promoting uniformity, which is beneficial in biomedical applications for efficient mass diffusion.
• The fluctuating patterns imply that the concentration distribution is extremely responsive to the parameter of Brownian motion. In procedures requiring exact concentration control, such as chemical reactions or material creation, this might be extremely important.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
ME: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. AA: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. YA: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. SA: conceptualization, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Abdullah Alsharif acknowledges Taif University, Saudi Arabia, for supporting this work through the project number TU-DSPP-2024-185. Sara I. Abdelsalam acknowledges Fundación Mujeres por África for supporting this work through the fellowship awarded to her.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdal, S., Mariam, A., Ali, B., Younas, S., Ali, L., and Habib, D. (2021). Implications of bioconvection and activation energy on Reiner-Rivlin nanofluid transportation over a disk in rotation with partial slip. Chin. J. Phys. 73, 672–683. doi:10.1016/j.cjph.2021.07.022
Akbar, N. S., and Nadeem, S. (2014). Carreau fluid model for blood flow through atapered artery with a stenosis. Ain Shams Eng. J. 5, 1307–1316. doi:10.1016/j.asej.2014.05.010
Akhtar, S., McCash, L. B., Nadeem, S., Saleem, S., and Issakhov, A. (2021). Mechanics of non-Newtonian blood flow in an artery having multiple stenosis and electroosmotic effects. Sci. Prog. 104 (3), 368504211031693–15. doi:10.1177/00368504211031693
Ali, B., Siddique, I., Ahmadian, A., Senu, N., Ali, L., and Haider, A. (2022). Significance of Lorentz and Coriolis forces on dynamics of water based silver tiny particles via finite element simulation. Ain Shams Eng. J. 13 (2), 101572. doi:10.1016/j.asej.2021.08.014
Ali, L., Liu, X., and Ali, B. (2020). Finite element analysis of variable viscosity impact on MHD flow and heat transfer of nanofluid using the cattaneo-christov model. Coatings 10 (4), 395. doi:10.3390/coatings10040395
Apostolidis, A. J., and Beris, A. N. (2014). Modeling of the blood rheology in steady-state shear flows. J. Rheology 58 (3), 607–633. doi:10.1122/1.4866296
Basha, H. T., Rajagopal, K., Ahammad, N. A., Sathish, S., and Gunakala, S. R. (2022). Finite difference computation of Au-Cu/Magneto-bio-hybrid nanofluid flow in an inclined uneven stenosis artery. Complexity 2022 (1), 2078372–18. doi:10.1155/2022/2078372
Berger, S. A., Talbot, L., and Yao, L. S. (1983). Flow in curved pipes. Annu. Rev. Fluid Mech. 15 (1), 461–512. doi:10.1146/annurev.fl.15.010183.002333
Bhatti, M. M., Abbas, M. A., and Muhammad, S. (2024). “Optimizing fluid flow efficiency: third-grade hybrid nanofluid flow with electro-magneto-hydrodynamics in confined vertical spaces,” in Nanofluids (Elsevier), 243–275. doi:10.1016/b978-0-443-13625-2.00012-7
Chakravarty, S., and Mandal, P. (1996). A nonlinear two dimensional model of blood flow in an overlapping arterial stenosis subjected to body acceleration. Math. Comput. Model. 24 (1), 43–58. doi:10.1016/0895-7177(96)00079-9
Cheng, C. P., Wilson, N. M., Hallett, R. L., Herfkens, R. J., and Taylor, C. A. (2006). In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion. J. Vasc. Interventional Radiology 17 (6), 979–987. doi:10.1097/01.rvi.0000220367.62137.e8
Das, S., Barman, B., Jana, R. N., and Makinde, O. D. (2021). Hall and ion slip currents impact on electromagnetic blood flow conveying hybrid nanoparticles through an endoscope with peristaltic waves. Bio Nanosci. 11, 770–792. doi:10.1007/s12668-021-00873-y
El-Dabe, N. T. M., and Mostapha, D. R. (2020). Hall current and Joule heating effects on peristaltic flow of a Sisko fluid with mild stenosis through a porous medium in a tapered artery with slip and convective boundary conditions. Sains Malays. 49 (5), 1175–1190. doi:10.17576/jsm-2020-4905-23
El Kot, M. A., and Abd Elmaboud, Y. (2021). Hybrid nanofluid flows through a vertical diseased coronary artery with heat transfer. J. Mech. Med. Biol. 21 (02), 2150012. doi:10.1142/s0219519421500123
El Kot, M. A., and Abd Elmaboud, Y. (2023). Model of LDL-C concentration of blood flow through a vertical porous microchannel with multiple stenoses: computational simulation. J. Taibah Univ. Sci. 17 (1), 2176194. doi:10.1080/16583655.2023.2176194
El Kot, M. A., and Abd Elmaboud, Y. (2024). Numerical simulation of electroosmotic sutterby hybrid nanofluid flowing through an irregularly mild stenotic artery with an aneurysm. Arabian J. Sci. Eng. 49, 2483–2498. doi:10.1007/s13369-023-08257-y
El-Masry, Y. A. S., Abd Elmaboud, Y., and Abdel-Sattar, M. A. (2020). Direct current/alternating current magnetohydrodynamic micropump of a hybrid nanofluid through a vertical annulus with heat transfer. J. Therm. Sci. Eng. Appl. 12 (4). doi:10.1115/1.4046058
Gijsen, F. J. H., van de Vosse, F. N., and Janssen, J. D. (1999). The influence of the non-Newtonian properties of blood on the flow in large arteries: steady flow in a carotid bifurcation model. J. Biomechanics 32 (6), 601–608. doi:10.1016/S0021-9290(99)00015-9
Haghighi, A. R., Kabdool, A. A., Asl, M. S., and Kiyatsatfar, M. C. (2006). Numerical investigation of pulsatile blood flow in stenosed artery. Int. J. Appl. Comput. Math. 2, 649–662. doi:10.1007/s40819-015-0084-0
Hassan, M., Chunwei, Z., Firdous, A., and Bhatti, M. M. (2024). Viscoelastic fluid flow on variable thickness sheets using a three-element viscous model. Int. J. Model. Simul., 1–12. doi:10.1080/02286203.2024.2338583
Huminic, G., and Huminic, A. (2018). Hybrid nanofluids for heat transfer applications - a state-of-the-art review. Int. J. Heat Mass Transf. 125, 82–103. doi:10.1016/j.ijheatmasstransfer.2018.04.059
Huo, Y., and Kassab, G. S. (2006). Pulsatile blood flow in the entire coronary arterial tree: theory and experiment. Am. J. Physiology-Heart Circulatory Physiology 302 (6), H1074–H1087. doi:10.1152/ajpheart.00200.2006
Johnston, B. M., Johnston, P. R., Corney, S., and Kilpatrick, D. (2004). Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J. Biomechanics 37 (5), 709–720. doi:10.1016/j.jbiomech.2003.09.016
LaDisa, J. F., Olson, L. E., Molthen, R. C., Hettrick, D. A., Pratt, P. F., Hardel, M. D., et al. (2005). Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am. J. Physiology-Heart Circulatory Physiology 288 (5), H2465–H2475. doi:10.1152/ajpheart.01107.2004
Long, Q., Xu, X. Y., Ramnarine, K. V., and Hoskins, P. (2001). Numerical investigation of physiologically realistic pulsatile flow through arterial stenosis. J. Biomechanics 34 (10), 1229–1242. doi:10.1016/s0021-9290(01)00100-2
Morris, P. D., Narracott, A., von Tengg-Kobligk, H., Silva Soto, D. A., Hsiao, S., Lungu, A., et al. (2016). Computational fluid dynamics modelling in cardiovascular medicine. Heart 102 (1), 18–28. doi:10.1136/heartjnl-2015-308044
Pal, D., and Mondal, H. (2011). Effects of Soret, Dufour, chemical reaction, and thermal radiation on MHD non-Darcy unsteady mixed convective heat and mass transfer over a stretching sheet. Commun. Nonlinear Sci. Numer. Simul. 16 (4), 1942–1958. doi:10.1016/j.cnsns.2010.08.033
Perktold, K., Resch, M., and Peter, R. O. (1991). Three-dimensional numerical analysis of pulsatile flow and wall shear stress in the carotid artery bifurcation. J. Biomechanics 24 (6), 409–420. doi:10.1016/0021-9290(91)90029-M
Pincombe, B., and Mazumdar, J. (1977). The effects of post-stenotic dilatations on the flow of a blood analogue through stenosed coronary arteries. Math. Comput. Model. 25 (6), 57–70. doi:10.1016/S0895-7177(97)00039-3
Quarteroni, A., Tuveri, M., and Veneziani, A. (2000). Computational vascular fluid dynamics: problems, models and methods. Comput. Vis. Sci. 2 (4), 163–197. doi:10.1007/s007910050039
Ramanamurthy, J. V., Prasad, K. M., and Narla, V. K. (2013). Unsteady peristaltic transport in curved channels. Phys. Fluids 25, 1903–1909. doi:10.1063/1.4821355
Rana, J., and Liao, S. (2019). A general analytical approach to study solute dispersion in non-Newtonian fluid flow. Eur. J. Mech. - B/Fluids 77, 183–200. doi:10.1016/j.euromechflu.2019.04.013
Sarkar, J., Ghosh, P., and Adil, A. (2015). A review on hybrid nanofluids: recent research, development and applications. Renew. Sustain. Energy Rev. 43, 164–177. doi:10.1016/j.rser.2014.11.023
Sundar, L. S., Farooky, Md. H., Sarada, S. N., and Singh, M. K. (2013). Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int. Commun. Heat Mass Transf. 41, 41–46. doi:10.1016/j.icheatmasstransfer.2012.11.004
Sundar, L. S., Singh, M. K., and Sousa, A. C. M. (2014). Enhanced heat transfer and friction factor of MWCNTF e3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 52, 73–83. doi:10.1016/j.icheatmasstransfer.2014.01.012
Tawade, J. V., Guled, C. N., Noeiaghdam, S., Fernandez-Gamiz, U., Govindan, V., and Balamuralitharan, S. (2022). Effects of thermophoresis and Brownian motion for thermal and chemically reacting Casson nanofluid flow over a linearly stretching sheet. Results Eng. 15, 100448. doi:10.1016/j.rineng.2022.100448
Tzirtzilakis, E. E. (2005). A mathematical model for blood flow in magnetic field. Phys. Fluids 17 (7), 077103. doi:10.1063/1.1978807
Wajihah, S. A., and Sankar, D. S. (2021). Effects of porosity in four-layered non-linear blood rheology in constricted narrow arteries with clinical applications. Comput. Methods Programs Biomed. 199, 105907. doi:10.1016/j.cmpb.2020.105907
Wang, X., Cheng, C., Wang, S., and Liu, S. (2009). Electroosmotic pumps and their applications in microfluidic systems. Microfluid. Nanofluidics 6 (2), 145–162. doi:10.1007/s10404-008-0399-9
Wang, X., Jiang, Y., Qiao, Y., Xu, H., and Qi, H. (2020). Numerical study of electroosmotic slip flow of fractional Oldroyd-B fluids at high zeta potentials. ELECTROPHORESIS 41 (10-11), 769–777. doi:10.1002/elps.201900370
Xu, F., Lu, T. J., Seffen, K. A., and Ng, E. (2009). Mathematical modeling of skin bioheat transfer. Appl. Mech. Rev. 62 (5). doi:10.1115/1.3124646
Young, D. F. (1968). Effect of a time-dependent stenosis on flow through a tube. J. Eng. Industrial Trans. ASME 90 (2), 248–254. doi:10.1115/1.3604621
Keywords: hybrid non-Newtonian nanofluid, electroosmotic flow, pulsatile flow, curved stenosed artery, finite-difference technique, cardiovascular treatment
Citation: El Kot MA, Alsharif AM, Abd Elmaboud Y and Abdelsalam SI (2024) Harnessing electroosmotic hybrid nanofluid dynamics in curved arteries: insights into biomedical flow enhancement. Front. Nanotechnol. 6:1520183. doi: 10.3389/fnano.2024.1520183
Received: 31 October 2024; Accepted: 29 November 2024;
Published: 23 December 2024.
Edited by:
Anwar Shahid, Quanzhou Institute of Information Engineering, ChinaReviewed by:
M. M. Bhatti, North West University, South AfricaBagh Ali, Harbin Institute of Technology, China
Copyright © 2024 El Kot, Alsharif, Abd Elmaboud and Abdelsalam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara I. Abdelsalam, c2FyYS5hYmRlbHNhbGFtQGJ1ZS5lZHUuZWc=
 M. A. El Kot1,2
M. A. El Kot1,2 Sara I. Abdelsalam
Sara I. Abdelsalam