- 1Department of Orthopaedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Class 6 of Year 2021 Clinical Medicine, School of Medicine, Shenzhen University, Shenzhen, Guangdong Province, China
Tanshinone IIA serves as a coenzyme for certain biochemical reactions, exhibiting various pharmacological effects in the treatment of neurological diseases including spinal cord injury (SCI), however, its working mechanism in the treatment of SCI is not clear. Based on previous research, we believe that tanshinone IIA promotes the survival and repair of nerves after spinal cord injury through its pharmacological effects such as anti-inflammatory, antioxidant, and prevention of cellular apoptosis in the spinal cord.
1. Introduction
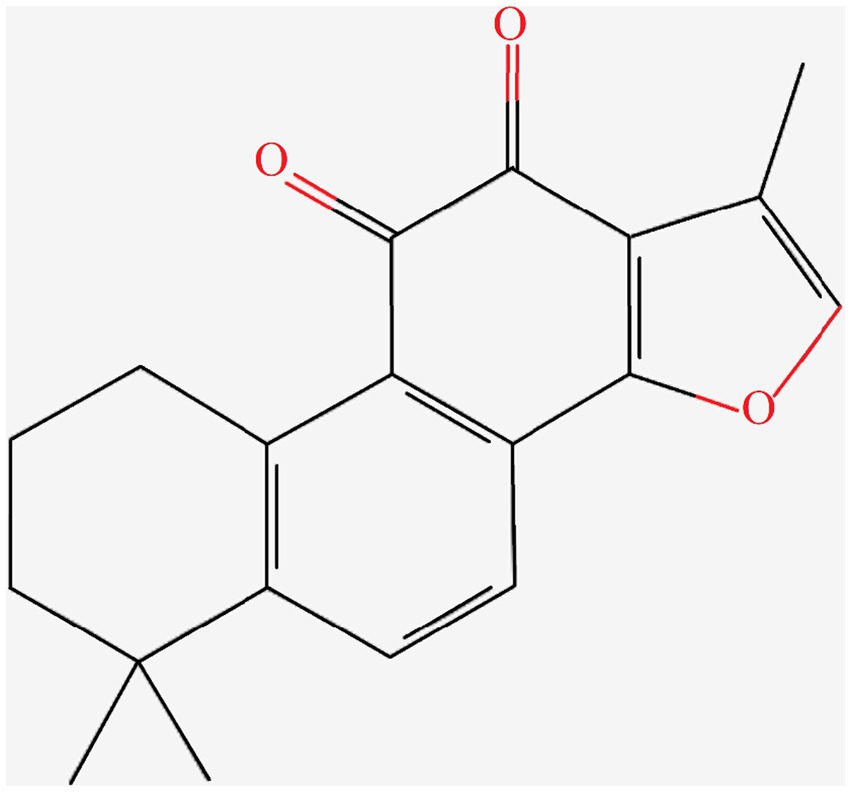
Danshen is a perennial herbaceous plant in the family Lamiaceae and the genus Salvia. Tanshinone IIA is the representative lipophilic component of Danshen, which is mainly distributed in the root of the plant. Tanshinone IIA is a cherry red needle-shaped crystal with a molecular weight of 294.34. It is insoluble or slightly soluble in water, and soluble in organic solvents such as dimethyl sulfoxide, ethanol, acetone, ether, and benzene. Supercritical CO2 extraction is used to extract tanshinone IIA from Danshen, with the extraction pressure of 25 MPa, extraction temperature of 40°C, extraction time of 2 h, and ethanol flow rate of 1.0 mL/min. The chemical structure of tanshinone IIA was obtained through searching the PubChem Compound database1 (Figure 1).
Tanshinone IIA has proven pharmacological effects on cardiovascular diseases, anti-tumor and anti-inflammatory effects, improvement of organ fibrosis as well as neuroprotection. Based on the previous literature research, we hypothesize that, tanshinone IIA promotes the survival and repair of nerves after spinal cord injury through following pharmacological effects:
2. Anti-inflammatory effect
Inflammation reaction is the main reason for secondary tissue injury and cellular apoptosis after spinal cord injury. There are studies confirming (Zhang et al., 2010; Yu et al., 2014) that tanshinone IIA has a significant anti-inflammatory effect. It can regulate the production of inflammatory factors IL-6, IL-10, IL-1β, and TNF-α, and weaken the activation of white blood cells. Zhou Yan et al. found that tanshinone IIA can exert anti-inflammatory effects in a mouse model of experimental colitis induced by sodium polysaccharide sulfate by reducing the activity of neutrophils and levels of inflammatory factors (Yan et al., 2017). It can also inhibit the activity of myeloperoxidase and adrenaline in neutrophils, thereby inhibiting joint swelling in mice with gouty arthritis (Xu et al., 2022). Huang et al. (2015) studied the feasibility of tanshinone IIA alleviating cardiac dysfunction, and found that pretreatment with tanshinone IIA significantly attenuates cardiac dysfunction by inhibiting inflammation. Tanshinone IIA was found to alleviate the upregulation of TNF-α and IL-1β induced by LPS, downregulate NADPH oxidase, and reduce phosphorylation levels of extracellular signal-regulated kinase 1/2 (ERK1/2) and mitogen-activated protein kinase (MAPK).
In in-vitro studies, tanshinone IIA was reported to inhibit the proliferation of RAW264.7 cells and the production of phospholipase A2, and promote the release of interleukin-10 in RAW264.7 cells, while reducing the production of interleukin-6 (IL-6) and its mRNA expression (Bzour et al., 2011; Fan et al., 2016). In addition, tanshinone IIA can inhibit mRNA expression of TLR4 and TNF-α in EA.hy926 cells stimulated by LPS (Jia et al., 2011), exerting its anti-inflammatory activity. Tang et al. found that tanshinone IIA can inhibit the production of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) and dose-dependently inhibit the adhesion of neutrophils to brain microvascular endothelial cells (BMVECs) induced by TNF-α. Tanshinone IIA can reduce the expression of macrophage migration inhibitory factor (MIF) and inflammatory factors (MIF), TNF-α and interleukin (IL)-6 in the brain of rats with cerebral ischemia/reperfusion, thereby alleviating the effects of cerebral ischemia/reperfusion (Chen et al., 2012). Other studies have confirmed that tanshinone IIA can reduce the release of radiation-induced pro-inflammatory cytokines and NF-κBp65 nuclear translocation, and its mechanism of action is to exert its anti-inflammatory properties by inhibiting the transcription of pro-inflammatory cytokine genes associated with the NF-κB signaling pathway, thereby slowing down the progression of Alzheimer’s disease in rats (Jiang et al., 2014). Jiang et al. further found that tanshinone IIA can reduce the production of pro-inflammatory cytokines induced by LPS in RAW264.7 cells and inhibit the phosphorylation of IκB-α in a dose-dependent manner. It can also inhibit the activation of NIK-IKK and MAPKs (p38, ERK1/2, JNK) signaling pathways, thereby inhibiting LPS-induced IκB-α degradation and NF-κB activation to exert anti-inflammatory activity (Jang et al., 2006). Tanshinone IIA was also reported to protect the human blood–brain barrier model from leukocyte-associated hypoxia-reoxygenation injury (Zhang et al., 2010).
Therefore, it is plausible to assume that tanshinone IIA alleviates secondary tissue injury and cellular apoptosis after spinal cord injury by inhibiting inflammatory reaction through TLR4- p38 MAPK and Erk–Jnk–p38-MAPK signaling pathways (Lanna et al., 2017).
3. Antioxidation effect
The oxidation stress is also an important cause of further tissue damage after spinal cord injury. It has been confirmed that tanshinone IIA inhibits the generation of DNA adducts and reduces cell toxicity by clearing lipid free radicals and interrupting the chain reaction of lipid peroxidation. It effectively inhibits the interaction between lipid peroxidation products and DNA inside cells, playing a protective role on DNA. Tanshinone IIA can enhance the activity of glutathione peroxidase in brain hypoxic lesions, reduce the content of malondialdehyde, and effectively alleviate oxidative stress (Niu et al., 2000; Tang et al., 2012). Tanshinone IIA was also reported to reduce the concentration of oxidized low-density lipoprotein (OxLDL) in plasma and decrease the generation of superoxide anions and malondialdehyde (MDA) (Tang et al., 2007). In a hypertensive rat model, tanshinone IIA inhibited the activity of NAD(P)H oxidase and the production of reactive oxygen species, exerting antioxidant effects and improving cardiac function (Wang et al., 2011). In both the hydrogen peroxide (H2O2)-induced J774 macrophage injury model and rat focal cerebral ischemia–reperfusion models, tanshinone IIA significantly increased the activity of glutathione peroxidase (Yu et al., 2008). Research by Wang et al. found that tanshinone IIA can dose-dependently reduce the production of MDA and LDH induced by H2O2 in human umbilical vein endothelial cells CRL21730, confirming the protective effect of tanshinone IIA from oxidative damage (Weirong and Rong, 2006). Chen et al. confirmed that tanshinone IIA can dose-dependently alleviate apoptosis of human umbilical vein endothelial cells (ECV304) induced by H2O2, reduce MDA production, increase the activity of superoxide dismutase (SOD), and inhibit the expression of endothelin-1 (ET-1) mRNA. It also increases the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) mRNA, indicating that tanshinone IIA has a significant protective effect on H2O2-induced injury in human umbilical vein endothelial cells (Haiming, 2008).
In animal studies, antioxidation effect of tanshinone IIA was used in the treatment of cerebral ischemia–reperfusion injuries 24 h after the onset of the condition. A study by Mingyan et al. (2004) found that tanshinone IIA can reduce the early accumulation of calcium ions ([Ca2+]) after ischemic hypoxic injury. In an experiment conducted by Longshan and Tao (2004), it was discovered that treatment with tanshinone IIA (25 mg·kg-1) significantly reduced the area of cerebral infarction in rats with cerebral ischemia. Tanshinone IIA also counteracted the decrease in superoxide dismutase (SOD) activity and the increase in malondialdehyde (MDA) content induced by cerebral ischemia–reperfusion. In the meanwhile, the molecular pathways for the antioxidation effect of tanshinone IIA are still needed to be further explored.
4. Inhibiting cellular apoptosis
Tanshinone IIA can protect neurons from amyloid β protein-induced cytotoxicity by activating cell apoptosis-related pathways (Qian et al., 2012). Tanshinone IIA can enhance the activity of adenosine triphosphatase and protein disulfide isomerase in neurons, improve the balance of energy metabolism, and maintain cellular homeostasis, thus protecting and repairing neurons (Wen et al., 2012). It can also increase the expression of Bcl-2 in ischemic spinal cord, reducing the damage caused by ischemia reperfusion through anti-apoptotic mechanisms (Chen et al., 2012).
In the case of cerebral ischemia, the proliferation and activation of astrocytes lead to the formation of glial scars around necrotic tissue, which can disrupt the normal growth of neuronal axons. Zhou et al. (2015) found that tanshinone IIA can inhibit the activation of astrocytes caused by cerebral ischemia, reduce the formation of glial scars, and thus play a neuroprotective role. Li et al. (2013) found that tanshinone IIA can effectively reduce the expression levels of NF-κB and IκB proteins in the brain tissue of a rat model of cerebral ischemia–reperfusion. Its possible mechanism of action is that tanshinone IIA can inhibit the positive feedback between NF-κB and IκB and reduce the loss of neurons. This is of great significance for further studying the neural protective effect and mechanism of tanshinone IIA administration. Yanhui et al. (2014) used the method of ligation of the abdominal aorta to make a rabbit spinal cord ischemia–reperfusion model. After random grouping, the spinal cord was taken at various time intervals, and venous blood was extracted to detect the glutamate content in the spinal cord and serum. It was found that tanshinone can reduce the local glutamate content in the spinal cord during reperfusion, thereby protecting spinal cord neurons and providing protection against spinal cord ischemia–reperfusion injury in rabbits (Shen et al., 2011).
Based on the above characteristics, tanshinone IIA has been applied to repair neural system injuries. Chen and Shasha (2018) used tanshinone IIA to hippocampal neurons in vitro and found that it can protect against radiation damage to the nervous system by reducing cell apoptosis, improving tissue hypoxia conditions, and inducing cell autophagy. In mice with radiation-induced brain damage, tanshinone IIA can inhibit the activation of the ATP-P2X7R axis, suppress the occurrence of inflammatory reactions and accumulation of free radicals, improve spatial learning and memory abilities, and reduce brain edema, thus exerting a neuroprotective effect (Youli et al., 2017). Yin et al. (2012) and Zhong et al. (2021) found that in mice with acute spinal cord injury, tanshinone IIA can decrease the expression of RhoA and ROCKII proteins, inhibit the phosphorylation of myosin light chain, and facilitate the recovery of nerve cells and the regeneration of neuronal axons.
5. Conclusion
Based on the results of its clinical effects, animal and in vitro experiments, we believe that tanshinone IIA promotes the survival and repair of nerves after spinal cord injury through its anti-inflammatory, antioxidant, anti-apoptosis effects (Figure 2).

Figure 2. Tanshinone IIA may promote the survival and repair of nerves after spinal cord injury through its anti-inflammatory, antioxidant, anti-apoptosis effects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZJ: Conceptualization, Investigation, Writing – original draft. TW: Funding acquisition, Supervision, Validation, Writing – review & editing. YZ: Investigation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The current research is supported by the National Natural Scientific Foundation of China (nos: 82074452, 82260252, and 81860235).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AM declared a past co-authorship with the author TW.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Bzour, J., Oran, S., Khaleel, M., Mashallah, S., and Bustanji, Y. (2011). Inhibitory effects of methanolic extract of Salvia fruticosa Mill. on pro-inflammatory cytokines production in RAW 264.7 in vitro cellular model and in Balb/c mice in vivo animal model. J. Med. Plants Res. 5, 6662–6671. doi: 10.5897/JMPR11.586
Chen, R., and Shasha, D. (2018). Tanshinone IIA protects hippocampal neuronal cells from radiation injury. Guangdong Med. J. 39, 807–810. doi: 10.3969/j.issn.1001-9448.2018.06.002
Chen, Y., Wu, X., Yu, S., Fauzee, N. J. S., Wu, J., Li, L., et al. (2012). Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/ reperfusion injury via anti-apoptotic pathway in rats. Biol. Pharm. Bull. 35, 164–170. doi: 10.1248/bpb.35.164
Chen, Y., Wu, X., Yu, S., Lin, X., Wu, J., Li, L., et al. (2012). Neuroprotection of tanshinone IIA against cerebral ischemia/reperfusion injury through inhibition of macrophage migration inhibitory factor in rats[J]. PLoS One 7:e40165. doi: 10.1371/journal.pone.0040165
Fan, G., Jiang, X., Wu, X., Fordjour, P. A., Miao, L., Zhang, H., et al. (2016). Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264. 7 macrophages via miRNAs and TLR4–NF-κB pathway[J]. Inflammation 39, 375–384. doi: 10.1007/s10753-015-0259-1
Haiming, C. (2008). Ye Pan: Tanshinone IIA protects vascular endothelial cells from oxidative stress damage. J. Chin. Med. Mat. 31, 569–572.
Huang, L., Zheng, M., Zhou, Y., Zhu, J., Zhu, M., Zhao, F., et al. (2015). Tanshinone IIA attenuates cardiac dysfunction in endotoxin-induced septic mice via inhibition of NADPH oxidase 2-related signaling pathway[J]. Int. Immunopharmacol. 28, 444–449. doi: 10.1016/j.intimp.2015.07.004
Jang, S. I., Kim, H. J., and Kim, Y. J. (2006). Tanshinone II a inhibits LPS -induced NF-kappaB activation in RAW 264.7 cells:possible involvement of the NIK -IKK,ERK1/2,p38 and JNKpathways[J]. Eur. J. Pharmacol. 542, 1–7. doi: 10.1016/j.ejphar.2006.04.044
Jia, L. Q., Feng, J. Y., Yang, G. L., Chen, W. N., and Chen, Y. (2011). Effect of tanshinone II a on TLR4 and TNF-α of endothelial cells induced by LPS[J]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 27, 733–735.
Jiang, P., Li, C., Xiang, Z., et al. (2014). Tanshinone IIA reduces the risk of Alzheimer's disease by inhibiting iNOS, MMP-2 and NF-κBp65 transcription and translation in the temporal lobes of rat models of Alzheimer's disease[J]. Mol. Med. Rep. 10, 689–694. doi: 10.3892/mmr.2014.2254
Lanna, A., Gomes, D. C., Muller-Durovic, B., McDonnell, T., Escors, D., Gilroy, D. W., et al. (2017). A sestrin-dependent Erk–Jnk–p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 18, 354–363. doi: 10.1038/ni.3665
Li, Z., Yanping, L., and Fang, W. (2013). The effect of Tanshinone IIa on the activity of NF-κB and IκB in the brain tissue of I/R rats. J. Chin. Med. Mat. 36, 1136–1139.
Longshan, Y., and Tao, X. (2004). Chen Feng: protective effect of Tanshinone IIA against focal cerebral ischemia reperfusion injury in rats. J. China Pharm. Univ. 35:267.
Mingyan, H., Ping, Y., and Taojin, K. (2004). Effects of Danshentong IIa on the brain intracellular free calcium in neonatal hypoxia-ischemic rats. J. Clin. Res. 21:876.
Niu, X. L., Ichimori, K., and Yang, X. (2000). Tanshinone IIA inhibits low density lipoprotein oxidation in vitro. Free Radic. Res. 33, 305–312. doi: 10.1080/10715760000301471
Qian, Y.-H., Xiao, Q., and Xu, J. (2012). The protective effects of tanshinone IIA on beta-amyloid protein (1-42)-induced cytotoxicity via activation of the Bcl-xL pathway in neuron. Brain Res. Bull. 88, 354–358. doi: 10.1016/j.brainresbull.2012.03.007
Shen, J. L., Chen, Y. S., Lin, J. Y., Tien, Y. C., Peng, W. H., Kuo, C. H., et al. (2011). Neuron regeneration and proliferation effects of danshen and tanshinone lla. Evi. Based Complem. Altern. Med. 2011:378907, 1–9. doi: 10.1155/2011/378907
Tang, Q., Han, R., Xiao, H., Shen, J., Luo, Q., and Li, J. (2012). Neuroprotective effects of tanshinone IIA and / or tetramethylpyrazine in cerebral ischemic injury in vivo and in vitro [J]. Brain Res. 1488, 81–91. doi: 10.1016/j.brainres.2012.09.034
Tang, F., Wu, X., Wang, T., and Wang, P. (2007). Tanshinone II a attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vasc. Pharmacol. 46, 427–438. doi: 10.1016/j.vph.2007.01.001
Wang, P., Wu, X., and Bao, Y. (2011). Tanshinone II a prevents cardiac remodeling through attenuating NAD (P)H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie 66, 517–524.
Weirong, W., and Rong, L. (2006). Peng Ning: Tanshinone IIA provides protective effects against hydrogen peroxide-induced damage to human vascular endothelial cells. J. Chin. Med. Mater. 29, 49–51.
Wen, P. Y., Yang, F. Z., Wang, F., Li, W. W., Zhou, L., Huang, L. H., et al. (2012). Study on regulation of tanshinone II(a) on GFAP and ATPase and PDI of cerebral ischemia reperfusion injury in rats. J. Chin. Med. Mater. 35, 1628–1632.
Xu, L., Liu, X., Zhang, Y., Jia, T., Li, L., du, Y., et al. (2022). Tanshinone IIA improves acute gouty arthritis in rats through regulating neutrophil activation and the NLRP3 Inflammasome[J]. Dis. Markers 2022, 1–12. doi: 10.1155/2022/5851412
Yan, Z., An, L., and Jun, Z. (2017). Tanshinone IIA protects against dextran sulfate sodium (DSS)-induced colitis by neutrophil modulation in mice. J. Toxicol. 31, 84–88.
Yanhui, Z., Linlin, L., and Linna, H. (2014). The protective effect of tanshinone on nerve cells in rabbits' spinal cord ischemia-reperfusion injury. J. Mudanjiang Med. Univ. 4, 71–73.
Yin, X., Yin, Y., Cao, F. L., Chen, Y. F., Peng, Y., Hou, W. G., et al. (2012). Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 7, 1–11. doi: 10.1371/journal.pone.0038381
Youli, S., Qingfeng, X., and Choayan, L. (2017). Protective effect of tan IIA on brain injury induced by radiation in mice and its molecular mechanism. Pharmacol. Clin. Chin. Mater. Med. 33, 66–70.
Yu, Q., Chen, H., Sheng, L., Liang, Y., and Li, Q. (2014). Sodium tanshinone IIA sulfonate prolongs the survival of skin allografts by inhibiting inflammatory cell infiltration and T cell proliferation[J]. Int. Immunopharmacol. 22, 277–284. doi: 10.1016/j.intimp.2014.07.002
Yu, L., Gary, E., and Renee, C. (2008). Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase[J]. Life Sci. 83, 557–562. doi: 10.1016/j.lfs.2008.08.003
Zhang, W., Feng, J., Zhou, R., Ye, L. Y., Liu, H. L., Peng, L., et al. (2010). Tanshinone IIA protects the human blood–brain barrier model from leukocyte-associated hypoxia-reoxygenation injury[J]. Eur. J. Pharmacol. 648, 146–152. doi: 10.1016/j.ejphar.2010.08.040
Zhong, C., Lin, Z., Ke, L., Shi, P., Li, S., Huang, L., et al. (2021). Recent research progress (2015-2021) and perspectives on the pharmacological effects and mechanisms of tanshinone IIA. Front. Pharmacol. 12:778847. doi: 10.3389/fphar.2021.778847
Keywords: spinal cord injury, axonal regeneration, tanshinone IIA, mechanism, hypothesis
Citation: Jia Z, Wen T and Zhang Y (2023) Possible mechanisms of treatment for spinal cord injury repair with tanshinone IIA. Front. Mol. Neurosci. 16:1277755. doi: 10.3389/fnmol.2023.1277755
Edited by:
Aikeremujiang Muheremu, Sixth Affiliated Hospital of Xinjiang Medical University, ChinaReviewed by:
Yuqing Sun, Beijing Jishuitan Hospital, ChinaJun Shang, Linfen People's Hospital, China
Copyright © 2023 Jia, Wen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianlin Wen, d2VudGlhbmxpbjIxQDE2My5jb20=; Yuning Zhang, bGlsbGlhbjAzMDQyOEAxNjMuY29t
 Zhiwei Jia
Zhiwei Jia Tianlin Wen1*
Tianlin Wen1*